Abstract
Associations between telomere length and cancer risk have been investigated in many epidemiological studies, but the results are controversial. These associations may be biased by reverse causation or confounded by environmental exposures. To avoid potential biases, we used Mendelian randomization method to evaluate whether TL is the causal risk factor for lung cancer. We conducted Mendelian randomization analysis in two published East Asian GWAS studies (7127 cases and 6818 controls). We used both weighted genetic risk score and inverse‐variance weighting method to estimate the relationship between TL and lung cancer risk. Nonlinear test also used to detect potential association trends. We observed that increased weight GRS was associated with increased risk of lung cancer (OR = 2.25, 95%CI: 1.81‐2.78, P = 1.18 × 10−13). In different subtypes, weight GRS was significantly associated with lung adenocarcinoma risk (OR = 2.69, 95% CI: 2.11‐3.42, P = 7.20 × 10−16); while lung squamous cell carcinoma showed a marginal association (OR = 1.45, 95% CI = 1.01‐2.10, P = .047). Nonlinear analysis suggested a log‐linear dose‐response relationship between increased weight GRS and lung cancer risk. Our results indicated that longer TL increases lung cancer risk. Those biological mechanisms changes caused by long TL may play an important role in lung carcinogenesis.
Keywords: Asian population, lung cancer, mendelian randomization, telomere length
Telomere length GRS indicated that longer TL increases LC risk. A log linear dose‐response relationship was observed between increased GRS and LC risk.
![]()
1. INTRODUCTION
Lung cancer is the most common malignant tumor worldwide, accounting for 11.6% of all diagnosed cancer and 18.4% of all cancer deaths in 2018.1 Consistent with world trends, lung cancer remains the most common cancer in Chinese population, as well as the leading cause of cancer‐related death.2 Tobacco smoking is the major risk factor of lung cancer, and approximately 90% cases can be attributed to smoking.3 Also, genetic factors play an important role in lung cancer carcinogenesis. In the past decade, genome‐wide association studies (GWAS) successfully identified lots of lung cancer susceptibility loci, such as CHRNA5‐CHRNA3‐CHRNB4 region of chromosome 15q25 and human leukocyte antigen region. Even so, at least 25% of lung cancer cases were never smokers and the heritability of lung cancer was also relatively low, nearly 18%.4, 5, 6, 7 The risk factors of lung cancer still need more exploration.
Telomeres, located at the end of each chromosome, are specialized DNA‐protein structures and play essential roles for life functions. Human telomeres consist of long tracts of double‐stranded TTAGGG repeats. During DNA replication, telomeres would prevent the ends of chromosomes shortening and help keep genome stability and integrity.8, 9 Previous studies show that telomere length (TL) may be a double‐edged sword. Short TL may lead to genetic instability, as well as cellular senescence and apoptosis.10 Long TL or telomerase activity up regulation may promote cell growth and proliferation.11, 12 Thus, telomeres are crucial in human carcinogenesis. A number of studies suggest that TL was associated with multiple cancer types, but associations are not consistent because of the dual role of telomeres in carcinogenesis. For example, many prospective studies measuring TL in peripheral leukocytes showed that lung cancer risk increased with longer telomeres.13, 14, 15, 16 However, a large prospective study based on Danish population found there was no association between TL and lung cancer.17
Because of the limitation of observational study, the true relationship between TL and cancer risk may be obscured by confounding factors, such as age at TL measurement. Mendelian randomization (MR), based on the random assortment of genetic variants during meiosis, is an effective method to test the causal effect in observational studies. MR uses instrumental variables to evaluate the relationship between the exposure and an outcome.18, 19 In MR analysis, using genetic variants associated with certain exposure or phenotype as instrumental variables can avoid potential confounding bias. Previous lung cancer MR studies also suggested increased lung cancer risk was associated with long leukocyte TL.20, 21 However, those studies were either in Western population or in East Asian never‐smoking women.
To elucidate causal effects for lung cancer risk, we conducted an MR method and selected genetic variants significantly associated with TL as instrumental variables to estimate the causal relationship between TL and lung cancer risk in a pooled East Asian population.
2. MATERIALS AND METHODS
2.1. Study subjects
The pool samples included two previous published lung cancer GWAS studies from East Asia. The details of subjects were described in the original studies.22, 23, 24 A total of 13 945 samples (7127 cases and 6818 controls) were enrolled from our previous Chinese population lung cancer GWAS study (NJMU, 5408 samples from China) and published GWAS from the Female Lung Cancer Consortium in Asia (FLCCA, 8537 samples from East Asia).22, 23, 24 There are 4773 lung adenocarcinoma cases and 1482 lung squamous cell carcinoma cases in pooled samples. The rest 872 lung cancer cases were classified in other histology types. Each study obtained informed consent from the participants and was approved by the respective Institutional Review Boards. The detailed information for all samples is shown in Table S1.
2.2. Genetic instrumental variables selection
We used TL‐related SNPs as MR instrumental variables. SNPs were selected from previously published TL GWAS studies, following these criteria: (a) reported SNP signals showed genome‐wide association significance level with TL (P ≤ 5×10−8); (b) minor allele frequency (MAF) for TL‐related SNPs more than 0.05 in East Asian population; (c) variants having low linkage disequilibrium (LD) between each SNP (r 2 < .5). MAF and LD information was calculated from the 1000 Genomes Project (Phase 3) ASN subjects. Finally, we chose nine SNPs identified in leukocyte TL GWAS and met selection criteria for further analysis.25, 26, 27 Based on previous studies, we obtained long TL allele as effect allele, as well as association estimate for the long allele (in terms of kb increase in TL per allele). Details for nine SNPs used in our studies are list in Table S2.
2.3. Quality control and genotype imputation
Quality control and genotype imputation for two GWAS studies have been fully discussed in previous articles.22, 23, 24 In brief, genotyping in NJMU data used Affymetrix Genome‐Wide Human SNP Array 6.0 chips. The FLCCA data was obtained from public database (the database of Genotypes and Phenotypes, Study Accession: phs000716.v1.p1) and genotyping was conducted in Illumina 610Q SNP microarray and Illumina 660W SNP microarray. For standing quality control procedures, we first used PLINK software (v1.90) to exclude low quality individuals and low quality SNPs. Samples with low call rates, extreme heterozygosity rates and familial relationships, as well as SNPs with low call rates, low MAF, and violating the Hardy‐Weinberg equilibrium, were all removed. The overlapped samples in FLCCA and NJMU GWAS data were excluded from the FLCCA GWAS samples. GWAS data imputation was performed by IMPUTE2 software (v2.3.2) using 1000 Genomes Project Phase 3 data as an imputation reference.
2.4. Statistical analysis
We applied two MR methods based on individual level and summary statistic level, respectively. Firstly, we used the nine selected TL‐associated SNPs to build genetic predicted leukocyte telomere length. We calculated weighted genetic risk scores (GRS) using the following formula:
Here, x represents the number of long alleles for the j th SNP in the i th subject (xij = 0, 1 or 2) and βj is the weight for the j th SNP. All weights were obtained from published TL GWAS studies (Table S2) and scaled to kb of TL per long allele to uniform weight scale. Finally, we used weighted GRS to predict individual telomere length, like an instrumental variable. We performed logistic regression to estimate the association between weighted GRS and lung cancer risk, adjusting for age, sex, pack‐years, first principal component, and different study. In addition to weighted GRS approach, we also used another summary data based MR method called inverse‐variance weighting (IVW) method to evaluate the association for TL and risk of lung cancer. This method has been fully described by Burgess et al28 and has been successfully used in many studies. In this study, we used the same nine SNPs’ summary statistics to estimate potential causal effects of TL. IVW method was conducted by “gtx” package (v0.0.8) in R software (v3.3.1). We also used aggregate test, which used log likelihood ratio test to compare a null model only including covariates with a model having all TL‐associated SNPs and all covariates, to calculate the total effect of all TL‐associated SNPs.
To better investigate the effect of TL on lung cancer risk, we categorized weighted GRS into 10 groups based on its decile distribution in all participants and tested association in each group to observe trends. What's more, we further used a restricted cubic spline analysis to examine whether there were potential nonlinear trends between TL and lung cancer risk.
2.5. Sensitivity analyses
For a causal interpretation of MR, instrumental variables need to meet several important assumptions. First, instrumental variables are associated with the exposure; Second, instrumental variables can affect the outcome only via the exposure; Third, instrumental variables are not associated with any confounders of the exposure‐outcome association.29 Violations of MR assumptions may lead to unreliable results. Since all the nine SNPs included in this analysis were significantly associated with leukocytes TL, which meet the first MR assumption. We further test if there is any violation of the rest assumptions. Under the second and third assumptions, TL‐related SNPs’ effect on TL should be proportional to their effect on lung cancer risk. We used “gtx” package pleiotropy test function to assess the second and third assumptions.
All statistical analyses were performed using PLINK (v1.90) and R (v3.3.1). Two‐sided P < .05 was considered statistically significant.
3. RESULTS
3.1. Association estimates for individual SNPs with LC
The associations between nine TL‐related SNPs and lung cancer risk in all participants was described in Table 1, suggesting that most of the TL‐related SNPs were not in observed significant association with lung cancer, except rs2736100 and rs10936599. Meanwhile, except rs2736100 and rs11125529, the rest seven SNPs did not show significant heterogeneity between two datasets (Table S3). Associations with P < .05 were found for lung adenocarcinoma (rs10936599, rs2736100), lung squamous cell carcinoma (rs2736100, rs7675998, rs755017). Aggregate test showed one or more TL‐related variants were in relation to lung cancer risk in aggregate (pooled P < 1×10−8). The results of NJMU and FLCCA data were similar with the overall results (Table S3).
Table 1.
Associations of telomere length‐associated variants and lung cancer risk
| SNP | Lung adenocarcinoma | Lung squamous cell carcinoma | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | P e | OR | 95%CI | P | OR | 95%CI | P | |
| rs10936599 | 1.12 | (1.06,1.19) | 2.42 × 10−5 | 1.05 | (0.96,1.14) | .300 | 1.10 | (1.04,1.15) | 2.51 × 10−4 |
| rs2736100 | 1.37 | (1.30,1.45) | 5.17 × 10−30 | 1.21 | (1.11,1.32) | 1.25 × 10−5 | 1.33 | (1.26,1.39) | 5.05 × 10−29 |
| rs7675998 | 1.06 | (0.98,1.14) | .126 | 1.13 | (1.01,1.27) | .034 | 1.07 | (1.00,1.14) | .052 |
| rs4387287 | 0.99 | (0.92,1.06) | .708 | 0.98 | (0.87,1.10) | .694 | 0.99 | (0.93,1.06) | .795 |
| rs8105767 | 1.05 | (0.99,1.11) | .131 | 0.97 | (0.89,1.06) | .523 | 1.02 | (0.97,1.08) | .385 |
| rs755017 | 0.99 | (0.94,1.05) | .809 | 0.89 | (0.82,0.97) | 8.44 × 10−3 | 0.97 | (0.93,1.02) | .297 |
| rs11125529 | 1.05 | (0.98,1.13) | .157 | 1.06 | (0.95,1.18) | .317 | 1.05 | (0.99,1.12) | .120 |
| rs3027234 | 1.07 | (0.93,1.23) | .368 | 1.04 | (0.83,1.30) | .729 | 1.05 | (0.92,1.18) | .482 |
| rs412658 | 1.03 | (0.97,1.09) | .367 | 0.99 | (0.91,1.08) | .809 | 1.03 | (0.97,1.08) | .331 |
| Aggregate testa | 3.43 × 10−29 | 7.79 × 10−5 | 1.51 × 10−27 | ||||||
| Genetic risk scoreb | 2.69 | (2.11,3.42) | 7.20 × 10−16 | 1.45 | (1.01,2.10) | .047 | 2.25 | (1.81,2.78) | 1.18 × 10−13 |
| MR(IVW)c | 2.82 | (2.21,3.61) | 1.32 × 10−16 | 1.51 | (1.03,2.22) | .037 | 2.37 | (1.90,2.96) | 1.85 × 10−14 |
| Heterogeneityd | 1.57 × 10−15 | 3.23 × 10−4 | 6.55 × 10−16 | ||||||
Aggregate test is a log likelihood ratio test comparing a model having all telomere length‐associated SNPs and covariates with a null model.
Genetic risk score ORs refer to a 1‐kb increase in telomere length.
Inverse‐variance weighted Mendelian randomization estimate for a 1‐kb increase in telomere length.
Pleiotropy test for significant heterogeneity across the nine SNP instruments used in the Mendelian randomization analysis.
P value was from logistic regression adjusting for age, sex, pack‐years, and first principal component.
3.2. MR estimates based on weight GRS
We then examined the relationship between the weighted GRS and lung cancer risk. Adjusting for potential confounders, we found that increased weighted GRS was associated with increased lung cancer risk (OR = 2.25, 95% CI: 1.81‐2.78, P = 1.18 × 10−13, Table 1). In subgroup analysis of lung cancer, weighted GRS was also significantly associated with lung adenocarcinoma (OR = 2.69, 95% CI: 2.11‐3.42, P = 7.20 × 10−16); while in lung squamous cell carcinoma, weighted GRS showed a marginal association (OR = 1.45, 95% CI = 1.01‐2.10, P = .047). Consistent results were also observed when analyzing two studies independently (Table S3). The decile of TL‐associated weight GRS result shows per decile increase in weighted GRS was significantly associated with increased risk of lung adenocarcinoma (OR = 1.05, 95% CI = 1.04‐1.07, P = 6.04 × 10−14), lung squamous cell carcinoma (OR = 1.02, 95% CI = 1.00‐1.04, P = .039), and combined lung cancer (OR = 1.04, 95% CI = 1.03‐1.06, P = 9.48 × 10−13). In lung adenocarcinoma type, compared with the first GRS decile, we found upper GRS deciles were in relation to increased lung cancer risk and we also observed a general rising trend. However, we did not observe any clear trend in lung squamous cell carcinoma (Figure 1).
Figure 1.
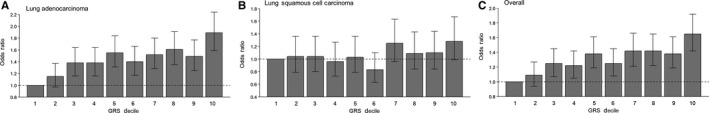
ORs for each telomere length‐associated GRS decile by lung adenocarcinoma (A), lung squamous cell carcinoma (B), and lung cancer overall (C). The lowest GRS decile is used as the reference of comparison
3.3. MR estimates based on summary data
The MR analysis based on summary data using the IVW method showed almost similar effect estimates with weighted GRS method (Table 1).The associations in NJMU GWAS and FLCCA GWAS were consistent with pooled participants (Table S3). Figure 2 showed all nine SNPs’ per long allele association with lung cancer risk, including two subtypes, plotted against the per long allele effect with kb of TL (vertical and horizontal black lines showing 95% CI for each SNP). The effects of TL on lung cancer were displayed as solid red lines with slopes meaning the MR estimates (dashed lines showing 95% CI). We found positive slopes in all lung cancers as well as in two histology subtypes, indicating that longer TL showed a significant positive association with lung cancer risk.
Figure 2.
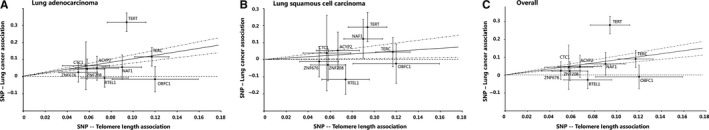
Scatter plot of the effect of each variant on telomere length and lung adenocarcinoma (A), lung squamous cell carcinoma (B), and lung cancer overall (C). Scatter plots show the per‐allele association with lung cancer risk plotted against the per‐allele association with kb of TL (with vertical and horizontal black lines showing 95% CI for each SNP). The scatter plot is overlaid with the Mendelian randomization estimate (slope of solid line with dashed lines showing 95% CI) of the effect of TL on lung cancer risk
3.4. Nonlinear associations test between weight GRS and lung cancer
In the analysis of the decile of TL‐associated GRS, we found an approximately log‐linear relationship between GRS and risk of lung cancer (Figure 1). Since “U shape” associations have been found in several studies, we used a restricted cubic spline analysis to fit the model to further investigate the liner trend between weight GRS and lung cancer risk. As shown in Figure 3, a significant log‐linear association was found in combined lung cancers subtypes (P‐linear < .001; P‐nonlinear = .821). In two subtypes of lung cancer, lung adenocarcinoma also demonstrated a significant log‐linear association (P‐linear < .001; P‐nonlinear = .102). However, lung squamous cell carcinoma showed a marginal nonlinear association (P‐linear = .022; P‐nonlinear = .062) between GRS and lung squamous cell carcinoma risk.
Figure 3.
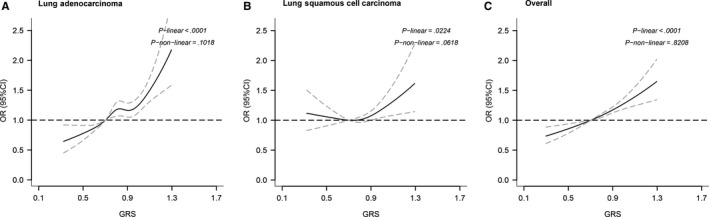
Nonlinear test between genetically increased TL and lung squamous cell carcinoma (A), lung adenocarcinoma (B), and lung cancer overall (C), based on restricted cubic spline function in the logistic regression model
3.5. Sensitivity analysis
In pleiotropy test (Table 1), we found that lung adenocarcinoma, lung squamous cell carcinoma and combined lung cancer, all showed a significant deviation from MR assumptions two and three (P for pleiotropy = 1.57 × 10−15, 3.23 × 10−4 and 6.55 × 10−16, respectively). It indicated that one or more TL‐related SNPs’ effects on TL were not proportional to their effects on lung cancer risk. When testing of the pleiotropic effect for each SNP, rs2736100 in the TERT region was only one that failed in the test. SNP rs2736100 was a known lung cancer risk SNP reported in many studies across different population, and it may affect lung cancer risk through different mechanisms instead of TL. After excluding SNP rs2736100 (Table 2), we can still find significant associations between TL‐related weight GRS and increased risk of lung adenocarcinoma (OR = 1.66, 95% CI = 1.27‐2.16, P = 1.65 × 10−4) and combined lung cancer (OR = 1.45, 95% CI = 1.15‐1.84, P = 1.70 × 10−3). However, there was no significant association between lung squamous cell carcinoma and weight GRS (OR = 1.06, 95% CI = 0.71‐1.58, P = .784). The IVW method showed the same results. Besides, no significant evidence for pleiotropy was found in all lung cancer (P = .091) and lung adenocarcinoma (P = .116), and lung squamous cell carcinoma showed a marginal pleiotropy effect (P = .049) (Table 2). In separate analyses for each study, we found that without rs2736100, the association between TL‐related weight GRS and lung cancer risk was only significant in FLCCA data (Table S3). However, we also found there was no significant heterogeneity between two studies. The fewer samples of NJMU data may limit detection of the association. When excluding pleiotropic SNP rs2736100, the TL‐related variants met all MR assumptions and the association between GRS and lung cancer (including lung adenocarcinoma) remained statistically significant suggesting that TL may be a causal factor for lung cancer risk.
Table 2.
Associations of telomere length‐associated variants and lung cancer risk after excluding rs2736100
| SNP | Lung adenocarcinoma | Lung squamous cell carcinoma | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | P | OR | 95%CI | P | OR | 95%CI | P | |
| Genetic risk score exclude rs2736100a | 1.66 | (1.27,2.16) | 1.65 × 10−4 | 1.06 | (0.71,1.58) | .784 | 1.45 | (1.15,1.84) | 1.70 × 10−3 |
| MR(IVW)exclude rs2736100b | 1.70 | (1.30,2.23) | 1.26 × 10−4 | 1.06 | (0.69,1.62) | .795 | 1.50 | (1.18,1.91) | 1.12 × 10−3 |
| Heterogeneityc | .116 | .048 | .091 | ||||||
Genetic risk score ORs refer to a 1‐kb increase in telomere length after excluding rs2736100.
Inverse variance weighted Mendelian randomization estimate for a 1‐kb increase in telomere length after excluding rs2736100.
Test for significant heterogeneity across the nine SNP instruments used in the Mendelian randomization analysis after excluding rs2736100.
4. DISCUSSION
In this study, we conducted a telomere length MR studies in East Asian population. Considering advantage of MR method, we can avoid confounding bias and estimate the causal relationship between telomere length and lung cancer risk. We found that longer TL showed positive association with increased lung cancer risk. After sensitivity analyses, positive association in lung adenocarcinoma was still significant. Using restricted cubic spline analysis, we observed a linear relationship between genetic predicted TL and the risk of lung cancer. We also validated the results in two studies independently and did not find significant heterogeneity, suggesting a reliable association result in East Asian population.
Leukocyte TL and lung cancer risk relationship have been investigated in many previous studies. Several retrospective case‐control studies reported negative associations between TL and lung cancer risk. For example, Jang JS et al found that individuals with short telomeres were at a significant higher risk of lung cancer than those with long telomeres in 243 lung cancer cases and 243 healthy controls.30 With small sample size and TL measuring on diagnosed cancer participants, those studies may be misled by reverse causation bias. Prospective studies with large sample size observed longer TL increased the risk of lung cancer in multiple populations.13, 14, 15, 16 However, another large prospective study including 47 102 participants found no significant association between TL and lung cancer risk.17 The previous inconsistent finding may be attributed to small sample size, confounding factors, such as age at TL measurement and accuracy of TL assessment.16 Using MR method, potential confounding bias may be avoided by choosing genetic variants which are significantly associated with TL as instrumental variables. A MR analysis of TL using multi‐SNP score in European population observed a significant association between long telomeres and lung adenocarcinoma (but not squamous cell carcinoma), which is accordance with our results.
The main function of telomeres is to maintain chromosome integrity and stability during cell division. Because of the dual role of telomeres in tumor development, the relationship between TL and the risk of cancer is still unclear.12, 31 Short telomeres could result in replicative senescence and apoptosis and may act as tumor suppressors. Contrarily, long telomeres may allow for extra cell division, which let cells have more chances to accumulate carcinogenesis somatic mutations, and finally resulted in malignant transformation.32, 33 In previous melanoma and B‐cell lymphoma studies, researchers found that long TL was associated with increased cancer risk.34, 35 It is suggested that long telomeres may have a stronger effect than short telomeres in carcinogenesis, with a proposed mechanism that long telomeres may promote cell growth and proliferation, thus delaying senescence and allowing further oncogenic mutations to accumulate. In the nonlinear test, we did find a significant linear trend in lung cancer, which meant increased GRS, presenting longer telomeres, was associated with increased lung cancer risk and the risk rose linearly. Together with other studies, we support that long telomeres are a risk factor of lung cancer.
Given its advantages, MR approach becomes an effective and reliable method for investigated relationships between TL and lung cancer risk. Genetic instrument shows its own advantage, that is, genetic risk score is more stable than other risk factors, considering genetic sequence is constant during whole life time.36 MR approach would not be influenced by confounding bias or reverse causation, because TL estimation is based on germline level and individual's genetic predicted TL exists before lung cancer. Moreover, after sensitivity analysis, no pleiotropic effects for genetic variants remained, which met the second and third assumptions. However, there are still some limitations in our study. Just like other studies using SNPs as instrumental variables, SNPs only explained small phenotype variance. Considering variance in measured TL explained by SNPs is approximately 1%,37 we may lose some power to detect the causal effects. Nevertheless, previous studies also used a few SNPs as surrogate measures of peripheral leukocyte TL and found significant association results.21, 38 In addition, we used leukocyte TL instead of TL from lung tissues due to lack of lung tissue‐specific TL GWAS studies. That may cause some biases, reducing the power to detect the causal association. However, previous studies have reported that TL measured in blood and lung was correlated, supporting the assumption that our SNPs can predict TL in lung tissue.39
In conclusion, our study provides evidence for a possible causal association between telomere length and lung cancer risk in East Asian population, consistent with Western population results. Further studies need to be undertaken to clarify specific mechanisms for telomere in lung cancer carcinogenesis. More efforts also need to combine telomeres with clinical application to improve lung cancer prediction and prevention.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
This work was supported by National Key Research and Development Program of China (2017YFC0907905), National Natural Science of China (81521004), Science Foundation for Distinguished Young Scholars in Jiangsu (BK20160046), the Innovation of Graduate Student Training Project in Jiangsu Province (KYZZ15_0268), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (Public Health and Preventive Medicine) and Top‐notch Academic Programs Project of Jiangsu Higher Education Institutions (PPZY2015A067).
Cao X, Huang M, Zhu M, et al. Mendelian randomization study of telomere length and lung cancer risk in East Asian population. Cancer Med. 2019;8:7469–7476. 10.1002/cam4.2590
Xuguang Cao and Mingtao Huang contributed equally to this work.
Contributor Information
Jiangbo Du, Email: dujiangbo@njmu.edu.cn.
Lin Xu, Email: xulin83cn@gmail.com.
Zhibin Hu, Email: zhibin_hu@njmu.edu.cn.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Sun K, Zheng R, et al. Cancer incidence and mortality in China, 2014. Chin J Cancer Res. 2018;30(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hecht SS. Tobacco carcinogens, their biomarkers and tobacco‐induced cancer. Nat Rev Cancer. 2003;3(10):733‐744. [DOI] [PubMed] [Google Scholar]
- 4. Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers–a different disease. Nat Rev Cancer. 2007;7(10):778‐790. [DOI] [PubMed] [Google Scholar]
- 5. Sampson JN, Wheeler WA, Yeager M, et al. Analysis of heritability and shared heritability based on genome‐wide association studies for thirteen cancer types. J Natl Cancer Inst. 2015;107(12):djv279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mucci LA, Hjelmborg JB, Harris JR, et al. Familial risk and heritability of cancer among twins in nordic countries. JAMA. 2016;315(1):68‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dai J, Shen W, Wen W, et al. Estimation of heritability for nine common cancers using data from genome‐wide association studies in Chinese population. Int J Cancer. 2017;140(2):329‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blackburn EH. Telomeres and their synthesis. Science. 1990;249(4968):489‐490. [DOI] [PubMed] [Google Scholar]
- 9. Blackburn EH. Structure and function of telomeres. Nature. 1991;350(6319):569‐573. [DOI] [PubMed] [Google Scholar]
- 10. Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6(8):611‐622. [DOI] [PubMed] [Google Scholar]
- 11. Kim N, Piatyszek M, Prowse K, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266(5193):2011‐2015. [DOI] [PubMed] [Google Scholar]
- 12. Hackett JA, Greider CW. Balancing instability: dual roles for telomerase and telomere dysfunction in tumorigenesis. Oncogene. 2002;21(4):619‐626. [DOI] [PubMed] [Google Scholar]
- 13. Shen M, Cawthon R, Rothman N, et al. A prospective study of telomere length measured by monochrome multiplex quantitative PCR and risk of lung cancer. Lung Cancer. 2011;73(2):133‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lan Q, Cawthon R, Gao Y, et al. Longer telomere length in peripheral white blood cells is associated with risk of lung cancer and the rs2736100 (CLPTM1L‐TERT) polymorphism in a prospective cohort study among women in China. PLoS ONE. 2013;8(3):e59230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seow WJ, Cawthon RM, Purdue MP, et al. Telomere length in white blood cell DNA and lung cancer: a pooled analysis of three prospective cohorts. Cancer Res. 2014;74(15):4090‐4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang X, Zhao Q, Zhu W, et al. The association of telomere length in peripheral blood cells with cancer risk: a systematic review and meta‐analysis of prospective studies. Cancer Epidemiol Biomarkers Prev. 2017;26(9):1381‐1390. [DOI] [PubMed] [Google Scholar]
- 17. Weischer M, Nordestgaard BG, Cawthon RM, Freiberg JJ, Tybjaerg‐Hansen A, Bojesen SE. Short telomere length, cancer survival, and cancer risk in 47102 individuals. J Natl Cancer Inst. 2013;105(7):459‐468. [DOI] [PubMed] [Google Scholar]
- 18. Hernan MA, Robins JM. Instruments for causal inference: an epidemiologist's dream? Epidemiology. 2006;17(4):360‐372. [DOI] [PubMed] [Google Scholar]
- 19. Sheehan NA, Didelez V, Burton PR, Tobin MD. Mendelian randomisation and causal inference in observational epidemiology. PLoS Medicine. 2008;5(8):e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Machiela MJ, Hsiung CA, Shu X‐O, et al. Genetic variants associated with longer telomere length are associated with increased lung cancer risk among never‐smoking women in Asia: a report from the female lung cancer consortium in Asia. Int J Cancer. 2015;137(2):311‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang C, Doherty JA, Burgess S, et al. Genetic determinants of telomere length and risk of common cancers: a Mendelian randomization study. Hum Mol Genet. 2015;24(18):5356‐5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu Z, Wu C, Shi Y, et al. A genome‐wide association study identifies two new lung cancer susceptibility loci at 13q12.12 and 22q12.2 in Han Chinese. Nat Genet. 2011;43(8):792‐796. [DOI] [PubMed] [Google Scholar]
- 23. Dong J, Hu Z, Wu C, et al. Association analyses identify multiple new lung cancer susceptibility loci and their interactions with smoking in the Chinese population. Nat Genet. 2012;44(8):895‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lan Q, Hsiung CA, Matsuo K, et al. Genome‐wide association analysis identifies new lung cancer susceptibility loci in never‐smoking women in Asia. Nat Genet. 2012;44(12):1330‐1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levy D, Neuhausen SL, Hunt SC, et al. Genome‐wide association identifies OBFC1 as a locus involved in human leukocyte telomere biology. Proc Natl Acad Sci USA. 2010;107(20):9293‐9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mangino M, Hwang S‐J, Spector TD, et al. Genome‐wide meta‐analysis points to CTC1 and ZNF676 as genes regulating telomere homeostasis in humans. Hum Mol Genet. 2012;21(24):5385‐5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Codd V, Nelson CP, Albrecht E, et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45(4):422‐427, 7e1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. VanderWeele TJ, Tchetgen Tchetgen EJ, Cornelis M, Kraft P. Methodological challenges in mendelian randomization. Epidemiology. 2014;25(3):427‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jang JS, Choi YY, Lee WK, et al. Telomere length and the risk of lung cancer. Cancer Sci. 2008;99(7):1385‐1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roos WP, Thomas AD, Kaina B. DNA damage and the balance between survival and death in cancer biology. Nat Rev Cancer. 2016;16(1):20‐33. [DOI] [PubMed] [Google Scholar]
- 32. Halaschek‐Wiener J, Vulto I, Fornika D, et al. Reduced telomere length variation in healthy oldest old. Mech Ageing Dev. 2008;129(11):638‐641. [DOI] [PubMed] [Google Scholar]
- 33. Bull CF, Mayrhofer G, O'Callaghan NJ, et al. Folate deficiency induces dysfunctional long and short telomeres; both states are associated with hypomethylation and DNA damage in human WIL2‐NS cells. Cancer Prev Res (Phila). 2014;7(1):128‐138. [DOI] [PubMed] [Google Scholar]
- 34. Iles MM, Bishop DT, Taylor JC, et al. The effect on melanoma risk of genes previously associated with telomere length. J Natl Cancer Inst. 2014;106(10):dju267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hosnijeh FS, Matullo G, Russo A, et al. Prediagnostic telomere length and risk of B‐cell lymphoma‐results from the EPIC cohort study. Int J Cancer. 2014;135(12):2910‐2917. [DOI] [PubMed] [Google Scholar]
- 36. Shen H, Jin G. Human genome epidemiology, progress and future. J Biomed Res. 2013;27(3):167‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cheng Y, Yu C, Huang M, et al. Genetic association of telomere length with hepatocellular carcinoma risk: a Mendelian randomization analysis. Cancer Epidemiol. 2017;50(Pt A):39‐45. [DOI] [PubMed] [Google Scholar]
- 38. Machiela MJ, Lan Q, Slager SL, et al. Genetically predicted longer telomere length is associated with increased risk of B‐cell lymphoma subtypes. Hum Mol Genet. 2016;25(8):1663‐1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saferali A, Lee J, Sin DD, Rouhani FN, Brantly ML, Sandford AJ. Longer telomere length in COPD patients with alpha1‐antitrypsin deficiency independent of lung function. PLoS ONE. 2014;9(4):e95600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


