Abstract
BACKGROUND
It has been suggested that chronic pancreatitis (CP) may be an independent risk factor for development of cardiovascular disease (CVD). At the same time, it seems that congestive heart failure (CHF) and CP share the responsibility for the development of important clinical conditions such as sarcopenia, cachexia and malnutrition due to development of cardiac cachexia and pancreatic exocrine insufficiency (PEI), respectively.
AIM
To explore the evidence regarding the association of CP and heart disease, more specifically CVD and CHF.
METHODS
A systematic search of MEDLINE, Web of Science and Google Scholar was performed by two independent investigators to identify eligible studies where the connection between CP and CVD was investigated. The search was limited to articles in the English language. The last search was run on the 1st of May 2019. The primary outcomes were: (1) Incidence of cardiovascular event [acute coronary syndrome (ACS), chronic coronary disease, peripheral arterial lesions] in patients with established CP; and (2) Incidence of PEI in patients with CHF.
RESULTS
Out of 1166 studies, only 8 were eligible for this review. Studies regarding PEI and CHF showed an important incidence of PEI as well as associated malabsorption of nutritional markers (vitamin D, selenium, phosphorus, zinc, folic acid, and prealbumin) in patients with CHF. However, after substitution of pancreatic enzymes, it seems that, at least, loss of appetite was attenuated. On the other side, studies investigating cardiovascular events in patients with CP showed that, in CP cohort, there was a 2.5-fold higher incidence of ACS. In another study, patients with alcohol–induced CP with concomitant type 3c diabetes had statistically significant higher incidence of carotid atherosclerotic plaques in comparison to patients with diabetes mellitus of other etiologies. Earlier studies demonstrated a marked correlation between the clinical symptoms in CP and chronic coronary insufficiency. Also, statistically significant higher incidence of arterial lesions was found in patients with CP compared to the control group with the same risk factors for atherosclerosis (hypertension, smoking, dyslipidemia). Moreover, one recent study showed that PEI is significantly associated with the risk of cardiovascular events in patients with CP.
CONCLUSION
Current evidence implicates a possible association between PEI and malnutrition in patients with CHF. Chronic pancreatic tissue hypoxic injury driven by prolonged splanchnic hypoperfusion is likely to contribute to malnutrition and cachexia in patients with CHF. On the other hand, CP and PEI seem to be an independent risk factor associated with an increased risk of cardiovascular events.
Keywords: Chronic, Pancreatitis, Pancreatic exocrine insufficiency, Heart failure, Cardiovascular diseases
Core tip: This systematic review explores the studies regarding the connection between chronic pancreatitis (CP) and cardiovascular disease, which seems to be a two-way street. On one hand, congestive heart failure may aid to development of at least mild pancreatic exocrine insufficiency (PEI) that, in return, contributes to development of loss of appetite, cachexia, and malnutrition in patients with heart failure. On the other hand, there is some evidence that CP with concomitant PEI may be an independent risk factor for cardiovascular events in terms of coronary disease, cerebrovascular disease and peripheral atherosclerotic plaques.
INTRODUCTION
Chronic pancreatitis (CP) is a persistent inflammation of the pancreas with pathological findings of the infiltration of immune cells and the development of fibrosis. Pancreatic exocrine insufficiency (PEI), caused by impaired secretion of pancreatic digestive enzymes due to loss of intact pancreatic acinar cells, represents one of the most frequent complications of CP[1]. Chronic heart failure describes the complex clinical syndrome where the heart is incapable of maintaining a cardiac output that is adequate to meet metabolic requirements and accommodate venous return[2,3]. There is evidence that CP is associated with an increased risk of cardiovascular diseases (CVD), which may be at least partially explained by sharing many risk factors among these patients[4-7]. Even association between CP and cerebrovascular disease was reported[8]. There is increasing evidence for the involvement of the gastrointestinal (GI) system in congestive heart failure (CHF)[9]. Furthermore, patients with CP and CHF share two other very important clinical conditions: sarcopenia and cachexia[10-13]. Muscle wasting, or sarcopenia, is characterized by loss of muscle mass, loss of muscle strength and impaired functional capacity[10]. The occurrence of malnutrition has been reported as a hallmark of muscle wasting especially in older patients[14,15]. Cardiac cachexia is the clinical entity occurring at the end of the chronic natural course of CHF with complex and multifactorial pathophysiology[16]. Association of PEI in patients with CHF with appetite loss was recently reported[17,18].
MATERIALS AND METHODS
Search strategy and study selection
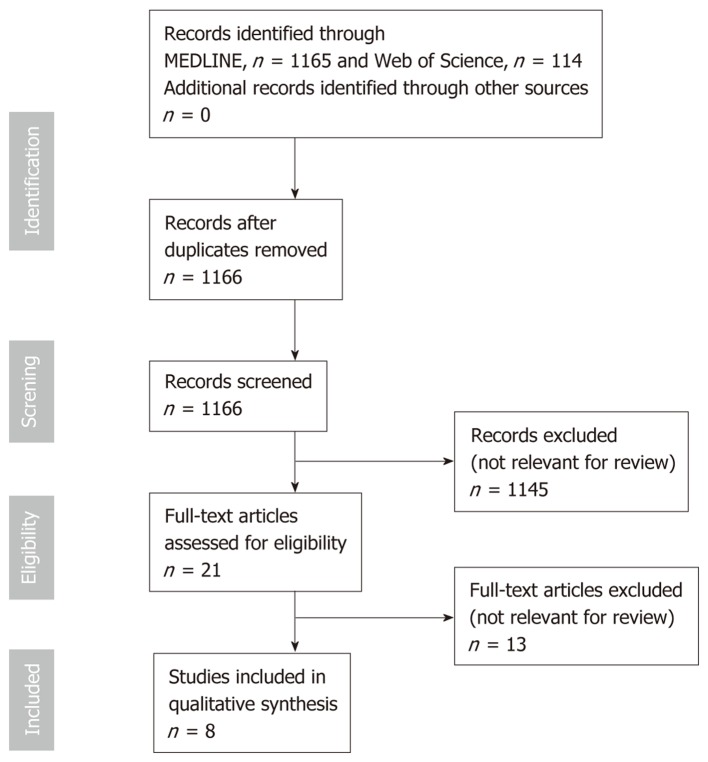
MEDLINE, Web of Science and Google Scholar databases were searched until May 1, 2019 using the following terms: [(Chronic pancreatitis OR pancreatic exocrine insufficiency) AND (heart failure OR cardiovascular risk OR cardiovascular disease)]. The search was limited to articles in the English language. Eligibility assessment was performed independently by screening the titles and, consequently, the abstracts by 2 reviewers, Nikolic S and Dugic A. All disagreements were resolved by Vujasinovic M and Löhr JM. Review articles, editorials, conference reports, comments on other studies, and animal studies were excluded. Studies on clinical association between CP and heart diseases were included. Of the selected articles, the full texts, as well as the reference lists, were reviewed independently (“snowball-strategy”) by three authors (Nikolic S, Dugic A and Vujasinovic M). The selection process of the articles for this review is summarized in Figure 1.
Figure 1.
Flow chart on study-selection process.
Data collection process and data items
This review was limited to the question of whether there is a connection between CP and CVD in patients older than 18 years at the onset of CVD. The following characteristics were extracted from the publications included: Year of publication, country of origin, study design, number of patients, demographics of patients included and study results.
Interventions made in all of the included studies were diagnostic measures needed for establishing CVD in patients with CP and vice versa. CHF was assessed by echocardiography[3]. Diagnosis of CP was made upon clinical data and pancreatic imaging[1]. Diagnosis of PEI was established by measuring fecal elastase in stool or a C-labeled mixed triglyceride breath test. Control patients were either healthy individuals or were matched with the study group. The exception is the study by de la Iglesia where controls had CP only, whereas the study group had CP and PEI[4].
RESULTS
Study selection
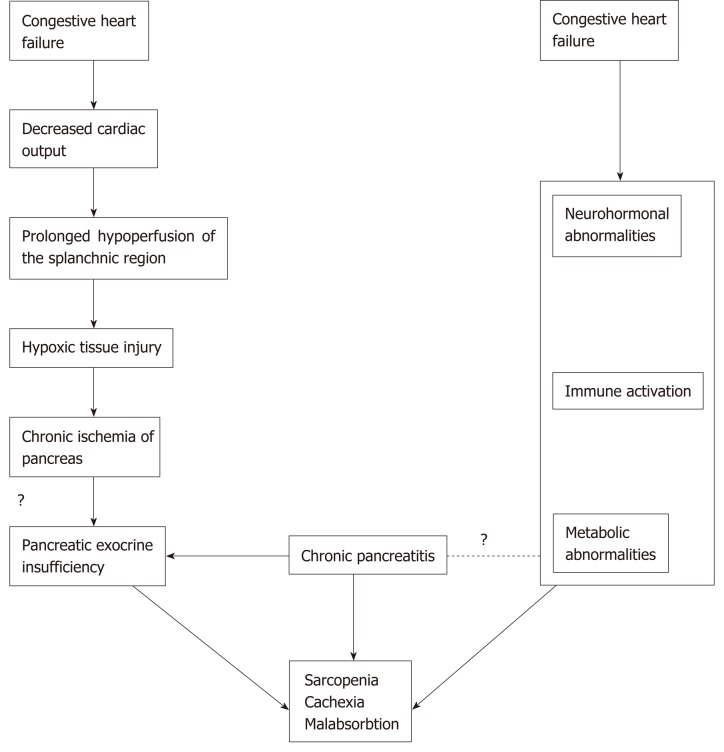
Overall, 1166 citations were retrieved; 1145 were rejected based on title, abstract relevance or duplication; 21 articles were fully reviewed. After further review, an additional 13 full-text articles were excluded due to reasons stated in Figure 1. Final analysis included 8 studies[4-6,17-21]. Characteristics of studies on PEI in patients with CHF are summarized in Table 1. Table 2 shows characteristics of studies on CVD in patients with CP. Figure 2 shows possible association between cardiovascular and pancreatic disease.
Table 1.
Studies on pancreatic exocrine insufficiency in patients with chronic heart failure
| Ref. | n | Mean age (yr) | Method used | PEI (%) |
| Xia et al[17], 2017, China | All patients: 104 (85.6% males) | 70.4 | FE-1 | All patients: n = 59 (56.7%) |
| NYHA I-II: Mild/moderate: n = 7, severe: n = 1; NYHA III: Mild/moderate: n = 15, severe: n = 14; NYHA IV: Mild/moderate: n = 10, severe: n = 12 | ||||
| NYHA I/II: 32 | ||||
| NYHA III: 42 | ||||
| NYHA IV: 30 | ||||
| Vujasinovicet al[18], 2016, Slovenia | All patients: 87 (64.4% males) | 74.7 | FE-1 | All patients: n = 6 (6.9%), mild/moderate PEI: n = 3, severe PEI: n = 3, NYHA II: n = 3, NYHA III: n = 3 |
| NYHA II: 54 | ||||
| NYHA III: 33 | ||||
| Özcan et al[19], 2015, Turkey | All patients: 52 (61,5% males) | 67.5 | FE-1 | All patients: n = 21 (40.4%) |
| NYHA I/II: Mild/moderate PEI: n = 3, severe PEI: n = 4 | ||||
| NYHA I/II: 32 | ||||
| NYHA III/IV: 20 | NYHA III/IV: Mild/moderate PEI: n = 4, severe PEI: n = 10 |
n: Number of patients; FE-1: Fecal elastase-1; PEI: Pancreatic exocrine insufficiency; NYHA: New York Heart Association classification.
Table 2.
Studies on cardiovascular diseases in patients with chronic pancreatitis
| Ref. | n | Mean age (yr) | Results |
| Tuzhilin et al[20], 1975, Union of Soviet Socialist Republics/United States | 98 | Not reported | Marked correlation between the clinical symptoms in CP and chronic coronary insufficiency |
| Pancreatic enzymes and their inhibitors profoundly affected blood coagulability and appear to influence the course of pancreatic inflammation | |||
| Gullo et al[5], 1982, Italy | 54 | 44.2 | Arterial lesions were found in 18 patients (33.3%) and in five controls (9.3%) (P < 0.01) |
| 49 (90.7%) males | |||
| No differences were found between the two groups for arterial hypertension, smoking habits, or blood lipid abnormalities | |||
| Hsu et al[6], 2016, Taiwan | 17405 | 48.3 | The overall incidence of acute coronary syndrome was 2.15-fold higher in the CP cohort than in the non-CP cohort (4.89 vs 2.28 per 10,000 person-years) with an adjusted hazard ratio of 1.40 (95% confidence interval 1.20-1.64) |
| 14418 (82.8%) males | |||
| Compared with individuals without CP, patients with CP aged ≤ 39 years exhibited the highest risk of acute coronary syndrome | |||
| CP may become an independent risk factor for acute coronary syndrome | |||
| Lee et al[21], 2018, United States | 32 | 61.7 | Statistically significant association between a diagnosis of alcohol-related CP and diabetes mellitus, and the presence of an atheroma (calcified carotid artery plaques) on the panoramic image, in comparison with the rate manifested by the historical general population cohort (25% vs 3%; P < 0.05) |
| (100%) males | |||
| de la Iglesia et al[4], 2018, Spain | 430 | 47.8 | Together with known major cardiovascular risk factors like smoking and hypertension, pancreatic exocrine insufficiency is significantly associated with the increased risk of cardiovascular events in patients with CP |
| 340 (79%) males |
CP: Chronic pancreatitis.
Figure 2.
Possible association between cardiovascular and pancreatic disease.
Results of the individual studies
Table 1 and Table 2 synthesize the main results of each individual study included in the systematic research.
Outcomes
Three studies assessed the incidence of PEI in patients with CHF as a primary outcome[17-19] and five studies assessed incidence of cardiovascular complication (acute or chronic coronary lesion, carotid atheroma, peripheral arterial lesion) in patients with CP[4-6,20,21]. Improvement of appetite loss by supplemented pancreatic enzymes was investigated as a secondary outcome in the study by Xia et al[17]. The study by de la Iglesia et al[4] examined the association of PEI and cardiovascular risk in patients with CP as a primary outcome. The primary outcome measure in most studies was the mean difference between control and patient groups. In studies by Hsu et al[6] and Wong et al[8], time to vascular event was measured and result represented as a hazard ratio.
DISCUSSION
Congestive heart failure is a progressive disease that causes ischemia and congestion in peripheral tissues and may result in function loss in many organs such as the kidney, liver, stomach and intestine[17]. Nausea and lack of appetite may also occur as blood is shifted from the GI tract to the more vital organs[2]. Susceptibility of the pancreas to ischemic injury in shock, malignant hypertension and after cardiac surgery is well known[22-25]. The splanchnic circulation normally receives ap-proximately 20%-30% of the cardiac output[26,27]. In patients with CHF, the splanchnic circulation is decreased, and if this state is prolonged, tissue damage to the splanchnic organs is possible, especially in pancreas that are highly vascularized organs[22,28].
Pancreatic exocrine insufficiency in patients with heart failure
Appetite loss and malnutrition are well known and highly prevalent in patients with CHF and an important risk factor for morbidity and mortality[29,30]. PEI refers to an insufficient secretion of pancreatic enzymes (acinar function) and/or sodium bicarbonate (ductal function), mostly associated with various pancreatic illnesses but could be associated even with extra pancreatic diseases[1].
Earlier, we performed a prospective study on 87 patients with CHF, using fecal elastase-1 (FE-1) as a diagnostic tool for diagnosis of PEI[18]. The mean time from the confirmation of chronic heart failure to inclusion in the study was 4 years and PEI was diagnosed in 6.9% of patients suggesting a possibility that PEI occurred because of decreased splanchnic circulation in CHF patients. Additionally, the clinical significance of PEI was assessed in this study by using a complete laboratory serum nutritional panel showing decreased levels in one or more nutritional serum markers (vitamin D, selenium, phosphorus, zinc, folic acid, and prealbumin) in all of the patients tested with PEI[18].
Özcan et al[19] investigated FE-1 levels (as an indicator of pancreatic exocrine function) and blood ghrelin levels (which affect eating, sleeping, cell proliferation, the cardiovascular system, and carbohydrate energy metabolism in patients with CHF as well as the pancreatic exocrine function) in 52 patients with acute decompensated heart failure and compared them with 31 healthy patients in the control group. The authors reported significant difference in FE-1 levels between the control and NYHA I/II patients vs NYHA III/IV group. In patients with advanced heart failure, ten patients (50%) had severe, and four patients (20%) moderate PEI, whereas two- thirds of the controls and patients with mild heart failure had normal pancreatic function. However, there was no statistically significant difference for ghrelin levels[19].
Xia et al[17] attempted to detect the association between PEI (measured by FE-1 levels) and CHF-induced appetite, which was tested by the simplified nutritional appetite questionnaire. PEI was diagnosed in 56.7% of 104 patients with CHF and in none within the control group (n = 20) of patients with normal heart function. In the very important second part of this study, patients with CHF and PEI were treated with pancreatic enzyme replacement therapy (PERT) in the form of pancreatin (30000 units per day) and compared with the CHF and PEI patients treated with a placebo. After a 4-wk treatment, pancreatin significantly improved the appetite loss in the treatment group, indicating that PERT can attenuate the appetite loss in CHF and give the strongest evidence so far in the association of PEI with appetite loss in patients with CHF[17].
Cardiovascular risk in patients with CP
The association between CP and CVD has received little attention in the past. In 1975, Tuzhilin et al[20] reported a study on cardiovascular lesions in 98 patients with CP and 32 control subjects, analyzing serum amylase, trypsin, trypsin inhibitor, elastase, plasma recalcification time, plasma heparin tolerance, blood fibrinogen level, fibrinolysis activity and capillary permeability to protein and water. They observed a marked correlation between the clinical symptoms in CP and chronic coronary insufficiency, probably because of pancreatic enzymes and their inhibitors that profoundly affected blood coagulability and appear to influence the course of pancreatic inflammation.
In 1982, Gullo et al[5] prospectively investigated 54 consecutive CP patients and 54 control subjects for the presence of cardiovascular lesions. Clinical and laboratory evidence of arterial involvement was found in 18 patients (33%) and in 5 controls (9%) (P < 0.01), concluding that patients with CP have more frequent cardiovascular lesions that tend to develop at an earlier age, compared to the general population (there were no differences between the two groups for major risk factors like arterial hypertension, smoking habits, or blood lipid abnormalities).
Lee et al[21] recently performed an interesting retrospective observational study on 32 patients with alcohol related CP and type 3 c diabetes mellitus (diabetes secondary to pancreatic disease) in whom panoramic (dental) images were taken and compared to a historical cohort of healthy patients. The prevalence rate of calcified carotid artery plaques (25%) was significantly higher than the rate (3%) in the control group.
Although DM is common in CP and is a well-known risk factor for arteriosclerosis, the link between CP and CVD seems to depend on other mechanisms[31]. Chronic inflammation has been found to be associated with accelerated atherosclerosis and increased risk of CVDs[32]. The pancreatic changes at an advanced age are considered to be related to atherosclerosis (of small vessels)[15]. This concept of “senile pancreatitis” was first described by Ammann et al[33] and in most cases has a silent and mild course[34].
In a prospective, longitudinal cohort study, Spanish colleagues evaluated the risk of cardiovascular events and the impact of PEI in a cohort of 430 CP patients with the mean follow-up of 8.6 years[4]. The study demonstrated, for the first time, that PEI is an independent risk factor significantly associated with an increased risk of cardiovascular events.
Hsu et al[6] conducted a nationwide retrospective cohort study in Taiwan to determine the risk of acute coronary syndrome (ACS) in patients with CP. In total, 17405 patients with CP and 69620 individuals without CP were followed for 84430 and 417426 person-years showing that overall ACS incidence was 2.15-fold higher in the CP cohort than in the non-CP cohort. Interestingly, the highest risk of ACS was observed in patients aged ≤ 39 years. Here the increased risk was thought to be caused by inflammation leading to endothelial dysfunction and progress of unstable plaque. In the similarly conducted retrospective population-based cohort study from Taiwan, Wong et al[8] reported association of CP with increased risk of subsequent cerebrovascular disease. The overall incidence of cerebrovascular disease among 16672 patients with CP was 1.24-fold greater than in the non-CP patients.
In conclusion, so far, research on association between heart and pancreas disease has received little attention and its role in pathogenesis is not fully elucidated. However, studies presented in this article indicate an important association that should be further investigated. Patients with CP/pancreatic exocrine insufficiency and chronic heart failure (especially right ventricular dysfunction) share similar clinical symptoms like abdominal pain, anorexia, nausea and bloating[1,2]. Interplay between malnutrition (intake driven) and cachexia (disease driven) can also be seen in both cardiac and pancreatic patients, as well as in sarcopenia (muscle wasting)[1,10,11,16,35]. Current evidence implicates possible association between PEI and malnutrition in patients with CHF. Chronic pancreatic tissue hypoxia and consequent injury is likely to contribute to malnutrition and cachexia in patients with CHF; however, too simplistic of an explanation should be avoided. Future prospective studies on this topic are necessary, especially using diagnostic methods for PEI other than FE-1, like the 13C-trygliceride breath test, secretin enhanced magnetic resonance cholangiopancreatography and serum nutritional markers[1].
On the other side, besides well-known risk factors, CP and PEI seem to be independent risk factors associated with an increased risk of CVD. However, most of the studies so far have been performed on patients with alcohol related CP, and in males. Future studies on CP patients with other etiologies and female patients would be of interest.
ARTICLE HIGHLIGHTS
Research background
Chronic pancreatitis (CP) is a persistent inflammation of the pancreas and with time fibrosis develops. Pancreatic exocrine insufficiency (PEI) due to loss of intact pancreatic acinar cells, represents one of the most frequent complications of CP. Chronic heart failure is a complex clinical syndrome where the heart is incapable of maintaining a cardiac output that is adequate to meet human metabolic requirements. Association between CP and heart disease was reported.
Research motivation
Despite sharing risk factors for atherosclerosis among patients with cardiovascular diseases (CVD) and CP, it has been suggested that CP may be an independent risk factor for development of CVD. At the same time, it seems that congestive heart failure (CHF) and CP share the responsibility for the development of important clinical entities such as sarcopenia, cachexia and malnutrition consequences of cardiac cachexia and PEI, respectively.
Research objectives
The objective of our systematic review was to explore all current evidence regarding the association between CP and heart disease such as CVD and CHF.
Research methods
MEDLINE, Web of Science and Google Scholar were independently searched by two investigators with the aim to identify eligible studies where the connection between CP and CVDs was researched. The primary outcomes were: (1) Incidence of cardiovascular event [acute coronary syndrome (ACS), chronic coronary disease, peripheral arterial lesions] in patients with established CP; and (2) Incidence of PEI in patients with CHF. The primary outcome measure in most studies was the mean difference between control and patient groups.
Research results
Eight studies were eligible for this review. Studies regarding PEI and CHF showed an important incidence of PEI as well as associated malabsorption of nutritional markers (vitamin D, selenium, phosphorus, zinc, folic acid, and prealbumin) in patients with CHF. However, after substitution of pancreatic enzymes, it seems that, at least, loss of appetite was attenuated. On the other side, studies investigating cardiovascular events in patients with CP showed that CP is associated with an increased risk of CVD (a 2.5-fold higher incidence of ACS). Also, CP with concomitant type 3c diabetes had statistically significant higher incidence of carotid atherosclerotic plaques in comparison to patients with diabetes mellitus of other etiologies. When other risk factors for atherosclerosis (hypertension, smoking, and dyslipidemia) were matched, patients with CP had significantly higher incidence of arterial lesions. Moreover, one recent study showed that PEI is significantly associated with the risk of cardiovascular events in patients with CP.
Research conclusions
Chronic pancreatic tissue hypoxic injury driven by prolonged splanchnic hypoperfusion is likely to contribute to malnutrition and cachexia in patients with CHF. On the other hand, CP and PEI seem to be an independent risk factor associated with an increased risk of cardiovascular events.
Research perspectives
Interplay between malnutrition (intake driven) and cachexia (disease driven) can be seen in both cardiac and pancreatic patients, as well as in sarcopenia (muscle wasting) Current evidence implicates possible association between PEI and malnutrition in patients with CHF as well as CP with or without PEI being an independent risk factor for CVD. However, too simplistic explanations should be avoided. Future prospective studies on this topic are necessary, especially using diagnostic methods for PEI other than fecal elastase-1, like the 13C-trygliceride breath test, secretin enhanced magnetic resonance cholangiopancreatography and serum nutritional markers.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Sweden
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Vujasinovic M and Löhr JM have worked as speakers for Mylan and Abbott Laboratories. Nikolic S has worked as a speaker for Mylan, Krka, Servier and Ferring. Authors declare no potential conflicts of interest and no financial support regarding presenting the manuscript.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Peer-review started: October 4, 2019
First decision: November 4, 2019
Article in press: November 16, 2019
P-Reviewer: Armellini E, Sahoo J S-Editor: Tang JZ L-Editor: A E-Editor: Zhang YL
Contributor Information
Sara Nikolic, Department of Medicine Huddinge, Karolinska Institute, Stockholm 14183, Sweden; Department of Gastroenterology, Division of Internal Medicine, University Medical Centre Maribor, Maribor 2000, Slovenia.
Ana Dugic, Department of Medicine, Klinikum Bayreuth, Bayreuth 95445, Germany.
Corinna Steiner, Department for Digestive Diseases, Karolinska University Hospital, Stockholm 14186, Sweden.
Apostolos V Tsolakis, Department for Digestive Diseases, Karolinska University Hospital, Stockholm 14186, Sweden; Department of Oncology and Pathology, Karolinska Institute, Stockholm 17176, Sweden; Cancer Centre Karolinska, CCK, Karolinska University Hospital, Stockholm 17176, Sweden.
Ida Marie Haugen Löfman, Unit of Cardiology, Heart and Vascular Theme, Karolinska University Hospital Huddinge, Stockholm 14186, Sweden; Department of Medicine Solna, Karolinska Institute, Stockholm 17176, Sweden.
J-Matthias Löhr, Department for Digestive Diseases, Karolinska University Hospital, Stockholm 14186, Sweden; Department of Clinical Science, Intervention, and Technology (CLINTEC), Karolinska Institute, Stockholm 17176, Sweden.
Miroslav Vujasinovic, Department of Medicine Huddinge, Karolinska Institute, Stockholm 14183, Sweden; Department for Digestive Diseases, Karolinska University Hospital, Stockholm 14186, Sweden. miroslav.vujasinovic@sll.se.
References
- 1.Löhr JM, Dominguez-Munoz E, Rosendahl J, Besselink M, Mayerle J, Lerch MM, Haas S, Akisik F, Kartalis N, Iglesias-Garcia J, Keller J, Boermeester M, Werner J, Dumonceau JM, Fockens P, Drewes A, Ceyhan G, Lindkvist B, Drenth J, Ewald N, Hardt P, de Madaria E, Witt H, Schneider A, Manfredi R, Brøndum FJ, Rudolf S, Bollen T, Bruno M HaPanEU/UEG Working Group. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU) United European Gastroenterol J. 2017;5:153–199. doi: 10.1177/2050640616684695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovasc Pathol. 2012;21:365–371. doi: 10.1016/j.carpath.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P Authors/Task Force Members; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 4.de la Iglesia D, Vallejo-Senra N, López-López A, Iglesias-Garcia J, Lariño-Noia J, Nieto-García L, Domínguez-Muñoz JE. Pancreatic exocrine insufficiency and cardiovascular risk in patients with chronic pancreatitis: A prospective, longitudinal cohort study. J Gastroenterol Hepatol. 2019;34:277–283. doi: 10.1111/jgh.14460. [DOI] [PubMed] [Google Scholar]
- 5.Gullo L, Stella A, Labriola E, Costa PL, Descovich G, Labò G. Cardiovascular lesions in chronic pancreatitis: a prospective study. Dig Dis Sci. 1982;27:716–722. doi: 10.1007/BF01393767. [DOI] [PubMed] [Google Scholar]
- 6.Hsu MT, Lin CL, Chung WS. Increased Risk of Acute Coronary Syndrome in Patients With Chronic Pancreatitis: A Nationwide Cohort Analysis. Medicine (Baltimore) 2016;95:e3451. doi: 10.1097/MD.0000000000003451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maisonneuve P, Lowenfels AB, Müllhaupt B, Cavallini G, Lankisch PG, Andersen JR, Dimagno EP, Andrén-Sandberg A, Domellöf L, Frulloni L, Ammann RW. Cigarette smoking accelerates progression of alcoholic chronic pancreatitis. Gut. 2005;54:510–514. doi: 10.1136/gut.2004.039263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong TS, Liao KF, Lin CM, Lin CL, Chen WC, Lai SW. Chronic Pancreatitis Correlates With Increased Risk of Cerebrovascular Disease: A Retrospective Population-Based Cohort Study in Taiwan. Medicine (Baltimore) 2016;95:e3266. doi: 10.1097/MD.0000000000003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krack A, Sharma R, Figulla HR, Anker SD. The importance of the gastrointestinal system in the pathogenesis of heart failure. Eur Heart J. 2005;26:2368–2374. doi: 10.1093/eurheartj/ehi389. [DOI] [PubMed] [Google Scholar]
- 10.Saitoh M, Rodrigues Dos Santos M, von Haehling S. Muscle wasting in heart failure : The role of nutrition. Wien Klin Wochenschr. 2016;128:455–465. doi: 10.1007/s00508-016-1100-z. [DOI] [PubMed] [Google Scholar]
- 11.Olesen SS, Büyükuslu A, Køhler M, Rasmussen HH, Drewes AM. Sarcopenia associates with increased hospitalization rates and reduced survival in patients with chronic pancreatitis. Pancreatology. 2019;19:245–251. doi: 10.1016/j.pan.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Singh S, Midha S, Singh N, Joshi YK, Garg PK. Dietary counseling versus dietary supplements for malnutrition in chronic pancreatitis: a randomized controlled trial. Clin Gastroenterol Hepatol. 2008;6:353–359. doi: 10.1016/j.cgh.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 13.Midha S, Singh N, Sachdev V, Tandon RK, Joshi YK, Garg PK. Cause and effect relationship of malnutrition with idiopathic chronic pancreatitis: prospective case-control study. J Gastroenterol Hepatol. 2008;23:1378–1383. doi: 10.1111/j.1440-1746.2008.05459.x. [DOI] [PubMed] [Google Scholar]
- 14.Htun NC, Ishikawa-Takata K, Kuroda A, Tanaka T, Kikutani T, Obuchi SP, Hirano H, Iijima K. Screening for Malnutrition in Community Dwelling Older Japanese: Preliminary Development and Evaluation of the Japanese Nutritional Risk Screening Tool (NRST) J Nutr Health Aging. 2016;20:114–120. doi: 10.1007/s12603-015-0555-3. [DOI] [PubMed] [Google Scholar]
- 15.Löhr JM, Panic N, Vujasinovic M, Verbeke CS. The ageing pancreas: a systematic review of the evidence and analysis of the consequences. J Intern Med. 2018;283:446–460. doi: 10.1111/joim.12745. [DOI] [PubMed] [Google Scholar]
- 16.Loncar G, Springer J, Anker M, Doehner W, Lainscak M. Cardiac cachexia: hic et nunc. J Cachexia Sarcopenia Muscle. 2016;7:246–260. doi: 10.1002/jcsm.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xia T, Chai X, Shen J. Pancreatic exocrine insufficiency in patients with chronic heart failure and its possible association with appetite loss. PLoS One. 2017;12:e0187804. doi: 10.1371/journal.pone.0187804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vujasinovic M, Tretjak M, Tepes B, Marolt A, Slemenik Pusnik C, Kotnik Kerbev M, Rudolf S. Is pancreatic exocrine insufficiency a result of decreased splanchnic circulation in patients with chronic heart failure? JOP.J Pancreas (Online) 2016;17:201–203. [Google Scholar]
- 19.Özcan M, Öztürk GZ, Köse M, Emet S, Aydın Ş, Arslan K, Arman Y, Akkaya V, Tükek T. Evaluation of malnutrition with blood ghrelin and fecal elastase levels in acute decompensated heart failure patients. Turk Kardiyol Dern Ars. 2015;43:131–137. doi: 10.5543/tkda.2015.06606. [DOI] [PubMed] [Google Scholar]
- 20.Tuzhilin DA, Dreiling DA. Cardiovascular lesions in pancreatitis. Am J Gastroenterol. 1975;63:381–388. [PubMed] [Google Scholar]
- 21.Lee UK, Chang TI, Polanco JC, Pisegna JR, Friedlander AH. Prevalence of Panoramically Imaged Carotid Atheromas in Alcoholic Patients With Chronic Pancreatitis and Comorbid Diabetes. J Oral Maxillofac Surg. 2018;76:1929.e1–1929.e7. doi: 10.1016/j.joms.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warshaw AL, O'Hara PJ. Susceptibility of the pancreas to ischemic injury in shock. Ann Surg. 1978;188:197–201. doi: 10.1097/00000658-197808000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feiner H. Pancreatitis after cardiac surgery; a morphologic study. Am J Surg. 1976;131:684–688. doi: 10.1016/0002-9610(76)90178-1. [DOI] [PubMed] [Google Scholar]
- 24.Hranilovich GT, Baggenstoss AH. Lesions of the pancreas in malignant hypertension; review of one hundred cases at necropsy. AMA Arch Pathol. 1953;55:443–456. [PubMed] [Google Scholar]
- 25.Fernández-del Castillo C, Harringer W, Warshaw AL, Vlahakes GJ, Koski G, Zaslavsky AM, Rattner DW. Risk factors for pancreatic cellular injury after cardiopulmonary bypass. N Engl J Med. 1991;325:382–387. doi: 10.1056/NEJM199108083250602. [DOI] [PubMed] [Google Scholar]
- 26.Ralevic V. Splanchnic circulatory physiology. Hepatogastroenterology. 1999;46 Suppl 2:1409–1413. [PubMed] [Google Scholar]
- 27.Takala J. Determinants of splanchnic blood flow. Br J Anaesth. 1996;77:50–58. doi: 10.1093/bja/77.1.50. [DOI] [PubMed] [Google Scholar]
- 28.Nyman LR, Wells KS, Head WS, McCaughey M, Ford E, Brissova M, Piston DW, Powers AC. Real-time, multidimensional in vivo imaging used to investigate blood flow in mouse pancreatic islets. J Clin Invest. 2008;118:3790–3797. doi: 10.1172/JCI36209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landi F, Picca A, Calvani R, Marzetti E. Anorexia of Aging: Assessment and Management. Clin Geriatr Med. 2017;33:315–323. doi: 10.1016/j.cger.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Rahman A, Jafry S, Jeejeebhoy K, Nagpal AD, Pisani B, Agarwala R. Malnutrition and Cachexia in Heart Failure. JPEN J Parenter Enteral Nutr. 2016;40:475–486. doi: 10.1177/0148607114566854. [DOI] [PubMed] [Google Scholar]
- 31.Szuszkiewicz-Garcia MM, Davidson JA. Cardiovascular disease in diabetes mellitus: risk factors and medical therapy. Endocrinol Metab Clin North Am. 2014;43:25–40. doi: 10.1016/j.ecl.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Steyers CM, 3rd, Miller FJ., Jr Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci. 2014;15:11324–11349. doi: 10.3390/ijms150711324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ammann R, Sulser H. ["Senile" chronic pancreatitis; a new nosologic entity? Studies in 38 cases. Indications of a vascular origin and relationship to the primarily painless chronic pancreatitis] Schweiz Med Wochenschr. 1976;106:429–437. [PubMed] [Google Scholar]
- 34.Nagai H, Ohtsubo K. Pancreatic lithiasis in the aged. Its clinicopathology and pathogenesis. Gastroenterology. 1984;86:331–338. [PubMed] [Google Scholar]
- 35.Aquilani R, Opasich C, Verri M, Boschi F, Febo O, Pasini E, Pastoris O. Is nutritional intake adequate in chronic heart failure patients? J Am Coll Cardiol. 2003;42:1218–1223. doi: 10.1016/s0735-1097(03)00946-x. [DOI] [PubMed] [Google Scholar]