Abstract
BACKGROUND
The human microRNA 375 (MIR375) is significantly downregulated in human colorectal cancer (CRC) and we have previously shown that MIR375 is a CRC-associated miRNA. The metadherin (MTDH) is a candidate target gene of MIR375.
AIM
To investigate the interaction and function between MIR375 and MTDH in human CRC.
METHODS
A luciferase reporter system was used to confirm the effect of MIR375 on MTDH expression. The expression levels of MIR375 and the target genes were evaluated by quantitative RT-PCR (qRT-PCR), western blotting, or immunohistochemistry.
RESULTS
MTDH expression was found to be upregulated in human CRC tissues compared to that in healthy controls. We show that MIR375 regulates the expression of many genes involved in the MTDH-mediated signal transduction pathways [BRAF-MAPK and phosphatidylinositol-4,5-biphosphate-3-kinase catalytic subunit alpha (PIK3CA)-AKT] in CRC cells. Upregulated MTDH expression levels were found to inhibit NF-κB inhibitor alpha, which further upregulated NFKB1 and RELA expression in CRC cells.
CONCLUSION
Our findings suggest that suppressing MIR375 expression in CRC regulates cell proliferation and angiogenesis by increasing MTDH expression. Thus, MIR375 may be of therapeutic value in treating human CRC.
Keywords: MicroRNA 375, Metadherin, Mitogen-activated protein kinase, Angiogenesis, Cell proliferation, Colorectal cancer
Core tip: The microRNA 375 (MIR375) is significantly downregulated in human colorectal cancer (CRC) tissues. In this study, we investigated that metadherin (MTDH) is a direct target gene of MIR375 and that MTDH expression levels were upregulated in CRC tissues. Upregulated MTDH expression levels were found to inhibit NF-κB inhibitor alpha expression, which further upregulated NFKB1 and RELA expression in CRC cells. MIR375 also regulate MTDH-mediated BRAF-MAPK and PIK3CA-AKT signal pathways in CRC cells. Consequently, MIR375 regulates cell proliferation, cell migration, and angiogenesis by suppressing MTDH expression in CRC progression.
INTRODUCTION
Colorectal cancer (CRC) is a common malignant tumor and is the third leading cause of cancer-related mortality worldwide[1,2]. The cause of CRC is multifactorial, which includes genetic variation as well as epigenetic factors[3]. Overall survival of patients with CRC has not much improved relative to significant advances in the management of CRC[4]. Thus, it is most importance to understand the molecular mechanisms underlying CRC tumorigenesis and recognize the fundamental genes responsible for such fatal cancer.
MicroRNAs (miRNAs) are endogenously expressed, small noncoding RNAs that bind at the 3′ untranslated region (3’-UTR) of their target mRNAs and promote mRNA degradation or inhibit translation[5]. miRNAs act as tumor suppressors or oncogenes by targeting the genes involved in cell proliferation, cell survival, apoptosis, and metastasis[6-8].
In humans, microRNA 375 (MIR375) is located on chromosomal band 2q35. MIR375 has been shown to have dual functions: As a tumor suppressor[9,10] and as an oncogene[11,12]. The dual characteristic of MIR375 depends on the target mRNA. In our previous study, we detected MIR375 in CRC[13] and dextran sulphate sodium (DSS)-induced mice colitis[14] via miRNA expression profiling of CRC tissues versus healthy colorectal tissues and DSS-induced colitis versus healthy colons, respectively. We found that MIR375 was significantly downregulated in both CRC and DSS-induced colitis tissue samples[13,14]. Additionally, we have shown that downregulation of MIR375 modulates epidermal growth factor receptor (EGFR) signaling pathways in human CRC cells and tissues by upregulating connective tissue growth factor (CTGF) expression[15].
Metadherin (MTDH, also known as AEG1, LYRIC, or LYRIC/3D3) is located on chromosome 8q22.1 and encodes for a 64 kDa protein. It was first detected as an upregulated transcript in primary human fetal astrocytes infected with human immunodeficiency virus 1 (HIV-1)[16]. Brown and Ruoslahti have shown that metadherin mediates tumor cell localization at the metastatic sites[17]. Several studies have shown the role of MTDH as an oncogene in different types of human malignant tumors[18] and revealed various functions such as increased tumor growth, invasion and metastasis, angiogenesis, and chemoresistance[19]. Furthermore, our previous research has shown that MTDH is one of the putative target genes of MIR375[15].
In this study, we show that MTDH is a target gene of MIR375 in CRC and analyze its functions in CRC tissues and cell lines. Additionally, we reveal that MIR375 regulates cell proliferation and migration in CRC progression by suppressing MTDH-mediated signaling pathways.
MATERIALS AND METHODS
Patients and tissue samples
The tissue samples used in this study were provided by Biobank of Wonkwang University Hospital, a member of National Biobank of Korea. On approval from the institutional review board and obtaining informed consent (WKIRB-201710-BR-012) from the patients, we collected 19 CRC tissue samples from 16 patients with colon cancer (10 males and 6 females) and 3 patients with rectal cancer (2 males and 1 female). Mean age of the patients with colon cancer and rectal cancer was 68.4 years and 67.0 years, respectively. Ten colon cancer tissue samples and matching healthy colon tissue samples (7 males and 3 females) were investigated to confirm the endogenous expression of MIR375. Additionally, 12 colon cancer tissue samples with matching healthy colon tissue samples and 3 rectal cancer tissue samples with matching healthy rectal tissue samples were assessed for MTDH expression levels. Four colon cancer tissue samples and matching healthy colon tissue samples (3 males and 1 female) were examined for immunohistochemistry analysis.
Cells culture and reagents
Human CRC cell lines; Caco2, HT29, LoVo, HCT116, and SW48 were obtained from Korea Cell Line Bank (KCLB, Seoul, South Korea) or American Type Culture Collection (ATCC, Manassas, VA, United States). SW48, HT29, Lovo, and HCT116 cells were cultured in RPMI 1640 (HyClone, Logan, UT, United States) supplemented with 10% FBS while Caco2 cells were cultured in α-MEM (HyClone, Logan, UT, United States) supplemented with 20% FBS in a humidified atmosphere containing 5% CO2 at 37 °C.
MTDH antibody and all secondary antibodies were purchased from Thermo Fisher Scientific (Waltham, MA, United States). NF-κB inhibitor alpha (NFKBIA/IκBα), nuclear factor κ B subunit 1 (NFκB1/p50), RELA (NFkB3/p65), protein kinase B (AKT), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies were obtained from Santa Cruz Biotechnology, Inc. (Dallas, Texas, United States). RAS, BRAF, p44/42 mitogen-activated protein kinase (MAPK) (Erk1/2), phospho-MAPK, phosphatidylinositol-4,5-biphosphate-3-kinase catalytic subunit alpha (PIK3CA), phospho-AKT, and GAPDH antibodies were purchased from Cell Signaling Technology (Danvers, MA, United States). β catenin (CTNNB1) antibody was purchased from Abcam (Cambridge, United Kingdom). Vascular endothelial growth factor A (VEGFA) antibody was purchased from Novus Biologicals (Centennial, CO, United States). Ez-cytox was obtained from DoGenBio (Seoul, South Korea) and dual luciferase reporter assay system was obtained from Promega (Madison, WI, United States). TRIzol and siPORT NeoFx transfection reagents were purchased from Ambion, Inc. (Waltham, MA, United States). Lipofectamine 2000 reagent was purchased from Invitrogen (Waltham, MA, United States) while Viromer blue transfection agent was purchased from Lipocalyx (Weinbergweg, Halle, Germany). RIPA buffer was obtained from Elpis biotech (Daejeon, South Korea) and DAB substrate kit was purchased from Pierce Biotechnology (Waltham, MA, United States).
RNA extraction and quantitative real-time polymerase chain reaction
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR) were performed according to our previously established protocol[13-15]. Total RNA was isolated using TRIzol reagent. After digesting with DNase and performing a sample clean-up, RNA samples were quantified, aliquoted, and stored at -80 °C. qRT-PCR was performed on total RNA samples that were isolated from tissue samples or cultured cells to synthesize cDNA using StepOne Real-time PCR system (Applied Biosystems, Foster City, CA, United States).
Differential miRNA expression patterns were validated by TaqMan qRT-PCR assay (Applied Biosystems, Foster City, CA, United States). qRT-PCR was performed using SYBR Green dye (ELPIS Biotech, Daejeon, Korea) to assess mRNA expression. RNU48 (for TaqMan qRT-PCR) or 5.8S (for SYBR qRT-PCR), and GAPDH served as endogenous controls for qRT-PCR of miRNA and mRNA, respectively. Each sample was analyzed in triplicates by qRT-PCR. Primers for qRT-PCR and TaqMan analysis are listed in Supplementary Table 1.
Transfection of oligonucleotides
Endogenous MIR375 mimic [hsa-miR-375, Pre-miR™ miRNA precursor (AM17100)], MTDH small interfering RNA (siRNA), and each of the negative controls were synthesized commercially (Ambion, Austin, TX, United States) and transfected at 50 nM. Transfection was performed according to our previously published protocols[13-15].
Luciferase reporter assay
Wild-type (WT) or mutant type (MT) fragments of the 3’-UTR of MTDH containing the predicted binding site for MIR375 were amplified using PCR. The primer set used for the experiment is shown in Supplementary Table 1. Plasmid constructions and analysis of the luciferase assay were executed following our previously published protocols[13-15].
Protein extraction and Western blot analysis
Protein extraction and western blot analysis were performed according to our earlier established methods[13-15]. Briefly, membranes were incubated overnight at 4 °C with primary antibodies to MTDH (1:250), NFKBIA (1:100), NFKB1 (1:50), RELA (1:100), KRAS (1:1000), BRAF (1:500), MAPK (1:1000), p-MAPK (1:500), CTNNB1 (1:2500), PIK3CA (1:1000), AKT (1:100), p-AKT (1:500), and VEGFA (1:500). Subsequently, the membranes were incubated with secondary antibodies (1:1000).
Cell proliferation assay
For cell proliferation assay, cells (2 × 104 cells/well) were transfected with MIR375 mimic, negative control siRNA, or MTDH siRNA (siMTDH) in 96-well plates. Cell growth was measured at 72 h after transfection using Ez-Cytox cell viability assay kit following manufacturer’s instructions. After incubating for 2 h, absorbance values were measured at 450 nm using SpectraMax (Molecular Devices, CA, United States). Percentage of viable cells was calculated by comparing to the number of viable cells in the untreated controls. Experiments were performed in triplicates. Cell proliferation assay was performed following our previously published protocols[15,20].
Immunohistochemistry
Immunohistochemistry assay was performed according to our previously established protocols[14,15]. The tissue slides were blocked with 3% BSA for 2 h at room temperature followed by overnight incubation at 4 °C with primary antibodies against MTDH (1:50) and RELA (1:50). The following day, the slides were incubated with SignalStain® Boost IHC detection reagent (Cell Signaling Technology; Danvers, MA, United States) for 2 h at room temperature. After washing, chromogenic substrate (Thermo Fisher Scientific; Waltham, MA, United States) was applied to visualize the staining of the target proteins. Following counterstaining with hematoxylin, the sections were dehydrated and mounted using a coverslip.
Statistical analysis
Sample size was estimated using the G*power software (Version 3.1., Heinrich Heine University, Duesseldorf, Germany). Each experiment was repeated at least three times and consistent results were obtained. Data are expressed as mean ± standard deviation (SD). The differences between the groups were evaluated using GraphPad Prism 5.0 statistical software (GraphPad Software Inc., San Diego, CA, United States) or Student’s t-test. Differences with P value less than 0.05 were considered as statistically significant.
RESULTS
Validation of MIR375 expression level in CRC tissues
Previously, we have shown MIR375 as a colon cancer-associated miRNA using miRNA microarray analysis of colon tumor tissues and matched healthy colon tissues[13]. Additionally, we have shown that MIR375 expression is downregulated in human CRC tissues. To confirm the result, we compared MIR375 expression in 10 human CRC tissues and matched healthy colon tissues by qRT-PCR. We found that MIR375 expression levels were significantly reduced in CRC tissues (P < 0.01; Supplementary Figure 1A).
Endogenous expression levels of MIR375 in CRC cell lines
To determine the endogenous expression levels of MIR375 in different cell lines, we performed qRT-PCR on the total RNA isolated from various cell lines including Caco2, SW480, HT29, HCT116, LoVo, and SW48 cells. As shown in Figure S1B, MIR375 expression level was highest in HT29 cells while it was lower in HCT116 and Caco2 cells (Supplementary Figure 1B).
MTDH is a direct target of MIR375
To determine the direct interaction between MTDH 3’-UTR and MIR375, we cloned the WT MTDH 3’-UTR region, the putative target sequence of MIR375, in a luciferase reporter vector (Figure 1A). We observed that luciferase activity was reduced by approximately 24% when cells were co-transfected with pre-MIR375 (P < 0.01, Figure 1B). As a control experiment, we cloned mutated MTDH 3’-UTR sequence which lacked ten of the total complementary bases (Figure 1A). As expected, repression of the luciferase activity was revoked when the interaction between MIR375 and its target 3’-UTR was disrupted (Figure 1B). Additionally, another control experiment was performed where pre-MIR1 (instead of pre-MIR375) was co-transfected with WT and mutated MTDH 3’-UTR constructs. We found that transfection with pre-MIR1 did not affect the luciferase activity of either of the constructs (Figure 1B).
Figure 1.
Metadherin is a direct target of microRNA 375. A: Sequence alignment of the wild type (WT) and mutated type (MT) microRNA 375 (MIR375) target site in the 3’-UTR of metadherin (MTDH); B: A luciferase reporter plasmid containing WT or MT MTDH 3’-UTR was co-transfected in HCT116 and Caco2 cells with pre-MIR1 as a negative control or pre-MIR375. Results are shown as relative firefly luciferase activity which is standardized to Renilla luciferase activity. Three independent experiments were conducted with duplicates; C: qRT-PCR analysis of MTDH mRNA expression in HCT116 and Caco2 cells. The data are presented as the fold change in MIR375 mimic transfected cells relative to non-transfected cells. Experiment was performed in duplicate and repeated 5 times; D: Cellular MTDH levels in MIR375 mimic-transfected and siMTDH-transfected HCT116, Caco2, and SW48 cells. Three independent experiments were conducted with duplicates. P values were calculated using Student’s t-test (aP < 0.05, bP < 0.01, cP < 0.001). MTDH: Metadherin; MIR375: MicroRNA 375; ns: Not significant.
MIR375 regulate MTDH expression in CRC cells
To validate the obtained data, we investigated whether MIR375 regulates MTDH mRNA levels in HCT116 and Caco2 cells. We found that MTDH mRNA expression levels were lower in HCT116 as well as Caco2 cells on transfection with MIR375 mimic compared with that in non-transfected control cells (P < 0.05; Figure 1C). Additionally, we investigated MTDH expression levels in MIR375 mimic or siMTDH-transfected HCT116, Caco2, and SW48 cells and found that cellular MTDH expression was significantly reduced in MIR375-overexpressing HCT116, Caco2, and SW48 cells. Furthermore, MTDH was significantly downregulated by siMTDH transfection (Figure 1D).
MIR375 regulates MTDH-mediated BRAF-MAPK signaling pathways
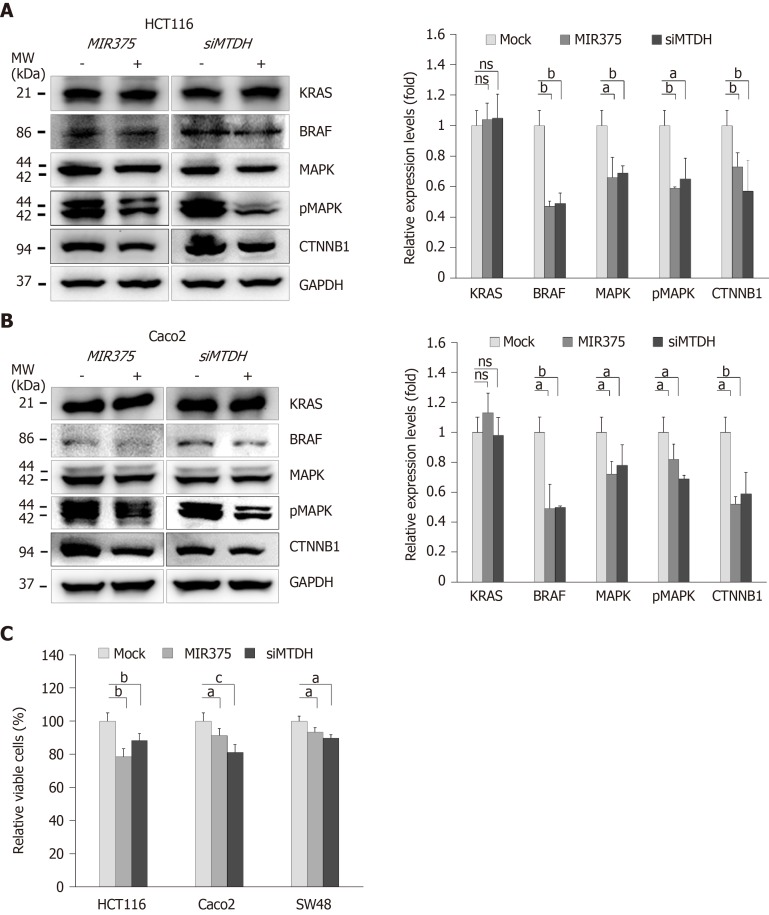
To determine the functional interaction between MIR375 and its target gene MTDH, we analyzed the expression levels of KRAS, BRAF, MAPK, pMAPK, and CTNNB1 in HCT116 and Caco2 cells on MIR375 mimic transfection. Earlier study has shown that HCT116 cells express WT BRAF and mutated KRAS while Caco2 cells express only WT KRAS and WT BRAF[21]. Although KRAS expression level was unaltered on MIR375 transfection, BRAF, MAPK, pMAPK, and CTNNB1 expression levels were significantly downregulated in HCT116 (Figure 2A) and Caco2 (Figure 2B) cells. We observed a similar expression trend in CRC cells on silencing MTDH with siMTDH (Figure 2A and B). These results suggested that MIR375 regulates the MTDH-mediated BRAF-MAPK signal pathway in CRC cells.
Figure 2.
MicroRNA 375 regulates metadherin-mediated BRAF-MAPK signaling in colorectal cancer cell lines. A: Western blot analysis of KRAS, BRAF, mitogen-activated protein kinase 3/1 (MAPK3/1), pMAPK3/1 and β catenin (CTNNB1) expression levels in colorectal cancer (CRC) cells. Except for KRAS; BRAF, MAPK3/1, pMAPK3/1 and CTNNB1 expression levels were downregulated on microRNA 375 (MIR375) mimic and siMTDH transfection in HCT116 cells; B: Western blot analysis of KRAS, BRAF, MAPK3/1, pMAPK3/1 and CTNNB1 expression levels in CRC cells. Except for KRAS; BRAF, MAPK3/1, pMAPK3/1 and CTNNB1 expression levels were downregulated on MIR375 mimic and siMTDH transfection in Caco2 cells. Five independent experiments were performed with duplicates; C: Effects of MIR375 and siMTDH on cell viability in HCT116, Caco2, and SW480 cells. Cell viability was determined by MTT assay. Three independent experiments were performed with duplicates and P values were calculated using Student’s t-test (aP < 0.05, bP < 0.01, cP < 0.001). ns: Not significant; MTDH: Metadherin; CTNNB1: β catenin; MAPK3/1: Mitogen-activated protein kinase 3/1; MIR375: microRNA 375; CRC: Colorectal cancer.
MIR375 inhibits CRC cells viability by inhibiting MTDH expression
We investigated the biological functions of MIR375 in CRC cells. MTT assay showed that cell viability was steadily reduced on MIR375 transfection in the CRC cell lines HCT116 (P < 0.01), Caco2 (P < 0.05), and SW48 (P < 0.05; Figure 2C) cells. Further, we found a similar trend on siMTDH transfection in HCT116 (P < 0.01), Caco2 (P < 0.001), and SW48 cells (P < 0.05; Figure 2C). These results suggested that MIR375 downregulates CRC cell proliferation by inhibiting MTDH expression. The rate of inhibition was lower in SW48 cells compared with that in HCT116 and Caco2 cells. This might be due to relatively high endogenous expression of MIR375 in SW48 cells than that observed in HCT116 and Caco2 cells (Supplementary Figure 1B).
MIR375 regulates MTDH-mediated PIK3CA-AKT signaling pathways
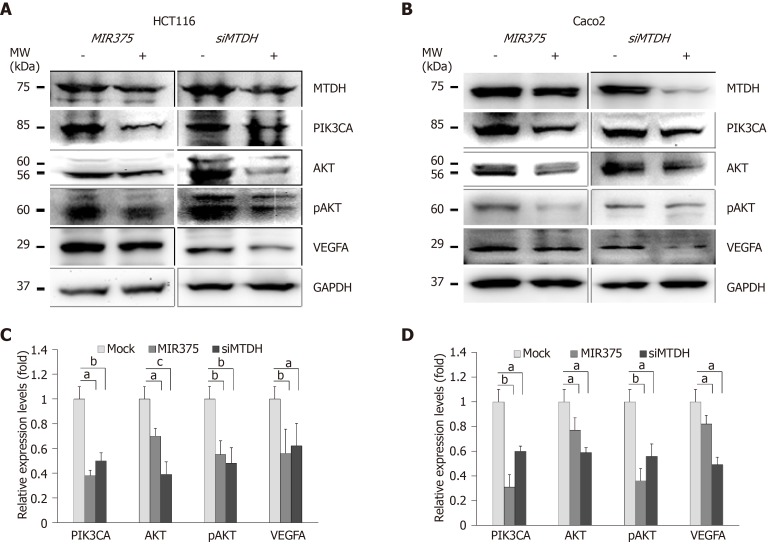
We investigated the functional correlation between MIR375 and MTDH expression in HCT116 and Caco2 cells. HCT116 cells are mutated for PIK3CA whereas Caco2 cells express wild type PIK3CA. We found that PIK3CA, AKT, pAKT, and VEGFA expression levels were downregulated on MIR375 mimic transfection in HCT116 as well as Caco2 cells (Figure 3A and 3B). Similar results were obtained on silencing MTDH in HCT116 and Caco2 cells (Figure 3C and 3D). Thus, the results suggested that MIR375 regulates MTDH-mediated PIK3CA-AKT signaling pathway by inhibiting MTDH expression levels in CRC cells.
Figure 3.
MicroRNA 375 regulates metadherin-mediated phosphatidylinositol-4,5-biphosphate-3-kinase catalytic subunit alpha-protein kinase B signaling in colorectal cancer cell lines. A: Western blot analysis of phosphatidylinositol-4,5-biphosphate-3-kinase catalytic subunit alpha (PIK3CA), protein kinase B (AKT), pAKT, and vascular endothelial growth factor A (VEGFA) expression levels in colorectal cancer (CRC) cells; B: Western blot analysis of PIK3CA, AKT, pAKT, and VEGFA expression levels in CRC cells; C: PIK3CA, AKT, pAKT, and VEGFA expression levels were downregulated on microRNA 375 (MIR375) mimic and siMTDH transfection in HCT116 cells; D: PIK3CA, AKT, pAKT, and VEGFA expression levels were downregulated on MIR375 mimic and siMTDH transfection in Caco2 cells. Five independent experiments were performed with duplicates and P values were calculated using Student’s t-test (aP < 0.05, bP < 0.01, cP < 0.001). MTDH: Metadherin; PIK3CA: Phosphatidylinositol-4,5-biphosphate-3-kinase catalytic subunit alpha; AKT: Protein kinase B; VEGFA: Vascular endothelial growth factor A; MIR375: microRNA 375; CRC: Colorectal cancer.
MIR375 regulates MTDH-mediated NFKB1 signaling pathways
Furthermore, we investigated another MTDH-mediated signaling pathway in CRC cells. To determine the role of MIR375 or MTDH-mediated pathways in NFKB1 signaling, we quantified the expression of relevant proteins in HCT116 and Caco2 cells on MIR375 mimic or siMTDH transfection. NFKB1 and RELA expression levels were found to be significantly downregulated in both the cell lines (HCT116 and Caco2 cells) on MIR375 transfection while NFKBIA expression levels were found to be upregulated (Figure 4A and B). We observed similar results on silencing MTDH using siMTDH in CRC cells (Figure 4C and D). Overall, the results evidently suggested that NFKBIA expression was upregulated on inhibiting MTDH in MIR375-overexpressing CRC cells which further leads to downregulation of NFKB1 and RELA expression in CRC cells. These results showed that MIR375 regulates MTDH-mediated NFKB1 signaling pathway.
Figure 4.
MicroRNA 375 regulates metadherin-mediated NFKB1 signaling in colorectal cancer cell lines. Western blot analysis of NF-κB inhibitor alpha (NFKBIA), NFKB1 (p50), and RELA (p65) expression levels in colorectal cancer (CRC) cells. A: NFKBIA expression levels were upregulated on microRNA 375 (MIR375) mimic and siMTDH transfection in HCT116 cells; B: NFKBIA expression levels were upregulated on MIR375 mimic and siMTDH transfection in Caco2 cells; C: NFKB1 and RELA levels were downregulated on MIR375 mimic and siMTDH transfection in HCT116 cells; D: NFKB1 and RELA levels were downregulated on MIR375 mimic and siMTDH transfection in Caco2 cells. Four independent experiments were performed with duplicates and P values were calculated using Student’s t-test (aP < 0.05, bP < 0.01). MTDH: Metadherin; MIR375: MicroRNA 375; NFKBIA: NF-κB inhibitor alpha; CRC: Colorectal cancer.
MTDH expression levels in human CRC tissues
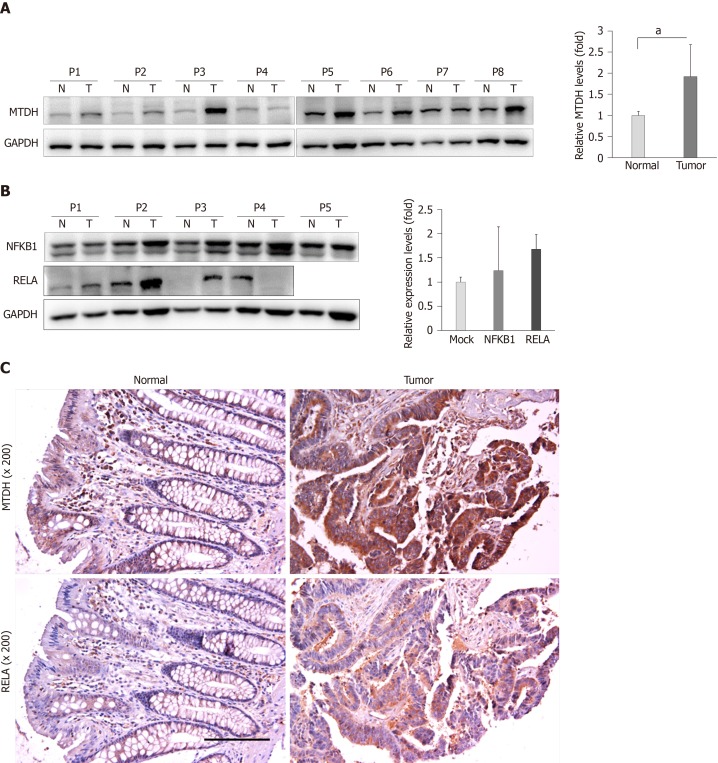
Based on the findings of this study, we evaluated MTDH expression in 15 human CRC tissues and matching healthy colon tissues. Western blot analysis showed that MTDH expression levels were upregulated (12 out of 15 samples) in CRC tissue samples compared with that in healthy colon tissues (P < 0.05, Figure 5A). Further, we investigated NFKB1 and RELA expression levels in five CRC tissues and four CRC tissue samples, respectively. NFKB1 expression level was significantly upregulated in all CRC tissues while RELA expression level was predominantly upregulated in three (75%) CRC tissues (Figure 5B).
Figure 5.
Endogenous metadherin expression in colorectal cancer tissues. A: metadherin (MTDH) expression levels were investigated in 15 colorectal cancer (CRC) tissue samples and matching healthy colorectal tissue samples. Results are shown for 8 colon cancer tissues in pairs. P1 to P8 indicate patients with colon cancer. Data are presented as fold change in the expression in tumor tissues relative to expression in matching healthy colon tissues. P values were calculated using Student’s t-test (aP < 0.05); B: Relative endogenous NFKB1 (n = 5) and RELA (n = 4) expression levels in colon cancer tissue samples and matching healthy colon tissue samples. Data are presented as fold change in the expression in tumor tissues relative to expression in matching healthy colon tissues; C: Immunostaining of MTDH in human CRC and adjacent healthy colorectal samples (200 × magnification). Experiments were independently performed three times in duplicates. MTDH: Metadherin.
Consistent with the results obtained, we investigated MTDH and RELA expression in four human CRC tissues and matching healthy colon tissues using immunohistochemical analysis. MTDH and RELA expression levels were significantly upregulated in CRC tissues (Figure 5C).
DISCUSSION
Many miRNAs have been detected as associated biomarkers and therapeutic targets in CRC. Several studies have shown that targeting specific miRNAs effectively inhibits cell proliferation and angiogenesis in CRC[22-24]. Thus, a better identification of CRC-associated miRNAs may contribute to the development of efficient miRNA-based therapy for CRC. In our previous study, we used miRNA expression profiling and showed MIR375 to be associated with human CRC tissue[13] as well as DSS-induced mice colitis[14] by comparing the expression pattern in CRC tissues versus matching healthy colorectal tissues and DSS-induced mice colitis versus healthy mice colons, respectively. Hyper-methylation of MIR375 has been demonstrated in CRC cell lines including HCT116 and SW480. Down regulation of MIR375 in HCT116 and SW480 cells compere to HT29 cells is due to hyper-methylation of MIR375 in HCT116 and SW480 cells[25]. In the present study, we confirmed the findings in a larger sample size and showed that MIR375 expression was downregulated in human CRC tissues compared with that in matching healthy colon tissues (Supplementary Figure 1A). The results of the current study are consistent with the earlier research work by Dai et al[26]. In addition to that in our study, MIR375 downregulation has been observed in several other types of cancer such as hepatocellular carcinoma[27], gastric cancer[28], and glioma[29]. However, some reports have revealed that MIR375 is upregulated in tumors of prostate cancer[12] and breast cancer[30]. Primarily, miRNA expression levels were believed to be cell type-specific in many cancer tissues[31].
In our previous study, we found that MIR375 regulates the CTGF-mediated EGFR-PIK3CA-AKT signaling pathway by directly downregulating CTGF expression in CRC cells. However, CTGF is not involved in EGFR-KRAS-BRAF-ERK1/2 signaling[15]. Although KRAS expression was unaffected by MIR375 overexpression, BRAF-ERK1/2 signaling was regulated on MIR375 overexpression in CRC cells[15]. These results guided us to investigate a novel MIR375 target gene that mediates the BRAF-ERK1/2 signaling pathway in CRC cells. In this study, we found that MTDH is a direct target gene of MIR375 in CRC cells (Figure 1). Further, we confirmed that MIR375 regulates the MTDH-mediated BRAF-ERK1/2 (MAPK3/1) signaling pathway (Figure 2A and 2B), which controls proliferation in CRC cells (Figure 2C). It is well known that MTDH contributes to the carcinogenic process of different tissues and organs by regulating multiple signaling pathways such as PI3K/AKT, NF-kB, and MAPK, which subsequently promotes tumorigenesis and metastasis[32,33].
MTDH promotes an invasive phenotype and angiogenesis via the PIK3CA-AKT signaling pathway. In addition, PIK3CA has been proven as a direct target of MIR375 in CRC cells[34]. MTDH expression is upregulated in many types of cancers, and is crucial in oncogenic transformation and angiogenesis[35-37]. The potential role of MTDH in angiogenesis has been correlated with VEGFA expression via the PIK3CA-AKT pathway in head and neck squamous cells[38]. Thus, PIK3CA-AKT-VEGFA signaling is affected on MIR375 overexpression or on VEGFA silencing (siVEGFA). We showed that PIK3CA-AKT-VEGFA signaling is downregulated on MIR375 overexpression and siVEGFA treatment in CRC cells (Figure 3). Our previous study using xenograft mouse model showed that the expression level of the angiogenic marker, CD31 was significantly decreased in xenograft tumors on MIR375 overexpression[15]. Consequently, these results suggest that MIR375 regulates angiogenesis via the MTDH-mediated PIK3CA-AKT-VEGFA signaling pathway in CRC progression.
Furthermore, MTDH has been shown to regulate the anchorage independent growth and invasion of HeLa cells via activation of the NF-κB pathway[35]. MTDH has also been shown to be upregulated during CRC development and liver metastasis through the NF-κB signaling pathway[39]. Similarly, in malignant glioma cells, MTDH has been found to mediate invasion and migration through activation of the NF-κB signaling pathway[40]. In this study, we showed that MIR375 regulates MTDH-mediated NFKB1 and RELA signaling by inhibiting NFKBIA expression in CRC cells (Figure 4).
In this study, MIR375 expression levels were downregulated in CRC tissues (Supplementary Figure 1A). Inversely, MTDH, NFKB1, and RELA expression levels were predominantly upregulated in CRC tumor tissues compared to their expression levels in matched healthy colon tissues (Figure 5A). Additionally, immunohistochemistry staining of the CRC tissues showed that MTDH and RELA expression were upregulated in CRC tumor tissues. Overall, these results suggested that MTDH expression levels were negatively correlated with MIR375 expression in CRC tissues.
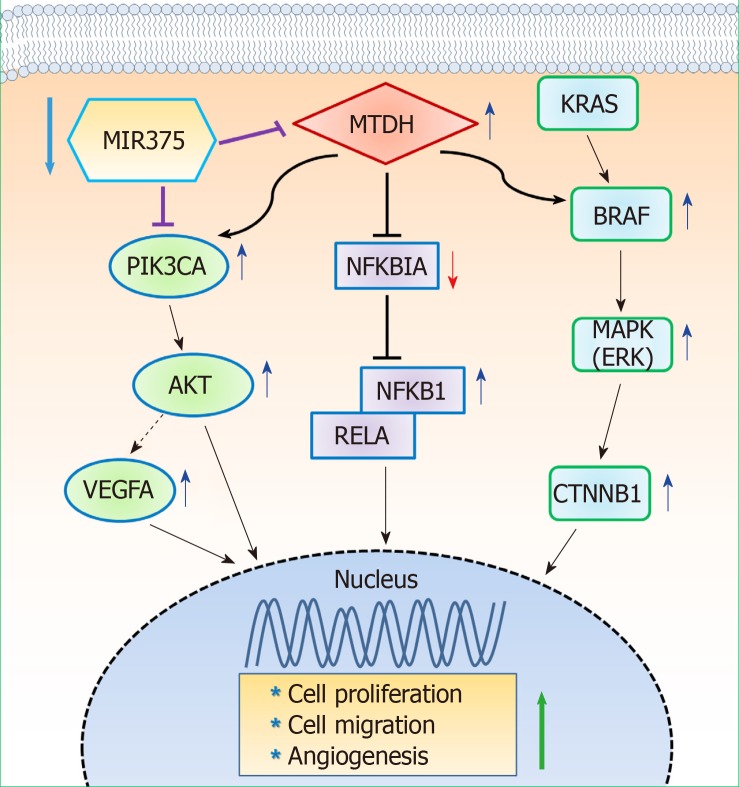
In summary, our study found that MIR375 expression is suppressed in tissues of patients with CRC and that MTDH is a direct target of MIR375. Furthermore, MTDH expression was upregulated in the tumors of CRC tissues on inhibiting MIR375 expression. Overall, our results suggest that MIR375 regulates MTDH-mediated signaling pathways such as MTDH-BRAF-MAPK, MTDH-PIK3CA-AKT, and MTDH-NFKBIA-NFKB1/RELA in CRC progression. Although we did not show MIR375-mediated VEGFA-VEGFR signaling in this study, our previous and present studies suggest that the generated VEGFA by MIR375-mediated PIK3CA-AKT or MTDH-PIK3CA-AKT signaling might effect to endothelial cell’s angiogenesis. Subsequently, MIR375 regulates cell proliferation, cell migration, and angiogenesis in CRC progression (Figure 6). Thus, we propose MIR375 to be a promising therapeutic target in inhibiting CRC tumorigenesis. However, this needs to be further investigated.
Figure 6.
Diagrammatic representations of putative mechanisms of microRNA 375 in regulating metadherin-induced cell proliferation, cell migration, and angiogenesis in human colorectal cancer. Decreased microRNA 375 (MIR375) expression in colorectal cancer (CRC) cells leads to upregulation of cellular metadherin (MTDH) levels. Subsequently, upregulated expression of MTDH promotes inhibition of NFKBIA and thus, NFKB1 and RELA expression is upregulated in CRC tissues and CRC cells. Upregulated MTDH expression level stimulates the BRAF-MAPK and PIK3CA-AKT signaling pathway. However, KRAS expression is unaltered by upregulation of MTDH. Consequently, downregulated MIR375 expression levels in CRC leads to upregulation of cell proliferation, cell migration, and angiogenesis. This simple hypothetical mechanism of MIR375-mediated upregulation of angiogenesis is based on the results of previous studies and our current study. MTDH: Metadherin; MIR375: MicroRNA 375; CRC: Colorectal cancer.
ARTICLE HIGHLIGHTS
Research background
Colorectal cancer (CRC) is the third most prevalent type of cancer worldwide. The cause of CRC is multifactorial including genetic variation and epigenetic and environmental factors. However, the precise molecular mechanism underlying the development and progression of CRC remains largely unknown. We previously found that microRNA 375 (MIR375) is significantly downregulated in CRC, and identified metadherin (MTDH) as a candidate target gene of MIR375.
Research motivation
MIR375 and their target MTDH will provide a new therapeutic information for human CRC.
Research objectives
To study the interaction and signaling between MIR375 and MTDH in human CRC pathogenesis.
Research methods
We constructed luciferase reporter plasmids to confirm the effect of MIR375 on MTDH gene expression. The expression levels of the MIR375 and MTDH were measured by qRT-PCR, Western blot, or immunohistochemistry. The effects of MIR375 on cell growth and angiogenesis were conducted by functional experiments in CRC cells. Assays were performed to explore functional correlation between MTDH and MIR375 in human CRC cells and tissues.
Research results
In the present study, we found that the expression levels of MTDH were significantly down-regulated in CRC cells by MIR375 mimic or siMTDH transfection. MTDH expression was up-regulated in human CRC tissues in comparing to match normal colon tissues. Upregulated MTDH expression levels were found to inhibit NF-κB inhibitor alpha (NFKBIA) expression, which further upregulated NFKB1 and RELA expression. We found that MIR375 regulate the expression levels of molecules in MTDH-mediated BRAF-MAPK and PIK3CA-AKT signal pathways in CRC cells.
Research conclusions
MIR375 regulates cell proliferation and angiogenesis by regulation of MTDH-mediated signaling pathways such as MTDH-BRAF-MAPK, MTDH-PIK3CA-AKT, and MTDH-NFKBIA-NFKB1/RELA in CRC progression.
Research perspectives
This study provides insight into the role of MIR375 in CRC pathogenesis by targeting MTDH. MIR375 might be a new therapeutic target for CRC.
ACKNOWLEDGEMENTS
Biospecimens used in this study were provided by Biobank of Wonkwang University Hospital, a member of the National Biobank of Korea which is supported by Ministry of Health and Welfare.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Review Board of Wonkwang University, South Korea (WKIRB-201710-BR-012).
Conflict-of-interest statement: The authors declare that they have no conflicts of interest.
ARRIVE guidelines statement: This study was prepared according to the ARRIVE guidelines.
Manuscript source: Invited Manuscript
Peer-review started: October 10, 2019
First decision: November 10, 2019
Article in press: November 23, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Donato R, Tarnawski AS, Yu B S-Editor: Wang J L-Editor: A E-Editor: Qi LL
Contributor Information
Seol-Hee Han, Department of Pathology, School of Medicine, Wonkwang University, Iksan, Chonbuk 54538, South Korea.
Ji-Su Mo, Department of Pathology, School of Medicine, Wonkwang University, Iksan, Chonbuk 54538, South Korea; Digestive Disease Research Institute, Wonkwang University, Iksan, Chonbuk 54538, South Korea.
Won-Cheol Park, Digestive Disease Research Institute, Wonkwang University, Iksan, Chonbuk 54538, South Korea.
Soo-Cheon Chae, Department of Pathology, School of Medicine, Wonkwang University, Iksan, Chonbuk 54538, South Korea; Digestive Disease Research Institute, Wonkwang University, Iksan, Chonbuk 54538, South Korea. chaesc@wku.ac.kr.
Data sharing statement
No data is shared.
References
- 1.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 4.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25–36. doi: 10.1016/s0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- 7.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 8.Su H, Yang JR, Xu T, Huang J, Xu L, Yuan Y, Zhuang SM. MicroRNA-101, down-regulated in hepatocellular carcinoma, promotes apoptosis and suppresses tumorigenicity. Cancer Res. 2009;69:1135–1142. doi: 10.1158/0008-5472.CAN-08-2886. [DOI] [PubMed] [Google Scholar]
- 9.Jung HM, Patel RS, Phillips BL, Wang H, Cohen DM, Reinhold WC, Chang LJ, Yang LJ, Chan EK. Tumor suppressor miR-375 regulates MYC expression via repression of CIP2A coding sequence through multiple miRNA-mRNA interactions. Mol Biol Cell. 2013;24:1638–1648, S1-S7. doi: 10.1091/mbc.E12-12-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang F, Li Y, Zhou J, Xu J, Peng C, Ye F, Shen Y, Lu W, Wan X, Xie X. miR-375 is down-regulated in squamous cervical cancer and inhibits cell migration and invasion via targeting transcription factor SP1. Am J Pathol. 2011;179:2580–2588. doi: 10.1016/j.ajpath.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giricz O, Reynolds PA, Ramnauth A, Liu C, Wang T, Stead L, Childs G, Rohan T, Shapiro N, Fineberg S, Kenny PA, Loudig O. Hsa-miR-375 is differentially expressed during breast lobular neoplasia and promotes loss of mammary acinar polarity. J Pathol. 2012;226:108–119. doi: 10.1002/path.2978. [DOI] [PubMed] [Google Scholar]
- 12.Szczyrba J, Nolte E, Wach S, Kremmer E, Stöhr R, Hartmann A, Wieland W, Wullich B, Grässer FA. Downregulation of Sec23A protein by miRNA-375 in prostate carcinoma. Mol Cancer Res. 2011;9:791–800. doi: 10.1158/1541-7786.MCR-10-0573. [DOI] [PubMed] [Google Scholar]
- 13.Mo JS, Alam KJ, Kang IH, Park WC, Seo GS, Choi SC, Kim HS, Moon HB, Yun KJ, Chae SC. MicroRNA 196B regulates FAS-mediated apoptosis in colorectal cancer cells. Oncotarget. 2015;6:2843–2855. doi: 10.18632/oncotarget.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mo JS, Alam KJ, Kim HS, Lee YM, Yun KJ, Chae SC. MicroRNA 429 Regulates Mucin Gene Expression and Secretion in Murine Model of Colitis. J Crohns Colitis. 2016;10:837–849. doi: 10.1093/ecco-jcc/jjw033. [DOI] [PubMed] [Google Scholar]
- 15.Alam KJ, Mo JS, Han SH, Park WC, Kim HS, Yun KJ, Chae SC. MicroRNA 375 regulates proliferation and migration of colon cancer cells by suppressing the CTGF-EGFR signaling pathway. Int J Cancer. 2017;141:1614–1629. doi: 10.1002/ijc.30861. [DOI] [PubMed] [Google Scholar]
- 16.Su ZZ, Kang DC, Chen Y, Pekarskaya O, Chao W, Volsky DJ, Fisher PB. Identification and cloning of human astrocyte genes displaying elevated expression after infection with HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid subtraction hybridization, RaSH. Oncogene. 2002;21:3592–3602. doi: 10.1038/sj.onc.1205445. [DOI] [PubMed] [Google Scholar]
- 17.Brown DM, Ruoslahti E. Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell. 2004;5:365–374. doi: 10.1016/s1535-6108(04)00079-0. [DOI] [PubMed] [Google Scholar]
- 18.Ying Z, Li J, Li M. Astrocyte elevated gene 1: biological functions and molecular mechanism in cancer and beyond. Cell Biosci. 2011;1:36. doi: 10.1186/2045-3701-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emdad L, Sarkar D, Su ZZ, Randolph A, Boukerche H, Valerie K, Fisher PB. Activation of the nuclear factor kappaB pathway by astrocyte elevated gene-1: implications for tumor progression and metastasis. Cancer Res. 2006;66:1509–1516. doi: 10.1158/0008-5472.CAN-05-3029. [DOI] [PubMed] [Google Scholar]
- 20.Mo JS, Han SH, Yun KJ, Chae SC. MicroRNA 429 regulates the expression of CHMP5 in the inflammatory colitis and colorectal cancer cells. Inflamm Res. 2018;67:985–996. doi: 10.1007/s00011-018-1194-z. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed D, Eide PW, Eilertsen IA, Danielsen SA, Eknæs M, Hektoen M, Lind GE, Lothe RA. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis. 2013;2:e71. doi: 10.1038/oncsis.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Lauriola M, Kim D, Francesconi M, D'Uva G, Shibata D, Malafa MP, Yeatman TJ, Coppola D, Solmi R, Cheng JQ. Adenomatous polyposis coli (APC) regulates miR17-92 cluster through β-catenin pathway in colorectal cancer. Oncogene. 2016;35:4558–4568. doi: 10.1038/onc.2015.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji S, Ye G, Zhang J, Wang L, Wang T, Wang Z, Zhang T, Wang G, Guo Z, Luo Y, Cai J, Yang JY. miR-574-5p negatively regulates Qki6/7 to impact β-catenin/Wnt signalling and the development of colorectal cancer. Gut. 2013;62:716–726. doi: 10.1136/gutjnl-2011-301083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang L, Gao C, Li Y, Sun M, Xu J, Li H, Jia L, Zhao Y. miR-125a-3p/FUT5-FUT6 axis mediates colorectal cancer cell proliferation, migration, invasion and pathological angiogenesis via PI3K-Akt pathway. Cell Death Dis. 2017;8:e2968. doi: 10.1038/cddis.2017.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christensen LL, Holm A, Rantala J, Kallioniemi O, Rasmussen MH, Ostenfeld MS, Dagnaes-Hansen F, Øster B, Schepeler T, Tobiasen H, Thorsen K, Sieber OM, Gibbs P, Lamy P, Hansen TF, Jakobsen A, Riising EM, Helin K, Lubinski J, Hagemann-Madsen R, Laurberg S, Ørntoft TF, Andersen CL. Functional screening identifies miRNAs influencing apoptosis and proliferation in colorectal cancer. PLoS One. 2014;9:e96767. doi: 10.1371/journal.pone.0096767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai X, Chiang Y, Wang Z, Song Y, Lu C, Gao P, Xu H. Expression levels of microRNA-375 in colorectal carcinoma. Mol Med Rep. 2012;5:1299–1304. doi: 10.3892/mmr.2012.815. [DOI] [PubMed] [Google Scholar]
- 27.He XX, Chang Y, Meng FY, Wang MY, Xie QH, Tang F, Li PY, Song YH, Lin JS. MicroRNA-375 targets AEG-1 in hepatocellular carcinoma and suppresses liver cancer cell growth in vitro and in vivo. Oncogene. 2012;31:3357–3369. doi: 10.1038/onc.2011.500. [DOI] [PubMed] [Google Scholar]
- 28.Ding L, Xu Y, Zhang W, Deng Y, Si M, Du Y, Yao H, Liu X, Ke Y, Si J, Zhou T. MiR-375 frequently downregulated in gastric cancer inhibits cell proliferation by targeting JAK2. Cell Res. 2010;20:784–793. doi: 10.1038/cr.2010.79. [DOI] [PubMed] [Google Scholar]
- 29.Chang C, Shi H, Wang C, Wang J, Geng N, Jiang X, Wang X. Correlation of microRNA-375 downregulation with unfavorable clinical outcome of patients with glioma. Neurosci Lett. 2012;531:204–208. doi: 10.1016/j.neulet.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 30.de Souza Rocha Simonini P, Breiling A, Gupta N, Malekpour M, Youns M, Omranipour R, Malekpour F, Volinia S, Croce CM, Najmabadi H, Diederichs S, Sahin O, Mayer D, Lyko F, Hoheisel JD, Riazalhosseini Y. Epigenetically deregulated microRNA-375 is involved in a positive feedback loop with estrogen receptor alpha in breast cancer cells. Cancer Res. 2010;70:9175–9184. doi: 10.1158/0008-5472.CAN-10-1318. [DOI] [PubMed] [Google Scholar]
- 31.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emdad L, Das SK, Dasgupta S, Hu B, Sarkar D, Fisher PB. AEG-1/MTDH/LYRIC: signaling pathways, downstream genes, interacting proteins, and regulation of tumor angiogenesis. Adv Cancer Res. 2013;120:75–111. doi: 10.1016/B978-0-12-401676-7.00003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Y, Li LP. Progress of cancer research on astrocyte elevated gene-1/Metadherin (Review) Oncol Lett. 2014;8:493–501. doi: 10.3892/ol.2014.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Tang Q, Li M, Jiang S, Wang X. MicroRNA-375 inhibits colorectal cancer growth by targeting PIK3CA. Biochem Biophys Res Commun. 2014;444:199–204. doi: 10.1016/j.bbrc.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 35.Emdad L, Lee SG, Su ZZ, Jeon HY, Boukerche H, Sarkar D, Fisher PB. Astrocyte elevated gene-1 (AEG-1) functions as an oncogene and regulates angiogenesis. Proc Natl Acad Sci USA. 2009;106:21300–21305. doi: 10.1073/pnas.0910936106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li C, Li R, Song H, Wang D, Feng T, Yu X, Zhao Y, Liu J, Yu X, Wang Y, Geng J. Significance of AEG-1 expression in correlation with VEGF, microvessel density and clinicopathological characteristics in triple-negative breast cancer. J Surg Oncol. 2011;103:184–192. doi: 10.1002/jso.21788. [DOI] [PubMed] [Google Scholar]
- 37.Long M, Dong K, Gao P, Wang X, Liu L, Yang S, Lin F, Wei J, Zhang H. Overexpression of astrocyte-elevated gene-1 is associated with cervical carcinoma progression and angiogenesis. Oncol Rep. 2013;30:1414–1422. doi: 10.3892/or.2013.2598. [DOI] [PubMed] [Google Scholar]
- 38.Zhu GC, Yu CY, She L, Tan HL, Li G, Ren SL, Su ZW, Wei M, Huang DH, Tian YQ, Su RN, Liu Y, Zhang X. Metadherin regulation of vascular endothelial growth factor expression is dependent upon the PI3K/Akt pathway in squamous cell carcinoma of the head and neck. Medicine (Baltimore) 2015;94:e502. doi: 10.1097/MD.0000000000000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gnosa S, Shen YM, Wang CJ, Zhang H, Stratmann J, Arbman G, Sun XF. Expression of AEG-1 mRNA and protein in colorectal cancer patients and colon cancer cell lines. J Transl Med. 2012;10:109. doi: 10.1186/1479-5876-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarkar D, Park ES, Emdad L, Lee SG, Su ZZ, Fisher PB. Molecular basis of nuclear factor-kappaB activation by astrocyte elevated gene-1. Cancer Res. 2008;68:1478–1484. doi: 10.1158/0008-5472.CAN-07-6164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data is shared.