Abstract
Cyclin-dependent kinase (CDK) 4/6 inhibitors have become standard of care in the treatment of hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer. They have been shown to double the efficacy of endocrine-based treatment. Three oral agents are available to date: palbociclib, ribociclib, and abemaciclib. The aim of this article is to give a short overview of the existing efficacy data, to summarize the recommended clinical monitoring procedures for patients under CDK4/6 inhibitors, and to shed light on the clinical management of the most common treatment-emergent adverse events. The hematological class side effect neutropenia as well as non-hematological toxicities (e.g., impaired liver function, prolonged QTc interval, and diarrhea) are discussed. In addition, the current knowledge about relevant drug interactions is reviewed.
Keywords: Advanced breast cancer, Palbociclib, Ribociclib, Abemaciclib, Cyclin-dependent kinase 4/6, Endocrine therapy, Cell cycle
Introduction
In January 2015, striking efficacy data for palbociclib, the first-in-class cyclin-dependent kinase (CDK) 4/6 inhibitor, were published for the first time [1]. One month later, based on these data, the US Food and Drug Administration (FDA) approved the first CDK4/6 inhibitor palbociclib (Ibrance®; Pfizer Inc.) for the treatment of postmenopausal women with estrogen receptor-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer as first-line therapy in combination with letrozole. Meanwhile, CDK4/6 inhibitors have found their way into the standard treatment of women with hormone receptor (HR)-positive, HER2-negative metastatic breast cancer (MBC). To date, three different CDK4/6 inhibitors have been approved by the European Medical Association (EMA): palbociclib (Ibrance®; Pfizer, New York, NY, USA), ribociclib (Kisquali®; Novartis, Basel, Switzerland), and abemaciclib (Verzenios®; Eli Lilly, Indianapolis, IN, USA). Each of them can be used in combination with an aromatase inhibitor as well as with fulvestrant, either as initial endocrine-based therapy or after disease progression following endocrine therapy.
The aim of this article is to give a condensed overview of existing evidence concerning the clinical characteristics of the three agents with a focus on monitor requirements and management of the most common and clinically relevant adverse events (AEs) during treatment with CDK4/6 inhibitors in breast cancer patients.
Efficacy Data of Endocrine-Based Therapy with CDK4/6 Inhibitors in MBC
There is remarkable consistency in the efficacy data of phase II and phase III trials dealing with first-line CDK4/6 inhibition in advanced/metastatic HR-positive MBC (Table 1). In patients with measurable disease, the objective response rate in all trials was over 50%. In all trials, the proportion of patients who had visceral metastases at the time of beginning CDK4/6inhibitor-based therapy was around 60%. The pronounced efficacy of CDK4/6 inhibitors is not limited to postmenopausal patients: In the MONALEESA-7 study, only pre- and perimenopausal patients were randomized to receive endocrine-based therapy alone versus in combination with ribociclib. With a prolongation of the median progression-free survival (PFS) from 13.0 to 23.8 months and a hazard ratio of 0.55, the MONALEESA-7 study results align seamlessly with the results of all other trials [2].
Table 1.
| Trial | Phase, treatment | n | ORR, % | PFS, months | HR |
|---|---|---|---|---|---|
| PALOMA-1 | II, letrozol/palbociclib | 165 | 39 vs. 55 | 10.2 vs. 20.2 | 0.49 |
| PALOMA-2 | III, letrozol/palbociclib | 666 | 44 vs. 55 | 14.5 vs. 24.8 | 0.58 |
| MONALEESA-2 | III, letrozol/ribociclib | 668 | 37 vs. 53 | 14.7 vs. NR | 0.56 |
| MONARCH-3 | III, NSAI/abemaciclib | 493 | 44 vs. 59 | 14.7 vs. NR | 0.54 |
| MONALEESA-7 | III, ET+OFS/ribociclib | 672 | 36 vs. 51 | 13.0 vs. 23.8 | 0.55 |
NSAI, nonsteroidal aromatase inhibitor; ET, endocrine therapy; OFS, ovarian function suppression; ORR, objective response rate; PFS, progression-free survival; HR, hazard ratio; NR, not reached.
Table 2 summarizes efficacy data of CDK4/6 inhibitors in the endocrine-resistant setting: in the PALOMA-3, MONARCH-2, and MONALEESA-3 studies, the CDK4/6 inhibitors were combined with fulvestrant. Similar to the endocrine-sensitive setting, the addition of a CDK4/6-inhibiting agent lead to a doubling of PFS in all three randomized trials. No conclusions can be drawn from the difference in the response rates of the different studies because other than in the MONARCH-2 and MONALEESA-3 studies, patients who had been treated with first-line chemotherapy were allowed to be included in the PALOMA-3 study. Of note, abemaciclib is the only CDK4/6 inhibitor, which has been tested as a single-agent therapy within a phase II trial and showed efficacy in this setting.
Table 2.
Efficacy of CDK4/6 inhibitors in selected trials in the second-line/endocrine-resistant setting [18]
| Trial | Phase, treatment | n | ORR, % | PFS, months | HR |
|---|---|---|---|---|---|
| PALOMA-3 | III, fulv/palbociclib | 521 | 11 vs. 25 | 4.6 vs. 9.5 | 0.46 |
| MONARCH-2 | III, fulv/abemaciclib | 669 | 21 vs. 48 | 9.3 vs. 16.4 | 0.55 |
| MONARCH-1 | II, abemaciclib | 132 | 20 | 6.0 |
Fulv, fulvestrant; ORR, objective response rate; PFS, progression-free survival; HR, hazard ratio.
Monitoring Requirements during the Treatment with CDK4/6 Inhibitors
In clinical routine, certain monitoring procedures have been established with the use of CDK4/6 inhibitors. These procedures include regular drawing of the complete blood count with palbociclib, ribociclib, and abemaciclib (Table 3), regular control of liver function tests (LFTs) with ribociclib and abemaciclib (Table 4), and regular control of electrocardiogram (ECG) together with monitoring of the QTc interval with ribociclib (Table 5). With ribociclib, appropriate monitoring of serum electrolytes (including potassium, calcium, phosphorous, and magnesium) should also be performed prior to the initiation of treatment, at the beginning of the first 6 cycles, and as clinically indicated.
Table 3.
Requirements for monitoring complete blood count during the treatment with CDK4/6 inhibitors [9, 19, 20]
| Cycle 1 |
Cycle 2 |
Cycles 3–4 | Cycles 5–6 | Cycles 6+ | |||||
|---|---|---|---|---|---|---|---|---|---|
| day 1 | day 14 | day 15 | day 1 | day 14 | day 15 | day 1 | day 1 | ||
| Palbociclib | × | × | × | × | × | × | day 1a | ||
| Ribociclib | × | × | × | × | × | × | as indicated | ||
| Abemaciclib | × | × | × | × | × | as indicated | as indicated | ||
For patients who experience a maximum of grade 1 or 2 neutropenia in the first 6 cycles, complete blood counts for subsequent cycles should be monitored every 3 months, prior to the beginning of a cycle, and as clinically indicated.
Table 4.
| Cycle 1 |
Cycle 2 |
Cycles 3–4 | Cycles 5–6 | Cycles 6+ | |||
|---|---|---|---|---|---|---|---|
| day 1 | day 14 | day 1 | day 14 | day 1 | day 1 | ||
| Ribocicliba | × | × | × | × | × | × | as indicated |
| Abemaciclib | × | × | × | × | × | as indicated | as indicated |
If grade ≥2 abnormalities are noted, more frequent monitoring is recommended.
Table 5.
ECG and QTc monitoring with ribociclib [20]
| Before treatment | Cycle 1 |
Cycle 2 |
Cycles 3+ | ||
|---|---|---|---|---|---|
| day 1 | day 14 | day 1 | day 14 | day 1 | |
| × | × | × | as indicated | ||
Management of Neutropenia during the Treatment with CDK4/6 Inhibitors
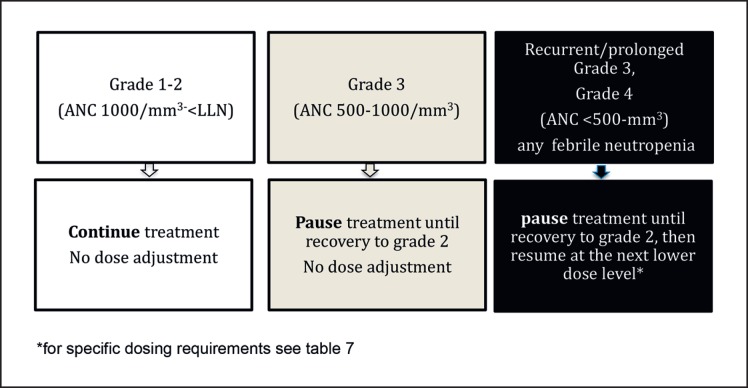
Neutropenia has been identified as the class side effect of CDK4/6 inhibitors by AE assessment within the PALOMA, MONALEESA, and MONARCH clinical trials [1, 2, 3, 4, 5]. Even though grade 3 and grade 4 neutropenia were very common in these trials, the rate of febrile neutropenia did not exceed 2% (Table 6). This is in contrast to the febrile neutropenia rates reported with chemotherapy. For example, in the HERNATA trial where first-line MBC patients had been treated with either docetaxel or vinorelbine, a febrile neutropenia rate up to 36% [6] was reported. The reason for this difference lies in the distinct biological mechanisms being responsible for neutropenia caused by CDK4/6 inhibitors versus cytotoxic chemotherapy agents: it has been shown in vitro that palbociclib causes cell cycle arrest but no death of proliferating neutrophil precursor cells, thus allowing for a rapid recovery of the neutrophil count. Unlike CDK4/6 inhibitors, chemotherapy induces DNA damage and apoptosis of proliferating neutrophil precursors, leading to a delayed recovery of the neutrophil count [7]. Because of these differences, granulocyte colony-stimulating factor should not be used for the management of CDK4/6 inhibitor-induced neutropenia. Neutropenia is managed by dose interruption and dose modification of the CDK4/6 inhibitor while continuing the endocrine agents (Fig. 1, Table 7). If febrile neutropenia occurs, the CDK4/6 inhibitor should be withheld and resumed at the next lower dose level.
Table 6.
| AE | Palbociclib |
Ribocliclib |
Abemaciclib |
|||
|---|---|---|---|---|---|---|
| any grade | grades 3/4 | any grade | grades 3/4 | any grade | grades 3/4 | |
| Neutropenia | 81 | 65 | 74 | 58 | 41–46 | 22–27 |
| Febrile neutropenia | 2 | 1 | 1 | NR | <1 | |
Values are presented as percentages. NR, not reached.
Fig. 1.
Management of neutropenia through dose modifications. For specific dosing requirements, see Table 7.
Table 7.
Dosing recommendations for CDK4/6 inhibitors in combination with endocrine therapy inhibitors [9, 19, 20]
| Dose level | Palbociclib | Ribociclib | Abemaciclib |
|---|---|---|---|
| Recommended starting dose First dose reduction Second dose reduction | |||
| 125 mg daily 3/4 weeks 100 mg daily 3/4 weeks 75 mg daily 3/4 weeks | |||
| 600 mg daily 3/4 weeks 400 mg daily 3/4 weeks 200 mg daily 3/4 weeks | |||
| 200 mg twice daily 150 mg twice daily 100 mg twice daily |
If neutropenia coincides with pancytopenia, bone marrow biopsy should be considered to detect possible bone marrow infiltration.
Management of Impaired LFTs during the Treatment with CDK4/6 Inhibitors
Early monitoring of LFTs when starting CDK4/6 inhibitor treatment is necessary to identify asymptomatic increase in liver enzymes and to differentiate between CDK4/6 inhibitor toxicity and other reasons such as hepatic progression. Since LFT abnormalities are more common with ribociclib and abemaciclib than with palbociclib, specific recommendations for dose modifications exist with ribociclib and abemaciclib [6, 20].
Management of Qtc Prolongation during the Treatment with Ribociclib
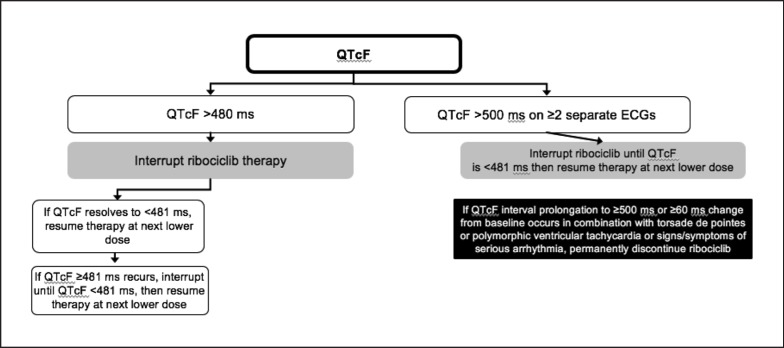
While no clinically significant effects on the QTc interval were observed with palbociclib [8] or abemaciclib [9], ribociclib has been shown to prolong the QTc interval [4]. Treatment with ribociclib should be initiated only in patients with QTcF values < 450 ms. ECG should be assessed before initiating the treatment with ribociclib and monitored thereafter according to Table 5. Any abnormality in electrolytes should be corrected before the start of ribociclib treatment. In the event of hypokalemia and/or hypomagnesemia, ribociclib should be interrupted until these are corrected. Management of Qtc prolongation during the treatment with ribociclib is shown in Figure 2. In case of QTcF prolongation during the treatment, more frequent ECG monitoring is recommended.
Fig. 2.
Management of QTc prolongation during the treatment with ribociclib [21].
Management of Diarrhea during the Treatment with Abemaciclib
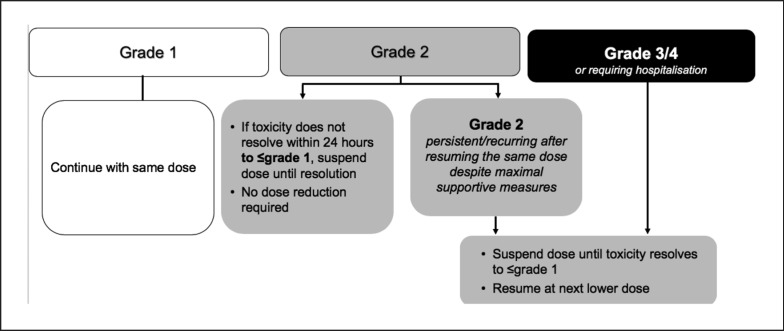
While palbociclib and ribociclib can be seen as highly selective inhibitors of CDK4 and CDK6, abemaciclib, on a molecular basis, has been shown to have more off-target effects on a variety of other kinases [10]. As a consequence, abemaciclib, unlike palbociclib and ribociclib, can lead to grade 3 diarrhea: in the MONARCH-2 trial, grade 3 diarrhea occurred in 13.4% of the patients treated with abemaciclib [11]. Management of diarrhea should be proactive: at first signs of loose stools, antidiarrheal medication (e.g., loperamide) should be initiated along with an increase in oral fluids. For further management of diarrhea, see Figure 3.
Fig. 3.
Management of diarrhea with abemaciclib [5].
Increase in Serum Creatinine during the Treatment with Abemaciclib
In MONARCH 2, increase in serum creatinine (SCr) was observed in 98.4% of the patients receiving abemaciclib [11]. This increase in SCr due to abemaciclib results from the inhibition of a molecular pump that transports creatinine from the blood to the urine. It occurs within the first 28-day cycle of abemaciclib, remains elevated but stable throughout the treatment period, and is reversible upon treatment discontinuation [12, 13]. Renal function (glomerular filtration rate [GFR]) is not affected by abemaciclib treatment, as are other measures of GFR that do not rely on SCr (such as cystatin C-calculated GFR) [14]. If the increase in SCr is progressive or there are other indications for renal injury (e.g., proteinuria), measures of renal function such as cystatin C GFR should be used as an alternative to either SCr or SCr-based calculations of GFR, since creatinine would not be an accurate method to assess renal function.
CDK4/6 Inhibitors and Surgical Procedures or Radiation Therapy
There are no evidence-based recommendations for dose modifications of CDK4/6 inhibitors during times around surgical procedures or radiation therapy. In our own clinical practice, we currently advise to withhold the CDK4/6 inhibitor 1 week before surgery or radiation therapy while continuing the endocrine agent. As soon as the wound-healing process is seen as satisfactory, the CDK4/6 inhibitor can be resumed again. In the case of radiation therapy, we would recommend withholding the CDK4/6 inhibitor for 2–4 weeks after the last irradiation, depending on the size of the irradiated region.
Drug Interactions with CDK4/6 Inhibitors
It is of great importance to take a detailed history of concomitant medications including prescription medicines, over-the-counter drugs, vitamins, and herbal products before starting a patient on CDK4/6 inhibitors. In addition to that, patients must be advised to double-check with their oncologist before starting any new medication.
Palbociclib, ribociclib, and abemaciclib are primari ly metabolized by CYP3A and sulfotransferase enzyme SULT2A1. In vivo, they are time-dependent inhibitors of CYP3A.
Agents That May Increase CDK4/6 Inhibitor Plasma Concentrations
Coadministration of a strong CYP3A inhibitor increases the plasma exposure of CDK4/6 inhibitors. Thus, concomitant use of strong CYP3A inhibitors (e.g., clarithromycin, grapefruit juice, indinavir, itraconazole, ketoconazole, lopinavir/ritonavir, nefazodone, nelfinavir, posaconazole, ritonavir, saquinavir, telaprevir, telithromycin, or voriconazole) should be avoided. If coadministration of a strong CYP3A inhibitor cannot be avoided, the dosage of the CDK4/6 inhibitor must be reduced.
Agents That May Decrease CDK4/6 Inhibitor Plasma Concentrations
Coadministration of a strong CYP3A inducer decreases the plasma exposure of CDK4/6 inhibitors. Thus, concomitant use of strong CYP3A inducers (e.g., phenytoin, rifampin, carbamazepine, enzalutamide, or St John's wort) should be avoided.
Drugs That May Have Their Plasma Concentrations Altered by CDK4/6 Inhibitors
The coadministration of CDK4/6 inhibitors and drugs that share the characteristics of being CYP3A substrates with a narrow therapeutic index should be avoided, since this can lead to a cumulation of these drugs and their toxicities. This is for example the case with the (commonly used) statins atorvastatin, lovastatin, and simvastatin. In fact, two cases (one of them being fatal) of statin-induced rhabdomyolysis in patients on palbociclib have been published to date [15, 16].
Drugs That Prolong the QTc Interval
Coadministration of ribociclib with drugs with a known potential to prolong the QTc, such as antiarrhythmic medicines (e.g., amiodarone, disopyramide, procainamide, quinidine, or sotalol) and others (e.g., chloroquine, halofantrine, clarithromycin, haloperidol, methadone, moxifloxacin, bepridil, pimozide, or ondansetron), must be avoided to minimize the risk of cardiac arrhythmias. In fact, in the MONALEESA-2 study, one sudden death of a patient who had taken a prohibited concomitant medication with a known risk for QT prolongation (methadone) while being on ribociclib in association with grade 3 hypokalemia and a grade 2 prolongation in the QTcF interval has been reported [17].
Conclusion
With palbociclib, ribociclib, and abemaciclib, a new class of antiproliferative drugs has become standard of care in the endocrine-based treatment of HR-positive, HER2-negative MBC. CDK4/6 inhibitors have led to clinically meaningful improvements in PFS regardless of endocrine sensitivity and menopausal status. On the basis of proper clinical monitoring (e.g., complete blood count, LFTs, ECG, or history of concomitant medications), typical treatment-emergent AEs (e.g., neutropenia, hepatotoxicity, diarrhea, or QTc prolongation) can be clearly identified and safely managed in routine clinical praxis. This makes CDK4/6 inhibitors generally well-tolerated oral agents for our patients with HR-positive, HER2-negative breast cancer.
Disclosure Statement
The author received honoraria from AstraZeneca, Celgene, Lilly, Novartis, Pfizer, and Roche and travel support from AstraZeneca, Novartis, and Pfizer.
References
- 1.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015 Jan;16((1)):25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 2.Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, Harbeck N, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018 Jul;19((7)):904–15. doi: 10.1016/S1470-2045(18)30292-4. [DOI] [PubMed] [Google Scholar]
- 3.Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016 Nov;375((20)):1925–36. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 4.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N Engl J Med. 2016 Nov;375((18)):1738–48. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 5.Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J Clin Oncol. 2017 Nov;35((32)):3638–46. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 6.Andersson M, Lidbrink E, Bjerre K, Wist E, Enevoldsen K, Jensen AB, et al. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: the HERNATA study. J Clin Oncol. 2011 Jan;29((3)):264–71. doi: 10.1200/JCO.2010.30.8213. [DOI] [PubMed] [Google Scholar]
- 7.Hu W, Sung T, Jessen BA, Thibault S, Finkelstein MB, Khan NK, et al. Mechanistic Investigation of Bone Marrow Suppression Associated with Palbociclib and its Differentiation from Cytotoxic Chemotherapies. Clin Cancer Res. 2016 Apr;22((8)):2000–8. doi: 10.1158/1078-0432.CCR-15-1421. [DOI] [PubMed] [Google Scholar]
- 8.Durairaj C, Ruiz-Garcia A, Gauthier ER, Huang X, Lu DR, Hoffman JT, et al. Palbociclib has no clinically relevant effect on the QTc interval in patients with advanced breast cancer. Anticancer Drugs. 2018 Mar;29((3)):271–80. doi: 10.1097/CAD.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.VERZENIO Prescribing iformation:abemaciclib. Indianapolis, IN: Eli Lilly and Company, September. 2017 [Google Scholar]
- 10.Chen P, Lee NV, Hu W, Xu M, Ferre RA, Lam H, et al. Spectrum and Degree of CDK Drug Interactions Predicts Clinical Performance. Mol Cancer Ther. 2016 Oct;15((10)):2273–81. doi: 10.1158/1535-7163.MCT-16-0300. [DOI] [PubMed] [Google Scholar]
- 11.Sledge GW, Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J Clin Oncol. 2017 Sep;35((25)):2875–84. doi: 10.1200/JCO.2017.73.7585. [DOI] [PubMed] [Google Scholar]
- 12.Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, Gandhi L, et al. Efficacy and Safety of Abemaciclib, an Inhibitor of CDK4 and CDK6, for Patients with Breast Cancer, Non-Small Cell Lung Cancer, and Other Solid Tumors. Cancer Discov. 2016 Jul;6((7)):740–53. doi: 10.1158/2159-8290.CD-16-0095. [DOI] [PubMed] [Google Scholar]
- 13.Dickler MN, Tolaney SM, Rugo HS, Cortés J, Diéras V, Patt D, et al. MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR+/HER2- Metastatic Breast Cancer. Clin Cancer Res. 2017 Sep;23((17)):5218–24. doi: 10.1158/1078-0432.CCR-17-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chappell JC, Turner PK, Pak YA, Bacon J, Chiang AY, Royalty J, et al. Abemaciclib Inhibits Renal Tubular Secretion Without Changing Glomerular Filtration Rate. Clin Pharmacol Ther. 2018 Nov;:cpt.1296. doi: 10.1002/cpt.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson KL, Stenehjem D, Driscoll M, Gilcrease GW. Fatal Statin-Induced Rhabdomyolysis by Possible Interaction with Palbociclib. Front Oncol. 2017 Jul;7:150. doi: 10.3389/fonc.2017.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gopalan PK, Pinder MC, Chiaporri A, Ivey AM, Gordillo Villegas A, Kaye FJ. A phase II clinical trial of the CDK 4/6 inhibitor palbociclib (PD 0332991) in previously treated, advanced non-small cell lung cancer (NSCLC) patients with inactivated CDKN2A. J Clin Oncol. 2014;32((suppl; abstr 8077)):5s. [Google Scholar]
- 17.Hortobagyi GN. Ribociclib for HR-Positive, Advanced Breast Cancer. N Engl J Med. 2017 Jan;376((3)):289. doi: 10.1056/NEJMc1615255. [DOI] [PubMed] [Google Scholar]
- 18.Turner NC, Ro J, André F, Loi S, Verma S, Iwata H, et al. PALOMA3 Study Group Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N Engl J Med. 2015 Jul;373((3)):209–19. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 19.IBRANCE . New York, NY: Pfizer Inc; 2017. Mar, Prescribing iformation: palbociclib. [Google Scholar]
- 20.KISQALI . East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2017. Mar, Prescribing iformation:ribociclib. [Google Scholar]
- 21.Novartis Pharma Kisqali® 200 mg Filmtabletten Fachinformation. 2018 Dec [Google Scholar]