Levels of evidence supporting recommendations in cardiovascular guidelines are known to be low, fewer than 15% of all recommendations in cardiovascular guidelines are supported by high-quality evidence from multiple randomized controlled trials (RCTs) and meta-analyses (level A evidence).1–3 One recent study showed that the evidence levels underlying the European and American guidelines did not change over the last decade.2 Considering that similar evidence levels have been found in most medical and surgical subspecialties, a grim outlook on the medical evidence base as a whole arises. However, light comes after the darkest hour.
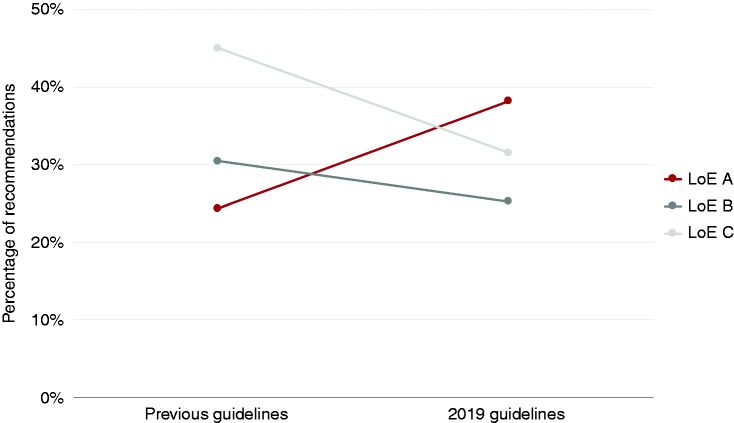
Evidence levels of the recently released 2019 guidelines of the European Society of Cardiology (ESC) prudently set forth a more commendable trend. In the five new 2019 ESC guidelines, the number of should and should not recommendations (class I and III) supported by level of evidence (LoE) A has increased from 24.4% (interquartile range (IQR) 18.9%–38.5%) to 38.2% (IQR 22.8%–45.9%), while the number of recommendations supported by LoE B and C has been reduced (Figure 1).4–8 Simultaneously, the number of recommendations has increased from a median of 96 (IQR 83–130) in previous guidelines to a median of 120 (IQR 120–138) recommendations in the 2019 guidelines.
Figure 1.
Overall evidence levels supporting should and should not (class I and III) recommendations.
These results suggest that the efforts of the last decade to improve the evidence on the management of cardiovascular disease might be starting to bear fruit. Indeed, these numbers represent just one year of guideline releases by one major cardiovascular society and should thus be interpreted with care. Nonetheless, it should not be unrecorded that steps were taken in the right direction by the authors of the 2019 guidelines. To further understand and improve the cardiovascular evidence base and identify where gaps exist, guidelines should be broken down into their recommended actions by, for example, categorizing recommendations by intervention (pharmaceutical/open surgical/lifestyle/etc.) and diagnostic (laboratory test/invasive imaging/risk stratification/etc.). Findings from such a recent breakdown analysis of the ESC guidelines showed that more than two-thirds of the 3531 recommendations issued between 2003 and 2018 were on therapeutic topics (largest groups: pharmaceutical (48.9%), open surgical (14.4%) and minimal-invasive (13.4%) interventions) and one-third on diagnostic topics (largest groups: non-invasive tests/imaging (45.5%), laboratory tests(18.9%) and invasive tests/imaging (13.0%) interventions). Pharmaceutical and lifestyle interventions were, as expected, substantially more grounded in level A evidence (class I/III 15.6% and 34.3%, respectively) than were open surgical (class I/III 4.1%) and diagnostic recommendations (range class I/III 2.3%–13.4%).3
Ideally, all care delivered would be supported by evidence from well-conducted RCTs. It should thus not be acceptable for high-quality evidence levels to remain around 40%. However, RCTs may not be the best methodology to, for example, demonstrate the value of diagnostic tools or prognostic assessments.9 Trials are inevitable when diagnostic and prognostic research suggest different intervention strategies or when adequate reference standards are missing.10 In reclassification, accuracy and cost-effectiveness studies, however, trials offer no surplus benefit over the results of cross-sectional and therapeutic studies combined. Hence, observational research is often the highest level of evidence viable for diagnostic and prognostic recommendations rendering the lower levels of evidence attached to these studies controversial.
Trials also have remained laborious and become increasingly expensive, barely innovating in their execution, limiting their numbers and leaving clinicians and guideline authors to rely on lower levels of evidence as a result.11 Innovation in trials to decrease their costs is sorely needed to allow them to add knowledge on evidence gaps where profitable business cases are lacking. Since most of the costs of trials are found to lie within (manual) patient accrual and follow-up, major opportunities to decrease these costs can be found by creating learning healthcare systems by leveraging routinely collected data, such as those found in electronic healthcare records.12 Until then, guideline authors are forced to decide whether or not to issue recommendations based on weaker evidence instead, keeping guidelines patient-centric and avoiding them becoming a mere recital of facts.13
By issuing recommendations substantiated by weaker evidence, however, guideline authors do not have the privilege of releasing just any recommendation. While a margin of appreciation for guideline authors remains necessary, as in some cases a recommendation supported only by trusted colleagues (LoE C) is better than no recommendation, guideline recommendations should remain focused on their goal of supporting clinicians in their practice. Recommendations on, for instance, political topics should therefore be considered carefully before they are issued.
Evidence levels supporting guidelines give insights into the status of the cardiovascular evidence base at a given time. To grow these insights, guidelines should be broken down into their actions (e.g. pharmaceutical interventions, surgical interventions, diagnostic imaging and prognostic stratification) to identify where to focus further efforts on improving the cardiovascular evidence base. Previously, for example, pharmaceutical and lifestyle interventions were found to be, as expected, more grounded in level A evidence than were diagnostic recommendations.3 Yet, these new guidelines represent the current status of guideline development, and, unburdened by the history of their predecessors, they suggest that improvements in guideline development are on their way.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Grobbee reported being a member of the Clinical Practice Guidelines Committee of the European Society of Cardiology. Dr Van Dijk reported no conflicts of interest.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by the Netherlands Organisation for Health Research and Development (ZonMW) (grant number 91217027).
References
- 1.Tricoci P, Allen JM, Kramer JM, et al. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA 2009; 301: 831–841. [DOI] [PubMed] [Google Scholar]
- 2.Fanaroff AC, Califf RM, Windecker S, et al. Levels of evidence supporting American College of Cardiology/American Heart Association and European Society of Cardiology guidelines, 2008–2018. JAMA 2019; 27715: 1069–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Dijk WB, Grobbee DE, de Vries MC, et al. A systematic breakdown of the levels of evidence supporting the European Society of Cardiology guidelines. Eur J Prev Cardiol. 2019; 26: 1944–1952. [DOI] [PMC free article] [PubMed]
- 4.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. Epub ahead of print 31 August 2019. DOI: 10.1093/eurheartj/ehz486. [DOI] [PubMed]
- 5.Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2019. Epub ahead of print 31 August 2019. DOI: 10.1093/eurheartj/ehz425. [DOI] [PubMed]
- 6.Brugada J, Katritsis DG, Arbelo E, et al. 2019 ESC Guidelines for the management of patients with supraventricular tachycardia The Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Eur Heart J. Epub ahead of print 31 August 2019. DOI: 10.1093/eurheartj/ehz467.
- 7.Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. Epub ahead of print 31 August 2019. DOI: 10.1093/eurheartj/ehz405. [DOI] [PubMed]
- 8.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2019. Epub ahead of print 31 August 2019. DOI: 10.1093/eurheartj/ehz455. [DOI] [PubMed]
- 9.Grobbee DE and Hoes AW. Clinical epidemiology: principles, methods, and applications for clinical research. Sudbury, MA: Jones and Bartlett Publishers, 2014.
- 10.Biesheuvel CJ, Grobbee DE and Moons KG. Distraction from randomization in diagnostic research. Ann Epidemiol 2006; 16: 540–544. [DOI] [PubMed]
- 11.Moore TJ, Zhang H, Anderson G, et al. Estimated costs of pivotal trials for novel therapeutic agents approved by the US Food and Drug Administration, 2015–2016. JAMA Intern Med 2018; 178: 1451–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bentley C, Cressman S, van der Hoek K, et al. Conducting clinical trials—costs, impacts, and the value of clinical trials networks: a scoping review. Clin Trials 2019; 16: 183–193. [DOI] [PubMed] [Google Scholar]
- 13.Piepoli MF and Ceconi C. Levels of evidence in the European Society of Cardiology Guidelines: gaps in knowledge? Eur J Prev Cardiol. 2019; 26: 1941–1943. [DOI] [PubMed]