Summary
Background
Atezolizumab is a humanised antiprogrammed death-ligand 1 (PD-L1) monoclonal antibody that inhibits PD-L1 and programmed death-1 (PD-1) and PD-L1 and B7–1 interactions, reinvigorating anticancer immunity. We assessed its efficacy and safety versus docetaxel in previously treated patients with non-small-cell lung cancer.
Methods
We did a randomised, open-label, phase 3 trial (OAK) in 194 academic or community oncology centres in 31 countries. We enrolled patients who had squamous or non-squamous non-small-cell lung cancer, were 18 years or older, had measurable disease per Response Evaluation Criteria in Solid Tumors, and had an Eastern Cooperative Oncology Group performance status of 0 or 1. Patients had received one to two previous cytotoxic chemotherapy regimens (one or more platinum based combination therapies) for stage IIIB or IV non-small-cell lung cancer. Patients with a history of autoimmune disease and those who had received previous treatments with docetaxel, CD137 agonists, anti-CTLA4, or therapies targeting the PD-L1 and PD-1 pathway were excluded. Patients were randomly assigned (1:1) to intravenously receive either atezolizumab 1200 mg or docetaxel 75 mg/m2 every 3 weeks by permuted block randomisation (block size of eight) via an interactive voice or web response system. Coprimary endpoints were overall survival in the intention-to-treat (ITT) and PD-L1-expression population TC1/2/3 or IC1/2/3 (≥1% PD-L1 on tumour cells or tumour-infiltrating immune cells). The primary efficacy analysis was done in the first 850 of 1225 enrolled patients. This study is registered with ClinicalTrials.gov, number .
Findings
Between March 11, 2014, and April 29, 2015, 1225 patients were recruited. In the primary population, 425 patients were randomly assigned to receive atezolizumab and 425 patients were assigned to receive docetaxel. Overall survival was significantly longer with atezolizumab in the ITT and PD-L1-expression populations. In the ITT population, overall survival was improved with atezolizumab compared with docetaxel (median overall survival was 13·8 months [95% CI 11·8–15·7] vs 9·6 months [8·6–11·2]; hazard ratio [HR] 0·73 [95% CI 0·62–0·87], p=0·0003). Overall survival in the TC1/2/3 or IC1/2/3 population was improved with atezolizumab (n=241) compared with docetaxel (n=222; median overall survival was 15·7 months [95% CI 12·6–18·0] with atezolizumab vs 10·3 months [8·8–12·0] with docetaxel; HR 0·74 [95% CI 0·58–0·93]; p=0·0102). Patients in the PD-L1 low or undetectable subgroup (TC0 and IC0) also had improved survival with atezolizumab (median overall survival 12·6 months vs 8·9 months; HR 0·75 [95% CI 0·59–0·96]). Overall survival improvement was similar in patients with squamous (HR 0·73 [95% CI 0·54–0·98]; n=112 in the atezolizumab group and n=110 in the docetaxel group) or non-squamous (0·73 [0·60–0·89]; n=313 and n=315) histology. Fewer patients had treatment-related grade 3 or 4 adverse events with atezolizumab (90 [15%] of 609 patients) versus docetaxel (247 [43%] of 578 patients). One treatment-related death from a respiratory tract infection was reported in the docetaxel group.
Interpretation
To our knowledge, OAK is the first randomised phase 3 study to report results of a PD-L1-targeted therapy, with atezolizumab treatment resulting in a clinically relevant improvement of overall survival versus docetaxel in previously treated non-small-cell lung cancer, regardless of PD-L1 expression or histology, with a favourable safety profile.
Introduction
Lung cancer remains the leading cause of cancer death globally, and outcomes for patients diagnosed with advanced non-small-cell lung cancer are poor despite recent advances in treatment.1 Docetaxel has been the standard of care for second-line or third-line treatment; however, its efficacy is offset by substantial toxic effects. The new development of antibodies that target the programmed death-ligand 1 (PD-L1) and programmed death-1 (PD-1) pathway represents an important advance in the management of metastatic non-small-cell lung cancer, with PD-1 inhibitors showing overall survival benefits over docetaxel. Compared with docetaxel, nivolumab has shown a median overall survival of 9·2 months versus 6·0 months (hazard ratio [HR] 0·59, 95% CI 0·44–0·79) in squamous non-small-cell lung cancer and 12·2 months versus 9·4 months (96% CI 0·73, 0·59–0·89) in non-squamous non-small-cell lung cancer.2,3 Additionally, pembrolizumab compared with docetaxel has shown a median overall survival of 10·4 months versus 8·5 months (HR 0·71, 95% CI 0·58–0·88) at the approved dose of 2 mg/kg in a patient population with non-small-cell lung cancer who expressed PD-L1 in 1% or more of tumour cells.4
PD-L1 is an immune checkpoint protein expressed on tumour cells and tumour-infiltrating immune cells. PD-L1 can mediate suppression of anticancer immunity by binding to its receptors PD-1 and B7–1 (also known as CD80).5–7 Atezolizumab is a humanised engineered IgG1 monoclonal antibody targeting PD-L1 and thus has a mechanism of action distinct from anti-PD-1 antibodies. In addition to blocking the PD-L1 and PD-1 interaction, which can reinvigorate suppressed immune cells to eliminate cancer cells,8–10 atezolizumab blocks PD-L1 and B7–1 binding, which might further enhance immune responses.11–14 Furthermore, direct targeting of PD-L1 leaves the PD-L2 and PD-1 interaction intact and might minimise autoimmunity.8,15,16
A phase 1 study17 of atezolizumab monotherapy has shown durable antitumour responses in non-small-cell lung cancer and has shown an association of PD-L1 expression on tumour cells and tumour-infiltrating immune cells with patients who had an objective response.9 In the phase 2, randomised POPLAR study,18,19 atezolizumab improved overall survival compared with docetaxel in patients with previously treated non-small-cell lung cancer (12·6 months vs 9·7 months; HR 0·69, 95% CI 0·52–0·92) in the intention-to-treat (ITT) population. Additionally, results from POPLAR suggested that there are two distinct subpopulations of non-small-cell lung cancer that can be identified through PD-L1 expression on tumour cells and tumour-infiltrating immune cells, with PD-L1 expression on tumour cells or tumour-infiltrating immune cells independently contributing to overall survival.18
We report the primary analysis of the OAK study, the first, to our knowledge, phase 3 study of a PD-L1-directed antibody (atezolizumab). OAK was designed to investigate the efficacy and safety of atezolizumab compared with docetaxel in patients with locally advanced or metastatic, previously treated non-small-cell lung cancer.
Methods
Study design
OAK is a randomised, open-label, international phase 3 study that was done in 194 academic medical centres and community oncology practices across 31 countries worldwide. The study was done in full accordance with the guidelines for Good Clinical Practice and the Declaration of Helsinki.
Patients
Patients had squamous or non-squamous non-small-cell lung cancer, were 18 years or older, had measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST; version 1.1), and had an Eastern Cooperative Oncology Group performance status of 0 or 1. Patients had received 1–2 previous cytotoxic chemotherapy regimens (≥1 platinum based combination therapy) for stage IIIB or IV non-small-cell lung cancer. Patients with EGFR mutations or an ALK fusion oncogene were additionally required to have received previous tyrosine kinase inhibitor therapy. Patients with treated asymptomatic supratentorial CNS metastases were eligible, whereas patients with a history of autoimmune disease and those who had received previous treatments with docetaxel, CD137 agonists, anti-CTLA4, or therapies targeting the PD-L1 and PD-1 pathway were excluded (appendix). All patients gave written informed consent.
Randomisation and masking
Patients were stratified by PD-L1 expression (IC0 vs IC1 vs IC2 vs IC3 level), number of previous chemotherapy regimens (one vs two), and histology (non-squamous vs squamous). PD-L1 expression was assessed centrally and prospectively in archival or fresh tumour samples according to previously published scoring criteria18 with the VENTANA SP142 PD-L1 immunohistochemistry assay (Ventana Medical Systems, Inc., Tucson, AZ, USA). TC1/2/3 or IC1/2/3 was defined as PD-L1 expression on 1% or more of tumour cells or tumour-infiltrating immune cells, TC2/3 or IC2/3 was defined as PD-L1 expression on 5% of these cells; TC3 was defined as PD-L1 expression on 50% or more of tumour cells and IC3 was defined as 10% or more of tumour-infiltrating immune cells; and TC0 as PD-L1 expression on less than 1% of tumour cells and IC0 on less than 1% of tumour-infiltrating immune cells (appendix). PD-L1 gene expression was assessed in tumour tissue with a Fluidigm-based gene-expression platform as previously described (Fluidigm; South San Francisco, CA, USA).18 Permuted block-randomisation (block size of eight) via an interactive voice or web response system (bracket) was used to assign patients in a 1:1 ratio to receive atezolizumab or docetaxel. The trial centres enrolled the patients. The study was open-label and allocation was unmasked.
Procedures
Atezolizumab was given as an intravenous 1200 mg fixed dose every 3 weeks; docetaxel was given intravenously at 75 mg/m2 every 3 weeks. Treatment was administered until unacceptable toxicity or disease progression, as assessed by the investigator. Atezolizumab treatment could continue beyond disease progression if the investigator deemed the patient to be receiving clinical benefit. No crossover to atezolizumab was allowed.
Tumour assessments were done at baseline, then every 6 weeks until week 36 and every 9 weeks thereafter. These assessments continued until disease progression, regardless of treatment discontinuation. For patients receiving atezolizumab beyond disease progression, tumour assessments continued until treatment discontinuation. Patients were followed up for survival throughout the study while receiving treatment and every 3 months after treatment discontinuation.
Outcomes
The primary endpoint was overall survival compared between treatment groups within the ITT and the PD-L1 TC1/2/3 or IC1/2/3 populations (PD-L1 expression on ≥1% of tumour cells or tumour-infiltrating immune cells18). Secondary endpoints included investigator-assessed progression-free survival, proportion of patients who had an objective response, duration of response, and safety.
Safety was assessed descriptively and based on all patients who received any dose of study treatment. The incidence, nature, and severity of adverse events and laboratory abnormalities were assessed by the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.20
Statistical analysis
OAK was initially designed to enrol 850 patients, and the sample size was later increased to enrol up to 1300 patients to power for an overall survival comparison in patients with high PD-L1 expression (TC3 or IC3, assuming a prevalence of approximately 20%); the final enrolment was 1225 patients. Data from the phase 2 randomised study POPLAR18 showed that the overall survival benefit extended to lower PD-L1 expression levels and that the assessment of this benefit required a relatively long follow-up because of the late separation of survival curves. Therefore, the OAK statistical design was amended on Jan 28, 2016, according to a prespecified modification plan to test overall survival in the ITT population and in the TC1/2/3 or IC1/2/3 population in a coprimary fashion (with α splitting between the ITT population [α=3%] and the TC1/2/3 or IC1/2/3 population [α=2%]) for which the initial 850 randomised patients provided sufficient power (95·3% in the ITT population and 98·6% in the TC1/2/3 or IC1/2/3 population) and follow-up time. Therefore, the primary efficacy analysis population comprises the first 850 patients who were randomly assigned to a treatment group.
The primary analysis of overall survival was planned when approximately 70% of patients in the primary efficacy analysis population had died. Overall survival was compared between treatment groups with a stratified log-rank test at the two-sided significance level. The Kaplan-Meier approach was used to estimate the median overall survival; the Brookmeyer-Crowley methodology was used to estimate 95% CIs. The HR was estimated with a stratified Cox regression analysis. Stratification factors were the same used for randomisation. Prespecified analyses were done to determine the consistency of the treatment effect according to key baseline characteristics and in different subgroups of patients according to their tumour PD-L1 expression level. Given the exploratory nature of subgroup analyses and potential small sample sizes in specific subgroups, the HRs from these analyses were estimated with an unstratified Cox regression analysis. Patients not reported as having died at the time of analysis were censored at the date they were last known to be alive. Patients without post-baseline information were censored at the randomisation date plus 1 day.
Progression-free survival and duration of response were analysed with the same methods as the overall survival analysis. The proportion of patients with an objective response and the corresponding 95% CIs for each treatment group were calculated with the Clopper-Pearson method and compared between treatment groups with the Cochran–Mantel–Haenszel test.
An independent data monitoring committee reviewed safety. Protocol approval was obtained from independent ethics committees for each site (listed in the appendix). Statistical analyses were done with the SAS version 9.2. This study is registered with ClinicalTrials.gov, number .
Role of the funding source
The funder of the study provided study drugs, was involved in the study design, data collection, data analysis, data interpretation, and writing of the report, and gave approval to submit for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
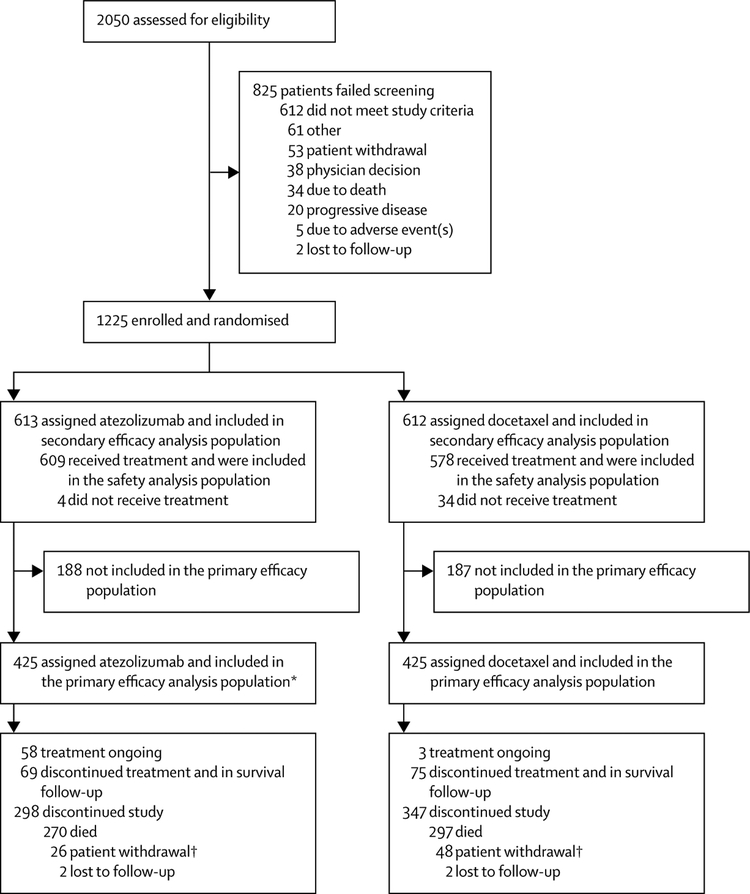
Between March 11, 2014, and Nov 28, 2014, 850 patients in the primary analysis population were recruited at 194 academic or community oncology centres across 31 countries; 425 patients were randomised to receive atezolizumab and 425 to receive docetaxel (ITT population; figure 1). Demographic and baseline characteristics were well balanced between groups (table 1; appendix). Enrolment of the final 375 patients took place until April 29, 2015. Of the final 1225 patients randomly assigned in the total patient population, 609 patients received atezolizumab and 578 patients received docetaxel (safety population). 125 (21%) of 609 patients in the atezolizumab group and 14 (2%) of 578 patients in the docetaxel group had a treatment duration longer than 12 months. Median treatment duration was 3·4 months (range 0–26) with atezolizumab and 2·1 months (range 0–23) with docetaxel. 40% of patients receiving atezolizumab were treated beyond progression, with a median treatment duration beyond progression of three cycles (range 1–34).
Figure 1: Trial profile.
*One patient randomly assigned to docetaxel received atezolizumab. †The deaths of one patient in the atezolizumab group and of one patient in the docetaxel group were collected via public records. This is why the number of deaths for the overall survival analysis is 271 in the atezolizumab group and 298 in the docetaxel group and not 270 vs 297 as shown. These two patients are shown as patient withdrawal.
Table 1: Baseline characteristics of the intention-to-treat primary population.
| Atezolizumab (n=425) | Docetaxel (n=425) | Overall (N=850) | |
|---|---|---|---|
| Age (years) | |||
| Median (range) | 63·0 (33·0–82·0) | 64·0 (34·0–85·0) | 64·0 (33·0–85·0) |
| Age ≥65 years | 190 (45%) | 207 (49%) | 397 (47%) |
| Sex | |||
| Male | 261 (61%) | 259 (61%) | 520 (61%) |
| Female | 164 (39%) | 166 (39%) | 330 (39%) |
| Race | |||
| White | 302 (71%) | 296 (70%) | 598 (70%) |
| Asian | 85 (20%) | 95 (22%) | 180 (21%) |
| Black | 5 (1%) | 11 (3%) | 16 (2%) |
| Other* | 13 (3%) | 9 (2%) | 22 (3%) |
| Unknown | 20 (5%) | 14 (3%) | 34 (4%) |
| ECOG performance status | |||
| 0 | 155 (36%) | 160 (38%) | 315 (37%) |
| 1 | 270 (64%) | 265 (62%) | 535 (63%) |
| Tobacco use history | |||
| Never | 84 (20%) | 72 (17%) | 156 (18%) |
| Current | 59 (14%) | 67 (16%) | 126 (15%) |
| Previous | 282 (66%) | 286 (67%) | 568 (67%) |
| EGFR mutation | |||
| Positive | 42 (10%) | 43 (10%) | 85 (10%) |
| Negative | 318 (75%) | 310 (73%) | 628 (74%) |
| Unknown | 65 (15%) | 72 (17%) | 137 (16%) |
| EML4-ALK translocation | |||
| Positive | 2 (<1%) | 0 | 2 (<1%) |
| Negative | 223 (52%) | 201(47%) | 424 (50%) |
| Unknown | 200(47%) | 224 (53%) | 424 (50%) |
| KRAS mutation | |||
| Positive | 26 (6%) | 33 (8%) | 59 (7%) |
| Negative | 99 (23%) | 104 (24%) | 203 (24%) |
| Unknown | 300 (71%) | 288 (68%) | 588 (69%) |
| Histology | |||
| Non-squamous | 313 (74%) | 315 (74%) | 628 (74%) |
| Squamous | 112 (26%) | 110 (26%) | 222 (26%) |
| PD-L1 subgroups | |||
| TC3 or IC3 | 72 (17%) | 65 (15%) | 137 (16%) |
| TC2/3 or IC2/3 | 129 (30%) | 136 (32%) | 265 (31%) |
| TC1/2/3 or IC1/2/3† | 241 (57%) | 222 (52%) | 463 (54%) |
| TC0 and IC0 | 180 (42%) | 199 (47%) | 379 (45%) |
| Number of previous therapies in the locally advanced or metastatic setting | |||
| 1 | 320 (75%) | 320 (75%) | 640 (75%) |
| 2 | 105 (25%) | 105 (25%) | 210 (25%) |
Data are median (range) and n (%), unless otherwise indicated. ECOG=Eastern Cooperative Oncology Group. IC=tumour-infiltrating immune cell. PD-L1=programmed death-ligand 1. TC=tumour cell.
Other includes American Indian, Alaska native, Hawaiian native, other Pacific Islander, other, and multiple.
Tumour tissue for eight patients was not evaluable for TC1/2/3 or IC1/2/3.
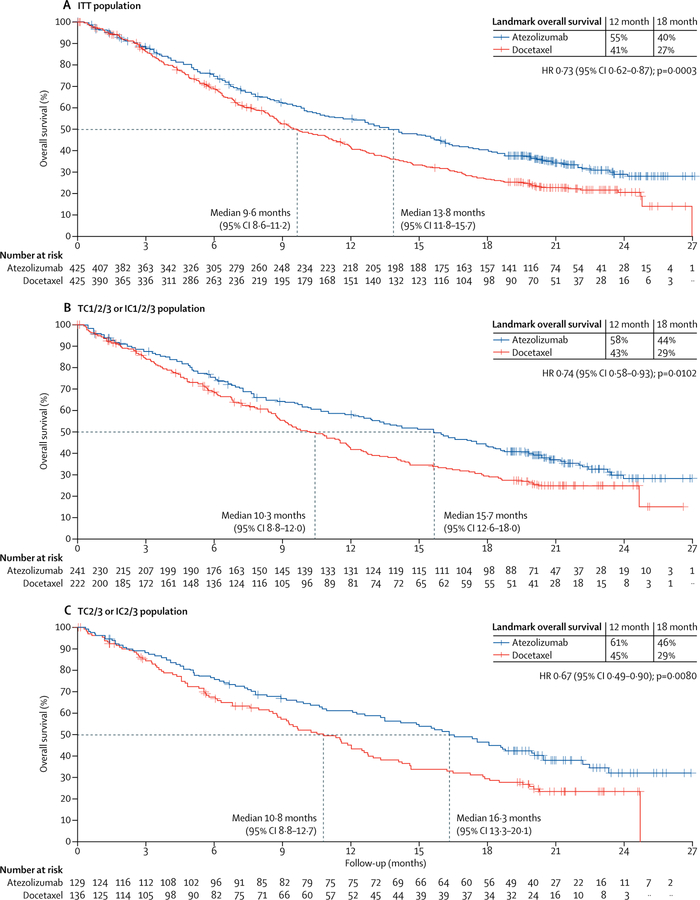
At the primary analysis (data cutoff July 7, 2016), the median follow-up was 21 months and 569 patients had died (271 in the atezolizumab group and 298 in the docetaxel group; event to patient ratio 67%). Compared with docetaxel, overall survival was better with atezolizumab in both the ITT and TC1/2/3 or IC1/2/3 populations (figures 2A, 2B). Overall survival was improved in the ITT population with atezolizumab (median 13·8 months [95% CI 11·8–15·7]) versus docetaxel (median 9·6 months [8·6–11·2]; HR 0·73 [95% CI 0·62–0·87], p=0·0003; figure 2A). At the cutoff date in the TC1/2/3 or IC1/2/3 population, 300 patients had died (149 [67%] of 222 patients in the docetaxel group and 151 [63%] of 241 patients in the atezolizumab group). Overall survival was significantly longer with atezolizumab than with docetaxel (median 15·7 months [95% CI 12·6–18·0] with atezolizumab vs 10·3 months [8·8–12·0] with docetaxel; HR 0·74 [95% CI 0·58–0·93], p=0·0102; figure 2B).
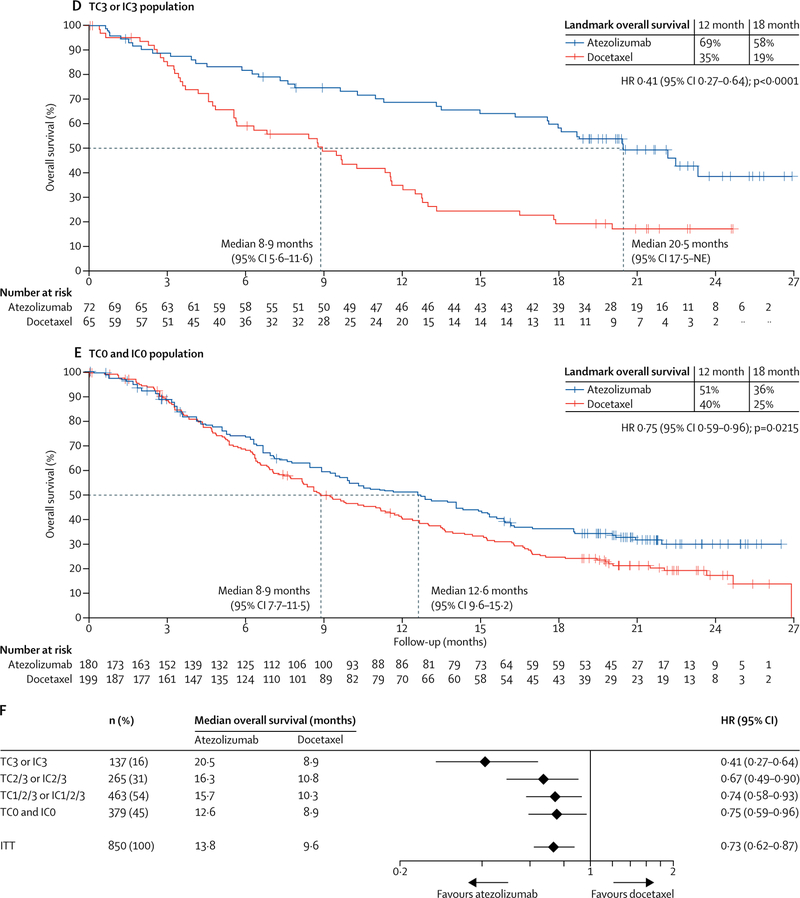
Figure 2: Overall survival in the ITT population and PD-L1 subgroups.
(A) Kaplan-Meier estimates in the ITT primary population, stratified according to PD-L1 expression on tumour-infiltrating immune cells (IC0 vs IC1 vs IC2 vs IC3), the number of previous chemotherapy regimens (one vs two), and histology (non-squamous vs squamous). (B) Kaplan-Meier estimates in theTC1/2/3 or IC1/2/3 group with the same strata. (C) Kaplan-Meier estimates in the TC2/3 or IC2/3 group (unstratified). (D) Kaplan-Meier estimates in the TC3 or IC3 group (unstratified). (E) Kaplan-Meier estimates in the TC0 and IC0 group (unstratified). (F) HRs for overall survival in PD-L1 subgroups. Median overall survival was estimated by Kaplan-Meier analysis. HR=hazard ratio. IC=tumour-infiltrating immune cells. ITT=intention-to-treat. NE=not evaluable. PD-L1=programmed death-ligand 1. TC=tumour cells.
After discontinuation of study treatment, 73 (17%) of 425 patients in the docetaxel group were known to have received immunotherapy (primarily nivolumab) compared with 19 (4%) of 425 patients in the atezolizumab group (appendix). The proportion of patients who received subsequent chemotherapy, predominantly docetaxel, was higher in the atezolizumab group (176 [41%] of 425 patients) than in the docetaxel group (131 [31%] of 425 patients). Subsequent targeted therapy was balanced between groups.
Progression-free survival was similar between treatment groups in the ITT population (HR 0·95 [95% CI 0·82–1·10]). Median progression-free survival was 2·8 months (95% CI 2·6–3·0) with atezolizumab and 4·0 months (3·3–4·2) with docetaxel (table 2, appendix). The proportion of patients with an objective response in the ITT population was also similar between treatment groups (table 2). However, median duration of response in the ITT population was notably longer in the atezolizumab group at 16·3 months (95% CI 10·0–not evaluable) compared with 6·2 months (4·9–7·6) in the docetaxel group (table 2). At the time of data cutoff, responses were ongoing in 30 (52%) of 58 patients in the atezolizumab group and in ten (18%) of 57 patients in the docetaxel group.
Table 2: Summary of key efficacy results.
| Atezolizumab (n=425) | Docetaxel (n=425) | HR (95% CI) | p value | |
|---|---|---|---|---|
| Progression-free survival (ITT population) | ||||
| Patients with event (%) | 380 (89%) | 375 (88%) | 0·95 (0·82–1·10) | 0·49 |
| Median (months; 95% CI) | 2·8 (2·6–3·0) | 4·0 (3·3–4·2) | .. | .. |
| Objective response rate (ITT population) | ||||
| Objective response (%) | 58 (14%) | 57 (13%) | .. | .. |
| Complete response (%) | 6 (1%) | 1 (<1%) | .. | .. |
| Partial response (%) | 52 (12%) | 56 (13%) | .. | .. |
| Stable disease (%) | 150 (35%) | 177 (42%) | .. | .. |
| Progressive disease (%) | 187 (44%) | 117 (28%) | .. | .. |
| Missing or unevaluable (%) | 30 (7%) | 74 (17%) | .. | .. |
| Duration of response (ITT population)* | ||||
| Median (months; 95% CI) | 16·3 (10·0–NE) | 6·2 (4·9–7·6) | 0–34 (0·21–0·55) | <0·0001 |
| Progression-free survival (TC1/2/3 or IC1/2/3) | ||||
| Patients with event (%) | 216/241 (90%) | 193/222 (87%) | 0–91 (0·74–1·12) | 0·38 |
| Median (months; 95% CI) | 2·8 (2·6–4·0) | 4·1 (2·9–4·3) | .. | .. |
| Objective response (TC1/2/3 or IC1/2/3) | ||||
| Objective response | 43/241 (18%) | 36/222 (16%) | .. | .. |
| Complete response | 5/241 (2%) | 1/222 (<1%) | .. | .. |
| Partial response | 38/241 (16%) | 35/222 (16%) | .. | .. |
| Stable disease | 79/241 (33%) | 85/222 (38%) | .. | .. |
| Progressive disease | 102/241 (42%) | 59/222 (27%) | .. | .. |
| Missing or unevaluable | 17/241 (7%) | 42/222 (19%) | .. | .. |
| Duration of response (TC1/2/3 orIC1/2/3)† | ||||
| Median (months; 95% CI) | 16–0 (9·7–NE) | 6·2 (4·9–9·2) | 0·38 (0·22–0·65) | 0·0003 |
HR was stratified for progression-free survival in the ITT and TC1/2/3 or IC1/2/3 populations; unstratified for other subgroups and duration of response.
n=58 for the atezolizumab group and n=57 for the docetaxel group.
n=43 for the atezolizumab group and n=36 for the docetaxel group. HR=hazard ratio. IC=tumour infiltrating immune cells. ITT=intention-to-treat. NE=not evaluable. TC=tumour cell.
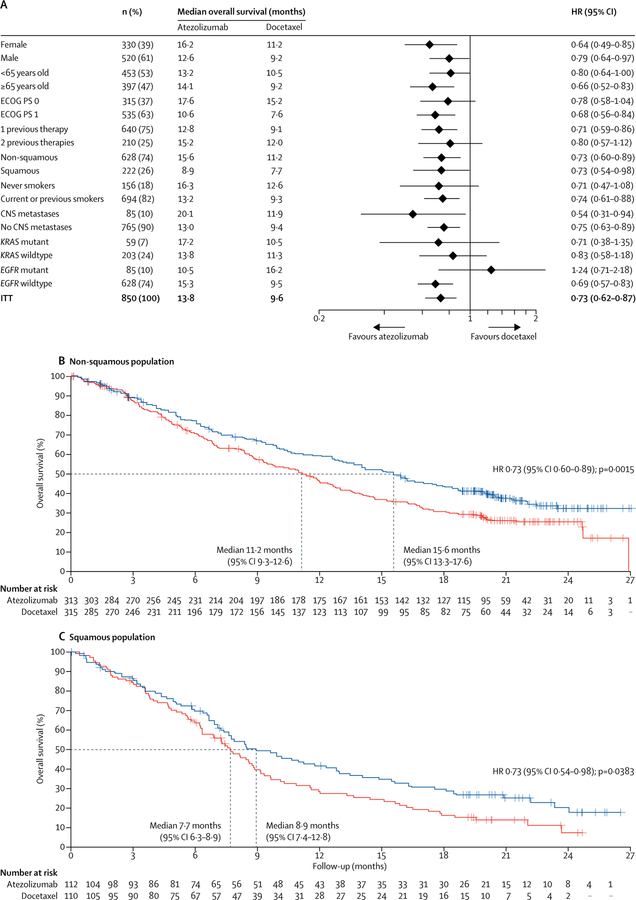
Overall survival was improved regardless of PD-L1 expression levels (figure 2C–F): patients in the PD-L1 low or undetectable subgroup (TC0 and IC0) derived benefit from atezolizumab treatment over docetaxel (median overall survival 12·6 months [95% CI 9·6–15·2] vs 8·9 months [7·7–11·5]; HR 0·75 [95% CI 0·59–0·96]; figure 2E). These results are consistent with our analysis of PD-L1 gene expression in tumour tissue, which showed an overall survival benefit in patients with lower than median expression of PD-L1 (50% prevalence; HR 0·74 [95% CI 0·58–0·96]; appendix). Patients with high PD-L1 expression (TC3 or IC3 subgroup) derived the greatest benefit from atezolizumab (median overall survival 20·5 months [95% CI 17·5–not evaluable] vs 8·9 months [5·6–11·6]; HR 0·41 [95% CI 0·27–0·64]; figure 2D). Although overall survival improvement was noted in all PD-L1 expression subgroups, including the PD-L1 low or undetectable subgroup, the interaction test analysis of mutually exclusive PD-L1 groups and treatment indicated that PD-L1 expression might be a modifier of treatment effect on overall survival (appendix); however, this finding might be attributed to the pronounced overall survival benefit at the highest expression level (TC3 or IC3; figure 2). To assess the independent contribution of PD-L1 expression on tumour cells or tumour-infiltrating immune cells we analysed non-overlapping subgroups. In the TC1/2/3 and IC0 subgroup, median overall survival was 13·2 months (95% CI 7·8–20·5) with atezolizumab and 12·0 months (3·7–14·7) with docetaxel (HR 0·72 [95% CI 0·36–1·45]). In the TC0 and IC1/2/3 subgroup, median overall survival was 14·3 months (95% CI 10·6–18·4) with atezolizumab and 9·8 months (7·3–13·7) with docetaxel (HR 0·73, 95% CI 0·52–1·02). Point estimates for the overall survival HR in both subgroups were similar to that noted for the ITT population, sample sizes were smaller and 95% CIs crossed 1; thus benefit was inconclusive. Overall survival HRs favoured atezolizumab across predefined subgroups, including in patients with squamous (HR 0·73 [95% CI 0·54–0·98]) or non-squamous disease (0·73 [0·60–0·89]; figure 3), patients with treated CNS metastases at baseline (0·54 [0·31–0·94]) and never smokers (0·71 [0·47–1·08]; figure 3). The exception was patients with EGFR mutation-positive status (HR 1·24 [95% CI 0·71–2·18]; figure 3).
Figure 3: Overall survival in prespecified subgroups.
(A) Median overall survival was estimated by Kaplan-Meier analysis. Stratified for ITT and unstratified for subgroups. (B) Kaplan-Meier estimates in the non-squamous histology subgroup (unstratified). (C) Kaplan-Meier estimates in the squamous histology subgroup (unstratified). ECOG PS=Eastern Cooperative Oncology Group performance status. HR=hazard ratio. ITT=intention-to-treat.
Progression-free survival was similar in both treatment arms in PD-L1 subgroups (including the TC1/2/3 or IC1/2/3 population; table 2, appendix), with the exception of the TC3 or IC3 group, which showed a greater benefit with atezolizumab than with docetaxel (progression-free survival HR 0·63 [95% CI 0·43–0·91], appendix). The proportion of patients with an objective response improved with atezolizumab versus docetaxel treatment in the TC3 or IC3 subgroup (22 [31%] of 72 patients vs seven [11%] of 65 patients), and was lowest in the TC0 and IC0 population (14 [8%] of 180 patients vs 21 [11%] of 199 patients). Duration of response improvement with atezolizumab compared with docetaxel was similar in all PD-L1 expression subgroups (table 2, appendix). PD-L1 expression status did not appear to be predictive for responses in patients treated with docetaxel.
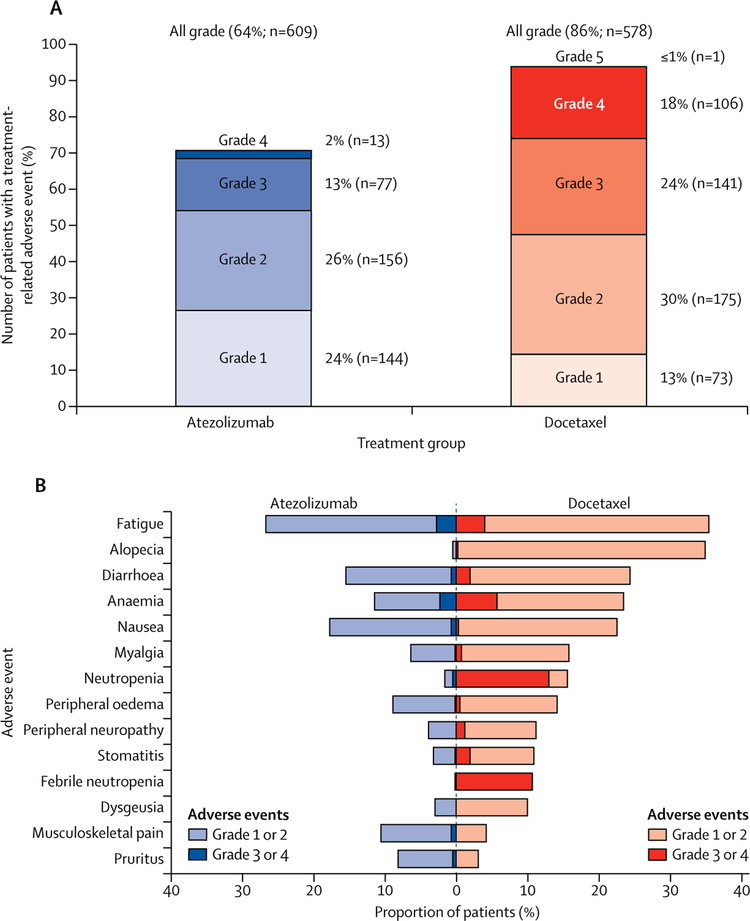
Grade 3 or 4 adverse events were reported in 227 (37%) of 609 patients treated with atezolizumab and 310 (54%) of 578 patients treated with docetaxel. There were fewer treatment-related adverse events with atezolizumab than with docetaxel, including grade 3 or 4 events (90 [15%] of 609 patients vs 247 [43%] of 578 patients, figure 4A; table 3). In the 609 patients, fatigue (87 [14%] patients), nausea (53 [9%] patients), decreased appetite (52 [9%] patients), and asthenia (51 [8%] patients) were the most common atezolizumab-related adverse events of any grade.
Figure 4: All-cause and treatment-related adverse events in the safety population.
(A) Adverse events that occurred within 30 days from the last study treatment were included in the analysis. Proportions of patients having treatment-related adverse events, by grade. (B) All-cause adverse events that differed by 5% or more between study groups. Additional adverse events with 5% or higher frequency in the docetaxel arm were not shown, and were neutrophil count decreased (10% vs <1% of patients in the docetaxel and atezolizumab arms, respectively), peripheral sensory neuropathy (7% vs 1%), mucosal inflammation (7% vs 1%), and nail disorder (5% vs 0%).
Table 3: Summary of adverse events in the safety population.
| Atezolizumab (n=609) | Docetaxel (n=578) | |
|---|---|---|
| All adverse events | 573 (94%) | 555 (96%) |
| Treatment-related adverse events | 390 (64%) | 496 (86%) |
| Grade 3 or 4 adverse events | 227 (37%) | 310 (54%) |
| Treatment-related grade 3 or 4 adverse events | 90 (15%) | 247 (43%) |
| All deaths | 10 (2%) | 14 (2%) |
| Treatment-related death | 0 | 1 (<1%)* |
| Serious adverse events | 194 (32%) | 181 (31%) |
| Adverse events leading to withdrawal from treatment | 46 (8%) | 108 (19%) |
| Adverse events leading to dose modification, delay, or interruption | 152 (25%) | 210 (36%) |
One death due to a respiratory tract infection.
Figure 4B shows all adverse events with a difference in incidence between groups of 5% or more. Of those, pruritus was more common with atezolizumab than with docetaxel. Musculoskeletal pain was more common with atezolizumab but rates of myalgia were higher with docetaxel. Adverse events occurring in 10% or more of patients in any group are shown in the appendix.
In the 609 patients in the safety analysis, immune-mediated adverse events reported with atezolizumab included pneumonitis (six [1%] patients at any grade; four [<1%] patients at grade 3, hepatitis (two [<1%] patients, both grade 4), and colitis (two [<1%] patients, both grade 2).
Adverse events leading to treatment discontinuation occurred in 46 (8%) of 609 patients with atezolizumab and in 108 (19%) of 578 patients with docetaxel. There were no deaths related to atezolizumab and one related to docetaxel (respiratory tract infection).
Discussion
To our knowledge, OAK, the first randomised phase 3 trial of a PD-L1-targeted therapy, met its coprimary endpoint, showing that atezolizumab treatment resulted in a significant improvement in overall survival compared with docetaxel in patients with advanced stage non-small-cell lung cancer (in the ITT and TC1/2/3 or IC1/2/3 populations) whose disease had progressed during or after platinum-based chemotherapy. These clinically meaningful data confirm the results of a phase 2 study (POPLAR),18 and both studies show improved survival irrespective of PD-L1 expression status and histology (squamous and nonsquamous), as well as increased durable responses with atezolizumab in non-small-cell lung cancer.
Of note, 17% of patients treated with docetaxel received subsequent cancer immunotherapies, predominantly the PD-1-targeted therapy nivolumab. Given the survival benefit provided by these agents in patients with previously treated non-small-cell lung cancer, this might have resulted in increased survival in the docetaxel group and a diminution in measured overall survival difference between groups.
Consistent with the POPLAR study, patients with tumours expressing high levels of PD-L1 (TC3 or IC3) derived the greatest benefit from atezolizumab. In this study, overall survival was also improved in patients with less than 1% PD-L1 expression (ie, TC0 and IC0 subgroup). These immunohistochemistry data are supported by a similar overall survival benefit noted in patients with low PD-L1 levels by gene expression analysis. By contrast, the proportion of TC0 and IC0 patients with an objective response is lower than that in those patients with higher PD-L1 expression, which is consistent with what was reported for anti-PD-1 inhibitors and in previous atezolizumab studies. Because low PD-L1 expression is associated with weak or no preexisting anticancer immunity,18 this observed survival benefit associated with atezolizumab in patients who are PD-L1-negative warrants additional investigation to better understand the mechanisms of response to therapy in this patient population. These include the biological hypothesis that atezolizumab increases anticancer immunity through enhanced priming of new anticancer immune responses.
The overall survival HRs favoured atezolizumab over docetaxel across other clinical subgroups, including the never smokers population. Although the confidence intervals for HR in this subgroup were too wide to show a conclusive benefit, this finding supports further evaluation given the low mutational heterogeneity and immunogenicity as well as minimal activity noted with PD-1 inhibitors in this population. In contrast to observations in PD-1 inhibitor studies,2,3 patients with treated CNS metastases at baseline seemed to derive benefit from atezolizumab treatment. Conversely, patients with EGFR mutation-positive disease received similar overall survival benefit with atezolizumab and docetaxel. This finding is similar to results reported with anti-PD-1 treatment in this clinical setting3 and might suggest decreased immunogenicity in this subgroup of patients. Subgroup analyses were not powered for formal efficacy comparisons and should be interpreted with caution.
As seen in other trials with PD-L1 and PD-1 antibodies,2–4,18 progression-free survival and the proportion of patients with an objective response in the ITT population were not improved with atezolizumab compared with docetaxel in OAK. The apparent discordance between progression-free survival and overall survival might be due to an initial increase in tumour volume from increased immune infiltration, delayed antitumour activity, or antitumour immune activation beyond progression that might be sustained by continued treatment.21 This discordance has been commonly observed in studies of this drug class. As such, at least for patients with lower PD-L1 expression levels, these data confirm that progression-free survival results underestimate the clinical benefit measured by overall survival for atezolizumab. The concept of post-progression prolongation of survival has been previously introduced for EGFR inhibitor therapies22 and the OAK results imply that this effect can occur with atezolizumab treatment. These observations also support further evaluation of the benefit and risk of continuing atezolizumab treatment until loss of clinical benefit.22
Overall, atezolizumab was well tolerated, with a favourable adverse event profile compared with docetaxel, and observed adverse events were consistent with those previously reported with atezolizumab.18 The proportion of patients who experienced grade 3 or 4 adverse events, treatment-related adverse events, and those leading to discontinuation of study treatment was lower with atezolizumab than with docetaxel. The incidence of specific immune-related adverse events was low, including pneumonitis (with 1% overall occurrence and less than 1% being grade 3, with no grade 4 events), which is of particular relevance to patients with lung cancer.
In conclusion, this phase 3 study of a PD-L1-directed antibody, atezolizumab, shows a clinically meaningful survival benefit over docetaxel in previously treated patients with non-small-cell lung cancer regardless of PD-L1 expression or histology, with a favourable safety profile compared with docetaxel. These clinically relevant data support atezolizumab as a new treatment option for patients with advanced non-small-cell lung cancer whose disease has progressed during or after platinum-based chemotherapy.
Supplementary Material
Research in context.
Evidence before this study
Docetaxel has been the standard of care for second-line or third-line treated, advanced, or metastatic non-small-cell lung cancer. Important advancements in the treatment of non-small-cell lung cancer have come from cancer immunotherapies that target the programmed death-ligand 1 (PD-L1) and programmed death-1 (PD-1) pathway. We searched PubMed from Sept 27, 2011, to Sept 27, 2016, for clinical trials with the terms “non-small cell lung cancer”, “programmed death-ligand 1”, “PD-L1”, “programmed death-1”, “PD-1”, and “cancer immunotherapy”, selecting relevant English language publications within the past 5 years. We identified eight studies (phases 1–3, all of which were international and open-label) of atezolizumab, pembrolizumab, or nivolumab. These studies indicated the therapeutic value of targeting of the PD-L1 and PD-1 pathway to treat non-small-cell lung cancer, and that atezolizumab shows durable responses and an overall survival benefit for this disease. These responses were associated with PD-L1 expression on tumour cells and tumour-infiltrating immune cells.
Added value of this study
To our knowledge, OAK is the first phase 3 randomised clinical trial to report results for an anti-PD-L1 antibody. In our study, atezolizumab showed a significant and clinically relevant improvement in overall survival compared with docetaxel in patients with advanced stage, previously treated non-small-cell lung cancer, regardless of histology or PD-L1 expression, with a favourable safety profile compared with docetaxel. Patients with tumours expressing high levels of PD-L1 (≥50% on tumour cells or ≥10% on tumour-infiltrating immune cells) derived the greatest benefit from atezolizumab. In contrast to data from PD-1 antibodies, overall survival was also improved in patients with little or no PD-L1 expression (<1% on tumour cells and tumour-infiltrating immune cells). There was a survival benefit of atezoliumab over docetaxel across clinical subgroups, including in patients with squamous and non-squamous disease, in the present and previous smokers population, and in the never smokers population, which has been associated with lower mutational heterogeneity and immunogenicity.
Implications of all the available evidence
Together with reports of the anti-PD1 antibodies pembrolizumab and nivolumab, our results affirm that not only the PD-1 receptor but also the ligand components (eg, PD-L1) of the pathway are valid targets for the treatment of lung cancer. Targeting of PD-L1 with atezolizumab results in a clinically relevant improvement of overall survival as well as a favourable safety profile compared with docetaxel in patients with previously treated non-small-cell lung cancer, regardless of PD-L1 expression or histology. Atezolizumab is the first checkpoint inhibitor to provide an overall survival benefit in patient populations who are historically less responsive to these agents, including patients with low or non-detectable levels of PD-L1 expression and never smokers.
Acknowledgments
The authors would like to acknowledge Gregg Fine and Cathi Ahearn of Genentech, Inc. for their contributions to the design of this study, Vilma Graupner of F. Hoffmann-La Roche Ltd for critical review of manuscript drafts, Jing Yi for contributions to statistical analysis, Dustin Smith, Susan Flynn, Ivette Estay, Natasha Miley, and Priti Hegde of Genentech, Inc. for contributions to the biomarker analyses, and Wei Zou of Genentech, Inc. for contributions to gene expression analyses. Qlabs (Livingston, United Kingdom) did the central PD-L1 testing. HistoGeneX (Antwerp, Belgium) did the PD-L1 scoring. Support for third-party writing assistance for this manuscript, by Daniel Clyde and Kshipra Desai of Health Interactions, was provided by Genentech, Inc.
Funding
F. Hoffmann-La Roche Ltd, Genentech, Inc.
Footnotes
Declaration of interests
AR received grants and personal fees from Roche, Eli Lilly, Bristol-Myers Squibb, AstraZeneca, MSD, Boehringer Ingelheim, and Pfizer. FB received personal fees from Roche. DW, MB, and AS are Genentech employees and holders of Roche stock. KP received personal fees and research funding from AstraZeneca, and personal fees from Astellas, AVEO, Boehringer Ingelheim, Clovis, Eli Lilly, Hanmi, KHK Novartis, Ono, and Roche. FC received personal fees from Merck Serono, AstraZeneca, Eli Lilly, Roche, and Bayer. JvP received advisory board fees paid to institution from Daiichi Sankyo, Clovis, Roche, Vertex, and Novartis. SMG received personal fees from Roche/Genentech, Pfizer, Bristol-Myers Squibb, Ariad, Boehringer Ingelheim, and AstraZeneca. TH received grants and personal fees from Chugai, Eli Lilly, Ono, Novartis, Taiho Pharmaceutical, Nippon Boehringer Ingelheim, Pfizer, Bristol-Myers Squibb, Clovis, and AstraZeneca, and grants from Eisai, Takeda, Dainippon Sumitomo Pharma, Abbvie, Merck, Kyowa Hakko Kirin, Daiichi Sankyo, and Astellas. DMK, MCD, DLC, JL, JP, FK, OAF, FDM, HT, J-SL declare no competing interests. CB received clinical research fees from Pfizer, Novartis, Amgen, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Roche/Genentech, Eli Lilly, Sanofi, Taiho Pharmaceutical, Mylan, Merrimack, Merck, Abbvie, Astellas, BioMarin, Bristol-Myers Squibb, Daiichi Sankyo, Abraxis BioScience, AB Science, Asana Biosciences, Medivation, Exelixis, ImClone Systems, LEO Pharma, Millennium, and personal fees from Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Roche (Genentech), Eisai, and Bioepis. MK and DSC are Genentech employees, holders of Roche stock, and have a Genentech patent pending for biomarkers and methods treating PD-1-related and PD-L1-related conditions. PH is a Genentech employee, holder of Roche and Amgen stock, and reports that a family member holds Gilead and Allergan stock. DRG received grants for serving as a consultant to Bristol-Myers Squibb, Genentech, and Merck.
References
- 1.Stinchcombe TE, Socinski MA. Considerations for second-line therapy of non-small cell lung cancer. Oncologist 2008; 13: 28–36. [DOI] [PubMed] [Google Scholar]
- 2.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540–50. [DOI] [PubMed] [Google Scholar]
- 5.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 2008; 8: 467–77. [DOI] [PubMed] [Google Scholar]
- 6.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013; 39: 1–10. [DOI] [PubMed] [Google Scholar]
- 7.Kim JM, Chen DS. Immune escape to PD-L1/PD-1 blockade: seven steps to success (or failure). Ann Oncol 2016; 27: 1492–504. [DOI] [PubMed] [Google Scholar]
- 8.Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy—inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res 2012; 18: 6580–87. [DOI] [PubMed] [Google Scholar]
- 9.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515: 563–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014; 515: 558–62. [DOI] [PubMed] [Google Scholar]
- 11.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7–1 costimulatory molecule to inhibit T cell responses. Immunity 2007; 27: 111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Riella LV, Chock S, et al. The novel costimulatory programmed death ligand 1/B7.1 pathway is functional in inhibiting alloimmune responses in vivo. J Immunol 2011; 187: 1113–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paterson AM, Brown KE, Keir ME, et al. The programmed death-1 ligand 1:B7–1 pathway restrains diabetogenic effector T cells in vivo. J Immunol 2011; 187: 1097–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park JJ, Omiya R, Matsumura Y, et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood 2010; 116: 1291–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumoto K, Fukuyama S, Eguchi-Tsuda M, et al. B7-DC induced by IL-13 works as a feedback regulator in the effector phase of allergic asthma. Biochem Biophys Res Commun 2008; 365: 170–75. [DOI] [PubMed] [Google Scholar]
- 16.Akbari O, Stock P, Singh AK, et al. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal Immunol 2010; 3: 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horn L, Spigel DR, Gettinger SN, et al. Clinical activity, safety and predictive biomarkers of the engineered antibody MPDL3280A (anti-PDL1) in non-small cell lung cancer (NSCLC): update from a phase Ia study. J Clin Oncol 2015; 33 (suppl): 8029 (abstr). [Google Scholar]
- 18.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016; 387: 1837–46. [DOI] [PubMed] [Google Scholar]
- 19.Smith DA, Vansteenkiste JF, Fehrenbacher L, et al. Updated survival and biomarker analyses of a randomized phase II study of atezolizumab vs docetaxel in 2L/3L NSCLC (POPLAR). J Clin Oncol 2016; 34 (suppl): 9028 (abstr). [Google Scholar]
- 20.National Cancer Institute. Common Terminology Criteria for Adverse Events version 4.0. May 29, 2009. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5×11.pdf (accessed July 14, 2016).
- 21.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009; 15: 7412–20. [DOI] [PubMed] [Google Scholar]
- 22.Gandara DR, Redman M, Hirsch FR. Postprogression prolongation of survival in EGFR-mutated lung cancer: reconciling the ASPIRATION and IMPRESS trials. JAMA Oncol 2016; 2: 300–01. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.