Abstract
Background & objectives:
Chronic exposure to pesticides can damage DNA and lead to cancer, diabetes, respiratory diseases and neurodegenerative and neurodevelopment disorders. The objective of this study was to determine the frequency of DNA damage through the comet assay and micronucleus (MN) test in two groups of children, under 10 yr of age living in rural Paraguay and in relation to pesticide exposure.
Methods:
Two groups of 5 to 10 yr old children were formed; the exposed group (group A, n=43), born and currently living in a community dedicated to family agriculture and surrounded by transgenic soybean crops, and the control group (group B, n=41), born and living in a community dedicated to family agriculture with biological control of pests. For each child, 2000 cells were studied for the MN test and 200 cells for the comet assay.
Results:
The comparison between exposed and control children revealed significant differences in biomarkers studied for the measurement of genetic damage (cell death and DNA damage). The median of MN was higher in the exposed group (6 vs. 1) (P<0.001). Binucleated cells (2.9 vs. 0.5, P<0.001); broken eggs (5.5 vs. 1.0, P<0.001); karyorrhexis (6.7 vs. 0.5, P<0.001); kariolysis (14.0 vs. 1.0, P<0.001); pyknosis (7.4 vs. 1.2, P<0.001) and condensed chromatin (25.5 vs. 7.0, P<0.001) were significantly higher in the exposed group. The values of tail length (59.1 vs 37.2 μm); tail moment (TM) (32.8 vs. 14.4 μm); TM olive (15.5 vs. 6); % DNA tail (45.2 vs. 27.6) and % DNA head (54.8 vs. 72.4), were significantly different between the two groups.
Interpretations & conclusions:
In children exposed to pesticides, a greater genotoxic and cytotoxic effect was observed compared to non-exposed children. Our findings suggest that monitoring of genetic toxicity in population exposed to pesticides and agrochemicals should be done.
Keywords: Biomonitoring, buccal micronucleus cytome assay, children, comet assay, DNA, pesticides
Paraguay is currently the world's fourth largest exporter and soy producer1. The area covered by this crop covers 80 per cent of agricultural land. Between August 2015 and July 2016, Paraguay officially imported almost 38,000 tons of pesticides; 64 per cent of the substances were herbicides, preferably for the monoculture of transgenics2. The application of agrochemicals is carried out in a context of low and ineffective environmental regulation3. Pesticides produce adverse effects on the environment and health. There is evidence of the relationship between exposure to pesticides and increased risk of cancers, neurodegenerative disorders and neurodevelopment and respiratory diseases and diabetes4. The importance of early detection of genetic damage is that it allows taking the necessary measures to reduce or suppress the exposure to the deleterious agent when it is still reversible and thereby prevent and reduce the risk of developing neoplasia and other pathological alterations5.
Micronucleus (MN) is a biomarker that is widely used in biomonitoring studies to determine the genetic risk associated with pesticide exposure. The oral MN assay is a cytogenetic method to measure genetic damage, cell proliferation, differentiation and death in exfoliated oral cells6,7. The comet assay is a sensitive technique to evaluate DNA damage of a variety of damaged cells caused by different physical and chemical agents. It is widely used in human biomonitoring to measure DNA damage as a marker of exposure to genotoxic agents or to investigate genoprotective effects8.
Among the exposed populations, children constitute a group that has particular exposure characteristics and special vulnerability to environmental toxics. Children may also differ from adults in levels of detoxification, in processes of DNA repair and cell proliferation. The most obvious difference between children and adults is the reduced impact of traditional confounding factors such as cigarette smoking and occupational exposure5,9.
The San Juan colony, located in the district of Francisco Alvarez Caballero, Department of Canindeyú, Paraguay, was officially authorized by the Rural Welfare Institute in 199710. At present, there are about 450 families dedicated to family agriculture. Surrounded by large areas of soybean, mostly transgenic, the population is exposed to aerial and terrestrial spraying, without any protection or precaution towards people3. The objective of this study was, therefore, to determine the frequency of DNA damage (evaluated through the comet assay and MN test in exfoliated cells of the buccal mucosa) and the alteration of plasma cholinesterase and its relation to pesticide exposures in two groups of children under 10 yr of age living in rural areas.
Material & Methods
This study was carried out at the Toxicological Genetics Laboratory of Faculty of Health Sciences of Catholic University “Nuestra Señora de la Asunción” between January 2016 to June 2017, after the protocol approval by the Ethics Committee of the Catholic University, Asuncion, Paraguay. Two groups of healthy children of 5-10 yr of age, of both sexes were formed. Group A (n=43) was considered as exposed and group B (n=41) was considered as not exposed. The exposed population (group A) of healthy children comprised those who were born in the place, and have been living on a permanent basis on that location for more than five years, of both sexes from 5 to 10 yr of age. They were potentially exposed (chronic exposure) to excessive and indiscriminate use11 of agrochemicals used in intensive fumigation practices of monocultures living less than 1 km away from the fumigated fields of the San Juan Colony in the Department of Canindeyú (Paraguay). This community was isolated, exposed to the practices of aerial and terrestrial fumigation of pesticides, and was surrounded by transgenic soybean crops. Fumigation with pesticides took place every 15 days during 150 days when the soybean crops mature (September-January); in the rainy season, the fumigation cycle for the transgenic soybeans was duplicated and further proceeded during the year due to the existence of transgenic corn and transgenic sunflower. The community had 450 families or less, mostly living on family agriculture for self consumption and as a means of income.
Unexposed population (group B) included children of both sexes, 5-10 yr of age, born in agricultural areas that perform biological pest control of the Sargent Baez County, of the First District of the Cordillera Department. Most of the adult population were sugar cane producers, members of the Manduvirá Cooperative (Cooperativa Manduvira)12. Families in the San Juan Colony as well as those of the Sargent Baez County lived in rural areas.
Exclusion criteria: Children without the consent of the parents or guardians, all those children who refused to take part in the study or those with physical disabilities, pubertal onset, chronic pathologies and congenital malformations and with a history of X-ray exposure in the past six months were excluded. Based on the suggestions given by Preston and Hoffman13 the simple size was calculated. For the present study, 43 cases and 41 controls were selected. The invitation to participate in the study was extended through communication with established community leaders. Subsequently, only with those individuals who read, understood and accepted the terms of the informed consent form and additionally were not excluded by the exclusion criteria, were studied. For the study of cell damage and MNs, the unit of analysis was constituted by oral mucosa cells and peripheral blood lymphocytes for the comet assay.
Measurements and instrument used to compile data: A questionnaire structured in six sections (risk factors, sociodemographic characteristics, anthropometric measurements, clinical profile, laboratory and biomarker study) with closed questions, was developed and validated. The quality control was done through a 'test-retest' in a subsample of ten individuals, with similar characteristics to the target population, that were administered through interviews. In those questions that presented difficulties of understanding, a deeper investigation was carried out to facilitate the analysis process and the corresponding adjustment of the items, thus ensuring an optimum version that was applied to the study sample.
To find about chronic exposure as a measure of the effect, the MN test and the comet assay were utilized. On the exposed population, the collection of genetic material was done at a time when the fumigation with pesticides was being carried out on a non-intensive basis. Acute intoxication from organic phosphates and carbamates was determined through plasma cholinesterase on both populations of interest.
Sample collection
Urine and blood (5 ml) samples taken in the morning (haemogram, proteinaemia, plasma cholinesterase, renal, hepatic and thyroid profile) were collected in January 2016 from group A children. Material for the comet assay and MN test was collected in August 2016. From group B children, urine and blood samples were collected in September 2016, for comet assay and MN test. The nutritional status was determined by anthropometry: weight and height14; proteinaemia, blood analysis, renal, hepatic, thyroid, plasma cholinesterase, simple urine and serial faeces. Anaemia was considered when Hb was below 11.5 g/dl15 and hypoproteinaemia below 6 g/dl16.
Genotoxicity biomarkers: The bioassays employed were carried out by the Laboratory of Genetic Toxicology of the Faculty of Health Sciences at the Catholic University Our Holy Mother of Asuncion.
Buccal micronucleus cytome (BMNcyt) assay: After performing a mouthwash with water, the inner surface of the cheek of the individuals was scraped with a sterile wooden tongue blade, pre-moistened in physiological serum. The sample was fixed on slide with 80 per cent methanol for 1 h and then allowed to dry at room temperature. The samples were stained according to the Feulgen technique17, for which the hydrolysis was done by immersing the sheets in 1N HCl at 60°C for 10 min. To cut off the hydrolysis, the samples were washed in cold distilled water, and then placed in the Schiff reagent (Sigma-Aldrich, USA) for 60 min.
For analysis, 2000 cells per individual were taken according to the Tolbert technique18. The frequency of MNs and other nuclear abnormalities such as karyorrhexis (KR), broken egg (BE), karyolysis (KL), pyknosis (PIk) and binucleated (BN) cells, were determined based on the considerations of Bolognesi et al6. The cells were evaluated with light-field Olympus microscopes, model CX21, from Japan, with a magnification of 1000×.
Comet assay: Evaluation of DNA damage was done by the comet assay19,20. Forty microlitre of peripheral blood was collected from the third finger of each participant in Eppendorf tubes, containing 1.5 ml of cell culture medium (RPMI, Roswell Park Memorial Institute), 20 mM EDTA (ethylenediaminetetraacetic acid) and 10 per cent dimethyl sulfoxide (DMSO), for correct assay. Initial sample (120 μl) was mixed with 240 μl of 0.7 per cent low melting point (LMP) agarose. These were plated on gelled sheets with one per cent normal melting point agarose, and coated with a third layer of LMP agarose. The gelled sheets were maintained at 4°C for 6 to 8 h immersed in a lysis solution (lysis buffer, Triton X-100 and DMSO), for membrane lysis. The plates were placed in an alkaline-buffered electrophoresis cuvette at pH 12.5 for 20 min to allow the decomposition of DNA and exposure of damaged alkali sites. After this, electrophoresis was performed for 20 min at 25 V and 300 mA and, the sheets were washed in a 0.4 M Trizma base at pH 7.5 neutralization buffer to remove excess alkali and the detergents. Ethidium bromide (80 μl of 10 μg/ml) was used for staining; 20 min later, deionized water was used to remove excess dye. The slides were examined under epifluorescence microscope (ZEISS AXIOS A1, Germany) with an increase of 200×, using a 590 nm filter.
Two hundred comets per participant were analyzed using the Comet Imager software v2.2 (MetaSystems, Germany). Cell damage was determine using the following parameters into account: Tail length (TL): Total length of the comet, from the nuclear centre to the end of the tail. Tail moment (TM): Migration length of the DNA outside the nucleus forming the tail of the comet. Olive TM (OTM): Tail size of the comet plus the total DNA fraction from the centre of the head to the tail end. Head DNA: the percentage of DNA in the head of the comet. Tail DNA: the percentage of DNA in the tail of the comet that indicates the amount of fragmented DNA that migrated on electrophoresis.
Statistical analysis: For data analysis related to demographic characteristics, nutritional status and anaemia, frequency measures, central tendency and dispersion, were used depending on the nature of the samples. Significance of the differences between groups was analysed using the Student's t test and Chi-square test. Non-parametric Mann-Whitney test was used for the evaluation of data related to DNA damage.
Results
The demographic characteristics are presented in the Table. The children in the two groups had similar age, haemoglobin level and nutritional status. They all had normal hepatic, renal and thyroid function. There was a significant difference in eosinophil count between the two groups. No child presented with chronic malnutrition (height/age); proteinaemia values were whitin the normal range in both the studied groups. One child (exposed group) was excluded from the study because of chronic renal failure, detected at the time of the study.
Table.
Demographic profile, nutritional status, eosinophilia and cholinesterase in the exposed and not exposed population
| Variables | Exposed (n=43) | Not exposed (n=41) |
|---|---|---|
| Age, mean±SD (yr) | 7.6±1.8 | 8.0±1.8 |
| Group, n (%) | ||
| 5-6 | 13 (30.2) | 10 (24.4) |
| 7-8 | 14 (32.6) | 13 (31.7) |
| 9-10 | 16 (37.2) | 18 (43.9) |
| Sex, n (%) | ||
| Male | 27 (62.7) | 19 (46.3) |
| Female | 16 (37.2) | 22 (53.7) |
| Nutritional status, n (%) | ||
| Suitable stature | 40 (93.0) | 33 (80.5) |
| Risk low stature | 3 (7.0) | 8 (19.5) |
| Low stature | - | - |
| Proteinaemia, g/dl (mean±SD) | 7.2±0.5*** | 7.5±1.1 |
| Eosinophilia, % median (rank) | 8.0±3.1*** | 3.5 (2.5) |
| Hb, g/dl (mean±SD) | 12.5±0.5 | 12.5±0.6 |
| Cholinesterase, U/l (mean±SD) | 9182±1830 | 9493±1623 |
***P<0.001 compared to not exposed group; Hb, haemoglobin
The results of the buccal MN cytome assay showed highly significant differences when comparing the exposed population with the unexposed population, as shown in Fig. 1. The frequency of MN was higher in the exposed group (6 vs 1) (P<0.001). Similar trends are observed with BN cells (2.9 vs 0.5, P<0.001); BE (5.5 vs 1.0, P<0.001), karyorrhexis (6.7 vs 0.5, P<0.001); karyolysis (14.0 vs 1.0, P<0.001); pyknosis (7.4 vs 1.2, P<0.001); and condensed chromatin (25.5 vs 7.0, P<0.001).
Fig. 1.
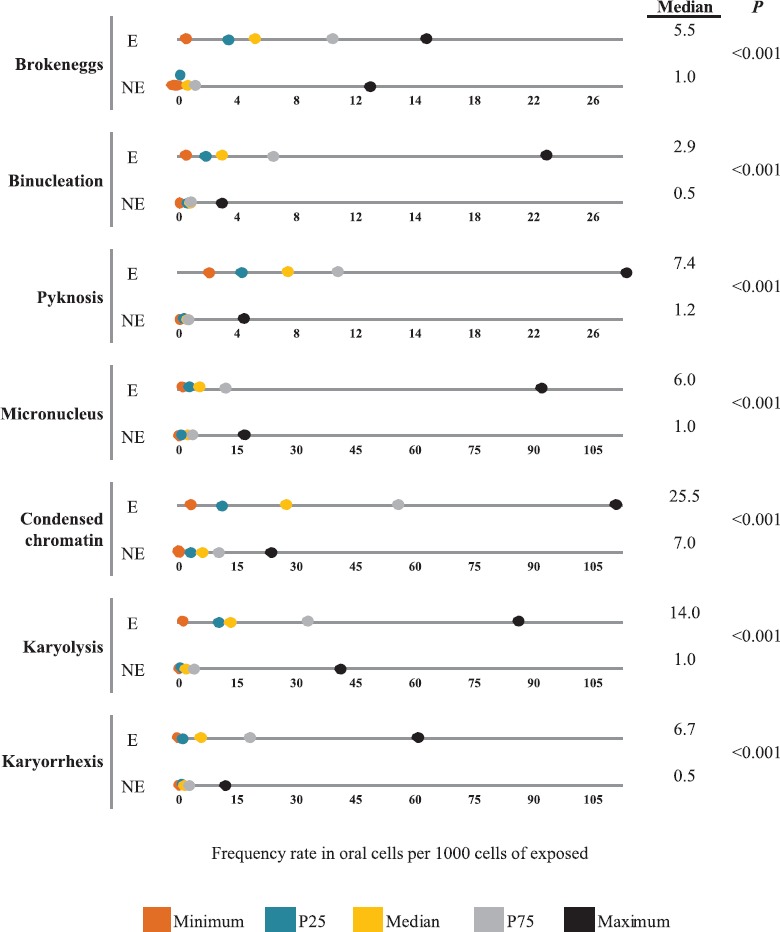
Anomaly rate in oral cells per 1000 cells of exposed (E) (n=43) and not exposed (NE) (n=41). P25, 25th percentile; P75, 75th percentile.
The mean values of TL, TM and OTM on the cells of the exposed children showed significant (P<0.001) differences compared to those not exposed, as shown in Fig. 2. The DNA percentage in the tails and heads of the comets of exposed and unexposed children showed a higher percentage of DNA in the head of the comets of unexposed children compared to the exposed group and higher percentage of DNA in the comet tails of exposed children's cells compared to the unexposed (Fig. 3).
Fig. 2.
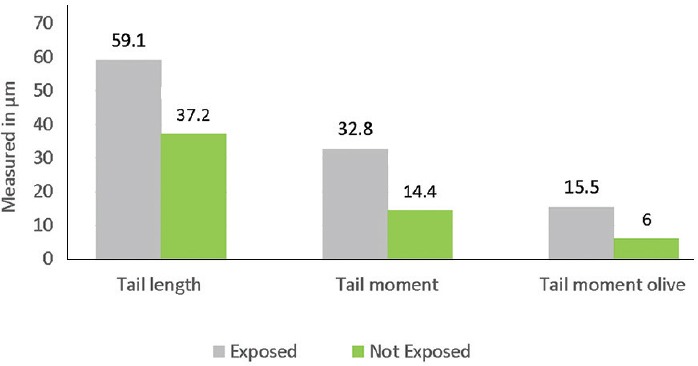
DNA damage, measured by length in μm of tail length, tail moment and tail moment olive parameters of the comet assay of exposed (n=43) and not exposed (n=41) populations.
Fig. 3.
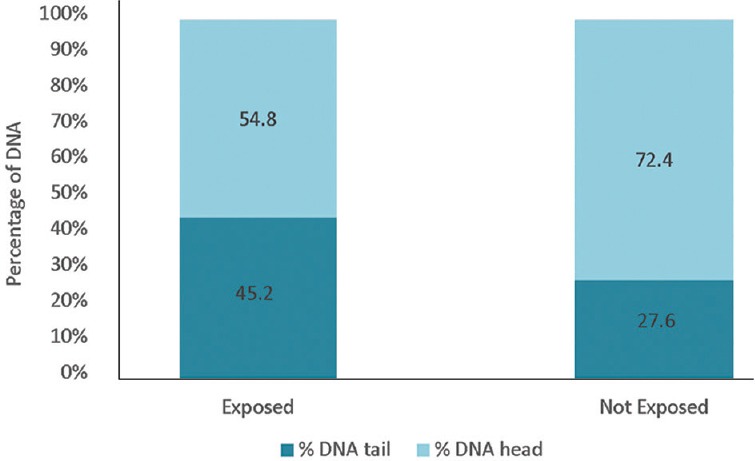
DNA distribution according to the comet assay for exposed (n=43) and not exposed (n=41) populations.
Discussion
The increase in TL, TM, OTM values and the percentage of DNA in the tail of the observed comets, in peripheral white blood cells of children exposed to different concentrations of agrochemicals, indicated that exposure to these compounds induced DNA damage. An earlier study from Brazil showed similar findings21. Studies in which the comet assay was used have reported that organophosphates and pyrethroids, commonly used as pesticides, have a high genotoxic potential22. Others have indicated glyphosate and organophosphates as pesticides that induce DNA damage23. Glyphosate has been reported to be safe for humans24. The continued exposure and persistence of unrepaired damage induced by pesticide and agrochemical components and the formation of free radicals may induce increased chromosomal aberrations, and the complex mixture of chemical components would interfere with DNA repair mechanisms25.
The buccal MN cytome test revealed damage in the genetic and cytotoxic material in the cells of exposed children, manifested by the increase of MN, BE and BN cells, compared to the population of unexposed children. The MN rate per 1000 cells counted in unexposed children was 0.22 per cent; according to Bonassi et al26, the baseline MN frequency for individuals not exposed to genotoxic agents is from 0.3 to 1.70. Though our study was conducted on children, our data for unexposed children were within the rank found by Bonassi et al26. Other nuclear figures have been observed in a large number in cells of exposed children, such as condensed chromatin, associated with apoptosis processes and corresponding to regions with little transcriptional activity6,27, karyolysis, which corresponds to the state of total disintegration of the nucleus and occurs in late states of apoptosis and necrosis28. These findings were similar to a previous investigation in which significant differences were found in the frequencies of MN, BN cells and karyorrhexis in a population of children exposed for six years to contaminants from an agrochemical factory, compared to the non-exposed population29.
The exposed population was surrounded by large soybean crops, at a distance of 20 km of the nearest urban centre with which it was connected through a dirt road, and air pollution due to vehicular use was scarce. This population was characterized by an overall good health and nutritional state. These children were exposed to intensive spraying and pesticide use in their homes. The level of damage at the DNA level, utilized as a marker of the effect in this study, was significant when comparing the exposed and non-exposed groups.
There were no significant differences in relation to the demographic profile of exposed group children when compared with the children of the unexposed population. The main confounders such as anaemia and malnutrition were not found. An increase in eosinophil count was identified in the exposed population that was not associated with infestations by helminthic parasites30. Verea et al31 described a significant increase in eosinophils and a decrease in white blood cells in a study carried out with a group of agricultural workers exposed to pesticides.
In children younger than two years, the mechanisms of hepatic detoxification mediated by glutathione S-transferase and glucuronyltransferase as well as by the cytochrome P450 enzyme system are immature, and their ability to inactivate and detoxify will determine whether a chemical compound will be toxic or not. Glyphosate can cause interference with these enzymes and alter their homeostasis.
The results found in several investigations of Latin America and Europe5,32 constitute evidence that indicate that the exposure to pesticides causes genotoxic damage, and this allows to establish the scientific bases to realize a preventive health intervention, especially in more vulnerable populations such as pregnant women and children. This genotoxic damage can initiate the process of carcinogenesis, mutagenesis and teratogenesis. Several pesticides have been characterized as probable human carcinogens by the International Agency for Research on Cancer33 associated with exposure to pesticides in epidemiological studies4.
In conclusion, a greater genotoxic and cytotoxic effect was observed in children exposed to pesticides compared to non-exposed children. There was a greater DNA damage in children exposed to pesticides compared to those not exposed. Further studies need to be done on exposure to the mixtures of pesticides and their effects on health over time.
Footnotes
Financial support & sponsorship: This investigation was conducted with the financial support of the National Council on Science and Technology (CONACYT) Project: 14INV180, Paraguay.
Conflicts of Interest: None.
References
- 1.The Community Action Program of East Central Oregon. Ranking mundial. [accessed on April 13, 2017]. Available from: http://capeco.org.py/ranking-mundial-es/
- 2.Franceschelli I. Importación de agroquímicos: La principal actividad económica nacional no es nacional. In: Marielle P, editor. Con la soja al cuello 2016: Informe sobre agronegocios en Paraguay. Asunción, Paraguay: BASE-Is; 2016. pp. 28–31. [Google Scholar]
- 3.Benítez-Leite S, Corvalán R, Avalos DS, Almada M, Corvalán A. Violated rights in rural populations exposed to transgenic soybean crop (Preliminary Study) Br J Med Med Res. 2016;16:1–8. [Google Scholar]
- 4.Bolognesi C, Holland N. The use of the lymphocyte cytokinesis-block micronucleus assay for monitoring pesticide-exposed populations. Mutat Res. 2016;770:183–203. doi: 10.1016/j.mrrev.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Aiassa D, Mañas F, Bosch B, Gentile N, Bernardi N, Gorla N. Biomarcadores de daño genético en poblaciones humanas expuestas a plaguicidas. Acta Biol Colomb. 2012;17:485–510. [Google Scholar]
- 6.Bolognesi C, Knasmueller S, Nersesyan A, Thomas P, Fenech M. The HUMNxl scoring criteria for different cell types and nuclear anomalies in the buccal micronucleus cytome assay – An update and expanded photogallery. Mutat Res. 2013;753:100–13. doi: 10.1016/j.mrrev.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Bolognesi C, Knasmueller S, Nersesyan A, Roggieri P, Ceppi M, Bruzzone M, et al. Inter-laboratory consistency and variability in the buccal micronucleus cytome assay depends on biomarker scored and laboratory experience: Results from the HUMNxl international inter-laboratory scoring exercise. Mutagenesis. 2017;32:257–66. doi: 10.1093/mutage/gew047. [DOI] [PubMed] [Google Scholar]
- 8.Collins A, Koppen G, Valdiglesias V, Dusinska M, Kruszewski M, Møller P, et al. The comet assay as a tool for human biomonitoring studies: The comNet project. Mutat Res Rev Mutat Res. 2014;759:27–39. doi: 10.1016/j.mrrev.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Neri M, Bonassi S, Knudsen LE, Sram RJ, Holland N, Ugolini D, et al. Children's exposure to environmental pollutants and biomarkers of genetic damage. I. Overview and critical issues. Mutat Res. 2006;612:1–3. doi: 10.1016/j.mrrev.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Valiente H. Asunción, Paraguay: BASE-Is; 2014. Comunidades en lucha: Cuatro demandas al Estado paraguayo por violación de Derechos Humanos; p. 128. [Google Scholar]
- 11.United Nations. Report of the Special Rapporteur on the right to food on her mission to Paraguay A/HRC/34/48/Add.2. Geneva: General Assembly; 2017. [Google Scholar]
- 12.Cooperativa Manduvirá. Primera Cooperativa productora y exportadora de azúcar orgánica del Paraguay. Asunción: Cooperativa Manduvira Ltda; 2012. [Google Scholar]
- 13.Preston RJ, Hoffmann GR. Genetic toxicology. In: Klaassen CD, editor. Cassaret & Doull's toxicology: The basic science of poisons. 7th ed. Nueva York: McGraw Hill; 2008. pp. 381–413. [Google Scholar]
- 14.World Health Organization. WHO anthro survey analyser and other tools. [accessed on April 30, 2017]. Available from: https://www.who.int/childgrowth/software/es/
- 15.World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity (WHO/NMH/NHD/MNM/11.1) Geneva: WHO; 2011. [Google Scholar]
- 16.Manary MJ, Trehan I. Protein-energy malnutrition. In: Goldman L, Shafer AI, editors. Goldman-Cecil Medicine. 25th ed. Philadelphia, PA: Elsevier Saunders; 2016. [Google Scholar]
- 17.Jalayer Naderi N. Reporting an experience: Improving the Feulgen staining technique for Better visualizing of nucleus. Iran J Pathol. 2018;13:106–7. [PMC free article] [PubMed] [Google Scholar]
- 18.Tolbert PE, Shy CM, Allen JW. Micronuclei and other nuclear anomalies in buccal smears: Methods development. Mutat Res. 1992;271:69–77. doi: 10.1016/0165-1161(92)90033-i. [DOI] [PubMed] [Google Scholar]
- 19.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–91. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 20.Hartmann A, Agurell E, Beevers C, Brendler-Schwaab S, Burlinson B, Clay P, et al. Recommendations for conducting the in vivo alkaline comet assay 4th International Comet Assay Workshop. Mutagenesis. 2003;18:45–51. doi: 10.1093/mutage/18.1.45. [DOI] [PubMed] [Google Scholar]
- 21.Benedetti D, Nunes E, Sarmento M, Porto C, Dos Santos CE, Dias JF, et al. Genetic damage in soybean workers exposed to pesticides: Evaluation with the comet and buccal micronucleus cytome assays. Mutat Res. 2013;752:28–33. doi: 10.1016/j.mrgentox.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Kaur R, Kaur S, Lata M. Evaluation of DNA damage in agricultural workers exposed to pesticides using single cell gel electrophoresis (comet) assay. Indian J Hum Genet. 2011;17:179–87. doi: 10.4103/0971-6866.92100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwiatkowska M, Reszka E, Woźniak K, Jabłońska E, Michałowicz J, Bukowska B. DNA damage and methylation induced by glyphosate in human peripheral blood mononuclear cells (in vitro study) Food Chem Toxicol. 2017;105:93–8. doi: 10.1016/j.fct.2017.03.051. [DOI] [PubMed] [Google Scholar]
- 24.Williams GM, Kroes R, Munro IC. Safety evaluation and risk assessment of the herbicide roundup and its active ingredient, glyphosate, for humans. Regul Toxicol Pharmacol. 2000;31:117–65. doi: 10.1006/rtph.1999.1371. [DOI] [PubMed] [Google Scholar]
- 25.Bolognesi C. Genotoxicity of pesticides: A review of human biomonitoring studies. Mutat Res. 2003;543:251–72. doi: 10.1016/s1383-5742(03)00015-2. [DOI] [PubMed] [Google Scholar]
- 26.Bonassi S, Coskun E, Ceppi M, Lando C, Bolognesi C, Burgaz S, et al. The Human micronucleus project on exfoliated buccal cells (HUMN(XL)): The role of life-style, host factors, occupational exposures, health status, and assay protocol. Mutat Res. 2011;728:88–97. doi: 10.1016/j.mrrev.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Oberhammer FA, Hochegger K, Fröschl G, Tiefenbacher R, Pavelka M. Chromatin condensation during apoptosis is accompanied by degradation of lamin A+B, without enhanced activation of cdc2 kinase. J Cell Biol. 1994;126:827–37. doi: 10.1083/jcb.126.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holland N, Bolognesi C, Kirsch-Volders M, Bonassi S, Zeiger E, Knasmueller S, et al. The micronucleus assay in human buccal cells as a tool for biomonitoring DNA damage: The HUMN project perspective on current status and knowledge gaps. Mutat Res. 2008;659:93–108. doi: 10.1016/j.mrrev.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Benítez-Leite S, Macchi ML, Fernández V, Franco D, Ferro EA, Mojoli A, et al. Prevalence of incidental finding eosinophilia in children in rural communities [Prevalencia de eosinofilia de hallazgo incidental en niños de comunidad rural] Pediatr (Asunción) 2016;43(Suppl):62. [Google Scholar]
- 30.Desvars P, Avalos DS, Galeano A, Benítez-Leite S, Campuzano A. Prevalence of incidental finding eosinophilia in children in rural communities [Prevalencia de eosinofilia de hallazgo incidental en niños de comunidad rural] Pediatr (Asunción) 2016;43(Suppl):62. [Google Scholar]
- 31.Verea MC, Masoero C, Gentile N, Bosch B, Aiassa D. Possible biomarkers to assess occupational exposure to pesticides [Biomarcadores posibles para evaluar la exposición laboral a plaguicidas] Retel Rev Toxicol Línea. 2016;45:13–27. [Google Scholar]
- 32.Koller VJ, Fürhacker M, Nersesyan A, Mišík M, Eisenbauer M, Knasmueller S, et al. Cytotoxic and DNA-damaging properties of glyphosate and roundup in human-derived buccal epithelial cells. Arch Toxicol. 2012;86:805–13. doi: 10.1007/s00204-012-0804-8. [DOI] [PubMed] [Google Scholar]
- 33.International Agency for Research on Cancer. Some organophosphate insecticides and herbicides: IARC monograph on the evaluation of carcinogenic risk to humans. 12. Geneva: World Health Organization; 2017. [PubMed] [Google Scholar]


