Abstract
Background
Tamoxifen (TAM) is the first-line drug for estrogen receptor-positive (ER+) breast cancer (BC) treatment. However, its resistance is a main obstacle in clinical practice. Thus, new therapeutic agents are urgently needed to fight TAM resistance.
Material/Methods
Here, we constructed TAM-resistant ER+BC cells with TAM resistance, named MCF-7-R. Western blot, quantitative real-time PCR (qRT-PCR), ALDH1 activity analysis, and spheroid-forming detection were used to detect the stemness of cells and the effects of napabucasin (NP) on BC cell stemness. Cell counting kit-8 (CCK8) assay was used to evaluate the effects of NP on cell viability.
Results
MCF-7-R cells exhibited higher stemness compared with the parental MCF-7 cells, which was evident by the increased spheroid formation ability at diluted concentration, aldehyde dehydrogenase (ALDH) activity, and expression of stemness critical biomarkers (Oct4, Nanog, and Sox2). Additionally, it was found that napabucasin (NP) specifically killed MCF-7-T cells, characterized by remarkably decreased IC50 value. Notably, NP reduced MCF-7-R cell stemness, which was evident as the decreased stemness marker expression, spheroid-forming capacity, and ALDH1 activity. Importantly, NP attenuated TAM resistance of MCF-7-R cells and enhanced sensitivity of MCF-7 cells to TAM. Mechanistic study showed that NP inhibited STAT3 activation, and overexpression of STAT3 rescued NP-mediated inhibition of the stemness-like characteristics of MCF-7-R cells.
Conclusions
NP might be used as an adjuvant therapy for ER+ BC patients with TAM resistance.
MeSH Keywords: Colony-Forming Units Assay, Crk-Associated Substrate Protein, Tamoxifen
Background
Endocrine therapy is an important part of comprehensive treatment for ER+breast cancer (BC) patients [1]. It mainly includes ER antagonist tamoxifen (TAM), aromatase inhibitors (AI) (e.g., anastrozole and letrozole). Use of drugs that downregulate ER (e.g., fulvestrant) has become increasingly common, and tamoxifen especially has become the first-line drug for ER+BC patients due to its low toxicity, affordability, and long-lasting effect, and has been used in early and late ER+BC treatment [2]. Although the early use of TAM has obvious benefits, clinical application has found that patients are prone to resistance to TAM, leading to cancer progression and metastasis, which is still a major clinical problem [3].
At present, the main mechanisms of TAM resistance include ER changes, enhanced growth factor pathways, nuclear receptor co-regulatory factors, hypersensitivity to estrogen, and drug metabolism changes [4]. In addition, studies have shown that cancer stem cells (CSCs) lead to the acquired drug resistance of BC [5]. CSCs are found in low numbers in cancer tissue, and have the ability to self-renew and differentiate [6]. Based on this ability, CSCs are considered to be the cells that produce cancer tissue. It has been found that BC CSCs (BCSCs) are resistant to radiotherapy and chemotherapy, and therefore result in the occurrence and development of BC [6]. Recent studies have shown that BCSCs are involved in TAM resistance. For example: the expression of stem cell markers is increased in ER+BC cells with endocrine resistance [7,8]; decreased expression of forkhead box protein A1 (FOXA1) was identified in TAM-resistant ER+ BC cells, which promotes BC cell stemness [9]; and Interleukin-33 (IL-33) induces tamoxifen resistance by promoting the stemness of BC cells [10]. Therefore, targeting BCSCs might overcome TAM resistance. Napabucasin (NP) is a small STAT3 inhibitor that has been shown to attenuate the stemness of other types of tumors [11]. Notably, inhibition of STAT3 has been shown to suppress BC stemness [12,13]. Here, we speculated that NP could attenuate the stemness of ER+cells with TAM resistance.
The present study found that MCF-7-R cells exhibited higher stemness compared with MCF-7 cells and NP decreased ALDH activity, spheroid formation ability, and stemness marker expression of MCF-7-R cells. Additionally, NP reversed the TAM resistance of MCF-7-R cells and enhanced the sensitivity of MCF-7 cells to TAM. Mechanistic research revealed that NP attenuated MCF-7-R cell stemness by regulating STAT3 activity. These data suggest that targeting BCSCs in the context of TAM-resistant ER+cells using NP, in combination with cisplatin, may be an effective and novel approach in ER+BC treatment.
Material and Methods
Cell culture and reagents
Human ER+ BC cell line MCF-7 cells were purchased from Gaining Biological (Shanghai, China). TAM-resistant MCF-7 cells (MCF-7-R) were constructed by culturing with 1 μM TAM (Selleck Chemicals, Houston, TX, USA) for 3 months and then cultured with 1 nM TAM. The resistance index was confirmed before use. Cell lines were maintained in DMEM medium (Hyclone), with 10% FBS (fetal bovine serum, Thermo Fisher Scientific) at 37°C in a humidified atmosphere with 5% CO2. Napabucasin, the inhibitor of STAT3, was purchased from MedChem Express (Monmouth Junction, NJ, USA).
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted using the Total RNA Extraction Kit (Solarbio, Beijing, China). Then, cDNA was reversely synthesized and qRT-PCR was performed using the Hifair™ III One-Step RT-qPCR Probe Kit (YEASEN). Primers sequences used were:
Nanog, forward, 5′-CACGCCAGACTTACCTGTCCTACT-3′,
reverse, 5′-TGTCAACATCCTCCTTATCTCCTT-3′;
Oct4, forward, 5′-GCAGGTATGGGTTCATAGAAGGGC-3′,
reverse, 5′-TGTGAGTGTCTGGTAGCAGGGATT-3′;
β-actin, forward, 5′-TTCGGGCTGGTGACAGGGAAGACA-3′,
reverse, 5′-TTTGCGGGACAAAGGGCAAGATTT-3′.
Western blot analysis
Whole protein was extracted using RIPA lysis buffer (Beyotime, Beijing, China). The BCA Protein Quantification Kit (Tiangen, Beijing, China) was used to measure the protein concentration. The procedure was performed according to the method described in a previous study [14]. The primary antibodies used were: Oct4 (cat. no. 11263-1-AP, ProteinTech, Wuhan, China, 1: 1000), Nanog (cat. no. 67255-1-Ig, ProteinTech, 1: 3000), Sox2 (cat. no. WL03273, Wanleibio, Shenyang, China, 1: 1500), Cleaved caspase 3 (ab32042, Abcam, 1: 3000), Caspase 3 (cat. no. 66470-2-Ig, Proteintech, 1: 1000), Cleaved PARP (ab32561, Abcam, 1: 3000), PARP (cat. no. WL0326, Wanleibio, 1: 1500), p-STAT3 (Tyr705) (ab76315, Abcam,1: 1000), p-STAT3 (Ser727) (ab32134, Abcam, 1: 1000), STAT3 (cat. no. WL01836, Wanleibio, 1: 1500), c-Myc (cat. no. WL01781, Wanleibio, 1: 1500), Bcl-xl (cat. no. WL03353, Wanleibio, 1: 1500), and β-actin (cat. no. WL01372, Wanleibio, 1: 200).
Plasmid construction and transfection
The STAT3 coding sequences were inserted into pcDNA3.1 (+) plasmid, denoted as pc-Stat3, which was confirmed by DNA-sequencing before use. Pc-Stat3 was transfected using EZ Trans (Shanghai Life iLAB Biotechnology Co., Shanghai, China).
Cell viability assay
Cells were seeded in 96-well plates and incubated with NP, TAM, NP, and TAM. Cell counting kit-8 (CCK8) was used to measure the number of viable cells. The IC50 values were calculated using GraphPad Prism.
Spheroid forming analysis
The procedure was performed according to the method described in a previous study [15].
ALDH1 activity assay
ALDH1 activity was determined using an ALDEFLUOR™ Kit.
Luciferase reporter activity analysis
The STAT3 reporter gene plasmid (pGL4.47(Luc2P/SIE/Hygro)) contains 5 copies of SIE (sis-inducing factor), which drives luc2P expression and transfects the plasmid into 293T cells. Throughout the experiment, pRL-TK Renilla luciferase plasmid was used to maintain constant total transfected DNA in each pore. After 72 h of transfection, the cells were harvested and the activity of luciferase was determined by use of a double-luciferase reporting and analysis system.
Statistical analysis
All results were denoted as mean±SD and analyzed using GraphPad Prism (Version X; La Jolla, CA, USA). Differences were analyzed using the t test or Tukey-Kramer post hoc test. Differences at P<0.05 were considered to be statistically significant.
Results
MCF-7-R cells showed stronger stemness than the wild-type MCF-7 cells
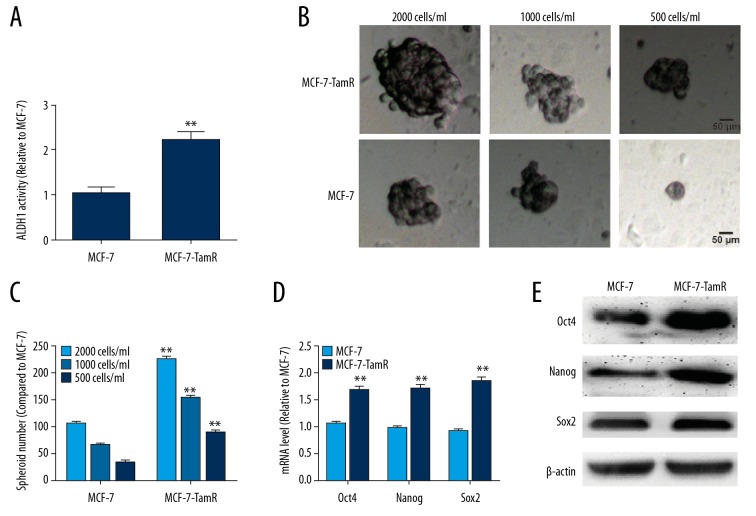
We first compared the stemness of MCF-7-R cells and MCF-7 cells. As shown in Figure 1A, MCF-7-R cells exhibited higher ALDH1 activity than MCF-7 cells. Additionally, a stronger spheroid formation capacity was observed in MCF-7-R cells than in MCF-7 cells at diluted concentrations (2000 cells/ml, 1000 cells/ml, and 500 cells/ml), which was evident by the increased sphere size and number (Figure 1B, 1C). Furthermore, the expression of critical regulators of stemness was examined in MCF-7-R and MCF-7 cells, and the expression levels of stemness markers displayed a higher level in MCF-7-R cells than in MCF-7 cells (Figure 1D, 1E). These results suggest that MCF-7-R cells have stronger stemness than the parental MCF-7 cells.
Figure 1.
MCF-7-R cells exhibited stronger stemness than did MCF-7 cells. (A) ALDH1 activity was examined in MCF-7-R and MCF-7 cells. (B, C) The spheroid forming ability was evaluated in MCF-7-R and MCF-7 cells at various dilutions. (D, E) QRT-PCR and western blot analysis of the expression of critical stemness regulators in MCF-7-R and MCF-7 cells. ** p<0.01 vs. MCF-7.
NP exerts stronger cytotoxicity on MCF-7-R cells than on MCF-7 cells
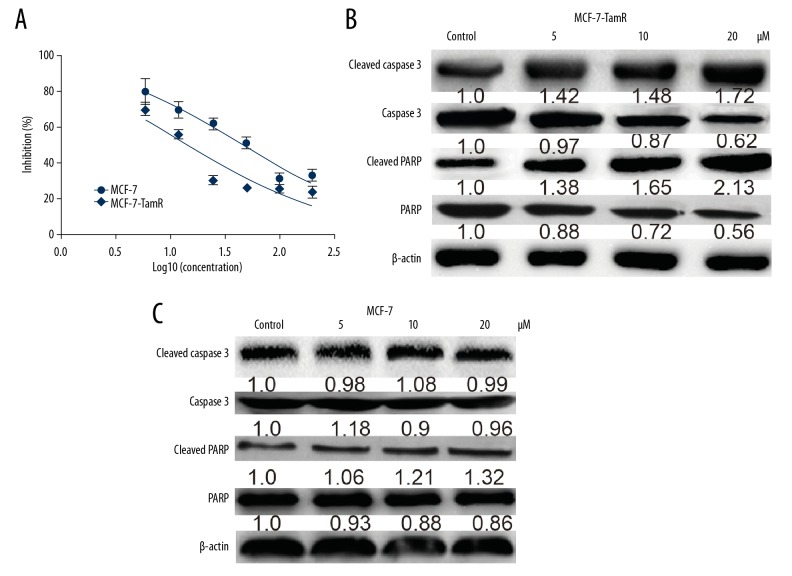
We assessed the effects of NP on MCF-7-R and MCF-7 cells. As shown in Figure 2A, NP exhibited a stronger inhibitory effect on MCF-7-R cell viability than on MCF-7 cells, characterized by lower IC50 value (15.74 μM for MCF-7-R vs. 49.91 μM for MCF-7). Then, we evaluated the effects of NP on MCF-7-R and MCF-7 cell apoptosis and found that NP increased the expression of apoptotic executors (Cleaved PARP and Cleaved caspase 3) in MCF-7-R cells but had little effect on MCF-7 cells (Figure 2B, 2C). Thus, our results demonstrated that NP selectively kills MCF-7-R cells but not MCF-7 cells.
Figure 2.
NP exerted stronger cytotoxicity in MCF-7-R cells than in MCF-7 cells. (A) The IC50 value of NP in MCF-7-R and MCF-7 cells was determined 48 h after cells were exposed to NP. (B, C) Western blot analysis of the expression of cleaved PARP and cleaved caspase 3 was examined in MCF-7-R and MCF-7 cells treated with different concentration of NP.
NP reduces the stemness of MCF-7-R cells
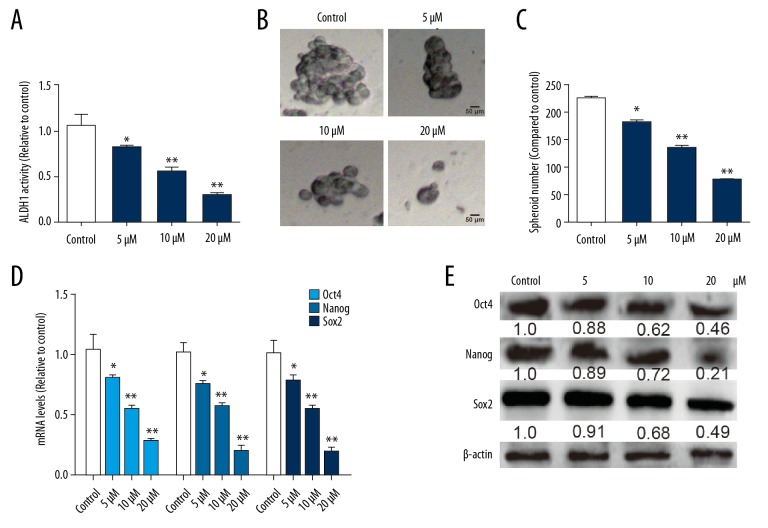
Since we confirmed that MCF-7-R cells exhibited a stronger stemness than MCF-7 cells, and because we found fewer CSCs in MCF-7 cells [16], we wondered whether NP specifically kills CSCs existing in these 2 cell lines so that NP exhibits a stronger cytotoxicity in MCF-7-R cells than in MCF-7 cells. Figure 3A shows that NP reduced the ALDH activity of MCF-7-R cells in a concentration-dependent fashion. Moreover, NP suppressed the self-renewal ability of MCF-7-R cells, as shown by decreasing spheroid size and numbers at various dilutions (Figure 3B, 3C). Moreover, the expression of stemness critical regulators (Oct4, Nanog, and Sox2) was decreased by NP in MCF-7-R cells in a concentration-dependent manner (Figure 3D, 3E). Collectively, these results indicate that NP attenuates the stem cell-like traits of MCF-7-R cells.
Figure 3.
NP reduced the stemness of MCF-7-R cells. (A) Analysis of ALDH activity in MCF-7-R cells treated with different concentrations of NP. (B, C) Analysis of spheroid formation ability was performed in MCF-7-R cells treated with different concentrations of NP. (D, E) Western blot analysis of the expression of critical stemness regulators was carried out in MCF-7-R cells treated with different concentrations of NP. * p<0.05, ** p<0.01 vs. control.
NP attenuates the stemness of MCF-7-R cells through suppressing STAT3 activation
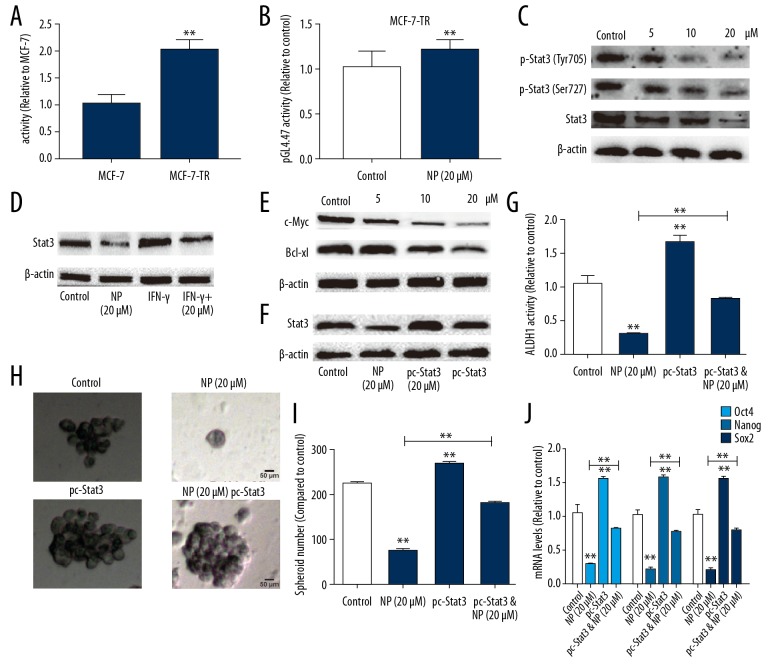
As NP has been shown to be an inhibitor of STAT3, we speculated that NP might suppress the stem cell-like traits of MCF-7-R cells through inhibiting STAT3 activation. First, we evaluated STAT3 activity by performing luciferase reporter analysis and showed that STAT3 activity was higher in MCF-7-R cells than in MCF-7 cells (Figure 4A). We also found that STAT3 activity was decreased by NP in MCF-7-R cells (Figure 4B). Western blot assay revealed that NP blocked the expression of phosphorylated STAT3 and total STAT3 in both Tyr705 and Ser727 sites (Figure 4C). It has been demonstrated that IFN-γ activates STAT3 phosphorylation and STAT3-dependent transcription. To further study the effect of NP on STAT3 activation in MCF-7-R cells, we assessed its effect on IFN-γ-dependent regulation and activation of STAT3. As shown in Figure 4D, NP also blocks IFN-γ-activated induction of STAT3 expression in MCF-7-R cells. As previous studies have shown that STAT3 serves as an upstream regulator of c-Myc and Bcl-xl, we further assessed the effects of NP on c-Myc and Bcl-xl expression. Western blot analysis showed that NP decreased c-Myc and Bcl-xl expression in MCF-7-R cells in a concentration-dependent manner (Figure 4E). We further assessed whether NP attenuates the stem cell-like traits of MCF-7-R cells through STAT3. We found that STAT3 expression was overexpressed in MCF-7-R cells after NP treatment, and the transfection efficiency was confirmed by Western blot analysis (Figure 4F). Notably, STAT3 overexpression rescued the inhibitory effects of NP on the stemness of MCF-7-R cells (Figure 4G–4J).
Figure 4.
NP attenuated MCF-7-R cell stemness through suppressing STAT3 activation. (A) The activity of pGL4.47 was measured in MCF-7-R and MCF-7 cells by luciferase reporter analysis. (B) The activity of pGL4.47 was assessed in MCF-7-R cells with or without NP treatment. (C) The expression of p-STAT3 was detected in MCF-7-R cells after treatment with different concentrations of NP. (D) Total STAT3 expression was examined in MCF-7-R cells with NP, IFN-γ, or NP plus IFN-γ treatment. (E) The expression of the downstream effectors of IFN-γ (c-Myc and Bcl-xl) was determined in MCF-7-R cells after treatment with different concentrations of NP. (F) The expression of total STAT3 was measured in MCF-7-R cells treated with NP, transfected with pc-STAT3, or with both. (G) Analysis of ALDH1 activity was performed in MCF-7-R cells treated with NP, transfected with pc-STAT3, or with both. (H, I) Spheroid forming ability was evaluated in MCF-7-R cells treated with NP, transfected with pc-STAT3, or with both. (J) The mRNA levels of stemness critical regulators were examined in MCF-7-R cells treated with NP, transfected with pc-STAT3, or with both. * p<0.05, ** p<0.01 vs. control.
NP reverses MCF-7-R resistance to TAM
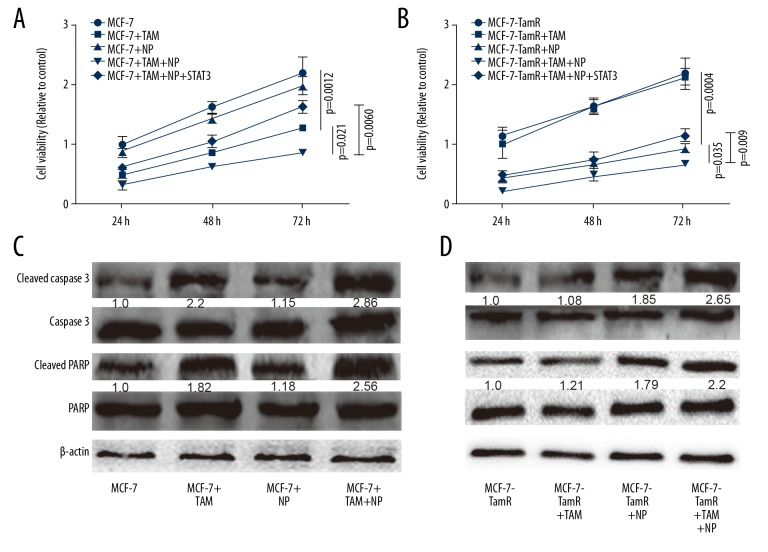
Finally, we evaluated the effects of NP on the resistance or sensitivity of MCF-7-R and MCF-7 cells, respectively. Cell viability assay showed that NP enhanced the sensitivity of MCF-7 cells to TAM (Figure 5A) and reversed TAM resistance of MCF-7-R cells (Figure 5B). Western blot analysis of the expression of apoptotic executors (Cleaved PARP and Cleaved caspase 3) produced consistent results (Figure 5C, 5D). Collectively, these results suggest that NP attenuates TAM resistance of ER+BC cells by reducing their stemness.
Figure 5.
NP reversed the resistance of MCF-7-R cells to TAM. (A) MCF-7 cells were treated with NP, TAM, or both, and cell viability assay was performed. (B) MCF-7-R cells were treated with TAM, NP, or both, and cell viability analysis was performed. (C, D) Apoptotic executor expression was measured in the cells described in (A) and (B). * p<0.05, ** p<0.01 vs. control.
Discussion
In the past 30 years, TAM, an ER antagonist, has become the most commonly used therapeutic drug in endocrine therapy of ER+BC [1]. However, TAM resistance has become a major problem in clinical practice. It has been found that BCSCs are resistant to radiotherapy and chemotherapy, and therefore are crucial to the occurrence and development of BC [17]. Studies have shown that ER+ MCF-7 cells are resistant to tamoxifen, accompanied by increased invasive ability and occurrence of EMT (epithelial-mesenchymal transition) [4]. The EMT process is tightly and positively associated with BCSC progression [18], which agrees with our results showing that MCF-7-R cells exhibit a stronger stemness than do MCF-7 cells. Additionally, a previous study has indicated a potential role of stem-like cells in TAM resistance [19], and expression of the embryonal stem cell transcription factor Oct4 predicts poor clinical outcome and tamoxifen resistance [8]. Moreover, CD44(+)CD24(−/low) BCSCs are resistant to TAM, and this resistance is mediated by STAT3 [20]. Our study is the first to show that NP reduces the stemness and reverses TAM resistance of MCF-7-R cells. This was evidenced by the following results: Firstly, MCF-7-R cells exhibited a stronger stemness than in the wild-type MCF-7 cells. Secondly, NP exerted a stronger and more specific cytotoxicity on MCF-7-R and MCF-7 cells. Third, NP attenuated MCF-7-R cell stemness in a concentration-dependent manner. Fourthly, NP enhanced the sensitivity of MCF-7 cells to TAM and reversed the resistance of MCF-7-R cells to TAM.
Mechanistically, the present study shows that NP attenuates MCF-7-R cell stemness through suppressing STAT3 transcriptional activity. STAT3 transcription activity regulates the proliferation, survival, apoptosis and drug resistance of cancer cells and CSCs [21,22]. Previous studies have shown that STAT3 is indispensable to these properties and functions of CSCs, and activation of STAT3 (phosphorylation of Y705 and S727) triggers gene expression that controls CSCs progression. For example, activation of STAT3 shifts non-CSCs dynamics to CSCs [23]; the IL-22 receptorhi population from pancreatic cancer cells has higher stemness and enhances the stemness and tumorigenicity through activating STAT3 signaling [24]; and Aquaporin 3 maintains the stem cell-like properties of hepatoma cells by activating STAT3 signaling [25]. Notably, STAT3 has become a potential drug target for cancer cells and CSCs. For example, NP was found to inhibit STAT3 activity and thus attenuate the stemness of lung cancer cells [26], colon cancer cells [11], and biliary tract cancer cells [27]. NP is currently being investigated in gastric cancer CSCs in the phase III BRIGHTER trial, based on the reported phase I and II results [28,29]; however, the roles of NP in ER+BC cells are still unclear. We are the first to confirm the roles of NP in attenuating the stemness of ER+BSCSs and TAM resistance. It must be noted that our conclusions need to be confirmed by in vivo experiments, and it is still unclear whether NP has similar effects on other types of BC and tumors.
Conclusions
Collectively, our results indicate that NP reduces the stemness of BCSCs and thus reverses the resistance of MCF-7-R cells to TAM, providing a potential drug for combined use in ER+BC treatment.
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Emanuel G, Henson KE, Broggio J, et al. Endocrine therapy in the years following a diagnosis of breast cancer: A proof of concept study using the primary care prescription database linked to cancer registration data. Cancer Epidemiol. 2019;61:185–89. doi: 10.1016/j.canep.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Im SA, Lu YS, Bardia A, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307–16. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 3.Rani A, Stebbing J, Giamas G, Murphy J. Endocrine resistance in hormone receptor positive breast cancer-from mechanism to therapy. Front Endocrinol (Lausanne) 2019;10:245. doi: 10.3389/fendo.2019.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng L, Meng X, Li X, et al. miR-125a-3p inhibits ERalpha transactivation and overrides tamoxifen resistance by targeting CDK3 in estrogen receptor-positive breast cancer. FASEB J. 2018;32(2):588–600. doi: 10.1096/fj.201700461RR. [DOI] [PubMed] [Google Scholar]
- 5.Tian J, Raffa FA, Dai M, et al. Dasatinib sensitises triple negative breast cancer cells to chemotherapy by targeting breast cancer stem cells. Br J Cancer. 2018;119(12):1495–507. doi: 10.1038/s41416-018-0287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke MF. Clinical and therapeutic implications of cancer stem cells. New Engl J Med. 2019;380(23):2237–45. doi: 10.1056/NEJMra1804280. [DOI] [PubMed] [Google Scholar]
- 7.Leung EY, Askarian-Amiri ME, Sarkar D, et al. Endocrine therapy of estrogen receptor-positive breast cancer cells: Early differential effects on stem cell markers. Front Oncol. 2017;7:184. doi: 10.3389/fonc.2017.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gwak JM, Kim M, Kim HJ, et al. Expression of embryonal stem cell transcription factors in breast cancer: Oct4 as an indicator for poor clinical outcome and tamoxifen resistance. Oncotarget. 2017;8(22):36305–18. doi: 10.18632/oncotarget.16750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaguchi N, Nakayama Y, Yamaguchi N. Down-regulation of Forkhead box protein A1 (FOXA1) leads to cancer stem cell-like properties in tamoxifen-resistant breast cancer cells through induction of interleukin-6. J Biol Chem. 2017;292(20):8136–48. doi: 10.1074/jbc.M116.763276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu H, Sun J, Wang C, et al. IL-33 facilitates endocrine resistance of breast cancer by inducing cancer stem cell properties. Biochem Biophys Res Commun. 2017;485(3):643–50. doi: 10.1016/j.bbrc.2017.02.080. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Rogoff HA, Keates S, et al. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc Natl Acad Sci USA. 2015;112(6):1839–44. doi: 10.1073/pnas.1424171112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rios-Fuller TJ, Ortiz-Soto G, Lacourt-Ventura M, et al. Ganoderma lucidum extract (GLE) impairs breast cancer stem cells by targeting the STAT3 pathway. Oncotarget. 2018;9(89):35907–21. doi: 10.18632/oncotarget.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thakur R, Trivedi R, Rastogi N, et al. Inhibition of STAT3, FAK and Src mediated signaling reduces cancer stem cell load, tumorigenic potential and metastasis in breast cancer. Sci Rep. 2015;5:10194. doi: 10.1038/srep10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng L, Li X, Gu Y, et al. The 3′UTR of the pseudogene CYP4Z2P promotes tumor angiogenesis in breast cancer by acting as a ceRNA for CYP4Z1. Breast Cancer Res Treat. 2015;150(1):105–18. doi: 10.1007/s10549-015-3298-2. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez HR, Gadre SM, Tan M, et al. The mitochondrial citrate carrier, SLC25A1, drives stemness and therapy resistance in non-small cell lung cancer. Cell Death Differ. 2018;25(7):1239–58. doi: 10.1038/s41418-018-0101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng L, Guo Q, Xiang C, et al. Transcriptional factor six2 promotes the competitive endogenous RNA network between CYP4Z1 and pseudogene CYP4Z2P responsible for maintaining the stemness of breast cancer cells. J Hematol Oncol. 2019;12(1):23. doi: 10.1186/s13045-019-0697-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bu W, Liu Z, Jiang W, et al. Mammary precancerous stem and non-stem cells evolve into cancers of distinct subtypes. Cancer Res. 2019;79(1):61–71. doi: 10.1158/0008-5472.CAN-18-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goncalves Ndo N, Colombo J, Lopes JR, et al. Effect of melatonin in epithelial mesenchymal transition markers and invasive properties of breast cancer stem cells of canine and human cell lines. PLoS One. 2016;11(3):e0150407. doi: 10.1371/journal.pone.0150407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin X, Li J, Yin G, et al. Integrative analyses of gene expression and DNA methylation profiles in breast cancer cell line models of tamoxifen-resistance indicate a potential role of cells with stem-like properties. Breast Cancer Res. 2013;15(6):R119. doi: 10.1186/bcr3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Wang G, Zhao Y, et al. STAT3 mediates resistance of CD44(+)CD24(−/low) breast cancer stem cells to tamoxifen in vitro. J Biomed Res. 2012;26(5):325–35. doi: 10.7555/JBR.26.20110050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulesza DW, Przanowski P, Kaminska B. Knockdown of STAT3 targets a subpopulation of invasive melanoma stem-like cells. Cell Biol Int. 2019;43(6):613–22. doi: 10.1002/cbin.11134. [DOI] [PubMed] [Google Scholar]
- 22.Tannous BA, Badr CE. A TNF-NF-kappaB-STAT3 loop triggers resistance of glioma-stem-like cells to Smac mimetics while sensitizing to EZH2 inhibitors. Cell Death Dis. 2019;10(4):268. doi: 10.1038/s41419-019-1505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SY, Kang JW, Song X, et al. Role of the IL-6-JAK1-STAT3-Oct-4 pathway in the conversion of non-stem cancer cells into cancer stem-like cells. Cell Signal. 2013;25(4):961–69. doi: 10.1016/j.cellsig.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He W, Wu J, Shi J, et al. IL22RA1/STAT3 signaling promotes stemness and tumorigenicity in pancreatic cancer. Cancer Res. 2018;78(12):3293–305. doi: 10.1158/0008-5472.CAN-17-3131. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Wu G, Fu X, et al. Aquaporin 3 maintains the stemness of CD133+ hepatocellular carcinoma cells by activating STAT3. Cell Death Dis. 2019;10(6):465. doi: 10.1038/s41419-019-1712-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacDonagh L, Gray SG, Breen E, et al. BBI608 inhibits cancer stemness and reverses cisplatin resistance in NSCLC. Cancer Lett. 2018;428:117–26. doi: 10.1016/j.canlet.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Beyreis M, Gaisberger M, Jakab M, et al. The cancer stem cell inhibitor napabucasin (BBI608) shows general cytotoxicity in biliary tract cancer cells and reduces cancer stem cell characteristics. Cancers (Basel) 2019;11(3) doi: 10.3390/cancers11030276. pii: E276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonbol MB, Bekaii-Saab T. A clinical trial protocol paper discussing the BRIGHTER study. Future Oncol. 2018;14(10):901–6. doi: 10.2217/fon-2017-0406. [DOI] [PubMed] [Google Scholar]
- 29.Bekaii-Saab T, El-Rayes B. Identifying and targeting cancer stem cells in the treatment of gastric cancer. Cancer. 2017;123(8):1303–12. doi: 10.1002/cncr.30538. [DOI] [PMC free article] [PubMed] [Google Scholar]