Abstract
The present phase II study of paclitaxel with an antiangiogenic agent for refractory urothelial cancer resulted in a high objective response rate with limited toxicity.
Introduction:
Currently, no standard treatments are available for relapsed or refractory urothelial carcinoma (UC). Paclitaxel has demonstrated efficacy in the treatment of UC when used alone or combined with other cytotoxic therapies. We designed a phase II trial combining paclitaxel with pazopanib, a commonly used antiangiogenic agent with significant antitumor activity in various solid tumors.
Patients and Methods:
We enrolled 32 patients with refractory UC who had demonstrated disease progression after 2 previous chemotherapeutic regimens. The patients received paclitaxel 80 mg/m2 on days 1, 8, and 15 of a 28-day cycle and oral pazopanib 800 mg daily. The primary endpoint was the overall response rate (ORR). The secondary endpoints included progression-free survival, overall survival, and a safety assessment of the combination.
Results:
Of the 28 evaluable patients, a complete response was observed in 3 patients and a partial response in 12, with an ORR of 54% (95% confidence interval, 33.9–72.5). The median progression-free and overall survival was 6.2 and 10 months, respectively. The most frequent side effects noted (all grades) were fatigue (63%), diarrhea (44%), and nausea and vomiting (41%). Hematologic toxicities were common and included (all grades) anemia (69%), neutropenia (38%), and thrombocytopenia (47%). Growth factor support was required for 44% of the patients.
Conclusion:
The combination of paclitaxel and pazopanib resulted in a promising ORR of 54% in patients with advanced pretreated UC. This represents a greater response rate and median survival than found with other existing second-line regimens for UC and is worthy of further study.
Keywords: Angiogenesis inhibitor, Chemotherapy, Combination, Second line, Targeted therapy
Introduction
Recurrent metastatic urothelial cancer (UC) is a challenging disease with few treatment options that improve patient survival. In 2015, cancer statistics estimated approximately 74,000 new cases of UC and 16,000 deaths from UC.1 Although platinum-based chemotherapy regimens result in high initial response rates ranging from 40% to 70%, they are generally not curative, with a median progression-free survival (PFS) of approximately 8 months and 5-year overall survival (OS) of 15%.2,3 Thus, most patients with UC will develop a relapse and require additional therapy.
Despite the evaluation of a number of cytotoxic and biologic agents for the treatment of UC in the second-line setting, currently, no standard treatment options are available. Only a few single agents have shown modest response rates in phase II and III trials. Taxanes are frequently implemented after cisplatin-based chemotherapy. In this setting, docetaxel has elicited a response rate of 13.3% and a median survival of 9 months.4 Weekly paclitaxel attained a response rate of 10%, with a median time to progression of 2.2 months and median OS time of 7.2 months. Nab-paclitaxel achieved a response rate of 27%.5,6 Combination therapy has demonstrated limited efficacy and increased toxicity.
Several preclinical studies have implicated the importance of angiogenesis in the pathogenesis and evolution of UC.7,8 The activity of pazopanib, an established multitargeted oral tyrosine kinase inhibitor targeting the vascular endothelial growth factor (VEGF) receptors 1, 2, and 3, platelet-derived growth factor receptors α and β, and stem cell factor receptor (c-Kit)9 was evaluated in a phase II clinical trial of patients with heavily pretreated metastatic UC.10 In this group, treatment with pazopanib produced an objective response rate (ORR) of 17%.
The combination of pazopanib with weekly paclitaxel in advanced solid tumors has been evaluated in a phase I trial. It was found that a dose of pazopanib at 800 mg once daily could be safely combined with a therapeutic dose of paclitaxel at 80 mg/m2 administered on days 1, 8, and 15, every 28 days.11 Of the 26 patients with advanced solid tumors enrolled in that study, 6 (23%) had a partial response (PR) and 15 (58%) had stable disease. The combination of 800 mg pazopanib and 80 mg/m2 paclitaxel resulted in a 26% greater geometric mean paclitaxel area under the curve, suggesting synergistic activity. Given the encouraging antitumor activity noted by combining these agents, we undertook a phase II study to evaluate the combination of pazopanib with weekly paclitaxel in patients with refractory UC.
Patients and Methods
The present phase II trial was conducted at Stanford Cancer Center (Stanford, CA) and the Karmanos Cancer Institute (Detroit, MI) after institutional review board approval. The eligible patients had histologically confirmed metastatic UC of the bladder, ureter, or renal pelvis, with documented recurrence after a maximum of 2 previous lines of chemotherapy, including previous perioperative chemotherapy. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 1 and measureable disease using the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1.12 Adequate hematologic, renal, and hepatic function were required: absolute neutrophil count ≥ 1.5 × 109/L, hemoglobin 9 g/dL, platelet count ≥ 100 × 109/L, prothrombin time or international normalized ratio ≤ 1.2 × upper limit of normal (ULN), total bilirubin ≤ 1.5 × ULN, aspartate aminotransferase and alanine aminotransferase ≤ 2.5 × ULN, serum creatinine ≤ 1.8 × ULN, and urine protein-to-creatinine ratio < 1. Other eligibility criteria included age ≥ 18 years and an ability to provide written informed consent and follow the study-specific procedures. Women of childbearing potential were required to have a negative serum pregnancy test 2 weeks before the first dose and to use adequate contraception during treatment.
The study excluded patients who had received taxane chemotherapy in the previous 12 months and those with evidence of central nervous system or leptomeningeal involvement in the previous 6 months. Patients with any significant cerebrovascular, cardiovascular, or thromboembolic event in the previous 6 months requiring hospitalization or intervention, an increased risk of gastrointestinal bleeding, poorly controlled hypertension, or prolonged QTc interval (> 480 ms), or any evidence of active bleeding or bleeding diathesis were also excluded. The patients were required to have completed radiation, surgery, tumor embolization, chemotherapy, immunotherapy, and/or any biologic or investigational therapy 14 days before the first dose of pazopanib. All patients provided written informed consent before study participation.
Procedures
The baseline assessment of all patients included a detailed history and physical examination, assessment of the ECOG PS, blood counts, coagulation status, electrolytes, renal and hepatic function, cardiac function (electrocardiogram and echocardiogram), computed tomography or positron emission tomography scans (thorax, abdomen, and pelvis), and bone scans (when clinically indicated). The patients received pazopanib at a starting dose of 800 mg daily plus paclitaxel dosed at 80 mg/m2 intravenously on days 1, 8, and 15 of a 28-day cycle. A detailed physical examination, determination of the ECOG PS, and blood tests were repeated at every cycle. The imaging studies were repeated every 2 cycles to assess the response to therapy using the RECIST, version 1.1, for measureable disease.
The study permitted a dose reduction of pazopanib to 600 mg/d, followed by a paclitaxel dose reduction to 65 mg/m2 and then to 51 mg/m2 for grade ≥ 2 neutropenia or thrombocytopenia. Any further reduction in the dose required the withdrawal of the patient from the study.
The treatment was continued until disease progression, as defined by the RECIST, version 1.1, unacceptable side effects from treatment, intercurrent illness, consent withdrawal, or the investigator deemed that the patient’s condition was unacceptable for further treatment. The imaging data were also assessed by Virtual Scopics, an independent reviewer for the study. All adverse events were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
Statistical Analysis
The primary endpoint of the present study was the ORR of the tumors measured using the RECIST, version 1.1. The secondary endpoints included an assessment of the PFS, OS, and safety and tolerability of the drug combination. The efficacy analysis included evaluable patients, defined as patients who had received ≥ 2 cycles of chemotherapy, and the safety parameters were assessed using the intent-to-treat principle.
The study was designed using Simon’s 2-stage design. With an estimated sample size of 32 patients, an interim analysis was planned for the first 9 evaluable patients. If ≥ 1 of the first 9 evaluable patients had a treatment response, the trial would proceed to complete enrollment. Of the 32 evaluable subjects, a response in ≥ 9 patients would reject the null hypothesis that the response rate would be ≤ 15%. This design would have 80% power for a true response rate of 35%, with a 5% (α) 1-sided significance level. Kaplan-Meier method was applied to generate survival curves for PFS and OS. All analyses were conducted using the STATA statistical package, version 11.0.13
Results
The study enrolled 32 patients from May 2010 to October 2014. The demographic and clinical characteristics of the study patients are listed in Table 1. Of the 32 patients, 41% had received chemotherapy for de novo metastatic disease. Most of the patients (59%) had received perioperative chemotherapy. The median interval from the last chemotherapy use was > 3 months for 53% of the patients. The patients received a median of 4 cycles of the combination of pazopanib and paclitaxel.
Table 1.
Demographic Characteristics of Enrolled Patients (n = 32)
| Characteristic | n (%) |
|---|---|
| Age (years) | |
| Median | 67 |
| Range | 29–89 |
| Sex | |
| Male | 23 |
| Female | 9 |
| Cycles | |
| Median | 4 |
| Range | 1–14 |
| Primary site | |
| Bladder | 15 (47) |
| Renal pelvis | 11 (34) |
| Ureter | 6 (19) |
| Previous chemotherapy regimens | |
| 1 | 13 (41) |
| 2 | 19 (59) |
| Metastatic sites | |
| Lymph nodes | 25 (78) |
| Lung | 15 (47) |
| Liver | 9 (28) |
| Bone | 6 (19) |
| Other | 11 (34) |
| ECOG PS | |
| 0 | 11 (34) |
| 1 | 21 (66) |
| Interval from last chemotherapy regimen (mo) | |
| <3 | 15 (47) |
| >3 | 17 (53) |
| Bellmunt score | |
| 0 | 7 (22) |
| 1 | 14 (41) |
| 2 | 11 (34) |
| 3 | 0 (0) |
Abbreviations: ECOG = Eastern Cooperative Oncology Group; PS = performance status.
All 32 patients received ≥ 1 cycle of treatment and were available for toxicity assessment. The most frequent side effects noted with this regimen (all grades) were fatigue (63%), diarrhea (44%), nausea and vomiting (41%), and neuropathy (24%). The most frequent grade 3 side effect was fatigue (13%); other grade ≥ 3 side effects observed included diarrhea, neuropathy, hypertension, pneumonitis, thrombosis, wound dehiscence, intracranial hemorrhage, and hand-foot syndrome (Table 2). No fistulizations of the gastrointestinal tract were noted with the combination of the 2 drugs.
Table 2.
Clinical and Laboratory Adverse Events Associated With Treatment (n = 32)
| Adverse Events | All Grades | Grade 3 | Grade 4 |
|---|---|---|---|
| Clinical adverse events | |||
| Fatigue | 20 (63) | 4 (13) | |
| Diarrhea | 14 (44) | 1 (3) | |
| Nausea/vomiting | 13 (41) | ||
| Neuropathy | 11 (34) | 1 (3) | |
| Epistaxis | 6 (19) | ||
| Rash | 6 (19) | ||
| Hypertension | 6 (19) | 2 (6) | |
| Pneumonitis | 2 (6) | 2 (6) | |
| Thrombosis | 2 (6) | 2 (6) | |
| Wound dehiscence | 2 (6) | 2 (6) | |
| CNS bleeding | 1 (3) | 1 (3) | |
| Hand foot syndrome | 1 (3) | 1 (3) | |
| Neutropenic fever | 2 (6) | 2 (6) | |
| Laboratory adverse events | |||
| Hematologic | |||
| Anemia | 22 (69) | 4 (13) | |
| Neutropenia | 12 (38) | 3 (9) | 2 (6) |
| Neutropenia/fever | 2 (6) | 2 (6) | |
| Neutropenia requiring growth factor support | 14 (44) | ||
| Thrombocytopenia | 15 (47) | 3 (9) | |
| Renal | |||
| Increase creatinine | 14 (44) | ||
| Proteinuria | 5 (16) | ||
| Hepatic | |||
| Elevated AST | 8 (25) | 1 (3) | |
| Elevated ALT | 8 (25) | 1 (3) | |
| Elevated bilirubin | 2 (6) | ||
| Elevated alkaline phosphatase | 11 (34) |
Abbreviations: ALT = alanine aminotransferase; AST = aspartate aminotransferase; CNS = central nervous system.
The most frequent laboratory abnormalities induced by this combination were hematologic and included (all grades) anemia (69%), neutropenia (38%), and thrombocytopenia (47%). Febrile neutropenia developed in 2 patients, and 14 patients required growth factor support during treatment. Only 1 patient experienced a grade 3 elevation in transaminases during treatment (Table 2). Owing to the hematologic toxicities observed, likely as a result of the greater paclitaxel concentration, 6 patients received a dose reduction of paclitaxel to 65 mg/m2 and pazopanib to 600 mg.
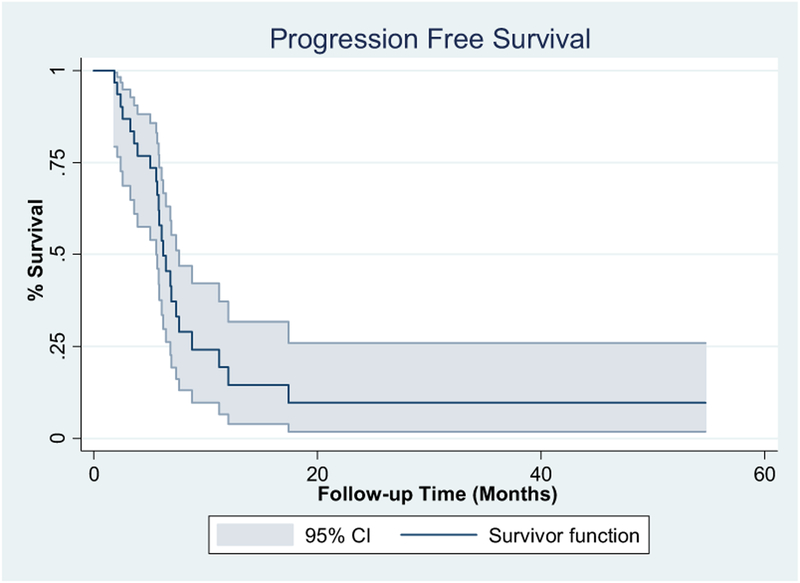
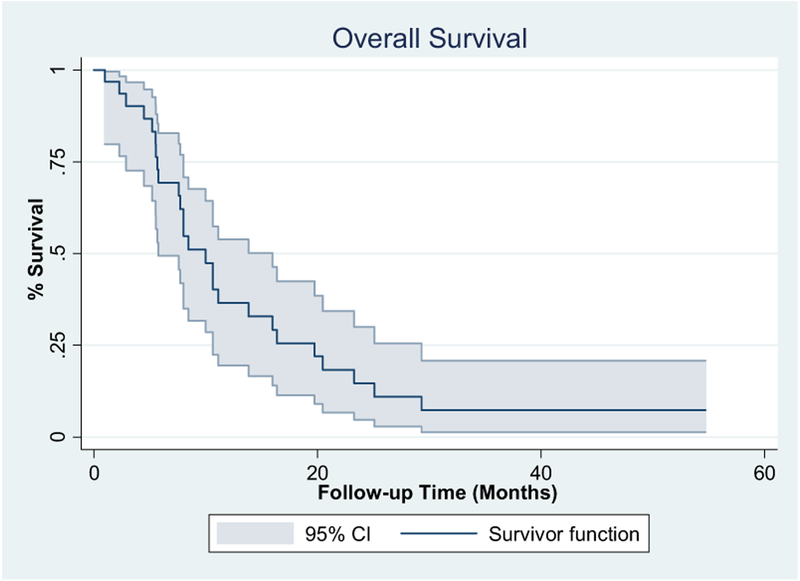
In the first 9 patients, 3 achieved a PR, allowing the study to move forward and complete enrollment. The patients were considered evaluable for response only if they had completed 2 cycles of therapy and subsequent imaging studies. Of the 28 evaluable patients, a complete response (CR) was observed in 3 patients and a PR in 12 patients, resulting in an ORR of 54% (95% confidence interval [CI], 36.7–70). Stable disease was observed in 11 patients and progressive disease in 2 patients. One of the 3 patients with a CR died during the subsequent follow-up period secondary to complications from myelodysplastic syndrome. Myelodysplastic syndrome was diagnosed after completion of the trial, with no evidence of UC. All 3 patients achieving a PR subsequently underwent consolidative radiation, and 1 of these patients remained disease free for > 55 months after treatment (Table 3). The median PFS was 6.2 months (95% CI, 5.6–7.6 months), and the median OS was 10 months (95% CI, 5.7–16 months; Figures 1 and 2).
Table 3.
Response to Treatment With Pazopanib and Paclitaxel (n = 28)
| Response | n (%) |
|---|---|
| Complete response | 3 (11) |
| Partial response | 12 (43) |
| Stable disease | 11 (39) |
| Progressive disease | 2 (7) |
| Not evaluable | 4 (14) |
| Response rate (complete plus partial response) | 15 (54) |
Figure 1. Progression-Free Survival.
Abbreviation: CI = confidence interval.
Figure 2. Overall Survival.
Abbreviation: CI = confidence interval.
The survival rates of the study patients were also assessed using the established prognostic factors for patients with UC progression after platinum-based chemotherapy, such as the ECOG PS, hemoglobin level, presence of hepatic metastases, and interval from the last chemotherapy regimen.14,15 Using the Bellmunt risk score, patients with 0, 1, and 2 risk factors had a survival of 13.8, 8, and 7.7 months, respectively. Using the interval from the last chemotherapy regimen as an additional adverse risk factor to this score, as proposed by Sonpavde et al,15 patients with 0, 1, 2, and 3 risk factors had survival of 10.6, 11.1, 8, and 5.5 months, respectively. It is noteworthy that 21% of the patients had a low-risk Bellmunt score of 0 in our study. Univariate analysis of the patient variables revealed that the screening ECOG PS was a significant variable indicating their response to therapy, with 82% of patients with an ECOG PS of 0% and 35% of patients with an ECOG PS of 1 responding to treatment with a CR or PR (P = .02; Table 4).
Table 4.
Univariate Analysis of Patient Variables in Response to Therapy
| Variable | CR/PR | SD/PD | P Value |
|---|---|---|---|
| Patients (n) | 15 | 13 | |
| Age (years) | .6600a | ||
| Median | 71 | 67 | |
| Range | 47–82 | 52–89 | |
| Sex | .6891b | ||
| Female | 4 (26.7) | 5 (38.5) | |
| Male | 11 (73.3) | 8 (61.5) | |
| Cycles | .1066a | ||
| Median | 4 | 3 | |
| Range | 3–14 | 2–12 | |
| Primary | .886b | ||
| Bladder | 5 (33.3) | 6 (46.2) | |
| Renal pelvis | 6 (40.0) | 5 (38.5) | |
| Ureter | 4 (26.7) | 2 (15.4) | |
| Previous therapy | .4757b | ||
| 1 | 8 (53.3) | 5 (38.5) | |
| 2 | 7 (46.7) | 8 (61.5) | |
| Metastatic sites | |||
| Lymph nodes | 13 (86.7) | 8 (61.5) | .1977b |
| Lung | 6 (40) | 7 (53.9) | .7051b |
| Liver | 5 (33.3) | 2 (15.4) | .3955b |
| Bone | 3 (20) | 2 (15.4) | 1.0000b |
| Interval from last chemotherapy | .1283b | ||
| <3 mo | 5 (33.3) | 9 (69.2) | |
| ≥3 mo | 10 (66.7) | 4 (30.8) | |
| Screening ECOG PS | .0238b | ||
| 0 | 9 (60.0) | 2 (15.4) | |
| 1 | 6 (40.0) | 11 (84.6) | |
| Bellmunt score | .0905b | ||
| 0 | 6 (40.0) | 1 (7.7) | |
| 1 | 4 (26.7) | 8 (61.5) | |
| 2 | 5 (33.3) | 4 (30.8) |
Data presented as n (%), unless otherwise noted.
Abbreviations: CR = complete response; ECOG = Eastern Cooperative Oncology Group; PD = progressive disease; PR = partial response; PS = performance status; SD = stable disease.
Wilcoxon rank sum test.
Fisher’s exact test.
Discussion
Currently, no agents for the treatment of UC in the second-line setting have been approved, which remains an unmet clinical need. Patients with advanced UC with progression after receiving ≥ 2 previous regimens are commonly frail with a poor PS and multiple comorbidities. Therefore, single agents such as the taxanes, gemcitabine, and pemetrexed have been most commonly used in this patient population, with response rates generally ranging from 10% to 27%.4–6,16–21 Combination regimens have been most often built on the backbone of gemcitabine, taxanes, and platinum-based agents. They have demonstrated better response rates (15%−60%) but have failed to improve survival and do result in increased toxicity.22–28
In our trial, 32 patients with advanced UC with progression after a maximum of 2 previous lines of chemotherapy, including their perioperative chemotherapy, were treated with pazopanib and weekly paclitaxel. This treatment regimen induced an ORR of 54%, with a median PFS of 6.2 months and OS of 10 months. These results were evaluated and confirmed by an independent reviewer.
Recently, clinical trials have evaluated targeted agents for the treatment of UC, especially agents targeting the angiogenesis (VEGF) and epidermal growth factor receptors, with variable, but encouraging, response rates (0%−70%).29–37 Our trial combined a cytotoxic chemotherapeutic agent with a targeted anti-VEGF tyrosine kinase inhibitor, and the regimen was well tolerated with a high response rate.
Our study regimen had a promising response rate in the second-line setting, especially in patients with adverse prognostic features. Of the 32 patients, 27 (84%) had ≥ 1 previously identified adverse prognostic factor (ECOG PS > 0, hemoglobin < 10 g/dL, presence of liver metastasis, interval from the last chemotherapy regimen < 3 months).14,15 Pooled data from phase II trials have shown that such patients have an overall survival of 3 to 6.7 months. Treatment with pazopanib and paclitaxel in our study resulted in a median OS of 5.5 to 11.1 months. Although all the patients had transitional cell histologic features, our study also included patients (34%) with upper tract disease.
The dosing of pazopanib at 800 mg daily and paclitaxel at 80 mg/m2 on days 1, 15, and 28 every 28 days was obtained from a previous phase I study evaluating this combination in multiple solid tumors.11 It was noted that the administration of 800 mg pazopanib resulted in 14% lower paclitaxel clearance and 31% greater paclitaxel maximal concentration than administration of paclitaxel alone at 15, 50, and 80 mg/m2, suggesting synergistic activity. The combination of pazopanib and paclitaxel was also well tolerated, with fatigue and hematologic toxicities the most prominent.
The common toxicities observed in our study were grade ≥ 3 aspartate aminotransferase and alanine aminotransferase elevation, fatigue, peripheral neuropathy, and neutropenia, similar to the toxicities noted in the phase I study combining these 2 agents.11 Supportive treatment with growth factors was required in 44% of our patients. Because of the hematologic toxicities, 6 patients received a dose reduction of paclitaxel to 65 mg/m2 and pazopanib to 600 mg. Of the 6 patients, 4 had stable disease and 2 were not evaluable for response. The pharmacokinetic studies of this lower dose combination are pending.
Multiple antiangiogenesis agents have been explored for the treatment of UC but with limited efficacy. In a recent phase II study, patients with advanced UC were treated with docetaxel (n = 44) versus docetaxel with ramucirumab (n = 46). The interim results reported that docetaxel and ramucirumab reduced the risk of disease progression and demonstrated an improving median PFS (5.1 vs. 2.4 mo). The ORR for the combination arm was 19.6% compared with 4.5% for the docetaxel arm (P = .0502), and the OS data are pending.38 The results of that trial, combined with those from our study, indicate a role of angiogenesis inhibitors for UC.
The limitations of the present phase II study included the small sample size and possible selection bias in the patient group enrolled. The enrollment to our trial required several years to complete in this challenging group of patients. Despite these limitations, the response rate obtained was superior to the results obtained in previous phase II studies evaluating the combination of targeted agents with cytotoxic chemotherapy in advanced UC (vandetanib and docetaxel, cetuximab and paclitaxel).29,34
Conclusion
The antiangiogenic agent pazopanib was shown to have significant clinical activity and to be tolerated when combined with paclitaxel in patients with refractory UC. The results obtained from our phase II study are encouraging and warrant further exploration, such as in a randomized phase III trial comparing this combination with single-agent paclitaxel. Although new immunotherapies, such as programmed cell death protein 1 blockade, are very promising, the disease of a subset of patients will not respond to such an approach. Thus, tolerable combinations with other mechanisms of action and promising efficacy, such as paclitaxel plus pazopanib, merit a larger study with incorporation of patients stratified by their PS, hemoglobin level, visceral metastasis, and interval from previous chemotherapy.
Clinical Practice Points.
Most patients with metastatic UC develop a relapse after responding to initial chemotherapy.
No approved drug or regimen is available for second-line therapy.
Our trial with paclitaxel and pazopanib resulted in a high ORR.
The toxicities observed were manageable with appropriate dose adjustments.
This combination is worthy of future study.
Acknowledgments
Funding for the present study was provided by GlaxoSmith Kline. The sponsor had no involvement in manuscript preparation.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29. [DOI] [PubMed] [Google Scholar]
- 2.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000; 18:3068–77. [DOI] [PubMed] [Google Scholar]
- 3.von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005; 23:4602–8. [DOI] [PubMed] [Google Scholar]
- 4.McCaffrey JA, Hilton S, Mazumdar M, et al. Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J Clin Oncol 1997; 15: 1853–7. [DOI] [PubMed] [Google Scholar]
- 5.Vaughn DJ, Broome CM, Hussain M, Gutheil JC, Markowitz AB. Phase II trial of weekly paclitaxel in patients with previously treated advanced urothelial cancer. J Clin Oncol 2002; 20:937–40. [DOI] [PubMed] [Google Scholar]
- 6.Ko YJ, Canil CM, Mukherjee SD, et al. Nanoparticle albumin-bound paclitaxel for second-line treatment of metastatic urothelial carcinoma: a single group, multi-centre, phase 2 study. Lancet Oncol 2013; 14:769–76. [DOI] [PubMed] [Google Scholar]
- 7.Bochner BH, Cote RJ, Weidner N, et al. Angiogenesis in bladder cancer: relationship between microvessel density and tumor prognosis. J Natl Cancer Inst 1995; 87:1603–12. [DOI] [PubMed] [Google Scholar]
- 8.Wu W, Shu X, Hovsepyan H, Mosteller RD, Broek D. VEGF receptor expression and signaling in human bladder tumors. Oncogene 2003; 22:3361–70. [DOI] [PubMed] [Google Scholar]
- 9.Hamberg P, Verweij J, Sleijfer S. (Pre-)clinical pharmacology and activity of pazopanib, a novel multikinase angiogenesis inhibitor. Oncologist 2010; 15:539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Necchi A, Mariani L, Zaffaroni N, et al. Pazopanib in advanced and platinum-resistant urothelial cancer: an open-label, single group, phase 2 trial. Lancet Oncol 2012; 13:810–6. [DOI] [PubMed] [Google Scholar]
- 11.Tan AR, Dowlati A, Jones SF, et al. Phase I study of pazopanib in combination with weekly paclitaxel in patients with advanced solid tumors. Oncologist 2010; 15: 1253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 13.StataCorp. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP; 2009. [Google Scholar]
- 14.Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol 2010; 28:1850–5. [DOI] [PubMed] [Google Scholar]
- 15.Sonpavde G, Pond GR, Fougeray R, et al. Time from prior chemotherapy enhances prognostic risk grouping in the second-line setting of advanced urothelial carcinoma: a retrospective analysis of pooled, prospective phase 2 trials. Eur Urol 2013; 63:717–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albers P, Siener R, Hartlein M, et al. Gemcitabine monotherapy as second-line treatment in cisplatin-refractory transitional cell carcinoma—prognostic factors for response and improvement of quality of life. Onkologie 2002; 25:47–52. [DOI] [PubMed] [Google Scholar]
- 17.Lorusso V, Pollera CF, Antimi M, et al. A phase II study of gemcitabine in patients with transitional cell carcinoma of the urinary tract previously treated with platinum. Italian Co-operative Group on Bladder Cancer. Eur J Cancer 1998; 34: 1208–12. [DOI] [PubMed] [Google Scholar]
- 18.Culine S, Theodore C, De Santis M, et al. A phase II study of vinflunine in bladder cancer patients progressing after first-line platinum-containing regimen. Br J Cancer 2006; 94:1395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaughn DJ, Srinivas S, Stadler WM, et al. Vinflunine in platinum-pretreated patients with locally advanced or metastatic urothelial carcinoma: results of a large phase 2 study. Cancer 2009; 115:4110–7. [DOI] [PubMed] [Google Scholar]
- 20.Bellmunt J, Theodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 2009; 27:4454–61. [DOI] [PubMed] [Google Scholar]
- 21.Sweeney CJ, Roth BJ, Kabbinavar FF, et al. Phase II study of pemetrexed for second-line treatment of transitional cell cancer of the urothelium. J Clin Oncol 2006; 24:3451–7. [DOI] [PubMed] [Google Scholar]
- 22.Meluch AA, Greco FA, Burris HA III, et al. Paclitaxel and gemcitabine chemotherapy for advanced transitional-cell carcinoma of the urothelial tract: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol 2001; 19: 3018–24. [DOI] [PubMed] [Google Scholar]
- 23.Sternberg CN, Calabro F, Pizzocaro G, Marini L, Schnetzer S, Sella A. Chemotherapy with an every-2-week regimen of gemcitabine and paclitaxel in patients with transitional cell carcinoma who have received prior cisplatin-based therapy. Cancer 2001; 92:2993–8. [DOI] [PubMed] [Google Scholar]
- 24.Sweeney CJ, Williams SD, Finch DE, et al. A phase II study of paclitaxel and ifosfamide for patients with advanced refractory carcinoma of the urothelium. Cancer 1999; 86:514–8. [DOI] [PubMed] [Google Scholar]
- 25.Krege S, Rembrink V, Borgermann C, Otto T, Rubben H. Docetaxel and ifosfamide as second line treatment for patients with advanced or metastatic urothelial cancer after failure of platinum chemotherapy: a phase 2 study. J Urol 2001; 165:67–71. [DOI] [PubMed] [Google Scholar]
- 26.Pagliaro LC, Millikan RE, Tu SM, et al. Cisplatin, gemcitabine, and ifosfamide as weekly therapy: a feasibility and phase II study of salvage treatment for advanced transitional-cell carcinoma. J Clin Oncol 2002; 20:2965–70. [DOI] [PubMed] [Google Scholar]
- 27.Vaishampayan UN, Faulkner JR, Small EJ, et al. Phase II trial of carboplatin and paclitaxel in cisplatin-pretreated advanced transitional cell carcinoma: a Southwest Oncology Group study. Cancer 2005; 104:1627–32. [DOI] [PubMed] [Google Scholar]
- 28.Pectasides D, Aravantinos G, Kalofonos H, et al. Combination chemotherapy with gemcitabine and ifosfamide as second-line treatment in metastatic urothelial cancer: a phase II trial conducted by the Hellenic Cooperative Oncology Group. Ann Oncol 2001; 12:1417–22. [DOI] [PubMed] [Google Scholar]
- 29.Choueiri TK, Ross RW, Jacobus S, et al. Double-blind, randomized trial of docetaxel plus vandetanib versus docetaxel plus placebo in platinum-pretreated metastatic urothelial cancer. J Clin Oncol 2012; 30:507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallagher DJ, Milowsky MI, Gerst SR, et al. Phase II study of sunitinib in patients with metastatic urothelial cancer. J Clin Oncol 2010; 28:1373–9. [DOI] [PubMed] [Google Scholar]
- 31.Dreicer R, Li H, Stein M, et al. Phase 2 trial of sorafenib in patients with advanced urothelial cancer: a trial of the Eastern Cooperative Oncology Group. Cancer 2009; 115:4090–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Twardowski P, Stadler WM, Frankel P, et al. Phase II study of aflibercept (VEGF-TRAP) in patients with recurrent or metastatic urothelial cancer, a California Cancer Consortium Trial. Urology 2010; 76:923–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pili R, Qin R, Flynn PJ, et al. A phase II safety and efficacy study of the vascular endothelial growth factor receptor tyrosine kinase inhibitor pazopanib in patients with metastatic urothelial cancer. Clin Genitourin Cancer 2013; 11:477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong YN, Litwin S, Vaughn D, et al. Phase II trial of cetuximab with or without paclitaxel in patients with advanced urothelial tract carcinoma. J Clin Oncol 2012; 30:3545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wulfing C, Machiels JP, Richel DJ, et al. A single-arm, multicenter, open-label phase 2 study of lapatinib as the second-line treatment of patients with locally advanced or metastatic transitional cell carcinoma. Cancer 2009; 115:2881–90. [DOI] [PubMed] [Google Scholar]
- 36.Petrylak DP, Tangen CM, Van Veldhuizen PJ Jr, et al. Results of the Southwest Oncology Group phase II evaluation (study S0031) of ZD1839 for advanced transitional cell carcinoma of the urothelium. BJU Int 2010; 105:317–21. [DOI] [PubMed] [Google Scholar]
- 37.Hussain MH, MacVicar GR, Petrylak DP, et al. Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor-2/neu-positive urothelial carcinoma: results of a multicenter phase II National Cancer Institute trial. J Clin Oncol 2007; 25:2218–24. [DOI] [PubMed] [Google Scholar]
- 38.Petrylak DP, Tagawa ST, Kohli M, et al. Interim results of a randomized phase 2 study of docetaxel with ramucirumab versus docetaxel in second-line advanced or metastatic urothelial carcinoma. J Clin Oncol 2015; 33(suppl 7), abstract 295. [Google Scholar]