Summary
U3–1565 is a monoclonal antibody directed against heparin-binding epidermal growth factor-like growth factor (HB-EGF), which mediates angiogenesis via induction of vascular endothelial growth factor (VEGF-A). This first-in-human study characterized the safety, tolerability, efficacy, pharmacokinetics, and pharmacodynamics of U3–1565 in subjects with advanced solid tumors. In Part 1 (dose escalation following a modified 3 + 3 design), Cohorts 1–4, U3–1565 was administered at 2, 8, 16, and 24 mg/kg every 3 weeks for Cycle 1 and every 2 weeks thereafter. In Part 1, Cohort 5, and in Part 2 (dose expansion), U3–1565 was administered at 24 mg/kg every week. Thirty-six subjects were enrolled and treated (15 in Part 1; 21 in Part 2). No subject experienced dose limiting toxicity and maximum tolerated dose was not reached. All drug-related events were Grade 1 or 2 in severity, with fatigue and rash predominating. Following treatment with U3–1565, 1 subject with metastatic colorectal cancer experienced partial response and 6 subjects achieved stable disease. Four subjects completed the study main phase (first 12 cycles) and entered the extension phase. Of the 6/36 subjects with high (> 1500 pg/ml) baseline VEGF-A levels, all showed a decrease in VEGF-A (median − 60% [−22% to −97%]). Of the remaining subjects, only 19/30 showed a decrease (median − 18% [−2% to −82%]). Subjects with high VEGF-A baseline levels remained on treatment longer (3/6 entered study extension phase versus 1/30), and were more likely to show disease control (3/6 versus 4/30). In conclusion, U3–1565 demonstrates both proof of mechanism and clinical activity across different tumor types.
Keywords: U3–1565, HB-EGF, Phase 1, Pharmacokinetics, VEGF-A
Introduction
Heparin-binding epidermal growth factor-like growth factor (HB-EGF) is a member of the EGF family of ligands that binds to and activates epidermal growth factor receptors 1 and 4 (EGFR/ErbB1/HER1 and EGFR4/ErbB4/HER4) [1, 2]. The EGFR family of transmembrane receptor tyrosine kinases (RTKs) forms homodimers or heterodimers upon ligand binding. Activation of these receptors initiates downstream signaling pathways, such as the mitogen-activated protein kinase (MAPK), the phosphoinositol 3 kinase (PI3K), and the signal transducers and activators of transcription (STAT) pathways, which are involved in the regulation of multiple cell functions, including growth, survival, and differentiation [3]. Dysregulation of these pathways by receptor overexpression or amplification of cognate ligands is observed in a variety of tumors, drives tumor processes such as metastatic dissemination and angiogenesis, and is associated with poor prognosis [4, 5].
RTK pathways are targeted by two major classes of therapeutics. First, tyrosine kinase inhibitors - such as gefitinib and erlotinib, which are small molecules that target the intracellular domains of RTKs - prevent activation of downstream signaling pathways in response to ligand binding [6, 7]. Second, monoclonal antibodies - such as cetuximab and panitumumab, which target the extracellular domains of RTKs - prevent ligand binding and subsequent dimerization required for receptor activation [8, 9]. HB-EGF expression is elevated in ovarian [10], gastric [11], and breast cancers [12], as well as in gliomas [13] and melanomas [14], and may contribute to tumor resistance to EGFR antagonist therapy [15].
U3–1565 is a fully human immunoglobulin subclass 2 (IgG2) monoclonal antibody directed against HB-EGF developed by Daiichi Sankyo, Co., Ltd. (Japan). Antigen specificity testing has demonstrated that U3–1565 specifically binds to HB-EGF, but does not recognize EGF-like ligands with sequence homology to HB-EGF, including amphiregulin, transforming growth factor-alpha, and neuregulins. In vitro studies have demonstrated that U3–1565 inhibits EGFR- and HER4-initiated signal transduction pathways, as well as tumor cell growth, tumor cell migration, and new vessel formation (angiogenesis) [16], thus supporting clinical evaluation.
The present first-in-human study was conducted to characterize the safety, tolerability, maximum tolerated dose (MTD) or maximum administered dose (MAD), efficacy, pharmacokinetics (PK), and pharmacodynamics (PD) of U3–1565 in subjects with advanced solid tumors. In particular, this study sought to evaluate the effect of U3–1565 on circulating levels of vascular endothelial growth factor-A (VEGF-A). As HB-EGF is known to promote the production of VEGF-A [17] and subsequent tumor angiogenesis [18], it was hypothesized that lower VEGF-A levels would be observed following treatment with U3–1565.
Methods
Subjects
This study was conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonization, and was approved by the institutional review board at each of the participating investigational sites. All subjects provided written informed consent prior to screening. The first subject was enrolled on January 11th 2011, and the last subject completed the main phase of the study (first 12 cycles of treatment, leading to the primary analysis) on January 7th, 2013. All subjects still on study at the end of the main phase were eligible to continue receiving U3–1565 in the extension phase.
Enrolled subjects met the following key eligibility criteria: Men and women aged ≥18 years old with a pathologically documented advanced solid malignant tumor refractory to standard treatment or for which no standard treatment was available; Eastern Cooperative Oncology Group (ECOG) performance status ≤1; the presence of an evaluable tumor for enrollment in all parts of the study and, only for Part 2, measurable tumor per RECIST Version 1.1 [19]. However, subjects with advanced ovarian cancer could be enrolled in Part 2 even if they did not have a tumor measurable per RECIST, as long as they had circulating levels of cancer antigen 125 (CA125) higher than 35 Units/mL.
Subjects were excluded from the study if they had: clinically active brain metastases, defined as symptomatic or requiring treatment with steroids or anticonvulsants; history of reactions to antibody drug products; history of stem cell or bone marrow transplantation; history of myocardial infarction within 6 months before enrollment, symptomatic congestive heart failure (New York Heart Association > Class II), unstable angina or unstable cardiac arrhythmia requiring medication; history of clinically significant pulmonary disease after receiving EGFR targeting agents; history of human immunodeficiency virus positivity; impaired bone marrow function defined as an absolute neutrophil count (ANC) < 1.5 × 109/L, platelet count <100 × 109/L, or hemoglobin <9 g/dL; impaired renal function defined as creatinine >1.5 × upper limit of normal (ULN) or creatinine clearance <60 mL/min, as calculated using the modified Cockroft-Gault equation; impaired hepatic function defined as aspartate aminotransferase (AST) > 3 × ULN (if liver metastases were present, > 5 × ULN), alanine aminotransferase (ALT) > 3 × ULN (if liver metastases were present, ≥ 5 × ULN), or bilirubin >1.5 × ULN; impaired coagulation defined as prothrombin time (PT) or partial thromboplastin time (PTT) > 1.5 × ULN; received anti-cancer therapy, including antibody, retinoid, or hormonal treatment, within 3 weeks prior to enrollment (prior and concurrent use of hormone replacement therapy, use of gonadotropin-releasing hormone modulators for prostate cancer, and use of somatostatin analogs for neuroendocrine tumors were permitted); received therapeutic radiation treatment within 4 weeks or palliative radiation treatment within 2 weeks before enrollment, as long as radiation toxicities had resolved to National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) Grade ≤ 1 or baseline values; received major surgery within 4 weeks before treatment; received an investigational drug within 3 weeks before enrollment; or were pregnant or breastfeeding.
Study design
This was a first-in-human, Phase 1, multicenter, open-label, non-randomized study consisting of two parts, dose escalation (Part 1), and dose expansion (Part 2).
The primary objectives of Part 1 were to assess the safety and tolerability of U3–1565 and to determine the MTD or to establish the safety and tolerability of the MAD. Key secondary objectives of Part 1 included assessment of the PK and preliminary efficacy signals of U3–1565. Exploratory PD biomarkers were also evaluated.
Part 1 (dose escalation) followed a modified 3 + 3 design and included five dose level cohorts. Dose escalation began with a dose of 2 mg/kg (Cohort 1) and continued with doses of 8, 16, and 24 mg/kg (Cohorts 2–4). A maximum of 2 new subjects received their first dose of U3–1565 per 24 h period during dose escalation. In Cohorts 1–4, the second dose was administered 3 weeks after the first dose, with subsequent doses administered every 2 weeks. The doses used in Cohorts 1–4 of Part 1 of this study (ie, 2, 8, 16, and 24 mg/kg given every 2 weeks) were respectively 200, 50, 24, and 16 times lower than the no observed adverse effect level found in rats and monkeys. Based on modeling and simulation studies and allometric scaling, 4 mg/kg was predicted to be the minimally active dose in humans, while 10 mg/kg was identified as the pharmacologically active dose, defined as the dose necessary to achieve the target concentration in humans (corresponding to the 90% effective dose [ED90] observed in severe combined immunodeficiency mice bearing EFO27-CL58 xenograft tumors) (unpublished data). In Cohort 5, a dose of 24 mg/kg was administered each week. In each cohort, a 21-day observation period followed the first infusion. At the end of this period, all relevant safety data were reviewed for pre-specified dose limiting toxicities (DLTs).
Dose escalation decisions were made by the Investigators and Sponsor, including the Medical Monitor, based on safety and other parameters obtained from subjects who had completed the 21-day observation period. MTD was defined as the highest dose that resulted in a DLT in less than one-third of the subjects enrolled in a cohort. A minimum of 6 subjects was required to define the MTD.
DLTs were defined as any AE, except those clearly related to the disease, that occurred during Cycle 1 (Days 1 through 21) and was classified as Grade 3 or higher according to the NCI CTCAE, Version 4.0, with specific requirements for the following toxicities: Febrile neutropenia with ANC < 1000/mm3 and a single finding of temperature ≥ 38.3 °C or a temperature ≥ 38 °C persisting for 1 h or more; Grade 4 AST/ALT; AST/ALT >5 × ULN if accompanied by ≥ Grade 2 elevation in bilirubin, or lasting >3 days in subjects without liver metastases, or lasting >3 days if the baseline value was ≤3 × ULN in subjects with liver metastases; AST/ALT >8 × ULN lasting >3 days if the baseline value was >3 × ULN but <5 × ULN in subjects with liver metastases. However, Grade 3 fatigue lasting <3 days, Grade 3 nausea, vomiting or diarrhea that had resolved to Grade 2 within 48 h after standard therapies, or isolated laboratory values not associated with symptoms were not considered DLTs.
The primary objectives of Part 2 were to confirm the safety and tolerability of U3–1565 at MTD or MAD in subjects with advanced solid malignant tumors (with a preference for subjects with advanced ovarian cancer) and to assess changes in tumor size and in PD biomarkers after U3–1565 treatment. Key secondary objectives included evaluation of the PK of U3–1565, formation of human anti-human antibodies (HAHA), and evaluation of tumor response using RECIST.
Part 2 (dose expansion) started upon completion of Part 1, once the MAD safety and tolerability was established. For Part 2, up to 30 subjects with advanced solid malignant tumors were to be enrolled. Since MTD was not reached in dose escalation, subjects in Part 2 were treated at the MAD of 24 mg/kg U3–1565 every week. This number of subjects allowed for the demonstration of anti-tumor activity of U3–1565 by showing treatment-induced changes in clinical activity and PD biomarkers. Preliminary safety analyses were conducted after the initial 6 subjects completed the first 21 days of treatment and again after the subjects either completed the first 42 days of treatment or were withdrawn from the study.
For all subjects, U3–1565 was diluted and administered by continuous IV infusion over 60 min. Infusion times could be extended to a maximum of 120 min if needed for tolerability.
Assessments
Safety parameters included DLTs, serious adverse events (SAEs), treatment emergent adverse events (TEAEs), physical examination findings, vital sign measurements, standard clinical laboratory results, and electrocardiogram (ECG) readings. Adverse events were coded using MedDRA (Version 13) and assigned grades (severities) based on NCI CTCAE, Version 4.0. The AE reporting period was from the time of signing the informed consent until 30 days after the last dose of U3–1565.
In both study parts, tumor assessment was performed by physical examination and computed tomography (CT) and/or magnetic resonance imaging (MRI) at screening and Day 1 of Cycle 4 and then every 3 cycles (6 weeks). Spiral CT or MRI with ≤5 mm cuts were used for tumor assessment unless another modality of disease assessment was necessary.
Blood samples for PK analyses were collected during Cycle 1 and Cycle 3 at baseline, end of infusion, 3, 6, 24, 72, and 168 h after infusion. In Part 1, Cohorts 1–4, additional samples were collected at 336 and 504 h after infusion during Cycle 1. U3–1565 concentrations were determined with a validated enzyme-linked immunosorbent assay (ELISA) by Covance Laboratories (Chantilly, VA).
Blood samples for HAHA analysis were collected during Part 1 at baseline, Cycle 1 Day 8, the beginning of Cycles 2, 4, and each cycle thereafter, and the end of treatment. Samples were collected during Part 2 at baseline, Cycle 1 Day 8, the start of Cycle 3 and of each cycle thereafter, and the end of treatment. Samples were analyzed using a validated electrochemiluminescent assay (ECLIA) by Intertek Pharmaceutical Services (San Diego, CA).
Blood samples for analysis of PD biomarkers were collected for all subjects at baseline, 24 h, Day 8, and Day 15 following the first infusion, then on Day 1 of Cycles 2 and 4, and on Day 1 every 3 cycles afterwards. All biomarkers were assayed by Medpace Inc. (Cincinnati, OH) using validated assays, using the CA125 assay kit by Roche (Basel, Switzerland); the Caspase-Glo 3/7 assay kit by Promega (Madison, WI); the human HB-EGF ELISA kit by RayBiotech (Norcross, GA); the VEGF-A ELISA kit by BioVendor (Brno, Czech Republic); and the VEGF-R2 ELISA kit by R&D Systems (Minneapolis, MN).
Statistics
The safety analysis set included all subjects who received any amount of study medication and had at least 1 safety assessment. The DLT-evaluable analysis set included all subjects in Part 1 who received at least 1 dose of U3–1565 and had an opportunity to be followed up for 3 weeks (21 days) or received 1 dose of U3–1565 and had a DLT within the first 3 weeks (21 days) on study. The PK analysis set included all subjects who received at least 1 dose of U3–1565 and had at least 1 evaluable PK assay sample.
Plasma concentration-time data were analyzed using non-compartmental methods in Phoenix WinNonlin (Version 6.2.1, Pharsight Corp., St. Louis, MO). Concentration values below the lower limit of quantification were set to zero. PK parameters calculated for U3–1565 included maximum (peak) observed concentration in plasma (Cmax), time of maximum observed concentration (Tmax), the area under the concentration versus-time curve (AUC) from time 0 to the last measured concentration (AUClast), as calculated by the linear trapezoidal method, and terminal half-life. Dose proportionality was assessed graphically with trend lines derived from linear regression, and by using a power model [20] using R (Version 3.4.4, R Foundation, Vienna, Austria).
Data availability
The data discussed in this manuscript are proprietary and has not been made available publically.
Results
Demographics and baseline characteristics
A total of 36 subjects were enrolled and treated with U3–1565 at one of 4 clinical sites in the United States. Fifteen subjects were enrolled in the dose escalation part (Part 1) and 21 in the dose expansion part (Part 2) of the study. Table 1 summarizes the demographics and baseline characteristics of all subjects enrolled in this study. Four of 36 (11%) subjects completed the 12 cycles of treatment comprising the main phase of the study and entered the extension phase. The reasons for discontinuation from the main phase of the study were: Radiographic evidence of progressive disease (25/36 [69%]) subjects), clinical disease progression (5/36 [14%] subjects), SAE (1/36 [3%] subject), and loss to follow-up after Study Day 45 (last study visit) (1/36 [3%] subject).
Table 1.
Demographics and baseline characteristics
| Characteristic | All Subjects (N = 36) |
|---|---|
| Gender, n (%) | |
| Male | 9 (25.0%) |
| Female | 27 (75.0%) |
| Age (Years) | |
| Mean ± SD | 58.3 ± 10.2 |
| Race, n (%) | |
| Caucasian | 31 (86.1%) |
| Black or African American | 3 (8.3%) |
| Asian | 2 (5.6%) |
| Weight (kg) | |
| Mean ± SD | 80.9 ± 22.4 |
| ECOG performance status, n (%) | |
| 0 | 20 (55.6%) |
| 1 | 16 (44.4%) |
| Primary tumor type, n (%) | |
| Colorectal | 15 (41.7%) |
| Ovarian | 14 (38.9%) |
| Non-small cell lung | 5 (13.9%) |
| Small cell lung | 1 (2.8%) |
| Small bowel | 1 (2.8%) |
| Prior radiotherapy, n (%) | |
| Yes | 11 (30.6%) |
| No | 25 (69.4%) |
| Number of prior chemotherapy regimens | |
| 2 | 4 (11.1%) |
| ≥3 | 32 (88.9%) |
SD standard deviation
Dose escalation, DLTs, MTD/MAD and dose expansion
Fifteen subjects (3 per Cohort) received at least one dose of U3–1565 in Part 1 (dose escalation). All subjects tolerated the U3–1565 infusion well. No DLTs were observed in any subject in any of the dose escalation cohorts, and therefore MTD was not reached. The highest dose of U3–1565 tested, 24 mg/kg per week, was determined to be the MAD. None of the 21 subjects enrolled and treated in Part 2 (dose expansion) experienced any adverse events that met the definition of DLT.
Safety profile
In total, 35 (97%) subjects reported a TEAE, with 17 (47%) subjects experiencing a TEAE attributed to the study drug. Among the 24 subjects across both parts treated at the MAD (24 mg/kg per week), 23 (96%) subjects experienced at least one TEAE, with 12 (50%) subjects experiencing TEAEs attributed to the study drug. No dose dependent increase in TEAEs was observed. The most common TEAEs (experienced by ≥10% of total subjects, irrespective of causality) are shown in Table 2. All drug-related TEAEs were Grade 1 or Grade 2 in intensity. Drug-related TEAEs reported by ≥10% of the subjects included fatigue (7 [19%]) and rash (4 [11%]). No clinically significant abnormal ECG results were observed at baseline or post-treatment in either part of the study.
Table 2.
Summary of treatment emergent adverse events experienced by at least 10% of subjects, irrespective of causality
| TEAEs | Part 1/Cohort 1 (N = 3) | Part 1/Cohort 2 (N = 3) | Part 1/Cohort 3 (N = 3) | Part 1/Cohort 4 (N = 3) | Part 1/Cohort 5 (N = 3) | Part 2/ 24 mg/kg (N = 21) | All Parts at 24 mg/kg (N = 24) | Total (N = 36) |
|---|---|---|---|---|---|---|---|---|
| All TEAEs | 3 (100.0%) | 3 (100.0%) | 3 (100.0%) | 3 (100.0%) | 3 (100.0%) | 20 (95.2%) | 23 (95.8%) | 35 (97.2%) |
| Fatigue | 1 (33.3%) | 0 | 0 | 1 (33.3%) | 1 (33.3%) | 8 (38.1%) | 9 (37.5%) | 11 (30.6%) |
| Decreased appetite | 2 (66.7%) | 0 | 1 (33.3%) | 0 | 0 | 5 (23.8%) | 5 (20.8%) | 8 (22.2%) |
| Nausea | 1 (33.3%) | 1 (33.3%) | 0 | 1 (33.3%) | 0 | 5 (23.8%) | 5 (20.8%) | 8 (22.2%) |
| Anaemia | 1 (33.3%) | 1 (33.3%) | 0 | 1 (33.3%) | 1 (33.3%) | 2 (9.5%) | 3 (12.5%) | 6 (16.7%) |
| Abdominal distension | 1 (33.3%) | 0 | 0 | 0 | 0 | 5 (23.8%) | 5 (20.8%) | 6 (16.7%) |
| Vomiting | 1 (33.3%) | 0 | 0 | 1 (33.3%) | 1 (33.3%) | 3 (14.3%) | 4 (16.7%) | 6 (16.7%) |
| Diarrhoea | 0 | 1 (33.3%) | 0 | 0 | 0 | 4 (19.0%) | 4 (16.7%) | 5 (13.9%) |
| Rash | 2 (66.7%) | 0 | 0 | 0 | 0 | 3 (14.3%) | 3 (12.5%) | 5 (13.9%) |
| Pyrexia | 1 (33.3%) | 0 | 1 (33.3%) | 0 | 0 | 3 (14.3%) | 3 (12.5%) | 5 (13.9%) |
| Abdominal pain | 0 | 1 (33.3%) | 0 | 0 | 0 | 3 (14.3%) | 3 (12.5%) | 4 (11.1%) |
| Constipation | 0 | 1 (33.3%) | 1 (33.3%) | 0 | 0 | 2 (9.5%) | 2 (8.3%) | 4 (11.1%) |
| Back pain | 0 | 1 (33.3%) | 0 | 0 | 1 (33.3%) | 2 (9.5%) | 3 (12.5%) | 4 (11.1%) |
| Musculoskeletal pain | 1 (33.3%) | 1 (33.3%) | 0 | 1 (33.3%) | 0 | 1 (4.8%) | 1 (4.2%) | 4 (11.1%) |
| Dyspnoea exertional | 0 | 0 | 0 | 1 (33.3%) | 0 | 3 (14.3%) | 3 (12.5%) | 4 (11.1%) |
| Pruritus | 2 (66.7%) | 0 | 0 | 0 | 0 | 2 (9.5%) | 2 (8.3%) | 4 (11.1%) |
n (%) is shown
Three subjects (8%) were discontinued from the study due to TEAEs. One subject in Part 1 (16 mg/kg dose cohort) was discontinued due to an SAE (small intestinal obstruction), which was determined to be unrelated to the study drug. Two subjects in Part 2 were discontinued due to TEAEs, one due to Grade 2 vomiting, Grade 1 nausea, and Grade 1 fatigue; and one due to Grade 2 fatigue. These TEAEs were deemed due to clinical disease progression and not related to the study drug. Two (8%) subjects experienced a TEAE leading to death within 30 days after the last administration of study drug. None of these deaths were considered to be related to the study drug.
Pharmacokinetics
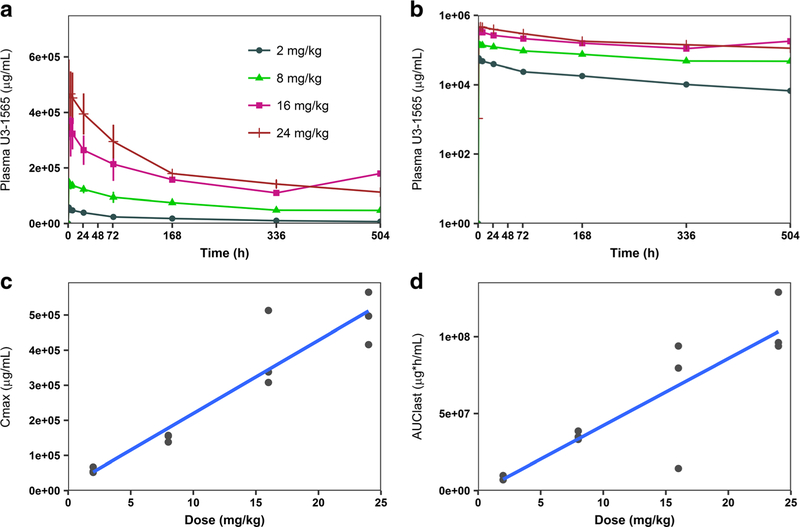
Mean concentration-time profiles after single ascending doses (2 mg/kg, 8 mg/kg, 16 mg/kg, and 24 mg/kg) of U3–1565 in Part 1 (dose escalation) are shown in Fig. 1a, b. PK parameters obtained following a single dose of U3–1565 in Part 1 are provided in Table 3, and those obtained from Cycles 1 and 3 of Part 2 (dose expansion) are provided in Table 4. Accumulation of U3–1565 was evident in Part 2 following administration of the 24 mg/kg/week dose with Cmax and AUC more than doubling.
Fig. 1. Concentration-time profiles and dose proportionality of single doses of U3–1565.
Mean (standard deviation) plasma concentration-time profiles of U3–1565 following the first infusion by dose level (Part 1, Cohorts 1–4). Both linear (a) and log (b) scales are shown. Dose proportionality was assessed graphically for Cmax and AUClast following the first infusion by dose level (c and d) following are shown for each subject in Part 1, Cohorts 1–4. Trend lines for dose linearity are shown for each graph
Table 3.
U3–1565 single dose plasma PK parameters in Part 1, Cycle 1
| Cohort 1 (2 mg/kg) | Cohort 2 (8 mg/kg) | Cohort 3 (16 mg/kg) | Cohort 4 (24 mg/kg) | Cohort 5 (24 mg/kg) | |
|---|---|---|---|---|---|
| n | 3 | 3 | 3 | 3 | 3 |
| Cmax (μg/mL) | 57.9 (8.17) | 150 (10.4) | 386 (111) | 493 (74.6) | 496 (147) |
| tmax (h) | 1.00 (0.98–1.03) | 1.03 (1.02–3.67) | 3.82 (0.97–6.72) | 1.07 (1.05–25.17) | 1.08 (0.97–2.05) |
| AUClast (mg h/mL)# | 7.95 (1.62) | 35.5 (2.79) | 62.6 (42.4) | 106 (19.5) | 47.2 (13.0) |
| Half-life (h)#* | 237 (18.5) | 304 (N/A) | 175 (N/A) | 404 (125) | 109 (10.5) |
PK parameters were calculated using time points collected following the first dose administration, and up until the second dose: Up to 3 weeks for Cohorts 1–4, and up to 1 week for Cohort 5.
For Cohorts 2 and 3, half-life was only evaluable for 2 subjects. Mean (standard deviation) is reported above. For tmax, median (min, max) is reported. n = number of subjects; N/A = no reportable value
Table 4.
U3–1565 Plasma PK Parameters in Part 2, Cycle 1 and Cycle 3
| Cycle 1 | Cycle 3 | |
|---|---|---|
| n | 21 | 21 |
| Cmax (μg/mL) | 486 (141) | 1121 (328) |
| tmax (h) | 3.93 (1.00–7.00) | 6.72 (0.97–74.4) |
| AUCtau (mg h/mL)# | 44.7 (11.8) | 147 (38.3) |
| Half-life (h)#* | 163 (60.8) | 469 (290) |
PK parameters are based on time points collected over 1 week.
Half-life was only evaluable for 15 subjects in Cycle 1, and for 10 subjects in Cycle 3. Mean (standard deviation) is reported above. For tmax, median (min, max) is reported. n = number of subjects
U3–1565 showed biexponential disposition with Cmax and AUC increasing approximately proportionally with the dose between 2 and 24 mg/kg administered every two weeks (Cycle 1 of Cohorts 1–4). Dose proportionality was examined graphically (Fig. 1c, d) and by applying a power model. Based on the goodness of fit statistic R2, there was a positive correlation between the exposure parameters Cmax and AUClast with dose (R2 = 0.88 and 0.75, respectively). Applying the power model, the slope of the regression line (β1) through Cmax and AUClast versus dose (on a natural log scale) ranged from 0.88 (Cmax, 90% CI: 0.77–1.00; p < 0.0001) to 0.98 (AUClast, 90% CI: 0.70–1.26; p < 0.0001).
Overall, a positive HAHA response was observed in 6 (17%) of 36 subjects. This is higher than the 5% false positive rate expected based on the cut point established using 50 lots of control serum without the addition of study drug. However, a robust HAHA response was not observed. Serum U3–1565 exposure was maintained throughout Cycle 3 in subjects that tested positive for HAHA, indicating that HAHA response did not have an impact on U3–1565 half-life. No subject developed signs or symptoms of an infusion reaction.
Assessment of therapeutic effects
Following exposure to U3–1565, clinical benefit was observed in 7/36 (19%) subjects - 1 with partial response (PR) and 6 with stable disease (SD) - remaining on study for at least 3 Cycles (7 weeks for Part 1, Cohorts 1–4; 6 weeks for Part 1, Cohort 5, & Part 2) without evidence of progressive disease. Table 5 summarizes data from these subjects. Four subjects (1 PR and 3 SD) completed the main phase of the study and entered the extension phase. Three subjects with SD were discontinued from the main phase due to clinical disease progression.
Table 5.
Summary of subjects with an overall response of stable disease or better
| Subject (Age/Sex) and Cohort (Dose) | Tumor type | Best response (Tumor response) | Weeks on treatment (Main phase/Extension phase) | VEGF-A Baseline (pg/mL) | Largest VEGF-A Decrease (% Change from baseline) |
|---|---|---|---|---|---|
| 55 / Female | Ovarian | SD (3.5%) | 16.4 / No | 300 | −13.0% |
| Part 1, Cohort 2 (8 mg/kg biw) 59 / Male | Colorectal | SD (−3.1%) | 15.1 / No | 753 | −22.4% |
| Part 1, Cohort 2 (8 mg/kg biw) 77 / Female# | Non-small cell lung | SD (−3.4%) | 24.0 / Yes | 2150 (High) | −75.9% |
| Part 1, Cohort 4 (24 mg/kg biw) 76 / Female# | Colorectal | PR (−37.5%) | 25.0/Yes | 3620 (High) | −96.9% |
| Part 1, Cohort 5 (24 mg/kg qw) 52 / Female | Ovarian | SD (2.6) | 22.0 / No | 982 | −3.9% |
| Part 2 (24 mg/kg qw) 76 / Female# | Ovarian | SD * | 23.9/Yes | 18,600 (High) | −45.0% |
| Part 2 (24 mg/kg qw) 29 / Female | Ovarian | SD (2.9) | 24.0 / Yes | 171 | 5.2% |
| Part 2 (24 mg/kg qw) |
Tumor response was determined by percent change from baseline in the sum of longest diameters of the target lesions. High baseline VEGF-A levels were defined as >1500 pg/mL.
Indicates the three notable subjects of interest (Subjects 1, 2, and 3) with high baseline VEGF-A and clinical benefit discussed further in the text.
Tumor size was not assessable by RECIST for this subject. Biw = every two weeks; qw = every week
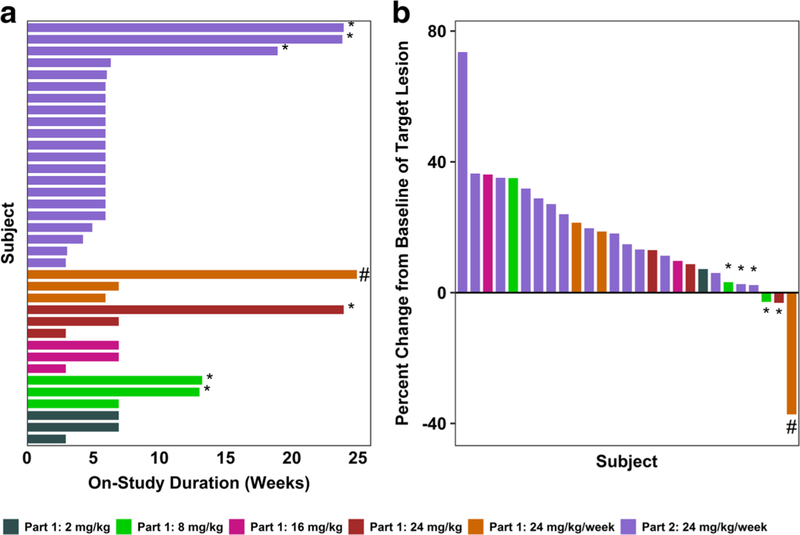
The type and duration of responses in all subjects on the study are shown in Fig. 2. Of the subjects enrolled in Part 1, 1/3 in Cohort 5 (24 mg/kg/week) achieved a best overall response of PR. Additionally, 2/3 subjects in Cohort 2 (8 mg/kg every 2 weeks) and 1/3 in Cohort 4 (24 mg/kg every 2 weeks) achieved a best overall response of SD. Of the subjects enrolled in Part 2 (24 mg/kg every week), 3/21 achieved a best overall response of SD.
Fig. 2. Duration of treatment and percent change in tumor burden following treatment with U3–1565.
a Duration of time in weeks that individual subjects at each dose level were treated during the study main phase. b Best percent change from baseline in the sum of longest diameters of target lesions by treatment group. Subjects achieving SD (*) or PR (#) are indicated. Nine subjects are not included in the waterfall plot for the following reasons: Discontinuation from the study before tumor size assessment occurred (7 subjects, 5 due to clinical disease progression, 1 due to an SAE, and 1 due to loss to follow up), and unavailability of tumor size data (2 subjects)
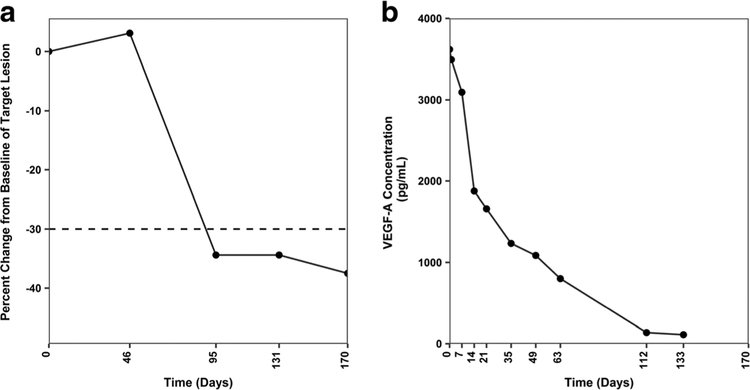
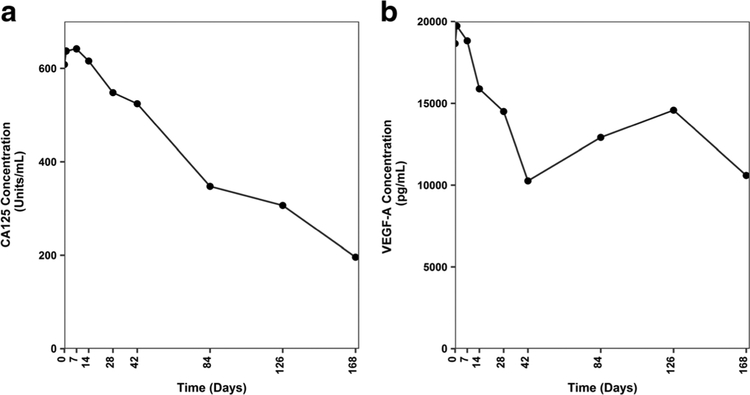
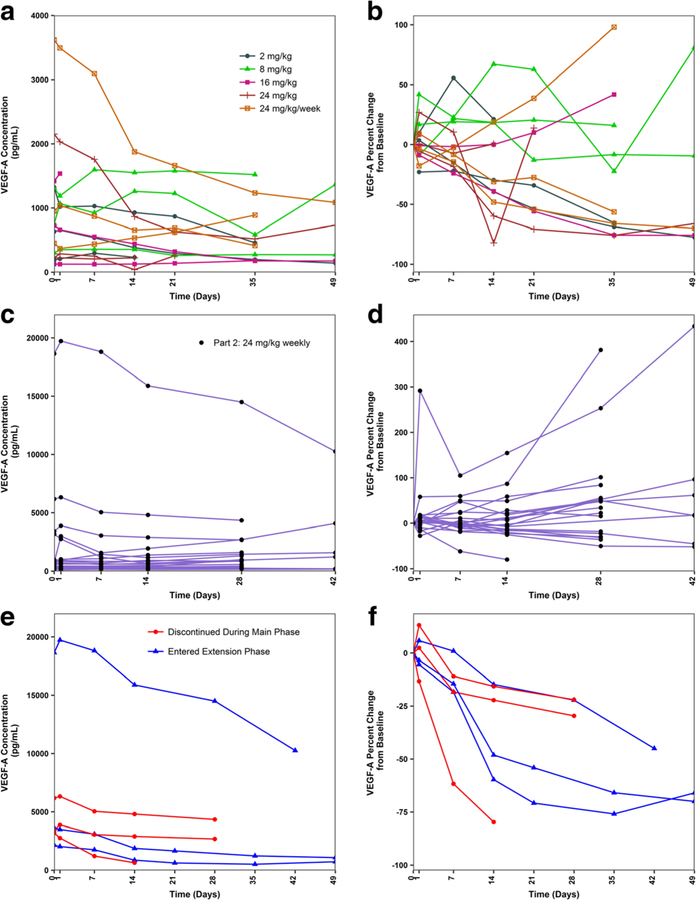
Three notable subjects, described briefly below, experienced clinical benefit following treatment with U3–1565, entered the study extension phase, and displayed substantial reductions in VEGF-A levels. Subject 1: A 76 year old female with metastatic colorectal cancer treated with U3–1565 at 24 mg/kg/week (Part 1, Cohort 5) achieved PR (−37.5% tumor size reduction during the study main phase). Fig. 3 shows the tumor response in this subject and accompanying reduction in circulating VEGF-A levels. Subject 2: A 77 year old female with non-small cell lung cancer treated with U3–1565 at 24 mg/kg every 2 weeks (Part 1, Cohort 4) achieved SD (−3.4% tumor size reduction). Subject 3: A 76 year old female with ovarian cancer treated with U3–1565 at 24 mg/kg/week (Part 2) achieved SD, as indicated by a sustained reduction of circulating CA125 (Fig. 4) (tumor size was not assessable by RECIST). Interestingly, these 3 subjects also showed a decrease in circulating VEGF-A upon treatment with U3–1565 (Figs. 3, 4, 5e–f, and Table 5).
Fig. 3. Partial response to U3–1565 in a subject with metastatic colorectal cancer following treatment with U3–1565.
A 76 year old female with metastatic colorectal cancer treated with U3–1565 at 24 mg/kg/week (Part 1, Cohort 5) experienced a sustained PR. a Percent change from baseline as determined by the sum of longest diameters of the target lesion. The cutoff for PR (−30%) is shown by the dotted line. b This subject had a high baseline VEGF-A level (3619 pg/mL), which remarkably decreased over time upon U3–1565 treatment
Fig. 4. Circulating levels of CA125 and VEGF-A in a subject with ovarian cancer following treatment with U3–1565.
A 76 year old female with ovarian cancer treated with U3–1565 at 24 mg/kg/week (Part 2) experienced prolonged SD, characterized by a sustained decrease in CA125 and VEGF-A levels. This subject had a high baseline VEGF-A level (18,645 pg/mL). Both CA125 (a) and VEGF-A (b) levels decreased over time upon U3–1565 treatment
Fig. 5. Plasma VEGF concentrations by subject following treatment with U3–1565.
Circulating levels of VEGF-A are shown for subjects in Part 1 (a), and Part 2 (c), with percent change from baseline shown in Panels B and D. VEGF-A levels are shown for those 6 subjects with high baseline VEGF-A levels (> 1500 pg/mL) in Panel E, with percent change from baseline shown in Panel F. These subjects appeared to have the greatest reductions in VEGF-A levels following treatment with U3–1565. Subjects in blue entered the study extension phase and are the three notable subjects of interest (Subjects 1, 2, and 3) discussed further in the text. Subjects in red did not enter the extension phase
Pharmacodynamics
Fig. 5 displays the circulating levels of VEGF-A at baseline and following treatment with U3–1565. Baseline VEGF-A levels displayed wide variability (Fig. 5a, c) and were consistent with those previously reported in cancer subjects [21]. Overall, no consistent trend in VEGF-A levels were observed following U3–1565 treatment at any dose level, in either Part 1 (Fig. 5a, b) or Part 2 (Fig. 5c, d). High (> 1500 pg/mL) baseline levels of VEGF-Awere observed in 6/36 (17%) subjects, 2 in Part 1, and 4 in Part 2 (Fig. 5e, f). All 6 subjects with high baseline VEGF-A levels showed a decrease (median − 60% [range − 22% to −97%]) in VEGF-A following treatment with U3–1565. Of the subjects with low (< 1500 pg/ml) baseline VEGF-A levels, 19/30 showed a decrease in VEGF-A (median − 18% [−2% to −82%]), while 11/30 showed an increase in VEGF-A. Subjects with high baseline VEGF-A levels remained on treatment longer, with 3/6 (50%) entering the extension phase compared to 1/30 (3%) subjects with low baseline VEGF-A levels. Finally, subjects with high baseline VEGF-A levels were more likely to show disease control, with 3/6 (50%) achieving either PR or SD compared to 4/30 (13%) subjects with low baseline VEGF-A levels, who achieved SD.
The 3 notable subjects (Subjects, 1, 2, and 3 briefly described above) who showed clinical activity and entered the study extension phase were among the 6 subjects with high baseline VEGF-A levels (Fig. 5e, f and Table 5). The other 3 subjects with high baseline VEGF-A levels included 2 with colorectal cancer, and 1 with ovarian cancer, all enrolled in Part 2 (Fig. 5e, f).
No significant changes were observed in circulating levels of HB-EGF, caspase 3/7 or VEGF-R2 in any subject.
Discussion
This first-in-human Phase 1 study examined the safety, tolerability, PK, PD biomarker response, and clinical efficacy of intravenous infusions of U3–1565 in subjects with advanced solid tumors.
Overall, U3–1565 was safe and well tolerated and no major safety issues were identified. No DLTs were observed up to the maximum administered dose and thus the MTD could not be established. Adverse events were dose independent and mild or moderate (Grades 1 or 2) in severity. No hypersensitivity reactions were observed and U3–1565 was not immunogenic. The safety and tolerability of U3–1565 stand in contrast to those observed in the first-inhuman Phase 1 study of KHK2866, another fully human anti-HB-EGF antibody. The KHK2866 study was stopped early due to neurotoxicity events including complex partial seizures, aphasia, and confusion [22]. The reasons for the difference in safety between these two antibodies remain to be elucidated. Hypersensitivity reactions were observed following the first dose of KHK2866, which could be mitigated with prophylactic medication regimens. Both U3–1565 and KHK2866 bind to soluble and membrane bound HB-EGF [23, 24]. It is not known if the two antibodies bind to the same epitope.
The PK profile of U3–1565 was similar to those observed with other IgG2 antibodies. Mean serum U3–1565 half-life in Cohorts 1–5 in Cycle 1 ranged from 109 to 404 h, for the first 1–3 weeks following dosing, which is generally consistent with other monoclonal antibodies in humans. Doses above 16 mg/kg appeared to be associated with drug accumulation. There was no indication of saturable or target-mediated clearance in this study, which was seen in preclinical studies (unpublished data). This may be due to the high relative starting dose of 2 mg/kg compared to some other studies with monoclonal antibodies. Following administration of 24 mg/kg per week dose, mean serum U3–1565 concentrations were maintained above 289 μg/mL, the U3–1565 concentration necessary to inhibit xenograft growth by 90% in mice (unpublished data). Five of 7 subjects who derived clinical benefit (PR and SD) received 24 mg/kg of U3–1565.
Both in vitro cell line studies and in vivo studies using mouse xenograft models have demonstrated that HB-EGF functions as a potent inducer of tumor growth and angiogenesis [25]. HB-EGF binds to EGF receptors inducing downstream activation of the MAPK and PI3K signaling pathways, which result in VEGF-A production, driving angiogenesis [17, 18]. Efficacy and PD results from the present study are in line with these observations.
U3–1565 demonstrated both proof of mechanism and clinical activity across different tumor types including colorectal, non-small cell lung, and ovarian cancer. Plausibly, there appeared to be a connection between clinical benefit from U3–1565 and reduction in VEGF-A. Subjects with high baseline VEGF-A levels appeared more likely to show disease control, complete the main phase of the study and enter the extension phase, and experience a reduction in VEGF-A levels in response to U3–1565. Three of the 4 subjects who entered the extension phase (2 SD and the PR) also experienced reductions in VEGF-A. Follow up studies will be needed to determine whether baseline VEGF-A levels may be a useful biomarker to select patients more likely to derive benefit from HB-EGF blockade.
In conclusion, the present first-in-human study shows that U3–1565 has a favorable safety profile with low potential for immunogenicity and displays preliminary evidence of clinical efficacy across different tumor types. Results from this study support continued exploration of HB-EGF as a potential clinical target.
Funding
The study described in this article was sponsored by Daiichi Sankyo.
Footnotes
Conflict of interest Kathleen N. Moore, Johanna C. Bendell, Patricia M. LoRusso, and Anthony J. Olszanski received funding from Daiichi Sankyo for the conduct of this this study. Esther Zwick-Wallasch and Mendel Jansen were employees of Daiichi Sankyo or its subsidiaries during the course of the study. Alexander G. Vandell and Giorgio Senaldi are current employees of Daiichi Sankyo.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M (1991) A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science 251(4996):936–939 [DOI] [PubMed] [Google Scholar]
- 2.Elenius K, Paul S, Allison G, Sun J, Klagsbrun M (1997) Activation of HER4 by heparin-binding EGF-like growth factor stimulates chemotaxis but not proliferation. EMBO J 16(6):1268–1278. 10.1093/emboj/16.6.1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yarden Y, Sliwkowski MX (2001) Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2(2):127–137. 10.1038/35052073 [DOI] [PubMed] [Google Scholar]
- 4.Lurje G, Lenz HJ (2009) EGFR signaling and drug discovery. Oncology 77(6):400–410. 10.1159/000279388 [DOI] [PubMed] [Google Scholar]
- 5.Salomon DS, Brandt R, Ciardiello F, Normanno N (1995) Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol 19(3):183–232 [DOI] [PubMed] [Google Scholar]
- 6.Moulder SL, Yakes FM, Muthuswamy SK, Bianco R, Simpson JF, Arteaga CL (2001) Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Res 61(24):8887–8895 [PubMed] [Google Scholar]
- 7.Pollack VA, Savage DM, Baker DA, Tsaparikos KE, Sloan DE, Moyer JD, Barbacci EG, Pustilnik LR, Smolarek TA, Davis JA, Vaidya MP, Arnold LD, Doty JL, Iwata KK, Morin MJ (1999) Inhibition of epidermal growth factor receptor-associated tyrosine phosphorylation in human carcinomas with CP-358,774: dynamics of receptor inhibition in situ and antitumor effects in athymic mice. J Pharmacol Exp Ther 291(2):739–748 [PubMed] [Google Scholar]
- 8.Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM (2005) Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 7(4):301–311. 10.1016/j.ccr.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 9.Foon KA, Yang XD, Weiner LM, Belldegrun AS, Figlin RA, Crawford J, Rowinsky EK, Dutcher JP, Vogelzang NJ, Gollub J, Thompson JA, Schwartz G, Bukowski RM, Roskos LK, Schwab GM (2004) Preclinical and clinical evaluations of ABX-EGF, a fully human anti-epidermal growth factor receptor antibody. Int J Radiat Oncol Biol Phys 58(3):984–990. 10.1016/j.ijrobp.2003.09.098 [DOI] [PubMed] [Google Scholar]
- 10.Miyata K, Yotsumoto F, Fukagawa S, Kiyoshima C, Ouk NS, Urushiyama D, Ito T, Katsuda T, Kurakazu M, Araki R, Sanui A, Miyahara D, Murata M, Shirota K, Yagi H, Takono T, Kato K, Yaegashi N, Akazawa K, Kuroki M, Yasunaga S, Miyamoto S (2017) Serum heparin-binding epidermal growth factor-like growth factor (HB-EGF) as a biomarker for primary ovarian Cancer. Anticancer Res 37(7):3955–3960. 10.21873/anticanres.11779 [DOI] [PubMed] [Google Scholar]
- 11.Murayama Y, Miyagawa J, Shinomura Y, Kanayama S, Isozaki K, Yamamori K, Mizuno H, Ishiguro S, Kiyohara T, Miyazaki Y, Taniguchi N, Higashiyama S, Matsuzawa Y (2002) Significance of the association between heparin-binding epidermal growth factor-like growth factor and CD9 in human gastric cancer. Int J Cancer 98(4):505–513 [DOI] [PubMed] [Google Scholar]
- 12.Ito Y, Takeda T, Higashiyama S, Noguchi S, Matsuura N (2001) Expression of heparin-binding epidermal growth factor-like growth factor in breast carcinoma. Breast Cancer Res Treat 67(1):81–85 [DOI] [PubMed] [Google Scholar]
- 13.Mishima K, Higashiyama S, Asai A, Yamaoka K, Nagashima Y, Taniguchi N, Kitanaka C, Kirino T, Kuchino Y (1998) Heparin-binding epidermal growth factor-like growth factor stimulates mitogenic signaling and is highly expressed in human malignant gliomas. Acta Neuropathol 96(4):322–328 [DOI] [PubMed] [Google Scholar]
- 14.Yotsumoto F, Yagi H, Suzuki SO, Oki E, Tsujioka H, Hachisuga T, Sonoda K, Kawarabayashi T, Mekada E, Miyamoto S (2008) Validation of HB-EGF and amphiregulin as targets for human cancer therapy. Biochem Biophys Res Commun 365(3):555–561. 10.1016/j.bbrc.2007.11.015 [DOI] [PubMed] [Google Scholar]
- 15.Wang F, Liu R, Lee SW, Sloss CM, Couget J, Cusack JC (2007) Heparin-binding EGF-like growth factor is an early response gene to chemotherapy and contributes to chemotherapy resistance. Oncogene 26(14):2006–2016. 10.1038/sj.onc.1209999 [DOI] [PubMed] [Google Scholar]
- 16.Pfeil I, Siepen Pad, Wagner T, Schramm J, Yvonne Riffner Y, Hettman T, Zwick-Wallasch E (2012) U3–1565, a fully human anti-HB-EGF monoclonal antibody, inhibits oncogenic signaling and tumor cell growth in vitro and in vivo. AACR 103rd Annual Meeting 2012, March 31-April 4, 2012; Chicago [Google Scholar]
- 17.Nakai K, Yoneda K, Moriue T, Igarashi J, Kosaka H, Kubota Y (2009) HB-EGF-induced VEGF production and eNOS activation depend on both PI3 kinase and MAP kinase in HaCaT cells. J Dermatol Sci 55(3):170–178. 10.1016/j.jdermsci.2009.06.002 [DOI] [PubMed] [Google Scholar]
- 18.Mehta VB, Besner GE (2007) HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways. Growth Factors 25(4):253–263. 10.1080/08977190701773070 [DOI] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 20.Smith BP, Vandenhende FR, DeSante KA, Farid NA, Welch PA, Callaghan JT, Forgue ST (2000) Confidence interval criteria for assessment of dose proportionality. Pharm Res 17(10):1278–1283 [DOI] [PubMed] [Google Scholar]
- 21.Kut C, Mac Gabhann F, Popel AS (2007) Where is VEGF in the body? A meta-analysis of VEGF distribution in cancer. Br J Cancer 97(7):978–985. 10.1038/sj.bjc.6603923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarantopoulos J, Mita MM, Birrer MJ, Cranmer LD, Campos LT, Zhang X, Bristow P, Kaito H, Strout V, Camacho LH (2016) Phase 1 study of Monotherapy with KHK2866, an anti-heparin-binding epidermal growth factor-like growth factor monoclonal antibody, in patients with advanced Cancer. Target Oncol 11(3):317–327. 10.1007/s11523-015-0394-5 [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto S, Iwamoto R, Furuya A, Takahashi K, Sasaki Y, Ando H, Yotsumoto F, Yoneda T, Hamaoka M, Yagi H, Murakami T, Hori S, Shitara K, Mekada E (2011) A novel anti-human HB-EGF monoclonal antibody with multiple antitumor mechanisms against ovarian cancer cells. Clin Cancer Res 17(21):6733–6741. 10.1158/1078-0432.CCR-11-1029 [DOI] [PubMed] [Google Scholar]
- 24.Kasai N, Yoshikawa Y, Enokizono J (2014) Effect of antigen-dependent clearance on pharmacokinetics of anti-heparin-binding EGF-like growth factor (HB-EGF) monoclonal antibody. MAbs 6(5):1220–1228. 10.4161/mabs.29792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ongusaha PP, Kwak JC, Zwible AJ, Macip S, Higashiyama S, Taniguchi N, Fang L, Lee SW (2004) HB-EGF is a potent inducer of tumor growth and angiogenesis. Cancer Res 64(15):5283–5290. 10.1158/0008-5472.CAN-04-0925 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data discussed in this manuscript are proprietary and has not been made available publically.