Abstract
The glycolytic phenotype of the Warburg effect is associated with acidification of the tumor microenvironment. In this review, we describe how acidification of the tumor microenvironment may increase the invasive and degradative phenotype of cancer cells. As a template of an extracellular acidic microenvironment that is linked to proteolysis, we use the resorptive pit formed between osteoclasts and bone. We describe similar changes that have been observed in cancer cells in response to an acidic microenvironment and that are associated with proteolysis and invasive and metastatic phenotypes. This includes consideration of changes observed in the intracellular trafficking of vesicles, i.e., lysosomes and exosomes, and in specialized regions of the membrane, i.e., invadopodia and caveolae. Cancer-associated cells are known to affect what is generally referred to as tumor proteolysis but little direct evidence for this being regulated by acidosis; we describe potential links that should be verified.
Keywords: Acidosis, Lysosomes, Exosomes, Invadopodia, Caveolae
1. Introduction
Hanahan and Weinberg [1] defined six common hallmarks underlying cancer development with an emphasis on genetic changes in the cancer cells. This is consistent with treatments for cancer primarily having targeted the cancer cells as if they existed and flourished independently of their surrounding microenvironment. Hanahan and Weinberg do however recognize that cancers are tissues that are comprised of cancer cells as well as normal cells that are recruited to the tumor microenvironment. They also propose that understanding the positive and negative interactions between cancer cells and normal cells in the tumor microenvironment might identify new and efficacious approaches for cancer therapies. Cancer tissues are complex and include, in addition to cancer cells and normal stromal fibroblasts and inflammatory cells that have infiltrated into the cancer, non-cellular components such as extracellular matrices, pathochemical entities such as hypoxia and acidosis, and a secretome containing signaling molecules and extracellular vesicles. The theory that the microenvironment or soil surrounding cancers is critical to the growth of cancer metastases dates back to Paget’s observations published in 1889 on breast cancer [2].
The microenvironment has been proposed to contribute to the six hallmarks of cancer defined by Hanahan and Weinberg [1] as well as the additional two emerging hallmarks and two enabling characteristics they defined in an updated review [3]. Indeed, the latter review [3] focused on the tumor microenvironment in regard to how interactions between normal cells associated with cancers and the cancer cells can exert either positive or negative influences on cancer development. A variety of reviews have postulated that various cellular and non-cellular components of the tumor microenvironment underpin cancer development, including stromal cells [4, 5], extracellular matrix [6], extracellular vesicles [7, 8], cancer metabolism [9], pH dynamics [10], hypoxia [11], and cancer-related inflammation/inflammatory cells [5, 12]. Among these, the reprogramming of energy metabolism was defined as an emerging hallmark of cancer in the updated Hanahan and Weinberg review [3]. The original findings of changes in energy metabolism were made in the 1920s by Warburg [13], who reported that tumors exhibit a high rate of fermentative glycolysis even in the presence of adequate oxygen. This switch of cancer cells to glycolysis occurs in parallel with intracellular alkalosis and extracellular acidosis [10, 14], with the acidification of the tumor microenvironment further enhancing metabolic reprogramming [15]. Gatenby, Gillies, and colleagues hypothesize that acidification of the tumor microenvironment by cancer cells is a niche engineering strategy that enhances the ability of the cancer cells to outcompete normal cells and thus invade and proliferate [16, 17]. Indeed, dysregulation of pH in cancers has been described as a “perfect storm” that promotes proliferation, evasion of apoptosis, metabolic adaptation, migration, and invasion [18]. In this chapter, we will discuss mechanisms by which acidification of the tumor microenvironment may increase the invasive and degradative phenotype of cancer cells.
2. Acidosis and extracellular proteolysis by non-cancer cells
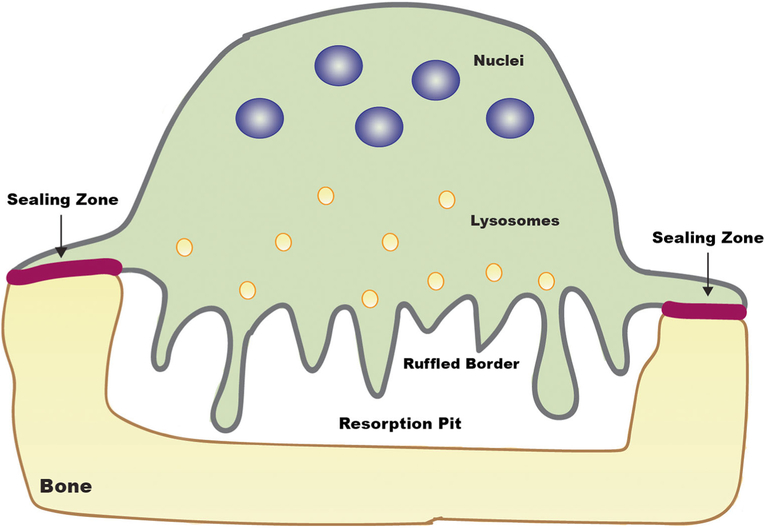
Acidosis is most dramatically linked to extracellular proteolysis in the normal biological process of bone resorption by osteoclasts [19] (Fig. 1). A sealing zone between the osteoclast and bone is formed by a peripheral belt of podosomes, an actin-rich protrusion of the plasma membrane associated with adhesion proteins [20, 21]. Inside this peripheral belt, the osteoclast plasma membrane becomes ruffled. This is associated with the transport of lysosomes to the membrane; incorporation of vacuolar H+-ATPases, i.e., proton pumps, into the membrane; and secretion of the lysosomal cysteine proteinase, cathepsin K. The specific form of vacuolar H+-ATPase that moves to the ruffled membrane during osteoclast differentiation is one that is present in the lysosomal membrane, i.e., the a3 isoform [22]. Acidification of the underlying resorptive pit occurs as a result of generation of H+ ions by carbonic anhydrase II, also present in the ruffled membrane, and their secretion into the pit via vacuolar H+-ATPases. Thus, the resorptive pit is essentially an extracellular lysosome. Bone resorption can be abrogated by interfering with acidification through deletion of carbonic anhydrase II or the vacuolar H+-ATPase or interfering with proteolysis through deletion of cathepsin K [19]. The complex resorptive pits formed by osteoclasts degrading bone, a difficult substrate, are not observed in other cell types in which punctate proteolysis occurs in association with podosomes: e.g., endothelial cells, vascular smooth muscle cells, macrophages, and dendritic cells. There is an ongoing debate on whether podosomes and their associated degradation of matrix in normal cells are distinct from invadopodia and their associated degradation of matrix observed in cancer cells (see [21] for further discussion).
Fig. 1.
Cartoon illustrating acid-mediated extracellular proteolysis of bone by osteoclasts. A resorption pit comparable to an extracellular lysosome is created between the ruffled border of a multi-nucleated osteoclast and underlying bone. Sealing zones formed by podosome belts isolate the resorption pit. Lysosomes move toward the ruffled border and fuse with the membrane resulting in release of the cysteine protease cathepsin K into the resorption pit and incorporation of the vacuolar H+ ATPase in the lysosomal membrane into the ruffled border membrane. Acidification of the resorptive pit occurs as a result of generation of H+ ions by carbonic anhydrase II, also present in the ruffled border, and secretion of H+ ions into the pit by the vacuolar H+-ATPase. The organic matrix of the bone is degraded by the secreted cathepsin K
3. Acidosis and extracellular proteolysis by cancer cells
Proteolysis and in particular extracellular proteolysis have been linked to cancer progression (for review, see [23]). There is an extensive literature on this topic with the vast majority of studies focusing on the matrix metalloproteases (MMPs). Solid cancers acidify their microenvironment to pH 6.4–7.0 (for review, see [15]). This is not as low as the pH 4.5 found in resorptive pits between osteoclasts and bone [19] or the pH between 4 and 5 found intracellularly in lysosomes [24]. Nonetheless, the acidosis surrounding cancers raises the possibility that proteases such as the lysosomal cysteine cathepsins, known to be secreted from cancers [25–27], might have a functional role in the tumor microenvironment. Acidosis increases degradation of the extracellular matrix surrounding cancer cells (for review, see [28]) as it does degradation of the organic matrix of bone (see Fig. 1). Studies in our laboratory on extracellular proteolysis and cancer progression have focused on the lysosomal cysteine proteinase cathepsin B, including how extracellular acidosis may affect the functions of cathepsin B in cancer [25–27, 29, 30]. We will concentrate on this enzyme in order to illustrate the effects of acidosis on proteolysis in the tumor microenvironment. We do, however, want to emphasize that we do not mean to indicate that cathepsin B acts alone; rather, it is one of many proteases interacting within a network of proteases [27, 31].
3.1. Acidosis and trafficking of lysosomes
Heuser [32] demonstrated that incubating macrophages and fibroblasts at a slightly acidic pH, i.e., pH 6.5, results in a redistribution of lysosomes from the region of the microtubule-organizing center to the cell periphery. We have shown that, in a wide variety of metastatic cancer cell lines, cathepsin B is distributed in both plasma membrane and lysosomal fractions; punctate immunostaining for cathepsin B is present peripherally and active cathepsin B is secreted (for review, see [25]). Others have also observed membrane-association of cathepsin B, e.g., Kobayashi et al. [33], in ovarian cancer cells in which they linked membrane association of cathepsin B to activation of a receptor-bound form of the serine protease, pro-urokinase plasminogen activator, and thereby activation of plasminogen to plasmin. Kobayashi et al. [33] propose that a membrane cathepsin B–initiated proteolytic network is responsible for the ability of ovarian tumor cells to degrade and invade through extracellular matrices. The redistribution of active cathepsin B to the surface of cancer cells is a phenomenon detected in many animal and human cancers. A redistribution of lysosomes to the cell periphery concomitant with membrane association of lysosomal proteases and their secretion has been seen in other cells that participate in diverse invasive processes, e.g., trypanosome invasion of epithelial cells [34], the inflammatory responses of macrophages in emphysema (for review, see [35]), elastinolysis of the arterial wall in atherosclerosis and aortic aneurysms (for review, see [35]), and bone degradation by osteoclasts (Fig. 1).
Translocation of lysosomes to the cell periphery as observed in cancer cells (Fig. 2) might be presumed to result in the release of all lysosomal enzymes; however, the lysosomal proteases found to play causal roles in extracellular proteolysis vary. This may reflect a heterogeneity in protease content of the lysosomes themselves; i.e., differential expression of specific lysosomal proteases in cancer cells of different origins or distinct substrate specificities of the secreted proteases. The latter would appear to be the case in osteoclasts as cathepsin K, unlike other lysosomal proteases, is able to degrade bone. Wound-induced repair of the plasma membrane is another process in which there is redistribution of lysosomes and release of lysosomal proteases [36]. In this case, the lysosomal proteases have been shown to have discrete functions. The wounding results in calcium influx and calcium-regulated exocytosis of the lysosomes. A rapid release of the cysteine cathepsins B and L, which are required for membrane repair, occurs and this is followed somewhat later by the release of the aspartic protease cathepsin D, which downregulates repair. This may indicate sequential exocytosis of two populations of lysosomes that differ in their protease content.
Fig. 2.
Cartoon illustrating acidosis-induced changes in trafficking of lysosomes and exosomes in cancer cells and in invadopodial and caveolar membrane structures associated with extracellular proteolysis in cancer cells. The invadopodium illustrated here is similar to the podosomal structures formed in normal cells such as osteoclasts (see Fig. 1) and associated with degradation of extracellular matrices. As in the osteoclast, lysosomes move into the invadopodium and fuse with the membrane resulting in the release of lysosomal proteases and incorporation of the vacuolar H+ ATPase in the lysosomal membrane into the invadopodial membrane. Similarly, secretion of exosomes occurs due to movement of multivesicular bodies (MVBs) into the invadopodium where they fuse with the membrane. This along with the plasma membrane sodium-hydrogen exchanger NHE1 in the invadopodial membrane results in local acidification and matrix degradation. Acidosis also increases secretion of lysosomes and exosomes from cancer cells in membrane regions other than invadopodia. Another membrane structure associated with acidosis and proteolysis is caveolae, which are dynamic membrane structures that transition in response to membrane stressors between flask-shaped invaginations as shown here and flat membranes. The invaginations are formed by oligomers of the major structural protein caveolin-1 (purple). Receptors, including those for proteases, clustered in caveolae facilitate signaling and proteolytic pathways at the surface of cancer cells. NHE1 and NaV1.5 sodium channels also are present in caveolae, leading to increased local acidification
We have shown that extracellular acidification results in human breast cancer cells of redistribution to the cell periphery of lysosomes that differ in their protease content. One population immunostains only for cathepsin B, a second only for cathepsin D, and a third for both proteases [37]. We confirmed by electron microscopy the peripheral redistribution of the three populations of lysosomes and surface labeling for both cathepsins B and D. Our results are consistent with those of Glunde et al. [38] who observed that acidification induced dramatic changes in lysosomal trafficking. In their studies, they followed the redistribution of lysosomes by staining for the lysosome membrane proteins LAMP1 and 2 in fixed breast cells or labeling with a dansylated glucosamine in living breast cells. In acid-adapted breast cancer cells, Damaghi et al. [39] found increased expression of lysosomal proteins including LAMP2, as well as redistribution of LAMP2 to the plasma membrane and secretion of cathepsin B. The mechanism for increased expression is not known but the two latter results would be consistent with exocytosis of lysosomes, fusion of lysosomal and plasma membranes, and release of soluble lysosomal enzymes. Membrane staining for LAMP2 associated with acid adaptation was also observed in patient samples and has been confirmed by 4 other labs [39–42]. In the lysosome, LAMP2 protects the membranes from degradation by the lysosomal proteases. Damaghi et al. propose that redistribution of LAMP2 to the plasma membrane of cancer cells serves a similar protective mechanism to ensure survival in an acidic microenvironment. Trafficking of lysosomes to the cell periphery has also been demonstrated in prostate cancer cells. In this case, anterograde trafficking of the lysosomes occurs in conjunction with induction of invasion by either epidermal [43] or hepatocyte [44] growth factors and extracellular acidification generated by Na+/H+ exchanger (NHE) activity. This is observed in cells grown in either 2D or more pathophysiologically relevant 3D cultures and is associated with the formation of cellular protrusions and secretion of cathepsin B.
Association of cathepsin B with the membrane of cancer cells has been shown to be causal in cancer progression and metastasis. Knockout of cathepsin B in mammary cancer cells driven by the murine polyoma middle T antigen results in compensatory upregulation of another cysteine cathepsin, i.e., cathepsin X/Z, on the membranes of the mammary cancer cells [45]. Unlike the other cysteine cathepsins, cathepsins B and X/Z have carboxypeptidase activity so these results suggest a critical function for exopeptidase cleavage on the cancer cell membrane. Further studies using the polyoma middle T model to determine the effects of single or double knockout of the two cysteine cathepsins have shown that cathepsin B can compensate for the loss of cathepsin X/Z, which is exclusively a carboxypeptidase [46]. In contrast, cathepsin X/Z cannot entirely compensate for the loss of cathepsin B, which has both carboxypeptidase and endopeptidase activities. Thus, the roles played by cathepsin B in cancer progression and metastasis seem to require both types of proteolytic activity.
3.2. Acidosis and invadopodia
Increases in a variety of membrane protrusions are induced by acidosis. Bhujwalla and colleagues [38] reported an increase in membrane protrusions (defined as filopodia) in highly metastatic human breast cancer cells maintained in 2D culture at an acidic extracellular pH. They speculated that exocytosis of lysosomal proteases occurs in the filopodia. They did not examine the breast cancer cells for invadopodia, ventral protrusions originally observed in 2D culture and associated with focal proteolysis of extracellular matrices (Fig. 2). Gould and Courtneidge [47] in a detailed review describe how acidosis, as well as various other aspects of the tumor microenvironment, affects the formation of invadopodia as well as functions of the invadopodia. McNiven [48] proposed that filopodia and invadopodia are invasive cell structures that are part of a “dynamic and distinct, but remarkably related” group that also includes lamellipodia, focal adhesions, and podosomes. He hypothesizes that all of these structures form a dynamic and multi-purpose invadosome that degrades the extracellular matrix. Others [49, 50], however, restrict the term “invadosome” to designate podosomes in normal cells and “invadopodia” to cancer cells. There is not yet consensus on either terminology or the in vivo equivalent of podosomal and invadopodial structures observed in 2D cultures.
Three classes of proteases have been localized to invadopodia: MMPs, serine proteases, and cysteine proteases. In v-Src-transformed fibroblasts, Tu et al. [51] have observed trafficking of lysosomes to invadopodia and secretion of the cysteine protease cathepsin B in parallel with increases in degradation of extracellular matrices. Kryczka et al. [52] have made similar observations in colon cancer cells engineered to overexpress Snail, an inducer of the epithelial to mesenchymal transition. In their studies, they found increases in expression and activity of cathepsin B in invadosomes and they propose that cathepsin B participates in invasion of the colon cancer cells by activating zymogens of MMPs. The pH optima for the two proteolytic activities of cathepsin B are acidic with that for the carboxydipeptidase activity more acidic than that for the endopeptidase activity [53]. The elegant studies by Busco et al. [54] show that the peri-invadopodial space of breast cancer cells is acidified by NHE1, a Na+/H+ exchanger isoform, which would be consistent with a local environment that enhances cathepsin B activity. Indeed, we have demonstrated in the same breast cancer cell line, i.e., MDA-MB-231, that an acidic pericellular environment dramatically increases the ability of cathepsin B to degrade the basement membrane protein type IV collagen [55]. Busco et al. [54] found that epidermal growth factor increases NHE1-dependent acidification and proteolysis in the MDA-MB-231 breast cancer cells, a finding similar to the observations by Cardelli and colleagues in prostate cancer cells although not linked to invadopodia in those cells [43]. Further analyses of invadopodial proteolysis in the MDA-MB-231 breast cancer cells [56] have established a close physical and functional association of NHE1 with cathepsin B and two MMPs, MMP-2 and MMP-9; NHE1-induced acidification and secretion of the three proteases; and acidification-enhanced proteolysis by all three proteases. Thus, acidosis has a direct effect on invadopodia-associated proteolysis in that it increases protease activity and an indirect effect in that it induces secretion of proteases.
3.3. Acidosis and trafficking of exosomes/microvesicles
Cancers have been reported for > 40 years to shed membrane vesicles both in vitro and in vivo [57–59]. The vesicles identified in those early studies had both platelet-aggregating [57] and procoagulant [58, 59] activities, the former shown to be induced by cathepsin B [60]. Lyden and colleagues have published a comprehensive review on the extracellular vesicles, including exosomes, secreted by cancer cells (see [61]), which discusses the critical roles played by exosomes in communication among cancer cells as well as communication between cancer cells and cells that infiltrate into the tumor microenvironment.
Exosome trafficking in cancer cells is increased by acidosis [62] as is lysosome trafficking in cancer cells (see above). Secretion of exosomes and their uptake and transfer of exosomal cargo have been found to be increased in melanoma cells grown in an acidic microenvironment. This acidic tumor microenvironment also increases the stability of the secreted exosomes, facilitating transfer of cargo and cell:cell communication [63]. One intriguing exosome cargo that is transferred is caveolin-1, a protein that has been shown to be either a tumor suppressor or promoter; most literature supports the concept that high expression of caveolin-1 in the stroma acts as a tumor suppressor (for review, see [64]). In contrast, in cancer cells, caveolin-1 expression is heterogeneous and has been demonstrated to function as either a tumor promoter or a tumor suppressor. This can depend on the type of cancer or the stage of an individual cancer. Studies in melanoma cells found that exosome uptake delivers caveolin-1 to less aggressive cells in concert with an increase in malignancy of these cells [65]. Caveolin-1 is thus acting as a tumor promoter in this context.
Exosomes have been implicated in intratumoral heterogeneity in part due to the transfer of metastatic molecules. In colon cancers, the uptake of exosomes from metastatic cells by non-metastatic cells results in a more aggressive phenotype, mediated by the serine protease thrombin [66]. Thrombin induces platelet aggregation, linking this recent observation on exosomes to the early reports on membrane vesicles shed from cancer cells. Acidosis is also linked to the transfer of metastatic molecules. Exosomes secreted by melanoma cells exposed to an acidic microenvironment increase migration and invasion of melanoma cells that were not exposed to an acidic microenvironment [67].
3.4. Acidosis and caveolae
Caveolae, originally observed in 1953 by George Palade in endothelial cells using electron microscopy [68], are flask-shaped invaginations that line the plasma membrane of most cells (for review, see [69]). Their structure is maintained by two main proteins, caveolin and cavins, which form a coat around the invaginated membrane. The literature on caveolae as well as that on caveolin-1, the main structural component of caveolae in cells other than skeletal muscle, is contradictory. Caveolae are often defined as specialized lipid rafts; however, Nichols [69] contends in recent reviews that caveolae are “likely to be entirely distinct from rafts” and rather than static invaginations of the membrane, they are dynamic structures that protect cells from mechanical stresses. Despite being described > 60 years ago, the functions of caveolae are still widely debated [70]. Roles in endocytosis have been postulated as have roles in signal transduction. The latter would be consistent with the reported presence of a variety of growth factor receptors in caveolae.
Receptors for proteolytic networks also co-sediment in caveolar fractions, suggesting that caveolae may function to localize proteolytic activity to specific regions on the cell surface. These receptors include plasminogen receptors such as S100A10 of the annexin II heterotetramer [71–73] and enolase-1 [74], plasminogen activator receptors [75–77], and the cathepsin B binding protein S100A10 [78]. Plasminogen and plasminogen activators are part of a cell surface proteolytic network initiated by cathepsin B that can lead to ovarian cancer cell invasion [33], activation of latent TGF-β by breast cells [79], and degradation of collagen IV and invasion by colon cancer cells [80]. In the latter study, downregulation of caveolin-1 reduced urokinase plasminogen activator, β1-integrin, cathepsin B, and S100A10 in caveolae as well as invasion and collagen IV degradation. Plasminogen activators activate plasminogen to plasmin, which in turn activates MMPs, growth factors, and cytokines and cleaves the transmembrane molecule CUB domain-containing protein 1 to induce outside-in signal transduction (for review, see [81]). This proteolytic network is linked to acidosis. Enolase-1, in addition to binding plasminogen, is a glycolytic enzyme associated with the Warburg effect [82], and urokinase plasminogen activator and its receptor have been shown to regulate aerobic glycolysis in melanoma cells in part through enolase-1 [83]. The Warburg effect in the melanoma cells involves a urokinase plasminogen activator receptor and epidermal growth factor receptor connection mediated by α5β1 integrin and leading to the activation of PI3K/AKT/mTOR signaling. A possible mechanism for acidification by caveolae and associated enhancement of proteolysis and invasion is the co-localization in caveolae of breast cancer cells of NHE1 and NaV1.5 sodium channels resulting in increased H+ efflux in parallel with increased invasiveness [84].
There is an emerging consensus that the function of caveolae is protective with caveolae being removed by endocytosis or disassembly and degradation in response to stresses on the plasma membrane [85]. Interestingly, caveolae were first observed to be dynamic rather than static structures > 40 years ago in a study by Dulhunty and Franzini-Armstrong [86] in skeletal muscle cells. Other functions for caveolae however are supported by literature demonstrating that caveolin-1 binds a variety of proteins, thus potentially localizing them to caveolae. This is controversial as some studies have shown that the putative binding site in the scaffolding domain of caveolin-1, which was identified in the purified protein, would not be accessible when caveolin-1 is integrated into the plasma membrane (for discussion, see [69, 70]). Nevertheless, downregulation of caveolin-1 does alter the localization of many of the proteins shown to be associated with caveolae, as we have shown for cathepsin B, which cosediments in caveolar fractions but does not bind to caveolin-1 [80]. Nwosu et al. [87] address some of the controversies surrounding caveolin-1 in cancer and propose roles in various types of cell metabolism. Many of the earlier studies on caveolae and caveolin-1 in cancer will need to be reassessed in light of the evidence for these structures serving as membrane sensors. In this regard, Shin et al. [88] have shown that membrane receptors localized to caveolae are protected from fluid shear stress, linking roles for caveolae as membrane protectors and sites of signal transduction.
4. Acidosis and extracellular proteolysis by cancer-associated cells
Stromal and inflammatory cells that infiltrate into cancers in vitro have long been known to contribute to what is designated as cancer proteolysis; e.g., stromal cells contribute matrix MMPs and inflammatory cells contribute cysteine cathepsins (for review, see [23, 89]). Acidification of the microenvironment surrounding cancers enhances proteolysis and invasion [28, 90]. There are, however, relatively few studies that have examined links between acidosis and proteolysis in cancer-associated cells. Dolo and colleagues [91] have studied microvesicles (not further defined) that are shed by ovarian cancer cells for their ability to increase the invasiveness of endothelial cells. MMP-2 and MMP-9 are found in the shed vesicles and, when the shed vesicles are exposed to an acidic pH, they induce endothelial cell invasion. This corresponds to an increase in activities of the two matrix metalloproteinases, which is mediated by cathepsin B in the shed vesicles. Note that, in these studies, the shed vesicles rather than the ovarian cancer cells were exposed to an acidic microenvironment. These studies are consistent with acidosis affecting proteolytic pathways in cancer-associated cells as well as those in cancer cells but are not definitive.
Cancer-associated fibroblasts (CAFs) are linked to a glycolytic phenotype that is induced by interactions with cancer cells; this has been termed the “reverse Warburg effect” by Lisanti and colleagues [92]. The CAFs in this case exhibit three characteristics: (1) increases in myofibroblast markers, (2) aerobic glycolysis with concomitant increases in glycolytic enzymes, and (3) loss of caveolin-1. This phenomenon has not been directly linked to changes in proteolysis. A “reverse Warburg effect” has been shown by Dhanasekaran and colleagues [93] to occur in response to interactions between ovarian cancer cells and fibroblasts. In this study, in which normal fibroblasts were incubated with media conditioned by ovarian cancer cells, induction of a glycolytic phenotype in the fibroblasts preceded induction of a CAF phenotype. This could be mimicked by lysophosphatidic acid, a bioactive phospholipid that has been implicated in cancer [94]. Lysophosphatidic acid has been shown to induce protease secretion and activation and invasion of cancer cells [95–97], providing a possible link between acidosis, extracellular proteolysis, and cancer-associated stromal cells.
5. Conclusions
Pericellular acidification in cancers has been termed a perfect storm that enhances processes integral to malignant progressions such as proliferation, invasion, and metastasis. These processes are mediated in part by proteolytic networks, yet few studies of proteolysis in cancer have examined how pericellular acidification might affect degradative phenotypes. There is a precedent for pericellular acidification increasing degradation, most notably in bone resorption by osteoclasts, which is associated with changes in trafficking of lysosomes and changes in degradation-associated structures in the plasma membrane of osteoclasts. Similar changes occur in cancer cells in response to pericellular acidification and, as in osteoclasts, result in increases in proteolysis. The changes in cancer cells include increases in exocytosis of lysosomes and secretion of lysosomal proteases, increases in the formation of invadopodia, and increases in the secretion of exosomes/microvesicles. There is also an association of caveolae with acidosis and proteolysis, but there are a number of controversies in the field of caveolae and caveolin-1 that need to be addressed to confirm an involvement in acidosis-associated proteolysis. Although many of the proteases linked to “tumor proteolysis” are derived from cells that have infiltrated into the cancers rather than the cancer cells themselves, there is not yet any direct evidence that acidosis affects extracellular proteolysis either by cancer-associated cells or resulting from interactions between cancer cells and cancer-associated cells.
Footnotes
Conflict of interest The authors declare that they have no competing interests.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hanahan D, & Weinberg RA (2000). The hallmarks of cancer. Cell, 100(1), 57–70. [DOI] [PubMed] [Google Scholar]
- 2.Paget S (1989). The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Reviews, 8(2), 98–101. [PubMed] [Google Scholar]
- 3.Hanahan D, & Weinberg RA (2011). Hallmarks of cancer: the next generation. Cell, 144(5), 646–674. 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Pietras K, & Ostman A (2010). Hallmarks of cancer: interactions with the tumor stroma. Experimental Cell Research, 316(8), 1324–1331. 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, & Coussens LM (2012). Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell, 21(3), 309–322. 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 6.Pickup MW, Mouw JK, & Weaver VM (2014). The extracellular matrix modulates the hallmarks of cancer. EMBO Reports, 15(12), 1243–1253. 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanada M, Bachmann MH, & Contag CH (2016). Signaling by extracellular vesicles advances cancer hallmarks. Trends Cancer, 2(2), 84–94. 10.1016/j.trecan.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Meehan K, & Vella LJ (2016). The contribution of tumour-derived exosomes to the hallmarks of cancer. Critical Reviews in Clinical Laboratory Sciences, 53(2), 121–131. 10.3109/10408363.2015.1092496. [DOI] [PubMed] [Google Scholar]
- 9.Pavlova NN, & Thompson CB (2016). The emerging hallmarks of cancer metabolism. Cell Metabolism, 23(1), 27–47. 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harguindey S, Orive G, Luis Pedraz J, Paradiso A, & Reshkin SJ (2005). The role of pH dynamics and the Na+/H+ antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin–one single nature. Biochimica et Biophysica Acta, 1756(1), 1–24. 10.1016/j.bbcan.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Ruan K, Song G, & Ouyang G (2009). Role of hypoxia in the hallmarks of human cancer. Journal of Cellular Biochemistry, 107(6), 1053–1062. 10.1002/jcb.22214. [DOI] [PubMed] [Google Scholar]
- 12.Colotta F, Allavena P, Sica A, Garlanda C, & Mantovani A (2009). Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis, 30(7), 1073–1081. 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 13.Warburg O (1925). The metabolism of carcinoma cells. Cancer Research, 9(1), 148–163. 10.1158/jcr.1925.148. [DOI] [Google Scholar]
- 14.White KA, Grillo-Hill BK, & Barber DL (2017). Cancer cell behaviors mediated by dysregulated pH dynamics at a glance. Journal of Cell Science, 130(4), 663–669. 10.1242/jcs.195297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peppicelli S, Andreucci E, Ruzzolini J, Margheri F, Laurenzana A, Bianchini F, & Calorini L (2017). Acidity of microenvironment as a further driver of tumor metabolic reprogramming. Journal of Clinical & Cellular Immunology, 8, 485 10.4172/2155-9899.1000485. [DOI] [Google Scholar]
- 16.Gatenby RA, Gawlinski ET, Gmitro AF, Kaylor B, & Gillies RJ (2006). Acid-mediated tumor invasion: a multidisciplinary study. Cancer Research, 66(10), 5216–5223. 10.1158/0008-5472.CAN-05-4193. [DOI] [PubMed] [Google Scholar]
- 17.Gillies RJ, & Gatenby RA (2015). Metabolism and its sequelae in cancer evolution and therapy. Cancer Journal, 21(2), 88–96. 10.1097/PPO.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webb BA, Chimenti M, Jacobson MP, & Barber DL (2011). Dysregulated pH: a perfect storm for cancer progression. Nature Reviews. Cancer, 11(9), 671–677. 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- 19.Teitelbaum SL (2000). Bone resorption by osteoclasts. Science, 289(5484), 1504–1508. [DOI] [PubMed] [Google Scholar]
- 20.Georgess D, Machuca-Gayet I, Blangy A, & Jurdic P (2014). Podosome organization drives osteoclast-mediated bone resorption. Cell Adhesion & Migration, 8(3), 191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy DA, & Courtneidge SA (2011). The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nature Reviews. Molecular Cell Biology, 12(7), 413–426. 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toyomura T, Murata Y, Yamamoto A, Oka T, Sun-Wada GH, Wada Y, & Futai M (2003). From lysosomes to the plasma membrane: localization of vacuolar-type H+ -ATPase with the a3 isoform during osteoclast differentiation. The Journal of Biological Chemistry, 278(24), 22023–22030. 10.1074/jbc.M302436200. [DOI] [PubMed] [Google Scholar]
- 23.Edwards D, Hoyer-Hansen G, Blasi F, & Sloane BF (2008). The cancer degradome: protease and cancer biology. New York: Springer. [Google Scholar]
- 24.DiCiccio JE, & Steinberg BE (2011). Lysosomal pH and analysis of the counter ion pathways that support acidification. The Journal of General Physiology, 137(4), 385–390. 10.1085/jgp.201110596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roshy S, Sloane BF, & Moin K (2003). Pericellular cathepsin B and malignant progression. Cancer Metastasis Reviews, 22(2–3), 271–286. [DOI] [PubMed] [Google Scholar]
- 26.Sloane BF, Yan S, Podgorski I, Linebaugh BE, Cher ML, Mai J, et al. (2005). Cathepsin B and tumor proteolysis: contribution of the tumor microenvironment. Seminars in Cancer Biology, 15(2), 149–157. 10.1016/j.semcancer.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Mohamed MM, & Sloane BF (2006). Cysteine cathepsins: multifunctional enzymes in cancer. Nature Reviews. Cancer, 6(10), 764–775. 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- 28.Corbet C, & Feron O (2017). Tumour acidosis: from the passenger to the driver’s seat. Nature Reviews. Cancer, 17(10), 577–593. 10.1038/nrc.2017.77. [DOI] [PubMed] [Google Scholar]
- 29.Podgorski I, & Sloane BF (2003). Cathepsin B and its role(s) in cancer progression. Biochemical Society Symposium, 70(70), 263–276. [DOI] [PubMed] [Google Scholar]
- 30.Aggarwal N, & Sloane BF (2014). Cathepsin B: multiple roles in cancer. Proteomics. Clinical Applications, 8(5–6), 427–437. 10.1002/prca.201300105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mason SD, & Joyce JA (2011). Proteolytic networks in cancer. Trends in Cell Biology, 21(4), 228–237. 10.1016/j.tcb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heuser J (1989). Changes in lysosome shape and distribution correlated with changes in cytoplasmic pH. The Journal of Cell Biology, 108(3), 855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi H, Moniwa N, Sugimura M, Shinohara H, Ohi H, & Terao T (1993). Effects of membrane-associated cathepsin B on the activation of receptor-bound prourokinase and subsequent invasion of reconstituted basement membranes. Biochimica et Biophysica Acta, 1178(1), 55–62. [DOI] [PubMed] [Google Scholar]
- 34.Andrade LO, & Andrews NW (2005). The Trypanosoma cruzi-host-cell interplay: location, invasion, retention. Nature Reviews. Microbiology, 3(10), 819–823. 10.1038/nrmicro1249. [DOI] [PubMed] [Google Scholar]
- 35.Chapman HA Jr., Munger JS, & Shi GP (1994). The role of thiol proteases in tissue injury and remodeling. American Journal of Respiratory and Critical Care Medicine, 150(6 Pt 2), S155–S159. 10.1164/ajrccm/150.6_Pt_2.S155. [DOI] [PubMed] [Google Scholar]
- 36.Castro-Gomes T, Corrotte M, Tam C, & Andrews NW (2016). Plasma membrane repair is regulated extracellularly by proteases released from lysosomes. PLoS One, 11(3), e0152583 10.1371/journal.pone.0152583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sameni M, Elliott E, Ziegler G, Fortgens PH, Dennison C, & Sloane BF (1995). Cathepsin B and D are localized at the surface of human breast cancer cells. Pathology Oncology Research, 1(1), 43–53. [DOI] [PubMed] [Google Scholar]
- 38.Glunde K, Guggino SE, Solaiyappan M, Pathak AP, Ichikawa Y, & Bhujwalla ZM (2003). Extracellular acidification alters lysosomal trafficking in human breast cancer cells. Neoplasia, 5(6), 533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Damaghi M, Tafreshi NK, Lloyd MC, Sprung R, Estrella V, Wojtkowiak JW, Morse DL, Koomen JM, Bui MM, Gatenby RA, & Gillies RJ (2015). Chronic acidosis in the tumour microenvironment selects for overexpression of LAMP2 in the plasma membrane. Nature Communications, 6, 8752 10.1038/ncomms9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dovmark TH, Saccomano M, Hulikova A, Alves F, & Swietach P (2017). Connexin-43 channels are a pathway for discharging lactate from glycolytic pancreatic ductal adenocarcinoma cells. Oncogene, 36, 4538–4550. 10.1038/onc.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bohn T, Rapp S, Luther N, Klein M, Bruehl TJ, Kojima N, Aranda Lopez P, Hahlbrock J, Muth S, Endo S, Pektor S, Brand A, Renner K, Popp V, Gerlach K, Vogel D, Lueckel C, Arnold-Schild D, Pouyssegur J, Kreutz M, Huber M, Koenig J, Weigmann B, Probst HC, von Stebut E, Becker C, Schild H, Schmitt E, & Bopp T (2018). Tumor immunoevasion via acidosis-dependent induction of regulatory tumor-associated macrophages. Nature Immunology, 19(12), 1319–1329. 10.1038/s41590-018-0226-8. [DOI] [PubMed] [Google Scholar]
- 42.Rohani N, Hao L, Alexis MS, Joughin BA, Krismer K, Moufarrej MN, Soltis AR, Lauffenburger DA, Yaffe MB, Burge CB, Bhatia SN, & Gertler FB (2019). Acidification of tumor at stromal boundaries drives transcriptome alterations associated with aggressive phenotypes. Cancer Research, 79, 1952–1966. 10.1158/0008-5472.CAN-18-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dykes SS, Steffan JJ, & Cardelli JA (2017). Lysosome trafficking is necessary for EGF-driven invasion and is regulated by p38 MAPK and Na+/H+ exchangers. BMC Cancer, 17(1), 672 10.1186/s12885-017-3660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steffan JJ, Williams BC, Welbourne T, & Cardelli JA (2010). HGF-induced invasion by prostate tumor cells requires anterograde lysosome trafficking and activity of Na+-H+ exchangers. Journal of Cell Science, 123(Pt 7, 1151–1159. 10.1242/jcs.063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vasiljeva O, Papazoglou A, Kruger A, Brodoefel H, Korovin M, Deussing J, et al. (2006). Tumor cell-derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Research, 66(10), 5242–5250. 10.1158/0008-5472.CAN-05-4463. [DOI] [PubMed] [Google Scholar]
- 46.Sevenich L, Schurigt U, Sachse K, Gajda M, Werner F, Muller S, Vasiljeva O, Schwinde A, Klemm N, Deussing J, Peters C, & Reinheckel T (2010). Synergistic antitumor effects of combined cathepsin B and cathepsin Z deficiencies on breast cancer progression and metastasis in mice. Proceedings of the National Academy of Sciences of the United States of America, 107(6), 2497–2502. 10.1073/pnas.0907240107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gould CM, & Courtneidge SA (2014). Regulation of invadopodia by the tumor microenvironment. Cell Adhesion & Migration, 8(3), 226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McNiven MA (2013). Breaking away: matrix remodeling from the leading edge. Trends in Cell Biology, 23(1), 16–21. 10.1016/j.tcb.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Martino J, Henriet E, Ezzoukhry Z, Goetz JG, Moreau V, & Saltel F (2016). The microenvironment controls invadosome plasticity. Journal of Cell Science, 129(9), 1759–1768. 10.1242/jcs.182329. [DOI] [PubMed] [Google Scholar]
- 50.Paterson EK, & Courtneidge SA (2018). Invadosomes are coming: new insights into function and disease relevance. The FEBS Journal, 285(1), 8–27. 10.1111/febs.14123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tu C, Ortega-Cava CF, Chen G, Fernandes ND, Cavallo-Medved D, Sloane BF, Band V, & Band H (2008). Lysosomal cathepsin B participates in the podosome-mediated extracellular matrix degradation and invasion via secreted lysosomes in v-Src fibroblasts. Cancer Research, 68(22), 9147–9156. 10.1158/0008-5472.CAN-07-5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kryczka J, Papiewska-Pajak I, Kowalska MA, & Boncela J (2019). Cathepsin B is upregulated and mediates ECM degradation in colon adenocarcinoma HT29 cells overexpressing snail. Cells, 8(3). 10.3390/cells8030203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stachowiak K, Tokmina M, Karpinska A, Sosnowska R, & Wiczk W (2004). Fluorogenic peptide substrates for carboxydipeptidase activity of cathepsin B. Acta Biochimica Polonica, 51(1), 81–92. [PubMed] [Google Scholar]
- 54.Busco G, Cardone RA, Greco MR, Bellizzi A, Colella M, Antelmi E, Mancini MT, Dell’Aquila ME, Casavola V, Paradiso A, & Reshkin SJ (2010). NHE1 promotes invadopodial ECM proteolysis through acidification of the peri-invadopodial space. The FASEB Journal, 24(10), 3903–3915. 10.1096/fj.09-149518. [DOI] [PubMed] [Google Scholar]
- 55.Rothberg JM, Bailey KM, Wojtkowiak JW, Ben-Nun Y, Bogyo M, Weber E, Moin K, Blum G, Mattingly RR, Gillies RJ, & Sloane BF (2013). Acid-mediated tumor proteolysis: contribution of cysteine cathepsins. Neoplasia, 15(10), 1125–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greco MR, Antelmi E, Busco G, Guerra L, Rubino R, Casavola V, et al. (2014). Protease activity at invadopodial focal digestive areas is dependent on NHE1-driven acidic pHe. Oncology Reports, 31(2), 940–946. 10.3892/or.2013.2923. [DOI] [PubMed] [Google Scholar]
- 57.Gasic GJ, Boettiger D, Catalfamo JL, Gasic TB, & Stewart GJ (1978). Aggregation of platelets and cell membrane vesiculation by rat cells transformed in vitro by Rous sarcoma virus. Cancer Research, 38(9), 2950–2955. [PubMed] [Google Scholar]
- 58.Dvorak HF, Quay SC, Orenstein NS, Dvorak AM, Hahn P, Bitzer AM, et al. (1981). Tumor shedding and coagulation. Science, 212(4497), 923–924. [DOI] [PubMed] [Google Scholar]
- 59.Dvorak HF, Van DeWater L, Bitzer AM, Dvorak AM, Anderson D, Harvey VS, et al. (1983). Procoagulant activity associated with plasma membrane vesicles shed by cultured tumor cells. Cancer Research, 43(9), 4434–4442. [PubMed] [Google Scholar]
- 60.Honn KV, Cavanaugh P, Evens C, Taylor JD, & Sloane BF (1982). Tumor cell-platelet aggregation: induced by cathepsin B-like proteinase and inhibited by prostacyclin. Science, 217(4559), 540–542. [DOI] [PubMed] [Google Scholar]
- 61.Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, & Lyden D (2016). Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell, 30(6), 836–848. 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, et al. (2009). Microenvironmental pH is a key factor for exosome traffic in tumor cells. The Journal of Biological Chemistry, 284(49), 34211–34222. 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ban JJ, Lee M, Im W, & Kim M (2015). Low pH increases the yield of exosome isolation. Biochemical and Biophysical Research Communications, 461(1), 76–79. 10.1016/j.bbrc.2015.03.172. [DOI] [PubMed] [Google Scholar]
- 64.Martinez-Outschoorn UE, Sotgia F, & Lisanti MP (2015). Caveolae and signalling in cancer. Nature Reviews. Cancer, 15(4), 225–237. 10.1038/nrc3915. [DOI] [PubMed] [Google Scholar]
- 65.Felicetti F, Parolini I, Bottero L, Fecchi K, Errico MC, Raggi C, Biffoni M, Spadaro F, Lisanti MP, Sargiacomo M, & Carè A (2009). Caveolin-1 tumor-promoting role in human melanoma. International Journal of Cancer, 125(7), 1514–1522. 10.1002/ijc.24451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schillaci O, Fontana S, Monteleone F, Taverna S, Di Bella MA, Di Vizio D, et al. (2017). Exosomes from metastatic cancer cells transfer amoeboid phenotype to non-metastatic cells and increase endothelial permeability: their emerging role in tumor heterogeneity. Scientific Reports, 7(1), 4711 10.1038/s41598-017-05002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boussadia Z, Lamberti J, Mattei F, Pizzi E, Puglisi R, Zanetti C, Pasquini L, Fratini F, Fantozzi L, Felicetti F, Fecchi K, Raggi C, Sanchez M, D’Atri S, Carè A, Sargiacomo M, & Parolini I (2018). Acidic microenvironment plays a key role in human melanoma progression through a sustained exosome mediated transfer of clinically relevant metastatic molecules. Journal of Experimental & Clinical Cancer Research, 37(1), 245 10.1186/s13046-018-0915-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palade GE (1953). Fine structure of blood capillaries. Journal of Applied Physics, 24, 1424. [Google Scholar]
- 69.Nichols B (2018). The mystery of caveolae. The Scientist, 42–47. [Google Scholar]
- 70.Cheng JPX, & Nichols BJ (2016). Caveolae: one function or many? Trends in Cell Biology, 26(3), 177–189. 10.1016/j.tcb.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 71.Cavallo-Medved D, Dosescu J, Linebaugh BE, Sameni M, Rudy D, & Sloane BF (2003). Mutant K-ras regulates cathepsin B localization on the surface of human colorectal carcinoma cells. Neoplasia, 5(6), 507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bydoun M, & Waisman DM (2014). On the contribution of S100A10 and annexin A2 to plasminogen activation and oncogenesis: an enduring ambiguity. Future Oncology, 10(15), 2469–2479. 10.2217/fon.14.163. [DOI] [PubMed] [Google Scholar]
- 73.Madureira PA, Bharadwaj AG, Bydoun M, Garant K, O’Connell P, Lee P, & Waisman DM (2016). Cell surface protease activation during RAS transformation: critical role of the plasminogen receptor, S100A10. Oncotarget, 7(30), 47720–47737. 10.18632/oncotarget.10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zakrzewicz D, Didiasova M, Zakrzewicz A, Hocke AC, Uhle F, Markart P, Preissner KT, & Wygrecka M (2014). The interaction of enolase-1 with caveolae-associated proteins regulates its subcellular localization. The Biochemical Journal, 460(2), 295–307. 10.1042/BJ20130945. [DOI] [PubMed] [Google Scholar]
- 75.Stahl A, & Mueller BM (1995). The urokinase-type plasminogen activator receptor, a GPI-linked protein, is localized in caveolae. The Journal of Cell Biology, 129(2), 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schwab W, Gavlik JM, Beichler T, Funk RH, Albrecht S, Magdolen V, et al. (2001). Expression of the urokinase-type plasminogen activator receptor in human articular chondrocytes: association with caveolin and beta 1-integrin. Histochemistry and Cell Biology, 115(4), 317–323. [DOI] [PubMed] [Google Scholar]
- 77.Kwon M, MacLeod TJ, Zhang Y, & Waisman DM (2005). S100A10, annexin A2, and annexin a2 heterotetramer as candidate plasminogen receptors. Frontiers in Bioscience, 10, 300–325. [DOI] [PubMed] [Google Scholar]
- 78.Mai J, Finley RL Jr., Waisman DM, & Sloane BF (2000). Human procathepsin B interacts with the annexin II tetramer on the surface of tumor cells. The Journal of Biological Chemistry, 275(17), 12806–12812. [DOI] [PubMed] [Google Scholar]
- 79.Guo M, Mathieu PA, Linebaugh B, Sloane BF, & Reiners JJ Jr. (2002). Phorbol ester activation of a proteolytic cascade capable of activating latent transforming growth factor-betaL a process initiated by the exocytosis of cathepsin B. The Journal of Biological Chemistry, 277(17), 14829–14837. 10.1074/jbc.M108180200. [DOI] [PubMed] [Google Scholar]
- 80.Cavallo-Medved D, Mai J, Dosescu J, Sameni M, & Sloane BF (2005). Caveolin-1 mediates the expression and localization of cathepsin B, pro-urokinase plasminogen activator and their cell-surface receptors in human colorectal carcinoma cells. Journal of Cell Science, 118(Pt 7), 1493–1503. 10.1242/jcs.02278. [DOI] [PubMed] [Google Scholar]
- 81.Deryugina EI, & Quigley JP (2012). Cell surface remodeling by plasmin: a new function for an old enzyme. Journal of Biomedicine & Biotechnology, 2012, 564259 10.1155/2012/564259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Capello M, Ferri-Borgogno S, Riganti C, Chattaragada MS, Principe M, Roux C, Zhou W, Petricoin EF, Cappello P, & Novelli F (2016). Targeting the Warburg effect in cancer cells through ENO1 knockdown rescues oxidative phosphorylation and induces growth arrest. Oncotarget, 7(5), 5598–5612. 10.18632/oncotarget.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Laurenzana A, Chilla A, Luciani C, Peppicelli S, Biagioni A, Bianchini F, et al. (2017). uPA/uPAR system activation drives a glycolytic phenotype in melanoma cells. International Journal of Cancer, 141(6), 1190–1200. 10.1002/ijc.30817. [DOI] [PubMed] [Google Scholar]
- 84.Brisson L, Gillet L, Calaghan S, Besson P, Le Guennec JY, Roger S, et al. (2011). Na(V)1.5 enhances breast cancer cell invasiveness by increasing NHE1-dependent H(+) efflux in caveolae. Oncogene, 30(17), 2070–2076. 10.1038/onc.2010.574. [DOI] [PubMed] [Google Scholar]
- 85.Parton RG, & del Pozo MA (2013). Caveolae as plasma membrane sensors, protectors and organizers. Nature Reviews. Molecular Cell Biology, 14(2), 98–112. 10.1038/nrm3512. [DOI] [PubMed] [Google Scholar]
- 86.Dulhunty AF, & Franzini-Armstrong C (1975). The relative contributions of the folds and caveolae to the surface membrane of frog skeletal muscle fibres at different sarcomere lengths. The Journal of Physiology, 250(3), 513–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nwosu ZC, Ebert MP, Dooley S, & Meyer C (2016). Caveolin-1 in the regulation of cell metabolism: a cancer perspective. Molecular Cancer, 15(1), 71 10.1186/s12943-016-0558-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shin H, Haga JH, Kosawada T, Kimura K, Li YS, Chien S, & Schmid-Schönbein GW (2019). Fine control of endothelial VEGFR-2 activation: caveolae as fluid shear stress shelters for membrane receptors. Biomechanics and Modeling in Mechanobiology, 18(1), 5–16. 10.1007/s10237-018-1063-2. [DOI] [PubMed] [Google Scholar]
- 89.Sloane BF, List K, Fingleton B, & Matrisian L (2013). Proteases: structure and function. New York: Springer. [Google Scholar]
- 90.Estrella V, Chen T, Lloyd M, Wojtkowiak J, Cornnell HH, Ibrahim-Hashim A, Bailey K, Balagurunathan Y, Rothberg JM, Sloane BF, Johnson J, Gatenby RA, & Gillies RJ (2013). Acidity generated by the tumor microenvironment drives local invasion. Cancer Research, 73(5), 1524–1535. 10.1158/0008-5472.CAN-12-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Giusti I, D’Ascenzo S, Millimaggi D, Taraboletti G, Carta G, Franceschini N, et al. (2008). Cathepsin B mediates the pH-dependent proinvasive activity of tumor-shed microvesicles. Neoplasia, 10(5), 481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, Martinez-Outschoorn UE, Sotgia F, & Lisanti MP (2009). The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle, 8(23), 3984–4001. 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 93.Radhakrishnan R, Ha JH, Jayaraman M, Liu J, Moxley KM, Isidoro C, Sood AK, Song YS, & Dhanasekaran DN (2019). Ovarian cancer cell-derived lysophosphatidic acid induces glycolytic shift and cancer-associated fibroblast-phenotype in normal and peritumoral fibroblasts. Cancer Letters, 442, 464–474. 10.1016/j.canlet.2018.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mills GB, & Moolenaar WH (2003). The emerging role of lysophosphatidic acid in cancer. Nature Reviews. Cancer, 3(8), 582–591. 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 95.Pustilnik TB, Estrella V, Wiener JR, Mao M, Eder A, Watt MA, et al. (1999). Lysophosphatidic acid induces urokinase secretion by ovarian cancer cells. Clinical Cancer Research, 5(11), 3704–3710. [PubMed] [Google Scholar]
- 96.Fishman DA, Liu Y, Ellerbroek SM, & Stack MS (2001). Lysophosphatidic acid promotes matrix metalloproteinase (MMP) activation and MMP-dependent invasion in ovarian cancer cells. Cancer Research, 61(7), 3194–3199. [PubMed] [Google Scholar]
- 97.Jeong KJ, Park SY, Cho KH, Sohn JS, Lee J, Kim YK, Kang J, Park CG, Han JW, & Lee HY (2012). The rho/ROCK pathway for lysophosphatidic acid-induced proteolytic enzyme expression and ovarian cancer cell invasion. Oncogene, 31(39), 4279–4289. 10.1038/onc.2011.595. [DOI] [PubMed] [Google Scholar]