Abstract
Biologic grafts used in hernia repair undergo rapid cellular infiltration and remodeling, but their premature degradation often results in hernia recurrence. We hypothesize that a temporary barrier that prevents infiltration of acute inflammatory cells into the graft during the initial 4 weeks of implantation could mitigate graft degradation. The purpose of this study is to design tyramine-substituted hyaluronan (THA) hydrogel coatings with tunable degradation properties, as a means to develop a resorbable barrier for human acellular dermis grafts (HADM). THA plugs prepared at different crosslinking densities, by varying crosslinking agent concentration (0.0001–0.0075% H2O2), demonstrated varying rates of in vitro degradation (25U/ml hyaluronidase, 48h). Based on these results, HADM grafts were coated with THA at three crosslinking densities (0.0001%, 0.00075%, 0.003% H2O2) and THA coating degradation was evaluated in vitro (25U/ml hyaluronidase, 48h) and in vivo (rat intraperitoneal implantation, 1–4 weeks). THA coatings degraded in vitro and in vivo, with the lowest crosslinking density (0.0001% H2O2) generally showing greater degradation as evidenced by significant decrease in coating cross-sectional area. However, all three coatings remained partially-degraded after 4 weeks of in vivo implantation. Alternate strategies to accelerate in vivo degradation of THA coatings are required to allow investigation of the study hypothesis.
Keywords: HADM, hyaluronan coating, hyaluronidase, biodegradation, hernia
Introduction
Ventral abdominal wall hernias occur in nearly one-third of the over two million patients undergoing laparotomies in the United States each year.1 Ventral hernia repairs are commonly performed using synthetic meshes and biologic grafts, but have demonstrated only limited success.2,3 Synthetic meshes have an increased risk of infection-related complications,4,5 and are generally not recommended when there is associated bacterial contamination or a high risk wound (Grade 2, 3 and 4 hernias).6,7 Biologic grafts, such as acellular dermis matrix (ADM), allow use in contaminated fields, have abdominal wall-like de novo mechanical properties,8,9 and are believed to foster a favorable host response and undergo a dynamic process of cell-mediated resorption and constructive remodeling.7–9
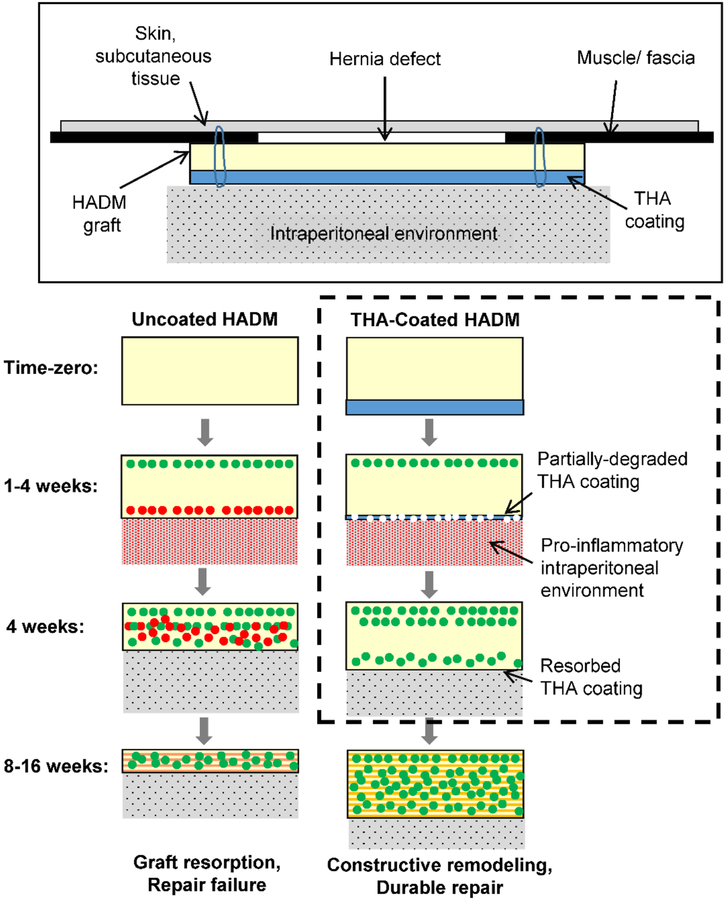
However, biologic grafts have poor long-term durability, which often results in the repair prematurely losing mechanical strength and integrity, and manifests as repair bulging, dehiscence and hernia recurrence in many patients.8,10–14 In general, for long-term durability, a biologic grafts must undergo constructive remodeling during which the rate of ECM deposition exceeds graft resorption, such that the graft always possesses sufficient strength and integrity during healing.15,16 Biologic grafts typically undergo rapid cellular infiltration17–19. The nature of cellular recruitment and infiltration into a biologic graft, in particular the initial mesothelial cell influx and downstream macrophage response, likely plays an important role in determining graft remodeling fate and repair outcomes.20–22 Mesothelial cells from the adjacent peritoneum and peritoneal space cover the intraperitoneally implanted biologic graft in 3–7 days.23 Adherent peritoneal tissues show peak levels of inflammation at 7 days,24 and the intraperitoneal environment remains pro-inflammatory and adhesiogenic for the first four weeks.25,26 While the local repair environment has been studied extensively in relation to synthetic hernia grafts, its influence on biologic grafts has not been adequately investigated.27–30 We envisage that transient inhibition of cellular infiltration from peritoneal tissues during the initial inflammatory phases of wound repair (1–4 weeks) may mitigate premature graft resorption, and subsequent cellular infiltration will allow constructive graft remodeling, leading to improved durability and ultimately repair outcomes (Figure 1).
Figure 1.
Schematic showing the hypothesized mechanism of resorption of HADM grafts in ventral hernia repair. We hypothesize that infiltration of cells (red dots) from the pro-inflammatory intraperitoneal environment during the first four postoperative weeks results in premature graft resorption and repair failure. A degradable THA coating on the graft would serve as a temporary barrier to inflammatory cells and mitigate premature graft resorption. Following degradation of the coating after 1–4 weeks, infiltration of pro-remodeling cells (green dots) would allow constructive graft remodeling leading to a durable repair. The objective of this study (represented by dashed box) was to develop biodegradable THA coatings for HADM grafts, with the goal of identifying THA coating formulations that largely resorb during 4 weeks after intraperitoneal implantation, in order to allow future investigations into relationships between cellular infiltration and biologic graft durability.
Hyaluronan (HA) is a naturally occurring glycosaminoglycan molecule in the ECM31. HA-based temporary barrier coatings have been used on synthetic hernia grafts to prevent the formation of peritoneal adhesions.32 We envisage that a HA-based coating on a biologic graft could act as a temporary barrier to cellular infiltration during the initial 4 weeks of implantation in order to mitigate premature graft resorption. Tyramine-substituted hyaluronan (THA) is a derivative of hyaluronan that can be cross-linked using hydrogen peroxide (H2O2, initiator) and horseradish peroxidase (HRP, catalyst)33. THA has been shown to be non-cytotoxic and biocompatible33, and influence host response when immobilized within a biologic graft.34 Further, the cross-linking density and degradation rate of THA hydrogels can be controlled by varying the H2O2 concentration.35,36 The objective of this study was to develop biodegradable THA hydrogel coatings for acellular dermis grafts, with the goal of identifying THA coating formulations that largely resorb during 4 weeks after intraperitoneal implantation, in order to allow future investigations into relationships between cellular infiltration and biologic graft durability.
Methods
THA hydrogel plugs were first prepared at varying crosslinking densities and their biodegradability was assessed in an in vitro enzyme degradation assay. Next, select THA hydrogel formulations were coated on the surface of human acellular dermis grafts (HADM) and their biodegradability was assessed in in vitro and in vivo assays. The study design is summarized in Table 1 and described in detail below.
Table 1.
Study design
| Group | Time-points | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. THA Plug - In vitro degradation | |||||||||
| 0h | 6h | 12h | 24h | 30h | 36h | 48h | |||
| 0.00010% | N=18 /group |
N=3 /group |
N=3 /group |
N=3 /group |
N=3 /group |
N=3 /group |
N=3 /group |
||
| 0.00075% | |||||||||
| 0.00100% | |||||||||
| 0.00300% | |||||||||
| 0.00450% | |||||||||
| 0.00750% | |||||||||
| 2. THA Coating | |||||||||
| • In vitro degradation | |||||||||
| 0h | 24h | 48h | |||||||
| 0.00010% | N=4 /group |
N=6 /group |
N=6 /group |
||||||
| 0.00075% | |||||||||
| 0.00300% | |||||||||
| • In vivo degradation | |||||||||
| 0 week | 1 week | 4 week | |||||||
| 0.00010% | N=3 /group |
N=3 /group |
N=3 /group |
||||||
| 0.00075% | |||||||||
| 0.00300% | |||||||||
THA plugs
Preparation of THA plugs
A 1% w/v solution of THA (5.5% substitution, 0.9–1 MDa MW, LifeCore Biomedical, Chaska, MN) was prepared in sterile phosphate buffered saline (PBS, 1X). After adding HRP (final concentration, 1 U/ml; Sigma-Aldrich, St. Louis, MO), 100 μl of the THA solution was cast into wells (7 mm diameter, 5mm depth) of a custom mold, and frozen at −20°C overnight. The frozen THA plugs were crosslinked in varying concentrations of H2O2 (0.0001%, 0.00075%, 0.001%, 0.003%, 0.0045%, and 0.0075% in PBS; n = 18/group) at room temperature (~25°C) for 30 minutes, and extruded into pre-weighed cell-culture inserts (pore size, 8um) for in vitro degradation tests. The range of H2O2 concentrations was chosen based on pilot studies that showed absence of gelation at H2O2 <0.0001% and no noticeable change in in vitro degradation profiles at H2O2 >0.0075%.
In vitro degradation of THA plugs
Cell-culture inserts containing the hydrogel plugs were rinsed in PBS for 30 minutes, blotted dry and weighed to determine their initial mass (n=18/group). The inserts were then placed in the wells of a 24-well plate containing 2.5ml of a digestion buffer (25 U/ml hyaluronidase in PBS), and incubated at 37°C. At various predetermined time-points over 48 hours, the inserts containing the hydrogel plugs (n=3/group/time, Table 1) were blotted dry and weighed. At each time-point, 500 μl of fresh digestion buffer was added to each well to maintain the buffer volume at ~2.5ml over 48 hours. The percentage of original hydrogel mass at each time point during the degradation period was calculated to determine the degradation rates of the different formulations.35,36
THA-coated HADM
Preparation of THA-coated HADM
Sterile acellular human dermis grafts (HADM, DermaMatrix™, 1.2–1.6 mm thickness, Musculoskeletal Transplant Foundation, NJ) were used. Circular pieces (1.5 cm diameter, for in vitro degradation studies) and square pieces (2×2 cm, for in vivo degradation studies) of HADM were cut out of larger grafts derived from a single donor. HADM samples were rinsed in sterile PBS overnight and again in fresh PBS for 30 minutes, and blotted dry with sterile gauze. Next, the epidermal surface of each graft was coated with 250μL (circular pieces) or 500μL (square pieces) of a 1% w/v THA solution containing 1U/ml HRP, by pipetting and spreading with a sterile flat spatula. After air-drying overnight in a biosafety cabinet, the coated HADM samples were suspended in varying concentrations of H2O2 (0.0001%, 0.00075%, 0.003% in sterile PBS) at room temperature (~25°C) for 30 minutes to crosslink the THA coating. Next, the coated HADM pieces were rinsed twice in PBS for 30 minutes each, and stored at 4°C until further use.
In vitro degradation of THA-coated HADM
Sterile 1.5 cm diameter circular pieces of THA-coated HADM were placed in the wells of a 24-well plate containing 2.5ml of a digestion buffer (25U/ml hyaluronidase in PBS), and incubated at 37°C for 24–48h. Samples were analyzed for THA coating cross-sectional area and thickness in histologic sections, and uronic acid content (a degradation component of HA) released into degradation buffer at 0h, 24h, and 48h of incubation (n=3–6/group/time, Table 1).
THA coating cross-sectional area and thickness:
At each time point, THA-coated HADM pieces were removed from digestion buffer, rinsed in PBS, bisected vertically, fixed in 10% neutral buffered formalin (NBF), and processed for routine paraffin embedding and histology. Five μm-thick sections were obtained at four 1mm step-levels spanning the entire width of each sample. After hematoxylin and eosin (H&E) and Alcian blue staining, the sections were scanned in their entirety at 20× magnification (Aperio AT2 scanner, Leica Microsystems, Wetzlar, Germany). The entire cross-sectional area of the THA coating was measured on each scanned section using Leica ImageScope (Version 12.1.0.5029, Aperio Technologies, Inc.) and ImageJ software, and averaged across the four step-level sections to represent the cross-sectional area of the THA coating for that sample. Additionally, in the time-zero samples, THA coating thickness was measured at five different locations on each section, and average measures across all four step-level sections of a sample were recorded.
Uronic acid release:
The amount of uronic acid released during incubation in digestion buffer was measured using a previously established carbazole reaction technique.37 Briefly, 50 μL of the digestion buffer (run in triplicates) from each sample at each time point was diluted 1:1 in 50 μl of distilled water, added to 750 μL of concentrated sulfuric acid/sodium tetraborate decahydrate solution (Sigma-Aldrich, St. Louis, MO), and heated to 100°C for 10 min. After adding 10 μL of 0.15% hydroxyphenyl reagent (m-phenylphenol (m-hydroxyphenol)) in 125 mM (0.5%) NaOH, 250 μL of the solution was transferred into wells (in duplicate) of a 96-well plate, and the absorbance at 530 nm was measured using a microplate reader (SpectraMax 250, Molecular Devices, San Jose, CA). Uronic acid concentration was determined from a standard curve using solutions with known concentrations of a 50 kDa HA standard, and digestion buffer with uncoated dermis (n=4/time point) was used as a baseline control. The amount of uronic acid released was calculated from the volume of the incubation buffer and the molecular weight (400 g/mol) of HA disaccharide units.
In vivo degradation of THA-coated HADM
Sterile 2×2 cm square pieces of THA-coated HADM (n=3/group/time, Table 1) were evaluated at 1 and 4 weeks after intraperitoneal implantation in 18 male Sprague Dawley rats (>450 g, Envigo, Indianapolis, IN) using a previously-described hernia repair model17. All procedures were performed in accordance with the National Institutes of Health (NIH) guidelines for care and use of laboratory animals and approved by the Cleveland Clinic Institutional Animal Care and Use Committee (IACUC, ARC 2016–1713). Briefly, each rat was anesthetized with an intraperitoneal injection of ketamine, xylazine, and acepromazine (30/6/1 mg/kg). A 1.2×1.2 cm full-thickness midline defect was created by resecting the rectus abdominis muscle and fascia. The defect was repaired using a 2×2 cm THA-coated HADM graft placed as an intraperitoneal underlay, with its THA coated surface facing the visceral peritoneum, and fixed using eight transfascial mattress sutures (5–0 Prolene). The skin was closed with interrupted subcuticular sutures (4–0 coated Vicryl).
Post-operatively, an E-collar was fitted to the rats to limit their access to the incision during the first week of the post-operative period. All animals were given buprenorphine hydrochloride (0.02–0.05 mg/kg, subcutaneously) twice a day for 3 days, and acetaminophen (2 mg/ml) and trimethoprim/sulfamethoxazole (0.2 & 1.0 mg/ml, respectively) in their water for 7 days. Animals were euthanized 1 week (n=3/group) and 4 weeks (n=3/group) by carbon dioxide asphyxiation. The entire abdominal wall was retrieved, bisected along the mid-line, fixed in 10% NBF for 72 hours, and routinely processed for paraffin embedding. Five μm-thick sections were obtained at 1mm step-levels to span the entire width of the specimen, and stained with H&E and Alcian blue. Three to five representative sections were selected, scanned in their entirety at 20X, and analyzed for THA coating cross-sectional area as in the in vitro degradation assays. Three additional grafts from each coating group were similarly processed to obtain the time-zero measures of coating cross-sectional area.
Statistical Analysis
Data are presented as mean ± standard error of mean (SEM). Considering the exploratory nature of the investigations, most experiments used small sample sizes (n=3–6; Table 1). Equal variances for all outcomes were confirmed with Levene’s test, so one-way ANOVA and Tukey tests were performed for pairwise comparisons. p < 0.05 was considered to be statistically significant. Statistical analysis was performed using SigmaStat for Windows v3.5 (Systat Software, San Jose, CA).
Results
In vitro degradation of THA plugs
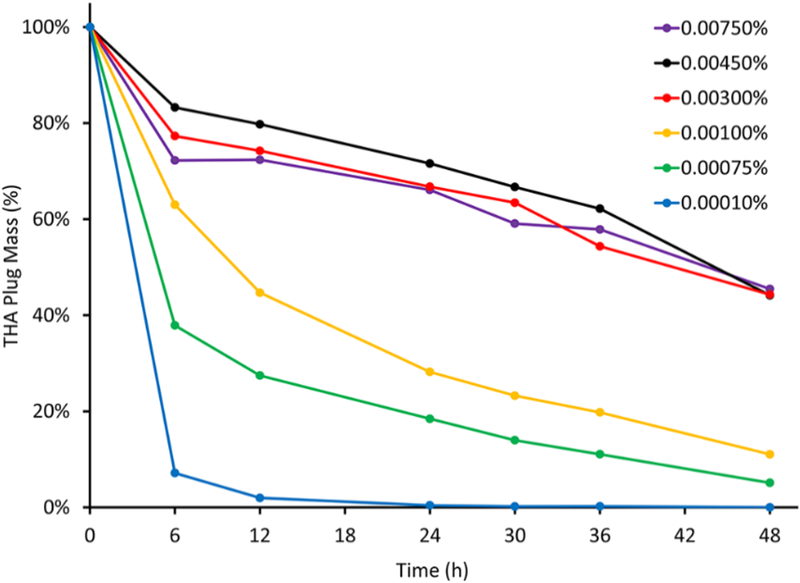
Figure 2 empirically demonstrates the difference in in vitro degradation profiles of THA plugs prepared at different crosslinking densities. Increasing crosslinking density by increasing H2O2 concentration from 0.0001% to 0.003% resulted in THA hydrogels with slower degradation rates. Crosslinking at H2O2 concentrations >0.003% did not result in further decrease in degradation rate.
Figure 2.
In vitro degradation assay results showing the difference in degradation profiles of THA hydrogel plug formulations prepared at different crosslinking densities corresponding to 0.0001% to 0.0075% H2O2 (n= 3/group/time-point). Empirically, increasing H2O2 concentration from 0.0001% to 0.003% resulted in hydrogels with progressively slower degradation rates; concentrations >0.003% H2O2 did not result in further decrease in degradation rate, suggesting that the THA hydrogels were maximally crosslinked at ~0.003% H2O2.
In vitro degradation of THA-coated HADM
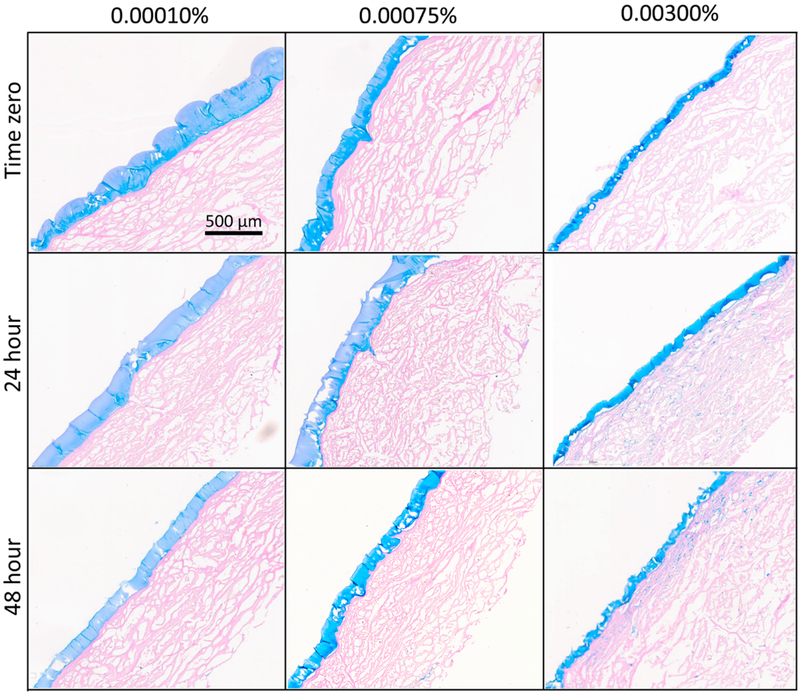
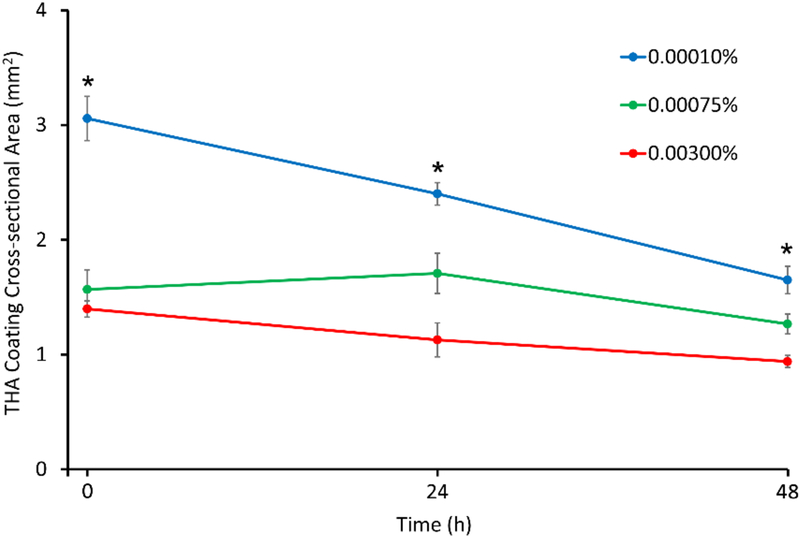
Figure 3 shows representative histological sections of THA-coated HADM grafts at time-zero, and after 24 and 48 hours of in vitro degradation. At time zero, the epidermal surface of HADM grafts were selectively coated with a uniform and continuous coat of THA at all three crosslinking densities (Figure 3, top row). THA coating thickness was significantly higher (176 ±10 μm) in the 0.0001% H2O2 group, compared to the 0.00075% H2O2 group (97 ±4 μm, p=0.003), and the 0.003% H2O2 group (93 ±12 μm, p=0.002). Because the same amount of hydrogel was used to coat each sample, these results indicate that hydrogels with lower crosslinking density have a greater swelling capacity. Similarly, the cross-sectional area of the entire THA coating at time zero was significantly higher (3.1±0.19 mm2) in the 0.0001% H2O2 group, compared to the 0.00075% H2O2 group (1.6 ±0.17 mm2, p<0.001) and the 0.003% H2O2 group (1.4 ±0.07 mm2, p<0.001) (Figure 4).
Figure 3.
In vitro degradation results of HADM coated with THA crosslinked using 0.0001%, 0.00075%, and 0.003% H2O2. Alcian blue stained histologic sections showed a continuous THA coating localized on the epidermal surface of HADM at time-zero. THA coatings appeared thinner, particularly in the 0.0001% H2O2 group after 24 and 48h of degradation in 25U/ml hyaluronidase.
Figure 4.
In vitro degradation assay results showing cross-sectional area of THA coatings on HADM. Compared to time-zero cross-sectional area, the 0.0001% H2O2 group showed a significant decrease after 24h (p=0.01) and 48h (p<0.001) of degradation, whereas the 0.00075% and 0.003% H2O2 groups did not show a significant change over time. (* indicates significant pairwise differences)
Figures 3 and 4 show the changes in THA coating cross-sectional area during in vitro degradation. Compared to time-zero cross-sectional area, the 0.0001% H2O2 group showed a significant decrease after 24h (p=0.01) and 48h (p<0.001) of degradation, whereas the 0.00075% and 0.003% H2O2 groups did not show a significant change in cross-sectional area over time. After 48h of in vitro degradation, the coatings had 54 ±4%, 81 ±6%, and 67 ±4% of their initial cross-sectional area in the 0.0001%, 0.00075%, and 0.003% H2O2 groups, respectively.
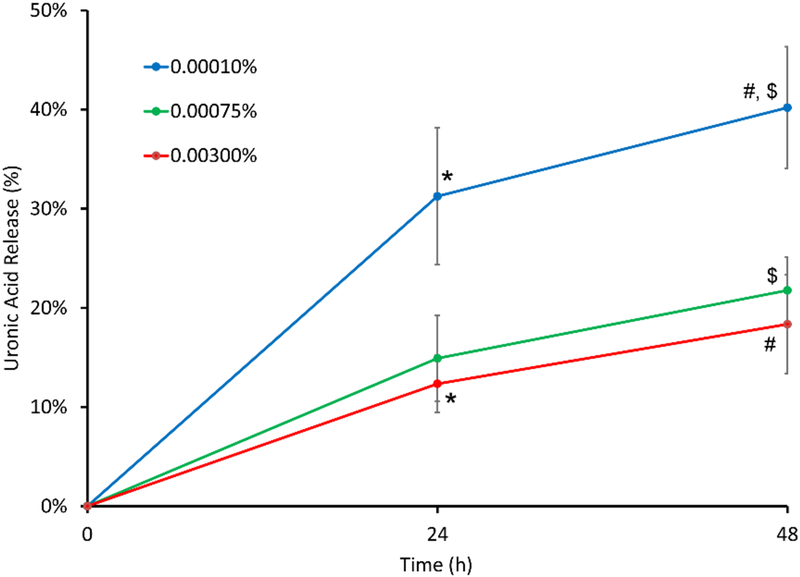
Uronic acid assay results also showed statistically significant differences in the amount of uronic acid released during in vitro degradation from THA-coated HADM crosslinked at the three different H2O2 concentrations (Figure 5). Specifically, significantly more uronic acid was released from the 0.0001% H2O2 group (31 ±7% at 24 hours, 40 ±6% at 48 hours) compared to the 0.003% H2O2 group (12 ±3%, p=0.043) at 24h, and compared to both 0.00075% (22 ±3%, p=0.048) and 0.003% H2O2 groups (18 ±5%, p=0.019) at 48h.
Figure 5.
In vitro degradation assay results showing higher release of uronic acid from THA-coated HADM crosslinked at 0.0001% H2O2 concentration compared to 0.00075% and 0.003% H2O2 concentrations. (* p =0.043, $ p = 0.048, # p = 0.019)
In vivo degradation of THA-coated HADM
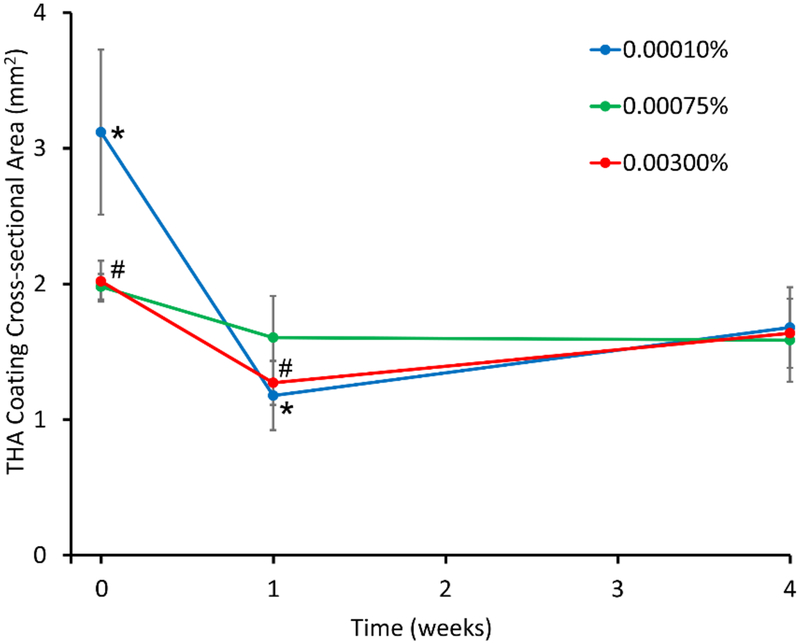
Figure 6 shows representative histological sections of THA-coated HADM grafts at time-zero, and after 1 and 4 weeks of implantation. THA coatings appeared to be largely intact in the three groups after 4 weeks of implantation. Figure 7 shows the changes in cross-sectional area of the THA coatings during in vivo degradation. A significant decrease in THA coating cross-sectional area was observed between 0–1 week of implantation in the 0.0001% H2O2 (p=0.038) and 0.003% H2O2 groups (p=0.015). After 1 week of implantation, the coatings had 38 ±8%, 81 ±16%, and 63 ±8% of their initial cross-sectional areas in the 0.0001%, 0.00075%, and 0.003% H2O2 groups, respectively. After 4 weeks of implantation, the coatings had 54 ±10%, 80 ±15%, and 81 ±1% of their initial cross-sectional areas in the 0.0001%, 0.00075%, and 0.003% H2O2 groups, respectively.
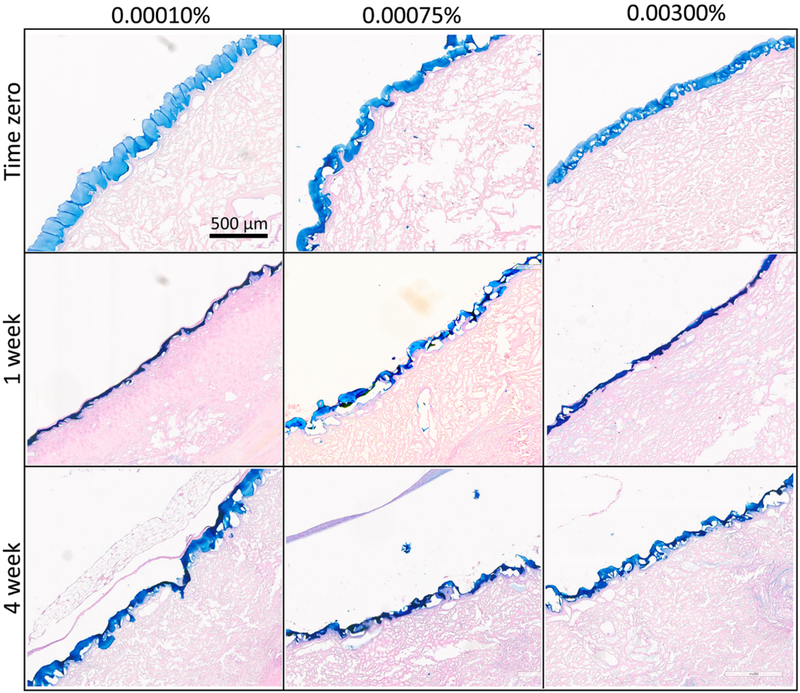
Figure 6.
In vivo degradation results. Alcian-blue stained sections of the rat ventral abdominal wall showing the THA coated HADM graft at time-zero, 1 week and 4 weeks following implantation. The THA coatings at the three different crosslinking densities appeared to be largely intact even at 4 weeks of implantation.
Figure 7.
In vivo degradation results showing change in cross-sectional area of THA coatings on HADM. A significant decrease in cross-sectional area was observed between 0–1 week of implantation in the 0.0001% H2O2 (*, p=0.038) and 0.003% H2O2 groups (#, p=0.015).
Discussion
The objective of this study was to develop biodegradable THA hydrogel coatings on acellular dermis grafts, with the goal of identifying THA coating formulations that largely resorb in 4 weeks after intraperitoneal implantation. THA hydrogel plugs and coatings with varying in vitro degradation rates were prepared by varying the crosslinking density of the hydrogel preparation. However, all three THA coatings investigated remained partially-degraded after 4 weeks of in vivo implantation in a rat model.
Increasing crosslinking density by increasing H2O2 concentration from 0.0001% to 0.003% resulted in THA hydrogel plugs with slower in vitro degradation rates, but H2O2 concentrations >0.003% did not result in further decrease in degradation rate, suggesting that the THA hydrogel plugs were likely maximally crosslinked at ~0.003% H2O2. In prior pilot experiments, we observed that H2O2 concentrations < 0.0001% did not consistently result in gelation of the frozen hydrogel plugs in the 25°C crosslinking solution. Similarly, gelation did not result at HRP concentrations < 1 U/ml in our prior pilot experiments. These results are in agreement with previous reports that have demonstrated the degradation tunability of THA hydrogels35,36. Similar to the findings reported here, THA hydrogels (1–2% w/v) formed using 0.001–0.3% H2O2 and 0.1–2.5 U/ml HRP were shown to be degradable in vitro over 24–48 hours35,36. In particular, H2O2 concentration was shown to influence THA crosslinking density and degradation rates of the THA hydrogel plugs, whereas HRP concentration was shown to influence gelation speed and spreadability of the hydrogel.35,36.
Three crosslinking densities (corresponding to 0.0001%, 0.00075%, or 0.003% H2O2 concentrations) that resulted in distinct degradation profiles of THA hydrogel plugs in the in vitro degradation study were chosen for preparing THA hydrogel coatings on HADM. We showed that a uniform, thin and continuous coat of THA hydrogel could be selectively applied on the surface of HADM at these three crosslinking densities. Like THA hydrogel plugs, THA coatings were also degradable in vitro, with lower crosslinking density associated with higher degradability. After 48h of degradation, coatings in the 0.0001% H2O2 group retained ~50% of their initial cross-sectional area (compared to 70–80% in the 0.00075% and 0.003% H2O2 groups), and ~60% of their initial uronic acid content (compared to ~80% in the 0.00075% and 0.003% H2O2 groups).
However, in vivo degradation of the THA coatings on HADM did not entirely correlate with the in vitro results. While coating cross-sectional area showed a drop during the 1st week of implantation (to 40% in the 0.0001% H2O2 group, and 60–80% in the 0.00075% and 0.003% H2O2 groups), there was no further change between 1 week and 4 weeks. At the end of 4 weeks, all three coatings retained ~50–80% of their initial cross-sectional area. In vitro incubation of select explanted specimens in 25U/ml hyaluronidase solution at 37°C for 72 hours confirmed that THA coatings remained completely degradable after implantation. Physiologic hyaluronidase concentration is known to vary from extremely low levels in plasma (0.006 U/ml) and various other tissues38,39 to high levels in ovary (38.5 U/ml)40, but is not known in the peritoneal fluid of hernia patients. While inflammation and tissue remodeling processes increase levels of hyaluronidase and macrophage-generated reactive oxygen and nitrogen species that are known to accelerate hyaluronic acid degradation41,42, our results suggest that hyaluronidase levels in the peritoneal environment in our animal model of acute hernia injury/repair were significantly lower than the concentration (25U/ml) used the in vitro degradation assay and likely too low to effect complete in vivo degradation of the THA-coatings. Extrinsic administration of hyaluronidase into the peritoneal cavity43,44 could be adopted as a strategy to accelerate in vivo degradation of the THA coating and allow further investigation of our overarching hypothesis that transient inhibition of cellular infiltration from visceral/peritoneal tissues during the initial 4 weeks after implantation may mitigate premature graft resorption but allow constructive graft remodeling, leading to improved durability and ultimately repair outcomes (Figure 1).
We posit that understanding and potentially modifying the mechanisms responsible for premature biologic graft resorption may result in strategies to improve biologic graft durability and thus reduce the incidence of complications and failure and improve clinical outcomes following ventral hernia repair.8,10–12 Currently, the only strategy to increase biologic graft durability involves chemical cross-linking of the endogenous collagen in the grafts. However, such cross-linking also reduces biocompatibility and causes a loss of “biologic” behavior in the grafts.16,45 Collagen cross-linking is associated with poor cellular infiltration, revascularization, remodeling and integration of biologic grafts with host tissues, which can result in adverse events such as severe foreign body reaction, acute mechanical failure and disintegration of the graft.16,45–47 There is currently an unmet need for innovative and improved biologic graft materials that are both biocompatible and mechanically durable to make them attractive over the newer and cheaper synthetic and biosynthetic meshes that have also shown some success in contaminated settings.3,12,48–51
The study has several limitations. First, relatively small sample sizes (n=3–6) were used, considering the exploratory nature of several investigations. Therefore, the study may be underpowered to detect some physiologically relevant differences. Second, limitations inherent to animal models not accurately representing the clinical scenario may exist. We used the rat intraperitoneal hernia repair model based on previous evidence of HADM thinning and hernia recurrence in this model17. Third, we did not perform a detailed characterization of cellular infiltrate in the HADM grafts in this study, since the THA coatings were largely intact and cellular infiltration across the coated surface was absent/minimal at the end of 4 weeks in all groups. Future studies will investigate longer implantation durations as well as rapidly degrading THA coating formulations (e.g., THA with lower rates of tyramine-substitution, extrinsic administration of hyaluronidase into the peritoneal cavity) to allow testing of the hypothesis that a resorbable THA coating would modulate cellular infiltration and improve graft durability.
In conclusion, THA hydrogel could be coated on acellular dermis as a uniform, thin and continuous coat. The THA coatings were demonstrated to be degradable in vitro, with lower hydrogel crosslinking associated with higher degradation rates. However, the coatings developed in this study remained un-degraded at the end of 4 weeks of implantation. Alternate strategies to accelerate in vivo degradation of THA coatings are required to investigate if resorbable THA coatings on acellular dermis grafts would modulate cellular infiltration and improve biologic graft durability.
Acknowledgments
Research reported in this publication was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number 5R21EB022668. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure
S.S. has patents/ patent applications on graft technologies, receives personal fees from Viscus Biologics, and owns stock in Becton Dickinson & Co, Medtronic Plc, and Johnson & Johnson. K.A.D. has patents/ patent applications on graft and hydrogel technologies, and receives personal fees from Viscus Biologics and Orthofix. M.J.R. has received funds from Bard Davol and W.L. Gore and Associates, Inc as a paid consultant or speaker, and serves on the board of Ariste Medical. Other authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.Poulose BK, Shelton J, Phillips S, Moore D, Nealon W, Penson D, Beck W, Holzman MD. Epidemiology and cost of ventral hernia repair: making the case for hernia research. Hernia 2012;16(2):179–83. [DOI] [PubMed] [Google Scholar]
- 2.Luijendijk RW, Hop WC, van den Tol MP, de Lange DC, Braaksma MM, JN IJ, Boelhouwer RU, de Vries BC, Salu MK, Wereldsma JC and others. A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med 2000;343(6):392–8. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery A. The battle between biological and synthetic meshes in ventral hernia repair. Hernia 2013;17(1):3–11. [DOI] [PubMed] [Google Scholar]
- 4.Butler CE, Langstein HN, Kronowitz SJ. Pelvic, abdominal, and chest wall reconstruction with AlloDerm in patients at increased risk for mesh-related complications. Plast Reconstr Surg 2005;116(5):1263–75; discussion 1276–7. [DOI] [PubMed] [Google Scholar]
- 5.Asfaw TS, Northington G. Synthetic and Biological Graft Materials: Biological Concepts. Seminars in Colon and Rectal Surgery 2009;20(3):112–117. [Google Scholar]
- 6.Kanters AE, Krpata DM, Blatnik JA, Novitsky YM, Rosen MJ. Modified hernia grading scale to stratify surgical site occurrence after open ventral hernia repairs. J Am Coll Surg 2012;215(6):787–93. [DOI] [PubMed] [Google Scholar]
- 7.Group VHW, Breuing K, Butler CE, Ferzoco S, Franz M, Hultman CS, Kilbridge JF, Rosen M, Silverman RP, Vargo D. Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery 2010;148(3):544–58. [DOI] [PubMed] [Google Scholar]
- 8.Bellows CF, Albo D, Berger DH, Awad SS. Abdominal wall repair using human acellular dermis. Am J Surg 2007;194(2):192–8. [DOI] [PubMed] [Google Scholar]
- 9.Eberli D, Rodriguez S, Atala A, Yoo JJ. In vivo evaluation of acellular human dermis for abdominal wall repair. J Biomed Mater Res A 2010;93(4):1527–38. [DOI] [PubMed] [Google Scholar]
- 10.Jin J, Rosen MJ, Blatnik J, McGee MF, Williams CP, Marks J, Ponsky J. Use of acellular dermal matrix for complicated ventral hernia repair: does technique affect outcomes? J Am Coll Surg 2007;205(5):654–60. [DOI] [PubMed] [Google Scholar]
- 11.Hiles M, Record Ritchie RD, Altizer AM. Are biologic grafts effective for hernia repair?: a systematic review of the literature. Surg Innov 2009;16(1):26–37. [DOI] [PubMed] [Google Scholar]
- 12.Smart NJ, Marshall M, Daniels IR. Biological meshes: a review of their use in abdominal wall hernia repairs. Surgeon 2012;10(3):159–71. [DOI] [PubMed] [Google Scholar]
- 13.Blatnik J, Jin J, Rosen M. Abdominal hernia repair with bridging acellular dermal matrix--an expensive hernia sac. Am J Surg 2008;196(1):47–50. [DOI] [PubMed] [Google Scholar]
- 14.Sahoo S, Greeson CB, McCarron JA, Milks RA, Aurora A, Walker E, Iannotti JP, Derwin KA. Effect of pretension and suture needle type on mechanical properties of acellular human dermis patches for rotator cuff repair. J Shoulder Elbow Surg 2012;21(10):1413–21. [DOI] [PubMed] [Google Scholar]
- 15.Cornwell KG, Landsman A, James KS. Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg 2009;26(4):507–23. [DOI] [PubMed] [Google Scholar]
- 16.De Silva GS, Krpata DM, Gao Y, Criss CN, Anderson JM, Soltanian HT, Rosen MJ, Novitsky YW. Lack of identifiable biologic behavior in a series of porcine mesh explants. Surgery 2014;156(1):183–9. [DOI] [PubMed] [Google Scholar]
- 17.Ma J, Sahoo S, Baker AR, Derwin KA. Investigating muscle regeneration with a dermis/small intestinal submucosa scaffold in a rat full-thickness abdominal wall defect model. J Biomed Mater Res B Appl Biomater 2015;103(2):355–64. [DOI] [PubMed] [Google Scholar]
- 18.Sahoo S, Baker AR, Haskins IN, Krpata DM, Rosen MJ, Derwin KA. Assessment of Human Acellular Dermis Graft in Porcine Models for Ventral Hernia Repair. Tissue Eng Part C Methods 2017;23(11):718–727. [DOI] [PubMed] [Google Scholar]
- 19.Monteiro GA, Rodriguez NL, Delossantos AI, Wagner CT. Short-term in vivo biological and mechanical remodeling of porcine acellular dermal matrices. J Tissue Eng 2013;4:2041731413490182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 2009;30(8):1482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown BN, Londono R, Tottey S, Zhang L, Kukla KA, Wolf MT, Daly KA, Reing JE, Badylak SF. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater 2012;8(3):978–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf MT, Dearth CL, Ranallo CA, LoPresti ST, Carey LE, Daly KA, Brown BN, Badylak SF. Macrophage polarization in response to ECM coated polypropylene mesh. Biomaterials 2014;35(25):6838–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellon JM, Contreras LA, Pascual G, Bujan J. Evaluation of the acute scarring response to the implant of different types of biomaterial in the abdominal wall. J Mater Sci Mater Med 2000;11(1):25–9. [DOI] [PubMed] [Google Scholar]
- 24.Gomez-Gil V, Garcia-Honduvilla N, Pascual G, Rodriguez M, Bujan J, Bellon JM. Peritoneal adhesion formation and reformation tracked by sequential laparoscopy: optimizing the time point for adhesiolysis. Surgery 2010;147(3):378–91. [DOI] [PubMed] [Google Scholar]
- 25.Schreinemacher MH, Emans PJ, Gijbels MJ, Greve JW, Beets GL, Bouvy ND. Degradation of mesh coatings and intraperitoneal adhesion formation in an experimental model. Br J Surg 2009;96(3):305–13. [DOI] [PubMed] [Google Scholar]
- 26.Gomez-Gil V, Pascual G, Garcia-Honduvilla N, Rodriguez M, Bujan J, Bellon JM. Characterizing omental adhesions by culturing cells isolated from a novel in vivo adhesion model. Wound Repair Regen 2009;17(1):51–61. [DOI] [PubMed] [Google Scholar]
- 27.Rosch R, Junge K, Schachtrupp A, Klinge U, Klosterhalfen B, Schumpelick V. Mesh implants in hernia repair. Inflammatory cell response in a rat model. Eur Surg Res 2003;35(3):161–6. [DOI] [PubMed] [Google Scholar]
- 28.Xu H, Wan H, Sandor M, Qi S, Ervin F, Harper JR, Silverman RP, McQuillan DJ. Host response to human acellular dermal matrix transplantation in a primate model of abdominal wall repair. Tissue Eng Part A 2008;14(12):2009–19. [DOI] [PubMed] [Google Scholar]
- 29.Adrales GL, Honigsberg E. Biologic Prosthetics: What Are They and How Do They Interact with the Body? The SAGES Manual of Hernia Repair: Springer; 2013. p 311–321. [Google Scholar]
- 30.Sadava EE, Krpata DM, Gao Y, Rosen MJ, Novitsky YW. Wound healing process and mediators: Implications for modulations for hernia repair and mesh integration. J Biomed Mater Res A 2014;102(1):295–302. [DOI] [PubMed] [Google Scholar]
- 31.Laurent TC, Laurent UB, Fraser JR. The structure and function of hyaluronan: An overview. Immunol Cell Biol 1996;74(2):A1–7. [DOI] [PubMed] [Google Scholar]
- 32.Brochhausen C, Schmitt VH, Rajab TK, Planck CN, Kramer B, Wallwiener M, Hierlemann H, Kirkpatrick CJ. Intraperitoneal adhesions--an ongoing challenge between biomedical engineering and the life sciences. J Biomed Mater Res A 2011;98(1):143–56. [DOI] [PubMed] [Google Scholar]
- 33.Darr A, Calabro A. Synthesis and characterization of tyramine-based hyaluronan hydrogels. J Mater Sci Mater Med 2009;20(1):33–44. [DOI] [PubMed] [Google Scholar]
- 34.Chin L, Calabro A, Rodriguez ER, Tan CD, Walker E, Derwin KA. Characterization of and host response to tyramine substituted-hyaluronan enriched fascia extracellular matrix. J Mater Sci Mater Med 2011;22(6):1465–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurisawa M, Chung JE, Yang YY, Gao SJ, Uyama H. Injectable biodegradable hydrogels composed of hyaluronic acid-tyramine conjugates for drug delivery and tissue engineering. Chem Commun (Camb) 2005(34):4312–4. [DOI] [PubMed] [Google Scholar]
- 36.Lee F, Chung JE, Kurisawa M. An injectable hyaluronic acid-tyramine hydrogel system for protein delivery. J Control Release 2009;134(3):186–93. [DOI] [PubMed] [Google Scholar]
- 37.Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem 1973;54(2):484–9. [DOI] [PubMed] [Google Scholar]
- 38.Fiszer-Szafarz B, Litynska A, Zou L. Human hyaluronidases: electrophoretic multiple forms in somatic tissues and body fluids. Evidence for conserved hyaluronidase potential N-glycosylation sites in different mammalian species. J Biochem Biophys Methods 2000;45(2):103–16. [DOI] [PubMed] [Google Scholar]
- 39.Garg HG, Hales CA. Chemistry and biology of hyaluronan: Elsevier; 2004. [Google Scholar]
- 40.Yeo Y, Highley CB, Bellas E, Ito T, Marini R, Langer R, Kohane DS. In situ cross-linkable hyaluronic acid hydrogels prevent post-operative abdominal adhesions in a rabbit model. Biomaterials 2006;27(27):4698–705. [DOI] [PubMed] [Google Scholar]
- 41.Cowman MK, Lee HG, Schwertfeger KL, McCarthy JB, Turley EA. The Content and Size of Hyaluronan in Biological Fluids and Tissues. Front Immunol 2015;6:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benedetti L, Cortivo R, Berti T, Berti A, Pea F, Mazzo M, Moras M, Abatangelo G. Biocompatibility and biodegradation of different hyaluronan derivatives (Hyaff) implanted in rats. Biomaterials 1993;14(15):1154–60. [DOI] [PubMed] [Google Scholar]
- 43.Carlsson O, Rosengren BI, Rippe B. Effects of peritoneal hyaluronidase treatment on transperitoneal solute and fluid transport in the rat. Acta Physiol Scand 2000;168(3):371–6. [DOI] [PubMed] [Google Scholar]
- 44.Stoehr BJ, Gutierrez JE, Close AS. Effect of intraperitoneal hyaluronidase on the reformation of intestinal adhesions. Am J Surg 1966;111(6):881–3. [DOI] [PubMed] [Google Scholar]
- 45.Novitsky YW, Orenstein SB, Kreutzer DL. Comparative analysis of histopathologic responses to implanted porcine biologic meshes. Hernia 2014;18(5):713–21. [DOI] [PubMed] [Google Scholar]
- 46.Sandor M, Xu H, Connor J, Lombardi J, Harper JR, Silverman RP, McQuillan DJ. Host response to implanted porcine-derived biologic materials in a primate model of abdominal wall repair. Tissue Eng Part A 2008;14(12):2021–31. [DOI] [PubMed] [Google Scholar]
- 47.Harth KC, Rosen MJ. Major complications associated with xenograft biologic mesh implantation in abdominal wall reconstruction. Surg Innov 2009;16(4):324–9. [DOI] [PubMed] [Google Scholar]
- 48.Shankaran V, Weber DJ, Reed RL 2nd, Luchette FA. A review of available prosthetics for ventral hernia repair. Ann Surg 2011;253(1):16–26. [DOI] [PubMed] [Google Scholar]
- 49.Sahoo S, Haskins IN, Huang LC, Krpata DM, Derwin KA, Poulose BK, Rosen MJ. Early Wound Morbidity after Open Ventral Hernia Repair with Biosynthetic or Polypropylene Mesh. J Am Coll Surg 2017;225(4):472–480 e1. [DOI] [PubMed] [Google Scholar]
- 50.Rosen MJ, Bauer JJ, Harmaty M, Carbonell AM, Cobb WS, Matthews B, Goldblatt MI, Selzer DJ, Poulose BK, Hansson BM and others. Multicenter, prospective, longitudinal study of the recurrence, surgical site infection, and quality of life after contaminated ventral hernia repair using biosynthetic absorbable mesh: the COBRA study. Ann Surg 2017;265(1):205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Majumder A, Winder JS, Wen Y, Pauli EM, Belyansky I, Novitsky YW. Comparative analysis of biologic versus synthetic mesh outcomes in contaminated hernia repairs. Surgery 2016;160(4):828–38. [DOI] [PubMed] [Google Scholar]