Abstract
Patients with transthyretin cardiac amyloidosis (TTR CA) suffer from impaired exercise capacity, have a poor quality of life (QoL), and approved treatments are lacking. Stimulators of the soluble guanylate cyclase are promising new pharmaceuticals in the treatment armamentarium of heart failure patients. The aim of the present study was to report on the safety and efficacy of riociguat administration in patients with TTR CA. TTR CA patients received riociguat for 4–6 months within the frames of a national named patient use (NPU) program. Parameters of interest included changes in submaximal exercise capacity, invasive hemodynamic parameters, and QoL. Between March 2012 and June 2017, 86 CA patients were screened for the NPU program, of whom 13 TTR CA patients were eligible for participation. In our study cohort, riociguat had an acceptable tolerability profile. At follow-up, we could detect slight improvements in median 6-min walk distance (396 m [interquartile range (IQR) = 340–518] vs. 400 m [IQR = 350–570], P = 0.045), New York Heart Association class ≥ III (n = 7 [53.9%] vs. n = 0 [0.0%], P = 0.031), cardiac output (4.3 L/min [IQR = 3.9–5.1] vs. 4.5 L/min [IQR = 4.2–5.1], P = 0.022), diastolic pressure gradient (1.0 mmHg [IQR = −1.5–3.0) vs. −1.0 mmHg [IQR = −3.0–1.0], P = 0.049), and QoL (50.0% [IQR = 40.0–58.0] vs. 60.0% [IQR = 50.0–75.0], P = 0.021). Pulmonary arterial pressures were not altered. The present case series of TTR CA patients indicates that riociguat administration was safe and associated with minor clinical as well as hemodynamic improvements.
Keywords: invasive hemodynamics, riociguat, quality of life
Introduction
Transthyretin (TTR) cardiac amyloidosis (CA) is caused by accumulation of misfolded TTR proteins within the extracellular space of the myocardium. Misfolding of the TTR protein may be caused by point mutations in the respective gene. The most prevalent form, however, is age-associated without a distinct genetic background.1 Irrespective of its etiology, myocardial deposition of misfolded TTR proteins eventually translates into the clinical picture of chronic heart failure (HF), a condition with a deep negative impact on quality of life (QoL) and survival.2–5 Though considered a rare disease, recent studies indicate that TTR CA affects a clinically relevant percentage of the HF population, as TTR amyloid deposits could be found in 20% of patients with HF and preserved ejection fraction (HFpEF),6 in 6% of patients with severe aortic stenosis,7 and in 5% of patients with hypertrophic cardiomyopathy of unknown origin.8
Currently, only one approved disease-modifying compound exists for TTR amyloidosis patients. However, this compound has only been approved for the treatment of mutant TTR polyneuropathy and clinical studies testing its efficacy in CA are currently underway (clinicaltrials.gov identifier NCT01994889).9 Therefore, therapeutic options are limited and often solely rely on diuretic agents. Standard HF therapies, such as beta-blockers or angiotensin converting enzyme (ACE) inhibitors, often lead to hemodynamic deterioration in these patients.3,10
Riociguat, a stimulator of the soluble guanylate cyclase (sGC), belongs to a relatively new class of drugs with pulmonary vasodilator and anti-fibrotic properties and is approved for the treatment of pulmonary hypertension (PH).11 Trials in chronic HF showed that riociguat administration is safe and leads to clinical as well as hemodynamic improvements.3,12,13 Following the hypothesis that riociguat may have beneficial effects on disease-specific hemodynamic characteristics and symptoms, we initiated a named patient use (NPU) program, where patients were evaluated at baseline as well as after 4–6 months of treatment. Here, we report on a single-center experience of riociguat administration in patients with TTR CA.
Methods
Setting and study design
The present study was conducted at the Division of Cardiology at the Medical University of Vienna, Austria, a tertiary care center with a dedicated CA outpatient clinic and a high-volume cardiac catheterization laboratory. All data were collected within the frames of a prospective HF registry, which was approved by the local ethics committee (EK no. 796/2010) and complies with the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from all patients.
Diagnosis of transthyretin cardiac amyloidosis
TTR CA was diagnosed either by histological assessment of endomyocardial biopsy samples with Congo red staining and subsequent immunohistochemical typing with AmY-kit amyloid antibodies (Martinsried, Germany) or non-invasively in accordance with the algorithm proposed by Gillmore et al.14 After confirmation of TTR CA, patients underwent genetic testing of the TTR gene.
Named patient use program
In order to participate in the NPU, the following inclusion criteria had to be met: definite diagnosis of TTR CA; New York Heart Association (NYHA) functional class (FC) ≥ II; stable HF symptoms for ≥ 1 month; baseline right heart catheterization (RHC); consent to the off-label treatment with riociguat; and ability to understand and follow instructions to complete all study procedures. Exclusion criteria were: other forms of CA (e.g. light-chain CA); NYHA FC I; estimated glomerular filtration rate < 30 mL/min/1.73 m2; symptomatic hypotension; systolic blood pressure (SBP) <100 mmHg at baseline; inability to perform 6-min walk tests (6MWT); and hepatic dysfunction (Child Pugh stage B or C).
Patients were provided with riociguat by Bayer Austria, Ges.m.b.H. (Vienna, Austria) from 2014 to 2015 and by Merck Sharp & Dohme Austria, Ges.m.b.H. (Vienna, Austria) from 2016 to 2017, for 4–6 months. After this initial treatment period, three public health insurance companies (insurance institution for railways and mining, regional medical insurances for Vienna and for Lower Austria) agreed to cover the costs for riociguat, if the following criteria were fulfilled: hemodynamic improvement defined as a decrease of pulmonary arterial pressures (PAP) and/or increase in cardiac output (CO) as assessed by RHC accompanied by clinical improvement defined as an improvement in 6-min walk distance (6MWD) and/or NYHA class.
Treatment regimen
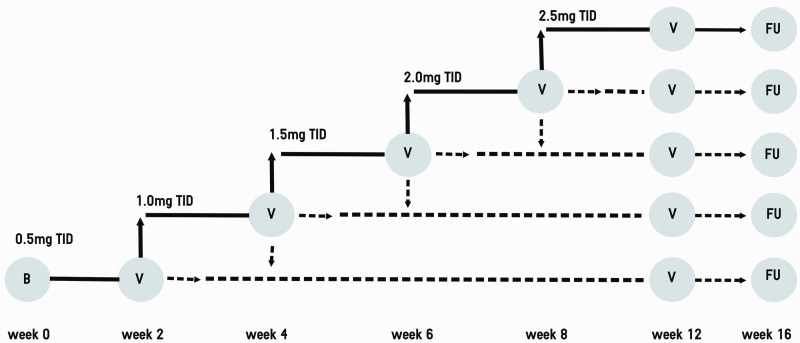
Study participants received riociguat in addition to standard care. Riociguat was administered orally, in dosages that were individually adjusted according to a predefined dosing regimen (Fig. 1). The starting dose was 0.5 mg three times daily (t.i.d.). The dose was adjusted according to the patients’ SBP and symptoms of hypotension at safety visits. If SBP was ≥110 mmHg, the dose could be up-titrated to 2.5 mg t.i.d. in 0.5 mg steps every two weeks. If SBP was 100–109 mmHg without symptoms of hypotension, the dose was maintained. The dose was reduced if SBP was <100 mmHg. Riociguat was stopped if SBP was <100 mmHg accompanied by clinical symptoms of hypotension (e.g. dizziness or presyncope) or in case of riociguat-related adverse events (AEs).
Fig. 1.
Titration scheme. Riociguat titration scheme in the named patient use program. Starting dose was 0.5 mg three times a day (t.i.d.). Up-titration and down-titration was possible every two weeks during on-site safety visits (V). Scheduled follow-up was after 16 weeks of riociguat intake. B, baseline; FU, follow-up.
Hemodynamic and clinical outcome measures
For RHC, a 7-F Swan-Ganz catheter (Baxter, Irvine, CA, USA) inserted via jugular or femoral access was used. Filling pressures were recorded using CathCorLX (Siemens AG, Berlin and Munich, Germany). Directly measured hemodynamic read-outs included CO, cardiac index (CI), stroke volume (SV), systolic/diastolic/mean pulmonary arterial pressure (mPAP), pulmonary artery wedge pressure (PAWP), right atrial pressure as well as arterial oxygen saturation and mixed venous oxygen saturation. Pulmonary vascular resistance (PVR), pulmonary pulse pressure, and the diastolic pressure gradient (DPG) were calculated according to standard formulae.15
Clinical read-outs were 6MWD, NYHA class, N-terminal prohormone of brain natriuretic peptide (NT-proBNP), EuroQoL visual analogue scale (EQ-VAS), and EuroQoL 5-dimensions three-level (EQ-5D-3 L) questionnaire. 6MWTs were performed according to a standardized protocol published by the American Thoracic Society.16 Borg dyspnea score was used to grade the level of dyspnea after 6MWT completion.
Safety measures
Safety measures were assessed at every outpatient visit and included AEs, physical examination, vital signs, electrocardiogram, and routine clinical laboratory parameters, including NT-proBNP and serum creatinine. AEs were reviewed monthly by an internal data monitoring committee consisting of DB and JM, which could terminate the study at any given point of time.
Statistical analysis
IBM SPSS version 21 (SPSS Inc.; Chicago, IL, USA) was used for statistical analysis. Continuous variables are expressed as median and interquartile ranges (IQR). Categorical variables are presented as n (%). Baseline and follow-up variables were compared using the Wilcoxon signed-rank test and McNemar’s test as appropriate. Patients who did not tolerate riociguat administration were excluded from final statistical analysis. Statistical significance was defined as P values from two-sided tests of <0.05.
Results
Patient population
Between March 2012 and June 2017, 86 patients were diagnosed with CA. Of them, 73 patients could not be offered a place in the NPU program because of a diagnosis of AL CA (n = 50), lack of baseline RHC (n = 11), NYHA FC I (n = 5), unwillingness to undergo follow-up RHC (n = 3), SBP < 100 mmHg (n = 2), and immobility (n = 1). One patient died before baseline evaluation. Of the 13 NPU program participants, 11 had been diagnosed with wild-type TTR CA and two had mutations in the TTR gene (His108Arg). Study patient flow is depicted in Fig. 2.
Fig. 2.
Patient flow chart. A total of 86 patients with a diagnosis of cardiac amyloidosis were screened for the NPU program; 73 patients were not eligible to participate. Exclusion criteria were a diagnosis of AL CA (n = 50), lack of baseline RHC (n = 11), NYHA FC I (n = 5), unwillingness to undergo follow-up RHC (n = 3), SBP < 100 mmHg (n = 2), immobility (n = 1), and one patient died before baseline evaluation. Thirteen patients were enrolled initially and two patients prematurely discontinued the NPU. Thus, 11 patients completed all study procedures. NPU, named patient use; RHC, right heart catheterization; NYHA, New York Heart Association; SBP, systolic blood pressure.
Baseline characteristics
Patient baseline characteristics are presented in Table 1. The median age of the study population was 75.0 years (IQR = 69.0–83.0) and 11 (84.6%) were men. The majority of patients were in NYHA FC ≥ III (n = 7, 53.9%) and NT-proBNP values were markedly elevated with a median level of 2923 pg/mL (IQR = 1722–6878). Median 6MWD was 396 m (IQR = 340–518). With regards to concomitant HF medication at baseline, seven patients (53.9%) were on beta-blockers, one patient (7.7%) had an ACE inhibitor, four (30.8%) had an angiotensin receptor blocker, nine (69.2%) had a loop diuretic, two (15.4%) had a thiazide diuretic, and six (46.2%) had a mineralocorticoid receptor blocker. Baseline invasive hemodynamic assessment revealed elevated cardiac filling pressures, with a mPAP of 33.0 mmHg (IQR = 29.0–37.0) and a median PAWP of 21.0 mmHg (IQR = 19.0–27.5). CO at baseline was 4.3 L/min (IQR = 3.9–5.1).
Table 1.
Patient characteristics and change from baseline to follow-up for clinical and hemodynamic parameters.
| Clinical parameters | Baseline (n = 13) | Follow-up (n = 11)* | P value |
|---|---|---|---|
| NYHA FC ≥ III | 7 (53.9) | 0 (0.0) | 0.031 |
| NT-proBNP (pg/mL) | 2923 (1772–6878) | 2584 (1804–7255) | 0.929 |
| 6MWD (m) | 396 (340–518) | 400 (350–570) | 0.045 |
| eGRF (mL/min/1.73m2) | 63.0 (43.6–80.4) | 61.6 (43.7–79.5) | 0.594 |
| Systolic arterial pressure (mmHg) | 125 (115–132) | 118 (114–128) | 0.328 |
| Diastolic arterial pressure (mmHg) | 82.0 (69.0–87.5) | 73.0 (61.0–79.0) | 0.021 |
| Mean arterial pressure (mmHg) | 95.0 (88.0–102) | 87.0 (81.0–96.0) | 0.119 |
| Concomitant medication | |||
| Beta-blocker | 7 (53.8) | 3 (27.3) | |
| Angiotensin converting enzyme inhibitor | 1 (7.7) | 0 (0.0) | |
| Angiotensin receptor blocker | 4 (30.8) | 0 (0.0) | |
| Antiarrhythmic agent | 1 (7.7) | 1 (9.1) | |
| Loop diuretic | 9 (69.2) | 8 (72.7) | |
| Thiazide diuretic | 2 (15.4) | 0 (0.0) | |
| Mineralocorticoid receptor antagonist | 6 (46.2) | 7 (63.6) | |
| Oral anticoagulant | 8 (61.5) | 7 (63.6) | |
| Antiplatelet agent | 4 (30.8) | 3 (27.3) | |
| Statin | 4 (30.8) | 2 (18.2) | |
| Invasive hemodynamic parameters | |||
| Systolic PAP (mmHg) | 45.0 (41.0–55.0) | 47.0 (40.0–53.0) | 0.350 |
| Diastolic PAP (mmHg) | 23.0 (21.0–25.0) | 19.0 (18.0–24.0) | 0.229 |
| Mean PAP (mmHg) | 33.0 (29.0–37.0) | 33.0 (28.0–38.0) | 0.476 |
| Right atrial pressure (mmHg) | 11.0 (10.0–16.0) | 11.0 (7.0–18.0) | 0.719 |
| Pulmonary artery wedge pressure (mmHg) | 21.0 (19.0–27.5) | 19.0 (17.0–27.0) | 0.449 |
| Cardiac output (L/min) | 4.3 (3.9–5.1) | 4.5 (4.2–5.1) | 0.022 |
| Cardiac index (L/min/m2) | 2.4 (1.9–2.6) | 2.4 (2.1–2.7) | 0.028 |
| SaO2 (mmHg) | 96.0 (93.1–98.0) | 93.5 (89.3–96.7) | 0.114 |
| SvO2 (mmHg) | 60.0 (57.5–63.4) | 62.0 (50.3–65.3) | 0.959 |
| Systemic vascular resistance (dyn·s·cm–5) | 331 (269–560) | 300 (267–419 | 0.130 |
| Pulmonary vascular resistance (dyn·s·cm–5) | 207 (142–266) | 200 (151–228) | 0.575 |
| Pulmonary pulse pressure (mmHg) | 22.0 (21.0–30.0) | 25.0 (19.0–29.0) | 0.929 |
| Diastolic pressure gradient (mmHg) | 1.0 (−1.5–3.0) | −1.0 (−3.0–1.0) | 0.049 |
| Quality of life | |||
| Health state (%) | 50.0 (40.0–58.0) | 60.0 (50.0–75.0) | 0.021 |
| Mobility | |||
| No problems | 5 (41.7) | 7 (63.6) | 0.625 |
| Problems | 7 (58.3) | 4 (36.4) | 0.625 |
| Self-care | |||
| No problems | 7 (58.3) | 10 (90.9) | 0.250 |
| Problems | 5 (41.7) | 1 (9.1) | 0.250 |
| Usual activities | |||
| No problems | 3 (25.0) | 5 (45.5) | 0.125 |
| Problems | 9 (75.0) | 6 (54.6) | 0.125 |
| Pain/discomfort | |||
| No problems | 3 (25.0) | 5 (45.5) | 0.500 |
| Problems | 9 (75.0) | 6 (54.6) | 0.500 |
| Anxiety/depression | |||
| No problems | 6 (50.0) | 7 (63.6) | 1.000 |
| Problems | 6 (50.0) | 4 (36.4) | 1.000 |
Values are presented as n (%) or median (IQR).
Two patient drop-outs.
NYHA, New York Heart Association; IQR, interquartile range; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; eGFR, estimated glomerular filtration rate; SaO2, arterial oxygen saturation; SvO2, mixed venous oxygen saturation; PAP, pulmonary arterial pressure.
EQ-VAS and EQ-5D-3 L questionnaires were completed by 12/13 NPU program participants. At baseline, median self-rated health status as assessed with the EQ-VAS was 50.0% (IQR = 40.0–58.0). With regards to EQ-5D-3 L parameters, a vast majority of our patients had difficulties with mobility (n = 7, 58.3%), usual activities (n = 9, 75.0%), and complained of pain or discomfort (n = 9, 75.0%). Approximately one-third had problems with self-care (n = 5, 41.7%) and half of our study cohort (n = 6, 50.0%) reported anxiety or depression.
Effects of riociguat administration
From a clinical perspective, there was a rather small but statistically significant increase in 6MWD from 396 m (IQR = 340–518) at baseline to 400 m (IQR = 350–570) at follow-up (P = 0.045; Tables 1 and 2, Fig. 3a). This was accompanied by significant improvements in NYHA FC (baseline: NYHA FC ≥ III: n = 7 (53.9%), follow-up: n = 0 (0.0%), P = 0.031; Tables 1 and 2, Fig. 3b), whereas NT-proBNP did not change from baseline (2923 pg/mL [IQR = 1722–6878]) to follow-up (2584 pg/mL [IQR = 1804–7255], P = 0.929; Tables 1 and 2).
Table 2.
Overview of individual changes from baseline.
| Patient | CO* | CO† | 6-MWD* | 6-MWD† | NYHA* | NYHA† | NT-proBNP* | NT-proBNP† |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | 4.3 | 4.4 | 60.0 | 80.0 | 3 | 2 | 9180 | 7573 |
| Patient 2 | 4.7 | 5.1 | 330 | 433 | 3 | 2 | 5843 | 3916 |
| Patient 3 | 5.6 | 5.6 | 396 | 383 | 2 | 2 | 1962 | 2584 |
| Patient 4‡ | 4.1 | NA | 400 | NA | 3 | NA | 3332 | NA |
| Patient 5 | 2.6 | 3.1 | 350 | 400 | 2 | 2 | 1462 | 872 |
| Patient 6 | 5.1 | 4.9 | 260 | 370 | 3 | 2 | 8087 | 11,583 |
| Patient 7 | 3.1 | 4.0 | 587 | 622 | 2 | 2 | 2174 | 2098 |
| Patient 8 | 5.1 | 6.0 | 370 | 350 | 3 | 2 | 2923 | 3940 |
| Patient 9‡ | 5.5 | NA | 510 | NA | 2 | NA | 1671 | NA |
| Patient 10 | 4.0 | 4.5 | 526 | 612 | 3 | 1 | 1773 | 1287 |
| Patient 11 | 4.8 | 4.5 | 367 | 558 | 2 | 1 | 7912 | 2415 |
| Patient 12 | 3.8 | 4.2 | 425 | 340 | 3 | 2 | 4556 | 7255 |
| Patient 13 | 3.9 | 4.6 | 534 | 570 | 2 | 2 | 1432 | 1804 |
Cardiac output is given in L/min, 6MWD in meters, and NT-proBNP in pg/mL.
Value at baseline.
Value at follow-up.
Premature discontinuation of riociguat NPU program.
CO, cardiac output; 6MWD, 6-min walk distance; NYHA, New York Heart Association; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; NA, not applicable; NPU, named patient use.
Fig. 3.
Individual changes in clinical and hemodynamic parameters after riociguat administration. Change in 6MWD (a*, P = 0.045), NYHA FC (b, P = 0.031), cardiac output (c, P = 0.022), and quality of life (d†, P = 0.021) from baseline to follow-up. Patients 4 and 9 are not depicted in (a–d) as no follow-up data were obtained due to premature study discontinuation. *Short 6MWD in patient 1 is attributed to frailty. †Ten lines visible due to identical quality of life values from patients 5 and 6.
By the time of follow-up, CO had slightly improved from 4.3 L/min (IQR = 3.9–5.1) to 4.5 L/min (IQR = 4.2–5.1, P = 0.022; Table 1). Table 2 and Fig. 3c show longitudinal changes in CO for individual patients. Furthermore, we could detect statistically significant changes in CI (baseline: 2.4 L/min/m2 [IQR = 1.9–2.6], follow-up: 2.4 L/min/m2 [IQR = 2.1–2.7], P = 0.028) and DPG (baseline: 1.0 mmHg [IQR = −1.5–3.0], follow-up: −1.0 mmHg [IQR = −3.0–1.0], P = 0.049). However, other hemodynamic parameters including systemic vascular resistance (baseline: 331 dyn·s·cm–5 [IQR = 269–560], follow-up: 300 dyn·s·cm–5 [IQR = 267–419], P = 0.130) remained unchanged (Table 1).
Self-reported health status on EQ-VAS slightly improved from 50.0% (IQR = 40.0–58.0) at baseline to 60.0% (IQR = 50.0–75.0) at follow-up (P = 0.021; Fig. 3d). However, no changes with regards to the EQ-5D-3 L questionnaire were detected (mobility: P = 0.625, self-care: P = 0.250, usual activities: P = 0.125, pain/discomfort: P = 0.500, anxiety/depression: P = 1.000).
Change of clinical and hemodynamic parameters from baseline to follow-up, excluding patient drop-outs, is provided in Supplemental Table 1.
Dosing and duration of riociguat administration
At the time of follow-up, five (45.5%) patients were on 2.0 mg riociguat t.i.d., four (36.4%) patients were on 1.5 mg riociguat t.i.d., one patient (9.1%) was on 1.0 mg riociguat t.i.d., and one (9.1%) patient was on 0.5 mg riociguat t.i.d. Patients were treated with riociguat 13.0–47.0 (median = 15.0 weeks, IQR = 14.0–22.0) before follow-up.
Change of concomitant medication
Compared to baseline, five (45.5%) patients had changes to their HF medication and diuretic regimen, two (18.2%) had changes to their HF medication alone, and two (18.2%) had changes to their diuretic scheme only. In detail, the percentage of patients who were on beta-blockers, ACE inhibitors, angiotensin receptor blockers, and thiazide diuretics was reduced from 53.8%, 7.7%, 30.8%, and 15.4% to 27.3%, 0.0%, 0.0%, and 0.0%, whereas usage of loop diuretics and mineralocorticoid receptor antagonists increased slightly from 69.2% and 46.2% to 72.7% and 63.6%, respectively (Table 1). Detailed dose changes are shown in Supplemental Table 3.
Safety and tolerability
Of the 13 patients who started the NPU program, 11 completed all study procedures. Two patients experienced AEs (symptomatic hypotension and leg edema in one patient, diarrhea and nausea in the other patient), which led to premature NPU termination. All AEs resolved within one week after riociguat intake had been stopped. In the remaining 11 patients, riociguat treatment was well tolerated. AEs during the study period are listed in Supplemental Table 2.
Discussion
In the present case series, we could detect marginal but statistically significant improvements in CO, CI, DPG, NYHA FC, 6MWD, and QoL.
Soluble guanylate cyclase stimulators in heart failure
Riociguat belongs to a relatively new class of drugs, namely stimulators of the sGC, and is approved for the treatment of pre-capillary forms of PH. Riociguat acts as a pulmonary and systemic vasodilator and has a dual mode of action. It sensitizes the sGC to endogenous nitric oxide (NO) via stabilizing NO–sGC binding and also directly stimulates the sGC independently of NO.17 In two previously published studies (LEPHT, DILATE-1), riociguat led to significant hemodynamic improvements and was well tolerated in HF patients. LEPHT, a phase IIb study, investigated the hemodynamic effects of riociguat in patients with HF and reduced ejection fraction (HFrEF) over a 16-week period. CI and SV increased. PVR could be reduced and QoL improved significantly.13 DILATE-1 was a proof-of-concept study that assessed the acute hemodynamic effects of single doses of riociguat in HFpEF.12 SV and CI improved after riociguat administration. Vericiguat, another stimulator of the sGC, was recently investigated in two phase IIb dose-finding studies, which included 477 HFpEF (SOCRATES-PRESERVED) and 456 HFrEF patients (SOCRATES-REDUCED).18,19 Over a 12-week treatment period, four different dose levels were compared against placebo. Vericiguat was well tolerated; however, both trials failed to meet the primary endpoint, change in NT-proBNP. A further study with vericiguat in HF is currently underway (clinicaltrials.gov identifier NCT02861534), as QoL had improved significantly in the HFpEF cohort and a significant NT-proBNP reduction in highest dose arms had been observed in the HFrEF cohort. Given the fact that we could detect improvements in hemodynamic and clinical parameters, our results are in line with previously published studies investigating sGC stimulators in HF patients.
Management of transthyretin cardiac amyloidosis
Due to the lack of approved disease-modifying drugs, treatment and management of patients with TTR CA is challenging. To date, the mainstay of treatment is the use of diuretics in order to relieve symptoms of HF.1,20 Standard HF therapies such as beta-blockers or ACE inhibitors can lead to hemodynamic deterioration and should therefore be avoided.1,21,22 In contrast to standard HF drugs, our results indicate that riociguat treatment was associated with minor hemodynamic and clinical improvements. Thus, riociguat may have the potential to provide a valuable therapeutic option in patients with TTR CA.
Exercise capacity in cardiac transthyretin amyloidosis
In a recent study, which investigated the natural course of TTR CA, Gillmore et al. found that 6MWD decreased rapidly in their patient population.23 Within six months, patients walked 36 m less. After 12 and 18 months, the decrease was 106 m and 140 m, respectively. A further study could also demonstrate a significant decline in 6MWD every six months (–26 m) in TTR CA patients.2 Contrary to these observations, 6MWD slightly increased in our study cohort over a median time period of 15.0 weeks.
In parallel with a decrease in 6MWD, NYHA FC has been shown to increase over time in TTR CA.23 In our case series, NHYA FC had improved over the observation period. Despite the lack of a placebo group, our findings suggest a possible beneficial effect of riociguat on exercise capacity.
While NYHA FC and 6MWD are important and well-established predictors of outcome in HF, the body of evidence concerning the prognostic significance in TTR CA is still limited, especially with regards to 6MWD.24–27 Therefore, it remains speculative, whether improvements in these rather simple measures of exercise capacity can translate into improved patient outcomes.
Safety and tolerability of riociguat in transthyretin cardiac amyloidosis
In our study cohort of TTR CA patients, riociguat had an acceptable safety profile. Nevertheless, two patients stopped the NPU program due to AEs likely related to riociguat (symptomatic hypotension and leg edema in one patient, diarrhea and nausea in the other patient).28 Both patients fully recovered within one week after riociguat discontinuation. Interestingly, despite the vasodilatatory effects of riociguat and the fact that CA patients are prone to develop hypotension,20 only one patient discontinued the NPU program due to symptomatic hypotension. Discontinuation rates observed in our cohort were higher when compared to drop-out rates in riociguat trials with HF or PH (15.4% vs. 3.0%, 2.9%, and 3.0%).13,29,30 However, when compared to drop-out rates in the ATTR-ACT Study, which tested a disease-modifying drug (Tafamidis) in TTR CA patients, discontinuation rates were lower (15.4% vs. 21.2%).31
Of the remaining 11 patients who finished the NPU program, seven had no AEs and four patients had 1–5 AEs each (Supplemental Table 1). AEs during the study period, such as cardiac decompensations or arrhythmias, were most probably reflective of the underlying condition rather than a result of riociguat administration. This is also supported by Ruberg et al., who investigated the natural disease progression in patients with wild-type as well as mutant TTR CA and frequently observed similar clinical conditions.2
Study limitations
The present study has several limitations. The study was conducted in a single center. Thus, a center-specific bias cannot be excluded. Nevertheless, limiting data to collection to one center has the advantages of constant clinical routine, constant quality of work-up, and constant follow-up. Furthermore, our sample size was small and a placebo-control group was lacking. Therefore, a major limitation of our study is that we cannot exclude that the observed hemodynamic and clinical changes were due to a placebo effect or due to changes in concomitant medications. Nevertheless, the detection of hemodynamic and clinical improvements after a median treatment period of 15.0 weeks argues against a placebo effect, as studies in PH indicate that placebo-related effects last for 4–8 weeks.30,32
Another limitation is that due to organizational reasons not all patients could perform the same study schedule leading to different durations of the follow-up period.
Despite these major limitations, results from our case series of TTR CA patients who were treated with riociguat are promising and could potentially lay the groundwork for studies of a stronger design.
Conclusion
In the present case series of 13 TTR CA patients, we found that riociguat had an acceptable tolerability profile and its administration led to small, but statistically significant hemodynamic as well as clinical improvements. Due to limitations in design, results of the present study can only be seen as hypothesis-generating. Nevertheless, given the positive signals and the urgent need for therapies in this patient population, further trials of stronger design are warranted to explore the therapeutic potential of riociguat in TTR CA.
Supplemental Material
Supplemental material, PUL849394 Supplemetal Material for Riociguat for the treatment of transthyretin cardiac amyloidosis: data from a named patient use program in Austria by Franz Duca, Stefan Aschauer, Caroline Zotter-Tufaro, Christina Binder, Andreas A. Kammerlander, Benedikt Börries, Hermine Agis, Renate Kain, Christian Hengstenberg, Julia Mascherbauer and Diana Bonderman in Pulmonary Circulation
Conflict of interest
FD received speaker fees and congress supports from Bayer, Novartis, and Pfizer. DB received speaker fees and honoraria for consulting from Actelion, Bayer, Boehringer Ingelheim, GSK, AOP Orphan, United Therapeutics, and Pfizer as well as research grants from Bayer. The other authors declare that there is no conflict of interest.
Funding
Bayer Austria Ges.m.b.H. (Vienna, Austria) and Merck Sharp & Dohme Austria Ges.m.b.H. (Vienna, Austria) provided riociguat for the NPU program, but had no influence on study design, data processing, or statistical analysis.
ORCID iD
Renate Kain https://orcid.org/0000-0002-2428-543X
References
- 1.Gertz MA, Benson MD, Dyck PJ, et al. Diagnosis, prognosis, and therapy of transthyretin amyloidosis. J Am Coll Cardiol 2015; 66: 2451–2466. [DOI] [PubMed] [Google Scholar]
- 2.Ruberg FL, Maurer MS, Judge DP, et al. Prospective evaluation of the morbidity and mortality of wild-type and V122I mutant transthyretin amyloid cardiomyopathy: the Transthyretin Amyloidosis Cardiac Study (TRACS). Am Heart J 2012; 164: 222–228.e221. [DOI] [PubMed] [Google Scholar]
- 3.Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation 2012; 126: 1286–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lane T, Bangova A, Fontana M, et al. Quality of life in ATTR amyloidosis. Orphanet J Rare Dis 2015; 10: O26. [Google Scholar]
- 5.Rapezzi C, Merlini G, Quarta CC, et al. Systemic cardiac amyloidoses. Circulation 2009; 120: 1203. [DOI] [PubMed] [Google Scholar]
- 6.Mohammed SF, Mirzoyev SA, Edwards WD, et al. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail 2014; 2: 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Treibel TA, Fontana M, Gilbertson JA, et al. Occult transthyretin cardiac amyloid in severe calcific aortic stenosis: prevalence and prognosis in patients undergoing surgical aortic valve replacement. Circ Cardiovascular Imaging 2016; 9: e005066. [DOI] [PubMed] [Google Scholar]
- 8.Damy T, Costes B, Hagege AA, et al. Prevalence and clinical phenotype of hereditary transthyretin amyloid cardiomyopathy in patients with increased left ventricular wall thickness. Eur Heart J 2016; 37: 1826–1834. [DOI] [PubMed] [Google Scholar]
- 9.Coelho T, Maia LF, Martins da Silva A, et al. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology 2012; 79: 785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gertz MA, Dispenzieri A, Sher T. Pathophysiology and treatment of cardiac amyloidosis. Nat Rev Cardiol 2015; 12: 91–102. [DOI] [PubMed] [Google Scholar]
- 11.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 12.Bonderman D, Pretsch I, Steringer-Mascherbauer R, et al. Acute hemodynamic effects of riociguat in patients with pulmonary hypertension associated with diastolic heart failure (DILATE-1): a randomized, double-blind, placebo-controlled, single-dose study. Chest 2014; 146: 1274–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonderman D, Ghio S, Felix SB, et al. Riociguat for patients with pulmonary hypertension caused by systolic left ventricular dysfunction: a phase IIb double-blind, randomized, placebo-controlled, dose-ranging hemodynamic study. Circulation 2013; 128: 502–511. [DOI] [PubMed] [Google Scholar]
- 14.Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016; 133: 2404–2412. [DOI] [PubMed] [Google Scholar]
- 15.Aschauer S, Kammerlander AA, Zotter-Tufaro C, et al. The right heart in heart failure with preserved ejection fraction: insights from cardiac magnetic resonance imaging and invasive haemodynamics. Eur J Heart Fail 2016; 18: 71–80. [DOI] [PubMed] [Google Scholar]
- 16.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. [DOI] [PubMed]
- 17.Grimminger F, Weimann G, Frey R, et al. First acute haemodynamic study of soluble guanylate cyclase stimulator riociguat in pulmonary hypertension. Eur Respir J 2009; 33: 785–792. [DOI] [PubMed] [Google Scholar]
- 18.Pieske B, Maggioni AP, Lam CSP, et al. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur Heart J 2017; 38: 1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gheorghiade M, Greene SJ, Butler J, et al. Effect of vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in patients with worsening chronic heart failure and reduced ejection fraction: The SOCRATES-REDUCED randomized trial. JAMA 2015; 314: 2251–2262. [DOI] [PubMed] [Google Scholar]
- 20.Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation 2005; 112: 2047–2060. [DOI] [PubMed] [Google Scholar]
- 21.Shah KB, Inoue Y, Mehra MR. Amyloidosis and the heart: a comprehensive review. Arch Intern Med 2006; 166: 1805–1813. [DOI] [PubMed] [Google Scholar]
- 22.Castaño A, Drachman BM, Judge D, et al. Natural history and therapy of TTR-cardiac amyloidosis: emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev 2015; 20: 163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilmore JD, Maurer MS, Vest J, et al. Analysis of disease progression in patients with transthyretin cardiac amyloidosis. Orphanet J Rare Dis 2015; 10: O10. [Google Scholar]
- 24.Pulido V, Doros G, Berk JL, et al. The six-minute walk test in patients with AL amyloidosis: a single centre case series. Br J Haematol 2017; 177: 388–394. [DOI] [PubMed] [Google Scholar]
- 25.Zotter-Tufaro C, Mascherbauer J, Duca F, et al. Prognostic significance and determinants of the 6-min walk test in patients with heart failure and preserved ejection fraction. JACC Heart Fail 2015; 3: 459–466. [DOI] [PubMed] [Google Scholar]
- 26.Pinney JH, Whelan CJ, Petrie A, et al. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc 2013; 2: e000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quarta CC, Solomon SD, Uraizee I, et al. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation 2014; 129: 1840–1849. [DOI] [PubMed] [Google Scholar]
- 28.Riociguat - Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/adempas-epar-product-information_en.pdf (accessed 12 Oct 2017).
- 29.Ghofrani HA, D’Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013; 369: 319–329. [DOI] [PubMed] [Google Scholar]
- 30.Ghofrani HA, Galie N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013; 369: 330–340. [DOI] [PubMed] [Google Scholar]
- 31.Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018; 379: 1007–1016. [DOI] [PubMed] [Google Scholar]
- 32.Channick RN, Simonneau G, Sitbon O, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet 2001; 358: 1119–1123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, PUL849394 Supplemetal Material for Riociguat for the treatment of transthyretin cardiac amyloidosis: data from a named patient use program in Austria by Franz Duca, Stefan Aschauer, Caroline Zotter-Tufaro, Christina Binder, Andreas A. Kammerlander, Benedikt Börries, Hermine Agis, Renate Kain, Christian Hengstenberg, Julia Mascherbauer and Diana Bonderman in Pulmonary Circulation