Abstract
Circular RNAs have continuous, stable, and covalently closed circular structures and are not easily degraded by nucleases, thus they are ideal serum biomarkers for detecting diseases. However, research is still lacking on circular RNAs as diagnostic and prognostic markers for idiopathic pulmonary arterial hypertension. This study investigated the potential role of serum circ_0068481 levels in idiopathic pulmonary arterial hypertension diagnosis and prognosis. This prospective cohort study enrolled 82 patients with idiopathic pulmonary arterial hypertension between January 2016 and July 2018 at Guangdong Provincial People’s Hospital. Serum circ_0068481 levels were measured using quantitative reverse transcription-polymerase chain reaction. Baseline data, including clinical background, hemodynamic variables, and biochemical variables, were collected. Receiver operating characteristic curves were used to investigate diagnostic effect, the Kaplan–Meier method was used to estimate survival rates, and univariate analysis of prognostic factors was performed with a Cox proportional hazard model. We found that serum circ_0068481 expression levels were significantly higher in patients with idiopathic pulmonary arterial hypertension and had higher sensitivity and specificity for predicting idiopathic pulmonary arterial hypertension. Additionally, we found that circ_0068481 expression correlated significantly with heart function, 6-min walk distance, serum N-terminal pro-B-type natriuretic peptide, serum H2S, the 6th World Symposium on Pulmonary Hypertension risk stratification, right heart failure, and patient death. Moreover, serum circ_0068481 levels were elevated in patients with idiopathic pulmonary arterial hypertension and right heart failure and were able to predict right heart failure. Serum circ_0068481 levels were also elevated in patients who died with idiopathic pulmonary arterial hypertension and were able to predict poorer clinical outcomes. Circ_0068481 is a novel and noninvasive biomarker for diagnosing idiopathic pulmonary arterial hypertension and predicting poor clinical outcome in patients with idiopathic pulmonary arterial hypertension.
Keywords: Circular RNAs (circRNA), right heart failure, biomarker
Introduction
Pulmonary arterial hypertension (PAH) is a progressive disorder that affects both pulmonary vasculature and the heart, leading to right ventricular hypertrophy, dilatation, and right heart failure (RHF).1 Right ventricular function is the most important predictor of morbidity and mortality in patients with PAH, and RHF is the leading cause of PAH mortality.2 Idiopathic pulmonary arterial hypertension (IPAH), a type of PAH, is characterized by a progressive increase in pulmonary vascular resistance that leads to heart failure. The early recognition and prognostic evaluation of IPAH may guide decisions regarding the use of more aggressive therapy; however, assessing IPAH prognosis is challenging and mostly based on invasive hemodynamics and subjective parameters such as right heart catheterization. There is currently a need for non-invasive biomarkers that reflect pathological changes in pulmonary arterial vessels to diagnose IPAH. Previous studies found chitinase 3-like 1, Growth Differentiation Factor-15, and red blood cell distribution width to be novel diagnostic and (or) prognostic biomarkers for IPAH;3–5 however, it is important to develop more novel, specific, and non-invasive biomarkers for IPAH diagnosis and prognosis.
Circular RNAs (circRNAs) have continuous, stable, and covalently closed circular structures; since they have no free 3′ or 5′ ends, they are not easily degraded by nucleases, making them ideal biomarkers for detecting diseases.6 CircRNAs are also involved in the development and progression of cardiovascular disease; for example, Myocardial Infarction-Associated Circular RNA (MICRA) is associated with the development of heart failure after myocardial infarction and can be used to predict the development of myocardial infarction,7 circRNA_010567 silencing upregulates miR-141 and downregulates transforming growth factor-β1 expression to regulate myocardial fibrosis,8 heart-related circRNA (HRCR) protects the heart from pathological hypertrophy and heart failure by targeting miR-223 and could serve as a new therapeutic target for heart disease,9 mitochondrial fission and apoptosis-related circRNA (MFACR) regulates cardiac mitochondrial dynamics and apoptosis by adsorbing miR-652-3p and may serve as a potential therapeutic approach for cardiovascular diseases,10 and circ-Foxo3 inhibits heart aging and provides myocardial protection.11,12 Previous circRNA studies have focused on left heart diseases and heart failure; thus, it remains unclear whether circRNAs can be used as markers to predict IPAH progression and clinical outcome.
In this study, we focused on hsa_circ_0068481, whose gene, ST6 beta-galactoside alpha-2,6-sialyltransferase 1, is located at chr3:186756529–186761098. A previous study found that circ_0068481 expression significantly increased in patients with chronic thromboembolic pulmonary hypertension compared with healthy controls.13 In this study, we investigated circ_0068481 expression in IPAH and its potential role as a novel diagnostic and prognostic biomarker in IPAH.
Methods
Clinical samples
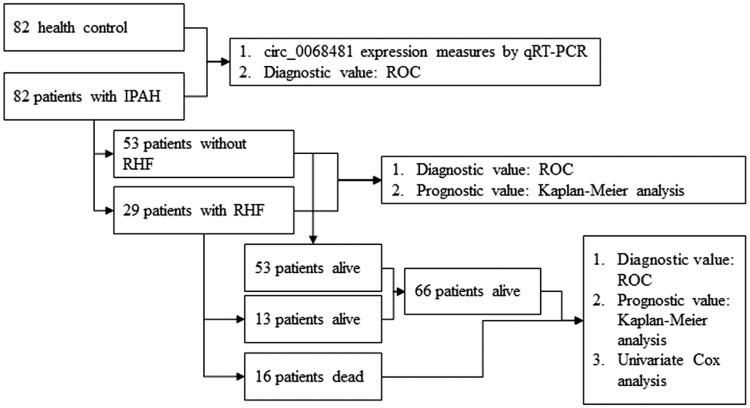
This study was approved by the Ethics Committee of Guangdong Provincial People’s Hospital (No. GDREC2016305H). All study participants received oral and written information about the objectives of the study and provided written informed consent. A total of 82 patients with IPAH were consecutively enrolled in the prospective cohort study between January 2016 and March 2018 at Guangdong Provincial People’s Hospital. All participants conformed to the inclusion and exclusion criteria and received no treatment before baseline clinical characteristics were collected. The inclusion and exclusion criteria for IPAH were the same as those used in previous studies.5,14 RHF was diagnosed as IPAH and have the symptoms of right ventricular fractional (RVF), including exertional dyspnea, fatigue, peripheral edema, and abdominal fullness/ascites, and also with cardiac index <2.5 L/(min × m2) as measured by right heart catheterization. Therapeutic schedule was given targeted drug therapy according to the 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology, International Society for Heart and Lung Transplantation and 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the ESC Developed with the special contribution of the Heart Failure Association of the ESC. The control group (82 health control) consisted of age- and sex-matched healthy volunteers with no medical conditions or medications. Flowchart demonstrating classification of participants is shown in Fig. 1.
Fig. 1.
Flowchart demonstrating classification of participants.
Clinical parameter collection
Data regarding the patients’ age, sex, height, weight, body mass index (BMI), and 6-min walk distance (6MWD) were collected. Biochemical markers, including serum N-terminal pro-B-type natriuretic peptide (NT-proBNP), homocysteine (HCY), and H2S, were measured using an Abbott Architect assay (Abbott Diagnostics, Abbott Park, IL). Right atrial dimension, right ventricular dimension, pulmonary artery width, pulmonary artery blood flow, tricuspid annular plane systolic excursion, tricuspid annular motion velocities, pulmonary systolic blood pressure, and tricuspid regurgitation were obtained from echocardiographic examinations using an ultrasound platform (Vivid 7; GE Healthcare, Milwaukee, WI) equipped with a phased-array transducer. Two-dimensional and Doppler measurements were obtained and evaluated according to the guidelines of the American Society of Echocardiography.15 All Doppler measurements were performed three times and the average value was obtained. Mean pulmonary arterial pressure, pulmonary capillary wedge pressure, pulmonary vascular resistance, pulmonary vascular resistance index, mean right atrial pressure, pulmonary vein oxygen saturation, and pulmonary artery oxygen saturation were evaluated using pulmonary hemodynamic measurements obtained by cardiac catheterization. The follow-up period was from the date of blood sampling to either death or June 2018. Follow-up evaluations consisted of hospital visits or telephone interviews. The median follow-up duration was 14 (range, 1–24) months. During the follow-up period, 29 patients had IPAH with RHF complications and 16 died.
Real-time quantitative reverse transcription polymerase chain reaction
Total RNA was isolated from serum samples using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.). After incubation with RNase R at 37℃ for 30 min to degrade linear RNA, RNA was reverse transcribed using an ImProm-IITM Reverse Transcription System (Promega) under the following conditions: 30℃ for 10 min, 42℃ for 60 min, and 85℃ for 10 min. circ_0068481 expression was measured by real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) using the Roche 480II system (Roche, Basel, Switzerland) and SYBR Premix Ex Taq II (Tli RNaseH Plus; Takara, Dalian, China). circ_0068481 and GAPDH primers were synthesized by Sangon Biotech (Shanghai, China) as follows: glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward 5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse 5′-TGGTGAAGACGCCAGTGGA-3′; hsa_circ_0068481 forward 5′-TATCTGCCCAAGGAGAGCAT-3′ and reverse 5′-TATTATCCATGGGAGGGAAGGT-3′. Data were analyzed using the comparative cycle threshold method with three independent experiments.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences, version 19.0 (IBM, Armonk, NY, USA and GraphPad Prism, version 7.0 for Windows (GraphPad Software, San Diego, CA, USA). Normally distributed data were expressed as the mean ± standard deviation, and differences between two groups were assessed using independent t-tests. Non-normally distributed data were expressed as the median (P25–P75) and differences between two groups were assessed using Mann–Whitney U tests. Data were presented as the number and percentage of patients, with differences between categorical variables evaluated using chi-square tests. We performed receiver operating characteristic (ROC) curve analysis to investigate serum circ_0068481 levels that predicted IPAH against healthy volunteers and used the Kaplan–Meier method to estimate survival rates. Univariate analyses of prognostic factors were performed with a Cox proportional hazards model. P values of <0.05 were considered statistically significant.
Results
Serum circ_0068481 levels were elevated in patients with IPAH and act as a diagnostic biomarker
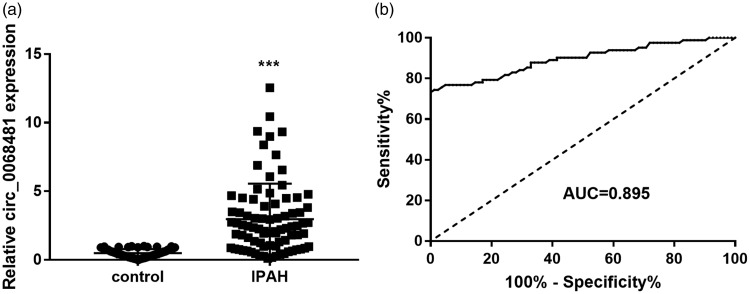
Serum circ_0068481 levels detected by qRT-PCR were significantly higher in patients with IPAH than in the control group (P < 0.05). The ability of serum circ_0068481 levels to diagnose IPAH was assessed using ROC curve analysis. At the optimal expression cutoff value of 0.995, the sensitivity and specificity were 74.39% and 98.78%, respectively (Fig. 2).
Fig. 2.
Serum circ_0068481 levels were elevated in patients with idiopathic pulmonary arterial hypertension (IPAH) and act as a diagnostic biomarker. (a) Serum circ_0068481 levels were detected by qRT-PCR. ***P < 0.001. (b) The ability of serum circ_0068481 levels to diagnose IPAH was assessed using ROC curve analysis.
AUC: area under the curve; qRT-PCR: Real-time quantitative reverse transcription polymerase chain reaction; ROC: receiver operating characteristic.
Serum circ_0068481 levels correlated with the clinicopathological features of patients with IPAH
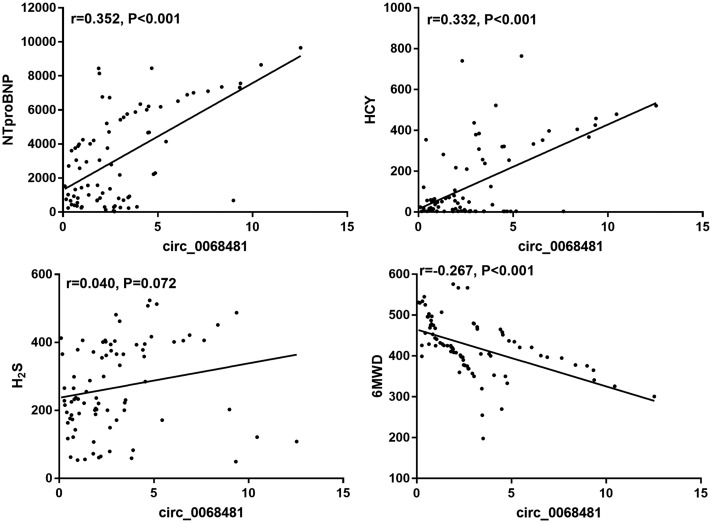
circ_0068481 levels correlated with the baseline clinical characteristics of patients with IPAH. The relationship between circ_0068481 levels and the baseline clinical characteristics of patients with IPAH is shown in Table 1. Patients with IPAH were divided into two groups according to the median circ_0068481 value (2.31): a low group (≤2.31, n = 41) and a high group (>2.31, n = 41). There was no correlation between circ_0068481 expression and age, sex, BMI, serum HCY, right atrial dimension, pulmonary artery width, pulmonary artery blood flow, tricuspid annular plane systolic excursion, mean pulmonary arterial pressure, pulmonary vascular resistance index, or pulmonary vein oxygen saturation (P > 0.05). Conversely, circ_0068481 expression correlated significantly with heart function, 6MWD, serum NT-proBNP, serum H2S, right ventricular dimension, the 6th World Symposium on Pulmonary Hypertension (WSPH) risk stratification, tricuspid annular motion velocities, pulmonary systolic blood pressure, tricuspid regurgitation, pulmonary capillary wedge pressure, pulmonary vascular resistance, mean right atrial pressure, pulmonary artery oxygen saturation, IPAH with RHF, and death (P < 0.05). Therapeutic schedule for IPAH had no significant change between two groups. Additionally, we found that circ_0068481 expression had significantly correlated with NT-proBNP level, HCY level, and 6MWD while it had no correlation with H2S (Fig. 3). These results showed that serum circ_0068481 levels may act as a novel diagnostic and prognostic biomarker for patients with IPAH and RHF and the death of patients with IPAH.
Table 1.
The relationship between circ_0068481 expression and baseline clinical characteristics of patients with IPAH.
| Characteristic | Circ_0068481 expression |
t/U/χ2 | P | |
|---|---|---|---|---|
| ≤2.31 (n = 41) | >2.31 (n = 41) | |||
| Age, years | 40.1 ± 10.55 | 39.7 ± 11.62 | 0.149a | 0.882 |
| Sex | 0.060c | 0.806 | ||
| Male | 12 | 11 | ||
| Female | 29 | 30 | ||
| Body mass index, kg/m2 | 20.77 ± 2.569 | 21.28 ± 3.081 | 0.818a | 0.416 |
| Heart function | 26.648 | <0.001* | ||
| 1–2 | 22 | 1 | ||
| 3–4 | 19 | 40 | ||
| NT-proBNP | 2207 ± 2138 | 4125 ± 2955 | 3.367a | 0.001* |
| HCY | 24.34 (6.515, 63.72) | 210.9 (4.254, 382.3) | 664b | 0.102 |
| H2S | 216.1 ± 101.2 | 318.2 ± 138.9 | 3.804a | 0.001* |
| Six-minute walking distance, m | 496.5 ± 51.45 | 387.3 ± 67.19 | 5.449a | <0.001* |
| Echocardiography | ||||
| Right atrial diameters, mm | 59.05 ± 8.264 | 59.44 ± 11.13 | 0.180a | 0.857 |
| Right ventricular diameters, mm | 59.27 ± 7.823 | 62.80 ± 7.192 | 3.498a | 0.001* |
| Width of pulmonary artery, mm | 34.05 ± 4.919 | 34.37 ± 5.783 | 0.267a | 0.790 |
| Pulmonary artery blood flow, m/s | 0.74 ± 0.177 | 0.66 ± 0.221 | 1.944a | 0.055 |
| Tricuspid annular place systolic excursion, mm | 14.61 ± 3.548 | 13.55 ± 3.619 | 1.341a | 0.183 |
| Tricuspid annular motion velocities, cm/s | 9.75 ± 2.655 | 7.74 ± 2.205 | 3.733a | 0.001* |
| Pulmonary systolic blood pressure, mm Hg | 93.12 ± 18.130 | 82.83 ± 15.240 | 2.782a | 0.007* |
| Tricuspid regurgitation, cm2 | 7.61 ± 4.344 | 12.69 ± 6.910 | 3.954a | <0.001* |
| Hemodynamic | ||||
| Mean pulmonary arterial pressure, mm Hg | 58.7 ± 11.70 | 63.9 ± 11.89 | 1.985a | 0.051 |
| Pulmonary capillary wedge pressure, mm Hg | 8.80 ± 2.939 | 11.34 ± 3.381 | 3.636a | 0.001* |
| Pulmonary vascular resistance, wood unit | 15.35 ± 5.317 | 19.21 ± 5.357 | 3.272a | 0.002* |
| Pulmonary vascular resistance index | 2.73 ± 0.614 | 2.54 ± 0.769 | 1.269a | 0.208 |
| Mean right atrial pressure, mm Hg | 6.44 ± 3.486 | 8.42 ± 4.647 | 2.177a | 0.032* |
| Pulmonary vein oxygen saturation, % | 97.1 ± 1.60 | 97.0 ± 1.97 | 0.308a | 0.759 |
| Pulmonary artery oxygen saturation, % | 66.3 ± 7.33 | 60.5 ± 9.29 | 3.155a | 0.002* |
| IPAH | 45.93c | <0.001* | ||
| Without RHF | 40 | 13 | ||
| With RHF | 1 | 28 | ||
| Therapeutic schedule | 1.942c | 0.379 | ||
| PDE5 inhibitor | 38 | 40 | ||
| ERA | 10 | 12 | ||
| Prostacyclin analogues | 4 | 10 | ||
| Death | 26.10c | <0.001* | ||
| No | 41 | 25 | ||
| Yes | 0 | 16 | ||
| WSPH risk stratification | 82.00c | <0.001* | ||
| Mild | 16 | 3 | ||
| Moderate | 14 | 25 | ||
| Severe | 11 | 13 | ||
Note: a,b, and c represent t, U, and χ2 test, respectively.
P < 0.05.
IPAH: idiopathic pulmonary arterial hypertension; RHF: right heart failure; NT-proBNP: N-terminal pro-B-type natriuretic peptide; HCY: homocysteine; WSPH: the 6th World Symposium on Pulmonary Hypertension; ERA: Endothelin Receptor Antagonist; PDE5: Phosphodiesterase type 5.
Fig. 3.
The correlation analysis between hsa_circ_0068481 and NT-proBNP, HCY, H2S, and 6MWD (six-min walking distance) were analyzed by Spearman’s correlation analysis.
NT-proBNP: N-terminal pro-B-type natriuretic peptide; HCY: homocysteine.
Serum circ_0068481 levels were elevated in patients with IPAH and RHF and act as a prognostic biomarker for RHF
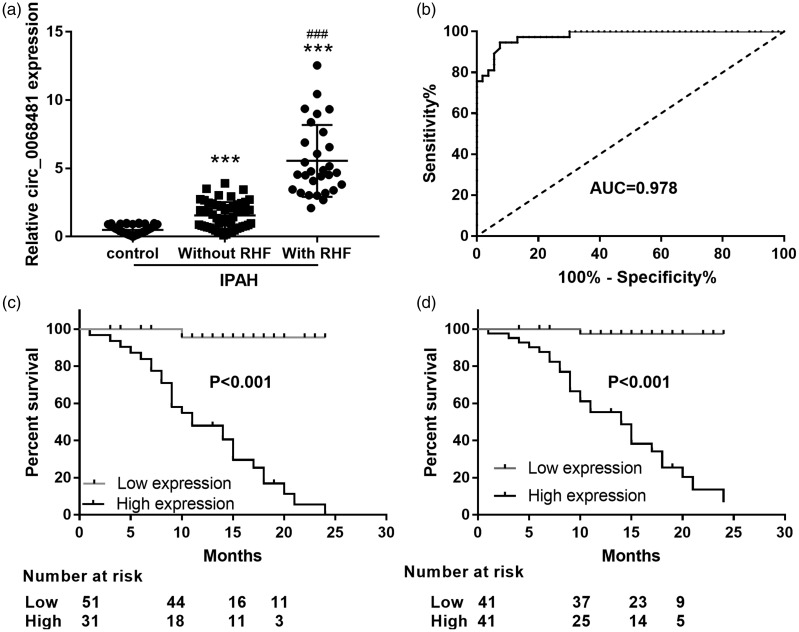
Next, we studied the diagnostic and prognostic effects of serum circ_0068481 levels in patients with IPAH and RHF. qRT-PCR showed that circ_0068481 expression was significantly higher in patients with IPAH without RHF than that in the control group (P < 0.05, Fig. 4a); circ_0068481 expression was significantly higher in patients with IPAH and RHF than patients with IPAH and no RHF (P < 0.05, Fig. 4a). The ability of serum circ_0068481 levels to diagnose IPAH with RHF was assessed using ROC curve analysis. At the optimal expression cutoff value of 2.97, the sensitivity and specificity were 94.59% and 92.45%, respectively (Fig. 4b). Furthermore, to assess whether circ_0068481 expression is a risk factor for IPAH with RHF, patients with IPAH were divided into two groups according to the optimal circ_0068481 expression cutoff value (2.97): low expression group, circ_0068481 expression <2.97 and high expression group: circ_0068481 expression ≥2.97. During the follow-up period, 29 patients had IPAH with RHF, all of whom were in the high expression group. The Kaplan–Meier analysis results are shown in Fig. 4c. Patients with IPAH in the high group exhibited higher RHF incidence than those in the low group (P < 0.05). Additionally, patients with IPAH were divided into two groups according to the median value of ocirc_0068481 expression (2.31): low expression group, circ_0068481 expression <2.31 and high expression group: circ_0068481 expression ≥2.31. During the follow-up period, 29 patients had IPAH with RHF, all of whom were in the high expression group. The Kaplan–Meier analysis result showed that patients with IPAH in the high group exhibited higher RHF incidence than those in the low group (P < 0.05, Fig. 4d).
Fig. 4.
Serum circ_0068481 levels were elevated in patients with idiopathic pulmonary arterial hypertension (IPAH) and right heart failure (RHF) and act as a diagnostic and prognostic biomarker for IPAH with RHF. (a) Serum circ_0068481 levels were detected by qRT-PCR in patients with IPAH with or without RHF. ***P < 0.001 vs control; ###P < 0.001 vs IPAH without RHF. (b) The ability of serum circ_0068481 levels to diagnose IPAH with RHF was assessed using ROC curve analysis. (c) Whether circ_0068481 expression was a risk factor for IPAH with RHF was assessed using Kaplan–Meier analysis. According to the optimal circ_0068481 expression cutoff value (2.97), patients with IPAH were divided into two groups: low group: circ_0068481 expression <2.97, n = 51; high group: circ_0068481 expression ≥2.97, n = 31. (d) Whether circ_0068481 expression was a risk factor for IPAH with RHF was assessed using Kaplan–Meier analysis. According to the median value of ocirc_0068481 expression (2.31), patients with IPAH were divided into two groups: low group: circ_0068481 expression <2.31, n = 41; high group: circ_0068481 expression ≥2.31, n = 41.
AUC: area under the curve; qRT-PCR: real-time quantitative reverse transcription polymerase chain reaction; ROC: receiver operating characteristic.
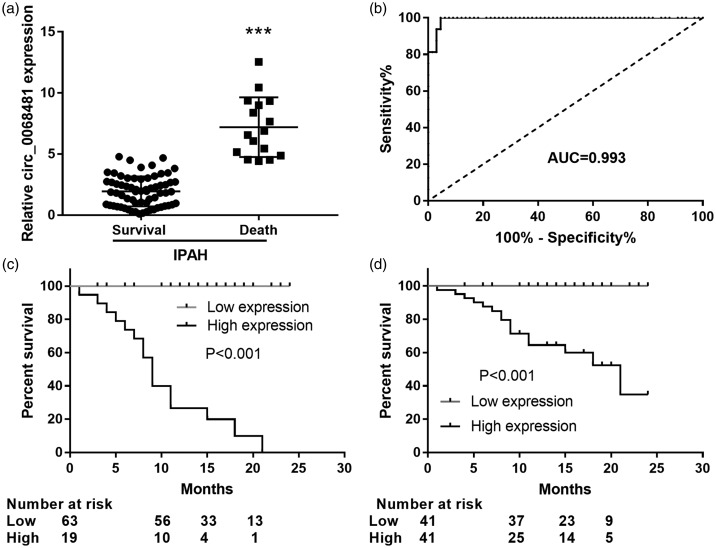
Serum circ_0068481 levels were elevated in patients with IPAH who died and act as a prognostic biomarker for clinical outcome
Finally, we studied the effects of serum circ_0068481 levels on clinical outcome in patients with IPAH. qRT-PCR showed that circ_0068481 expression was significantly higher in patients with IPAH who died than in those who survived (P < 0.05, Fig. 5a). The ability of serum circ_0068481 levels to diagnose the clinical outcome of IPAH was analyzed using ROC curve analysis. At the optimal expression cutoff value (4.25), the sensitivity and specificity were 100 and 95.45%, respectively (Fig. 5b). To assess whether circ_0068481 expression was a risk factor for clinical outcome in IPAH, patients with IPAH were divided into two groups according to the optimal circ_0068481 expression cutoff value (4.25): low expression group, circ_0068481 expression <4.25 and high expression group: circ_0068481 expression ≥4.25. During the follow-up period, 16 patients died, all of whom were in the high expression group. The Kaplan–Meier analysis results are shown in Fig. 5c. Patients with IPAH in the high group exhibited a poorer clinical outcome than those in the low group (P < 0.05). Additionally, patients with IPAH were divided into two groups according to the median value of ocirc_0068481 expression (2.31): low expression group, circ_0068481 expression <2.31 and high expression group: circ_0068481 expression ≥2.31. During the follow-up period, 16 patients died, all of whom were in the high expression group. The Kaplan–Meier analysis result showed that patients with IPAH in the high group exhibited a poorer clinical outcome than those in the low group (P < 0.05, Fig. 5d). Univariate analyses were conducted to evaluate whether circ_0068481 expression and various non-invasion clinicopathological parameters were prognostic factors of patient outcome (Table 2). The results suggested that NT-proBNP, HCY, H2S, circ_0068481 expression, and 6MWD were prognostic factors that indicate poor prognosis in patients with IPAH.
Fig. 5.
Serum circ_0068481 levels were elevated in patients with idiopathic pulmonary arterial hypertension (IPAH) who died and act as a diagnostic and prognostic biomarker for poorer clinical outcome in IPAH. (a) Serum circ_0068481 levels were detected by qRT-PCR in patients with IPAH who survived and died. ***P < 0.001. (b) The ability of serum circ_0068481 levels to diagnose the death of IPAH patients was assessed using ROC curve analysis. (c) Whether circ_0068481 expression was a risk factor for the death of IPAH patients was assessed using Kaplan–Meier analysis. According to the optimal circ_0068481 expression cutoff value (4.25), patients with IPAH were divided into two groups: low group: circ_0068481 expression <4.25, n = 63; high group: circ_0068481 expression ≥4.25, n = 19. (d) Whether circ_0068481 expression was a risk factor for the death of IPAH patients was assessed using Kaplan–Meier analysis. According to the median value of ocirc_0068481 expression (2.31), patients with IPAH were divided into two groups: low group: circ_0068481 expression <2.31, n = 41; high group: circ_0068481 expression ≥2.31, n = 41.
AUC: area under the curve; qRT-PCR: real-time quantitative reverse transcription polymerase chain reaction; ROC: receiver operating characteristic.
Table 2.
Results of the univariate analyses of different parameters in patients with IPAH by Cox regression.
| Characteristic | Risk ratio | 95% CI | P |
|---|---|---|---|
| Age, years | 1.009 | 0.996–1.022 | 0.180 |
| Sex | 0.346 | 0.078–1.526 | 0.161 |
| Body mass index | 1.113 | 0.942–1.315 | 0.207 |
| NT-proBNP | 1.000 | 1.000–1.001 | <0.001* |
| HCY | 1.004 | 1.002–1.006 | <0.001* |
| H2S | 1.013 | 1.007–1.019 | <0.001* |
| Circ_0068481 | 2.581 | 1.892–3.514 | <0.001* |
| 6MWD | 0.993 | 0.986–0.999 | 0.030* |
Note: *P < 0.05.
IPAH: idiopathic pulmonary arterial hypertension; NT-proBNP: N-terminal pro-B-type natriuretic peptide; HCY: homocysteine; 6MWD: six-minute walking distance.
Discussion
IPAH can induce RHF and patient death. Currently, specific and non-invasive biomarkers for IPAH diagnosis and prognosis are scarce. In this study, we found that elevated serum circ_0068481 expression had high sensitivity and specificity for diagnosing IPAH. Additionally, serum circ_0068481 levels were elevated in patients with IPAH and RHF and in patients with IPAH who died, thus act as a biomarker for clinical outcome.
circRNAs have continuous, stable, and covalently closed circular structures and are not easily degraded by nucleases, thus are ideal serum biomarkers for detecting diseases. circRNAs are considered diagnostic and prognostic biomarkers in patients with tumors, hypertension, and cardiovascular diseases.16–18 In hypoxia-induced PAH, 74 abnormally expressed circRNAs, including mmu_circRNA_004592 and mmu_circRNA_018351, display potential as diagnostic biomarkers for PAH.19 Additionally, 351 circRNAs are abnormally expressed in chronic thromboembolic PAH, with hsa_circ_0002062 and hsa_circ_0022342 potentially being key circRNAs for diagnosing chronic thromboembolic PAH.13 However, studies researching circRNAs as diagnostic and prognostic biomarkers for IPAH are lacking. In this study, we found that serum circ_0068481 expression was significantly higher in patients with IPAH than in healthy volunteers. Elevated serum circ_0068481 levels had high sensitivity (74.39%) and specificity (98.78%) for diagnosing IPAH, suggesting that circ_0068481 is a novel and noninvasive biomarker for diagnosing IPAH. Additionally, we found that circ_0068481 expression was significantly higher in patients with IPAH without RHF than that in the control group, and suggested that circ_0068481 expression was induced by IPAH.
Additionally, we found that circ_0068481 expression correlated significantly with heart function, 6MWD, serum NT-proBNP, serum H2S, WSPH risk stratification, right ventricular dimension, tricuspid annular motion velocities, pulmonary systolic blood pressure, tricuspid regurgitation, pulmonary capillary wedge pressure, pulmonary vascular resistance, mean right atrial pressure, pulmonary artery oxygen saturation, and IPAH with RHF. Right ventricular dimension, tricuspid annular motion velocities, and tricuspid regurgitation have been associated with right ventricular enlargement and dysfunction,20 whilst pulmonary systolic blood pressure, pulmonary capillary wedge pressure, pulmonary vascular resistance, mean right atrial pressure, and pulmonary artery oxygen saturation have been associated with IPAH development. Serum NT-proBNP, H2S, and 6MWD have been reported to correlate with IPAH progression and RHF,21–23 suggesting that circ_0068481 may be associated with RHF in patients with IPAH. In this study, we found that circ_0068481 expression correlated significantly with RHF and the death of patients with IPAH, further suggesting that circ_0068481 plays an important role in regulating IPAH progression. We also found that serum circ_0068481 levels were higher in patients with IPAH and RHF than in those without RHF and that elevated circ_0068481 levels could diagnose and predict RHF. Moreover, serum circ_0068481 levels were higher in patients with IPAH who died than in those who survived, with elevated circ_0068481 levels able diagnose and predict poor clinical outcome. These results suggest that circ_0068481 can predict poor clinical outcome in patients with IPAH.
This study has several limitations. First, as an observational study, a cause-and-effect relationship between circ_0068481 and IPAH prognosis cannot be established. Second, we did not analyze the effect of treatment schedule on circRNA expression in patients with IPAH, which may affect the prognosis judgment. Third, circ_0068481 levels were not serially assessed during follow-ups. Additionally, the sample size and follow-up period were relatively limited; therefore the magnitude of this relationship must be interpreted with caution due to the low number of IPAH in this cohort and the potential limited generalizability of these results. Large-scale prospective studies are required to confirm the prognostic ability of circ_0068481 in IPAH.
In conclusion, circ_0068481 is a novel and noninvasive biomarker for diagnosing IPAH and predicting poor clinical outcome in patients with IPAH.
Availability of data and material
Datasets analyzed during the present study are available from the corresponding author on reasonable request.
Ethical approval
This study was approved by the Ethics Committee of Guangdong Provincial People’s Hospital (No. GDREC2016305H).
Contributorship
YZ, YC, HT, and YZ conceived and designed the present study and developed the methodology. YZ, YC, HY, ZL, HT, and YZ completed the experiment and collected the data. YZ, YC, GC, HT, and YZ analyzed and interpreted the data. YZ and YC drafted the manuscript. All authors read and approved the final manuscript.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This study was supported by Grants from the Scientific Technology Project of Guangdong Province (No. 2016A020215227), the Scientific Technology Project of Guangzhou (No. 201707010432), and the Natural Science Foundation of Guangdong Province (No. 2016A030313798).
References
- 1.Vonk-Noordegraaf A, Haddad F, Chin KM, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol 2013; 62: D22–D33. [DOI] [PubMed] [Google Scholar]
- 2.Haddad F, Doyle R, Murphy DJ, et al. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation 2008; 117: 1717–1731. [DOI] [PubMed] [Google Scholar]
- 3.Nickel N, Kempf T, Tapken H, et al. Growth differentiation factor-15 in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2008; 178: 534–541. [DOI] [PubMed] [Google Scholar]
- 4.Hampole CV, Mehrotra AK, Thenappan T, et al. Usefulness of red cell distribution width as a prognostic marker in pulmonary hypertension. Am J Cardiol 2009; 104: 868–872. [DOI] [PubMed] [Google Scholar]
- 5.Chen G, Yang T, Gu Q, et al. Elevated plasma YKL-40 as a prognostic indicator in patients with idiopathic pulmonary arterial hypertension. Respirology 2014; 19: 608–615. [DOI] [PubMed] [Google Scholar]
- 6.Holdt LM, Kohlmaier A, Teupser D. Molecular roles and function of circular RNAs in eukaryotic cells. Cell Mol Life Sci 2018; 75: 1071–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salgado-Somoza A, Zhang L, Vausort M, et al. The circular RNA MICRA for risk stratification after myocardial infarction. Int J Cardiol Heart Vasc 2017; 17: 33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou B, Yu JW. A novel identified circular RNA, circRNA_010567, promotes myocardial fibrosis via suppressing miR-141 by targeting TGF-beta1. Biochem Biophys Res Commun 2017; 487: 769–775. Research Support, Non-U S Gov’t. [DOI] [PubMed] [Google Scholar]
- 9.Wang K, Long B, Liu F, et al. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur Heart J 2016; 37: 2602–2611. [DOI] [PubMed] [Google Scholar]
- 10.Wang K, Gan TY, Li N, et al. Circular RNA mediates cardiomyocyte death via miRNA-dependent upregulation of MTP18 expression. Cell Death Differ 2017; 24: 1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du WW, Yang W, Chen Y, et al. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur Heart J 2017; 38: 1402–1412. [DOI] [PubMed] [Google Scholar]
- 12.Du WW, Yang W, Liu E, et al. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res 2016; 44: 2846–2858. Research Support, Non-U S Gov’t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miao R, Wang Y, Wan J, et al. Microarray expression profile of circular RNAs in chronic thromboembolic pulmonary hypertension. Medicine 2017; 96: e7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). The Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39.e14. [DOI] [PubMed] [Google Scholar]
- 16.Chaichian S, Shafabakhsh R, Mirhashemi SM, et al. Circular RNAs: a novel biomarker for cervical cancer. J Cell Physiol Epub ahead of print 27 June 2019. DOI: 10.1002/jcp.29009. [DOI] [PubMed]
- 17.Zaiou M. Circular RNAs in hypertension: challenges and clinical promise. Hypertens Res 2019; 42: 1653–1663. [DOI] [PubMed] [Google Scholar]
- 18.Li JJ, Wang W, Wang XQ, et al. A novel strategy of identifying circRNA biomarkers in cardiovascular disease by meta-analysis. J Cell Physiol 2019; 234: 21601–21612. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Zhu MC, Kalionis B, et al. Characteristics of circular RNA expression in lung tissues from mice with hypoxiainduced pulmonary hypertension. Int J Mol Med 2018; 42: 1353–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vezzosi T, Domenech O, Costa G, et al. Echocardiographic evaluation of the right ventricular dimension and systolic function in dogs with pulmonary hypertension. J Vet Intern Med 2018; 32: 1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suntharalingam J, Goldsmith K, Toshner M, et al. Role of NT-proBNP and 6MWD in chronic thromboembolic pulmonary hypertension. Respir Med 2007; 101: 2254–2262. [DOI] [PubMed] [Google Scholar]
- 22.Mirsaeidi M, Omar HR, Baughman R, et al. The association between BNP, 6MWD test, DLCO% and pulmonary hypertension in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2016; 33: 317–320. [PubMed] [Google Scholar]
- 23.Lopez V, Moraga FA, Llanos AJ, et al. Plasmatic concentrations of ADMA and homocystein in llama (Lama glama) and regulation of arginase type II: an animal resistent to the development of pulmonary hypertension induced by hypoxia. Front Physiol 2018; 9: 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets analyzed during the present study are available from the corresponding author on reasonable request.