Abstract
Spheroid culture is a widely used three-dimensional culture technology that simulates the three-dimensional structure of tumors in vivo and has been considered a good model for tumor research. However, current commercialized spheroid culture tools have the shortcomings of high cost or relatively poor spheroid-forming results for some special cells. To solve such problems, we designed a 3D printed, reusable, stamp-like resin mold that could shape microstructures for spheroid culture of tumor cells on the surface of agarose substrate in a 96-well plate. We applied this homemade three-dimensional culture tool in spheroid formation for hepatocellular carcinoma cells. The experimental data show that the effect of spheroid culture on four hepatocellular carcinoma cell lines in our homemade spheroid culture plate is better than that of the commercialized ultralow attachment spheroid culture plate, and compared to two-dimensional culture, three-dimensional culture improves cell functions. In addition, the drug-sensitive test based on patient-derived hepatocellular carcinoma cells showed a different pattern between spheroid and two-dimensional cultures. In conclusion, our spheroid culture tool is characterized by its low cost, reusability, low cell consumption, convenience in medium exchange, and good effect of spheroid formation, suggesting that this technique could be widely used in individual treatment and high-throughput drug screening.
Keywords: 3D culture, spheroids, agarose, drug screening, primary hepatocarcinoma cells
Introduction
The progress of antitumor drug screening has been greatly hampered by the shortage of reproducible in vitro cell-based models by which to assess the efficacy of candidate therapeutic agents.1,2 Because of the convenience in appliance, two-dimensional (2D) cultured cell lines are still dominant in the research of antitumor agents. Recent studies have shown that 2D culture fails to recapitulate the critical features of tumors growing in vivo, especially their three-dimensional (3D) organization.3 This implies that the drug response of 2D-cultured cancer cells may not reflect the actual response of cancer cells in vivo.
Because of advances in cell biology, microfabrication technology, and tissue engineering, new 3D cell culture models have emerged in recent years. With the gradual maturation of 3D culture techniques, artificial organic microtissues provide good news for the screening and efficacy evaluation of antineoplastic drugs. At present, there are various 3D culture techniques, including multicellular spheroids, organoids, scaffolds, hydrogels, organs-on-chips, and 3D bioprinting, each with its own advantages. Although the principles and protocols of these 3D culture techniques are different, compared with 2D culture, these methods can better simulate the morphology, functions, and microenvironment of cells in vivo.4
Spheroid culture is a widely used 3D culture technology and has been developed for nearly three decades.5 This technique is characterized by its low technical difficulty and cost, and it can well imitate the 3D structure of tumors in vivo.6,7 Moreover, the spheroid model compensates for the many deficiencies observed in monolayer cultures. For instance, spheroids can create heterogeneous cell populations (e.g. hypoxic vs normoxic, quiescent vs replicating cells) by developing gradients of oxygen, nutrients, metabolites, and soluble signals. In addition, spheroids have a well-defined geometry and undergo optimal physiological cell–cell and cell–extracellular matrix (ECM) interactions.8 A recent study revealed that spheroids composed of patient-derived tumor cells could maintain tumor characteristics in vitro for a long time.9 These findings indicate that spheroid culture has great application value in individualized treatment and screening new anti-cancer drugs.
At present, the commercialized tools for spheroid culture are mainly hanging drop spheroid culture plates and ultralow attachment cell culture plates. The operation of a hanging drop spheroid culture plate (e.g. GravityPLUS Kit, ISP-06-010; InSphero, Schlieren, Switzerland) is complex, and the formed cell spheroids need to be transferred to a new culture plate. The ultralow attachment cell culture plate (e.g. Ultralow Attachment Round-Bottom 96-Well Plate, 3603; Corning, New York, USA) is not suitable for certain cell lines or primary cells that are not inclined to form spheroids. In addition, the cell spheroids were easily sucked away when pipetting the medium. And the high cost of these commercialized spheroid culture tools hinders their application in high-throughput drug screening. Therefore, it is urgent to develop inexpensive, convenient, and efficient tools for spheroid culture for high-throughput drug tests.
Agarose is a kind of biomaterial with good biocompatibility, low price, and low attachment that could promote cell spheroidization.10 In a previous study using 3D printing technology, we developed a new resin mold to create agarose concave Petri dishes for the mass production of spheroids with hepatocellular carcinoma (HCC) cell lines.11 However, the fabrication of this agarose-based mold is comparatively complex and is more suitable for creating agarose concave 12- or 24-well plates, which does not meet the requirements of a high-throughput drug test. In this model, each dish contained 121 wells, which required relatively high cell-seeding densities (1.45 × 105 per dish). Patient-derived tumor cells are necessary for experiments of individualized drug screening or searching for new antitumor drugs; however, the number of primary tumor cells from the removed tissue is limited. Therefore, an in vitro cell-based spheroid model with features of low seeding cell consumption, high-throughput tests, and easy use should be considered. In this study, we developed a new cell spheroid culture tool: a stamp-like resin mold with six rows of tiny hemispherical protuberances fabricated with the assistance of 3D printing technology. This tool could be used to create agarose microwells for spheroid culture (1200 cells or less in every well) in ordinary commercial 96-well cell culture plates, which is efficient in spheroid formation from different cell lines or primary solid tumor cells. Moreover, this tool has the characteristics of low cost and reusability and is suitable for high-throughput antitumor agent sensitivity tests.
To test the function of our new tool, we first applied it in building HCC cell spheroids. The reason we chose HCC cells is that HCC is one of the most lethal malignancies worldwide.12 Due to the relatively high chemoresistance of HCC, transarterial chemoembolization (TACE), which can greatly increase the drug concentration in tumor, is now the first-line treatment for patients with intermediate stage HCC.13 However, the chemotherapy regimens of TACE for HCC patients were mainly based on the clinical experience of the doctor. Theoretically, HCC is a complex entity; each HCC patient is unique. Therefore, a personalized chemotherapy strategy based on the drug sensitivity data using a patient-derived HCC tumor model should be recommended.
In this study, we evaluated the effect of homemade agarose microwells on spheroid formation in HCC cell lines and tested their cell functions and features compared to 2D culture. Furthermore, we used the agarose microwell plate in a chemotherapy drug sensitivity assay based on primary HCC cells from patients. Our data show that agarose microwell plates made by the stamp-like resin mold are a promising tool for building high-throughput in vitro models of 3D tumor spheroids.
Materials and methods
Fabrication of agarose microwells in a 96-well plate
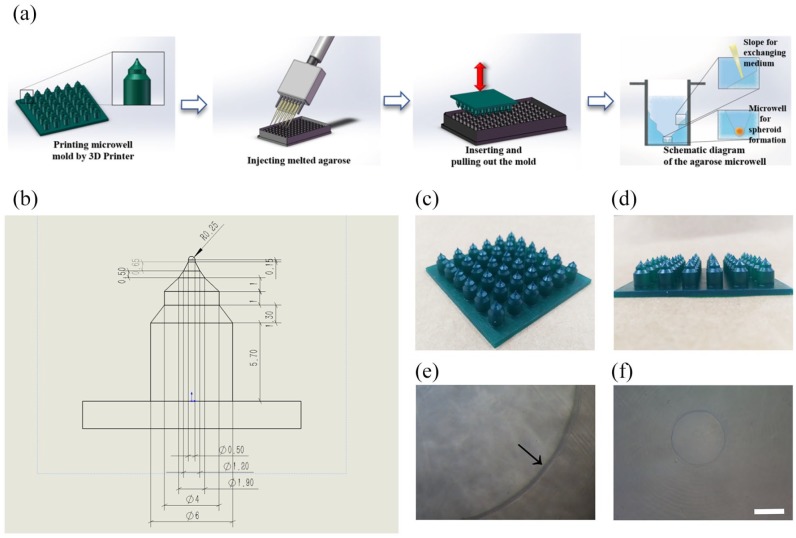
A schematic diagram of the fabrication process for agarose microwell 96-well plates using the newly designed resin molds is illustrated in Figure 1(a). The detailed fabrication process is as follows: (1) the stamp-like molds were designed and created using SolidWorks software (Dassault Systèmes, Concord, USA). The mold consists of a flat plate (60 mm × 60 mm × 2 mm) and six rows of columns for agarose shaping (the height of each column is 9 mm, and the interval between the centers of the adjacent columns is 9 mm) (Figure 1(a)). Each column contains three gradually contracting cylinders connected by two slopes with a hemisphere on the top. The specific parameters are shown in Figure 1(b); (2) a Formlabs Phoenix Touch Pro UV-LED 3D Printing System (Full Spectrum Laser, Las Vegas, USA) was used to fabricate the photosensitive resin (SpeedCast Green Resin; Full Spectrum Laser) molds; (3) after printing, the resin molds were immersed in pure alcohol for 20 min and then the resin residue was rinsed from the mold surface; (4) before use, the resin mold was sterilized with 75% alcohol or under an ultraviolet lamp for 1 h; (5) 2% (w/v, agarose/deionized water) melted (80°C–100°C) agarose (111860; Biowest Regular, Hong Kong, China) was added to the 96-well plate (Black Plate, Clear Bottom 96-Well Assay Plate, 3603; Corning) (170 μL/well); (6) before agarose solidification, the mold was immediately clamped with tweezers and then inserted, convex face downward, into the cell culture wells. After cooling for 5 min at room temperature until agarose solidification, the molds were pulled out with tweezers. Then, the structures of the medium exchange slope and multicellular spheroid culture microwells were formed (Figure 1(a)); (7) before using the agarose microwells, 170 μL of Dulbecco’s Modified Eagle’s Medium (DMEM, C11995500BT; Gibco, Beijing, China) was added to saturate each agarose well and avoid the adsorption of nutrients from medium by the agarose during cell culture. After 15 min, the culture medium was removed, and the process was repeated 3 times. The agarose concave 96-well culture plate was then ready for use.
Figure 1.
Fabrication of the agarose microwell 96-well plate. (a) Schematic diagram of the fabrication process for agarose microwell 96-well plates. (b) Parameters of the resin column (unit: mm). (c, d) Resin mold printed by 3D printer. (e) Black arrow points to the agarose inclined platform for medium exchange. (f) The microwell diameter in each cell culture well is approximately 400 μm. Scale bar = 200 μm.
Tests of spheroid formation efficiency and cell function
HCC cell line culture and spheroid formation
The human HCC cell lines PLC/PRF/5, HepG2, Hep3b, and SK-Hep1 were purchased from ATCC (American Type Culture Collection). All cell lines were cultured in DMEM with 10% fetal bovine serum (A3160801; Gibco). For spheroid formation, cells at different densities (300, 600, 1200, and 2400 cells/well) were seeded onto a homemade spheroid culture plate or ultralow attachment round-bottom 96-well plate (7007; Corning). HCC cells were then cultured in DMEM with 10% fetal bovine serum and incubated at 37°C under a humidified atmosphere with 5% CO2.
Cell proliferation test
The cells were seeded at a density of 1200 cells/well. After culturing for 1 day, 3 days, 6 days, 9 days, 12 days, and 15 days, the viability of the cells seeded into microwells was counted using the CellTiter-Glo® 3D Cell Viability Assay (G9683; Promega Corporation, Madison, USA) according to the manufacturer’s instructions, and the number of spheroids at different times was evaluated using a standard curve.
Calcein-AM/propidium iodide staining
The cells were incubated in phosphate-buffered saline (PBS, C10010500BT; Gibco) with 2 μM Calcein-AM and 2.5 μg/mL PI (C326 and P346; Dojindo Molecular Technologies, Shanghai, China) for 10 min at room temperature, and then the spheroids were examined and photographed under a fluorescence microscope.
RNA isolation and quantitative reverse-transcription polymerase chain reaction
Total RNA was extracted from HepG2 cells cultured under monolayer or spheroid conditions using TRIzol Reagent (15596018; Thermo Fisher Scientific, Shanghai, China) in accordance with the manufacturer’s instructions. For quantitative reverse-transcription polymerase chain reaction (qRT-PCR), total RNA was reverse-transcribed to cDNA. Next, cDNA was quantified by real-time PCR with a SYBR Green qPCR Master Mix (4309155; Thermo Fisher Scientific). These tested genes include albumin synthesis gene (ALB), phase-I enzyme genes (CYP2C9 and CYP3A4), phase-II enzyme genes (SUT2A1 and UGT1A1), and a drug transporter gene (ABCG2). GAPDH was used as a reference gene. The primers used in this experiment are shown in Table 1.
Table 1.
Primers for qRT-PCR.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| ALB | CAAAGATGACAACCCAAACCTC | GGATGTCTTCTGGCAATTTCA |
| CYP2C9 | CCAAAGAACCTTGACACCACTC | AATGCCCCAGAGGAAAGAGAG |
| CYP3A4 | GTGGTGATGATTCCAAGCTATGC | TCCTTGTTCTTCTTGCTGAATC |
| UGT1A1 | GGAATCAACTGCCTTCACCA | GCAATTGCCATAGCTTTCTTCT |
| SUT2A1 | GATGTCCAATTATTCCCTCCTG | TCTGCCATCTTCTCTTGGAAC |
| ABCG2 | CCTGTGGAGGAACTGGGTA | TAAGGATGTAAATGTTGGGATG |
| GAPDH | AGCCACATCGCTCAGACACC | ACCCGTTGACTCCGACCTT |
qRT-PCR: quantitative reverse-transcription polymerase chain reaction.
Phalloidin-iFluor 488 reagent staining of the cell spheroids
To observe the structure of the spheroids, HepG2 and PLC/PRF/5 spheroids were fixed with 4% paraformaldehyde (P0099; Beyotime Biotechnology, Shanghai, China) for 24 h, permeabilized with 0.1% Triton X-100 (ST795; Beyotime Biotechnology) for 2 h at room temperature and then washed 3 times with PBS. The spheroids were incubated with Phalloidin-iFluor 488 Reagent (ab176753; Abcam, Cambridge, UK) and DAPI (ab228549; Abcam) for 2 h in the dark and then washed 3 times with PBS. Fluorescence images were obtained using multiphoton excitation laser scanning microscope (FV1200MPE; OLYMPUS, Tokyo, Japan).
3D culture of primary HCC cells in a homemade spheroid culture plate and drug sensitivity testing
Isolation and culture of human primary HCC cells
The HCC specimens were obtained from patients in the Department of Hepatobiliary Surgery II, Zhujiang Hospital of Southern Medical University, Guangzhou, China. Relative information on HCC patients is shown in Table 2. The samples were obtained with the consent of patients and the hospital ethics committee (approval document number: 2017-GDEK-004). Immediately after surgery, we first cut the HCC tumor into 3–4 slices and obtained specimens (total volume is 3–9 cm3 according to the size of the tumor) from different areas of each tumor slice, avoiding the necrotic area. Then, the specimens were immersed in DMEM and transported to the laboratory at 0°C. The specimens were collected under sterile conditions and rinsed 2–3 times with DMEM to remove the blood. After removing the blood, the tumor samples were cut into small fragments (1 mm3 or smaller), gently dispersed, and the cut specimens were placed in Hank’s Balanced Salt Solution (HBSS, 14025076; Gibco) containing 0.1% type-IV collagenase (17104-019; Gibco) and digested for 1–2 h at 37°C. The resulting suspension was filtered through a 100-μm nylon filter and centrifuged at 50 × g for 3 min at 4°C. Then, the pellet was washed twice with HBSS. The final cell suspensions were cultured in T25 flasks (TCF001050; JETBIOFIL, Guangzhou, China) and hepatocyte culture medium (CC-3198; Lonza, Basel, Switzerland) at 37°C in a humidified incubator with 5% CO2. The medium was changed at 24 h after seeding to remove the dead cells and debris. After 2–3 days of culture, the primary cells were harvested and seeded onto ordinary 96-well plates or homemade spheroid culture plates at a density of 1200 cells/well.
Table 2.
Donor characteristics at the time of HCC resection.
| Patient characteristics | ID: 1188890 | ID: 2880826 |
|---|---|---|
| Age (years)/sex | 27/female | 69/male |
| HBsAg | Positive | Positive |
| Serum HBV DNA | 7.08 × 102 IU/mL | 3.05 × 105 IU/mL |
| Anti-HCV antibody | Negative | Negative |
| Serum α-fetoprotein | 26.8 μg/L | 4 μg/L |
| HCC pathologic diagnosis | Poorly differentiated HCC | Highly differentiated HCC |
| Vascular invasion | No vascular invasion | |
| No cirrhosis | Cirrhosis |
HCC: hepatocellular carcinoma; HBsAg: hepatitis B virus surface antigen; HBV: hepatitis B virus; HCV: hepatitis C virus.
Immunofluorescence analysis
An immunofluorescence analysis was performed to confirm that the isolated primary cells were HCC cells. The primary cells were fixed with 4% paraformaldehyde for 10 min at room temperature, permeabilized with 0.1% Triton X-100 for 20 min at room temperature, and then washed 3 times with PBS. To reduce background non-specific staining and permeabilize the sample, the cells were incubated with 5% bovine serum albumin (BSA) and 0.5% Triton in Tris-buffered saline (TBS) solution for 1 h. The following primary antibodies were purchased from Abcam: anti-human serum albumin (ALB, ab207327), anti-human keratin 18 (CK-18, ab668), anti-human Arginase-1 (ARG-1, ab212522), and anti-human alpha fetoprotein (AFP, ab169552). The samples were incubated with the primary antibodies for 16 h at 4°C and then washed 3 times for 10 min with PBS. The secondary antibodies used for staining were goat anti-mouse IgG conjugated with Alexa® Fluor 488 (ab150078; Abcam) and goat anti-rabbit IgG conjugated with Alexa Fluor 555 (ab150117; Abcam). The samples were then incubated with secondary antibodies for 1 h at room temperature in the dark and washed 5 times for 10 min with PBS. The cells were incubated with DAPI (Abcam) for 10 min at room temperature in the dark and quickly washed twice with PBS to stain the nucleus. All fluorescent images were obtained using a Leica fluorescence microscope (Dmi8+DFC7000T; Leica Microsystems, Wetzlar, Germany).
Drug sensitivity test in 2D and spheroid culture
The primary cells were incubated for 2 days in monolayer or 3D culture plates (1200 cell/well). A drug sensitivity test was then performed to compare the cell response to chemotherapeutic agents between the 2D and the 3D cultures. The chemotherapeutic drugs used in this experiment include adriamycin (ADM), cisplatin (DDP), gemcitabine (GEM), oxaliplatin (OXA), and 5-fluorouracil (5-FU) as a single drug treatment, and ADM + DDP, GEM + OXA, and 5-FU + OXA as a combination, which are commonly applied in clinical treatment for HCC. The highest concentrations of drugs were set according to the preliminary experimental results. Specifically, antitumor agents were serially diluted to six concentrations with culture medium and then added into the cell culture well after 2–3 days of culture. Cell viability was measured using a 3D cell viability assay after incubating for 24 h with the drugs. Finally, the inhibitory concentration of 50% (IC50) and inhibitory concentration of 90% (IC90) of each drug scheme were calculated using the Prohibit method with SPSS 20.0 software (IBM SPSS software, New York, USA). In general, the categories of in vitro sensitivity/resistance of tumor to systemic drugs could be predicted based on the relationship between the IC50 value and the peak plasma concentration (PPC).14 However, TACE treatment was recommended for these two HCC patients in our study. Considering TACE is the local treatment, and the intertumoral drug concentration by TACE is approximately 50 times higher than intravenous injection.15 We modified the criteria of the drug sensitivity according to the method reported previously.16 The criteria of TACE chemotherapy sensitivity is shown in Table 3. The PPCs of drugs used in this study are listed as follows: adriamycin, 0.5 µg/mL; cisplatin, 2.4 µg/mL; gemcitabine, 5 µg/mL; oxaliplatin, 3 µg/mL; 5-fluorouracil, 27 µg/mL.17–21 Each assay was repeated in triplicate.
Table 3.
Criteria of the sensitivity.
| High sensitivity | IC50 < 25 ivPPC |
| Sensitivity | 25 ivPPC ⩽ IC50 ⩽ 50 ivPPC |
| Resistance | IC50 > 50 ivPPC |
ivPPC: drug peak plasma concentration by intravenous injection.
Histology and staining
After 6 days of culturing, the multicellular spheroids in the microwells were collected and washed 3 times with PBS. The spheroids were then fixed in 2% agarose solution. Spheroids and their corresponding liver cancer tissues were fixed with 4% formalin and dehydrated with gradient alcohol and xylene. The spheroids and tissues were embedded in paraffin and cut into 4-µm-thin sections. For hematoxylin–eosin (HE) staining, the sections were stained with hematoxylin and eosin. For immunohistological staining, paraffin slides were deparaffinized and subjected to antigen retrieval using citrate sodium solution (pH 6.0). To reduce non-specific staining and permeabilize the sample, slides were incubated in TBS solution containing 1% BSA and 0.5% Triton for 1 h at room temperature. Slides were then washed 3 times with PBS and incubated with primary antibodies anti-liver arginase antibody (ARG-1, ab239731; Abcam) at 1:500 dilution overnight at 4°C. Endogenous peroxidase activity was blocked in a 3% hydrogen-peroxide/methanol buffer for 15 min at room temperature. Detection of bound antibody was accomplished with the Streptavidin Peroxidase Kit (SP-9001; ZSGB-BIO, Beijing, China).
Statistical analysis
All experiments were performed at least 3 times. The results are presented as mean ± standard deviation. Statistical analysis was performed using a two-tailed unpaired Student’s t-test. The value of p less than 0.05 was considered statistically significant.
Results
Preparation of agarose microwell 96-well plates
The photosensitive resin mold printed by a 3D printer is shown in Figure 1. The surface of the mold is smooth, and the structure is clear without defects, as show in Figure 1(c) and (d). The agarose 3D culture chambers were fabricated using this mold in a commercial 96-well plate. The hemispherical concave holes with a diameter of approximately 400 μm (Figure 1(f)) and an inclined platform for medium exchange were formed in each cell culture well, as show in Figure 1(e).
HCC cell lines form uniformed spheroids in agarose microwell 96-well plates
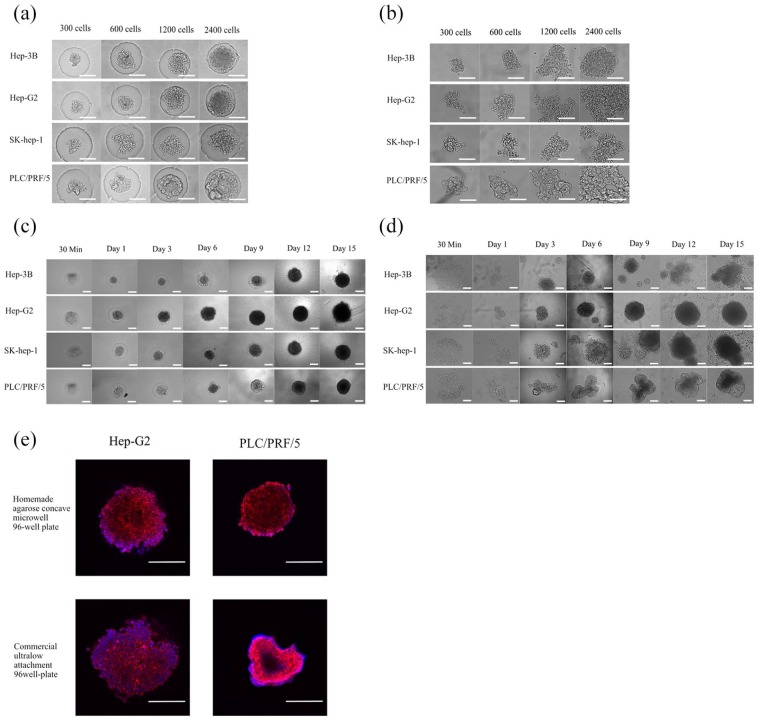
To compare the effect of spheroid formation in a homemade or commercial spheroid culture system, four HCC cell lines—PLC/PRF/5, HepG2, Hep3b, and SK-Hep1—were seeded onto agarose microwell 96-well plates or ultralow attachment round-bottom 96-well plates at different densities (300, 600, 1200, and 2400 cells/well) for 15 days of culture, and their morphological changes were observed. We found that compared with the commercial spheroid culture plate, the homemade spheroid culture plate could restrict the distribution of cells and agglomerate the cell into agarose micropores. In the homemade spheroid culture plate, most of the HCC cell lines at different seeding densities could form relatively uniform and regular spheres within 24 h, except for SK-Hep1 cells at an initial seeding density of 600 cells/well (Figure 2(a)), and all cells could maintain regular spheres during the later stage of cell culture (Figure 2(c)). In the ultralow attachment round-bottom 96-well plate, the spheroid-forming effect of different cell lines was inconsistent. The effect of spheroid formation on HepG2, PLC/PRF/5, and SK-Hep1 cells was poor in the first 24 h, and the morphology of formatted microtissues was irregular (Figure 2(b)). At the later stage of culture (9–15 days), the irregularity of the formatted microtissues in the ultralow attachment culture plate was aggravated (except for HepG2 cells), and abundant scattered single cells were observed around the bottom of the cell culture wells (Figure 2(e)). Immunofluorescence showed that the microstructures of HepG2 and PLC/PRF/5 cell lines were spherical and regular in the homemade spheroid culture system, while the microstructures formed on the commercialized ultralow attachment round-bottom 96-well plate were relatively irregular (Figure 2(c)).
Figure 2.
Hepatocellular carcinoma cell lines cultured in homemade or commercialized spheroid culture plates. (a) HCC cell lines were seeded at different densities (300–2400 cells/well) in agarose microwell 96-well plates, and pictures were taken after incubating for 24 h. (b) HCC cell lines cultured in ultralow attachment round-bottom 96-well plates at different initial seeding densities (300–2400 cells/well), and images were taken after incubation for 24 h. (c) HCC cell lines were cultured in homemade agarose microwell 96-well plates at an initial seeding density of 1200 cells/well and cultured for 15 days. (d) HCC lines were cultured in ultralow attachment round-bottom 96-well plates at an initial seeding density of 1200 cells/well and cultured for 15 days. (e) Immunofluorescence images of HCC cell spheroids cultured in homemade or commercialized spheroid culture plates for 6 days. Red is Phalloidin, and blue is DAPI. Scale bar = 200 μm.
Proliferation of HCC cell lines in agarose microwell 96-well plates
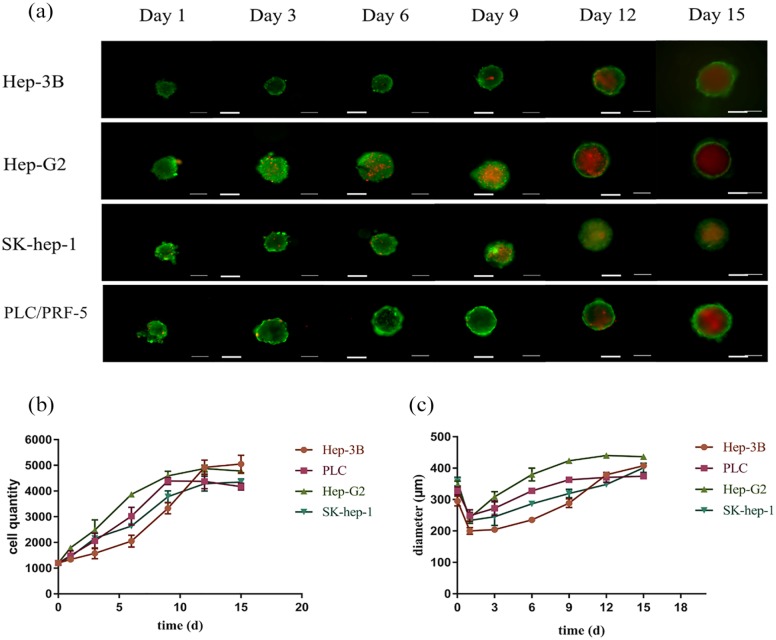
Four kinds of HCC cells were seeded onto agarose microwell culture plates at a density of 1200 cells/well. As shown in Figure 3(c), the cells could aggregate into spheroids within 24 h, and the diameter of the spheroids increased continuously in the subsequent culture process. The CellTiter-Glo 3D Cell Viability Assay was used to measure the cell viability and count the number of cells in spheroids on the 1st, 3rd, 6th, 9th, 12th, and 15th days (Figure 3(c)). We found that HepG2 cells with faster proliferation rates reached the proliferative plateau stage on the 9th day, while the other three cell lines reached the proliferative plateau stage on the 12th day. After reaching the proliferative plateau stage, the cell spheroid viability began to decline. In the Calcein-AM/PI staining experiment, HepG2 cell spheres showed obvious central necrosis area on the 9th day, and the other three cell spheroids appeared on the 12th day, which was consistent with the cell viability test results (Figure 3(a)).
Figure 3.
Proliferation of HCC cell lines in agarose microwell 96-well plates. (a) Calcein-AM/PI staining of HCC cell spheroids at different time points. (b) Cell counts of HCC cell spheroids at different time points. (c) Diameter of HCC cell spheroids at different time points. Data are shown as the mean ± SD of triplicate experiments. Scale bar = 200 μm.
Gene expression of hepatocyte-related functions in HCC cells enhanced by 3D spheroid culture
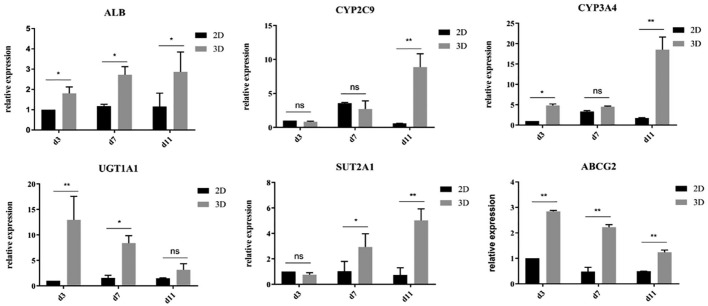
Low levels of drug metabolism enzymes in 2D-cultured HepG2 cells contribute to the misclassification of chemical entities that form toxic metabolites.22 Improving the gene expression of hepatocyte-related functions in HCC cells cultured in vitro may help cells better reflect the drug responses of cells in vivo. In this experiment, we compared the functional gene expression of ALB (albumin), CYP2C9 ( phase-I metabolic enzyme), CYP3A4 ( phase-I metabolic enzyme), UGT1A1 (phase-II metabolic enzyme), SUT2A1 (phase-II metabolic enzyme), and ABCG2 (ATP-binding cassette transporter) in HepG2 cells at different time points (3, 7, and 11 days) in 2D and spheroid culture systems (Figure 4). Through qRT-PCR, we found that the expression levels of albumin, UGT1A1, and ABCG2 in 3D spheroids were significantly higher than those in 2D culture at all time points. Compared with the 2D culture group, the 3D culture group showed significantly higher expression levels of SUT2A1 in the middle stage of culture (7 days) and significantly higher expression levels of CYP2A9 and CYP3A4 in the later stage of culture (11 days).
Figure 4.
Gene expression of hepatocyte-related functions in hepatocellular carcinoma cells enhanced by 3D spheroid culture. *p < 0.05; **p < 0.001.
Drug sensitivity test of primary HCC cells cultured in agarose microwell 96-well plates or ordinary 96-well plates
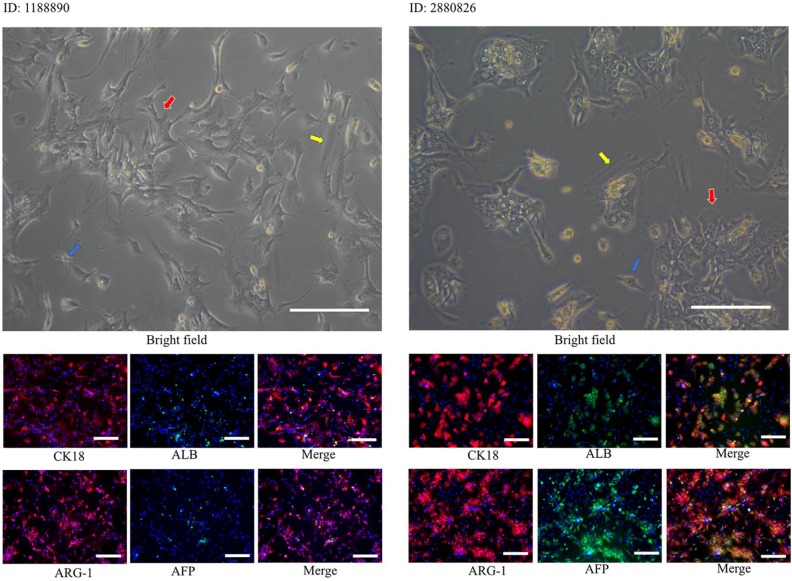
We successfully isolated primary cells from fresh tumor specimens obtained from two patients with HCC and cultured these cells in monolayer or spheroids systems. To identify whether the primary cells obtained were HCC cells, we immunostained the primary cells for several relatively specific hepatocyte antigens (ALB, AFP, ARG-1, and CK-18). As shown in Figure 5, ARG-1 and CK-18 were strongly expressed in two primary HCC cell lines, while ALB and AFP were moderately expressed in highly differentiated HCC cell line 2880826 and weakly expressed in poorly differentiated HCC cell line 1188890, which was consistent with the histopathological results. Notably, immunofluorescence images showed that a small number of cells did not express any markers of hepatocyte cells, which indicated that the primary cell components obtained were not single type of cells. In addition to HCC cells, other cells, such as fibroblasts or endothelial cells, might also be contained in HCC tumors. As shown in Figure 5, the isolated cells included more than one type of cell. In addition to HCC cells, which account for the largest proportion in cell groups, flat, long, spindle-shaped fibroblasts and small spindle-shaped endothelial cells could also be observed. This finding was consistent with the immunofluorescence results.
Figure 5.
Bright field and immunofluorescence photographs of primary cells. In bright field pictures, the red arrow points to the hepatocellular carcinoma cells, the yellow arrow points to the flat, long, spindle-shaped fibroblasts, and the blue arrow points to small spindle-shaped endothelial cells. AFP, albumin, ARG-1, and CK-18 immunostaining of primary cells to examine the cellular origin of primary HCC cells.
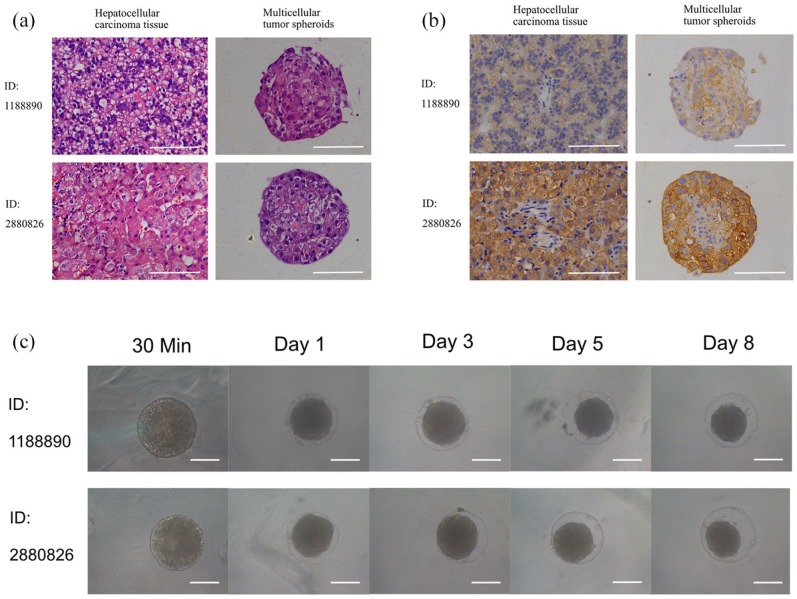
Studies have shown that the response of HCC cells to chemotherapeutic drugs is closely related to the microenvironment of cells. In addition to the 3D structure, stromal cells, such as tumor-related fibroblasts and endothelial cells, can also affect the killing effect of chemotherapeutic drugs on cancer cells. In our primary HCC tumor spheroid culture method, both HCC cells and mesenchymal cells, such as cancer-associated fibroblasts and endothelial cells, were contained in one spheroid. HE staining showed that HCC cells aggregated into regular spheres under 3D culture conditions (Figure 6(a)). It has been proved that ARG-1 is a sensitive and specific marker for HCC, and the expression level of ARG-1 declined with the decrease in tumor differentiation.23 Immunohistochemical staining of ARG-1 in this study revealed that spheroids preserve ARG-1 expression of their original tumors (Figure 6(b)). Both primary tissue and spheroids from the highly differentiated HCC (ID: 2880826) had a much higher expression than that from the poorly differentiated tumor (ID: 1188890), confirming that ARG-1 expression level of spheroids was associated with the differentiation of corresponding tissues. The cell morphology was also similar to that of HCC cells in vivo, indicating that the 3D structure of HCC cells in vivo was well simulated by our in vitro spheroid model.
Figure 6.
Images of spheroid culture of primary cells. (a) HE staining of tumor tissue and corresponding multicellular tumor spheroids. (b) immunohistological staining of tumor tissue and corresponding tumor spheroids with ARG-1. (c) Primary cells cultured in agarose microwells 96-well plates at different time points. Scale bar = 200 μm.
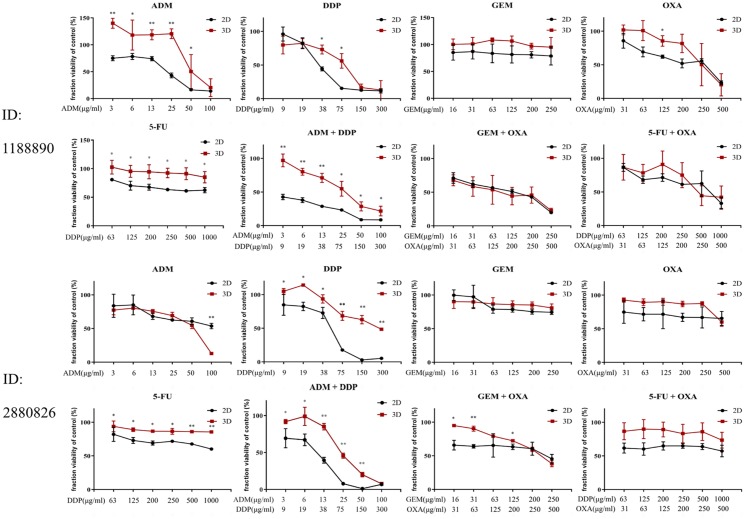
Our results showed that primary HCC cells formed uniform spheroids in agarose microwell culture plates within 1 day. Unlike cell lines, the diameter of primary cell spheroids continuously decreased during subsequent culture in wells without drugs (Figure 6(c)). This effect may be due to the weak proliferation ability of primary cells and the tightening of cell binding in the sphere. In addition, we found that HCC 1188890 and HCC 2880826 in 2D or 3D culture showed completely different drug sensitivity patterns (Figure 7). The highly differentiated HCC 2880826 was more resistant than the poorly differentiated HCC 1188890. In addition, 2D-cultured HCC 2880826 cells were sensitive to GEM + OXA and highly sensitive to DDP and ADM + DDP, but no drug regimen used in this experiment could inhibit HCC 2880826 cells under spheroid culture conditions. The 2D-cultured HCC 1188890 cell line was sensitive to ADM and OXA and highly sensitive to DDP, ADM + DDP, GEM + OXA, and 5-Fu + OXA, but spheroid-cultured HCC 1188890 cells were only sensitive to DDP, ADM + DDP, and 5-Fu + OXA and highly sensitive to GEM + OXA. It seems that 3D-cultured primary HCC cells were more resistant to antitumor agents than monolayer cultured cells (Tables 4 and 5).
Figure 7.
Fraction viability of primary HCC cells cultured in 2D or 3D with different concentrations of antitumor drugs compared with the control group. The chemotherapeutic drugs used in this experiment include adriamycin (ADM), cisplatin (DDP), gemcitabine (GEM), oxaliplatin (OXA), and 5-fluorouracil (5-FU) as single drug treatments, and ADM + DDP, GEM + OXA, and 5-FU + OXA as combination treatments. The data are shown as mean ± SD of triplicate experiments. *p < 0.05; **p < 0.001.
Table 4.
Half maximal inhibitory concentrations (IC50) of anti-cancer drugs in primary HCC.
| ID: 1188890 |
ID: 2880826 |
|||||||
|---|---|---|---|---|---|---|---|---|
| 2D |
3D |
2D |
3D |
|||||
| IC50
(μg/mL) |
IC90
(μg/mL) |
IC50
(μg/mL) |
IC90
(μg/mL) |
IC50
(μg/mL) |
IC90
(μg/mL) |
IC50
(μg/mL) |
IC90
(μg/mL) |
|
| ADM | 17.43 | 100 | >100 | >100 | 77.26 | >100 | 42.30 | >100 |
| DDP | 40.31 | 218.75 | 60.92 | >300 | 40.56 | 168.04 | 208.12 | >300 |
| GEM | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| OXA | 132.07 | >500 | 322.64 | >500 | >500 | >500 | >500 | >500 |
| 5-FU | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| ADM | 2.93 | 61.12 | 24.20 | >100 | 7.77 | 32.90 | 25.50 | >100 |
| +DDP | 8.92 | 183.00 | 72.78 | >300 | 23.51 | 98.86 | 76.53 | >300 |
| GEM | 46.67 | >250 | 40.06 | >250 | 113.05 | >250 | >250 | >250 |
| +OXA | 92.99 | >500 | 79.77 | >500 | 225.82 | >500 | >500 | >500 |
| 5-FU | 487.69 | >1000 | 777.90 | >1000 | >1000 | >1000 | >1000 | >1000 |
| +OXA | 244.07 | >500 | 388.98 | >500 | >500 | >500 | >500 | >500 |
A + B indicates a combination; ADM: adriamycin; DDP: cisplatin; GEM: gemcitabine; OXA: oxaliplatin; 5-FU: 5-fluorouracil; 2D: two-dimensional; 3D: three-dimensional.
Table 5.
Prediction of drug sensitivities of patient-derived cells in 2D culture and 3D spheroid culture.
| ID: 1188890 |
ID: 2880826 |
|||
|---|---|---|---|---|
| 2D | 3D | 2D | 3D | |
| ADM | + | – | – | – |
| DDP | ++ | + | ++ | – |
| GEM | – | – | – | – |
| OXA | + | – | – | – |
| 5-FU | – | – | – | – |
| ADM + DDP | ++ | + | ++ | – |
| GEM + OXA | ++ | ++ | + | – |
| 5-FU + OXA | ++ | + | – | – |
ADM: adriamycin; DDP: cisplatin; GEM: gemcitabine; OXA: oxaliplatin; 5-FU: 5-fluorouracil; A + B indicates a combination; –, resistant; +, sensitive; ++, highly sensitive.
For combination regimen, the sensitivity for each drug was graded, and the higher sensitivity was chosen as the sensitive level for combination of drugs.
Discussion
In recent years, a paradigm shift from 2D to 3D cell culture techniques has occurred because culturing cells in 3D results in the formation of natural cell–cell attachment and the microstructure of cells in 3D culture is closer to tissue in vivo.24 Accordingly, compared with monolayer culture, 3D cell culture has been shown to have dramatic effects on cell polarity and differentiation as well as signaling cascades and gene expression profiles.25–27 Moreover, primary cells isolated from patients cultured in 3D could maintain some special characteristics of cells in vivo for a long time in vitro.9 All these findings imply that the response of 3D-cultured cells to antitumor drugs could better reflect the response of cells in vivo.
3D culture techniques are various, and spheroids are one of the most commonly used 3D culture approaches. Spheroids are spherical, heterogeneous aggregates of proliferating, quiescent, and necrotic cells in culture that retain 3D architecture and tissue-specific functions.28 Such aggregates are analogous to tissues but have no animal-derived or synthetic scaffolds for aiding cell attachment,29 which well avoids the influence of batch difference and animal-derived chemicals derived from scaffolds and benefits the shape control of the microtissue, inducing the formation of uniform spheres. Necrosis is a typical feature of tumors growing in vivo, and cell spheroids could well restore this feature and form necrotic areas in the center of spheroids.30 Thus, spheroids are a particularly useful tool for studying tumor growth. Recent studies involve the analysis of radiotherapy, chemotherapy, radioimmunotherapy, cell- and antibody-based immunotherapy, gene therapy delivery, and photodynamic treatment, as well as studies involving proliferation, viability, metabolism, invasion, and cell–cell interactions.31
Multicellular spheroid culture, as a kind of mature 3D culture technique, can well simulate the 3D structure of tumors and is inexpensive with less technical difficulty, which is beneficial for high-throughput drug screening and individualized treatment. In our previous research, we developed an approach to fabricate agarose concave Petri dishes for the mass production of tumor spheroids, and we found that an agarose hemispheric micropore with a 400-μm width and 400-μm thickness is best for spheroid culture.11 However, it is important to reduce the cell consumption in high-throughput drug screening for primary cells. Hence, the agarose concave Petri dish we previously described,11 which is used in the mass production of spheroids, is not suitable for high-throughput drug screening and individualized treatment. Based on a previous study, we designed an easily used stamp-like mold to create agarose microwells in an ordinary 96-well cell culture plate, which is efficient in forming uniform high-throughput cell spheroids and greatly reduces the number of cells used.
The reason we chose agarose as the substrate for 3D culturing is that agarose is a low-cost, non-adhesive, and non-toxic biomaterial for cells. The curved agarose surface of the microwell prevents the adhesion of cells and effectively accelerates the formation of self-assembled spheroids.32 We designed a stamp-like mold that could insert into the hot liquid agarose in a 96-well plate. After the agarose cools, the tool could be pulled out. Arranged tiny wells could be formed on the agarose substrate, and the cells could aggregate and form spheroids. We hope that the seeding cell number could be minimized and spheroids could be formed very effectively on the homemade agarose plate. An inclined platform is also designed above the multicellular spheroid culture chamber, which might be helpful for exchanging medium and avoiding sucking up the spheroids by pipetting. As a highly innovative technology, rapid prototyping with 3D printing is a quick, easy, and cost-effective way to turn great ideas into successful products. With the help of highly innovative 3D printing technology, in this study, we printed the designed resin mold and fabricated an agarose 96-well plate using the resin mold.
Theoretically, this homemade stamp-like tool is convenient in forming spheroids for various types of solid tumors. In this experiment, we first evaluated the features of the spheroids of four HCC cell lines using our homemade tool. The data show that the effect of the agarose microwells on spheroid formation is remarkable. HCC cell lines could form spheroids of uniform size and shape within 24 h, which is conducive for quality control and the reproducibility of experiments. Tumor cell proliferation in solid tumors in vivo generates concentration gradients of nutrients, oxygen, and catabolites.33 These conditions impact gene and protein expression profiles and the distribution and penetration of soluble factors, including drugs, possibly resulting in poor response to treatment, as typically described in avascular tumors where the formation of necrotic areas occurs.34–37 The 3D spheroids could mirror the structures of solid tumors, including hypoxic/necrotic areas. The size and shape of spheroids are two crucial factors that will greatly affect the generation of hypoxic/necrotic areas in spheroids. Therefore, it is important to control the size and shape of spheroids in a unified form to avoid the impact of these factors on the penetration of drugs and the generation of hypoxic/necrotic areas. Our experimental data showed that the shape of cell spheroids cultured in a homemade 3D culture plate was more uniform than that in a commercialized ultralow attachment 96-well plate in a wide range of initial seeding densities (300–2400 cells/well). This result suggested that the stamp-like spheroid culture mold is a good tool to control experimental confounding factors when screening antitumor drugs.
Tumoral processes such as drug metabolism and drug transport should be considered as the important factors involved in mechanism of resistance to chemotherapy and means of achieving optimal therapy.38 The expression of genes related with drug metabolism and drug transport in HCC cells cultured in vitro may help to better reflect the drug responses of these cells in vivo. We therefore compared the expression of hepatocyte-specific functional genes involved in phase-I metabolism (CYP2C9 and CYP3A4), phase-II metabolism (UGT1A1 and SUT2A1), and drug transport (ALB and ABCG2) in HCC cell lines between 2D and 3D spheroid cultures. We found that the expression levels of all the tested genes were enhanced in spheroid cultures compared to 2D cultures at different culture time points, which is consistent with previous studies.39 This finding implies that our spheroid culture tool could well maintain cell functions. We consider that the main reason for the enhanced functional gene expression in spheroids might be related with the higher differentiation of cells in 3D culture. 2D culture initiates a rapid dedifferentiation of cells with degradation in phenotypic characteristics, collapse of biological functionalities, and decreased capacity in cell metabolism.40 Compared to 2D culture, spheroids restore the 3D structure of cells and provide a bio-mimic 3D microenvironment, which might promote the differentiation of cells in vitro.39 However, the underlying mechanisms involved in the improved function of cell spheroids still need further investigation.
In addition, in our research, we successfully isolated and cultured two patient-derived primary HCC cell lines. In our homemade spheroid culture plate, we restored the tumor microstructure and heterogeneity to a certain extent. HCC is a highly heterogeneous disease in terms of its molecular profiles, as genome variation and expression are diverse in different parts of tumors, and this feature is one of the most important reasons leading to drug resistance.41 In the primary HCC cell spheroid culture method described above, we obtained several specimens from different parts of the tumor to obtain as many heterogeneous HCC cells as possible. After digestion, HCC cells in specimens with different genic backgrounds or molecular profiles were scattered. These cells were then reorganized into a mini tumor in the process of spheroid culture, which made every tumor spheroid a heterogeneous tumor microtissue. Furthermore, the histological structure and features of tumor were well simulated in the primary cell spheroids. Tumor microenvironmental components, such as cancer-associated fibroblasts and endothelial cells, directly or indirectly affect the response of tumor cells to drugs.42,43 In our spheroid culture method, we obtained not only HCC cells but also fibroblasts and endothelial cells, indicating that our method of primary liver cell isolation can retain the cell components of tumor tissues.
The other advantages of our homemade spheroid culture plate are the shortened spheroid formation time and the low consumption of cells, in which the cell-seeding density in each cell culture well ranged from 300 to 2400 and a regular spheroid could form within 24 h. This feature is very beneficial for personalized treatment because the primary cells are so precious that it is hard to obtain a large number. In addition, a shorter spheroid culture time could accelerate the drug screening process and help patients obtain drug sensitivity results on time. Our experimental data show that primary HCC 1188890 and HCC 2880826 cells cultured in 2D or 3D have completely different drug sensitivity patterns. Highly differentiated HCC 2880826 cells were more resistant than poorly differentiated HCC 1188890 cells. This difference may be because highly differentiated HCC cells produces a higher level of drug metabolism and transporting proteins, such as transporter protein ABCG2, and possesses stronger damage repair ability.44 In addition, 3D-cultured primary HCC cells were more resistant to antitumor agents than monolayer cultured cells, which was consistent with previous primary studies.9,45,46 In summary, primary HCC cells showed different drug sensitivities depending on the culture methods, and the experimental data indicating the results of drug sensitivity in the spheroid culture method may better reflect the actual drug responses of HCC cells in vivo because this kind of tumor spheroid strikingly mirrors the 3D cellular context and heterogeneity of in vivo tumors. The results of chemotherapy sensitivity assay in this study may provide guidance on personalized TACE treatment for HCC patients. However, it is worth mentioning that our drug tests of clinic samples are preliminary; more profound research on how to correlate the in vitro drug test with clinic benefit is still needed.
Conclusion
Our study demonstrates an approach to fabricate a new stamp-like spheroid culture tool and use it to 3D culture tumor cells for screening sensitive antitumor drugs. Our homemade spheroid culture devise has the following advantages: (1) convenient to use, low cost (the cost of a resin mold is less than US$4), and reusable (the mold is reusable, and even the ordinary 96-well plate used to fabricate agarose microwells could be reused after removing the shaped agarose substrate); (2) shortened spheroid formation time; (3) controllable size and shape of the spheroids; (4) less cell consumption; (5) convenient in exchanging medium; (6) free of animal components and no synthetic scaffold to alter cell physiology; and (7) viable for mass production. We suggest that our tumor spheroid culture device could be applied in tumor patients for individualized treatment and high-throughput drug screening. However, limited by the short time of agarose solidification and the size of printed objects, we could only design a mold with six rows of tiny hemispherical protuberances in this study. In the future, we hope to modify the stamp-like tool to meet various experimental conditions and industrialize the production of agarose-based microwell 96-well plates to extend its application in hepatotoxicity drug testing and new antitumor drug screening.
Acknowledgments
The authors thank Professor Du from the Department of Pediatrics and the staff of the Heart Center Laboratory, Zhujiang Hospital, for providing some of the experimental equipments.
Footnotes
Authors’ note: Yi Gao is also affiliated with State Key Laboratory of Organ Failure Research, Southern Medical University, Guangzhou, China.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was financially supported by the National Key R&D Program of China (Nos 2018YFC1106400 and 2018YFA0108200); Science and Technology Planning Project of Guangdong Province (No. 2015B020229002); The National Natural Science Foundation of China (Nos 81470875, 81701580, 81600489, and 31972926); The Natural Science Foundation of Guangdong Province (Nos 2014A030312013, 2018A030313128, and 2018A030313214); Guangdong Key Research and Development Plan (No. 2019B020234003); and Science and Technology Program of Guangzhou (Nos 201604020002 and 201803010086).
ORCID iD: Yi Gao  https://orcid.org/0000-0002-4536-6191
https://orcid.org/0000-0002-4536-6191
References
- 1. Sharma SV, Haber DA, Settleman J. Cell line-based platforms to evaluate the therapeutic efficacy of candidate anticancer agents. Nat Rev Cancer 2010; 10(4): 241–253. [DOI] [PubMed] [Google Scholar]
- 2. De Minicis S, Kisseleva T, Francis H, et al. Liver carcinogenesis: rodent models of hepatocarcinoma and cholangiocarcinoma. Dig Liver Dis 2013; 45(6): 450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shamir ER, Ewald AJ. Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat Rev Mol Cell Biol 2014; 15(10): 647–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fang Y, Eglen RM. Three-dimensional cell cultures in drug discovery and development. SLAS Discov 2017; 22(5): 456–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Landry J, Bernier D, Ouellet C, et al. Spheroidal aggregate culture of rat liver cells: histotypic reorganization, biomatrix deposition, and maintenance of functional activities. J Cell Biol 1985; 101(3): 914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dilworth C, Hamilton GA, George E, et al. The use of liver spheroids as an in vitro model for studying induction of the stress response as a marker of chemical toxicity. Toxicol Vitro 2000; 14(2): 169–176. [DOI] [PubMed] [Google Scholar]
- 7. Li AP, Colburn SM, Beck DJ. A simplified method for the culturing of primary adult rat and human hepatocytes as multicellular spheroids. In Vitro Cell Dev Biol 1992; 28A(9–10): 673–677. [DOI] [PubMed] [Google Scholar]
- 8. Edmondson R, Broglie JJ, Adcock AF, et al. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol 2014; 12(4): 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Song Y, Kim JS, Kim SH, et al. Patient-derived multicellular tumor spheroids towards optimized treatment for patients with hepatocellular carcinoma. J Exp Clin Cancer Res 2018; 37(1): 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Norotte C, Marga FS, Niklason LE, et al. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 2009; 30(30): 5910–5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang B, Li Y, Wang G, et al. Fabrication of agarose concave petridish for 3D-culture microarray method for spheroids formation of hepatic cells. J Mater Sci Mater Med 2018; 29(5): 49. [DOI] [PubMed] [Google Scholar]
- 12. Petrick JL, Braunlin M, Laversanne M, et al. International trends in liver cancer incidence, overall and by histologic subtype, 1978-2007. Int J Cancer 2016; 139(7): 1534–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raoul JL, Forner A, Bolondi L, et al. Updated use of TACE for hepatocellular carcinoma treatment: how and when to use it based on clinical evidence. Cancer Treat Rev 2019; 72: 28–36. [DOI] [PubMed] [Google Scholar]
- 14. Matsuoka H, Nagaya M, Tsukikawa S, et al. Repeated hepatic intra-arterial chemotherapy based on results of anticancer drug sensitivity test in patients with synchronous hepatic metastases from colorectal cancer. Surgery 2006; 140(3): 387–395. [DOI] [PubMed] [Google Scholar]
- 15. Liang B, Zhao D, Liu Y, et al. Chemoembolization of liver cancer with doxorubicin-loaded CalliSpheres microspheres: plasma pharmacokinetics, intratumoral drug concentration, and tumor necrosis in a rabbit model. Drug Deliv Transl Res. Epub ahead of print 3 September 2019. DOI: 10.1007/s13346-019-00672-9. [DOI] [PubMed] [Google Scholar]
- 16. Kearsley JH, Hurst T, Khoo SK. Chemosensitivity testing of primary cultures of Merkel cell cancer. Anticancer Drugs 1993; 4(5): 571–575. [DOI] [PubMed] [Google Scholar]
- 17. Zhang Y, Kuchimanchi M, Zhu M, et al. Assessment of pharmacokinetic interaction between rilotumumab and epirubicin, cisplatin and capecitabine (ECX) in a Phase 3 study in gastric cancer. Br J Clin Pharmacol 2017; 83(5): 1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kurbacher CM, Cree IA, Brenne U, et al. Heterogeneity of in vitro chemosensitivity in perioperative breast cancer cells to mitoxantrone versus doxorubicin evaluated by a microplate ATP bioluminescence assay. Breast Cancer Res Treat 1996; 41(2): 161–170. [DOI] [PubMed] [Google Scholar]
- 19. Peters GJ, Clavel M, Noordhuis P, et al. Clinical phase I and pharmacology study of gemcitabine (2,’ 2’-difluorodeoxycytidine) administered in a two-weekly schedule. J Chemother 2007; 19(2): 212–221. [DOI] [PubMed] [Google Scholar]
- 20. Borner MM, Schoffski P, de Wit R, et al. Patient preference and pharmacokinetics of oral modulated UFT versus intravenous fluorouracil and leucovorin: a randomised crossover trial in advanced colorectal cancer. Eur J Cancer 2002; 38(3): 349–358. [DOI] [PubMed] [Google Scholar]
- 21. Gori S, Lunardi G, Inno A, et al. Pharmacokinetics of oxaliplatin in a hemodialyzed patient: chemotherapy dose adjustment and timing of dialysis. Clin Colorectal Cancer 2014; 13(4): 260–263. [DOI] [PubMed] [Google Scholar]
- 22. Wilkening S, Stahl F, Bader A. Comparison of primary human hepatocytes and hepatoma cell line Hepg2 with regard to their biotransformation properties. Drug Metab Dispos 2003; 31(8): 1035–1042. [DOI] [PubMed] [Google Scholar]
- 23. Sang W, Zhang W, Cui W, et al. Arginase-1 is a more sensitive marker than HepPar-1 and AFP in differential diagnosis of hepatocellular carcinoma from nonhepatocellular carcinoma. Tumour Biol 2015; 36(5): 3881–3886. [DOI] [PubMed] [Google Scholar]
- 24. Bhatia SN, Underhill GH, Zaret KS, et al. Cell and tissue engineering for liver disease. Sci Transl Med 2014; 6(245): 245sr2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sakai Y, Yamagami S, Nakazawa K. Comparative analysis of gene expression in rat liver tissue and monolayer- and spheroid-cultured hepatocytes. Cells Tissues Organs 2010; 191(4): 281–288. [DOI] [PubMed] [Google Scholar]
- 26. Luebke-Wheeler JL, Nedredal G, Yee L, et al. E-cadherin protects primary hepatocyte spheroids from cell death by a caspase-independent mechanism. Cell Transplant 2009; 18(12): 1281–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takabatake H, Koide N, Tsuji T. Encapsulated multicellular spheroids of rat hepatocytes produce albumin and urea in a spouted bed circulating culture system. Artif Organs 1991; 15(6): 474–480. [PubMed] [Google Scholar]
- 28. Fitzgerald KA, Malhotra M, Curtin CM, et al. Life in 3D is never flat: 3D models to optimise drug delivery. J Control Release 2015; 215: 39–54. [DOI] [PubMed] [Google Scholar]
- 29. Hamilton G. Multicellular spheroids as an in vitro tumor model. Cancer Lett 1998; 131: 29–34. [DOI] [PubMed] [Google Scholar]
- 30. Proskuryakov SY, Gabai VL. Mechanisms of tumor cell necrosis. Curr Pharm Des 2010; 16: 56–68. [DOI] [PubMed] [Google Scholar]
- 31. Kunz-Schughart LA, Freyer JP, Hofstaedter F, et al. The use of 3-D cultures for high-throughput screening: the multicellular spheroid model. J Biomol Screen 2004; 9(4): 273–285. [DOI] [PubMed] [Google Scholar]
- 32. Fennema E, Rivron N, Rouwkema J, et al. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol 2013; 31(2): 108–115. [DOI] [PubMed] [Google Scholar]
- 33. Friedrich J, Eder W, Castaneda J, et al. A reliable tool to determine cell viability in complex 3-d culture: the acid phosphatase assay. J Biomol Screen 2007; 12(7): 925–937. [DOI] [PubMed] [Google Scholar]
- 34. Timmins NE, Maguire TL, Grimmond SM, et al. Identification of three gene candidates for multicellular resistance in colon carcinoma. Cytotechnology 2004; 46(1): 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou Y, Arai T, Horiguchi Y, et al. Multiparameter analyses of three-dimensionally cultured tumor spheroids based on respiratory activity and comprehensive gene expression profiles. Anal Biochem 2013; 439(2): 187–193. [DOI] [PubMed] [Google Scholar]
- 36. Weiswald LB, Richon S, Massonnet G, et al. A short-term colorectal cancer sphere culture as a relevant tool for human cancer biology investigation. Br J Cancer 2013; 108(8): 1720–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Daster S, Amatruda N, Calabrese D, et al. Induction of hypoxia and necrosis in multicellular tumor spheroids is associated with resistance to chemotherapy treatment. Oncotarget 2017; 8(1): 1725–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Michael M, Doherty MM. Tumoral drug metabolism: overview and its implications for cancer therapy. J Clin Oncol 2005; 23(1): 205–229. [DOI] [PubMed] [Google Scholar]
- 39. Ramaiahgari SC, den Braver MW, Herpers B, et al. A 3D in vitro model of differentiated HepG2 cell spheroids with improved liver-like properties for repeated dose high-throughput toxicity studies. Arch Toxicol 2014; 88(5): 1083–1095. [DOI] [PubMed] [Google Scholar]
- 40. Kaur P, Robin Mehta RG, et al. Progression of conventional hepatic cell culture models to bioengineered HepG2 cells for evaluation of herbal bioactivities. Biotechnol Lett 2018; 40(6): 881–893. [DOI] [PubMed] [Google Scholar]
- 41. Gao Q, Wang ZC, Duan M, et al. Cell culture system for analysis of genetic heterogeneity within hepatocellular carcinomas and response to pharmacologic agents. Gastroenterology 2017; 152(1): 232–242. e4. [DOI] [PubMed] [Google Scholar]
- 42. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer 2016; 16(9): 582–598. [DOI] [PubMed] [Google Scholar]
- 43. Tredan O, Galmarini CM, Patel K, et al. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst 2007; 99(19): 1441–1454. [DOI] [PubMed] [Google Scholar]
- 44. Chen YL, Chen PM, Lin PY, et al. ABCG2 overexpression confers poor outcomes in hepatocellular carcinoma of elderly patients. Anticancer Res 2016; 36(6): 2983–2988. [PubMed] [Google Scholar]
- 45. Ding Y, Liu W, Yu W, et al. Three-dimensional tissue culture model of human breast cancer for the evaluation of multidrug resistance. J Tissue Eng Regen Med 2018; 12(9): 1959–1971. [DOI] [PubMed] [Google Scholar]
- 46. Khawar IA, Park JK, Jung ES, et al. Three dimensional mixed-cell spheroids mimic stroma-mediated chemoresistance and invasive migration in hepatocellular carcinoma. Neoplasia 2018; 20(8): 800–812. [DOI] [PMC free article] [PubMed] [Google Scholar]