Abstract
The aim of this study was to determine practice patterns and inter-institutional variability in how acute vasoreactivity testing (AVT) is performed and interpreted in pediatrics throughout the world. A survey was offered to physicians affiliated with the Pediatric & Congenital Heart Disease Taskforce of the Pulmonary Vascular Research Institute (PVRI), the Pediatric Pulmonary Hypertension Network (PPHNET), or the Spanish Registry for Pediatric Pulmonary Hypertension (REHIPED), from February to December 2016. The survey requested data about the site-specific protocol for AVT and subsequent management of pediatric patients with idiopathic pulmonary arterial hypertension (IPAH) or heritable PAH (HPAH). Twenty-eight centers from 13 countries answered the survey. AVT is performed in most centers using inhaled nitric oxide (iNO). Sitbon criteria was used in 39% of the centers, Barst criteria in 43%, and other criteria in 18%. First-line therapy for positive AVT responders in functional class (FC) I/II was calcium channel blocker (CCB) in 89%, but only in 68% as monotherapy. Most centers (71%) re-evaluated AVT-positive patients hemodynamics after 6–12 months; 29% of centers re-evaluated based only on clinical criteria. Most centers (64%) considered a good response as remaining in FC I or II, with near normalization of pulmonary arterial pressure and pulmonary vascular resistance, but a stable FC I/II alone was sufficient criteria in 25% of sites. Protocols and diagnostic criteria for AVT, and therapeutic approaches during follow-up, were highly variable across the world. Reported clinical practice is not fully congruent with current guidelines, suggesting the need for additional studies that better define the prognostic value of AVT for pediatric IPAH patients.
Keywords: Pediatric pulmonary hypertension, idiopathic pulmonary arterial hypertension, cardiac catheterization, acute vasoreactivity testing, calcium channel blockers
Introduction
Calcium channel blockers (CCB) were the first drugs to improve hemodynamics and survival for a specific subset of adult patients with idiopathic pulmonary arterial hypertension (IPAH) and a positive response to acute vasoreactivity testing.1–3 In the absence of a widely available genetic or molecular biomarker for this favorable phenotype,4 a clinical definition is used, based on the acute hemodynamic response to pulmonary vasodilators, and on the long-term response to CCB. This subset of IPAH patients may benefit from treatment with a low-cost oral drug that is available in most countries.5–10
The criteria to define a positive acute vasodilator response are well-established in the adult guidelines. In pediatrics, different definitions for positive acute vasoreactivity testing (AVT) have been used:
– Barst criteria11: decrease in mean pulmonary arterial pressure (mPAP) of 20%, with an increase or no change in cardiac index (CI), and a decrease or no change in the pulmonary vascular resistance (PVR) to systemic vascular resistance ratio;
– Sitbon criteria12: decrease in mPAP ≥ 10 mmHg, reaching ≤40 mmHg, with an increase or no change in cardiac output;
– UK National Service for pediatric pulmonary hypertension (PH): almost normalization of PAP, with pulmonary vascular resistance index (PVRI) <4.5 WU.m2.13,14
Current guidelines for pediatric PH are based on level of evidence C (mostly expert opinion); CCB treatment is largely restricted to patients with a positive AVT. Despite published consensus documents with specific recommendations for AVT in children,18–21 data from the TOPP registry reflects a lack of consistency in practice of selected referral centers across the world.10 This inconsistency likely accounts for some portion of the wide range of reported positive AVT responders in pediatric IPAH at 6.5–42%.6–14,17 Only recently, the last published guidelines from the 2018 World Congress Pediatric Taskforce have clearly recommended the use of Sitbon criteria also for pediatric PH.24
The aims of our study were to evaluate: (1) how AVT is performed and interpreted in pediatric patients diagnosed with IPAH, in pediatric PH centers across the world; (2) the treatment algorithms used in those considered vasoresponders; and (3) regional differences in the protocols used for the catheterization, the AVT, and the pharmacological treatment chosen for the vasoresponders.
Methods
A cross-sectional survey was conducted from February to December 2016, in pediatric PH centers identified as members of the Pediatric and Congenital Heart Disease Task Forces of the Pulmonary Vascular Research Institute (PVRI), members of the Pediatric Pulmonary Hypertension Network (PPHNET), which includes major PH centers in Canada and the United States (Appendix is available as supplementary material online), and members of the Spanish Registry for Pediatric Pulmonary Hypertension (REHIPED). The survey was completed on paper by those attending the PVRI conference (Rome, January 2016) and by others online. The survey was completed by the physician responsible of the pediatric PH unit in each center.
The survey (see Supplement) comprised three sections, investigating the center’s geographic information, AVT protocol specifics, and approach in managing pediatric patients with IPAH or heritable PAH (HPAH) with a positive vasodilator testing response.
Statistics
Survey results were imported into SPSS version 22.0 statistical software for analysis. Qualitative variables were summarized in absolute and relative frequencies, and quantitative variables were summarized in medium-sized and interquartile ranges, frequency distribution tables, pie charts, and bar graphs for summarizing the univariate analysis of the distribution by region and workplace, stacked bar diagrams for the distribution of workplace events and criteria used for reactive PH, error bar diagrams, to represent the frequency of patients seen with reactive PH.
Results
We received 28 survey responses from 28 centers representing 13 countries and four continents (50.9% of the centers invited to participate). The distribution of participant centers by countries and continents is shown in Fig. 1. Nine of the 28 centers (32%) had enrolled patients into the TOPP registry, from which a sub-analysis was previously performed on AVT and use of CCB in pediatric IPAH.10 Three other centers enrolled patients into the Spanish REHIPED.17
Fig. 1.
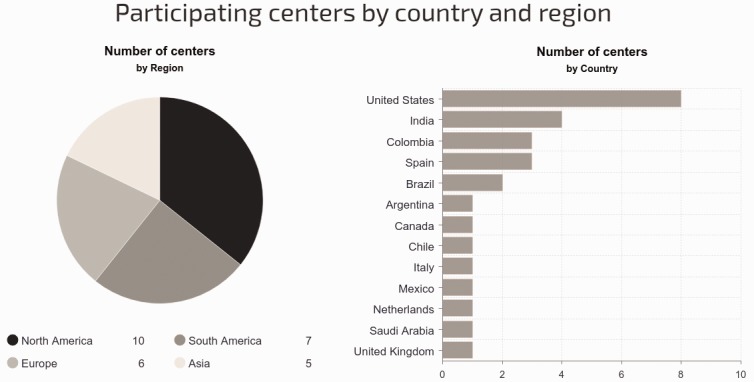
Distribution of participant centers by country and continent (North America included Canada, USA, and Mexico).
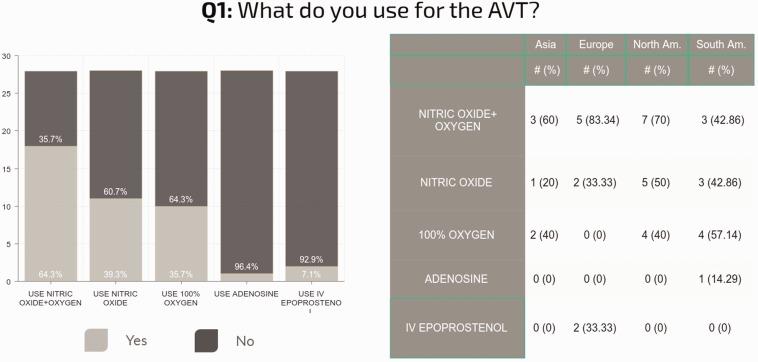
AVT agents
The choice of vasodilators delivered during AVT varied among sites and even within sites among individuals. iNO and supplemental oxygen were most commonly used “in at least some cases” (n = 18, 64%), followed by iNO alone (n = 11, 39%), oxygen alone (n = 10, 36%), epoprostenol (n = 2, 7%), and adenosine (n = 1, 3.5%). Although many centers employ multiple vasodilators for AVT, it remains unclear which criteria are used to determine the actual application in a specific patient. Responses varied by continent (Fig. 2). The answers in each continent do not represent the practice in every center of that continent, but the practice in the centers of that continent who answered the survey.
Fig. 2.
Regional differences in the protocol and drug for the AVT (multiple answers were possible and, as such, the total may be > 100%). Some centers reported the use of a complex approach testing different drugs in a single patient, or use different agents according to the patient-specific diagnosis/clinical situation, or to the planned therapeutic strategy. The answers in each continent do not represent the practice in every center of that continent, but only the practice in the centers of that continent who answered the survey.
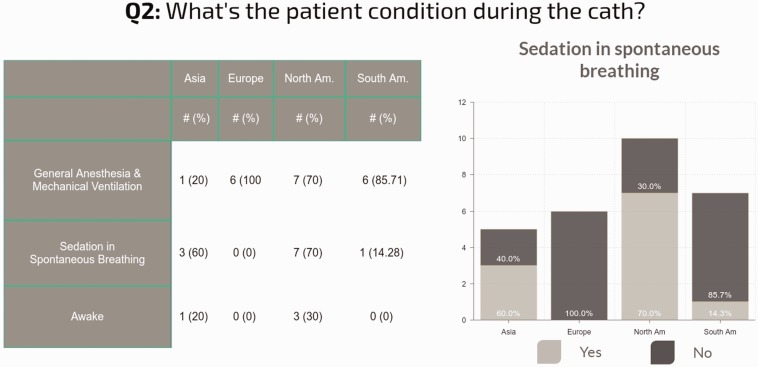
Approach to cardiac catheterization: Not surprisingly, the anesthesia techniques varied, but most centers use general anesthesia for performing cardiac catheterization and AVT in some patients (73% of centers), while sedation with spontaneous breathing was used at least occasionally at 42% of the centers. Patients were awake for some cases at only 15% of centers (Fig. 3).
Fig. 3.
Regional differences in the conditions used for cardiac catheterization. The answers in each continent do not represent the practice in every center of that continent, but only the practice in the centers of that continent who answered the survey.
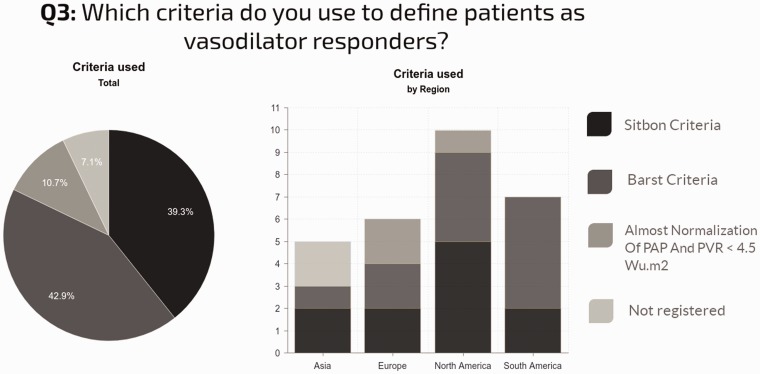
Criteria to define positive response to AVT and patient selection: Most centers used either Barst (43%) or Sitbon (39%) criteria to define a positive AVT response. Near-normalization of mPAP with PVRI < 4.5 WU.m2 (10%) or other criteria (8%) were far less commonly employed (Fig. 4). AVT was performed regardless of etiology in 46% of centers. Regional differences in practice were observed, as shown in Fig. 4.
Fig. 4.
Criteria used to interpret the AVT and regional differences in its use.
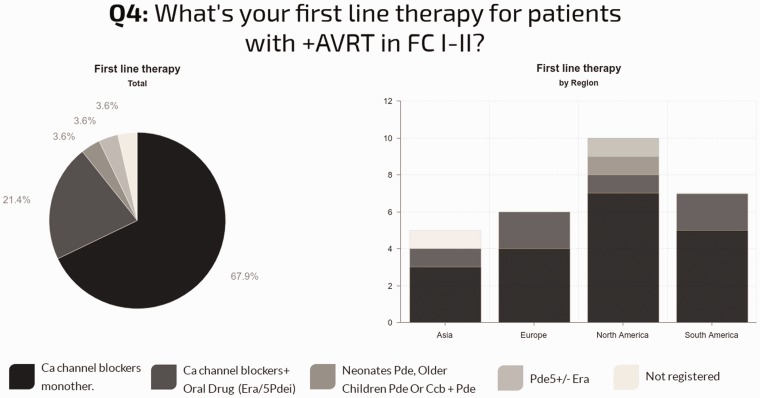
Fig. 5.
Drug therapy used for functional vasoresponders in FC I/II and regional differences.
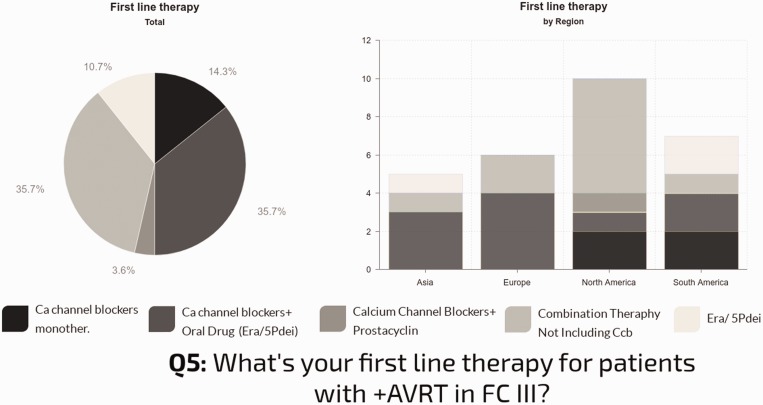
First line drug therapy for patients with positive AVT: In individuals with functional class (FC) I–II, nearly all centers used CCBs (89%), 68% as monotherapy. The use of CCBs as monotherapy in this group was quite consistent: 70% in North America; 67% in Europe; 71% in South America; and 60% in Asia. Combination therapy with a CCB was reported in 21% of centers. In individuals with FC III, only 53% of the centers use CCBs: 14% as monotherapy; 36% combined with oral drugs; and 4% in combination with prostanoids. The other centers report combination therapy not including CCBs (36%) or oral drugs (endothelin receptor antagonists [ERA] or type 5 phosphodiesterase inhibitors [PDE5i] monotherapy [11%]). Geographic differences in practice were observed, as shown in Fig. 6.
Fig. 6.
Left: First-line therapy used for vasoresponders in FC III/IV. Right: Regional differences in first-line therapy for vasoresponders.
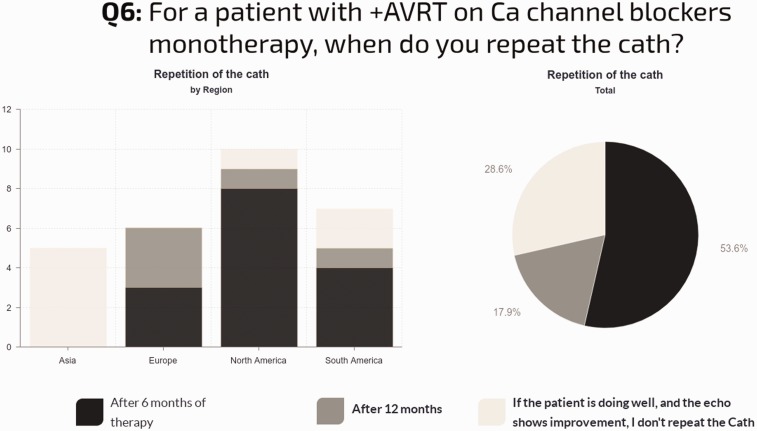
Repeat cardiac catheterization for AVT-positive patients on CCB monotherapy: The majority of sites (59%) perform repeat cardiac catheterization after six months, 18% after 12 months, and 29% do not repeat the catheterization if the patient is doing well clinically with stable echocardiographic findings. In North America, 80% of sites repeat catheterization: 70% after six months and 10% after one year. In Europe, all centers repeat catheterization: 50% after six months and 50% after one year. In South America, 71% repeat catheterization, with 57% after six months. In Asia, none of the centers routinely repeat the catheterization if the patient is clinically well (Fig. 7).
Fig. 7.
Timing of repeat catheterization for acute vasoresponders, and regional differences to this question.
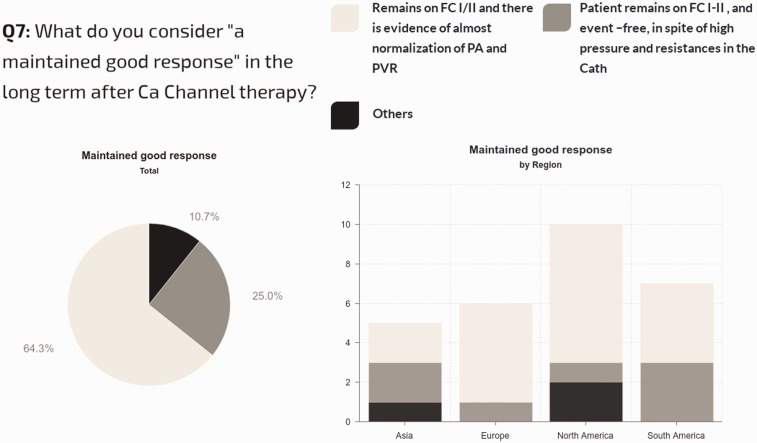
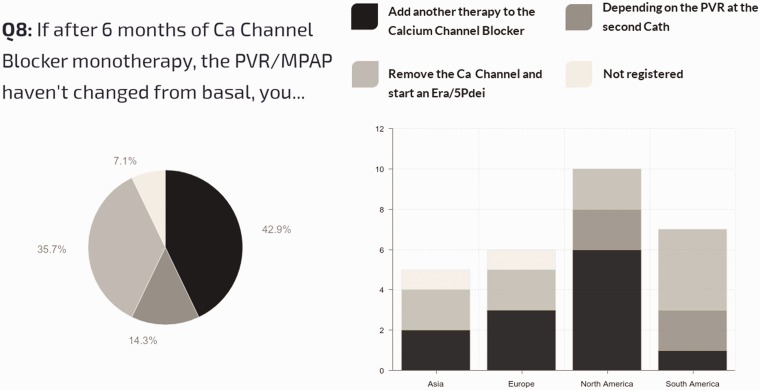
Definition of long-term sustained responses to CCB therapy: Of all centers, 64% use a hemodynamic and clinical definition for a sustained response to CCB (patients in FC I/II with evidence of near-normalization of mPAP and PVRi), while 25% simply require stable FC I–II status, regardless of hemodynamics. Regional differences are shown in Fig. 8. Management strategy for acute vasoresponders not showing improvement in pulmonary pressures and resistances after CCB therapy is shown in Fig. 9.
Fig. 8.
Criteria used to classify the patient as having a maintained good response with CCB therapy.
Fig. 9.
Management strategy for acute vasoresponders not showing improvement in pulmonary pressures and resistances after CCB therapy.
Percentage of IPAH with positive AVT and sustained long-term response to CCB: When the centers were asked how many of their IPAH pediatric patients have had a positive AVT and shown a long-term favorable response to CCB (5–10 years follow-up), the responses were highly variable, in the range of 1–70%, with median values of 4% in North America, 8% in Europe, 10% in South America, and only 4% in Asia (Table 1, Supplemental Material).
The use of AVT in other etiologies of PH (associated with congenital heart defects, lung disease, etc.) is shown in Fig. 1 (Supplemental Material).
Discussion
The results of this survey show that the acute vasoreactivity testing in the pediatric population with IPAH/HPAH is performed with very different protocols and interpreted in a heterogenous way in pediatric PH units across the world. In addition, the treatment and follow-up of those patients diagnosed as “vasoresponders” are not standardized in all centers. Although our survey data may not precisely reflect actual practice in every center across the world, it suggests that either better distribution of the guidelines is required or there exists a need for additional studies that better define the impact and value of AVT on long-term outcomes of pediatric PAH patients.
Differences in the protocols by which AVT is performed, especially as they relate to the use of sedation and anesthetic agents, may contribute to differences in AVT responsiveness. TOPP registry data10 suggested that patients undergoing catheterization using sedation alone were more likely to have positive AVT compared with infants/children tested under general anesthesia. Our survey showed that general anesthesia was used in most European and South American centers that answered the survey; in North American centers, both general anesthesia and sedation were equally reported, and in Asian centers, sedation was most frequently reported. Third, drugs used for AVT also vary between centers; some centers even reported using different drugs at their own site, the use of a complex approach testing different drugs in a single patient, the use of different agents according to the patient specific diagnosis/clinical situation, or to the planned therapeutic strategy. Therefore, this survey shows the lack of a standardized approach common to all centers. Although most centers reported the use of iNO and oxygen or only iNO, a significant proportion of centers (34%), especially in America and Asia, reported only the use of 100% oxygen. Variable responses to AVT may vary depending on the agent used for study, which would make future studies on the association of AVT with late outcomes even more difficult.
Regarding the criteria used to consider a patient “vasoresponder,” the adult PH guidelines had consistently recommended the use of Sitbon criteria;22 however, different pediatric guidelines had been recommending either Sitbon or Barst criteria: the Pediatric PH guidelines published after the 5th World Symposium on Pulmonary Hypertension in Nice18 and a combined AHA/ATS society-supported manuscript,20 both had supported the use of either Barst or Sitbon criteria to define a positive AVT response. A group forming the European Pediatric Pulmonary Vascular Disease Network cited the Barst definition, with a recommendation level Class IIa, and level of evidence.21 Apitz et al., in a document focused on the hemodynamic evaluation in pediatric PH,26 mentioned both Sitbon and Barst criteria, and suggested a modification of the Barts criteria: “ > 20% fall in mean PAP and indexed pulmonary vascular resistance (PVRi)/indexed systemic vascular resistance (SVRi) ratio without a decrease in cardiac output (‘AVR in IPAH/HPAH’). In the presence of a positive AVR, a fall of the ratio of PVRi/SVRi (or as the authors suggest alternatively, the ratio of the diastolic PAP/SAP) below 0.4 due to the AVT might be an indication for calcium-channel blocker therapy.” It has only been recently that the Paediatric Taskforce of the World Congress held in Nice in 2018 has clearly pointed out that the Sitbon criteria should be used also in pediatrics,24 based on the evidence from the TOPP registry.10,15 TOPP investigators reported that when the Barst criteria were used, the percentage of responders was higher, but these “Barst responders” did not show a beneficial prognosis on CCB compared to non-responders. In contrast, the percentage of responders identified using Sitbon criteria was smaller, but this “Sitbon responder” group had a beneficial response to CCB compared to non-responders, suggesting that Sitbon criteria would be superior to Barst’s to predict long-term responses to CCB in the pediatric population with IPAH/HPAH.
The management of IPAH patients considered vasoresponders is also heterogeneous and not always according to the recommendations of the published guidelines. Each of the pediatric guidelines18,20,21 recommended ongoing CCB therapy for pediatric patients aged >1 year with positive AVT with a sustained beneficial clinical response. Nevertheless, in our survey, although most centers (89%) reported the use of CCBs for IPAH/HPAH patients in FC I/II, only 68% used CCB as monotherapy, as current guidelines recommend: CCBs were frequently used combined with PDE5i or ERA and, in a small proportion of patients, ERA or PDE5i were used instead of a CCB. The management of vasoresponders in advanced FC (e.g. III or IV) is not so well-established and was therefore quite different between institutions.
The reported protocols for follow-up in patients deemed vasoresponders are also different among institutions. Most (71%) centers typically repeat cardiac catheterization with AVT 6–12 months after the start of CCB, as recommended by published guidelines. However, 28% of the centers, mostly in Asia and South America, do not routinely repeat the catheterization if the patient has shown clinical improvement. More striking was the fact that 28% of the centers, distributed across the four continents, considered a maintained positive response to CCB to be defined by stable FC I/II, despite persistence of high PAP and PVRI. Nevertheless, some centers use even stricter criteria, including the need for near normalization of PAP and lower PVRI. The ESC guidelines published in 201523 support the approach that adults with positive AVT should be catheterized 3–4 months after the start of CCB to evaluate the response. Specific data and recommendations supporting this practice in the pediatric population remain lacking.
The percentage of IPAH with positive AVT and maintained favorable response to the CCB during long-term follow-up was variable between the different institutions (4%–70%), but the median was 13% (IQR = 4–10). This figure is comparable to data from the UK registry (8%),14 the Spanish registry (6%),17 or the 14% reported in the Reveal registry.11
Only half of the PH centers that answered the survey used AVT in pediatric PH disorders that differ from IPAH, likely reflecting uncertainty regarding the clinical significance of AVT in other settings
This study has some limitations. The answers of the survey were based on the written answers given by the responding physician, but not based on reported sets of data, as is done for registries. The answers reported in each continent or region only reflect the practice in the centers that answered the survey and so could not entirely reflect the clinical practice in that region or continent.
In conclusion, we have found that the use of AVT in pediatric IPAH/HPAH is not standardized regarding protocols for catheterization, performing and interpreting the AVT response, or long-term management of the patient, especially regarding re-assessment of AVT during follow-up care. Standardization of the approach to AVT is needed to implement therapeutic decisions based on the response and to better study and evaluate the potential role of AVT on survival and long-term outcomes. In addition, we recommend future studies of AVT in settings beyond IPAH/HPAH to determine the potential utility for identifying children who are better responders to pharmacologic therapy and risk for poor outcomes in specific diseases associated with PH.
Supplemental Material
Supplemental material, PUL857533 Supplemetal Material1 for Acute vasoreactivity testing in pediatric idiopathic pulmonary arterial hypertension: an international survey on current practice by Lina Caicedo, Rachel Hopper, Humberto Garcia Aguilar, Dunbar Ivy, Dora Haag, Jeff Fineman, Tillman Humpl, Omar Al-Tamimi, Jeff A. Feinstein, Rolf Berger, Erika Rosenzweig, Tarek Kashour, Gabriel Fernando Diaz, Alberto Mendoza, Usha Krishnan, Prashant Bobhate, Stephanie Handler, Antonio Augusto Lopes, Manoj Kumar Rahit, Parag Barward, Carlos Labrandero de Lera, Ian Adatia, Shahin Moledina, Steven Abman, Maria Jesus del Cerro and on behalf of PVRI Pediatric & CHD Taskforce, PPHNET, and REHIPED registry in Pulmonary Circulation
Supplemental Material
Supplemental material, PUL857533 Supplemetal Material2 for Acute vasoreactivity testing in pediatric idiopathic pulmonary arterial hypertension: an international survey on current practice by Lina Caicedo, Rachel Hopper, Humberto Garcia Aguilar, Dunbar Ivy, Dora Haag, Jeff Fineman, Tillman Humpl, Omar Al-Tamimi, Jeff A. Feinstein, Rolf Berger, Erika Rosenzweig, Tarek Kashour, Gabriel Fernando Diaz, Alberto Mendoza, Usha Krishnan, Prashant Bobhate, Stephanie Handler, Antonio Augusto Lopes, Manoj Kumar Rahit, Parag Barward, Carlos Labrandero de Lera, Ian Adatia, Shahin Moledina, Steven Abman, Maria Jesus del Cerro and on behalf of PVRI Pediatric & CHD Taskforce, PPHNET, and REHIPED registry in Pulmonary Circulation
Acknowledgments
The authors thank the members of the Pediatric and Congenital Heart Disease Taskforce of the PVRI, the PPHNET, and the REHIPED registry for their collaboration in this survey. They also thank Dr. Ornella Milanesi for her contribution to the survey during her participation in the Pediatric & CHD Taskforce Meeting in Rome, January 2016.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs
Alberto Mendoza https://orcid.org/0000-0002-1359-451X
Antonio Augusto Lopes https://orcid.org/0000-0002-4448-4911
References
- 1.Rich S, Brundage BH, Levy PS. The effect of vasodilator therapy on the clinical outcome of patients with primary pulmonary hypertension. Circulation 1985; 71(6): 1191–1196. [DOI] [PubMed] [Google Scholar]
- 2.Rich S, Brundage BH. High-dose calcium channel-blocking therapy for primary pulmonary hypertension: evidence for long-term reduction in pulmonary arterial pressure and regression of right ventricular hypertrophy. Circulation 1987; 76(1): 135–141. [DOI] [PubMed] [Google Scholar]
- 3.Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med 1992; 327: 76–81. [DOI] [PubMed] [Google Scholar]
- 4.Hemnes AR, Trammell AW, Archer SL, et al. Peripheral blood signature of vasodilator-responsive pulmonary arterial hypertension. Circulation 2015; 131: 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medarov BI, Judson MA. The role of calcium channel blockers for the treatment of pulmonary arterial hypertension: How much do we actually know and how could they be positioned today? Respir Med 2015; 109: 557–564. [DOI] [PubMed] [Google Scholar]
- 6.Barst RJ, Maislin G, Fishman AP. Vasodilator therapy for primary pulmonary hypertension in children. Circulation 1999; 99: 1197–1208. [DOI] [PubMed] [Google Scholar]
- 7.Barst RJ, Mcgoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2004; 43(12): S40–47. [DOI] [PubMed] [Google Scholar]
- 8.Ploegstra M, Zijlstra WMH, Douwes JM, et al. Prognostic factors in pediatric pulmonary arterial hypertension: A systematic review and meta-analysis. Int J Cardiol 2015; 184: 198–207. [DOI] [PubMed] [Google Scholar]
- 9.Manes A, Palazzini M, Leci E, et al. Current era survival of patients with pulmonary arterial hypertension associated with congenital heart disease: a comparison between clinical subgroups. Eur Heart J 2014; 35(11): 716–724. [DOI] [PubMed] [Google Scholar]
- 10.Douwes JM, Humpl T, Bonnet D, et al. Acute vasodilator response in pediatric pulmonary arterial hypertension: current clinical practice from the TOPP Registry. J Am Coll Cardiol 2016; 67(11): 1312–1323. [DOI] [PubMed] [Google Scholar]
- 11.Barst RJ, McGoon MD, Elliott CG, et al. Survival in childhood pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Circulation 2012; 125(1): 113–122. [DOI] [PubMed] [Google Scholar]
- 12.Sitbon O, Humbert M, Jaïs X, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation 2005; 111: 3105–3111. [DOI] [PubMed] [Google Scholar]
- 13.Cerro MJ, Moledina S, Haworth SG, et al. Cardiac catheterization in children with pulmonary hypertensive vascular disease: consensus statement from the Pulmonary Vascular Research Institute, Pediatric and Congenital Heart Disease Task Forces. Pulm Circ 2016; 6(1): 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moledina S, Hislop AA, Foster H, et al. Childhood idiopathic pulmonary arterial hypertension: a national cohort study. Heart 2010; 96(17): 1401–1406. [DOI] [PubMed] [Google Scholar]
- 15.Douwes JM, van Loon RL, Hoendermis ES, et al. Acute pulmonary vasodilator response in paediatric and adult pulmonary arterial hypertension: occurrence and prognostic value when comparing three response criteria. Eur Heart J 2011; 32(24): 3137–3146. [DOI] [PubMed] [Google Scholar]
- 16.Fraisse A, Jais X, Schleich JM, et al. Characteristics and prospective 2-year follow-up of children with pulmonary arterial hypertension in France. Arch Cardiovasc Dis 2010; 103(2): 66–74. [DOI] [PubMed] [Google Scholar]
- 17.del Cerro Marín MJ, Sabaté Rotés A, Rodriguez Ogando A, et al. Assessing pulmonary hypertensive vascular disease in childhood. Data from the Spanish registry. Am J Respir Crit Care Med 2014; 190(12): 1421–1429. [DOI] [PubMed] [Google Scholar]
- 18.Ivy DD, Abman SH, Barst RJ, et al. Pediatric pulmonary hypertension. J Am Coll Cardiol 2013; 62(25 Suppl): D117–126. [DOI] [PubMed] [Google Scholar]
- 19.Hansmann G, Apitz C, Abdul-Khaliq H, et al. Executive summary. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart 2016; 102(Suppl): ii86–ii100. [DOI] [PubMed] [Google Scholar]
- 20.Lammers AE, Apitz C, Zartner P, et al. Diagnostics, monitoring and outpatient care in children with suspected pulmonary hypertension/paediatric pulmonary hypertensive vascular disease. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart 2016; 102(Suppl 2): ii1–13. [DOI] [PubMed] [Google Scholar]
- 21.Abman SH, Hansmann G, Archer SL, et al. Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation 2015; 132(21): 2037–2099. [DOI] [PubMed] [Google Scholar]
- 22.Galié N, Corris P, Frost A, et al. Updated treatment algorithm for pulmonary arterial hypertension. J Am Coll Cardiol 2013; 62: D60–D72. [DOI] [PubMed] [Google Scholar]
- 23.Galiè N, Humbert M, Vachiery JL, et al. ESC 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37(1): 67–119. [DOI] [PubMed] [Google Scholar]
- 24.Rosenzweig EB, Abman SH, Adatia I, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J 2019; 53: 1801916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Apitz C, Hansmann G, Schranz D. Hemodynamic assessment and acute pulmonary vasoreactivity testing in the evaluation of children with pulmonary vascular disease. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart 2016; 102(Suppl 2): ii23–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, PUL857533 Supplemetal Material1 for Acute vasoreactivity testing in pediatric idiopathic pulmonary arterial hypertension: an international survey on current practice by Lina Caicedo, Rachel Hopper, Humberto Garcia Aguilar, Dunbar Ivy, Dora Haag, Jeff Fineman, Tillman Humpl, Omar Al-Tamimi, Jeff A. Feinstein, Rolf Berger, Erika Rosenzweig, Tarek Kashour, Gabriel Fernando Diaz, Alberto Mendoza, Usha Krishnan, Prashant Bobhate, Stephanie Handler, Antonio Augusto Lopes, Manoj Kumar Rahit, Parag Barward, Carlos Labrandero de Lera, Ian Adatia, Shahin Moledina, Steven Abman, Maria Jesus del Cerro and on behalf of PVRI Pediatric & CHD Taskforce, PPHNET, and REHIPED registry in Pulmonary Circulation
Supplemental material, PUL857533 Supplemetal Material2 for Acute vasoreactivity testing in pediatric idiopathic pulmonary arterial hypertension: an international survey on current practice by Lina Caicedo, Rachel Hopper, Humberto Garcia Aguilar, Dunbar Ivy, Dora Haag, Jeff Fineman, Tillman Humpl, Omar Al-Tamimi, Jeff A. Feinstein, Rolf Berger, Erika Rosenzweig, Tarek Kashour, Gabriel Fernando Diaz, Alberto Mendoza, Usha Krishnan, Prashant Bobhate, Stephanie Handler, Antonio Augusto Lopes, Manoj Kumar Rahit, Parag Barward, Carlos Labrandero de Lera, Ian Adatia, Shahin Moledina, Steven Abman, Maria Jesus del Cerro and on behalf of PVRI Pediatric & CHD Taskforce, PPHNET, and REHIPED registry in Pulmonary Circulation