Abstract
Objective:
To evaluate the utility of the quick Sepsis-related Organ Failure Assessment (qSOFA) score to predict risks for emergency department (ED) and hospital mortality among patients in a sub-Saharan Africa (SSA) setting.
Methods:
This retrospective cohort study was carried out at a tertiary-care hospital, in Kigali, Rwanda and included patients ≥15 years, presenting for ED care during 2013 with an infectious disease (ID). ED and overall hospital mortality were evaluated using multivariable regression, with qSOFA scores as the primary predictor (reference: qSOFA = 0), to yield adjusted relative risks (aRR) with 95% confidence intervals (CI). Analyses were performed for the overall population and stratified by HIV status.
Results:
Among 15,748 cases, 760 met inclusion (HIV infected 197). The most common diagnoses were malaria and intra-abdominal infections. Prevalence of ED and hospital mortality were 12.5% and 25.4% respectively. In the overall population, ED mortality aRR was 4.8 (95% CI 1.9–12.0) for qSOFA scores equal to 1 and 7.8 (95% CI 3.1–19.7) for qSOFA scores ≥2. The aRR for hospital mortality in the overall cohort was 2.6 (95% 1.6–4.1) for qSOFA scores equal to 1 and 3.8 (95% 2.4–6.0) for qSOFA scores ≥2. For HIV infected cases, although proportional mortality increased with greater qSOFA score, statistically significant risk differences were not identified.
Conclusion:
The qSOFA score provided risk stratification for both ED and hospital mortality outcomes in the setting studied, indicating utility in sepsis care in SSA, however, further prospective study in high-burden HIV populations is needed.
Keywords: Sepsis, qSOFA, HIV, Mortality, Emergency care, Rwanda, Africa
1. Introduction
There are over 30 million cases of sepsis annually, with the majority occurring in low- and middle- income countries (LMICs) [1]. Sepsis treatment can require substantial resources, that are often limited in LMICs, and also confers disproportionately higher mortality in these settings as compared to High Income Countries (HICs) [2–6]. In resource-constrained settings, there is immense clinical potential for scoring systems and protocols to assist in identifying patients with sepsis, as doing so could improve patient identification, allocation of scarce assets and outcomes [7–10].
In 2016, the quick Sepsis-related Organ Failure Assessment (qSOFA) score was developed as a rapid evaluation method to identify patients with organ dysfunction due to a dysregulated host response to infection, and subsequently at increased risk for adverse outcomes [11]. In HICs, greater qSOFA scores have been correlated with increased risks for in-hospital mortality [12]. Additionally, in HIC emergency department (ED) populations qSOFA scores have been shown to have prognostic accuracy in identifying patients in need of intensive care unit (ICU) treatment and those with greater mortality risks [13,14].
As the qSOFA score is derived solely from physical exam, it has even greater potential application in resource-limited settings, where barriers exist to laboratory and device-based methods used in HICs for sepsis risk stratification [15–18]. There is however, limited data on the utility of qSOFA scores in LMICs, where patient and facility factors differ substantially from HICs [19,20]. Furthermore, the clinical utility of the qSOFA score in HIV-infected patients, which are more prevalent in LMICs [21], commonly suffer from distinct infectious etiologies (i.e. fungal and mycobacteria), have altered and impaired immune response mechanisms [22] and have higher mortality from sepsis [23–26], has been minimally studied [19]. Compounding these gaps in the evidence, there is no research evaluating ED-specific outcomes from sepsis in LMICs from sub-Saharan Africa (SSA), where mortality risks are up to 100 fold greater than in HIC EDs [8,27], and as such representing a population with a great margin for benefit from rapid and appropriate identification of the highest risk sepsis patients.
This study aimed to evaluate the utility of the qSOFA score to predict ED and overall hospital mortality outcomes among patients presenting for emergency care, with and without HIV infection, in the SSA setting of Kigali, Rwanda.
2. Materials and Thethods
The research was approved by the University Teaching Hospital of Kigali (UTH-K) ethics committee (EC/CHUK/463/2017) and the institutional review board of Rhode Island Hospital (Reference number: 414114; 45 CFR 46.110.5). Study funding was provided via grants from the University Emergency Medicine Foundation, Providence, Rhode Island and the International Respiratory and Severe Illness Center at the University of Washington (INTERSECT), Seattle, Washington. The funders had no role in the study design, data collection or reporting processes.
2.1. Study design, setting and population
This retrospective cohort study was carried out at the UTH-K in Kigali, Rwanda. The site serves Rwanda as the primary national public referral center for healthcare needs. UTH-K is an urban, tertiary-care institution with approximately 40 ED and 500 inpatient beds and access to specialty services, laboratory medicine and radiologic capabilities. Patients fifteen years of age or older presenting to the ED for care from 1 January to 31 December 2013 with a final ED diagnosis of an infectious disease (ID) were eligible for inclusion.
2.2. Data management
Cases were identified and data was queried from hardcopy medical records via protocolized methods, as previously described [28–30]. Briefly, using a multipoint composite index, all ED cases during the accruement period were identified and the corresponding medical records were screened. Protocol-trained personnel reviewed records for inclusion and exclusion criteria and data were abstracted using a standardized instrument. A local data manager continually oversaw data collection and protocol adherence. Cases were identified primarily by a Rwandan physician (Z.A.M) and uncertainties were assessed by a second reviewer (A.R.A) such that adjudicated consensus for inclusion or exclusion were reached. Data procedures conformed to quality practices for chart review research in emergency medicine [31].
Data included information on demographics, comorbidities, clinical presentation, ED care, ID diagnoses, care duration, and ED and inpatient outcomes. ED treatments of interest included intravenous fluids (IVF), antimicrobials, vasopressors, and the provision of respiratory support (supplemental oxygen and mechanical ventilation). Ambiguous elements were coded as missing. Data were entered into an electronic password-protected database. To evaluate the reliability of the data extraction process, 10% of records were randomly selected and double entered by personnel blinded to the initial abstraction.
HIV status was identified either via serologic testing occurring during the index care period or through prior documentation of infection. Cases with an HIV-negative result during the index care period were classified as uninfected. In order to be representative of clinical practice in LMICs, where HIV results may not be available, cases were assumed to be uninfected if no HIV testing was undertaken. HIV assessments were based on national guidelines and performed using standard immunoassays (Alere Determine™ HIV-1/2 and/or Uni-Gold™ HIV-1/2) [32]. In addition to HIV status, ID diagnoses (multiple if present) were categorized based on either etiological pathogens (i.e. S. typhi) or clinical syndromes (i.e. uncomplicated malaria) as determined by the available data and based on a standard emergency medicine reference text [33]. For cases discharged from the ED, the ID diagnoses at ED discharged were used, and in cases admitted to the hospital the final inpatient ID diagnoses were used.
2.3. Data analysis
Data analysis was performed using STATA version 15.0 (StataCorp; College Station, USA). Descriptive analyses were undertaken for the overall cohort and stratified by HIV status. Variables were described using frequencies with percentages or medians with associated interquartile ranges (IQR). Characteristics base on HIV status were compared using Pearson X2 or Fisher’s exact tests for categorical variables and by Mann-Whitney or t-tests for non-normally and normally distributed continuous variables, respectively. To account for multiple testing, a Bonferroni correction with a significance level of p < 0.003 was utilized in comparative analyses by HIV status [34].
The primary outcomes of interest were all-cause ED and overall hospital mortality. Hospital mortality aggregated ED and inpatient data. The primary predictor variable was the qSOFA score. The qSOFA score, which assigns one point for each of the following: systolic blood pressure (SBP) ≤ 100 millimeters of mercury (mm Hg), respiratory rate (RR) ≥ 22 breaths/min and Glasgow Coma Scale (GCS) < 15, were calculated based on assessments at ED presentation [12]. Only cases with complete data for all qSOFA variables had scores calculated. Due to only fifteen cases (2.5%) having qSOFA scores of three, and prior research demonstrating a score of ≥2 as a threshold for increased risk [12], strata two and three were merged such that scores were coded as 0, 1 or ≥2.
Mortality outcomes based on qSOFA scores were explored using proportions with associated 95% CIs. Significance trends across qSOFA scores were assessed using Spearman’s rho. Logistic regression models yielding relative risks (RR) were used to quantify magnitudes of effect for the outcomes of interest. Multivariable regression analyses, adjusted a priori for covariates known to be associated with sepsis mortality, were used to calculate adjusted relative risks (aRR). Covariates adjusted for included: age [35,36], comorbidities [36,37], IVF treatment [38,39], and antimicrobial treatment [40,41]. In overall hospital mortality models, further adjustment for duration of care was performed to control for temporal trends in sepsis outcomes [15,42]. Analyses were performed for the overall study population and, as HIV impacts mortality outcomes in LMICs, analyses were performed separately for HIV uninfected and HIV infected cases as pre-planned subgroups [23,24,43]. Due to the multiple models developed, a pre-defined significance level of p b 0.005 was used [34].
The test characteristics for mortality outcomes based on qSOFA scores of sensitivity, specificity, positive predictive value and negative predictive value were calculated. The accuracy for ED and hospital mortality was assessed using area under the receiver-operating characteristic curves (AUC) with associated 95% confidence intervals (CI), in accordance with standards on evaluating diagnostic accuracy [44]. The test characteristics were analyzed for the overall cohort and stratified by HIV status.
To evaluate the representativeness of patients with qSOFA scores in relation to the larger sample meeting inclusion, characteristics of cases with and without qSOFA scores calculated were evaluated for statistical differences. Additionally, covariates were compared between cases coded as HIV uninfected based on documented HIV testing and those assumed to be HIV uninfected to assess validity in the methods. In the qSOFA score and HIV uninfected analyses, significance levels of p < 0.005 were used to account for multiple testing [34]. Sensitivity analyses to evaluate more proximate outcomes were undertaken. In the sensitivity analyses, the same regression models were used however the outcome of interest was 7-day mortality. For internal data quality assessment using the double-entered records, inter-rater reliability (IRR) was calculated via Cohen’s κappa (κ), and interpreted according to established criteria [45].
3. Results
3.1. Characteristics of the study population
Among 20,861 ED cases treated during the study period, 5113 medical records were unable to be located, resulting in 15,748 cases being identified and screened using the predefined criteria. Of these, 760 were included in the analysis. Within this cohort, 197 cases (25.9%) were HIV infected and 563 cases (74.1%) were HIV uninfected (Fig. 1).
Fig. 1.
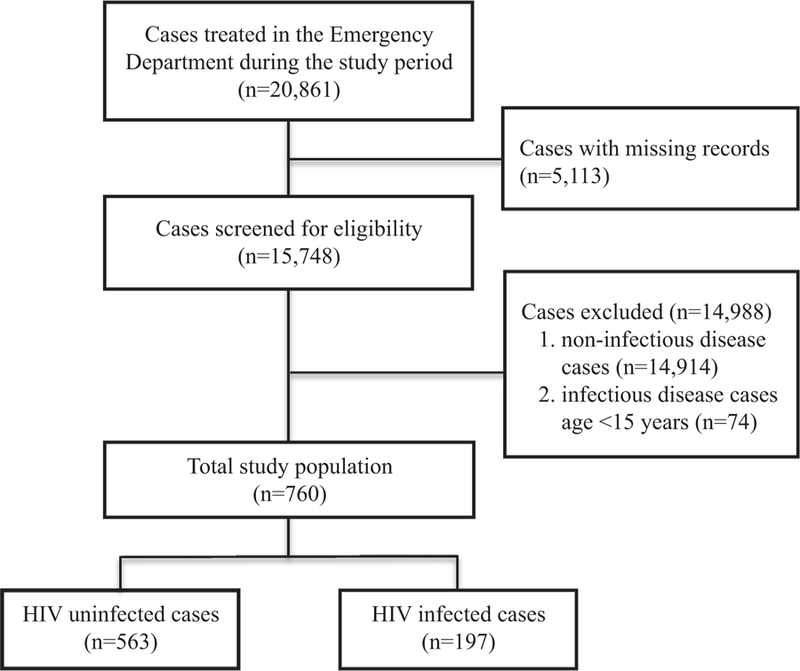
Study population.
In the overall study population, the median age was 36 years (26, 51) and 45.9% of cases were female. Among those with sufficient documentation, approximately half had preexisting comorbidities (Table 1). The most common comorbidities were HIV (44.3%) and non-communicable diseases, of diabetes mellitus (DM), chronic kidney disease (CKD) and/or cardiovascular (CV) disease, which were present in 40.1% of cases. The most common ID diagnoses were malaria (22.8%), intra-abdominal infections (19.2%), Tuberculosis (TB) species infections (18.4%), non-TB pulmonary infections (11.7%) and genitourinary (GU) infections (5.1%).
Table 1.
Study population characteristics.
| Characteristics | n (%)/median (IQR) |
|---|---|
| Gender | |
| Male | 411 (54.1%) |
| Female | 349 (45.9%) |
| Age (years) | 36 (26, 51) |
| Preexisting comorbidities | |
| No | 258 (34.0%) |
| Yes | 413 (54.3%) |
| Unknown | 89 (11.7%) |
| qSOFAb criteria | |
| Systolic blood pressure ≤ 100 mm Hg | 164 (23.5%) |
| Respiratory rate ≥ 22 breaths/min | 267 (39.7%) |
| Glasgow coma score < 15 | 159 (24.8%) |
| qSOFAa score | |
| 0 | 217 (28.6%) |
| 1 | 249 (32.7%) |
| ≥2 | 129 (17.0%) |
| Unknown | 165 (21.7%) |
| Emergency department treatments | |
| Intravenous fluids | 497 (65.4%) |
| Antimicrobial medications | 560 (73.7%) |
| Respiratory support | 175 (23.0%) |
| Vasopressors | 46 (6.1%) |
| Emergency department outcome | |
| Discharged | 158 (20.8%) |
| Admitted to hospital | 470 (61.9%) |
| Transferred | 4 (0.5%) |
| Died | 95 (12.5%) |
| Eloped | 0 (0.0%) |
| Unknown | 33 (4.3%) |
| Inpatient outcomeb | |
| Discharged | 356 (75.7%) |
| Transferred | 13 (2.8%) |
| Died | 98 (20.9%) |
| Eloped | 1 (0.2%) |
| Unknown | 2 (0.4%) |
| Overall outcome | |
| Discharged | 514 (67.6%) |
| Transferred | 17 (2.2%) |
| Died | 193 (25.4%) |
| Eloped | 1 (0.1%) |
| Unknown | 35 (4.6%) |
| Care duration (days) | 7 (2, 17) |
qSOFA abbreviates, quick Sepsis-related Organ Failure.
Represents cases admitted to inpatient care.
There was sufficient data to calculate qSOFA scores for 595 cases. Among these; 217 (36.5%) had a qSOFA score of 0, 249 (41.8%) had a qSOFA score of 1, and 129 (21.7%) had qSOFA score of ≥2. There were no significant differences identified between cases with sufficient data to calculate qSOFA scores and those without (Appendix A). Most cases received ED treatments with IVF (65.4%) and antimicrobials (73.7%). Respiratory support and vasopressors were used in 23.0% and 6.1% of cases, respectively (Table 1). The most frequently provided antimicrobials were third-generation cephalosporins (74.7%). For ED outcomes, 470 cases (61.9%) were admitted and 95 (12.5%) died in the ED. Among admitted cases, 98 (20.9%) died. The overall hospital mortality prevalence was 25.4%, and the median duration of care was 7 days (2, 17) (Table 1).
3.1.1. Characteristics based on HIV status
For HIV infected cases, 183 were identified via prior diagnosis and fourteen were incident diagnoses. There were no significant differences in gender, age, qSOFA score, ED treatment with IVF, respiratory support or vasopressors between HIV uninfected and HIV infected cases. HIV infected cases as compared to the uninfected were more likely to receive antimicrobials (84.8% versus 69.8%, p < 0.001), had longer median care durations (14 versus 6 days, p < 0.001), and greater hospital mortality (33.5% versus 22.6%, p = 0.002) (Table 2). In the HIV uninfected subgroup, the most common diagnoses were malaria, intra-abdominal infections, and TB species infections. For HIV infected cases, non-TB pulmonary infections, central nervous system (CNS) infections, and TB species infections were the most frequent diagnoses. Among HIV negative cases, there were no significant differences identified based on method of HIV status categorization (Appendix B).
Table 2.
Comparative characteristics of cases by HIV status.
| Characteristics | HIV uninfected (n = 563) |
HIV infected (n = 197) |
p |
|---|---|---|---|
| Gender | |||
| Male | 307 (54.5%) | 104 (52.8%) | |
| Female | 256 (45.5%) | 93 (47.2%) | 0.674 |
| Unknown | 0 (0.0%) | 0 (0.0%) | |
| Age (years) | 35 (24, 55) | 37 (31, 45) | 0.355 |
| Preexisting comorbidities | |||
| No | 253 (44.9%) | 5 (2.5%) | |
| Yes | 225 (40.0%) | 188 (95.5%) | <0.001 |
| Unknown | 85 (15.1%) | 4 (2.0%) | |
| qSOFAb criteria | |||
| Systolic blood pressure ≤ 100 mm Hg | 98 (17.4%) | 66 (33.5%) | <0.001 |
| Respiratory rate ≥ 22 breaths/min | 191 (33.9%) | 76 (38.6%) | 0.453 |
| Glasgow coma score < 15 | 125 (22.2%) | 34 (17.3%) | 0.171 |
| qSOFAa score | |||
| 0 | 178 (31.6%) | 39 (19.8%) | |
| 1 | 180 (32.0%) | 69 (35.0%) | 0.015 |
| ≥2 | 91 (16.2%) | 38 (19.3%) | |
| Unknown | 114 (20.2%) | 51 (25.9%) | |
| Emergency department treatments | |||
| Intravenous fluids | 358 (63.4%) | 139 (70.6%) | 0.077 |
| Antimicrobial medications | 393 (69.8%) | 167 (84.8%) | <0.001 |
| Respiratory support | 131 (23.4%) | 44 (22.3%) | 0.789 |
| Vasopressors | 32 (5.7%) | 14 (7.1%) | 0.422 |
| Emergency department outcome | |||
| Discharged | 135 (24.0%) | 23 (11.7%) | |
| Admitted to hospital | 322 (57.2%) | 148 (75.1%) | |
| Transferred | 4 (0.7%) | 0 (0.0%) | <0.001 |
| Died | 71 (12.6%) | 24 (12.2%) | |
| Eloped | 0 (0.0%) | 0 (0.0%) | |
| Unknown | 31 (5.5%) | 2 (1.0%) | |
| Inpatient outcomeb | |||
| Discharged | 259 (80.4%) | 97 (65.5%) | |
| Transferred | 7 (2.2%) | 6 (4.1%) | |
| Died | 56 (17.4%) | 42 (28.4%) | 0.002 |
| Eloped | 0 (0.0%) | 1 (0.7%) | |
| Unknown | 0 (0.0%) | 2 (1.3%) | |
| Overall outcome | |||
| Discharged | 394 (70.0%) | 120 (60.9%) | |
| Transferred | 11 (1.9%) | 6 (3.1%) | |
| Died | 127 (22.6%) | 66 (33.5%) | 0.003 |
| Eloped | 0 (0.0%) | 1 (0.5%) | |
| Unknown | 31 (5.5%) | 4 (2.0%) | |
| Care duration (days) | 6 (2, 13) | 14 (5, 26) | <0.001 |
qSOFA abbreviates, quick Sepsis-related Organ Failure Assessment.
Represents cases admitted from in patient care.
3.2. Mortality outcomes by qSOFA scores
Proportional mortality outcomes by qSOFA score for the overall study population and stratified by HIV status are depicted in Fig. 2. Trends in ED mortality significantly increased with higher qSOFA score in the overall cohort and were 3.0% for those with a score of 0, 15.6% with a score of 1 and 27.3% with a score ≥ 2 (p < 0.001). These trends were maintained in the HIV uninfected (p < 0.001) and HIV infected (p = 0.002) subgroups. Similarly, hospital mortality in the overall study population was 10.3%, 30.9% and 43.0% for cases with a qSOFA score of 0, 1 and ≥2, respectively (p < 0.001). The significant trend in hospital mortality was also found in both HIV infected (p = 0.004) and uninfected (p < 0.001) subgroups (Fig. 2).
Fig. 2.
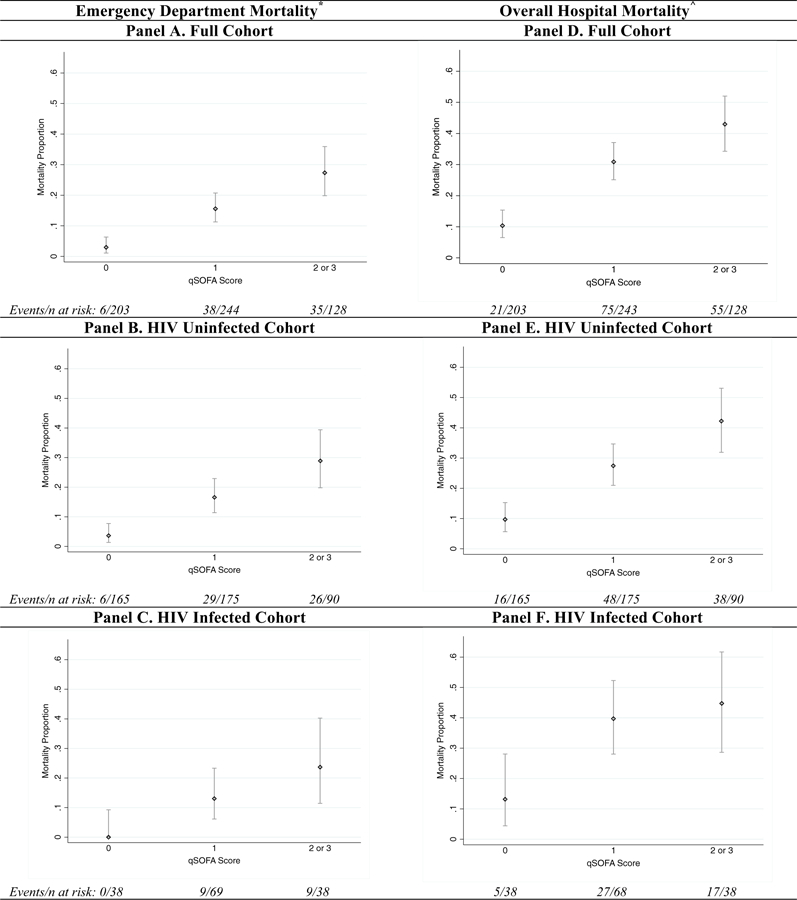
Proportional mortality outcomes. * Emergency department mortality outcomes by qSOFA (quick Sepsis-related Organ Failure Assessment) score with associated 95% CIs. ^ Cumulative outcomes for emergency department and inpatient mortality by qSOFA score with associated 95% CIs.
3.2.1. ED mortality risks
The risk for ED mortality increased with higher qSOFA score across all regression models. In the overall study population in multivariable analyses, as compared to cases with qSOFA scores of 0, the aRR of ED mortality was 4.8 (95% CI 1.9–12.0) for those with qSOFA score of 1 and 7.8 (95% CI 3.1–19.7) for cases with qSOFA score of ≥2. For HIV uninfected patients, the aRR for ED mortality was 3.9 (95% CI 1.5–9.8) for cases with a qSOFA score of 1 and 6.2 (95% CI 2.1–15.9) for those with a qSOFA score ≥ 2 (Table 3). Risks for HIV infected ED mortality were not calculated as there were no mortality events in the reference stratum.
Table 3.
Emergency department mortality risks based on qSOFAa scores.
| qSOFAa score | Full cohort |
|||
|---|---|---|---|---|
| RRb (95% CI) | p | aRRb,c (95% CI) | p | |
| 0 | Reference | Reference | ||
| 1 | 5.3 (2.3–12.2) | <0.001 | 4.8 (1.9–12.0) | 0.001 |
| ≥2 | 9.3 (4.0–21.4) | <0.001 | 7.8 (3.1–19.7) | <0.001 |
| qSOFAa score | HIV uninfected cohort |
|||
| RRb (95% CI) | P | aRRb,c (95% CI) | p | |
| 0 | Reference | Reference | ||
| 1 | 4.6 (1.9–10.7) | <0.001 | 3.9 (1.5–9.8) | 0.004 |
| ≥2 | 7.9 (3.4–18.6) | <0.001 | 6.2 (2.4–15.9) | <0.001 |
qSOFA abbreviates, quick Sepsis-related Organ Failure Assessment.
RR abbreviates Relative Risk and aRR abbreviates adjusted Relative Risk.
Multivariate models adjusted for patient age, pre-existing co-morbidities, emergency department treatment with intravenous fluids and emergency department treatment with antimicrobials.
3.2.2. Hospital mortality risks
In regression models, risk for hospital mortality was significantly increased with higher qSOFA score in the overall cohort. In multivariable analysis of the overall cohort, the aRR as compared to the reference stratum (qSOFA score of 0) was 2.6 (95% CI 1.6–4.1) for cases with a score of 1 and 3.8 (95% CI 2.4–6.0) for qSOFA score ≥ 2. In the HIV uninfected subgroup, the aRR were similar. Among cases that were HIV infected, although mortality risks increased with higher qSOFA scores, there were no significant differences identified in regression models based on the predefined significance threshold of p < 0.005 (Table 4).
Table 4.
Overall hospital mortality risks based on qSOFAa scores.
| qSOFAa score | Full cohort |
|||
| RRb (95% CI) | p | aRRb,c (95% CI) | p | |
| 0 | Reference | Reference | ||
| 1 | 3.0 (1.9–4.7) | <0.001 | 2.6 (1.6–4.1) | <0.001 |
| ≥2 | 4.2 (2.7–6.5) | <0.001 | 3.8 (2.4–6.0) | <0.001 |
| qSOFAa score | HIV uninfected cohort |
|||
| RRb (95% CI) | p | aRRb,c (95% CI) | p | |
| 0 | Reference | Reference | ||
| 1 | 2.8 (1.6–4.8) | <0.001 | 2.3 (1.3–4.1) | 0.003 |
| ≥2 | 4.4 (2.6–7.4) | <0.001 | 3.4 (1.9–6.0) | <0.001 |
| qSOFAa score | HIV infected cohort |
|||
| RRb (95% CI) | p | aRRb,c (95% CI) | p | |
| 0 | Reference | Reference | ||
| 1 | 3.0 (1.3–7.2) | 0.013 | 3.0 (1.3–7.1) | 0.014 |
| ≥2 | 3.4 (1.4–8.3) | 0.007 | 3.4 (1.4–8.3) | 0.007 |
qSOFA abbreviates, quick Sepsis-related Organ Failure Assessment.
RR abbreviates Relative Risk and aRR abbreviates adjusted Relative Risk.
Multivariate models adjusted for patient age, pre-existing co-morbidities, emergency department treatment with intravenous fluids, emergency department treatment with antimicrobials and care duration.
3.3. Diagnostic characteristics
In the full cohort of cases for ED mortality qSOFA scores of ≥1 and ≥2 had sensitivities of 92% and 44% and specifies of 40% and 81% respectively. The AUC for ED morality was 0.73 (95% CI 0.67–0.78). For overall hospital morality sensitivity was found to be 86% for a qSOFA score of ≥1 and 36% for a score ≥ 2, while specificities were 43% for a qSOFA score of ≥1 and 83% for cases with scores ≥2. The corresponding AUC for overall hospital morality was 0.70 (95% CI 0.65–0.75) (Fig. 3). Similar trends in diagnostic characteristics were found when the cohort was stratified by HIV status and analyzed (Appendix C).
Fig. 3.
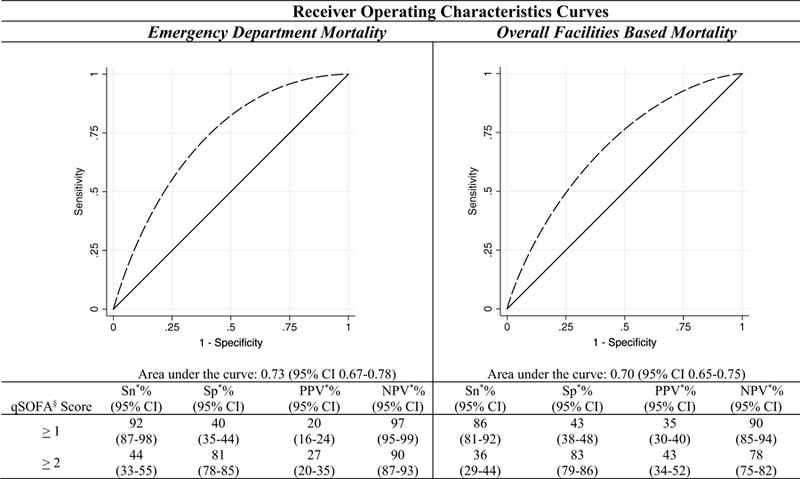
Diagnostic accuracy of qSOFA§ score (overall cohort). § qSOFA abbreviates, quick Sepsis-related Organ Failure Assessment. * Abbreviations: Sn; sensitivity, Sp; specificity, PPV; positive predictive value, NPV; negative predictive value.
3.4. Sensitivity and validity analyses
Sensitivity models evaluating hospital mortality through day 7 for the overall study population reproduced the same significant risk trends as in the primary analyses (Appendix D). Among double entered records, IRR was excellent, κ = 0.87 (standard error 0.09).
4. Discussion
This study provides the first available data on the utility of the qSOFA score in a population presenting for emergency care of IDs in a SSA setting. The results demonstrate that among a diverse case mix, the qSOFA score may provide useful risk stratification for both ED and overall hospital mortality outcomes. However, for HIV infected cases, although greater qSOFA scores correlated with larger proportional mortality burdens, the higher score did not yield statistically significantly increased mortality risks in adjusted analyses based on predetermined significance thresholds. These findings highlight that the qSOFA score, which is derived from easily attainable clinical information, may have application in resource-limited settings, both in the ED and inpatient arenas, to assist in rapidly and appropriately identifying the highest risk patients with sepsis. However, given the retrospective design and findings in the HIV infected subgroup, larger prospective studies in LMICs with high HIV prevalence are needed to better inform care provision.
Although there are three prior reports from urban ED settings in China [46–48], the current results represent the only available data on the utility of the qSOFA score to inform ED specific mortality risk from a LMIC in Africa. The results show that among a large cohort of patients presenting with a broad spectrum of IDs and substantial burdens of malaria, TB and HIV, higher qSOFA scores at triage were associated with significantly increased risks for ED mortality. EDs in LMICs care for high-risk and underserved populations; a systematic review and meta-analysis found that the median ED mortality was 4.8% among reports from east, central and west Africa, a figure one-hundred fold greater than summary metrics on ED mortality from the United States [8,27]. Adding to this, the limited access to trained emergency personnel and resources in LMICs [8,49], the potential for simple clinical tools, such as the qSOFA score, to identify patients at greatest risk and improve the allocation of scarce resources may be substantial. In HICs, up to two-thirds of patients with sepsis access care through EDs [50]. Although no similar data exist for LMICs, given the less developed primary care and referral systems [51], it is likely an even greater proportion. Therefore assessing these large numbers of patients for those with higher qSOFA scores, and as such at potentially greater risk for mortality, could facilitate more expeditious treatment in LMICs. The illustrated utility in mortality risk stratification suggests that implementation of qSOFA assessments could be beneficial, however identification is only a first-step in improving sepsis care in the minimally studied and complex milieus of LMICs. As exemplified by an interventional trial from Zambia published in 2017, in which protocol based resuscitation increased in-hospital mortality compared with usual practice [52], application of sepsis care paradigms from HICs is not sufficient, and future research evaluating the use of qSOFA scores in conjunction with setting-appropriate treatment algorithms in LMICs is imperative.
All prior published qSOFA assessment and sepsis treatment research from SSA has utilized solely inpatient data and outcomes [19,20,24,52]. Although such selection makes performing studies easier, it disregards the initial emergency care period and, given the high mortality burdens in that phase [8], results may be biased and misinform practice. To provide a more complete evaluation, mortality across the continuum of care was used as an outcome of interest in the current research. Subsequently, the presented cohort is the largest from SSA assessing the utility of the qSOFA score for sepsis risk assessment, and shows that higher qSOFA scores are associated with increased ED and hospital mortality risks. These results agree with findings from an inpatient cohort in Gabon in which scores ≥2 were associated with greater odds of mortality, and a study of admitted patients in Malawi in which the mortality proportions increased from 3% to 14% to 40% for qSOFA scores of 0, 1 and ≥2 respectively [19,20]. Furthermore, the current results are consistent with the increased mortality risk associated with higher qSOFA score reported in the original derivation cohort of non-ICU patients [12]. This concordance across multiple research venues suggests validity in the findings presented. However, given secular trends in healthcare and outcomes in LMIC contexts, coupled to the crucial needs for research specific to LMICs, additional contemporary prospective studies are required to validate these results.
Unlike the previously reported increased risk of poor outcomes with sepsis in HIV infection [24,26], the present study did not demonstrate statistically significant increased risks of mortality with greater qSOFA scores among the HIV infected. However, as proportional mortality increased with higher qSOFA scores in HIV infected cases, this finding is likely secondary to insufficient power to be able to discriminate significant inter-strata qSOFA risk differences. An alternative possibility is that patient heterogeneity in immune statuses existed which may have mitigated outcomes in the HIV infected sub-population. As the available data could not control for these patient-level factors, this hypothesis could not be assessed. Taking into account that information on patient immune status is often unavailable in LMICs during the acute care period, the analysis performed is pragmatic and represents an applicable interpretation of the available data.
The AUCs for diagnostic accuracy in the current data corresponded with prior studies from HICs from both ED [14] and inpatient [53] populations, and as well with research in LMICs from SSA settings [19,20], supporting validity in the results. Of note the sensitivity for mortality outcomes was highest with a cut-point at a qSOFA score ≥ 1 while specificity was maximized at scores ≥2 across the overall population and when stratified by HIV status. As concern regarding the sensitivity of the qSOFA score has been expressed [54], the current data suggests that thresholds for screening and treatment of high-risk sepsis patients in LMICs may differ from those put forth in HICs, and further study of this is warranted. Additionally, though not evaluated in the present analysis, it is postulated that the qSOFA sensitivity could be augmented through serial assessments, which is a realistic programmatic goal in LMICs and may be an important implementation component to improve care in such settings [47].
This study must be interpreted with certain limitations. Although rigorous methods for the study type were used [31], the retrospective design resulted in missing data for the primary predictor variable, which may have introduced bias. As there were no statistically significant differences in variables between cases with calculated qSOFA scores and those without, it is likely that the results are representative of the overall study population. Contrary to prior retrospective studies, which used antimicrobial treatment as the indicator of an ID [14,19], the current report used final diagnosis. This methodology could limit the application of the results, as such information is not known at the time of patient presentation. However, as provision of antimicrobials in LMICs can be inhibited by systems and resource factors, final diagnosis represents a strategy less vulnerable to such externalities and provides a more inclusive population for study. Additionally, the research was performed at a single tertiary-care hospital, which may limit the generalizability of the findings. Despite these limitations, the results allow reasonable conclusions to be drawn pertaining to the utility of the qSOFA score to stratify patients with IDs at risk of both ED and overall hospital mortality from sepsis and should be used to inform future research and subsequent practice.
This research provides the first available data on the utility of the qSOFA score from a LMIC emergency care population from SSA, and demonstrates that the easily attainable qSOFA metric provides useful risk stratification for both ED and overall hospital mortality outcomes. With appropriate implementation the use of the qSOFA score could translate into improved allocation of healthcare resources and prediction of patient outcomes among populations with the greatest margin for gains through enhanced sepsis care. However, given the findings pertaining to the HIV infected subgroup and the overall limited available evidence on sepsis care from resource-limited settings, appropriate implementation necessitates further prospective studies in LMICs with large HIV burdens to better inform health policy and practice.
Acknowledgments
Funding
Funding was provided through grants from the University Emergency Medicine Foundation, Providence Rhode Island and the International Respiratory and Severe Illness Center at the University of Washington (INTERSECT), Seattle, Washington. The funders had no role in the study design, data collection or reporting processes.
Abbreviations:
- aRR
adjusted relative risk
- AUC
area under the receiver-operating characteristic curves
- CV
cardiovascular
- CNS
central nervous system
- CKD
chronic kidney disease
- CI
confidence intervals
- DM
diabetes mellitus
- ED
emergency department
- GU
genitourinary
- GCS
Glasgow Coma Scale
- HICs
High Income Countries
- IVF
intravenous fluids
- ICU
intensive care unit
- IRR
inter-rater reliability
- IQR
interquartile ranges
- LMICs
low- and middle- income countries
- mm Hg
millimeters of mercury
- qSOFA
quick Sepsis-related Organ Failure Assessment
- RR
respiratory rate
- SSA
sub-Saharan Africa
- SBP
systolic blood pressure
- TB
Tuberculosis
- UTH-K
University Teaching Hospital of Kigali
Appendix A. Comparison of cases by availability of qSOFA1 scores
| Characteristics | Cases without qSOFA scores (n = 165) | Cases with qSOFA scores (n = 595) | p |
|---|---|---|---|
| Gender | |||
| Male | 92 (55.8) | 319 (53.6%) | |
| Female | 73 (44.2%) | 276 (46.4%) | 0.625 |
| Age (years) | 34 (25, 50) | 36 (27, 51) | 0.270 |
| Preexisting comorbidities | |||
| No | 46 (27.9%) | 212 (35.6%) | |
| Yes | 99 (60.0%) | 314 (52.8%) | 0.169 |
| Unknown | 20 (12.1%) | 69 (11.6%) | |
| HIV status | |||
| HIV uninfected | 114 (69.1%) | 449 (75.5%) | 0.100 |
| HIV infected | 51 (30.9%) | 146 (24.5%) | |
| Emergency department treatments | |||
| Intravenous fluids | 102 (61.8%) | 395 (66.4%) | 0.275 |
| Antimicrobial medications | 123 (74.6%) | 437 (73.5%) | 0.776 |
| Respiratory support | 32 (19.4%) | 143 (24.0%) | 0.210 |
| Vasopressors | 10 (6.1%) | 36 (6.1%) | 0.544 |
| Overall outcome | |||
| Discharged | 104 (63.0%) | 410 (68.9%) | |
| Transferred | 5 (3.0%) | 12 (2.0%) | |
| Died | 42 (25.5%) | 151 (25.4%) | 0.077 |
| Eloped | 0 (0.0%) | 1 (0.2%) | |
| Unknown | 14 (8.5%) | 21 (3.5%) | |
Appendix B. Comparison of HIV uninfected cohort by diagnostic Thethod
| Characteristics | HIV uninfected by history (n = 395) | HIV uninfected by test during index care period (n = 168) | p |
|---|---|---|---|
| Gender | |||
| Male | 215 (54.4) | 92 (54.8%) | |
| Female | 180 (45.6%) | 76 (45.2%) | 0.942 |
| Age (years) | 33 (24, 52) | 38 (28, 61) | 0.011 |
| Preexisting comorbidities | |||
| No | 171 (51.6%) | 82 (55.8%) | |
| Yes | 160 (48.3%) | 65 (44.2%) | 0.405 |
| qSOFA1 score | |||
| 0 | 134 (43.4%) | 44 (31.4%) | 0.024 |
| 1 | 121 (39.1%) | 59 (42.1.5%) | |
| ≥2 | 54 (17.5%) | 37 (26.4%) | |
| Emergency department treatments | |||
| Intravenous fluids | 246 (62.3%) | 112 (66.7%) | 0.322 |
| Antimicrobial medications | 268 (67.9%) | 125 (74.4%) | 0.121 |
| Respiratory support | 89 (22.5%) | 42 (25.0%) | 0.526 |
| Vasopressors | 22 (1.0%) | 10 (6.0%) | 0.544 |
| Overall outcome | |||
| Discharged | 266 (67.3%) | 128 (76.2%) | |
| Transferred | 7 (1.8%) | 4 (2.4%) | |
| Died | 92 (23.3%) | 35 (20.8%) | 0.006 |
| Eloped | 0 (0.0%) | 0 (0.0%) | |
| Unknown | 30 (7.6%) | 1 (0.6%) | |
Appendix C. Diagnostic accuracy of qSOFA1 scores by HIV status
| Emergency department mortality |
|||||
|---|---|---|---|---|---|
| qSOFA1 score | HIV uninfected |
HIV infected |
|||
| ≥1 | ≥2 | ≥1 | ≥2 | ||
| Sna% (95% CI) | 90 (83–98) | 43 (30–55) | 100 (100−100) | 50 (27–73) | |
| Spa% (95% CI) | 43 (38–48) | 83 (79–87) | 30 (22–38) | 77 (70–84) | |
| PPVa% (95% CI) | 21 (16–26) | 29 (20–38) | 17 (10–24) | 24 (10–37) | |
| NPVa% (95% CI) | 96 (94–99) | 90 (86–93) | 100 (100–100) | 92 (86–97) | |
| AUCb (95% CI) | 0.71 (0.65–0.77) | 0.71 (0.61–0.81) | |||
| Overall facilities based mortality |
|||||
| qSOFA1 score | HIV uninfected |
HIV infected |
|||
| ≥1 | ≥2 | ≥1 | ≥2 | ||
| Sna% (95% CI) | 84 (77–91) | 37 (28–47) | 90 (81–98) | 35 (21–48) | |
| Spa% (95% CI) | 45 (40–51) | 84 (80–88) | 35 (25–44) | 78 (70–86) | |
| PPVa% (95% CI) | 32 (27–38) | 42 (32–52) | 42 (32–51) | 45 (29–61) | |
| NPVa% (95% CI) | 90 (86–95) | 81 (77–85) | 87 (76–98) | 70 (61–79) | |
| AUCb (95% CI) | 0.68 (0.63–0.74) | 0.64 (0.55–0.72) | |||
Abbreviations: Sn; sensitivity, Sp; specificity, PPV; positive predictive value, NPV; negativepredictive value.
AUC abbreviates area under the receiver-operating characteristic curve, and CI abbreviates confidence interval.
Appendix D. Seven day hospital Thortality risk by qSOFA1 score
| qSOFA1 score | Overall study population |
|||
|---|---|---|---|---|
| RRa (95% CI) | p | aRRa,b (95% CI) | p | |
| 0 | Reference | Reference | ||
| 1 | 4.0 (2.0–8.0) | <0.001 | 3.8 (1.9–8.0) | <0.001 |
| ≥2 | 7.2 (3.6–14.4) | <0.001 | 5.7 (2.8–11.6) | <0.001 |
RR abbreviates Relative Risk and aRR abbreviates adjusted Relative Risk.
Multivariate models adjusted for patient age, pre-existing co-morbidities, emergency department treatment with intravenous fluids, emergency department treatment with antimicrobials and care duration.
Footnotes
Prior presentations: Preliminary results from this work were presented at the Society for Academic Emergency Medicine meeting, Indianapolis, USA. 15–18 May 2018.
Declarations of interest
None.
qSOFA abbreviates, quick Sepsis-related Organ Failure Assessment.
References
- [1].Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 2016;193:259–72. [DOI] [PubMed] [Google Scholar]
- [2].Cheng AC, West TE, Limmathurotsakul D, Peacock SJ. Strategies to reduce mortality from bacterial sepsis in adults in developing countries. PLoS Med 2008;5:e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tanriover MD, Guven GS, Sen D, Unal S, Uzun O. Epidemiology and outcome of sepsis in a tertiary-care hospital in a developing country. Epidemiol Infect 2006;134: 315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Vincent JL, Marshall JC, Namendys-Silva SA, Francois B, Martin-Loeches I, Lipman J, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med 2014;2:380–6. [DOI] [PubMed] [Google Scholar]
- [5].Cerro G, Checkley W. Global analysis of critical care burden. Lancet Respir Med 2014; 2:343–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD. Critical care and the global burden of critical illness in adults. Lancet (London, England) 2010;376:1339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Murthy S, Leligdowicz A, Adhikari NK. Intensive care unit capacity in low-income countries: a systematic review. PLoS One 2015;10:e0116949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Obermeyer Z, Abujaber S, Makar M, Stoll S, Kayden SR, Wallis LA, et al. Emergency care in 59 low- and middle-income countries: a systematic review. Bull World Health Organ 2015;93:577–86G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mullan PC, Torrey SB, Chandra A, Caruso N, Kestler A. Reduced overtriage and undertriage with a new triage system in an urban accident and emergency department in Botswana: a cohort study. Emerg Med J 2014;31(5):356–60. [DOI] [PubMed] [Google Scholar]
- [10].Kesinger MR, Nagy LR, Sequeira DJ, Charry JD, Puyana JC, Rubiano AM. A standardized trauma care protocol decreased in-hospital mortality of patients with severe traumatic brain injury at a teaching hospital in a middle-income country. Injury 2014;45:1350–4. [DOI] [PubMed] [Google Scholar]
- [11].Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Freund Y, Lemachatti N, Krastinova E, Van Laer M, Claessens YE, Avondo A, et al. Prognostic accuracy of sepsis-3 criteria for in-hospital mortality among patients with suspected infection presenting to the emergency department. JAMA 2017; 317:301–8. [DOI] [PubMed] [Google Scholar]
- [14].Singer AJ, Ng J, Thode HC Jr, Spiegel R, Weingart S. Quick SOFA scores predict mortality in adult emergency department patients with and without suspected infection. Ann Emerg Med 2017;69:475–9. [DOI] [PubMed] [Google Scholar]
- [15].Aluisio AR, Jain A, Baron BJ, Sarraf S, Sinert R, Legome E, et al. The prognostic role of non-critical lactate levels for in-hospital survival time among ED patients with sepsis. Am J Emerg Med 2016;34:170–3. [DOI] [PubMed] [Google Scholar]
- [16].Karon BS, Tolan NV, Wockenfus AM, Block DR, Baumann NA, Bryant SC, et al. Evaluation of lactate, white blood cell count, neutrophil count, procalcitonin and immature granulocyte count as biomarkers for sepsis in emergency department patients. Clin Biochem 2017;50:956–8. [DOI] [PubMed] [Google Scholar]
- [17].Saugel B, Huber W, Nierhaus A, Kluge S, Reuter DA, Wagner JY. Advanced hemodynamic management in patients with septic shock. Biomed Res Int 2016; 2016:8268569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Baelani I, Jochberger S, Laimer T, Otieno D, Kabutu J, Wilson I, et al. Availability of critical care resources to treat patients with severe sepsis or septic shock in Africa: a self-reported, continent-wide survey of anaesthesia providers. Crit Care 2011;15: R10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Huson MAM, Katete C, Chunda L, Ngoma J, Wallrauch C, Heller T, et al. Application of the qSOFA score to predict mortality in patients with suspected infection in a resource-limited setting in Malawi. Infection 2017;45(6):893–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Huson MA, Kalkman R, Grobusch MP, van der Poll T. Predictive value of the qSOFA score in patients with suspected infection in a resource limited setting in Gabon. Travel Med Infect Dis 2017;15:76–7. [DOI] [PubMed] [Google Scholar]
- [21].Global AIDS. Update. at http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf; 2016, Accessed date: 20 January 2017.
- [22].Silva JM Jr, dos Santos Sde S. Sepsis in AIDS patients: clinical, etiological and inflammatory characteristics. J Int AIDS Soc 2013;16:17344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Green S, Kong VY, Odendaal J, Sartorius B, Clarke DL, Brysiewicz P, et al. The effect of HIV status on clinical outcomes of surgical sepsis in KwaZulu-Natal Province, South Africa. S Afr Med J 2017;107:702–5. [DOI] [PubMed] [Google Scholar]
- [24].Jacob ST, Moore CC, Banura P, Pinkerton R, Meya D, Opendi P, et al. Severe sepsis in two Ugandan hospitals: a prospective observational study of management and outcomes in a predominantly HIV-1 infected population. PLoS One 2009;4:e7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Xiao H, Siddiqui J, Remick DG. Mechanisms of mortality in early and late sepsis. Infect Immun 2006;74:5227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Japiassu AM, Amancio RT, Mesquita EC, Medeiros DM, Bernal HB, Nunes EP, et al. Sepsis is a major determinant of outcome in critically ill HIV/AIDS patients. Crit Care 2010;14:R152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tang N, Stein J, Hsia RY, Maselli JH, Gonzales R. Trends and characteristics of US emergency department visits, 1997–2007. JAMA 2010;304:664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kearney A, Kabeja L, George N, Karim N, Aluisio AR, Mutabazi Z, et al. Development of a trauma and emergency database in Kigali, Rwanda. Afr J Emerg Med December 31 2016;6(4):185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mbanjumucyo G, George N, Kearney A, Karim N, Aluisio AR, Mutabazi Z, et al. Epidemiology of injuries and outcomes among trauma patients receiving prehospital care at a tertiary teaching hospital in Kigali, Rwanda. Afr J Emerg Med December 31 2016;6(4):191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Aluisio AR, Umuhire OF, Mbanjumucyo G, George N, Kearney A, Karim N, et al. Epidemiologic characteristics of pediatric trauma patients receiving prehospital care in Kigali, Rwanda. Pediatr Emerg Care 2017. 10.1097/PEC.0000000000001045. [DOI] [PubMed]
- [31].Kaji AH, Schriger D, Green S. Looking through the retrospectoscope: reducing bias in emergency medicine chart review studies. Ann Emerg Med 2014;64:292–8. [DOI] [PubMed] [Google Scholar]
- [32].Republic of Rwanda, Ministry of Health. National guidelines for prevention and management of HIV, STIs & other blood borne Infections; 2013. http://www.hivst.org/files1/Rwanda-guidelines-2013.pdf, Accessed date: 20 January 2017.
- [33].Marx John A, Rosen Peter. Rosen’s emergency medicine: concepts and clinical practice 8th ed. Philadelphia, PA: Elsevier/Saunders; 2014. [Print]. [Google Scholar]
- [34].Streiner DL. Best (but oft-forgotten) practices: the multiple problems of multiplicity-whether and how to correct for many statistical tests. Am J Clin Nutr 2015;102: 721–8. [DOI] [PubMed] [Google Scholar]
- [35].Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med 2006;34:15–21. [DOI] [PubMed] [Google Scholar]
- [36].Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence 2014;5:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sarnak MJ, Jaber BL. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int 2000;58:1758–64. [DOI] [PubMed] [Google Scholar]
- [38].Raghunathan K, Shaw A, Nathanson B, Sturmer T, Brookhart A, Stefan MS, et al. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis*. Crit Care Med 2014;42:1585–91. [DOI] [PubMed] [Google Scholar]
- [39].Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 2017;45:486–552. [DOI] [PubMed] [Google Scholar]
- [40].Ferrer R, Martin-Loeches I, Phillips G, Osborn TM, Townsend S, Dellinger RP, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med 2014;42:1749–55. [DOI] [PubMed] [Google Scholar]
- [41].Gaieski DF, Mikkelsen ME, Band RA, Pines JM, Massone R, Furia FF, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med 2010;38:1045–53. [DOI] [PubMed] [Google Scholar]
- [42].Otto GP, Sossdorf M, Claus RA, Rodel J, Menge K, Reinhart K, et al. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit Care 2011;15:R183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Aluisio AR, Rege S, Stewart BT, Kinuthia J, Levine AC, Mello MJ, et al. Prevalence of HIV-seropositivity and associated impact on mortality among injured patients from low-and middle-income countries: a systematic review and meta-analysis. Curr HIV Res 15 (5), 2017, 307–317. [DOI] [PubMed] [Google Scholar]
- [44].Bossuyt PM, Cohen JF, Gatsonis CA, Korevaar DA, group S. STARD 2015: updated reporting guidelines for all diagnostic accuracy studies. Ann Transl Med 2016;4:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74. [PubMed] [Google Scholar]
- [46].Chen YX, Wang JY, Guo SB. Use of CRB-65 and quick sepsis-related organ failure assessment to predict site of care and mortality in pneumonia patients in the emergency department: a retrospective study. Crit Care 2016;20:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hwang SY, Jo IJ, Lee SU, Lee TR, Yoon H, Cha WC, et al. Low accuracy of positive qSOFA criteria for predicting 28-day mortality in critically ill septic patients during the early period after emergency department presentation. Ann Emerg Med 7 (1), 2017, 1–9.e2. [DOI] [PubMed] [Google Scholar]
- [48].Wang JY, Chen YX, Guo SB, Mei X, Yang P. Predictive performance of quick sepsis-related organ failure assessment for mortality and ICU admission in patients with infection at the ED. Am J Emerg Med 2016;34:1788–93. [DOI] [PubMed] [Google Scholar]
- [49].Hsia RY, Mbembati NA, Macfarlane S, Kruk ME. Access to emergency and surgical care in sub-Saharan Africa: the infrastructure gap. Health Policy Plan 2012;27: 234–44. [DOI] [PubMed] [Google Scholar]
- [50].Perman SM, Goyal M, Gaieski DF. Initial emergency department diagnosis and management of adult patients with severe sepsis and septic shock. Scand J Trauma Resusc Emerg Med 2012;20:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mendis S, Al Bashir I, Dissanayake L, Varghese C, Fadhil I, Marhe E, et al. Gaps in capacity in primary care in low-resource settings for implementation of essential noncommunicable disease interventions. Int J Hypertens 2012;2012:584041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Andrews B, Semler MW, Muchemwa L, Kelly P, Lakhi S, Heimburger DC, et al. Effect of an early resuscitation protocol on in-hospital mortality among adults with sepsis and hypotension: a randomized clinical trial. JAMA 2017;318:1233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Finkelsztein EJ, Jones DS, Ma KC, Pabon MA, Delgado T, Nakahira K, et al. Comparison of qSOFA and SIRS for predicting adverse outcomes of patients with suspicion of sepsis outside the intensive care unit. Crit Care 2017;21:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Churpek MM, Snyder A, Han X, Sokol S, Pettit N, Howell MD, et al. Quick sepsis-related organ failure assessment, systemic inflammatory response syndrome, and early warning scores for detecting clinical deterioration in infected patients outside the intensive care unit. Am J Respir Crit Care Med 2017;195:906–11. [DOI] [PMC free article] [PubMed] [Google Scholar]


