Abstract
Introduction:
Extracapsular extension (ECE) in regional lymph nodes and positive surgical margins (PSM) are considered high-risk adverse pathologic features in patients with oropharyngeal squamous cell carcinoma (OPSCC) that each constitute an indication for postoperative adjuvant chemoradiation. We identify pre-operative clinical factors that can predict post-operative ECE and/or PSM and create a nomogram to help clinical decision making.
Methods:
Adult patients with non-metastatic OPSCC with initial surgical treatment and confirmed HPV status diagnosed between 2010 and 2014 were selected from the National Cancer Database. Clinical staging was modified to American Joint Committee on Cancer 8th edition parameters. Logistic regression was used for multivariate analysis to identify predictors of pathologic ECE and/or PSM.
Results:
5065 patients were included. 47.5% of the 3336 HPV-positive (HPV+) patients had ECE/PSM. 40.4% of the 1729 HPV-negative (HPV–) patients with had ECE/PSM. A model was built that included age, clinical ECE, tumor grade, and clinical T and N staging for HPV+ patients. Increasing N-classification was highly predictive of pathologic ECE and/or PSM (N1 OR = 3.6, N2 OR = 7.0, N3 OR = 11.2, p < 0.01). Clinical ECE (OR = 4.1, p < 0.01), tumor grade (ORs 2.2–4.4 with p < 0.05), and increasing clinical T-classification (ORs 1.2–1.8, p < 0.05) were also associated with ECE and/or PSM. A similar model was built for HPV– with similar predictive capability. Two internally validated nomograms were designed that demonstrated good discrimination (HPV+ AUC = 0.66, 95% CI: 0.64–0.68, and HPV– AUC = 0.70, 95% CI: 0.67–0.72) and good calibration (goodness-of-fit statistic of HPV+ 6.32, p = 0.61 and HPV– 11.66, p = 0.17).
Conclusions:
These are the first nomograms designed to help predict ECE or PSM for both HPV+ and HPV–OPSCC. The nomograms can facilitate shared decision-making between clinicians and patients as they consider upfront treatment selection for OPSCC.
Keywords: Head and neck cancer, Oropharyngeal cancer, Chemoradiotherapy, Adjuvant, Radiation therapy, Decision making, Carcinoma, Squamous cell, Human papillomavirus (HPV), Surgical margins, Lymph nodes
Introduction
The management of oropharyngeal squamous cell carcinoma (OPSCC) has undergone a significant transformation over the last two decades, reflecting the increasing incidence of human papillomavirus (HPV) that some experts have called an epidemic [1]. Patients with HPV-associated OPSCC are primarily younger, healthier individuals with little or no tobacco exposure [2]. They tend to have increased response to treatment compared to patients with OPSCC associated with tobacco and alcohol use and thus have much better oncologic outcomes [2,3]. With these improved outcomes in patients with HPV-associated OPSCC, the American Joint Committee on Cancer (AJCC) 7th edition staging algorithm lost its ability to differentiate outcomes between stages, thus reducing its predictive capacity, and a new staging system was designed with HPV status distinguishing the OPSCC subtypes [4].
Historically, before the advent of sophisticated radiation techniques and transoral robotic surgery, OPSCC was managed surgically with invasive procedures that often-required reconstruction with a vascularized flap. Despite this aggressive locoregional therapy, many patients still needed post-operative radiation. Definitive radiation and subsequently chemoradiation (CRT) were shown to be just as effective as surgical resection with decreased morbidity and mortality [5,6]. Despite its efficacy, CRT itself has been found to be associated with long-term toxicity and functional impairment, including feeding tube dependence and pharyngeal or laryngeal dysfunction [7–11]. The short and long-term side effects of radiation and chemotherapy, coupled with the younger average age of HPV-driven OPSCC patients, has led to an increase in surgical management, particularly minimally invasive transoral surgery [12–14]. Today, many clinicians have a keen interest in de-escalation therapy, especially for the mostly young and healthy population of HPV-positive (HPV+) OPSCC patients [14,15].
Despite an interest in de-escalation therapy, extracapsular extension (ECE) or positive surgical margins (PSM) remain a poor prognostic sign in head and neck squamous cell carcinoma. Since the seminal trials from the late 1990s that demonstrated adjuvant CRT over adjuvant radiation therapy alone improved locoregional control and overall survival for patients with PSM and/or ECE, these two risk factors have constituted indications for adjuvant CRT [5,6,16]. While this continues to be the unopposed status quo for HPV-negative (HPV–) patients, recent studies from several institutions have suggested that for surgically treated HPV+ patients with OPSCC, ECE does not predict poor clinical behavior [17,18]. Other studies have indicated that ECE and/or PSM still portend high risk for metastatic disease [19]. Studies have also not been conclusive when it comes to PSM. While surgical margin status is dependent on numerous factors including the skill of the surgeon and type of surgery, studies have noted greater local recurrence rates and worse survival among patients with head and neck squamous cell carcinoma (HNSCC) with positive margins [20,21]. However, other authors have not found margin status to be prognostic within OPSCC [22,23].
Currently, expert guidelines continue to consider ECE and/or PSM as indications for adjuvant CRT, irrespective of HPV status [24,25]. Patients undergoing this triple modality therapy would be expected to have worse long-term functional outcomes than either unimodality or bimodality therapy [7]. In the European Organization for Research and Treatment of Cancer Trial 22931, the increased 13% 5-year survival benefit with CRT came at the cost of significant increased grade 3 mucosal toxicity from 21% to 41%. Preoperative risk stratification of patients according to the risk of pathologic ECE or PSM may, therefore, offer tremendous benefit in guiding treatment selection and avoiding the toxicity of triple modality therapy. However, to our knowledge, there is currently no reliable method of preoperatively predicting the presence of ECE or likelihood of PSM for either HPV+ or HPV– patients [26,27]. Using the National Cancer Database (NCDB), we sought first to identify preoperative clinical characteristics that predict pathologic ECE and/or PSM in HPV– and HPV+ patients with OPSSC and then to build a prediction model and nomogram to guide clinical decision-making in the era of AJCC 8th edition staging between the providers, and provider and patient.
Methods
Data source and patient selection
The NCDB, a joint project of the American College of Surgeons Commission on Cancer and the American Cancer Society, is a hospital-based registry capturing approximately 70% of incident cancer cases in the United States and drawing data from > 1500 Commission on Cancer accredited cancer programs. The NCDB contains detailed information regarding demographic, clinical, and treatment-related factors. The current analysis was performed with the approval of our local Institutional Review Board.
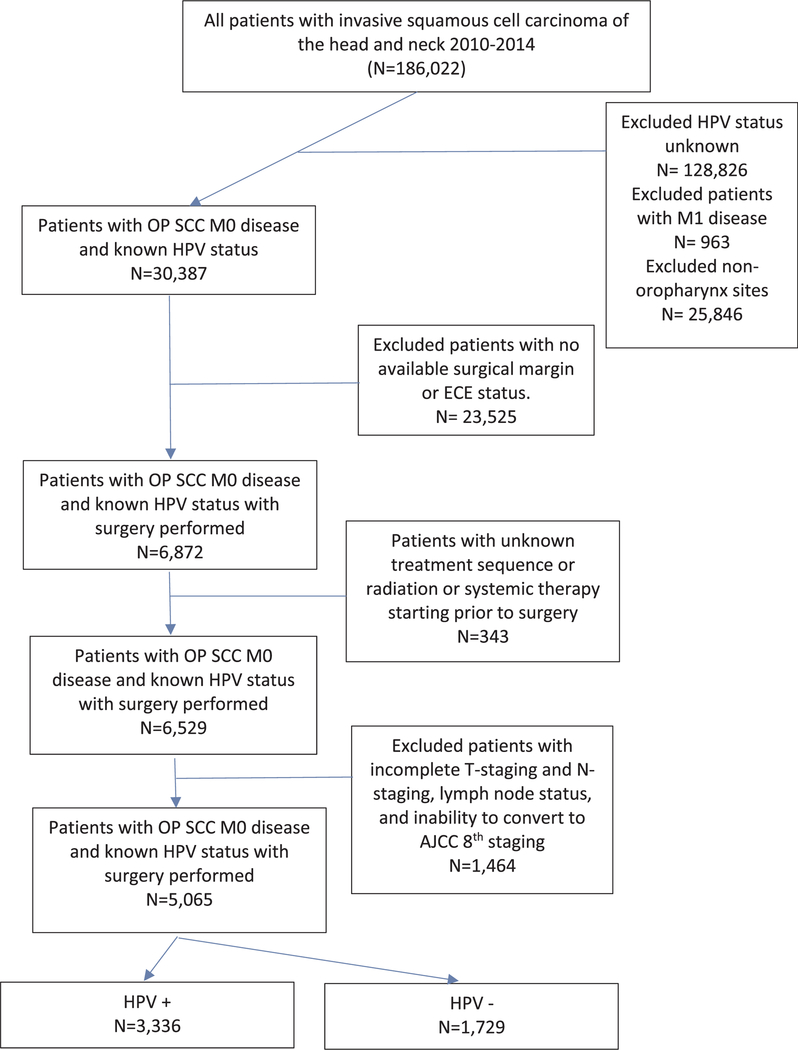
We queried the NCDB for all cases of oropharyngeal SCC diagnosed from 2010 through 2014 among patients aged ≥18 years as HPV status was sparsely recorded prior to this date. The cohort was limited to squamous cell carcinoma histologic codes (International Classification of Diseases for Oncology [third edition] histology codes 8051–8052, 8070–8075, 8083–8084, and 8094), patients with known HPV status, and patients with initial primary surgical intervention including a neck dissection. We excluded patients with distant metastases (M1), incomplete clinical T and N staging, and incomplete post-operative lymph node assessment. Patients with high-risk HPV status were classified as HPV+ and those with low-risk or negative HPV were classified as HPV–. Patients included in our study had a known pathological ECE status and surgical margin status. See Fig. 1 for a flow diagram.
Fig. 1.
Flowchart illustrating patient selection for the final analysis and exclusion criteria.
Patient demographics, outcome, and treatment variables
Age was categorized as 18–69 years or ≥70 years as this is a commonly used cutoff to denote “older” patients in HNSCC [28,29]. Race was categorized as White, Black, or other. Insurance status was classified as private, Medicaid, Medicare, government, and uninsured. Residence zip code was coded according to US Department of Agriculture Economic Research Service as within, near, or distant from a metropolitan area. The median household income in each patient’s zip code was assessed as quartiles with respect to the US population in 2012. The institution type was classified as an academic program versus nonacademic. Morbidity was classified according to the Charlson-Deyo comorbidity score, dichotomized as 0 or ≥1 morbidity [30]. Patients were assigned T-classification and N-classification corresponding to the AJCC 8th edition by using HPV status, staging information according to AJCC 7th edition, and clinical evidence of extracapsular extension (defined as radiologic evidence of fixed or matted nodes) [4]. Given the strong association between p16-positive disease and HPV-associated OPSSC, unknown primary tumors were categorized as T0/TX. Clinical T-staging was stratified to group T3 and T4 tumors together. Tumor grade was defined by NCDB as “well differentiated,” “moderately differentiated,” “poorly differentiated,” undifferentiated,” and “unknown.” The primary outcome, ECE and/or PSM, was classified in a binary fashion as either present or absent. Pathologic ECE was defined as either microscopic or macroscopic extension. PSM was defined as either microscopic or macroscopic gross tumor involvement.
Statistical analysis
All statistical analyses were performed with Stata 13.1 (StataCorp LP, College Station, TX). Pearson chi-square tests were used to assess associations between variables and ECE and/or PSM by HPV status. Univariable logistic regression was performed on ECE and/or PSM by HPV status. Variables were selected a priori and those with significant associations or a trend towards significance (p < 0.10) were incorporated in multivariable logistic regression models. Stepwise backward selection was performed manually to obtain a predictive model. Only variables that remained significantly associated with outcome in the multivariate analysis remained in the final model. Then, we used the nomolog program by Zlotnik et al to generate a Kattan-style nomogram [31]. Two methods, discrimination and calibration, were used to conduct internal validation for estimating the predictive accuracy of the model. Discrimination was evaluated using the area under the receiver-operating-characteristic curve (AUC ROC) [32]. Calibration was evaluated with a Hosmer-Lemeshow goodness of fit test. All internal validations were performed using bootstrapping with 1000 resamples.
Results
Patient characteristics
We identified a total of 5065 patients with known HPV status, M0 disease, and OPSCC who underwent primary surgery with surgical margin and ECE status recorded. Of these, 3336 patients were HPV+ and 1729 were HPV–. HPV+ individuals were more likely to be male, younger, healthier, white, and of higher socioeconomic status (based on insurance and income zip code). Patients with HPV+ tumors were also more likely to be treated at an academic/research center. Their tumors tended to have higher grades and were more likely to have pathological ECE (OR 1.5, 95% CI 1.3–1.7). PSM did not vary significantly by HPV status. Clinical ECE was only noted in 4% of patients and, like PSM, did not vary significantly by HPV status. Post-operative treatment modality did differ by HPV status with more HPV– patients undergoing surgery alone. The number of patients varied with treatment modality with 874 (26.2%) HPV+ patients having undergone surgery alone, 1059 (32.7%) underwent adjuvant RT, and 1372 (41.2%) underwent adjuvant CRT versus 637 (36.9%), 437(25.3%), and 631(36.5%), respectively, among HPV– patients (see Table 1 for details).
Table 1.
Clinical characteristics of the cohort by HPV status and ECE and/or PSM.
| HPV– Negative ECE/PSM (N = 1030) No (%) |
HPV– Positive ECE/PSM (N = 699) No (%) |
P-value | HPV+ Negative ECE/PSM (N = 1749) No (%) |
HPV+ Positive ECE/PSM (N = 1587) No (%) |
P-value | |
|---|---|---|---|---|---|---|
| Sex | <0.01 | <0.01 | ||||
| Male | 737 (71.5) | 537 (76.8) | 1441 (82.4) | 1362 (85.8) | ||
| Female | 293 (28.5) | 162 (23.2) | 308 (17.6) | 225 (14.2) | ||
| Age | <0.01 | <0.01 | 0.76 | |||
| <70 years | 823 (79.9) | 595 (85.1) | 1563 (89.4) | 1413 (89.0) | ||
| ≥70 years | 207 (20.1) | 104 (14.9) | 186 (10.6) | 174 (11.0) | ||
| Race | 0.04 | 0.70 | ||||
| White | 875 (85.0) | 601 (86.0) | 1617 (92.5) | 1475 (92.9) | ||
| Black | 90 (8.7) | 41 (5.9) | 50 (2.9) | 47 (3.0) | ||
| Other | 65 (6.3) | 57 (8.1) | 82 (4.7) | 65 (4.1) | ||
| Insurance | 0.34 | 0.38 | ||||
| Private | 514 (49.9) | 382 (54.7) | 1188 (67.9) | 1032 (65.0) | ||
| Medicare | 357 (34.7) | 228 (32.6) | 392 (22.4) | 388 (24.5) | ||
| Medicaid | 90 (8.7) | 52 (7.4) | 81 (4.6) | 89 (5.6) | ||
| Government/Other | 32 (3.1) | 17 (2.4) | 45 (2.6) | 42 (2.7) | ||
| Uninsured | 37 (3.6) | 20 (2.9) | 43 (2.5) | 36 (2.3) | ||
| Zip code Income | 0.26 | 0.03 | ||||
| ≤38,000 | 181 (17.6) | 111 (15.9) | 184 (10.5) | 180 (11.3) | ||
| 38–48 K | 233 (22.6) | 138 (19.7) | 331 (18.9) | 350 (22.1) | ||
| 48 K-63 K | 269 (26.1) | 200 (28.6) | 471 (26.9) | 445 (28.0) | ||
| ≥63 K | 340 (33.0) | 248 (35.5) | 758 (43.3) | 608 (38.3) | ||
| Urban | 0.18 | 0.17 | ||||
| Lives in Metro | 882 (79.8) | 567 (81.1) | 1419 (81.1) | 1275 (80.3) | ||
| Near Metro | 123 (11.9) | 63 (9.0) | 193 (11.0) | 171 (10.8) | ||
| Far from Metro | 61 (5.9) | 52 (7.4) | 97 (5.6) | 114 (7.2) | ||
| Geography | 0.55 | <0.01 | ||||
| East | 226 (21.9) | 149 (21.3) | 438 (25.0) | 296 (18.7) | ||
| Midwest | 296 (28.7) | 215 (30.8) | 500 (28.6) | 555 (35.0) | ||
| South | 319 (31.0) | 194 (27.8) | 416 (23.8) | 395 (24.9) | ||
| West | 168 (16.3) | 123 (17.6) | 365 (20.9) | 320 (20.2) | ||
| Facility Type | 0.19 | 0.00 | ||||
| Non-Academic | 364 (35.3) | 273 (39.1) | 527 (30.1) | 586 (36.9) | ||
| Academic | 645 (62.6) | 408 (58.4) | 1192 (68.2) | 980 (61.8) | ||
| Charlson-Deyo Score | 0.41 | 0.41 | ||||
| 0 | 784 (76.1) | 544 (77.8) | 1443 (82.5) | 1292 (81.4) | ||
| ≥1 | 246 (23.9) | 155 (22.2) | 306 (17.5) | 295 (18.6) | ||
| Tumor Differentiation | <0.01 | <0.01 | ||||
| Well | 82 (8.0) | 30 (4.3) | 47 (2.7) | 16 (1.0) | ||
| Moderate | 496 (48.2) | 306 (43.8) | 561 (32.1) | 483 (30.4) | ||
| Poor | 349 (33.9) | 292 (41.8) | 899 (51.4) | 838 (52.8) | ||
| Undifferentiated | 7 (0.7) | 9 (1.3) | 25 (1.4) | 43 (2.7) | ||
| Clinical T Stage | <0.01 | 0.01 | ||||
| T1 | 384 (37.3) | 201 (28.8) | 717 (41.0) | 576 (36.3) | ||
| T2 | 395 (38.4) | 267 (38.2) | 639 (36.5) | 589 (37.1) | ||
| T3 | 95 (9.2) | 67 (9.6) | 86 (4.9) | 103 (6.5) | ||
| T4 | 64 (6.2) | 70 (10.0) | 38 (2.2) | 51 (3.2) | ||
| T0/TX | 92 (8.9) | 70 (13.5) | 269 (15.4) | 268 (16.9) | ||
| Clinical N Stage | <0.01 | <0.01 | ||||
| N0 | 513 (49.8) | 131 (18.7) | 468 (26.7) | 136 (8.6) | ||
| N1 | 200 (19.4) | 182 (26.0) | 1224 (70.0) | 1290 (81.3) | ||
| N2 | 300 (29.1) | 329 (47.1) | 39 (2.2) | 90 (5.7) | ||
| N3 | 16 (1.7) | 57 (8.2) | 18 (1.0) | 71 (4.5) | ||
| Treatment Modality | <0.01 | <0.01 | ||||
| Surgery alone | 502 (48.7) | 135 (19.3) | 652 (37.3) | 222 (14.0) | ||
| Surgery + RT | 303 (29.4) | 134 (19.2) | 716 (40.9) | 343 (21.6) | ||
| Surgery + Chemo | 9 (0.9) | 15 (2.2) | 9 (0.5) | 22 (1.4) | ||
| Surgery + CRT | 216 (21.0) | 415 (59.4) | 372 (21.3) | 1000 (63.0) | ||
Of the 3336 patients with HPV+ disease, 35.1% had pathologic ECE, and 20.7% had PSM; among those with HPV– disease, 27.0% had pathologic ECE, and 20.1% had PSM. Among HPV+ patients in the univariate analysis, gender, income, geographical location, treatment facility type, tumor grade, clinical ECE, and clinical T and N stage were all significantly associated with ECE and/or PSM (P < 0.05). Among HPV– patients, gender, age, tumor grade, clinical T-stage, and clinical N- stage were significantly associated with ECE and/or PSM (P < 0.05). Geographic location, income of resident zip code, and treatment facility type were not associated with ECE and/or PSM at a significance level of 0.05 as it was for HPV+ HPV+ patients. A complete comparison of demographic, clinical, and treatment variables with odds ratios and 95% confidence intervals are presented in Tables 1 and 2.
Table 2.
Unadjusted and adjusted logistic regression for ECE and/or PSM outcome.
| Characteristic | HPV– N = 1729 | HPV+ N = 3336 | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| Odds Ratio (95% CI) | P-value | Adjusted OR | P-value | Odds Ratio (95% CI) | P-value | Adjusted OR | P-value | |
| Age | ||||||||
| <70 year | 1.00 (Ref) | 0.01 | 1.00 (Ref) | 0.22 | 1.00 (Ref) | 0.76 | 1.00 (Ref) | 0.40 |
| ≥70 years | 0.69 (0.54–0.90) | 0.84 (0.63–1.11) | 1.03 (0.83–1.29) | 1.11 (0.88–1.40) | ||||
| Tumor Differentiation | ||||||||
| Well | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | ||||
| Moderately | 1.69 (1.08–2.62) | 0.02 | 1.49 (0.93–2.39) | 0.09 | 2.53 (1.42–4.52) | <0.01 | 2.19 (1.19–4.02) | 0.01 |
| Poorly | 2.29 (1.46–3.57) | <0.01 | 1.75 (1.08–2.81) | 0.02 | 2.74 (1.54–4.87) | <0.01 | 2.36 (1.29–4.32) | <0.01 |
| Undifferentiated | 3.51 (1.2–10.27) | 0.02 | 1.85 (0.60–5.72) | 0.28 | 5.05 (2.38–10.71) | <0.01 | 4.39 (2.00–9.62) | 0.01 |
| Clinical T Stage | ||||||||
| T1 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | ||||
| T2 | 1.29 (1.03–1.63) | 0.03 | 1.38 (1.08–1.76) | 0.01 | 1.15 (0.98–1.34) | 0.09 | 1.23 (1.04–1.45) | 0.01 |
| T3 | 1.35 (0.94–1.92) | 0.10 | 1.45 (1.00–2.02) | 0.05 | 1.49 (1.10–2.03) | 0.01 | 1.51 (1.09–2.10) | 0.01 |
| T4 | 2.09 (1.4–3.05) | <0.01 | 3.61 (1.41–3.18) | <0.01 | 1.67 (1.01–1.52) | 0.02 | 1.79 (1.11–2.88) | 0.02 |
| T0/Tx | 1.95 (1.4–2.73) | <0.01 | 1.67 (1.18–2.38) | <0.01 | 1.24 (1.01–1.52) | 0.04 | 1.24 (1.01–1.53) | 0.04 |
| Clinical N Stage | ||||||||
| N0 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | ||||
| N1 | 3.56 (2.7–4.7) | <0.01 | 3.43 (2.58–4.55) | <0.01 | 3.63 (2.95–4.46) | <0.01 | 3.56 (2.89–4.40) | <0.01 |
| N2 | 4.29 (3.35–5.5) | <0.01 | 4.00 (3.10–5.15) | <0.01 | 7.94 (5.21–12.1) | <0.01 | 6.96 (4.53–10.70) | <0.01 |
| N3 | 13.13 (7.39–23.32) | <0.01 | 12.44 (6.95–22.27) | <0.01 | 13.57 (7.82–23.56) | <0.01 | 11.20 (6.38–19.65) | <0.01 |
| Clinical ECE | ||||||||
| No | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | |||||
| Yes | 5.26 (2.81–9.83) | <0.01 | 5.52 (3.59–8.48) | <0.01 | 4.14 (2.68–6.40) | <0.01 | ||
| Sex | ||||||||
| Male | 1.00 (Ref) | 1.00 (Ref) | ||||||
| Female | 0.76 (0.61–0.95) | 0.01 | 0.77 (0.64–0.93) | 0.01 | ||||
| Race | ||||||||
| White | 1.00 (Ref) | 1.00 (Ref) | ||||||
| Black | 0.66 (0.45–0.97) | 0.04 | 1.03 (0.69–1.54) | 0.88 | ||||
| Other | 1.28 (0.88–1.85) | 0.20 | 0.87 (0.62–1.21) | 0.41 | ||||
| Insurance | ||||||||
| Private | 1.00 (Ref) | 1.00 (Ref) | ||||||
| Medicare | 0.86 (0.69–1.06) | 0.16 | 1.14 (0.97–1.34) | 0.12 | ||||
| Medicaid | 0.78 (0.54–1.12) | 0.18 | 1.26 (0.93–1.73) | 0.14 | ||||
| Government/Other | 0.71 (0.39–0.28) | 0.28 | 1.07 (0.70–1.65) | 0.74 | ||||
| Uninsured | 0.73 (0.42–1.27) | 0.27 | 0.96 (0.61–1.51) | 0.87 | ||||
| Urban | ||||||||
| Lives in Metro | 1.00 (Ref) | 1.00 (Ref) | ||||||
| Near Metro | 0.74 (0.54–1.02) | 0.07 | 0.99 (0.79–1.23) | 0.90 | ||||
| Far From Metro | 1.24 (0.84–1.82) | 0.28 | 1.31 (0.99–1.73) | 0.06 | ||||
| Geography | ||||||||
| East | 1.00 (Ref) | 1.00 (Ref) | ||||||
| Midwest | 1.10 (0.84–1.45) | 0.48 | 1.64 (1.36–1.99) | <0.01 | ||||
| South | 0.92 (0.7–1.21) | 0.56 | 1.41 (1.15–1.72) | <0.01 | ||||
| West | 1.11 (0.81–1.52) | 0.51 | 1.3 (1.05–1.6) | 0.02 | ||||
| Zip code Income | ||||||||
| ≤38,000 | 1.00 (Ref) | 1.00 (Ref) | ||||||
| 38–48K | 0.97 (0.7–1.33) | 0.83 | 1.08 (0.84–1.39) | 0.55 | ||||
| 48 K-63 K | 1.21 (0.9–1.63) | 0.21 | 0.97 (0.76–1.23) | 0.78 | ||||
| ≥63 K | 1.19 (0.89–1.59) | 0.24 | 0.82 (0.65–1.03) | 0.09 | ||||
| Facility Type | ||||||||
| Non-Academic | 1.00 (Ref) | 1.00 (Ref) | ||||||
| Academic | 0.84 (0.69–1.03) | 0.10 | 0.74 (0.64–0.85) | <0.01 | ||||
| Charlson-Deyo Score | ||||||||
| 0 | 1.00 (Ref) | 1.00 (Ref) | ||||||
| >1 | 0.91 (0.72–1.14) | 0.41 | 1.08 (0.9–1.28) | 0.41 | ||||
Separate multivariable logistic regression models for HPV+ and HPV– patients were constructed as described in the methods. Among both HPV+ and HPV– patients, increasing clinical N-classification was the strongest predictor of ECE and/or PSM, followed by increasing clinical T-classification (see Table 2 for values). While clinical ECE was also a significant predictor among HPV+ patients, its incorporation into AJCC 8th staging for HPV– patients introduces the potential for collinearity, and this variable was therefore excluded from the analysis dedicated to HPV– disease. Tumor grade remained significantly associated with positive ECE and/or PSM. Age ≥ 70 years was not associated with positive ECE and/or PSM for HPV+ or HPV– patients.
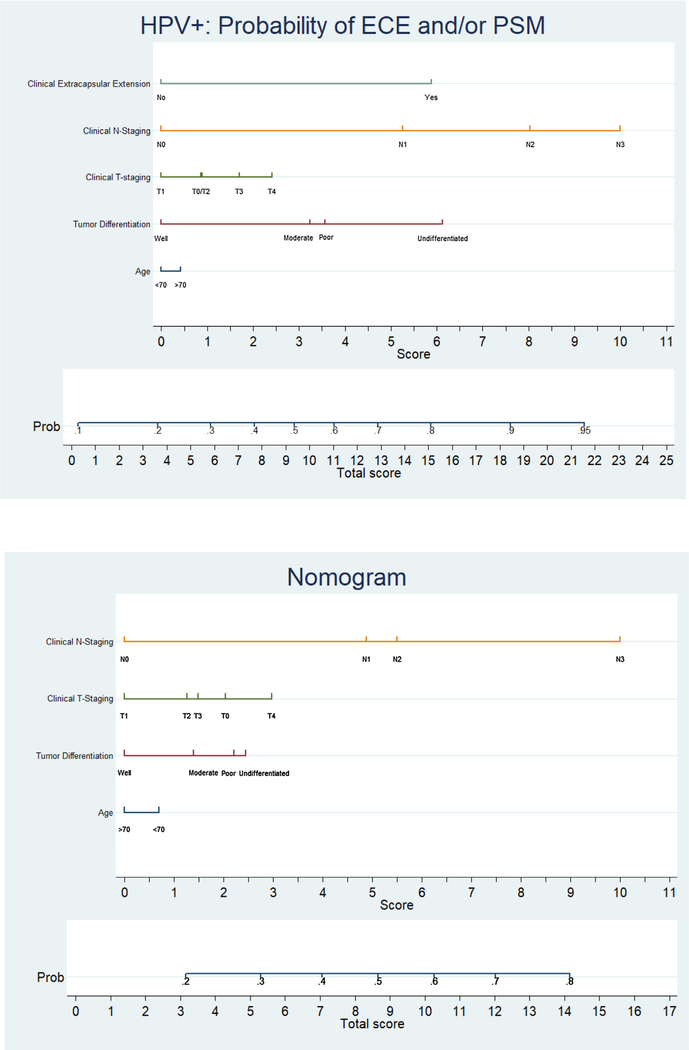
Using this MVA logistic regression, a Katten-style nomogram was built and is presented in Fig. 2 for HPV+ and HPV– OPSCC patients. Each nomogram is characterized by one scale corresponding to each variable, a score scale, a total score scale, and a probability scale. To use the nomogram, first obtain the value for each variable on the scale. Second, sum up the total score from the previous step and identify it on the total score scale. Lastly, identify the probability using the corresponding total score obtained previously. For example, a 75-year-old patient with HPV+ OPSCC of clinical T2N1M0 stage and moderate differentiation on biopsy without overt clinical ECE will have roughly a 50% probability of having pathologic ECE and/or PSM. See Supplementary Tables 1–2 for specific point values associated with each nomogram.
Fig. 2.
Nomogram by HPV status. (a) Nomogram for HPV + patients. (b) Nomogram for HPV − patients.
Validation of the pre-operative nomograms was conducted internally using discrimination and calibration. Receiver-operating characteristic analysis indicated good discrimination by both the HPV+ model (AUC ROC = 0.66, p < 0.01, 95% CI 0.64–0.68) and the HPV– model (AUC ROC = 0.70, p < 0.01, 95% CI 0.68–0.72). The models were calibrated with a Hosmer–Lemeshow goodness of fit test statistic of 6.32 (p = 0.61) and11.66 (p = 0.17), respectively, indicating good calibration. The AUC ROC is presented in Supplementary Figs. 1–2.
Discussion
To our knowledge, these are the first nomograms attempting to predict the high-risk pathologic features of ECE and/or PSM in patients with HPV+ or HPV– OPSCC. This is the largest dedicated analysis of surgically treated HPV+ OPSCC and the first to incorporate the new AJCC 8th staging criteria in the analysis [4]. Internal validation of the nomograms demonstrated an AUC of 66% for the HPV+ model and AUC of 70% for the HPV– model, indicating good discrimination. These nomograms offer a simple means to predict high-risk pathologic features in individual patients by incorporating readily available preoperative clinical parameters, and therefore the likelihood of needing adjuvant CRT [24,25]. We hope that these nomograms may prove helpful to clinicians by providing a tool to that enables the patient to engage in treatment decision making. We believe this will give patients better realistic expectations of their potential oncologic care. For example, some patients may opt out of tri-modality therapy with surgical treatment if they have a high likelihood of receiving adjuvant CRT anyhow, and instead opting for definitive bi-modality CRT. On the other hand, there may patients who are ambivalent about surgery versus organ-preservation but are concerned about toxicity. They would likely appreciate knowing their pre-operative risk of requiring tri-modality therapy, since a high risk could lead them to consider organ preservation, whereas low risk could lead them to consider surgery.
Some may question the clinical utility of predicting high-risk pathologic features in the setting of HPV disease in light of recent studies arguing for de-escalation. However, until prospective randomized trials unequivocally demonstrate the efficacy of single- or dual-modality therapy in comparison to standard-of-care triple-modality therapy for patients with high-risk pathologic features, postoperative CRT remains the preferred adjuvant approach, even for patients with HPV+ disease. This is based on level-one evidence from the seminal meta-analysis of two large randomized controlled trials, where ECE and PSM were each identified as independent risk factors that predict for a survival benefit from postoperative CRT over postoperative RT alone [6]. These results are the basis for adjuvant CRT in surgically-treated OPSCC with ECE and/or PSM, irrespective of HPV status, by the National Cancer Control Network and American College of Radiology Appropriate Criteria [24,25].
In recent years, however, multiple retrospective series have shown that in HPV+ OPSCC, PSM may not adversely impact overall survival [22,23,33]. Select studies analyzing ECE have similarly demonstrated no adverse effect on overall survival [18]. Critics of the aforementioned meta-analysis and its constituent trials will argue that patients with OPSCC constituted only a small portion of patients enrolled [5], and that their HPV status is unknown. Many of these studies advocate prospective trials for de-escalation, and we applaud their efforts to avoid the acute and chronic toxicity that comes with triple-modality therapy. However, despite the comparatively favorable outcomes of HPV+ OPSCC in comparison to tobacco- and alcohol- driven HPV–cancers, it is critical that we do not overcorrect and undertreat these patients. Prospective data to guide the management of HPV+ patients are eagerly anticipated, such as that to be obtained from the ongoing PATHOS trial (NCT02215265).
Given the relevance of adjuvant CRT in the setting of ECE and/or PSM, it is worthwhile to attempt to risk-stratify patients pre-operatively using a nomogram. Even our advanced current imaging technology cannot reliably distinguish the presence of microscopic ECE, rendering pathological examination of lymph nodes paramount in determining the need for adjuvant therapies [18,34]. Our study predicting ECE and/or PSM in OPSCC is analogous to predicting high-risk pathologic features in prostate cancer. Clinical features, namely T-classification, prostate-specific antigen, and Gleason score, are often used in the pre-operative setting to predict the risk of extraprostatic extension, seminal vesicle invasion, or positive lymph nodes [35–37]. Predictive tools linking clinical to pathologic factors are useful for clinicians and patients deciding between surgical versus non-surgical treatment approaches, as the discovery of any of these pathologic features in prostate cancer constitutes an indication for adjuvant RT. In the present analysis, we have similarly created a predictive tool that uses clinical features to predict the discovery of high-risk pathologic features in OPSCC.
The value of nomograms lies in providing individualized risk estimates that can facilitate patient management-related decisions. Our nomogram is comprised of 5 variables for HPV+ patients and 4 variables for HPV– patients: age, tumor grade, clinical T-classification, and clinical N-classification, with clinical ECE only for HPV+ patients. While clinical ECE may be considered an unreliable variable in NCDB as there is no gold standard for radiographically assessing ECE [26], this factor has been incorporated into the AJCC 8th staging guidelines for HPV– patients [4]. The strongest predictor of ECE and/or PSM in our models was N-classification, followed by T-classification, which makes theoretical sense. Clinical extracapsular extension was also a significant predictor in the HPV+ subset. The nomogram generated from our prediction model will allow a clinician to preoperatively estimate the probability of pathologic ECE and/or PSM for a specific patient and thereby provide insight into the patient’s likelihood of needing triple-modality treatment.
The nomograms also enable a shared-decision making approach to treatment, as they function as decision aids that allow the patient and physician to discuss the possible treatments and associated toxicities. Treatment of HNSCC has traditionally been paternalistic, with the provider unilaterally making treatment decisions without consideration of the patient’s preferences or values [38]. Historically, the majority of HNSCC patients have been socially disadvantaged and vulnerable, with poor health behaviors, and have hence tolerated this type of “doctor-knows-best” attitude [38]. However, healthcare has recently shifted overall towards a shared-decision making approach, particularly in oncology, allowing patients to take an active role in treatment selection [39]. In the world of prostate cancer, nomograms have been shown to be beneficial in enabling patients to engage in shared-decision making. Studies suggest that these decision aids promote a perception of decreased decisional conflict, increased treatment satisfaction, and reduced levels of anxiety and distress among men with newly diagnosed prostate cancer [40,41]. It would make sense that these decision aids can be extrapolated to the world of OPSCC. However, to our knowledge, there are no existing tools to predict pathologic ECE or PSM at surgery, which constitutes a barrier to shared decision-making with these patients. Of course, our nomograms are meant to provide information, not answers. The use of nomograms does not preclude the element of uncertainty inherent in counseling HNSCC patients. They do, however, allow evidence-based guidance and discussion with a patient and enables participation in their own care.
It is important to recognize the limitations of this study. Chiefly, this was a retrospective study and is therefore subject to the availability of clinical information in the database. The NCDB is also limited by the potential for miscoding and missing data. Retrospective studies are unable to adequately control for treatment selection biases due to unmeasured confounding variables. Smoking status, a critical prognostic feature for young and old patients with OPSCC, was unknown for this patient population. In addition, while our nomograms have good accuracy, they have limitations in their prediction capability. Furthermore, while we internally validated the nomograms, external validation of this nomogram needs to be performed for it to be applied as standard care, which would require a separate surgical cohort. Optimal validation would be performed using another independent dataset with sufficient sample size.
Conclusion
In conclusion, we used a large nationwide cohort to create nomograms for both HPV+ and HPV– patients that can pre-operatively detect the probability of having ECE and/or PSM in OPSCC. These accurate and easy-to-use tools can be used to facilitate the counseling of patients regarding treatment modalities and can be used a tool to enhance shared-decision making.
Supplementary Material
Footnotes
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.oraloncology.2018.06.005.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
References
- [1].Lewis A, Kang R, Levine A, Maghami E. The new face of head and neck cancer: the HPV epidemic. Oncology (Williston Park) 2015;29:616–26. [PubMed] [Google Scholar]
- [2].Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Haughey BH, Sinha P. Prognostic factors and survival unique to surgically treated p16+ oropharyngeal cancer. Laryngoscope 2012;122(Suppl. 2):S13–33. [DOI] [PubMed] [Google Scholar]
- [4].Lydiatt WM, Patel SG, O’Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, et al. Head and neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:122–37. [DOI] [PubMed] [Google Scholar]
- [5].Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 2005;27:843–50. [DOI] [PubMed] [Google Scholar]
- [6].Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefebvre JL, Greiner RH, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004;350:1945–52. [DOI] [PubMed] [Google Scholar]
- [7].Hunter KU, Schipper M, Feng FY, Lyden T, Haxer M, Murdoch-Kinch CA, et al. Toxicities affecting quality of life after chemo-IMRT of oropharyngeal cancer: prospective study of patient-reported, observer-rated, and objective outcomes. Int J Radiat Oncol Biol Phys 2013;85:935–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Guo GZ, Sutherland KR, Myers C, Lambert P, Loewen SK, Quon HC. Prospective swallowing outcomes after IMRT for oropharyngeal cancer: Dosimetric correlations in a population-based cohort. Oral Oncol 2016;61:135–41. [DOI] [PubMed] [Google Scholar]
- [9].Eisbruch A, Kim HM, Feng FY, Lyden TH, Haxer MJ, Feng M, et al. Chemo-IMRT of oropharyngeal cancer aiming to reduce dysphagia: swallowing organs late complication probabilities and dosimetric correlates. Int J Radiat Oncol Biol Phys 2011;81:e93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wong AT, Lai SY, Gunn GB, Beadle BM, Fuller CD, Barrow MP, et al. Symptom burden and dysphagia associated with osteoradionecrosis in long-term oropharynx cancer survivors: a cohort analysis. Oral Oncol 2017;66:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Head MDA, Neck Cancer Symptom Working Group, Eraj SA, Jomaa MK, Rock CD, Mohamed ASR, et al. Long-term patient reported outcomes following radiation therapy for oropharyngeal cancer: cross-sectional assessment of a prospective symptom survey in patients > / = 65 years old. Radiat Oncol 2017;12:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kaczmar JM, Tan KS, Heitjan DF, Lin A, Ahn PH, Newman JG, et al. HPV-related oropharyngeal cancer: Risk FACTORS for treatment failure in patients managed with primary transoral robotic surgery. Head Neck 2016;38:59–65. [DOI] [PubMed] [Google Scholar]
- [13].Hurtuk A, Agrawal A, Old M, Teknos TN, Ozer E. Outcomes of transoral robotic surgery: a preliminary clinical experience. Otolaryngol Head Neck Surg 2011;145:248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Weinstein GS, Quon H, O’Malley BW Jr., Kim GG, Cohen MA. Selective neck dissection and de-intensified postoperative radiation and chemotherapy for oropharyngeal cancer: a subset analysis of the University of Pennsylvania transoral robotic surgery trial. Laryngoscope 2010;120:1749–55. [DOI] [PubMed] [Google Scholar]
- [15].Owadally W, Hurt C, Timmins H, Parsons E, Townsend S, Patterson J, et al. PATHOS: a phase II/III trial of risk-stratified, reduced intensity adjuvant treatment in patients undergoing transoral surgery for Human papillomavirus (HPV) positive oropharyngeal cancer. BMC Cancer 2015;15:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 2004;350:1937–44. [DOI] [PubMed] [Google Scholar]
- [17].An Y, Park HS, Kelly JR, Stahl JM, Yarbrough WG, Burtness BA, et al. The prognostic value of extranodal extension in human papillomavirus-associated oropharyngeal squamous cell carcinoma. Cancer 2017;123:2762–72. [DOI] [PubMed] [Google Scholar]
- [18].Maxwell JH, Ferris RL, Gooding W, Cunningham D, Mehta V, Kim S, et al. Extracapsular spread in head and neck carcinoma: impact of site and human papillomavirus status. Cancer 2013;119:3302–8. [DOI] [PubMed] [Google Scholar]
- [19].Routman DM, Funk RK, Tangsriwong K, Lin A, Keeney MG, Garcia JJ, et al. Relapse rates with surgery alone in human papillomavirus-related intermediate- and high-risk group oropharynx squamous cell cancer: a multi-institutional review. Int J Radiat Oncol Biol Phys 2017. [DOI] [PubMed] [Google Scholar]
- [20].Kurita H, Nakanishi Y, Nishizawa R, Xiao T, Kamata T, Koike T, et al. Impact of different surgical margin conditions on local recurrence of oral squamous cell carcinoma. Oral Oncol 2010;46:814–7. [DOI] [PubMed] [Google Scholar]
- [21].Woolgar JA, Scott J, Vaughan ED, Brown JS, West CR, Rogers S. Survival, metastasis and recurrence of oral cancer in relation to pathological features. Ann R Coll Surg Engl 1995;77:325–31. [PMC free article] [PubMed] [Google Scholar]
- [22].McMahon J, O’Brien CJ, Pathak I, Hamill R, McNeil E, Hammersley N, et al. Influence of condition of surgical margins on local recurrence and disease-specific survival in oral and oropharyngeal cancer. Br J Oral Maxillofac Surg 2003;41:224–31. [DOI] [PubMed] [Google Scholar]
- [23].Barry CP, Ahmed F, Rogers SN, Lowe D, Bekiroglu F, Brown JS, et al. Influence of surgical margins on local recurrence in T1/T2 oral squamous cell carcinoma. Head Neck 2015;37:1176–80. [DOI] [PubMed] [Google Scholar]
- [24].Adelstein D, Gillison ML, Pfister DG, Spencer S, Adkins D, Brizel DM, et al. NCCN guidelines insights: head and neck cancers, version 2.2017. J Natl Compr Canc Netw 2017;15:761–70. [DOI] [PubMed] [Google Scholar]
- [25].Expert Panel on Radiation O-H, Neck, Salama JK, Saba N, Quon H, Garg MK, et al. ACR appropriateness criteria(R) adjuvant therapy for resected squamous cell carcinoma of the head and neck. Oral Oncol 2011;47:554–9. [DOI] [PubMed] [Google Scholar]
- [26].Geltzeiler M, Clayburgh D, Gleysteen J, Gross ND, Hamilton B, Andersen P, et al. Predictors of extracapsular extension in HPV-associated oropharyngeal cancer treated surgically. Oral Oncol 2017;65:89–93. [DOI] [PubMed] [Google Scholar]
- [27].Prabhu RS, Magliocca KR, Hanasoge S, Aiken AH, Hudgins PA, Hall WA, et al. Accuracy of computed tomography for predicting pathologic nodal extracapsular extension in patients with head-and-neck cancer undergoing initial surgical resection. Int J Radiat Oncol Biol Phys 2014;88:122–9. [DOI] [PubMed] [Google Scholar]
- [28].Gugic J, Strojan P. Squamous cell carcinoma of the head and neck in the elderly. Rep Pract Oncol Radiother. 2012;18:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pignon JP, le Maitre A, Maillard E, Bourhis J, Group M-NC. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. [DOI] [PubMed] [Google Scholar]
- [30].Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–9. [DOI] [PubMed] [Google Scholar]
- [31].Zlotnik A, Abraira V. A general-purpose nomogram generator for predictive logistic regression models. Stata J 2015;15:537–46. [Google Scholar]
- [32].Alba AC, Agoritsas T, Walsh M, Hanna S, Iorio A, Devereaux PJ, et al. Discrimination and calibration of clinical prediction models: users’ guides to the medical literature. JAMA 2017;318:1377–84. [DOI] [PubMed] [Google Scholar]
- [33].Molony P, Kharytaniuk N, Boyle S, Woods RSR, O’Leary G, Werner R, et al. Impact of positive margins on outcomes of oropharyngeal squamous cell carcinoma according to p16 status. Head Neck 2017;39:1680–8. [DOI] [PubMed] [Google Scholar]
- [34].Chai RL, Rath TJ, Johnson JT, Ferris RL, Kubicek GJ, Duvvuri U, et al. Accuracy of computed tomography in the prediction of extracapsular spread of lymph node metastases in squamous cell carcinoma of the head and neck. JAMA Otolaryngol Head Neck Surg 2013;139:1187–94. [DOI] [PubMed] [Google Scholar]
- [35].Nguyen PL, Chen MH, Hoffman KE, Katz MS, D’Amico AV. Predicting the risk of pelvic node involvement among men with prostate cancer in the contemporary era. Int J Radiat Oncol Biol Phys 2009;74:104–9. [DOI] [PubMed] [Google Scholar]
- [36].Roach M 3rd, Chen A, Song J, Diaz A, Presti J Jr., Carroll P. Pretreatment prostate-specific antigen and Gleason score predict the risk of extracapsular extension and the risk of failure following radiotherapy in patients with clinically localized prostate cancer. Semin Urol Oncol 2000;18:108–14. [PubMed] [Google Scholar]
- [37].Tosoian JJ, Chappidi M, Feng Z, Humphreys EB, Han M, Pavlovich CP, et al. Prediction of pathological stage based on clinical stage, serum prostate-specific antigen, and biopsy Gleason score: Partin Tables in the contemporary era. BJU Int 2017;119:676–83. [DOI] [PubMed] [Google Scholar]
- [38].van Linden van den Heuvell C, van Zuuren F, Wells M, van der Laan G, Reintsema H. Paradigm shift in head and neck oncology patient management. J Otolaryngol Head Neck Surg 2017;46:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Brom L, Pasman HR, Widdershoven GA, van der Vorst MJ, Reijneveld JC, Postma TJ, et al. Patients’ preferences for participation in treatment decision-making at the end of life: qualitative interviews with advanced cancer patients. PLoS One. 2014;9:e100435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Volk RJ, Hawley ST, Kneuper S, Holden EW, Stroud LA, Cooper CP, et al. Trials of decision aids for prostate cancer screening: a systematic review. Am J Prev Med 2007;33:428–34. [DOI] [PubMed] [Google Scholar]
- [41].Stacey D, Bennett CL, Barry MJ, Col NF, Eden KB, Holmes-Rovner M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2011:CD001431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.