Abstract
Adversity during development is a reliable predictor of psychiatric disorders such as depression and anxiety which are increasingly recognized to have an immune component. We have previously demonstrated that chronic adolescent stress (CAS) in rats leads to depressive-like behavior in adulthood along with long-lasting changes to the hypothalamic-pituitary-adrenal axis and pro-inflammatory cytokine induction in the hippocampus. However, the mechanisms by which CAS promotes hippocampal inflammation are not yet defined. Here we tested the hypothesis that a history of CAS exaggerates induction of the pro-inflammatory NFκB pathway in adult rat hippocampus without compromising the peripheral immune response. We also assessed potential sex differences because it is unclear whether females, who are twice as likely to suffer from mood disorders as males, are disproportionally affected by stress-primed inflammation. Male and female adolescent rats underwent a CAS paradigm or received no stress. Six weeks following the last stressor, all rats received a single systemic injection of either lipopolysaccharide or vehicle to unmask possible immune-priming effects of CAS. An NFκB signaling PCR array demonstrated that CAS exaggerated the expression of NFκB-related genes in the hippocampus of both males and females. Interestingly, targeted qPCR demonstrated that CAS potentiated the induction of hippocampal IL1B and REL mRNA in female rats only, suggesting that some immune effects of CAS are indeed sex-specific. In contrast to the hippocampal findings, indices of peripheral inflammation such as NFκB activity in the spleen, plasma IL-1β, IL-6, TNF-α, and corticosterone were not impacted by CAS in female rats. Despite showing no pro-inflammatory changes to hippocampal mRNA, male CAS rats displayed lower plasma corticosterone response to LPS at 2 hours after injection followed by an exaggerated plasma IL-1β response at 4 hours. This potentially blunted corticosterone response coupled with excessive innate immune signaling in the periphery is consistent with possible glucocorticoid resistance in males. In contrast, the effects of CAS manifested as excessive hippocampal immune reactivity in females. We conclude that while a history of exposure to chronic adolescent stress enhances adult immune reactivity in both males and females, the mechanism and manifestation of such alterations are sex-specific.
Keywords: neuroinflammation, stress, adolescence, sex differences, NFκB, IL-1β, IL-6, TNF-α, female, rat
1. Introduction
Early life adversity is associated with 25–32% of adult-onset psychiatric illnesses (Green et al., 2010), and can account for more than 10% of low-grade inflammation in adults (Danese et al., 2007), making it a risk factor for numerous disorders characterized by heightened inflammation (Miller et al., 2009a). Accumulating evidence suggests that in adult rodents, exposure to stressors can lead to immune priming, which refers to a sensitized inflammatory response to a subthreshold or secondary stimulus (Perry and Holmes, 2014). While the physiology and behavior of those with a history of stress may appear normal at baseline, a secondary stressor or challenge encountered subsequently can unmask the priming effects of prior stress (Frank et al., 2012; Hudson et al., 2014; Munhoz et al., 2006). Although adolescence was originally believed to be beyond the window of susceptibility to early life adversity, more recent findings have demonstrated that adversity during adolescence also increases the risk of psychiatric and somatic disorders (Blakemore et al., 2010), especially disorders with an inflammatory component (Mills et al., 2013). The adolescent brain and the hypothalamic-pituitary-adrenal axis may be particularly vulnerable to stressors (Holder and Blaustein, 2014; Neigh et al., 2013) due to the concurrent maturation of the HPA axis and the hypothalamic-pituitary-gonadal (HPG) axis during puberty (Romeo, 2003). When experienced during adolescence, chronic mixed-modality stress, consisting of isolation, restraint, and social defeat, has been shown to lead to enduring impairments to the HPA axis (Bourke et al., 2013) and depressive-like behavior (Bourke and Neigh, 2011). Notably, both the physical (restraint) and psychosocial (social defeat) modalities comprising this chronic adolescent stress (CAS) paradigm have been shown to promote neuroinflammation (Audet et al., 2011; Caso et al., 2008). Consistently, we have previously demonstrated that a history of exposure to CAS sensitizes the rat hippocampal immune profile to react more strongly when challenged with lipopolysaccharide weeks removed from the initial stress experience (Pyter et al., 2013). This suggests that while the acute sequelae of a stress response may dissipate soon after the stressor ends, chronic developmental stress is able to create a long-term impact by programming the way immune-related gene expression is regulated. Such broad changes are likely to be mediated by transcription factors; yet the role of transcription factors in stress-primed inflammation have not been fully elucidated.
Nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), a pro-inflammatory transcription factor, is ideally suited to mediate stress-induced immune alterations due to its role as a central regulator of immunity (Bekhbat et al., 2017). NFκB controls the induction of hundreds of immune and inflammatory genes in tissues of multiple organ systems including the central nervous system and immune organs (Hoffmann and Baltimore, 2006; Kaltschmidt and Kaltschmidt, 2009). Alterations in cytokines and other immune mediators that follow acute NFκB activation are involved in homeostatic functions under non-pathological conditions (Kaltschmidt et al., 1994); however, prolonged or excessive inflammation can cause mood and behavioral deficits (Raison et al., 2006). In healthy humans who endure a brief psychosocial stressor called the Trier Social Stress Test (TSST), NFκB is induced within minutes of stressor onset, and its activation is resolved within an hour thereafter (Bierhaus et al., 2003; Kuebler et al., 2015; Wolf et al., 2009). Repeated or chronic exposure to stressors appears to derail this healthy pattern of NFκB induction by increasing its magnitude and delaying its resolution (Pace et al., 2006). Indeed, a transcriptional profile characterized by excessive NFκB activity and blunted HPA axis signaling is one of the hallmarks of chronic stress (Miller et al., 2009a; Miller et al., 2008). Furthermore, the degree of chronic stress at a given time can predict the prospective expression of NFκB in white blood cells up to six months later (Miller et al., 2009b), suggesting feed-forward loops of inflammatory signaling. Here, in an effort to unearth the potential mechanisms underlying neuro-immune-priming following adolescent exposure to chronic stress, we investigated whether CAS primes NFκB-related inflammation in rats.
Identifying the organ tissue from where exaggerated immune responses originate is a first step to understanding the mechanisms underlying stress-primed inflammation. While many rodent stress paradigms such as social defeat alter immune processes in both the brain and peripheral organ tissue (Reader et al., 2015), the extent of immune-priming in the central nervous system and the periphery do not always correlate following stressors (Gibb et al., 2011). It is currently unclear as to whether CAS primes immune reactivity in both tissue types, and whether individual characteristics such as sex influence the extent of immune-priming following CAS in either the brain or the periphery. Despite a well-established female bias in stress-related psychiatric illnesses (Breslau et al., 1997; Piccinelli and Wilkinson, 2000), it is not clear whether females are particularly vulnerable to stress-induced immune alterations (Bekhbat and Neigh, 2017). Importantly, the onset of increased prevalence in females coincides with the onset of puberty (Bangasser and Valentino, 2014). This suggests that developmental stress impacts male and female brains potentially to different extents and/or via distinct mechanisms, leading to sex differences in future psychiatric and somatic dysfunction (Tak et al., 2015). All studies to date that examined NFκB signaling following stressors have done so in male subjects only. Thus, an additional goal of the present study was to investigate whether CAS-associated changes in NFκB-related inflammation are sex-specific.
2. Methods
Animals
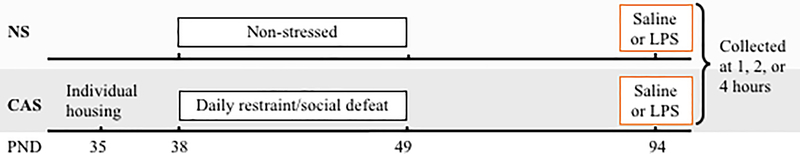
Male and female offspring (n=5–7/group, 98 total, see experimental design in Table 1 and Fig 1) of Wistar rats (Charles River) were culled to four male and four female pups per litter. No more than two pups from a litter were assigned to a group to control for litter effects. Pups were weaned into same-sex pairs on post-natal day (PND) 22 and housed on a 14:10 reverse light:dark cycle (on 0000 h, off 1400 h). Standard rat chow and water were provided ad libitum. The body weight of all animals was recorded at weaning, isolation, Day 1, 5, and 10 of the CAS paradigm, and weekly following the end of CAS until the terminal endpoint. Institutional Animal Care and Use Committees at Emory University and Virginia Commonwealth University approved all animal use procedures. Animal experimentation was carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Table 1. Experimental design.
Male and female adolescent rats were either non-stressed (NS) or subjected to chronic adolescent stress (CAS) during post-natal days (PNDs) 38-49. In adulthood on PND 94, all rats received a single, systemic injection of either saline or lipopolysaccharide (Sheppard et al.), and hippocampal tissue was collected at 1, 2, or 4 hours following injection (n=5-7/group).
| Female | Male | |||||||
|---|---|---|---|---|---|---|---|---|
| NS | CAS | NS | CAS | |||||
| Saline | LPS | Saline | LPS | Saline | LPS | Saline | LPS | |
| 1 hr | n = 6 | n = 6 | n = 6 | n = 5 | ||||
| 2 hr | n = 5 | n = 6 | n = 6 | n = 7 | n = 7 | n = 6 | n = 7 | n = 7 |
| 4 hr | n = 5 | n = 6 | n = 6 | n = 7 | ||||
Fig 1.
A graphical depiction of the experimental timeline.
Chronic adolescent stress
A graphical depiction of the experimental timeline is shown in Fig 1. On PND 35 rats were randomly assigned to non-stressed (NS) or chronic adolescent stress (CAS) groups. CAS rats were individually housed, and shortly after underwent CAS as detailed previously (Pyter et al., 2013). Briefly, our mixed-modality chronic stress paradigm consists of 6 random exposures to each social defeat and restraint stress which take place across twelve days spanning the mid-adolescent period in the rat (PND 38–49). In the social defeat paradigm, rats are exposed to a larger same-sex adult rat that is trained to demonstrate aggressive behavior toward the experimental rat. Aggressive behavior by male Long Evans rats is typically described in terms of “pinning” behavior whereby the juvenile Wistar rat is pinned to the floor in a supine, immobilized position by the adult Long Evans rat (Weathington et al., 2012). Typical aggressive behaviors by female Long Evans rats include kicking, defensive burying, and occasional pinning. Despite a lack of physical injury to the experimental rat, repeated exposure to an older defeater elicits a sustained stress response (Bourke and Neigh, 2011). Upon completion of the stress paradigm, rats were allowed to mature into adulthood without further stressor exposure. On PND 94, all rats received a single intraperitoneal injection of either saline or LPS (E. Coli, O:B123, L3880, Sigma Aldrich, 0.25 mg/kg=750,000 Endotoxin units). One, two or four hours following injection, rats were euthanized via rapid decapitation. Immediately thereafter, the brain and the spleen were harvested and flash frozen on dry ice, and stored at −80°C until further use. All experimental procedures were completed at least two hours before the end of the light cycle to avoid the diurnal peak in corticosterone concentrations which takes place at the beginning of the dark cycle. Order of collections was counterbalanced across experimental groups to normalize possible influences of the circadian rhythm.
NFκB PCR signaling array
Rat NFκB signaling array (PARN-025Z) containing 84 key genes related to NFκB-mediated signal transduction was purchased from Qiagen (Valencia, CA, USA). Hippocampal cDNA from n=3/group were randomly selected and pooled, and one pooled sample for each group was assayed on the PCR array. Our previous study using the same CAS paradigm (Pyter et al. 2013) revealed that the pro-inflammatory effects of CAS were evident only in LPS-, but not saline-injected rats. Therefore, we only included LPS-injected rats in this preliminary assay. Groups compared were: 1) Female/NS/2 hours post-LPS, 2) Female/CAS/2 hours post-LPS, 3) Male/NS/2 hours post-LPS, and 4) Male/CAS/2 hours post-LPS. Assays were performed according to the manufacturer’s recommendation. A list of all genes on the array along with their fold changes is shown in Supplementary Table 1.
Quantitative RT-PCR
The hippocampus was dissected under RNAse-free conditions, homogenized with Trizol (ThermoScientific, Wilmington, DE, USA), and RNA was extracted using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. RNA integrity was assessed by a NanoDrop One spectrophotometer (ThermoScientific, Wilmington, DE, USA) and RNA samples were reverse transcribed using the High Capacity RNA to cDNA Kit (Applied Biosystems, Foster City, CA, USA). Primers for rat IL1B (forward: AGCAGCTTTCGACAGTGAGG, reverse: CTCCACGGGCAAGACATAGG), IL6 (forward: AGCGATGATGCACTGTCAGA, reverse: GGAACTCCAGAAGACCAGAGC), TNF (forward: ATGGGCTCCCTCTCATCAGT, reverse: GCTTGGTGGTTTGCTACGAC), EGR1 (forward: ACCTGACCACAGAGTCCTTTTC, reverse: GTTGGTCATGCTCACAAGGC), FOS (forward: GGGAGCTGACAGATACGCTC, reverse: TCAAGTCCAGGGAGGTCACA), JUN (forward: GCACATCACCACTACACCGA, reverse: TATGCAGTTCAGCTAGGGCG), and REL (forward: GGCCGGGAAAGAAGAGGA, reverse: TTATATCCGCTCGAGGCCA) were designed using NCBI’s Primer Blast tool, and purchased from Invitrogen (Carlsbad, CA). Primer specificity was checked via melt curve analysis. The universal two-step RT-PCR cycling conditions used on the QuantStudio Flex 6 (Applied Biosystems, Foster City, CA) were: 50°C (2 min), 95°C (2 min), followed by 40 cycles of 95°C (15 s) and 60°C (1 min). Samples were run in triplicate, and the coefficient of variation within the triplicates was no more than 4%. Among the five internal control genes assessed (HPRT1, GAPDH, B2M, ACTB, LDHA), none displayed <15% variability across the eight experimental groups. Fold changes in gene expression were therefore calculated by the comparative 2−ΔCT quantification method relative to the female, NS, Saline group.
p65 DNA-binding assay
Splenic nuclear protein extract was prepared using the Nuclear Extract Kit (#40010) from ActiveMotif (Carlsbad, CA). Because the most common form of NFκB is the heterodimer consisting of p65/p50, p65’s DNA-binding activity was assessed in the spleen as a measure of activated NFκB using the TransAM NFκB p65 Chemi kit (#40097, ActiveMotif, Carlsbad, CA). Recombinant p65 protein (#31102) was purchased from ActiveMotif (Carlsbad, CA) and used to create a standard curve.
Plasma cytokine ELISA
Immediately following decapitation, trunk blood was collected into EDTA-treated glass tubes (Vacutainer, BD Biosciences, Franklin Lakes, NJ). Blood was centrifuged at 1800 rcf for 20 min at 4°C, and plasma was collected, aliquoted, and stored at −80°C until further use. Plasma cytokine concentrations were measured via Rat IL-1β, IL-6 and TNF-α ELISA kits purchased from R&D Systems (Minneapolis, MN) (product numbers RLB00, R6000B, and RTA00). Assays were performed in duplicate according to the manufacturer’s instructions. The coefficient of variance among the duplicates was less than 15%, and inter-assay variability was less than 15%.
Plasma corticosterone ELISA
Plasma was obtained as described previously. Measurement of plasma corticosterone was conducted using an ELISA kit (#ADI-900–097) from Enzo Life Sciences (Farmingdale, NY) according to the manufacturer’s instructions. The coefficient of variance among the duplicates was less than 15%. The plates were read on a Synergy HTX plate reader (Biotek, Winooski, VT), and its built-in four-parameter logistic regression software was used for plotting the standard curve and data extrapolation.
Statistical Analysis
Body weight data were analyzed within each sex via two-way repeated measures ANOVAs (within-subjects: time and between-subjects: stress) using SPSS 25.0. NFκB PCR array data were analyzed through the web-based software GeneGlobe Data Analysis Center (Qiagen, Valencia, USA), which uses standard ΔΔCt approach without adjustment for multiple comparisons. Corticosterone data did not meet assumptions of normality and equal variances as assessed by Kolmogorov-Smirnov (Lilliefors corrected) and Levene’s tests. Plasma corticosterone data were thus square root-transformed to meet the assumption of normality. Gene expression, spleen, and cytokine data were analyzed via three-way ANOVAs by modeling the main effects and interactions of sex, stress, and LPS. Significant interactions in the three-way ANOVA were followed up with 2×2 ANOVAs. Sidak’s posthoc test and a-priori one-way t-test were conducted where indicated. Body weight and peripheral endpoint data are expressed as mean ± SEM. Gene expression data are presented as mean fold change ± SEM. Significance threshold was set to α=0.05 for all analyses, and p-values between 0.05 and 0.10 were referred to as “trends” and interpreted cautiously.
3. Results
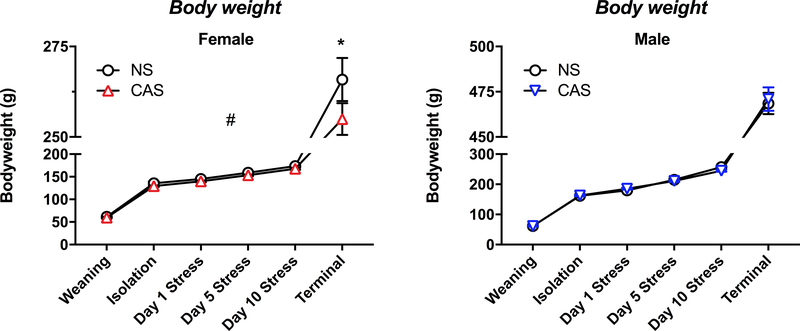
3.1. CAS leads to decreased body weight in female rats
CAS led to a decrease in body weight in females throughout the duration of the paradigm (F(1,204)=12.86, p<0.001), and this decrease was significant at the terminal time point (t(204)=2.674, p=0.047) (Fig 2A). CAS did not significantly impact the body weight of males throughout the paradigm (F(1,240)=0.321, p=0.571) (Fig 2B).
Fig 2. Chronic adolescent stress (CAS) led to a decrease in bodyweight in females.
Bodyweight was assessed in females (A) and males (B) separately using a 2×2 ANOVA with time and stress as main factors. CAS led to a decrease in bodyweight in CAS females throughout the duration of the paradigm (p<0.001, #), and this decrease was statistically significant at the terminal time point (p=0.047, *). # main effect of CAS within females, * CAS effect across the indicated comparison.
3.2. CAS exaggerates NFκB-related inflammatory gene expression in the adult hippocampus
An NFκB signaling PCR array was performed as an initial assessment to determine whether activation of the overall NFκB pathway was altered by CAS when assessed in adulthood. Within each sex, several genes from the array were found to be upregulated at least 1.5 fold in the CAS animals compared to NS controls 2 hours following an LPS injection (Table 2). No gene on the array was found to be downregulated at least 1.5 fold by CAS in either female or male rats. Based on this result and demonstration of these cytokines as the primary mediators of the innate immune response (Dantzer, 2004), we chose to evaluate the following panel of pro-inflammatory cytokines by targeted qPCR: IL1B, IL6, and TNF. In addition, based on array results we measured targeted expression of the top genes upregulated by CAS in females (REL) and males (EGR1) along with two other genes (FOS and JUN) that encode subunits of the transcription factor activator protein-1 (AP-1) which, similar to NFκB, is regulated by both cytokines (Krappmann et al., 2004) and stress (Nagabhushan et al., 2001; Webster et al., 2002).
Table 2. Genes from the NFκB PCR array that were upregulated at least 1.5 fold in CAS versus NS rats at 2 hours following LPS.
Functional classification information was obtained from the array manufacturer’s product manual. Hippocampal gene expression was assessed in female and male rats of NS and CAS backgrounds at 2 hours following a systemic LPS injection in adulthood. NFκB PCR array data were analyzed through the web-based software GeneGlobe Data Analysis Center (Qiagen, Valencia, USA) using standard ΔΔCt approach.
| Genes Over-Expressed in | |||||
|---|---|---|---|---|---|
| Female CAS vs. NS | Male CAS vs. NS | ||||
| Gene Symbol | Functional classification | Fold Change | Gene Symbol | Functional classification | Fold Change |
| Rel | NFκB Transcription Factor | 2.2043 | Egr1 | Transcription Factor (Other) | 1.6948 |
| Raf1 | Kinase (Other) | 2.0626 | Ccl2 | NFκB Signaling Ligands & Receptors | 1.6916 |
| Casp8 | Other NFκB Signaling Gene | 2.0339 | Fos | Transcription Factor (Other) | 1.6513 |
| Il1r1 | NFκB Signaling Ligands & Receptors | 1.9968 | Irf1 | Signaling Downstream of NFκB | 1.6244 |
| Il1b | NFκB Signaling Ligands & Receptors | 1.9578 | Kat2b | Other NFκB Signaling Gene | 1.5708 |
| Fos | Transcription Factor (Other) | 1.945 | Jun | Transcription Factor (Other) | 1.5548 |
| Tlr2 | NFκB Signaling Ligands & Receptors | 1.7714 | Traf3 | Signaling Downstream of NFκB | 1.5396 |
| Ccl2 | NFκB Signaling Ligands & Receptors | 1.7249 | Nfkbia | Cytoplasmic Sequestering / Releasing of NFκB | 1.5378 |
| Tnf | NFκB Signaling Ligands & Receptors | 1.5279 | LOC687813 | Signaling Downstream of NFκB | 1.5332 |
| Tnfrsf10b | NFκB Signaling Ligands & Receptors | 1.5154 | |||
| Ccl5 | NFκB Responsive Gene (Immune Response) | 1.5058 | |||
| Bcl2a1 | NFκB Responsive Gene (Apoptosis) | 1.5026 | |||
| Casp1 | Other NFκB Signaling Gene | 1.5007 | |||
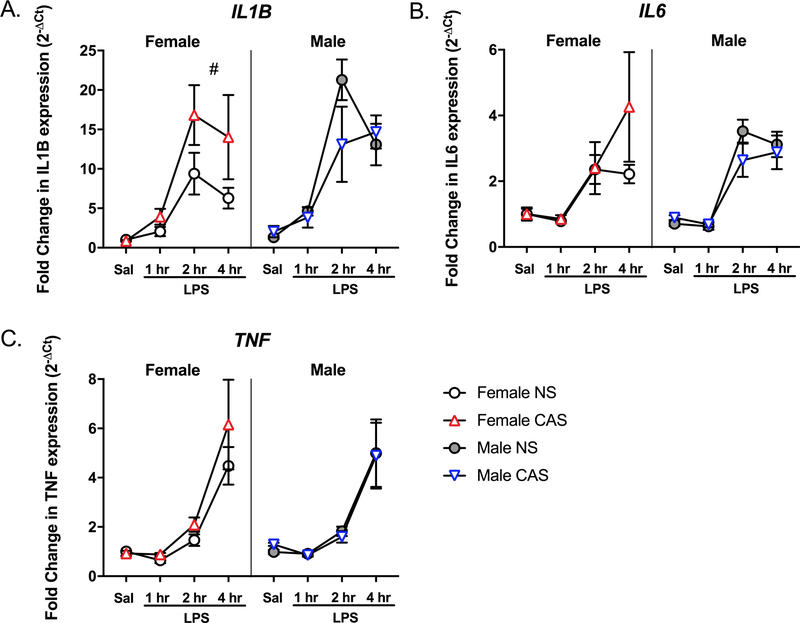
3.3. CAS enhances hippocampal IL1B mRNA expression in adult female rats
To assess CAS-induced alterations in neuroimmune reactivity, we performed qPCR using total hippocampal RNA from male and female rats after either saline or LPS injections. LPS injection significantly upregulated the hippocampal mRNA expression of IL1B (F(3,77)=75.853, p<0.001), IL6 (F(3,77)=51.621, p<0.001) and TNF (F(3,77)=74.667, p<0.001) (Fig 3). Specifically, mRNA expression of all three cytokines were significantly elevated at 2 and 4 hours post-LPS compared to that following saline injection (p<0.001). Males displayed a greater expression of IL1B mRNA compared to females (F(1,77)=6.652, p=0.012). While there was no main effect of CAS on IL1B (F(1,77)=1.514, p=0.222), sex and CAS interacted (F(1,77)=4.985, p=0.028) such that female CAS rats displayed greater expression of IL1B compared to female NS controls (F(1,38) = 4.956, p=0.032). The expression of IL6 and TNF were not impacted by sex (IL6: F(1,77)=0.855, p=0.358; TNF: F(1,77)=0.377, p=0.541) or CAS (IL6: F(1,77)=1.495, p=0.225; TNF: F(1,77)=1.732, p=0.192), and no significant interactions were present (p>0.05).
Fig 3. CAS led to priming of hippocampal IL1B gene expression in female rats only.
Male and female rats of NS or CAS backgrounds received a single systemic injection of saline or LPS in adulthood to unmask potential immune priming due to stressor exposure (n = 5–7/group). Targeted qPCR experiments confirmed that LPS led to a significant mRNA induction of all three cytokines (p<0.001) in the hippocampus. While there was no main effect of CAS, sex and CAS interacted such that CAS exaggerated hippocampal mRNA expression of A) IL1B in female (p=0.032, #), but not male, rats (p>0.05). CAS did not impact the mRNA expression of B) IL6 or C) TNF in the hippocampus of either males or females (p>0.05). Data are presented as mean fold change ± SEM. # main effect of CAS within females.
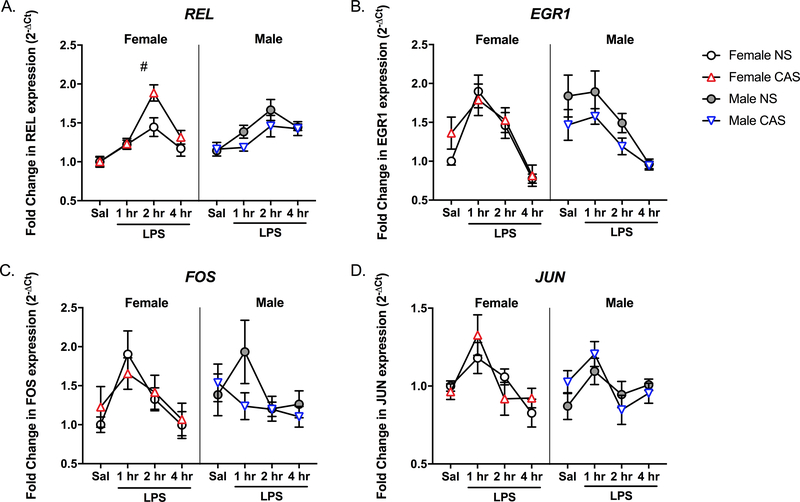
To confirm the preliminary data obtained from the NFκB array, mRNA expression of several other targets including REL, EGR1, FOS, and JUN were assessed via targeted qPCR. LPS injection significantly upregulated the hippocampal mRNA expression of REL (F(3,77)=24.771, p<0.001), EGR1 (F(3,77)=22.7, p<0.001), FOS (F(3,77)=3.734, p=0.015) and JUN (F(3,77)=7.797, p<0.001) (Fig 4). At 2 hours post-LPS, the mRNA expression of REL, but not EGR1, FOS, or JUN, was significantly higher compared to that following saline injection (p<0.001). Females displayed greater expression of REL mRNA compared to males (F(1,77)=4.011, p=0.049). While there was no main effect of CAS on REL (F(1,77)=0.145, p=0.705), sex and CAS interacted (F(1,77)=6.166, p=0.015) such that female CAS rats displayed greater expression of REL compared to female NS controls (F(1,38) = 4.124, p=0.049). Similarly, while EGR1 was not impacted by a main effect of CAS (F(1,77)=0.403, p=0.528) or sex (EGR1: F(1,77)=1.952, p=0.166), CAS and sex interacted (F(1,77)=4.095, p=0.046). A follow-up test within males revealed a trend towards greater expression of REL in male NS rats compared to the male CAS group (F(1,39) = 3.883, p=0.056). The expression of FOS and JUN were not impacted by sex (FOS: F(1,77)=0.161, p=0.689); JUN: F(1,77)=0.367, p=0.546) or CAS (FOS: F(1,77)=0.166, p=0.685); JUN: F(1,77)=0.171, p=0.681), and no significant interactions were present (p>0.05).
Fig 4. CAS leads to priming of hippocampal REL gene expression in female rats.
Male and female rats of NS or CAS backgrounds received a single systemic injection of saline or LPS in adulthood to unmask potential immune priming due to stressor exposure (n = 5–7/group). Targeted qPCR experiments confirmed that LPS led to a significant mRNA induction of all four NFκB-related genes (p<0.001) in the hippocampus. While there was no main effect of CAS, sex and CAS interacted such that CAS exaggerated the hippocampal mRNA expression of A) REL in female (p=0.049, #), but not male, rats (p>0.05). B) Similarly, sex and CAS interacted such that CAS males displayed a trend toward lower EGR1 expression compared to non-stressed males (p=0.056). CAS did not impact the mRNA expression of C) FOS or D) JUN in the hippocampus of either males or females (p>0.05). Data are presented as mean fold change ± SEM. # main effect of CAS within females.
3.4. CAS exaggerates peripheral immune reactivity in adult male rats
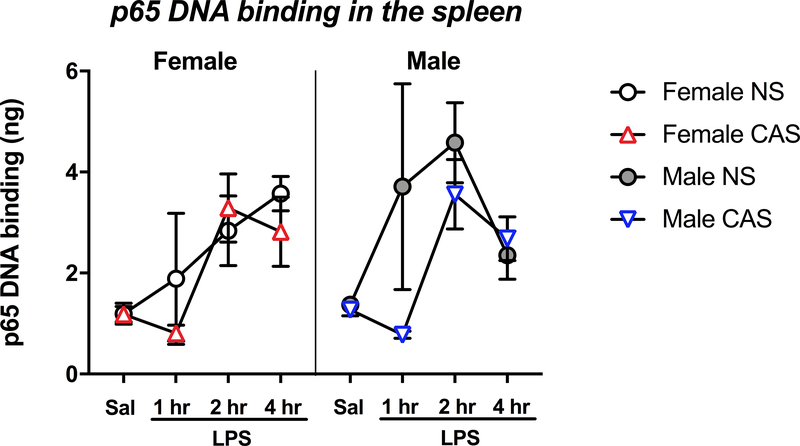
To determine whether CAS-driven increases in adult hippocampal NFκB induction were paralleled in peripheral immune tissue, we assessed the transcriptional activity of p65, the main subunit of the NFκB complex, in the adult spleen (Fig 5). LPS injection induced an increase in the binding of p65 to DNA (F(3,77)=8.702, p<0.001), and the LPS 2 and 4 hour time points were each significantly different from the saline condition (p<0.01). However, there was no main effect of sex (F(1,77)=1.126, p=0.292). CAS did not impact p65’s DNA binding activity in the spleen (F(1,77)=1.989, p=0.162).
Fig 5. CAS does not impact the splenic p65 activity at baseline or following LPS.
Male and female rats of NS or CAS backgrounds received a single systemic injection of saline or LPS in adulthood to unmask potential immune priming due to stressor exposure (n = 5–7/group). Splenic p65 activity was analyzed with sex * stress * LPS as main factors. While LPS led to a significant induction of p65 (p<0.001), neither sex nor stress impacted p65 activity (p>0.05). Data are presented as mean ± SEM.
IL-1β
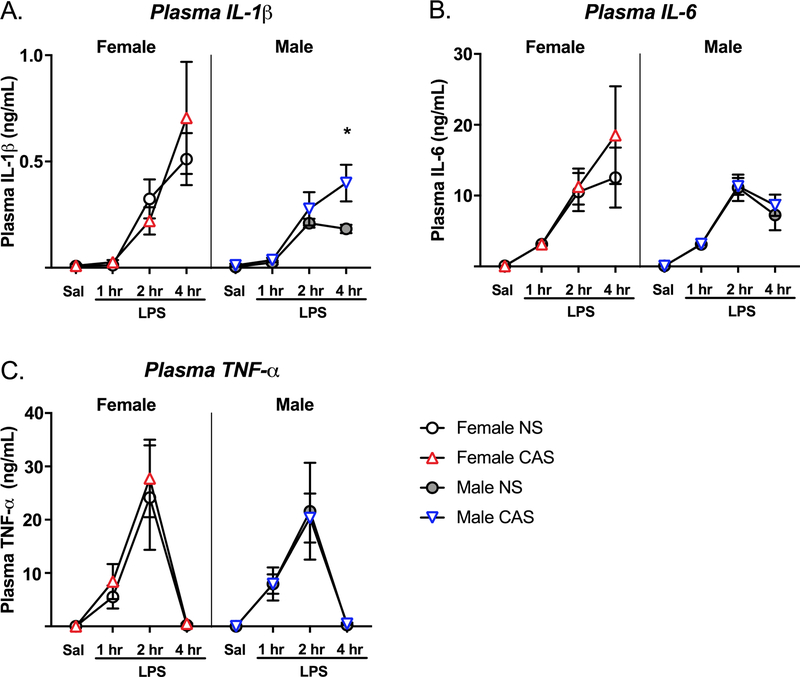
Fourteen of twenty five saline-injected rats displayed IL-1β concentrations below the limit of detection (assay sensitivity <5 pg/mL), and were not included in analysis. As expected, LPS significantly increased plasma IL-1β concentrations (F(3,62)=15.748, p<0.001) (Fig 6A), and the LPS 2 and 4 hour time points were each significantly different from the saline condition (p<0.05). While there was no main effect of sex (F(1,62)=2.375, p=0.128), there was a trend toward a sex * LPS interaction (F(3,62)=2.444, p=0.072). Because we found a sex-specific impact of CAS on hippocampal IL1B expression, we tested for the effect of CAS on plasma IL-1β in males and females separately using a 2×2 ANOVA. There was a trend toward a main effect of CAS in males (F(1,32)=3.931, p=0.056) which was mostly driven by increased IL-1β concentrations at 4 hours post-LPS compared to NS males (t(32)= 3.065, p=0.017) as revealed by a Sidak’s posthoc test. There was no main effect of CAS in females (F(1,30)=0.063, p=0.803).
Fig 6. CAS leads to enhanced plasma IL-1β in males following LPS.
Male and female rats of NS or CAS backgrounds received a single systemic injection of saline or LPS in adulthood to unmask potential immune priming due to stressor exposure (n = 5–7/group). Plasma cytokine concentrations were analyzed with sex, stress, and LPS as main factors. While LPS led to a significant induction of all three cytokines (p<0.001) in plasma, sex did not impact cytokine concentrations (p>0.05). CAS males displayed greater plasma IL-1β compared to NS males at 4 hours post-LPS (p=0.017, *). Data are presented as mean ± SEM. * CAS effect across the indicated comparison.
IL-6 and TNF-α
Eight of twenty five saline-injected rats displayed TNF-α concentrations below the limit of detection (assay sensitivity <5 pg/mL), and were not included in analysis. LPS significantly increased IL-6 (F(3,76)=22.126, p<0.001) and TNF-α (F(3,69)=20.922, p<0.001) concentrations, and the LPS 2 hour time point was significantly different from the saline condition (p<0.001). (Fig 6B–C). Sex did not impact plasma IL-6 (F(1,76)=1.788, p=0.185) or TNF-α (F(1,69)=0.048, p=0.826). However, there was a sex * LPS interaction on IL-6 (F(3,76)=2.725, p=0.050) such that females that received LPS displayed the greatest IL-6 concentrations. There were no main effects of CAS on plasma IL-6 (F(1,76)=1.085, p=0.301) and TNF-α (F(1,69)=0.250, p=0.619) concentrations.
3.5. CAS blunts corticosterone response to adult LPS challenge in males
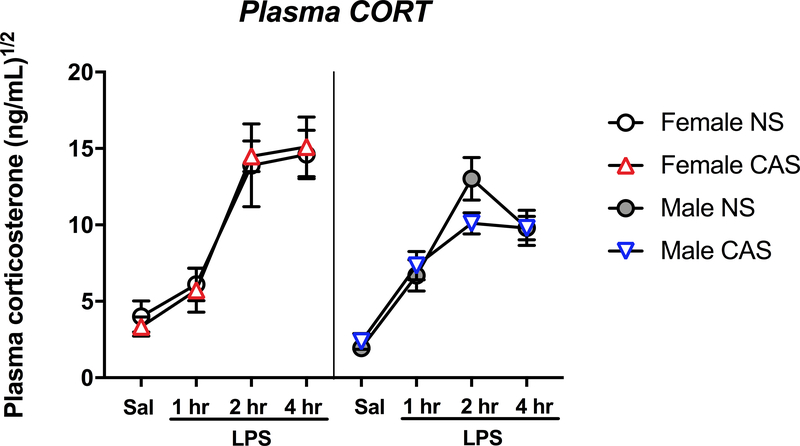
We next investigated altered HPA axis response to LPS as a potential mechanism underlying the exaggerated peripheral immune reactivity in CAS males. LPS led to a significant increase in plasma corticosterone (F(3,78)=58.651, p<0.001), and the LPS 1, 2, and 4 hour time points were each significantly different from the saline condition (p<0.001). (Fig 7). As expected, females displayed greater plasma corticosterone compared to males (F(1,78)=10.248, p=0.002), and there was a significant sex * LPS interaction (F(3,78)=3.919, p=0.012). There was no main effect of CAS on plasma corticosterone (F(1,78)=0.125, p=0.725). Because we previously observed exaggerated plasma IL-1β concentrations in CAS males at 4 hours post-LPS, we assessed whether plasma corticosterone was blunted in CAS males at an immediately preceding time point (2 hours post-LPS). A planned t-test indicated that CAS males displayed a blunted plasma corticosterone compared to NS males at 2 hours post-LPS (t(10)=1.87, p=0.045).
Fig 7. CAS male rats display a trend toward blunted corticosterone release following LPS.
Male and female rats of NS or CAS backgrounds received a single systemic injection of saline or LPS in adulthood to unmask potential immune priming due to stressor exposure (n = 5–7/group). Plasma corticosterone concentrations were analyzed with sex * stress * LPS as main factors. LPS led to a significant induction of all three cytokines (p<0.001). Females displayed greater corticosterone concentrations compared to males (p=0.002). A planned comparison at 2 hours post-LPS revealed lower corticosterone concentrations in CAS male rats compared to NS male rats (p=0.045). Data are presented as mean ± SEM.
4. Discussion
Due to its role as a hub of the immune system and its involvement in the stress response, NFκB could be part of a mechanism by which developmental stress sensitizes the neuroimmune profile. Here we demonstrated that experiencing chronic stress during adolescence led to sustained changes in the immune response as demonstrated by the priming of NFκB-related gene transcription in the hippocampus of both male and female adult rats. In addition, we showed that induction of the pro-inflammatory cytokine IL1B and the NFκB subunit REL was potentiated in the hippocampus of adult female CAS rats following LPS, suggesting that neuroimmune priming due to stress can manifest in a sex-specific manner when assessed weeks removed from the initial stressor exposure. Our results therefore augment previous literature in which chronic unpredictable stress was found to exacerbate NFκB’s transcriptional activity in the brain of male rats proximate to the stressor (Munhoz et al., 2006). Because LPS dosing was based on bodyweight, females received less LPS in total compared to males, and CAS females less than non-stressed females. Despite this, CAS females still displayed exaggerated pro-inflammatory cytokine induction in the hippocampus, thus highlighting the robustness of CAS effects.
Conversely, we found that the hippocampal expression of EGR1, which encodes early growth response 1, displayed a trend toward decrease in male CAS rats compared to male non-stressed controls. EGR1 is an immediate early gene induced by a variety of physiological processes including stress, and among its regulators are transcription factors such as extracellular signal-regulated kinase (ERK), mitogen-activated protein kinases (MAPK), and cyclic AMP-response element binding protein (CREB) (Duclot and Kabbaj, 2017). Although a CAS-associated decrease in EGR1 is unexpected given NFκB’s demonstrated role in inducing EGR1 (Thyss et al., 2005), EGR1 has been also shown to inhibit NFκB signaling (Chapman and Perkins, 2000), indicating complex mutual regulation between these transcription factors. Consistent with our result, there is some evidence that prior exposure to repeated stress downregulates acute inflammation-induced EGR1 expression in the adult male rat brain (Imbe and Kimura, 2017). The fact that EGR1 was indicated to be upregulated in male CAS rats based on the PCR array is likely due to the limitation of the assay, which was underpowered and used pooled samples. As some of the other targets identified from the array (FOS and JUN, subunits of the transcription factor AP-1) were subsequently determined to be unchanged by CAS using qPCR, these data suggest that the current PCR array results should be interpreted as preliminary only, thus confirming the need to verify array findings using targeted approaches with more power. Another target gene that was identified as upregulated by CAS in both males and females was the chemokine CCL2. In light of reports that social defeat stress promotes immune-to-brain leukocyte trafficking (Wohleb et al., 2013), this result suggests that altered rates of leukocyte infiltration to the hippocampus of CAS rats could contribute to the differences in neuroinflammation and should be a focus of future investigation.
We investigated the peripheral immune system as a potential source of the exaggerated hippocampal cytokine response in rats with a history of CAS. Previously, male mice (Gibb et al., 2011; Wohleb et al., 2012) and rats (Carobrez et al., 2002) subjected to social defeat have been shown to display exaggerated plasma cytokine concentrations when challenged with LPS within hours or up to a week after stressor exposure. Our results confirm and extend previous literature by demonstrating no alterations in plasma IL-1β in female rats with a history of CAS while indicating a potential CAS-related increase in plasma IL-1β in adult CAS males at four hours following LPS injection. These data further support sex differences in the manifestation of how CAS alters the adult innate immune response. It should be noted, however, that this potential male-specific impact of CAS on the adult peripheral immune response needs to be interpreted with caution as it derived from an exploratory statistical analysis. These results contrast with findings from Fonken et al. (2018) who found sensitized serum IL-1β responses in female, but not male, rats subjected to tail shock stress. Several causes, including differences in the nature of stressors, proximity of the stressor to immune assessments, and dose and/or time course of LPS, likely contribute to these divergent outcomes. Notably, a history of stress has been shown to accelerate the time course of plasma pro-inflammatory factors elicited by a secondary challenge (Cheng et al., 2015). Therefore, here we differentiated between CAS effects on time course versus magnitude of immune responses. Importantly, we did not see an effect of CAS on IL-6 and TNF-α concentrations at baseline, 1, 2 or 4 hours following LPS, suggesting that this lack of potentiation is likely not due to an altered time course of immune response. The current study cannot rule out the possibility that had the time course following LPS been continued beyond 4 hours, the peripheral immune potentiation in CAS males may have influenced hippocampal outcomes after the 4 hour time point.
Limited evidence exists regarding stress-induced changes in NFκB activity in peripheral tissue. We chose to examine the spleen because splenocytes of socially stressed mice have been demonstrated to exhibit potentiated NFκB activity ex vivo (Quan, 2003). Here we found that a history of CAS did not alter NFκB DNA binding in the spleen, which is consistent with Munhoz et al. (2006)’s report showing that chronic unpredictable stress did not exacerbate the LPS-induced increase in NFκB activity in the heart of rats. In the current study, at one hour following LPS injection, three of the twelve male and female NS rats displayed splenic NFκB activity that was much greater in magnitude compared to the remaining nine NS rats, suggesting a possible bimodal response among NS rats. In contrast, none of the CAS rats displayed an upregulation of splenic NFκB activity at one hour post-LPS. While the difference in splenic NFκB activity between NS and CAS rats was not statistically significant, it is possible that the lack of splenic activity seen in CAS rats reflects an altered splenic cellular composition due to differential splenocytic egress. In fact, some evidence suggests that chronic stress suppresses lymphocyte redeployment required for effective wound healing (Dhabhar and McEwen, 1997). Furthermore, repeated stress has also been documented to promote monocytic egress from the spleen (McKim et al., 2016). Therefore it is possible that the processing of the whole spleen in the current study as opposed to assessing specific subsets of splenocytes may have diluted the possible splenocyte-priming effects of CAS.
To determine whether exaggerated peripheral immune responses could be driven by a deficit in glucocorticoid-mediated anti-inflammatory signaling, we assayed plasma corticosterone, the primary output of the HPA axis. Immune activation such as that induced by LPS is known to activate the HPA axis, and therefore plasma corticosterone concentrations induced by LPS can be viewed as a proxy of the immune response (Lenczowski et al., 1997; Serrats et al., 2017). However, corticosterone is a powerful anti-inflammatory mediator, and therefore LPS-induced corticosterone could also indicate an anti-inflammatory compensation. Indeed, high baseline corticosterone is predictive of lower LPS-induced corticosterone (Perez-Nievas et al., 2010). In females, CAS did not impact baseline or LPS-induced plasma concentrations of corticosterone, which is in agreement with previously published CAS effects (Bourke et al., 2013; Pyter et al., 2013). However, at two hours following LPS injection, male CAS rats displayed a blunted corticosterone response compared to NS males. While plasma corticosterone levels elicited by systemic LPS have been reported to be exaggerated by stress in much the same manner that cytokine concentrations have been (Gibb et al., 2008; Gibb et al., 2011), the plasma corticosterone response has also been reported to be diminished in mice that have experienced repeated social defeat (Audet et al., 2011). Taken together, potentially blunted corticosterone release following LPS at two hours, followed by exaggerated plasma IL-1β at 4 hours, is consistent with a glucocorticoid resistance model in which regulation of the peripheral immune response by the HPA axis has been compromised by exposure to CAS. Surprisingly, CAS did not alter the peripheral inflammatory response to a secondary LPS challenge in female CAS rats, suggesting that the neuroimmune impact of CAS was uninfluenced by changes to peripheral inflammation.
One limitation of the current study is that estrous cycle was not accounted for at the time of collections. As demonstrated by Arakawa et al. (2014) stress-induced IL-1 in the brain varies by estrous stage, with significant upregulation during diestrus, proestrus, and estrus with no change seen in metestrus. While estrous stage can potentially influence CAS-related inflammatory gene expression in the brain, due to randomization and counterbalancing within each litter, all stages of estrous cycle are expected to be represented in each experimental group. Consistently, we have previously published that 17β-estradiol and progesterone were not impacted by CAS among saline-injected females (Pyter et al., 2013).
Overall, these data suggest that exposure to a history of chronic stress during adolescent development can impact the neuro-immune reactivity of both males and females when assessed later in adulthood. Furthermore, sex dictated exact manifestation of CAS effects on immune function with exaggerated hippocampal neuroimmune signaling in females and peripheral immune changes in males. As recently reviewed (Bekhbat and Neigh, 2018), the extent to which sex differences in stress-induced neuroinflammation contributes to the overall female bias in psychiatric illnesses is still undetermined. A majority of the rodent literature on stress-related neuroinflammation has identified pro-inflammatory consequences of stress in male, but not female, rodents – a conclusion perhaps at odds with the immune effect of stress in humans (Bekhbat and Neigh, 2018). It is unclear whether such results are an outcome of male-biased behavioral and pharmacological paradigms; however, more nuanced experimental approaches to assessing sex differences in stress-related neuroinflammation have emerged more recently as well (Fonken et al., 2018). Our current results extend the literature and begins to elucidate the mechanisms via which adolescent stress can alter the immune profile in the adult brain and body.
Supplementary Material
Acknowledgements
The authors receive funding from NIH: NR014886. SAR was supported by NIH training grant T32-GM008602.
Footnotes
Declaration of interest
The authors declare no conflict of interest.
References
- Arakawa K, Arakawa H, Hueston CM, Deak T, 2014. Effects of the estrous cycle and ovarian hormones on central expression of interleukin-1 evoked by stress in female rats. Neuroendocrinology 100, 162–177. [DOI] [PubMed] [Google Scholar]
- Audet MC, Jacobson-Pick S, Wann BP, Anisman H, 2011. Social defeat promotes specific cytokine variations within the prefrontal cortex upon subsequent aggressive or endotoxin challenges. Brain Behav Immun 25, 1197–1205. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ, 2014. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol 35, 303–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat M, Neigh GN, 2017. Sex differences in the neuro-immune consequences of stress: Focus on depression and anxiety. Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat M, Neigh GN, 2018. Sex differences in the neuro-immune consequences of stress: Focus on depression and anxiety. Brain Behav Immun 67, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat M, Rowson SA, Neigh GN, 2017. Checks and balances: The glucocorticoid receptor and NFkB in good times and bad. Front Neuroendocrinol 46, 15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP, 2003. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A 100, 1920–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Burnett S, Dahl RE, 2010. The role of puberty in the developing adolescent brain. Human brain mapping 31, 926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Neigh GN, 2011. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm Behav 60, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Stowe ZN, Neigh GN, Olson DE, Owens MJ, 2013. Prenatal exposure to escitalopram and/or stress in rats produces limited effects on endocrine, behavioral, or gene expression measures in adult male rats. Neurotoxicol Teratol 39, 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P, Peterson EL, Schultz LR, 1997. Sex differences in posttraumatic stress disorder. Arch Gen Psychiatry 54, 1044–1048. [DOI] [PubMed] [Google Scholar]
- Carobrez SG, Gasparotto OC, Buwalda B, Bohus B, 2002. Long-term consequences of social stress on corticosterone and IL-1beta levels in endotoxin-challenged rats. Physiol Behav 76, 99–105. [DOI] [PubMed] [Google Scholar]
- Caso JR, Pradillo JM, Hurtado O, Leza JC, Moro MA, Lizasoain I, 2008. Toll-like receptor 4 is involved in subacute stress-induced neuroinflammation and in the worsening of experimental stroke. Stroke 39, 1314–1320. [DOI] [PubMed] [Google Scholar]
- Chapman NR, Perkins ND, 2000. Inhibition of the RelA(p65) NF-kappaB subunit by Egr-1. J Biol Chem 275, 4719–4725. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Jope RS, Beurel E, 2015. A pre-conditioning stress accelerates increases in mouse plasma inflammatory cytokines induced by stress. BMC Neurosci 16, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R, 2007. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A 104, 1319–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, 2004. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol 500, 399–411. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS, 1997. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun 11, 286–306. [DOI] [PubMed] [Google Scholar]
- Duclot F, Kabbaj M, 2017. The Role of Early Growth Response 1 (EGR1) in Brain Plasticity and Neuropsychiatric Disorders. Front Behav Neurosci 11, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Frank MG, Gaudet AD, D’Angelo HM, Daut RA, Hampson EC, Ayala MT, Watkins LR, Maier SF, 2018. Neuroinflammatory priming to stress is differentially regulated in male and female rats. Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Thompson BM, Watkins LR, Maier SF, 2012. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav Immun 26, 337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb J, Hayley S, Gandhi R, Poulter MO, Anisman H, 2008. Synergistic and additive actions of a psychosocial stressor and endotoxin challenge: Circulating and brain cytokines, plasma corticosterone and behavioral changes in mice. Brain Behav Immun 22, 573–589. [DOI] [PubMed] [Google Scholar]
- Gibb J, Hayley S, Poulter MO, Anisman H, 2011. Effects of stressors and immune activating agents on peripheral and central cytokines in mouse strains that differ in stressor responsivity. Brain Behav Immun 25, 468–482. [DOI] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC, 2010. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry 67, 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, Baltimore D, 2006. Circuitry of nuclear factor kappaB signaling. Immunol Rev 210, 171–186. [DOI] [PubMed] [Google Scholar]
- Holder MK, Blaustein JD, 2014. Puberty and adolescence as a time of vulnerability to stressors that alter neurobehavioral processes. Front Neuroendocrinol 35, 89–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson SP, Jacobson-Pick S, Anisman H, 2014. Sex differences in behavior and pro-inflammatory cytokine mRNA expression following stressor exposure and re-exposure. Neuroscience 277, 239–249. [DOI] [PubMed] [Google Scholar]
- Imbe H, Kimura A, 2017. Attenuation of pCREB and Egr1 expression in the insular and anterior cingulate cortices associated with enhancement of CFA-evoked mechanical hypersensitivity after repeated forced swim stress. Brain Res Bull 134, 253–261. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt B, Kaltschmidt C, 2009. NF-kappaB in the nervous system. Cold Spring Harb Perspect Biol 1, a001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt C, Kaltschmidt B, Neumann H, Wekerle H, Baeuerle PA, 1994. Constitutive NF-kappa B activity in neurons. Mol Cell Biol 14, 3981–3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krappmann D, Wegener E, Sunami Y, Esen M, Thiel A, Mordmuller B, Scheidereit C, 2004. The IkappaB kinase complex and NF-kappaB act as master regulators of lipopolysaccharide-induced gene expression and control subordinate activation of AP-1. Mol Cell Biol 24, 6488–6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuebler U, Zuccarella-Hackl C, Arpagaus A, Wolf JM, Farahmand F, von Kanel R, Ehlert U, Wirtz PH, 2015. Stress-induced modulation of NF-kappaB activation, inflammation-associated gene expression, and cytokine levels in blood of healthy men. Brain Behav Immun 46, 87–95. [DOI] [PubMed] [Google Scholar]
- Lenczowski MJ, Van Dam AM, Poole S, Larrick JW, Tilders FJ, 1997. Role of circulating endotoxin and interleukin-6 in the ACTH and corticosterone response to intraperitoneal LPS. Am J Physiol 273, R1870–1877. [DOI] [PubMed] [Google Scholar]
- McKim DB, Patterson JM, Wohleb ES, Jarrett BL, Reader BF, Godbout JP, Sheridan JF, 2016. Sympathetic Release of Splenic Monocytes Promotes Recurring Anxiety Following Repeated Social Defeat. Biol Psychiatry 79, 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS, 2009a. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci U S A 106, 14716–14721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, Ma R, Cole SW, 2008. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry 64, 266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Rohleder N, Cole SW, 2009b. Chronic interpersonal stress predicts activation of pro- and anti-inflammatory signaling pathways 6 months later. Psychosom Med 71, 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills NT, Scott JG, Wray NR, Cohen-Woods S, Baune BT, 2013. Research review: the role of cytokines in depression in adolescents: a systematic review. J Child Psychol Psychiatry 54, 816–835. [DOI] [PubMed] [Google Scholar]
- Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, Lima Lde S, Avellar MC, Sapolsky RM, Scavone C, 2006. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci 26, 3813–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagabhushan M, Mathews HL, Witek-Janusek L, 2001. Aberrant nuclear expression of AP-1 and NFkappaB in lymphocytes of women stressed by the experience of breast biopsy. Brain Behav Immun 15, 78–84. [DOI] [PubMed] [Google Scholar]
- Neigh GN, Ritschel LA, Kilpela LS, Harrell CS, Bourke CH, 2013. Translational reciprocity: bridging the gap between preclinical studies and clinical treatment of stress effects on the adolescent brain. Neuroscience 249, 139–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM, 2006. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry 163, 1630–1633. [DOI] [PubMed] [Google Scholar]
- Perez-Nievas BG, Madrigal JL, Garcia-Bueno B, Zoppi S, Leza JC, 2010. Corticosterone basal levels and vulnerability to LPS-induced neuroinflammation in the rat brain. Brain Res 1315, 159–168. [DOI] [PubMed] [Google Scholar]
- Perry VH, Holmes C, 2014. Microglial priming in neurodegenerative disease. Nat Rev Neurol 10, 217–224. [DOI] [PubMed] [Google Scholar]
- Piccinelli M, Wilkinson G, 2000. Gender differences in depression. Critical review. Br J Psychiatry 177, 486–492. [DOI] [PubMed] [Google Scholar]
- Pyter LM, Kelly SD, Harrell CS, Neigh GN, 2013. Sex differences in the effects of adolescent stress on adult brain inflammatory markers in rats. Brain Behav Immun 30, 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N, 2003. Molecular mechanisms of glucocorticoid resistance in splenocytes of socially stressed male mice. Journal of Neuroimmunology 137, 51–58. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH, 2006. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 27, 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader BF, Jarrett BL, McKim DB, Wohleb ES, Godbout JP, Sheridan JF, 2015. Peripheral and central effects of repeated social defeat stress: monocyte trafficking, microglial activation, and anxiety. Neuroscience 289, 429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD, 2003. Puberty: a period of both organizational and activational effects of steroid hormones on neurobehavioural development. J Neuroendocrinol 15, 1185–1192. [DOI] [PubMed] [Google Scholar]
- Serrats J, Grigoleit JS, Alvarez-Salas E, Sawchenko PE, 2017. Pro-inflammatory immune-to-brain signaling is involved in neuroendocrine responses to acute emotional stress. Brain Behav Immun 62, 53–63. [DOI] [PubMed] [Google Scholar]
- Sheppard KA, Phelps KM, Williams AJ, Thanos D, Glass CK, Rosenfeld MG, Gerritsen ME, Collins T, 1998. Nuclear integration of glucocorticoid receptor and nuclear factor-kappaB signaling by CREB-binding protein and steroid receptor coactivator-1. J Biol Chem 273, 29291–29294. [DOI] [PubMed] [Google Scholar]
- Tak LM, Kingma EM, van Ockenburg SL, Ormel J, Rosmalen JG, 2015. Age- and sex-specific associations between adverse life events and functional bodily symptoms in the general population. J Psychosom Res 79, 112–116. [DOI] [PubMed] [Google Scholar]
- Thyss R, Virolle V, Imbert V, Peyron JF, Aberdam D, Virolle T, 2005. NF-kappaB/Egr-1/Gadd45 are sequentially activated upon UVB irradiation to mediate epidermal cell death. EMBO J 24, 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathington JM, Arnold AR, Cooke BM, 2012. Juvenile social subjugation induces a sex-specific pattern of anxiety and depression-like behaviors in adult rats. Horm Behav 61, 91–99. [DOI] [PubMed] [Google Scholar]
- Webster JI, Tonelli L, Sternberg EM, 2002. Neuroendocrine regulation of immunity. Annu Rev Immunol 20, 125–163. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP, 2012. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology 37, 1491–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Powell ND, Godbout JP, Sheridan JF, 2013. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci 33, 13820–13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf JM, Rohleder N, Bierhaus A, Nawroth PP, Kirschbaum C, 2009. Determinants of the NF-kappaB response to acute psychosocial stress in humans. Brain Behav Immun 23, 742–749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.