Matched donor hematopoietic stem cell transplantation (HSCT) cures most patients with inborn hematopoietic disorders, but haploidentical HSCT was historically associated with graft rejection, graft-versus-host disease (GvHD) and infections. In vivo depletion of haploidentical alloreactive T cells with post-transplant cyclophosphamide (haplo pTCy) has been shown to result in outcomes comparable to those with matched related or unrelated donors in adults with malignant diseases.1 Published results of this approach in patients with nonmalignant diseases are still relatively scarce.2 We therefore analyzed the results of haplo pTCy in combination with upfront serotherapy and a myeloablative conditioning regimen in patients with non-malignant diseases at our center, showing good overall survival, little GvHD, and few infectious complications.
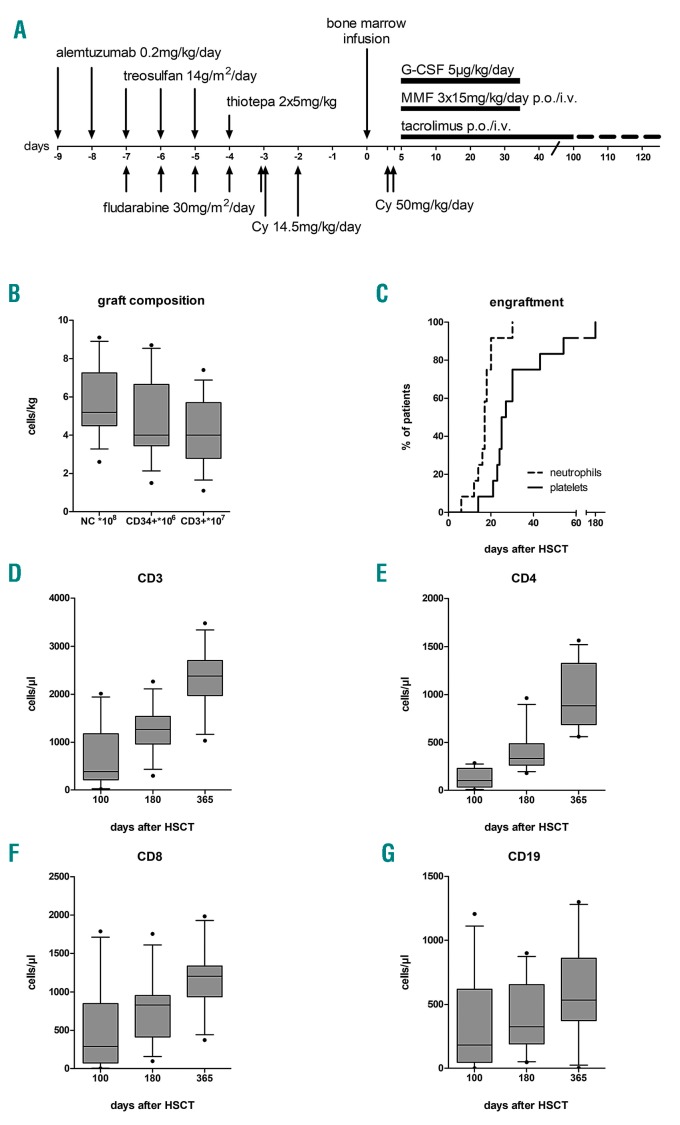
Between 2013 and 2018, all patients with inborn errors of hematopoiesis who lacked a suitable matched related or 10/10 HLA matched unrelated donor, or who had failed a previous HSCT, were treated according to this institutional protocol. The graft was unmanipulated bone marrow from familial HLA-haploidentical donors. Conditioning consisted of alemtuzumab 2×0.2 mg/kg/day (days ܋9 to ܋8), fludarabine 5×30 mg/m2/d (days ܋7 to ܋3), treosulfan 3×14 g/m2/d (days ܋7 to ܋5), thiotepa 2×5 mg/kg (day ܋4), and cyclophosphamide 2×14.5 mg/kg/d (days ܋3 to ܋2) in all patients except patient n. 5 who received submyeloablative busulfan [total Area Under the Curve (AUC) 55,000 ng × hour/mL] instead of treosulfan/thiotepa with the intention to enable central nervous system engraftment of donor-derived microglial cells.3,4 GvHD prophylaxis consisted of cyclophosphamide 50 mg/kg/d on days +3 and +4, followed by mycophenolate mofetil (days +5 to +35), and tacrolimus (days +5 to +100 with consecutive tapering) (Figure 1A and Online Supplementary Appendix).
Figure 1.
Conditioning regimen, graft composition, engraftment and immune reconstitution. (A) Schematic diagram of the conditioning regimen and immunosuppression based on the original Baltimore protocol.8 (B) Composition of the bone marrow grafts. (C) Cumulative incidence of neutrophil and platelet engraftment. (D-G) Immune reconstitution of CD3, CD4, CD8 and CD19 positive cells. Solid line: median; gray shaded boxes: interquartile range; whiskers: 10-90 percentile; dots: outliers. Cy: cyclophosphamide; G-CSF: granulocyte colony stimulating factor; MMF: mycophenolate mofetil; NC: nucleated cells; p.o.: oral administration; i.v.: intravenous.
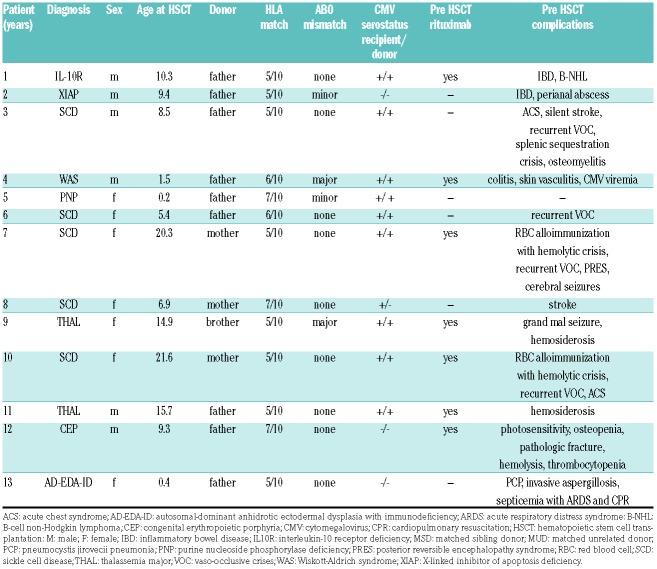
We included thirteen patients with a median age of 9.3 years at HSCT (range: 0.2-21.6). All patients received T-cell replete HLA-haploidentical bone marrow grafts. Five patients suffered from immunodeficiencies, seven from sickle cell disease (SCD) or thalassemia, and one from congenital erythropoietic porphyria (Table 1). Three patients (ns. 3, 6, 7) have been published previously.5 Patients n. 9 [matched sibling donor (MSD)] and n. 12 [matched unrelated donor (MUD)] had undergone a previous unsuccessful allogeneic HSCT after a myeloablative regimen. The grafts contained a median of 5.2×108/kg (range: 2.6-9.1) total nucleated, 4.0×106/kg (range: 1.5-8.7) CD3+ cells and 4.0×107/kg (range: 1.1-7.4) CD3+ cells (Table 1 and Figure 1B).
Table 1.
Patients’ and donor characteristics.
After a median follow up of 34.8 months (range: 0.2-72.0) overall survival is 92%, and event-free survival (event defined as disease recurrence or death) is 77% (Online Supplementary Figure S1). Neutrophil and platelet engraftment occurred in all evaluable patients on day +17 (median: range 6-30) and +26 (range: 14-180), respectively (Figure 1C). There was no impact of AB0 matching on the degree of erythroid engraftment. Patients were discharged from the ward at median day+34 (range: 24-47).
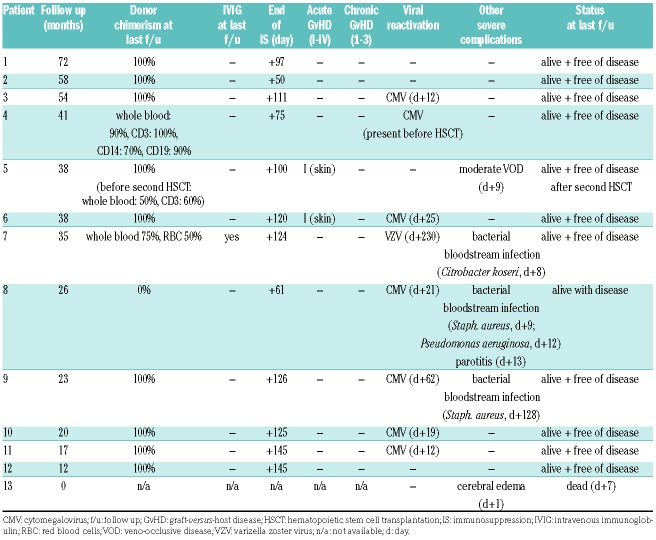
Two patients are stable mixed chimeras but remain disease free: Patient n. 3 has stable donor chimerism ≥90% and has no signs of his original disease; Patient n. 7 (SCD) has 75% donor chimerism in whole blood and 50% in the erythroid lineage. With a sickle hemoglobin (HbS) level of 45% at last follow up, she has no symptoms of SCD, and is off hydroxycarbamide. The other SCD patients with complete donor chimerism all have HbS levels comparable to their heterozygous donors (approx. 35%). Patient n. 5 experienced partial autologous reconstitution and disease recurrence 13 months after HSCT. She was successfully retransplanted from the same donor and has full donor chimerism. Patient n. 8 experienced secondary graft loss three months after HSCT and is alive with 0% donor chimerism. Thus, 77% of patients have ≥90% donor chimerism at last follow up and 85% are disease free (Table 2).
Table 2.
Hematopoietic stem cell transplantation outcome and complications.
Anti-donor HLA antibodies have been recognized as a risk factor for graft failure in haploidentical HSCT.6 In this cohort, four patients (ns. 7, 9, 10, 11) had such antibodies, in three of whom a reduction in antibody titers with plasmapheresis and rituximab was attempted (Online Supplementary Table S1). All patients primarily engrafted, including patient n. 11 who declined plasmapheresis.
Cellular immune reconstitution was timely with a median of 384/μL CD3+ cells (range: 17-2013), 103/μL CD4+ (range: 4-284), 291/μL CD8+ (range: 4-1786) and 182/μL CD19+ (range: 0-1206) on day +100, after HSCT (Figure 1D-G). Patient n. 7 remained severely B-lymphocytopenic at one year after HSCT (3/μL) and is still on intravenous immunoglobulin (IVIG) substitution at 35 months of follow up. She had received six doses of rituximab preceding HSCT due to severe hemolytic crisis. All other patients were off immunosuppression and off IVIG at last follow up.
One patient died on day +7 after HSCT from unexplained neurotoxicity; no infectious agent was identified, leading to the interpretation of fludarabine-associated toxicity in this infant patient. Acute GvHD was limited to transient disease of the skin (overall grade I) in two patients. Higher grade acute or chronic GvHD was not observed. Patient n. 4 had cytomegalovirus (CMV) viremia before and after HSCT. Reactivation of CMV occurred in six other patients, but no overt CMV disease was observed. Patient n. 7 developed varizella zoster virus (VZV) reactivation (shingles) after cessation of acyclovir prophylaxis. No adenovirus or Epstein-Barr virus reactivations were observed. Patient n. 5 experienced veno-occlusive disease of moderate severity on day+9 after HSCT despite an AUC of 55,000 ng × hour/mL which was successfully treated with defibrotide for two weeks.7 No renal or hepatic toxicity greater than Common Terminology Criteria for Adverse Events grade 2 was observed. No patient developed hemorrhagic cystitis.
Bolanos-Meade et al. provided proof of concept that haplo pTCy was a feasible therapeutic strategy for patients with SCD lacking a matched donor, but the submyeloablative, irradiation-containing preparative regimen resulted in high rates of autologous reconstitution.8 Treosulfan/fludarabine conditioning had been reported to result in limited acute and possibly little long-term toxicity in matched donor HSCT.9 We added thiotepa to increase myeloablation and a low dose of cyclophosphamide and alemtuzumab for additional recipient immunosuppression in the face of a greater HLA barrier in the haploidentical setting. The addition of either agent had previously been found not to add significant acute toxicities to treosulfan/fludarabine.10 In the future, pharmacokinetic monitoring of treosulfan may mean that targeting the degree of myeloablation can be improved even more, and possibly thiotepa could be omitted. The myeloablative conditioning with the addition of serotherapy may have enabled us to spare ionizing radiation, which we believe should be avoided in pediatric patients with non-malignant diseases due to its inherent risk for long-term sequelae. Overt immune-mediated rejection was not observed in our cohort, but two patients experienced graft loss through autologous reconstitution probably due to incomplete myeloablation. Durable complete donor engraftment after the first haploidentical HSCT was achieved in 75% of evaluable patients, indicating that even more myeloablation (e.g. with pharmacokinetically monitored busulfan or treosulfan) may be necessary to achieve full engraftment in patients in whom a complete chimerism is desired, and that the required degree of myeloablation may be disease specific. In theory, the tolerizing effect of pTCy obviates the need for serotherapy. We still chose to add alemtuzumab as an immunoablative agent directed at recipient cells to the conditioning regimen. We used a lower dose than is commonly used in T-cell replete matched transplants11 and applied it on days ܋9 and ܋8 in order not to compromise donor T-cell immunity. We observed viral reactivations in 67% of patients, but no overt viral disease. T-cell immune reconstitution did not seem to be negatively affected by the serotherapy. In fact, median CD3+ T-cell and CD19+ B-cell numbers on day +180 were higher than in two studies using HLA-haploidentical grafts and TCRαβ depletion.12,13 Future studies should address the pharmacokinetics of alemtuzumab in order to use this potent drug in the most safe and effective way in this setting.
Our results compare well with other published experience in similar patient populations. Shah et al. describe their experience with haplo pTCy in seven patients with dedicator of cytokinesis 8 (DOCK8) deficiency after conditioning with reduced-dose busulfan, fludarabine, cyclophosphamide and low-dose total body irradiation (200 cGy), resulting in full engraftment in all patients, 86% survival, and no chronic GvHD.14 Balashov et al. reported their experience with haploidentical TCRαβ/CD19-depleted peripheral blood stem cell transplantation in ten primary immunodeficiency disease (PID) patients with an incidence of grade III-IV acute GvHD of 10%, extensive chronic GvHD of 10%, and graft failure of 20%.12 Bertaina et al. used a similar strategy in 23 patients with non-malignant diseases, and saw no grade III-IV acute GvHD or extensive chronic GvHD, 17% graft failure, and 9% transplant-related mortality.13 A recent paper from the UK reporting on 24 PID patients who underwent a haploidentical TCRαβ/CD19-depleted HSCT described 84% overall survival, 4% graft failure, 4% grade III-IV acute GvHD, no chronic GvHD, and an infection-related mortality of 16%.15 TCRαβ/CD19 depletion is demanding from a technical, logistic and economic perspective, and may thus not be available to all centers.
While the number of treated patients in this single-center retrospective analysis is relatively low, the diseases are heterogeneous and the conditioning not entirely uniform, it still represents a larger cohort of patients treated with a uniform conditioning regimen and haplo pTCy in a variety of non-malignant diseases than in any other published report that is known to us. It remains unclear whether our approach is applicable to all inborn error disease categories and in all age groups. Even though a direct comparison to other studies is difficult, due to the heterogeneity of patients and conditioning regimens, none of the previously reported approaches with either in vivo or ex vivo depletion of alloreactive T cells seem to be superior to our experience. Myeloablative conditioning and haplo pTCy were safe and resulted in good disease-free survival in this cohort of patients with inborn errors in the absence of relevant GvHD. In general, haplo pTCy is an inexpensive T-cell depletion strategy that could be easily transferrable to centers with limited access to graft manipulation resources. However, larger prospective, and preferably comparative studies, are warranted to evaluate this approach in comparison to other donors, such as, for example, mismatched unrelated donors.
Footnotes
Funding: Part of this study was supported by the Care-for-Rare Foundation for children with rare diseases, Munich, Germany. MHA reports travel support by medac.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Raiola AM, Dominietto A, di Grazia C, et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant. 2014;20(10):1573-1579. [DOI] [PubMed] [Google Scholar]
- 2.Rastogi N, Katewa S, Thakkar D, Kohli S, Nivargi S, Yadav SP. Reduced-toxicity alternate-donor stem cell transplantation with posttransplant cyclophosphamide for primary immunodeficiency disorders. Pediatr Blood Cancer. 2018;65(1). [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson FL, Sergijenko A, Langford-Smith KJ, Malinowska M, Wynn RF, Bigger BW. Busulfan conditioning enhances engraftment of hematopoietic donor-derived cells in the brain compared with irradiation. Mol Ther. 2013;21(4):868-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15(3):367-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiebking V, Hutker S, Schmid I, Immler S, Feuchtinger T, Albert MH. Reduced toxicity, myeloablative HLA-haploidentical hematopoietic stem cell transplantation with post-transplantation cyclophosphamide for sickle cell disease. Ann Hematol. 2017;96(8):1373-1377. [DOI] [PubMed] [Google Scholar]
- 6.Kongtim P, Cao K, Ciurea SO. Donor Specific Anti-HLA Antibody and Risk of Graft Failure in Haploidentical Stem Cell Transplantation. Adv Hematol. 2016;2016:4025073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corbacioglu S, Carreras E, Ansari M, et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: a new classification from the European society for blood and marrow transplantation. Bone Marrow Transplant. 2018;53(2):138-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolanos-Meade J, Fuchs EJ, Luznik L, et al. HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood. 2012;120(22):4285-4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slatter MA, Rao K, Abd Hamid IJ, et al. Treosulfan and Fludarabine Conditioning for Hematopoietic Stem Cell Transplantation in Children with Primary Immunodeficiency: UK Experience. Biol Blood Marrow Transplant. 2018;24(3):529-536. [DOI] [PubMed] [Google Scholar]
- 10.Slatter MA, Boztug H, Potschger U, et al. Treosulfan-based conditioning regimens for allogeneic haematopoietic stem cell transplantation in children with non-malignant diseases. Bone Marrow Transplant. 2015;50(12):1536-1541. [DOI] [PubMed] [Google Scholar]
- 11.Inborn Errors Working Party of the European Group for Blood and Marrow Transplantation. EBMT/ESID GUIDELINES FOR HAEMATOPOIETIC STEM CELL TRANSPLANTATION FOR PRIMARY IMMUNODEFICIENCIES. 2012. [cited; Available from: https://www.ebmt.org/sites/default/files/migration_legacy_files/document/Inborn%20Errors%20Working%20Party%20ESID%20EBMT%20HSCT%20Guidelines%202017.pdf
- 12.Balashov D, Shcherbina A, Maschan M, et al. Single-Center Experience of Unrelated and Haploidentical Stem Cell Transplantation with TCRalphabeta and CD19 Depletion in Children with Primary Immunodeficiency Syndromes. Biol Blood Marrow Transplant. 2015;21(11):1955-1962. [DOI] [PubMed] [Google Scholar]
- 13.Bertaina A, Merli P, Rutella S, et al. HLA-haploidentical stem cell transplantation after removal of alphabeta+ T and B cells in children with nonmalignant disorders. Blood. 2014;124(5):822-826. [DOI] [PubMed] [Google Scholar]
- 14.Shah NN, Freeman AF, Su H, et al. Haploidentical Related Donor Hematopoietic Stem Cell Transplantation for Dedicator-of-Cytokinesis 8 Deficiency Using Post-Transplantation Cyclophosphamide. Biol Blood Marrow Transplant. 2017;23(6):980-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah RM, Elfeky R, Nademi Z, et al. T-cell receptor alphabeta(+) and CD19(+) cell-depleted haploidentical and mismatched hematopoietic stem cell transplantation in primary immune deficiency. J Allergy Clin Immunol. 2018;141(4):1417-1426.e1. [DOI] [PubMed] [Google Scholar]