Smoldering myeloma (SMM) is a pre-malignant monoclonal gammopathy with an annual risk of about 10% of progressing to active multiple myeloma (MM).1 The International Myeloma Working Group (IMWG) has recently updated the diagnostic criteria for SMM.2 The previously defined “ultra high-risk” SMM (characterized by specific biomarkers associated with a greater than 80% risk of progression to symptomatic MM within 2 years) has now been incorporated in active MM. SMM is biologically heterogeneous and encompasses a condition with a very low rate of progression to symptomatic MM, behaving similarly to monoclonal gammopathy of uncertain significance, as well as a disease with acquired organ damage and progression to active MM within 5 years from diagnosis.3
The molecular mechanisms involved in the progression of SMM to MM are still far from being fully understood. Genomic studies indicate that the genetic aberrations that characterize MM patients are already present in those with SMM,1,4 who present similar mutational and copy number alteration loads.5 Moreover, a four-gene score integrated with clinical features has been identified as a putative predictor of high-risk SMM.6 However, few data are available on the transcriptional profiles of SMM patients in relationship to the progression to active MM7-9 and, overall, the data indicate minimal differential expression in either coding or non-coding RNA. To date, the transcriptional profiles of plasma cells from paired samples obtained at the time of SMM and at the onset of MM are lacking.
Herein, for the first time we compare the transcriptomes of purified bone marrow CD138+ plasma cells from paired samples taken from patients with SMM who then progressed to active MM (P-SMM). The purpose was to identify any possible common transcriptional discrepancies that may help to understand intra-patient disease evolution. Contemporaneously, we investigated transcriptional differences between patients with P-SMM and a subset of those with non-progressed SMM (NP-SMM) for a median follow up of more than three times the time to progression of P-SMM.
To this aim a total of 21 patients with SMM (Table 1A, B), admitted to the Hematology Unit of Parma Hospital over the last 11 years, were considered: 11 with NP-SMM and ten with P-SMM. Paired samples, taken at the time of diagnosis of SMM and active MM, were available for the NP-SMM patients. SMM was diagnosed according to the IMWG revised criteria2 and patients were stratified by known risk factors for progression,3 as previously described.10 None of the patients enrolled in this study had previously received anti-MM therapy. The study was approved by the local Ethics Committee and written informed consent was obtained from all the patients involved in the study.
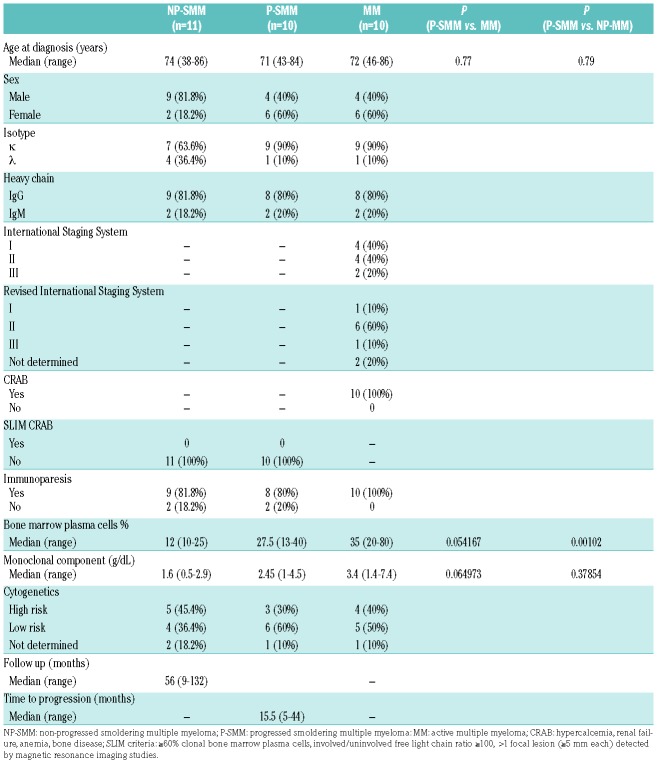
Table 1A.
Clinical characteristics of the patients enrolled in the study.
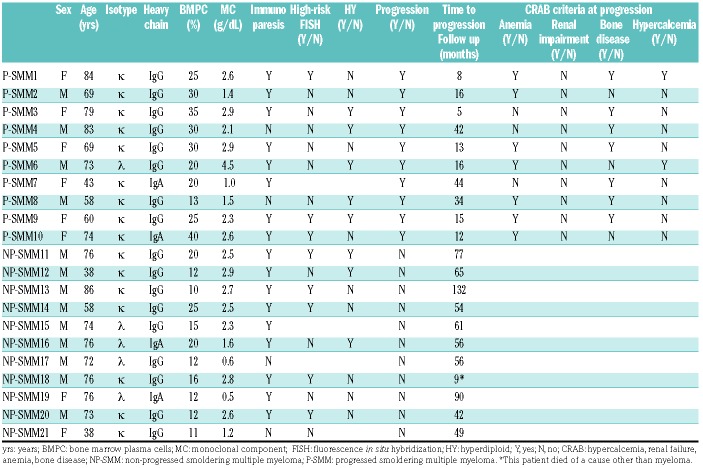
Table 1B.
Clinical characteristics of the individual patients enrolled in the study.
The median age at diagnosis was 71 years (range, 43-84) for the patients with P-SMM and 74 years (range, 38-86) for those with NP-SMM. The median percentage of bone marrow plasma cells at diagnosis in the ten patients with P-SMM was 27.5% (range, 13-40%). One of these ten patients had a monoclonal M component ≥3 g/dL, whereas 80% of patients presented with immunoparesis; high-risk cytogenetic features - either del(17p), or t(4;14) - were detected in three of nine cases. The median time to progression was 15.5 months and all patients progressed with the onset of CRAB features (hypercalcemia, renal failure, anemia, bone disease). The median percentage of bone marrow plasma cells in the 11 patients with NP-SMM was 12% (range, 10-25%) and 82% of them presented with immunoparesis; high-risk cytogenetic features were detected in five of the nine patients with enough bone marrow plasma cells to allow examination. According to the Mayo score,3 available for eight of the patients with NP-SMM, half of the patients were classified as having intermediate-risk disease and the other half as having low-risk disease. The median follow-up of the NP-SMM patients was 56 months. Primary CD138+ plasma cells were purified from bone marrow aspirates with an immunomagnetic method using anti-CD138 monoclonal antibody-coated microbeads (MACS, Miltenyi Biotec, Bergisch-Gladbach, Germany). Total RNA was extracted using an RNeasy kit (Qiagen, Hilden, Germany) and global expression profiles of 19,012 protein-coding and 13,972 long non-coding RNA (lncRNA) were extracted from GeneChip® ClariomD arrays (Affymetrix, Thermo Fisher Scientific, USA) analyzed using a robust microarray average (RMA) normalization procedure9 and annotations based on Gencode project (version 26) provided by the University of Michigan (http://brainarray.mbni.med.umich.edu/Brainarray/Database/CustomCDF/22.0.0/genecodeg.asp). Data are publicly available in the National Center for Biotechnology Information Gene Expression Omnibus (NCBI GEO) repository under accession number GSE117847. Rank product9 and gene set enrichment analysis (GSEA) were used for differential and functional analyses.
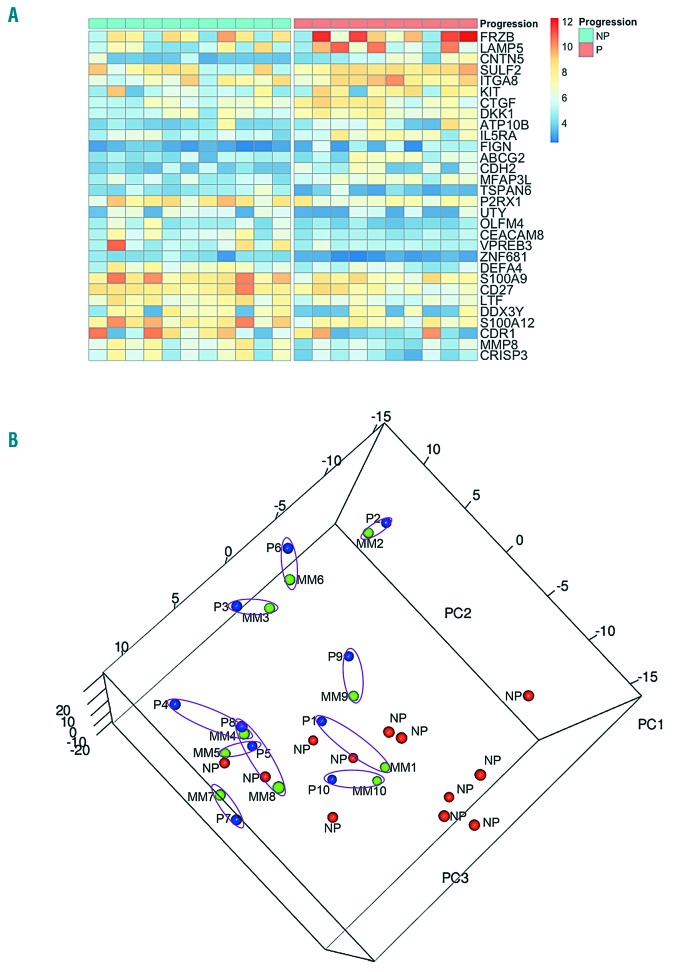
GSEA analyses on the global gene expression profiles of P-SMM compared to NP-SMM cases revealed a down-regulation of the antigen-processing gene set (Online Supplementary Figure S1A), whereas genes specifically associated with MM proliferation and hyperdiploidy were positively modulated in P-SMM cases (Online Supplementary Figure S1B). Interestingly, this observation was consistent with previously published data showing that, among the gene expression-based molecular subtypes,11 the proliferation subtype correlated significantly with progression in patients with asymptomatic myeloma.12 Additionally, we found 273 genes significantly modulated in P-SMM compared to NP-SMM (Online Supplementary Table S1). Specifically, among the 30 genes with at least a 2-fold change in expression levels, the Wnt inhibitors FRZB and DKK1, the adhesion molecule CDH2 and the pro-angiogenic CTGF were upregulated in association with progression to MM (Figure 1A). Subsequently, we confirmed the significant upregulation of DKK1 (Hs00183740_m1), FRZB (Hs00173503_m1), CDH2 (Hs00983056_m1) and CTGF (Hs00170014_m1) mRNA in bone marrow CD138+ cells from patients with P-SMM compared to CD138+ cells from patients with NP-SMM, by quantitative real-time polymerase chain reaction (TaqMan Assay, Life Technology, USA) performed on a Light Cycler 480 (Roche Diagnostics, Italy) following a standard protocol. In these experiments, GAPDH (Hs99999905_m1) was used as a housekeeping gene and the 2−ΔΔCt method was applied to calculate the mRNA fold changes (Online Supplementary Figure S2). Moreover, enzyme-linked immunosorbent assays for FRZB protein (Abbexa, UK), namely Secreted Frizzled Related Protein (sFRP)-3, were performed on frozen bone marrow plasma samples, when available, from the same cohorts of patients. The median sFRP-3 level in NP-SMM patients was significantly lower than in the P-SMM group (P=0.032) (Online Supplementary Figure S3). We also recently demonstrated that bone marrow DKK-1 protein levels were significantly higher in SMM patients who progressed to active MM than in those who did not progress.10
Figure 1.
Differential transcriptomic profile between non-progressed and progressed smoldering multiple myeloma and paired smoldering and active myeloma samples. (A) Heatmap of the 30 most variable coding genes (highlighted in light blue in Online Supplementary Table S1) with at least a 2-fold change in expression levels in samples from ten patients whose smoldering multiple myeloma progressed (P-SMM) versus samples from 11 whose smoldering disease had not progressed (NP-SMM). (B) Three-dimensional visualization of the results of principal component analysis on the most variable transcripts across the whole dataset. NP-SMM samples (red dots) agglomerated in a distinguishable cloud from P-SMM samples (blue dots), which tended to aggregate with their active multiple myeloma (MM) (green dots) paired samples. The P-SMM and MM samples from the same patient share the same number and are highlighted by a purple circle. NP: non-progressed smoldering multiple myeloma, P: progressed smoldering multiple myeloma; MM: active myeloma.
Additionally, a specific expression pattern of 65 lncRNA (7 with a >2-fold change) was observed in the comparison between P-SMM and NP-SMM cases (Online Supplementary Table S2), as also recently reported.9 The majority of the most differentially expressed lncRNA still have unknown functions in MM cell biology and pathophysiology, and little information concerning them has been provided in the literature. Modulation of the lncRNA AC092611.2 (antisense to the proximal GATB gene) has been described in juvenile myelomonocytic leukemia,13 whereas AL138899.1 was reported to be downregulated in T-cell acute lymphoblastic leukemia tumors compared with immature thymocytes.14 There is more evidence regarding the lncRNA XIST, whose involvement in progression and poorer outcome has been reported in several tumors.15
Conversely, the major finding of our analysis was that very similar expression profiles were observed between the ten paired SMM - MM samples. In fact, no significant differentially expressed coding genes or lncRNA were observed in the comparison between paired cases, thus suggesting that the progression of SMM to active MM was not associated with a substantial modification of the transcriptional profiles of plasma cells. A general picture of the most variable protein-coding genes and lncRNA throughout the entire dataset was offered by principal component analysis, which evidenced that NP-SMM samples agglomerated into a cloud that was reasonably distinguishable from samples from P-SMM patients, which tended to aggregate with their paired MM samples (Figure 1B). Of note, in the paired P-SMM and MM samples, no further deregulation was observed in the gene expression levels of the previously described 30 genes, including the Wnt inhibitors FRZB and DKK1 which were differentially expressed between P-SMM and NP-SMM cases.
Overall, our findings on the upregulation of Wnt inhibitors, such as DKK-1 and FRZB by CD138+ MM cells in P-SMM patients sustains the hypothesis that high levels of these molecules, produced by MM cells16 and also by bone marrow mesenchymal stromal cells,17,18 may influence the microenvironment, exerting a possible immunosuppressive effect19 that leads to the progression of SMM towards active MM. Moreover, our data from a cohort of patients with SMM, whose disease progressed to active MM in a short time, indicate that the transcriptome of the plasma cells of these patients did not change significantly during the progression. Although a larger study cohort and longer follow up would undoubtedly be desirable for confirmation, our data strongly suggest that the transcriptional alterations of plasma cells observed in MM patients are already present at the stage of smoldering disease. This adds support to the notion that alterations in microenvironmental cells could be critical in the progression from SMM to active MM.3
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
Funding: the authors would like to thank the “Associazione Italiana per la Ricerca sul Cancro” (AIRC) IG20229 (NG), IG16722 and IG10136 (AN), the International Myeloma Foundation 2018 Brian D. Novis Research Award (NG). KT was supported by a fellowship from Fondazione Umberto Veronesi. We thank the “Associazione Italiana Contro le Leucemie” (AIL) Parma section for providing a fellowship and for its support.
References
- 1.Pawlyn C, Morgan GJ. Evolutionary biology of high-risk multiple myeloma. Nat Rev Cancer. 2017;17(9):543-556. [DOI] [PubMed] [Google Scholar]
- 2.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-548. [DOI] [PubMed] [Google Scholar]
- 3.Rajkumar SV, Landgren O, Mateos MV. Smoldering multiple myeloma. Blood. 2015;125(20):3069-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker BA, Wardell CP, Melchor L, et al. Intraclonal heterogeneity is a critical early event in the development of myeloma and precedes the development of clinical symptoms. Leukemia. 2014;28(2):384-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Nieuwenhuijzen N, Spaan I, Raymakers R, Peperzak V. From MGUS to multiple myeloma, a paradigm for clonal evolution of pre-malignant cells. Cancer Res. 2018;78(10):2449-2456. [DOI] [PubMed] [Google Scholar]
- 6.Khan R, Dhodapkar M, Rosenthal A, et al. Four genes predict high risk of progression from smoldering to symptomatic multiple myeloma (SWOG S0120). Haematologica. 2015;100(9):1214-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez-Corral L, Corchete LA, Sarasquete ME, et al. Transcriptome analysis reveals molecular profiles associated with evolving steps of monoclonal gammopathies. Haematologica. 2014;99(8):1365-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong L, Chen CY, Ning B, et al. Pathway-based network analysis of myeloma tumors: monoclonal gammopathy of unknown significance, smoldering multiple myeloma, and multiple myeloma. Genet Mol Res. 2015;14(3):9571-9584. [DOI] [PubMed] [Google Scholar]
- 9.Ronchetti D, Agnelli L, Taiana E, et al. Distinct lncRNA transcriptional fingerprints characterize progressive stages of multiple myeloma. Oncotarget. 2016;7(12):14814-14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalla Palma B, Marchica V, Pedrazzoni M, et al. Bone marrow Dikkopf-1 levels are a new independent risk factor for progression in patients with smouldering myeloma. Br J Haematol. 2017; 183(5):812-815. [DOI] [PubMed] [Google Scholar]
- 11.Zhan F, Huang Y, Colla S, et al. The molecular classification of multiple myeloma. Blood. 2006;108(6):2020-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhodapkar MV, Sexton R, Waheed S, et al. Clinical, genomic, and imaging predictors of myeloma progression from asymptomatic monoclonal gammopathies (SWOG S0120). Blood. 2014;123(1):78-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofmans M, Lammens T, Helsmoortel HH, et al. The long non-coding RNA landscape in juvenile myelomonocytic leukemia. Haematologica. 2018;103(11):e501-e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallaert A, Durinck K, Van Loocke W, et al. Long noncoding RNA signatures define oncogenic subtypes in T-cell acute lymphoblastic leukemia. Leukemia. 2016;30(9):1927-1930. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Q, Hu W, Zhu W, et al. Long non coding RNA XIST as a prognostic cancer marker - a meta-analysis. Clin Chim Acta. 2018;482:1-7. [DOI] [PubMed] [Google Scholar]
- 16.Tian E, Zhan F, Walker R, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349(26):2483-2494. [DOI] [PubMed] [Google Scholar]
- 17.Fowler JA, Mundy GR, Lwin ST, Edwards CM. Bone marrow stromal cells create a permissive microenvironment for myeloma development: a new stromal role for Wnt inhibitor Dkk1. Cancer Res. 2012;72(9):2183-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corre J, Mahtouk K, Attal M, et al. Bone marrow mesenchymal stem cells are abnormal in multiple myeloma. Leukemia. 2007;21(5):1079-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Amico L, Mahajan S, Capietto AH, et al. Dickkopf-related protein 1 (Dkk1) regulates the accumulation and function of myeloid derived suppressor cells in cancer. J Exp Med. 2016;213(5):827-840. [DOI] [PMC free article] [PubMed] [Google Scholar]