Abstract
Changes in the intracellular concentration of calcium ([Ca2+]i) represent a vital signaling mechanism enabling communication between and among cells as well as with the environment. Cells have developed a sophisticated set of molecules, “the Ca2+ toolkit,” to adapt [Ca2+]i changes to specific cellular functions. Mammalian oocytes and eggs, the subject of this review, are not an exception, and in fact the initiation of embryo devolvement in all species is entirely dependent on distinct [Ca2+]i responses. Here, we review the components of the Ca2+ toolkit present in mammalian oocytes and eggs, the regulatory mechanisms that allow these cells to accumulate Ca2+ in the endoplasmic reticulum, release it, and maintain basal and stable cytoplasmic concentrations. We also discuss electrophysiological and genetic studies that have uncovered Ca2+ influx channels in oocytes and eggs, and we analyze evidence supporting the role of a sperm-specific phospholipase C isoform as the trigger of Ca2+ oscillations during mammalian fertilization including its implication in fertility.
An increase in the intracellular concentration of calcium ([Ca2+]i) underlies the initiation, progression, and/or completion of a wide variety of cellular processes, including fertilization, muscle contraction, secretion, cell division, and apoptosis (Berridge et al. 2000, 2003; Clapham 2007). For Ca2+ to perform these duties, cells create and maintain ionic gradients between extracellular and intracellular milieus and within the cellular content, thereby rendering this divalent ion into a signaling messenger. Cells have at their disposal a myriad of proteins with different affinities to bind Ca2+, which allows them to interpret and transform these elevations into cellular functions. This review will examine how mammalian oocytes and eggs, namely mouse gametes, which are the most widely studied, have adopted and adapted a variety of molecules involved in establishing cellular Ca2+ homeostasis, the “Ca2+ toolkit,” to serve their precise physiological functions. These include the processes of maturation, fertilization, and embryo development until the birth of an offspring.
In the egg, the activating Ca2+ signal that initiates development has been broadly studied in many mammalian and nonmammalian species (Miyazaki 2006; Whitaker 2006; Horner and Wolfner 2008), as their large size and precise cell-cycle stage made them an ideal model to learn about the cellular Ca2+ homeostasis machinery when the pharmacological and imaging tools were less sophisticated (Ridgway et al. 1977). Nevertheless, changes in Ca2+ homeostasis also occur during maturation and are of pivotal importance for the success of fertilization (Jones et al. 1995; Deguchi et al. 2005; Wakai et al. 2012). These mechanisms have not been well studied, but the recent discoveries in this area will be reviewed here in detail. Similarly, the intracellular Ca2+ release channel(s) that underpin most of the Ca2+ release during oocyte maturation and fertilization have been broadly investigated and characterized. Conversely, the plasma membrane (PM) channels responsible for the Ca2+ influx that is required to support the oscillations and filling of the internal stores in these cells have not been as carefully examined. Recent electrophysiological studies identified the first channels in these cells, and their contribution to Ca2+ homeostasis will be covered here. Last, we will discuss evidence supporting the role of phospholipase C ζ (PLCζ), the sperm-specific PLC isoform (Saunders et al. 2002), as the responsible factor for the majority of Ca2+ release at fertilization, and the recent insights gained from genetics and mutational studies.
THE Ca2+ TOOLKIT IN MAMMALIAN OOCYTES/EGGS
Changes in the concentrations of Ca2+ represent one of the oldest and more widespread modes of cellular communication in life, from bacteria to animals. The relentless pace of evolution that resulted in the appearance of eukaryotes, and subsequently, in the emergence of complex multicellular organisms has caused molecular adaptations and the development of diverse and complex Ca2+-signaling systems (Cai and Clapham 2012). This expanded capacity made possible for Ca2+ signaling to operate within different cellular compartments, between cells, and between cells and their extracellular environment (Clapham 2007). To accomplish this, the concentration of free Ca2+ must be parsed between the subcellular compartments and the surrounding environment. In most cell types, the basal cytoplasmic [Ca2+]i under resting conditions is 100 nm, which is 104 times lower than the extracellular Ca2+ concentration ([Ca2+]o) of ∼1 mm (Clapham 2007; Bagur and Hajnoczky 2017). Within the cell, the endoplasmic reticulum (ER), which is the main Ca2+ reservoir of nonexcitable cells, ([Ca2+]ER), accumulates Ca2+, reaching luminal Ca2+ concentrations >100 µm. [Ca2+]ER is maintained via the inositol 1,4,5-trisphosphate receptor (IP3R), which is the main intracellular Ca2+-release channel of almost all mammalian cell types and is located in the ER (for review, see Berridge et al. 2000; Bootman et al. 2001; Berridge 2009). Other organelles, such as mitochondria and the Golgi apparatus, also sequester Ca2+ and are involved in cellular homeostasis and Ca2+ signaling (Bagur and Hajnoczky 2017), as is discussed in detail below.
To create these concentration gradients between organelles and the cytoplasm and between the cell and the extracellular medium, cells possess a combination of pumps, channels, exchangers, and buffering mechanisms in the PM and membranes of intracellular organelles known as the Ca2+ toolkit, which makes possible the generation of oscillatory [Ca2+]i transients with precise spatiotemporal organization (Berridge et al. 2003; Cai and Clapham 2012). These key Ca2+-signaling molecules make Ca2+ entry into the cell possible mostly by various types of channels. Ca2+ release from intracellular organelles into the cytoplasm is mainly mediated by ion channels, whereas the clearing of the cytosolic Ca2+ increases is mainly mediated by adenosine triphosphatase (ATPase) transporters and ion exchangers (Fig. 1; described in further detail below). Further, each cell type presents a particular combination of the Ca2+ toolkit components to create a cell type– and stimulus-specific Ca2+ signal that suits their physiological requirements (Table 1; Cai and Clapham 2012). The fertilization-associated Ca2+ oscillations, which vary among species in duration, amplitude, number of rises, and the stage of meiosis at which they occur are notable examples of this notion (Stricker 1999; Miyazaki 2006). In mammals, the responses to fertilization are known as Ca2+ oscillations, as they unfold in a pattern of brief, periodic rises that last for several hours after sperm entry (Miyazaki 2006). The spatiotemporal complexity of the Ca2+ oscillations that unfold in mature, metaphase II–arrested (MII-arrested) oocytes, henceforth referred to as eggs, is in part the consequence that Ca2+ from two sources, extracellular media and intracellular stores, must be integrated to produce stereotypical responses. Importantly, the molecules and regulatory mechanisms that underlie the Ca2+ oscillations and homeostasis in these cells are largely unknown.
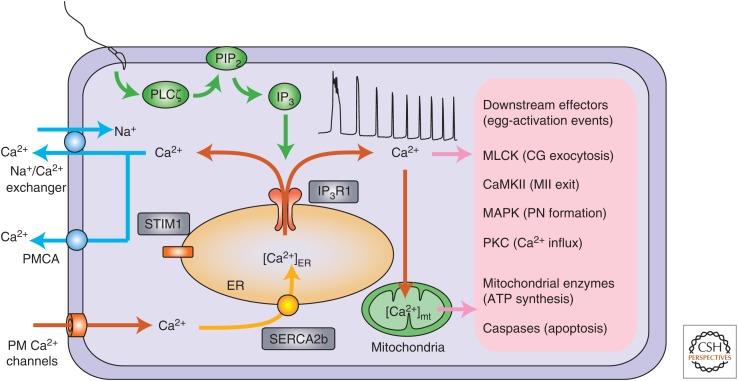
Figure 1.
Ca2+-signaling toolkit in mammalian oocytes/eggs. At fertilization, the sperm delivers phospholipase C ζ (PLCζ), which hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3) (green). IP3 binds its receptor, IP3R1, causing Ca2+ release out of the endoplasmic reticulum (ER). In mammals, the Ca2+ signal unfolds in brief but periodic rises termed Ca2+ oscillations (red). The spatiotemporal information provided by the pattern of these Ca2+ responses is decoded by downstream effectors that underpin the distinct cellular events of egg activation (pink), which include cortical granule (CG) exocytosis, exit from metaphase II (MII) arrest, and pronuclear (PN) formation. Part of the cytosolic Ca2+ is transduced into the mitochondria where it stimulates mitochondrial enzyme, resulting in adenosine triphosphatase (ATP) synthesis, whereas mitochondrial Ca2+ ([Ca2+]mt) overload results in a loss of mitochondrial membrane potential, release of cytochrome c, and activation of caspases causing apoptosis. Following Ca2+ release, elevated Ca2+ levels can be returned to baseline by the actions of plasma membrane (PM) Ca2+ ATPases (PMCAs) and/or Na+/Ca2+ exchanger (NCX), which extrudes Ca2+ into the external milieu (blue). The sarco-ER Ca2+ATPases (SERCAs) reuptake Ca2+ into the ER stores in anticipation of the next Ca2+ rise (yellow). Nevertheless, to consistently replenish ER Ca2+ ([Ca2+]ER) stores and maintain oscillations, extracellular Ca2+ must enter into the egg/oocyte across the PM by a variety of channels and mechanisms (Fig. 2). One proposed Ca2+ influx mechanism is the store-operated Ca2+ entry (SOCE) mechanism. SOCE consists of a two-component system whereby a PM protein, ORAI1, is recruited upon store depletion into clusters with ER-associated, stromal interaction molecule 1 (STIM1), which aggregates toward the PM upon sensing low [Ca2+]ER stores. SOCE seems redundant in mouse oocytes and eggs, but it might be more important in other mammalian species. In mice, other channels are the main carriers of Ca2+ from the external milieu. MLCK, Myosin light chain kinase; CaMKII, Ca2+/calmodulin-dependent protein kinase II; MAPK, mitogen-activated protein kinase; PKC, protein kinase C.
Table 1.
Components of the Ca2+ tool kit in mouse oocytes/eggs
| Mechanism | Protein | Expression | Phenotypic changes caused by molecular inhibition | References |
|---|---|---|---|---|
| Ca2+ release | PLCZl | Sperm | KO male mice were subfertile and Ca2+ oscillations after fertilization were disturbed; ICSI with KO sperm did not trigger oscillations | Saunders et al. 2002; Hachem et al. 2017; Nozawa et al. 2018 |
| IP3R1 | Oocytes/eggs | Functional blocking antibody prevents Ca2+ oscillations and egg activation | Miyazaki et al. 1992; Parrington et al. 1998; Jellerette et al. 2000 | |
| Ca2+ influx | Cav3.2 | Oocytes > eggs | KO mice showed normal Ca2+ oscillations but mild decrease of ER Ca2+ store content in eggs | Bernhardt et al. 2015; Carvacho et al. 2018 |
| TRPV3 | Eggs > oocytes | KO mice failed to Sr2+-induce egg activation | Carvacho et al. 2013 | |
| TRPM7-like | Oocytes and lesser in eggs | Pharmacological inhibition prevents spontaneous Ca2+ oscillations in GV oocytes; cKO eggs showed reduced oscillations after fertilization | Carvacho et al. 2016; Bernhardt et al. 2018 | |
| ORAI1 | Oocytes/eggs | KO mice showed normal fertilization-induced oscillations and fertility | Cheon et al. 2013; Bernhardt et al. 2017 | |
| STIMl/2 | Oocytes/eggs | KO mice showed normal fertilization-induced oscillations and fertility | Cheon et al. 2013; Bernhardt et al. 2017 | |
| Ca2+ pumps | SERCA2b | Oocytes/eggs | Pharmacological inhibition prevents/prematurely terminates Ca2+ oscillations in eggs | Kline and Kline 1992; Lawrence and Cuthbertson 1995; Wakai et al. 2013 |
| PMCAs | Unknown | Pharmacological inhibition enhances Ca2+ oscillations in eggs | Miao et al. 2012; Wakai et al. 2013 | |
| Ca2+ buffering | NCX | Unknown | Pharmacological inhibition enhances Ca2+ oscillations in oocytes/eggs | Carroll 2000 |
| MCU | Unknown | Unknown | ||
| VDAC | Unknown | Unknown |
PLCZl, phospholipase Cζl; KO, knockout; ICSI, intra-cytoplasmic sperm injection; ER, endoplasmic reticulum; IP3R1, inositol 1,4,5-trisphosphate receptor; TRPV3, transient receptor potential (TRP) vanilloid, member 3; TRPM7, TRP melastatin 7; GV, germinal vesicle; cKO, conditional knockout; STIM1/2, stromal interaction molecule 1/2; SERCA2b, sarco-endoplasmic reticulum Ca2+-ATPases; NCX, Na+/Ca2+ exchanger; PMCA, plasma membrane Ca2+-ATPases; MCU, mitochondrial Ca2+ uniporter; VDAC, voltage-dependent anion-selective channel.
Before ovulation, during maturation, oocytes undergo nuclear and cytoplasmic modifications in preparation for fertilization. Immature oocytes resume meiosis and transition from the germinal vesicle (GV) stage to the MII stage. Remarkably, the precise machinery required to develop the characteristic spatiotemporal pattern of the sperm-induced Ca2+ oscillations at the MII stage is acquired during maturation. In fact, in vitro fertilized GV oocytes show fewer [Ca2+]i oscillations and these oscillations show a shorter duration and amplitude than those observed in fertilized MII eggs (Carroll and Swann 1992; Wakai et al. 2012). Despite this knowledge, the mechanisms underlying the enhanced Ca2+-releasing ability of MII eggs versus GV oocytes are not well understood, although multiple parameters (e.g., IP3R1 posttranslational modifications, Ca2+ store content, and ER reorganization) are believed to be involved in this process (Ajduk et al. 2008). Spontaneous oscillations cease within a few hours after resumption of meiosis and the content of the [Ca2+]ER steadily increases until the MII stage, suggesting that the molecules responsible for these adjustments in Ca2+ homeostasis experience similar dynamic modifications. It is therefore possible that several features of Ca2+ signaling in GV oocytes change during maturation so that some of the Ca2+ mechanisms/channels active at the GV stage may not be so at the MII stage and vice versa. Moreover, these distinct and changing features of Ca2+ homeostasis during maturation are likely to underpin the acquisition of both maturation and developmental competence as well as fulfill stage-specific cellular functions.
THE MECHANISM OF INOSITOL 1,4,5-TRIPHOSPHATE RECEPTOR (IP3R)-MEDIATED Ca2+ RELEASE
The IP3R is a large protein (>250 kDa) that functions as a tetramer (>1000 kDa). There are three IP3R isoforms, denoted IP3R1–3, each with similar, albeit slightly distinctive, properties. Each monomer broadly contains three functional regions, a cytosolic amino-terminal domain that binds IP3, a regulatory domain with multiple regulatory sites for Ca2+, ATP, and other modulatory molecules/proteins, and a carboxy-terminal channel domain that contains six transmembrane domains and a short cytosolic tail. The activation and opening of the IP3R requires binding by both Ca2+ and IP3, and the regulation of IP3-induced Ca2+ release (IICR) by Ca2+ adopts a bell-shaped form, as IICR is stimulated at low [Ca2+]i and inhibited at high [Ca2+]i (Iino 1990). This dual regulation of IP3R by Ca2+ and IP3 makes it especially suited to support long-lasting oscillations.
Mammalian oocytes and eggs and their surrounding cells express all three IP3R isoforms (Fissore et al. 1999), although oocytes and eggs overwhelmingly express the IP3R1 isoform (Fig. 1; Parrington et al. 1998; Jellerette et al. 2000). The initial suggestion that IP3R may play a role during fertilization arose from studies in sea urchin eggs that showed that phosphoinositide metabolism via hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into IP3 increases after fertilization (Turner et al. 1984), an observation that was soon followed by the demonstration that injection of IP3 triggered Ca2+ release (Clapper and Lee 1985). Similar studies in hamster oocytes (Miyazaki 1988) further defined the realization of the role of IP3R in mammalian fertilization, which was later firmly established with the demonstration that injection of a functional blocking antibody prevented both the initiation of Ca2+ oscillations and egg activation (Miyazaki et al. 1992; Xu et al. 1994). Subsequent studies confirmed the role of IP3R1 in fertilization in other species (for review, see Parys et al. 1994; Thomas et al. 1998; Yoshida et al. 1998; Runft et al. 1999; Goud et al. 2002; Iwasaki et al. 2002; Wakai et al. 2011).
Several modifications of IP3R1 may explain some of the idiosyncratic changes of the Ca2+ signaling in mammalian oocytes and eggs. The fertilization-associated Ca2+ oscillations in mouse zygotes become less frequent as they transition from the MII stage into interphase before ceasing altogether at the time of pronuclear (PN) formation (Kono et al. 1996; Deguchi et al. 2000). During this transition, ∼50% of the IP3R1 mass is lost through ligand-induced degradation (Parrington et al. 1998; Jellerette et al. 2000). Likewise, the organization of IP3R1s is affected as the ER changes from a predominantly cortical and clustered organization at the MII stage, which is believed to enhance IP3R1's sensitivity (Ullah et al. 2014), to a more homogenous distribution that modifies the channel's properties (FitzHarris et al. 2003). Phosphorylation also affects IP3R1 function in eggs. Cell-cycle kinases play a prominent role in the MII arrest and phosphorylate IP3R1 (Jellerette et al. 2004). IP3R1 phosphorylation was first detected in mouse oocytes using an anti-MPM-2 antibody, which recognizes a variety of proteins that are phosphorylated during mitosis, and when in mouse oocytes during maturation, showed maximal reactivity at the MII stage followed by a gradual and persistent decline after egg activation (Lee et al. 2006). The MPM-2 epitope (peptides containing leucine-threonine-proline-leucine-lysine [LTPLK] and phenylalanine-threonine-proline-leucine-glutamine [FTPLQ] domains) can be modified by several mitosis (M-phase) kinases such as polo-like kinase 1 (Plk1), mitogen-activated protein kinase (MAPK), and cyclin-dependent kinase 1 (Cdk1) (Joughin et al. 2009), although it is presently unknown whether one or several of these kinases contribute to the modification of this epitope in IP3R1 in mammalian eggs. Importantly, subsequent studies using phosphospecific antibodies showed that serine 421 (S421) and threonine 799 (T799), which are Cdk1 consensus sites, are phosphorylated in mouse eggs at the MII stage (Wakai et al. 2012). In somatic cells, for example, phosphorylation of IP3R1 at these sites, both under in vitro and in vivo conditions, enhanced IP3 binding and Ca2+ (Malathi et al. 2005). Further, in mouse eggs, expression of a heterologous IP3R1 with phosphomimetic mutations corresponding to three M-phase motifs (which should be phosphorylated by M-phase kinases in MII eggs) resulted in greater Ca2+ oscillatory activity and sensitivity to IP3 compared with expression of wild-type (WT) IP3R1 (Zhang et al. 2015). Besides M-phase kinases, studies in somatic cells have shown that IP3R isoforms can be phosphorylated by more wide-ranging kinases (Bezprozvanny 2005). The most commonly implicated kinases include protein kinase A (PKA), protein kinase C (PKC), and CaMKII, all of which have important physiological functions in oocytes and eggs (Ducibella and Fissore 2008). Extensive phosphopeptide mapping combined with substrate-specific antibodies in Xenopus laevis oocytes found that IP3R1 is uniformly phosphorylated throughout maturation in both PKA consensus motifs (Ser1589 and Ser1755 within the central cytosolic domain of IP3R1 are the only sites phosphorylated by PKA), whereas IP3R1 is not phosphorylated on PKC sites (Sun et al. 2009). In mouse oocytes, phosphorylation by PKA, but not by PKC, was shown during maturation, with maximal PKA phosphorylation occurring at the GV stage (Wakai et al. 2012), which is consistent with the high level of cyclic adenosine monophosphate (cAMP) at this stage (Norris et al. 2009). It is therefore possible that down-regulation and reorganization of IP3R1 along with modifications of its phosphorylation status all contribute to shape the Ca2+ oscillations at fertilization in mammals.
An important remaining consideration is the site of IP3 production during fertilization in mammalian oocytes and eggs. Whereas canonical PLCs find most of their substrate, PIP2, at the PM, this does not appear to be the case with PLCζ1, which is the PLC that accounts for most of the Ca2+ release in mouse fertilization (see below for specific discussion of PLCζ function and regulation). PIP2 can be found in the PM of mouse eggs using specific probes (Halet 2004), although its levels there do not appear to change during fertilization, and in fact it might actually increase (Halet et al. 2002; Halet 2004). Further, depletion of PIP2 from the PM does not seem to affect Ca2+ oscillations (Sanders et al. 2018). Recent research shows that mouse eggs appeared to also store PIP2 in small cytoplasmic vesicles distributed throughout the ooplasm (Sanders et al. 2018), and a treatment that prevents the utilization of this PIP2, either by reducing its levels and/or by disrupting its distribution, causes rapid but reversible termination of the Ca2+ oscillations (Sanders et al. 2018). Thus, it appears that during mouse fertilization, IP3 might be generated throughout the ooplasm, which may be a logical choice given the diameter of eggs and the reported speeds of the fertilization-induced Ca2+ oscillations in these species. The typical speed of the fertilization Ca2+ wave is >50 µm/sec, crossing the egg in ∼1 sec, which cannot be simply explained by the diffusion coefficient of IP3 in intact cells that is only of <5 µm2/sec (Dickinson et al. 2016). Future studies should identify the nature of the vesicles that contain IP3 in mouse eggs, determine whether this strategy extends to other species, and uncover how the sperm's PLC finds the correct localization.
THE REGULATION OF [Ca2+]ER CONTENT
So far, we have discussed fertilization-induced Ca2+ oscillations in the context of IP3 production and regulation of IP3R1 function. Nevertheless, the regulation of Ca2+ oscillations in eggs is far more complex, as the components of the Ca2+ toolkit involved in Ca2+ homeostasis impart different spatial and temporal properties to the responses in oocytes and eggs. For example, for Ca2+ oscillations to continue uninterrupted and to avoid cellular toxicity following a [Ca2+]i increase, [Ca2+]i levels need to be returned to baseline and stores refilled in anticipation of the next transient. To bring [Ca2+]i to baseline, cells either return free cytosolic Ca2+ into the ER by the action of the sarco-ER Ca2+ ATPases (SERCAs), and/or extrude it by the action of PM Ca2+ ATPases (PMCAs) and Na+/Ca2+ exchangers (NCXs) (Berridge et al. 2003). The mitochondria and Golgi apparatus also contribute to shape [Ca2+]i, as mitochondrial calcium uniporter (MCU) and secretory-pathway Ca2+-transport ATPases (SPCAs) uptake Ca2+ into organelles. Three different SERCA genes (ATP2A1–3) encode three main isoforms (SERCA1–3), each of which undergoes tissue-specific splicing, further increasing the diversity of these pumps. SERCA1a and SERCA1b variants are expressed in skeletal muscle (Brini and Carafoli 2009). The SERCA2a variant is expressed in cardiac muscle, whereas the SERCA2b variant is expressed nearly ubiquitously and is thus considered the housekeeping isoform. SERCA3 is instead expressed in a limited number of nonmuscle cells. The expression of SERCA proteins in mammalian eggs was surmised by earlier reports using pharmacological inhibition of SERCA, as the exposure of eggs to thapsigargin (a specific SERCA inhibitor) prevents continuation of Ca2+ oscillations (Kline and Kline 1992; Lawrence and Cuthbertson 1995). A follow-up study revealed the presence of the SERCA2b protein (Wakai et al. 2013), and found that it was expressed throughout oocyte maturation and attained a conspicuous cortical cluster organization in mature eggs (Wakai et al. 2013). The colocalization of SERCA2b with the IP3R1 and ER cortical clusters may facilitate the initiation of robust Ca2+ oscillations at the time of fertilization.
Whether the [Ca2+]ER stores undergo changes in content simultaneously with the fertilization-initiated Ca2+ oscillations remain unknown. Recent studies, however, using fluorescence resonance energy transfer (FRET)-based Ca2+ indicators have provided evidence that indeed this is the case (Takahashi et al. 2013; Wakai and Fissore 2013). It was shown that [Ca2+]ER displays oscillatory responses such that with each Ca2+ oscillation, the [Ca2+]ER undergoes a simultaneous but opposite change in its content. Further, each Ca2+ oscillation is accompanied by a rapid decline in [Ca2+]ER levels, which suggests direct contribution of the [Ca2+]ER content to the cytosolic [Ca2+]i transient. Nevertheless, unlike the quick return to baseline that cytosolic [Ca2+]i transients experience, [Ca2+]ER level's return to basal levels is protracted, suggesting that other Ca2+ buffering systems contribute to cytosolic [Ca2+]i clearance. It is worth noting that for approximately the first three Ca2+ oscillations after sperm entry, the upstroke of the next Ca2+ oscillation occurs before [Ca2+]ER levels are fully recovered, showing that the [Ca2+]ER levels progressively decrease during this time. Thereafter, [Ca2+]ER levels seemingly reach a steady state in which each Ca2+ oscillation occurs from the same [Ca2+]ER level, which represents a partly refilled [Ca2+]ER, suggesting that [Ca2+]ER levels set the pace of the oscillations during the mid- to late-stage oscillations.
The filling status of [Ca2+]ER depends on Ca2+ influx, Ca2+ efflux, and sequestering mechanisms. Given our previous demonstration that only a fraction of Ca2+ from each Ca2+ oscillation is recycled into the [Ca2+]ER by SERCA, [Ca2+]o must be taken in to replenish [Ca2+]ER. Evidence for the need of Ca2+ influx to support Ca2+ oscillations during fertilization is long-standing, as sperm-initiated [Ca2+]i and [Ca2+]ER oscillations cease prematurely in the absence of [Ca2+]o (Kline and Kline 1994). Nevertheless, the identity of the channels responsible for Ca2+ influx in mouse and other mammalian eggs remains to be determined (see below for up-to-date detailed description of Ca2+ influx in mechanisms in these eggs). In terms of extrusion mechanisms, elevated [Ca2+]i can also be returned to baseline by the actions of PMCA and/or of the NCX, which release Ca2+ into the external milieu. There is functional evidence for both mechanisms in rodent oocytes and eggs. The functional activity of the NCX was evidenced by elimination of Na+ from the external media (i.e., to block the NCX from operating in “forward mode” and extruding Ca2+), which caused Ca2+ oscillations or accelerated existing ones, suggesting that a reverse mode of operation of the NCX (i.e., Ca2+ influx and Na+ efflux) can be induced in these cells (Carroll 2000; Machaty et al. 2002; Cui et al. 2003). Importantly, even in the absence of external Na+, [Ca2+]i returned to baseline levels (Carroll 2000), implying that the action of other mechanisms, especially PMCAs, may be physiologically more relevant. Despite these initial findings, the participation of PMCA in Ca2+ homeostasis in mammalian oocytes had not been tested. Demonstration of its functional presence was recently accomplished using high concentrations of gadolinium (Gd3+), which besides inhibiting Ca2+ channels, blocks PMCA activity, effectively preventing Ca2+ influx and efflux. This “Ca2+ insulation” method resulted in increased amplitude and duration of the sperm-initiated Ca2+ oscillations, suggesting the functional presence of PMCA(s) in mouse eggs (Miao et al. 2012). Nonetheless, the molecular identification of PMCA in mammalian oocytes/eggs has yet to be shown.
THE ROLES OF MITOCHONDRIAL Ca2+ ([Ca2+]mt) UPTAKE
Besides the ER, mitochondria also contribute to the shaping of Ca2+ oscillations during oscillations (Duchen 2000; Rizzuto et al. 2000), as they take up Ca2+ into the matrix, thereby alleviating the overall cytosolic Ca2+ load (Rizzuto et al. 2000). Despite early evidence to the contrary (Liu et al. 2001), a role as a Ca2+ reservoir does not seem to be the main function of these organelles in eggs, as inhibition of Ca2+ intake by the mitochondria does not immediately terminate the sperm-initiated oscillations (Dumollard et al. 2004). Importantly, besides contributing to Ca2+ buffering, propagation of Ca2+ signals from the ER into the mitochondria promote several events that sustain ATP levels in cells (Hajnoczky et al. 1995). Given the proximity between the ER and IP3R1s, the Ca2+-driven ATP output may be the mitochondria's most critical contribution to Ca2+ homeostasis in mammalian eggs. Simultaneous measurement of [Ca2+]i and mitochondrial Ca2+ ([Ca2+]mt) showed that Ca2+ oscillations are accompanied by parallel increases in [Ca2+]mt, which stimulates ATP output (Dumollard et al. 2008). To this end, inhibition of mitochondrial function with oligomycin reduced [Ca2+]ER levels by impairing its refilling (Wakai et al. 2013), suggesting that Ca2+-driven ATP output is required to sustain SERCA activity and the sperm-triggered Ca2+ oscillations.
The contributions of [Ca2+]mt to Ca2+ homeostasis have been known for decades, but the molecular identities of the channels and transporters responsible for the influx of Ca2+ into the mitochondria have not been identified until very recently. Cytosolic Ca2+ reaches the mitochondrial matrix by permeating the outer mitochondrial membrane through the voltage-dependent anion-selective channel (VDAC) (Blachly-Dyson et al. 1993) and the inner mitochondrial membrane (IMM) via the Ca2+ selective MCU (Baughman et al. 2011; De Stefani et al. 2011). The close association between the mitochondria and ER at contact sites (MERCS; also known as mitochondria-associated ER membranes [MAMs]) creates localized sites of high Ca2+ concentration that favor its influx into the mitochondrial matrix. Despite the pivotal role that Ca2+ oscillations play in mitochondrial ATP production and cellular homeostasis in mammalian oocytes/eggs, no studies have examined the function and regulation of the underlying molecules that mediate its influx into this organelle in oocytes and eggs. Future studies should identify their regulation and function using genetic approaches, because thus far conventional and nonspecific inhibitors of [Ca2+]mt intake such as ruthenium red appear to be ineffective in eggs (Dumollard et al. 2008).
Ca2+ INFLUX THROUGH THE PUTATIVE PLASMA MEMBRANE (PM) Ca2+ CHANNELS
The relationship between Ca2+ influx and spontaneous oscillations was first noted in the legend to Figure 2. Nevertheless, the molecules that mediate Ca2+ influx, and their regulation, remain poorly characterized. During maturation, the [Ca2+]ER stores in mouse oocytes must be filled in preparation for fertilization (Jones et al. 1995; Wakai et al. 2012), then to sustain Ca2+ oscillations after fertilization, the [Ca2+]ER stores need to be refilled between oscillations in anticipation of the next Ca2+ rise. These processes share dependency on sequestration and extrusion mechanisms as well as on the influx of external Ca2+, the latter of which is accomplished by Ca2+ channels and exchangers. For instance, store-operated Ca2+ entry (SOCE) is one of these proposed mechanisms that is activated by the depletion of [Ca2+]ER (Putney 2011). Eggs and somatic cells alike use several mechanisms to accomplish Ca2+ influx including receptor-operated channels (ROCs), voltage-gated channels (VGCs), as well as other PM channels such as ORAI 1–3, which is the channel component of SOCE (Bernhardt et al. 2017; Putney 2017), and the transient receptor potential (TRP) family of channels, which includes six subfamilies and nearly 30 human members that are expressed in multiple cell types and tissues (Wortzman-Show et al. 2007).
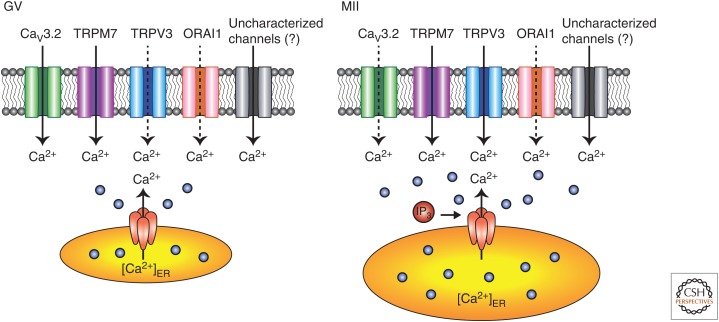
Figure 2.
Differential expression of plasma membrane (PM) Ca2+ channels in mammalian oocytes/eggs. Ca2+ influx plays a pivotal role in both spontaneous Ca2+ oscillations in germinal vesicle (GV) oocytes (left) and fertilization-induced oscillations in MII eggs (right). During oocyte maturation, the putative active channel(s) are progressively inactivated, which causes down-regulation of Ca2+ influx and termination of spontaneous Ca2+ oscillations by the GV breakdown stage, thereby avoiding parthenogenesis at the MII stage. A high [Ca2+]ER in MII eggs is important for robust IP3-induced Ca2+ release at the time of fertilization. Note that a variety of Ca2+ channels have been functionally detected in the PM of mouse oocytes, including the low-voltage-activated (LVA) T-type calcium channel 3.2 (Cav3.2 T-type), transient receptor potential (TRP) family members vanilloid member 3 (TRPV3), and TRP melastatin 7 (TRPM7) as well as ORAI1 channels. These channels are proposed to mediate the majority of the influx, although the PM channels that mediate Ca2+ influx during oocyte maturation and fertilization remain to be fully identified, and may be different between these two stages. In mouse GV oocytes, the persistently low [Ca2+]ER levels serve as the natural trigger for Ca2+ influx, which appears mostly operated through Cav3.2 channels. All the other channels are likely also to mediate additional influx, although of lesser magnitude. The only channel whose functional expression clearly increases during maturation in mouse oocytes is TRPV3, as it is nearly absent at the GV stage with its maximal expression being at the MII stage. TRPM7 also seems to change during maturation, as there is higher current density in GV oocytes than in MII eggs. However, fertilization reactivates influx, although how this is accomplished is presently unknown. Thick complete arrows, active influx; broken arrows, reduced expression and/or function.
VGCs are classified according to the voltage needed to activate them: high-voltage activated (HVA) and low-voltage activated (LVA). The mouse egg predominantly expresses an LVA VGC known as CaV3.2 (T-type) (Peres 1986; Kang et al. 2007; Bernhardt et al. 2015). Evidence suggests that the functional expression of these channels changes during maturation, as mouse GV oocytes display larger CaV3.2-mediated currents than MII eggs (Bernhardt et al. 2015; Carvacho et al. 2018), although the nature of these changes and mode of regulation remains to be well established. It is apparent, however, that mice null for the CaV3.2 gene, Cacna1h, only show mild subfertility and sperm-induced oscillations are largely unaffected (Bernhardt et al. 2015). Thus, the function of CaV3.2, at least in mouse oocytes and eggs, is believed to support Ca2+ influx during the GV stage and to prepare oocytes for fertilization, as after it, the current magnitude and functional properties vary (Yamashita 1982; Kang et al. 2007; Bernhardt et al. 2015). Besides, mouse oocytes/eggs are largely nonexcitable cells, and in contrast to invertebrate species, experience only a small change in membrane potential during fertilization (Igusa et al. 1983; Jaffe and Cross 1984). Thus, it remains to be determined why these VGCs are functionally expressed in mammalian oocytes and eggs. It is possible that their function as “window currents”—operating in a window of voltage in which activation and inactivation overlap, and that allows a sustained but low flux of Ca2+ into the cell (Rossier 2016)—is their most important contribution, especially in steady-state situations such as GV-stage oocytes of growing follicles (Bernhardt et al. 2015).
There is growing evidence for TRP channel expression in mouse oocytes and eggs and of their importance in the regulation of Ca2+ homeostasis in these cells. The first TRP channel to be reported in eggs was TRP vanilloid, member 3 (TRPV3) (Carvacho et al. 2013), although its expression is predominant in skin cells. TRP channels are modulated by a myriad of stimuli including chemical, mechanical, and temperature. Besides Ca2+, TRPV3 permeates other divalent cations such as strontium (Sr2+), which is essential for triggering parthenogenetic embryonic development (Carvacho et al. 2013). Remarkably, TRPV3 is differentially expressed during maturation, as it is nearly absent at the beginning of maturation with its maximal expression being at the MII stage, although it is not required for normal fertility either, as null females are fertile (Chen et al. 2003; Carvacho et al. 2013). The role of TRPV3 needs reexamination as well, as single knockout (KO) Trpv3−/− eggs do not show changes in oscillation frequency postfertilization, whereas elimination of both TRPV3 and CaV3.2 channels has devastating effects on the eggs’ Ca2+ store content and oscillations postfertilization (A Mehregan, G Ardestani, I Carvacho, et al., unpubl.).
The reason for the differential expression of CaV3.2 and TRPV3 channels in mouse oocytes and eggs also requires further investigation, but their simultaneous deletion as noted greatly impacts Ca2+ homeostasis in oocytes and eggs, and compromises the ability to initiate regularly spaced, frequent Ca2+ transients after fertilization. Females of this double KO group displayed subfertility, but were fertile nonetheless, suggesting that these two channels could potentially have redundant, compensatory functions that sustain normal oscillations in the loss of a single channel (A Mehregan, G Ardestani, I Carvacho, et al., unpubl.). It is also plausible that there are undetermined endogenous modulators of TRPV3 and CaV3.2 that are present in oocytes and eggs, but how their activity is regulated requires further investigation.
Another TRP channel member, melastatin 7 (TRPM7), was shown to be required for early embryonic development, as its global disruption resulted in embryonic lethality before day 7 (Jin et al. 2008). TRPM7 is a bifunctional protein known as a chanzyme with both ion channel and serine/threonine kinase activities, which can be modulated by Mg2+, pH, and PIP2 (Wu et al. 2010; Fleig and Chubanov 2014). A recent report has shown the functional expression of TRPM7-like channels in mouse oocytes and eggs (Carvacho et al. 2016), and like CaV3.2 and TRPV3, the functional expression of TRPM7 seems to change during maturation, as there is higher current density in GV oocytes than in MII eggs. Further, its functional expression is reactivated after fertilization, as noted by increased current in two-cell zygotes, which is consistent with its reported requirement during early embryogenesis and the survival of pluripotent stem cells alike; it is, however, not required after embryonic day 14 or for the survival of many differentiated cells (Jin et al. 2008).
The modulation of TRPM7 permeability and function by intracellular and extracellular Mg2+ confirmed its role in the regulation of physiological Mg2+ homeostasis. Disruption of such a function can lead to developmental problems such as impaired gastrulation in Xenopus, hypomagnesemia, and birth defects such as spina bifida in humans (Schmitz et al. 2003; Ryazanova et al. 2010; Liu et al. 2011). In mammalian oocytes and eggs, TRPM7 may also be mediating Mg2+ homeostasis, as it has recently been shown that fertilization-induced embryo development in several species is increased in media with lower concentrations of Mg2+ (Herrick et al. 2015), and that sperm-initiated Ca2+ oscillations were increased when measurements were performed in the presence of low levels of extracellular Mg2+ (Ozil et al. 2017).
Interestingly, the Mg2+-sensitive properties of TRPM7 are what differentiated it from Ca2+ release-activated current ([CRAC], the ion current that underlies SOCE). Initial studies in Xenopus oocytes revealed the molecular mechanism of SOCE in these cells, which manifests around the GV breakdown (GVBD) phase by the uncoupling of Ca2+ store depletion and influx (Machaca and Haun 2002). SOCE consists of a two-component system whereby a PM protein, ORAI1 (the Ca2+ influx channel), is recruited upon [Ca2+]ER store depletion into clusters with ER-associated protein, STIM1, which aggregates toward the PM upon sensing low [Ca2+]ER (Cheon et al. 2013; Putney et al. 2017). Evidence for SOCE in mammalian oocytes was first observed after the application of thapsigargin in Ca2+-free media (to empty the [Ca2+]ER store and thereby activate SOCE), causing a large influx of Ca2+ after adding Ca2+ back to the media (Kline and Kline 1992; Machaty et al. 2002). Coexpression of human STIM1 and ORAI1 induces a persistent increase in basal [Ca2+]i only in GV oocytes, but enhances Ca2+ influx at all stages of maturation (Cheon et al. 2013). Nevertheless, the magnitude of the Ca2+ influx decreases as maturation progresses, suggesting a dispensable role for this mechanism at the fertilization stage and/or that it is reactivated by fertilization. Recent studies appear to support the first possibility, as oocyte-specific conditional knockout (cKO) mice for Stim1 and Stim2, or Stim1/2 double cKO mice as well as Orai1-null mice showed normal fertilization-induced oscillations and fertility (Bernhardt et al. 2017). The role of SOCE may be more prominent in porcine oocytes, as knockdown of STIM1 and ORAI1 significantly disturbed sperm-induced Ca2+ oscillations in these species (Lee et al. 2012; Zhang et al. 2018). Whether these results extend to other large mammalian species is not known, as it is also unknown how inactivation of SOCE affects fertility in these species.
Alternatively, TRPM7 could be mediating a SOCE-like Ca2+ influx in mammalian oocytes. It was shown that TRPM7 can be a mediator of SOCE-like Ca2+ influx in somatic cells, such as in macrophages, which use TRPM7-mediated Ca2+ influx to support downstream-signaling pathways independent of STIM and ORAI proteins (Schappe et al. 2018). A similar pattern may be occurring in oocytes and eggs, as oocytes and eggs of cKO Trpm7 mice displayed greatly reduced Ca2+ influx induced by emptying the internal stores (Bernhardt et al. 2018). Nevertheless, these eggs were able to mount persistent Ca2+ oscillations, and the females were only mildly subfertile (Bernhardt et al. 2018). A stronger Ca2+ oscillation phenotype was observed following deletion of Trpm7 and Cacna1h channels, as the sperm-induced oscillations were of shorter duration and displayed an abnormal pattern (Bernhardt et al. 2018), suggesting that the oscillations of fertilization are supported by multiple channels.
Aside from these channels, there are other players mediating Ca2+ influx, although to a lesser extent, in mouse oocytes and eggs. Ca2+-activated chloride channels (CACCs) are activated in response to sperm-induced Ca2+ release, which causes the channel to conduct Cl– out of the cell, thereby inducing membrane depolarization to effectively block polyspermy (Cross and Elinson 1980; Jaffe et al. 1983; Wozniak et al. 2018). Although this is the predominant mechanism of polyspermy prevention at fertilization in Xenopus eggs (Wozniak et al. 2018), a CACC was also observed in two-cell-stage mouse embryos, in which exposure to a selective CACC inhibitor, niflumic acid (NFA), blocked anion-driven outward currents and impaired development (Li et al. 2007, 2009).
Ca2+-activated potassium (K(Ca)) channels have been reported in hamster eggs and human eggs. Fertilization and activation in hamster eggs is driven by large, hyperpolarizing currents that have been implicated with the activity of K(Ca) channels (Miyazaki and Igusa 1981, 1982), and were abolished by addition of the Ca2+ chelator, egtazic acid (EGTA) (Georgiou et al. 1983; Igusa and Miyazaki 1983). Similar to the hamster, in unfertilized human eggs, the bell-shaped current after application of Ca2+ ionophore, A23187, was blocked by a selective inhibitor of big conductance (BK) K(Ca) channels, iberiotoxin (Homa and Swann 1994). However, the mechanism in mouse eggs remains different from the hamster and human, whereby a voltage-gated K+ channel (KV) that is not mediated by cytosolic Ca2+ and whose activity is dependent on the cell-cycle stage of the oocyte, was reported (Day et al. 1993). The contributions of these channels to fertilization and early development are not well characterized.
In conclusion, the identification of ion channels in mammalian oocytes remains a technical challenge. There are few commercially available antibodies and there is a lack of specific pharmacological agents. Thus, we must look to the generation of KO models to study the effect of functional loss of a channel. Although current reports indicate that these KO models can elicit a change in the behavior of expression of the remaining channels, in which eggs may consequently develop a compensatory mechanism to maintain such a crucial process as Ca2+ influx. Thus, only the simultaneous development of electrophysiological methods, genetic models, and pharmacological reagents will allow for the initial discovery and subsequently, the manipulation of all ion channels in mammalian oocytes and eggs.
DISTINCT REGULATION OF Ca2+ HOMEOSTASIS DURING OOCYTE MATURATION
A peculiar aspect of the Ca2+ stores of GV-stage oocytes is that they fail to accumulate Ca2+, despite the presence of spontaneous Ca2+ oscillations and persistent Ca2+ influx (Carroll and Swann 1992; Carroll et al. 1994). In contrast to the sperm-induced Ca2+ oscillations in MII eggs, the spontaneous, repetitive Ca2+ rises in GV oocytes are of small amplitude and occur every 1–3 min. These Ca2+ oscillations appear to be stimulus-independent and are constitutive, although the mechanisms that underpin them and their functions remain poorly investigated. It is noteworthy that these spontaneous Ca2+ oscillations persist for a few hours and cease around the time of GVBD, which is when oocytes simultaneously undergo the most drastic increase in [Ca2+]ER store content and experience a sharp down-regulation in Ca2+ influx (Cheon et al. 2013; Wakai and Fissore 2013). The temporal coincidence of these phenomena implicates [Ca2+]ER-associated mechanisms in the occurrence of spontaneous Ca2+ oscillations. A later study uncovered a unique regulatory mechanism of [Ca2+]ER in GV oocytes, which is reversed in MII eggs; namely, in GV oocytes, [Ca2+]ER levels are maintained persistently low by a constitutive Ca2+ “leak” through IP3R1s that is manifested in the form of spontaneous Ca2+ oscillations (Wakai and Fissore 2019). These Ca2+ oscillations are actuated by nearly constitutive Ca2+ influx and its down-regulation and inactivation of the [Ca2+]ER leak, which together terminate the oscillations, and appear to be linked to the progressive increase in the content of the [Ca2+]ER that occurs during oocyte maturation.
The Ca2+ oscillations of mouse GV oocytes may be important for cellular homeostasis. The mitochondria are a common downstream target of Ca2+ increases and [Ca2+]mt homeostasis is important for the regulation of mitochondrial enzymes associated with the production of oxidizable substrates. Basal uptake of Ca2+ by the mitochondria in resting cells is important to maintain the reduced form of nicotinamide adenine dinucleotide (NADH) production to support oxidative phosphorylation (Cardenas et al. 2010). As noted above, after fertilization, the function of the mitochondria is indispensable for Ca2+ oscillations because it supplies the ATP necessary for the Ca2+ pumps required to maintain [Ca2+]ER levels provably through SERCA activity. Remarkably, the spontaneous Ca2+ oscillations in GV oocytes and their counterparts in the mitochondria have a seemingly similar function in these cells. During the spontaneous Ca2+ oscillations in GV oocytes part of the cytosolic Ca2+ signal is transduced into the mitochondria where it stimulates mitochondrial metabolism, thereby increasing the levels of ATP (Wakai and Fissore 2019). Future studies should ascertain whether and how enhanced ATP content affects oocyte maturation and developmental competence.
The [Ca2+]ER store content increases markedly during maturation (Jones et al. 1995; Wakai et al. 2012). This increase in [Ca2+]ER contributes to Ca2+ oscillations in MII eggs, as established using cyclopiazonic acid (CPA), a reversible SERCA inhibitor. CPA-treated GV oocytes advanced to the MII stage without delay or gross abnormalities, although without increase in [Ca2+]ER (Wakai et al. 2012). In this condition, CPA-matured eggs injected with PLCζ complementary RNA (cRNA) showed a shortened first Ca2+ oscillation even though CPA was washed away before initiating the oscillations. These results suggest that the increase in [Ca2+]ER directly impacts fertilization-initiated Ca2+ oscillations, especially the robust first Ca2+ oscillation (Wakai et al. 2012). How the shape, amplitude, and number of Ca2+ oscillations postfertilization impacts embryo development remains to be firmly established.
[Ca2+]i Oscillations Postfertilization: The Role and Function of PLCζ
How the sperm initiates the signal responsible for egg activation has interested researchers for >100 years. There have been comprehensive reviews on this topic in the last few years (Machaty 2016; Swann and Lai 2016; Parrington et al. 2019) and here we will only highlight the most salient points, recent advances, and unanswered questions. Suffice it to say that the impossibility to replicate the pattern of fertilization-induced Ca2+ oscillations in mammals following experimentation with the growing list of proposed hypotheses, resulted in the re-emergence in the late 1980s of the sperm factor (SF) idea originally articulated by Jacques Loeb in the early 1900s (Loeb 1913). This theory proposed that the sperm contains a soluble factor that, upon gamete fusion, enters the egg triggering the initiation of development. Subsequent studies showed that the trigger for development is a [Ca2+]i increase, which largely originates from the intracellular Ca2+ stores (for review, see Stricker 1999). The first evidence supporting this notion in mammals was provided by Karl Swann, who injected SF extracts into eggs, which induced Ca2+ oscillations similar to those of fertilization (Swann 1990), and later studies showed that the extracts induced all the events of egg activation (Stice and Robl 1990). The physiological significance of this concept was strengthened when it was realized that egg activation could be induced by injection of a sperm into the ooplasm without interaction of the gametes’ PMs (Uehara and Yanagimachi 1976; Yanagida et al. 1991). The intra-cytoplasmic sperm injection (ICSI) method, as it became known, was later perfected for application in humans and represents an essential tool to treat human male infertility (Palermo et al. 1992). Studies showed that ICSI induces Ca2+ oscillations similar to those initiated by in vitro fertilization (IVF), albeit with slight differences in the initiation of oscillations and in the propagation of the first rise (Tesarik et al. 1994; Nakano et al. 1997). Collectively, these results advanced the idea that a soluble SF carried in by the sperm caused egg activation. Despite this evidence, the molecular identity of the SF remained unclear for many years, including several misidentifications (Parrington et al. 1996; Sette et al. 1997; Wolosker et al. 1998; Wolny et al. 1999).
Logically, because PLCs are directly responsible for IP3 production, they were always potential SF candidates. It was a study of the Ca2+-releasing properties of mammalian sperm extracts using the cell-free sea urchin egg homogenate as the readout that first suggested that the SF might be a PLC (Jones et al. 1998). This was followed by a demonstration that the ability of mammalian SF fractions to trigger [Ca2+]i oscillations after chromatographic fractionation coincided with a PLC activity (Parrington et al. 1999). Multiple PLC isoforms are expressed in sperm (Mehlmann et al. 1998; Fukami et al. 2001; Parrington et al. 2002), although more in-depth in vitro PLC assays revealed that the sperm extracts’ PLC activity was highly sensitive to Ca2+ (Rice et al. 2000). A subsequent genomic search for testis/sperm-specific PLCs led to the discovery of a novel sperm-specific PLC, PLCζ, which was first reported in the mouse (Saunders et al. 2002) and then in other mammals (Cox et al. 2002). PLCζ was shown to be ∼100-fold more sensitive to Ca2+ compared with PLCδ and displays a half-maximal PLC activity at a [Ca2+]i concentration around the reported resting levels in mammalian eggs (Kouchi et al. 2004, 2005; Nomikos et al. 2005). Other distinct features of PLCζ were that it showed sperm-specific expression, injection of its messenger RNA (mRNA) triggered fertilization-like Ca2+ oscillations in a variety of mammalian eggs (Cox et al. 2002), and it was predominantly localized to the equatorial/postacrosomal region of mammalian sperm heads (Grasa et al. 2008; Yoon et al. 2008). Electron microscopy (EM) studies later found it to be in close association with the inner acrosomal membrane (Escoffier et al. 2015).
PLCζ has a basic domain structure consisting of four EF hands, an X and Y catalytic domain, and a C2 domain, but lacks the amino-terminal pleckstrin homology domain, which is present in all other PLCs to target membrane-bound substrate PIP2 (Saunders et al. 2002). EF hands are a Ca2+-binding motif that allows proteins to sense and respond to changes in Ca2+ levels. The X and Y domains are the conserved catalytic domains of PLCζ (Nomikos et al. 2011), but the discrete linker region that separates the X and Y domains in PLCζ contains a distinct cluster of basic amino acid residues not found in other PLCs, and a predicted nuclear localization signal (Kuroda et al. 2006; Ito et al. 2008). The C2 domain, which is believed to play an important role in the Ca2+-dependent subcellular membrane targeting in a variety of lipid-metabolizing enzymes (Clark et al. 1991), appears to play a similar role in PLCζ, as in vitro studies showed that the C2 domain of PLCζ binds to membrane phospholipids such as phosphatidylinositol 3-phosphate (PI3P) and phosphatidylinositol 5-phosphate (PI5P) (Kouchi et al. 2005; Nomikos et al. 2017). Disturbing the C2 domain may affect the function of PLCζ, which may explain the infertility caused in humans by the naturally occurring Ile489Phe mutation (Escoffier et al. 2016).
A question that has remained unanswered for some time is how PLCζ changes the intracellular concentration of IP3 ([IP3]i) during the Ca2+ oscillations. Do basal [IP3]i levels oscillate with each [Ca2+]i rise, or are the Ca2+ oscillations driven by persistently elevated levels of IP3? A recent study appears to have resolved this question using a FRET construct capable of detecting the low magnitude changes in [IP3]i that occur after mammalian fertilization (Matsu-Ura et al. 2019). It is shown that following fertilization, a persistent increase in the [IP3]i is observed, which remains high throughout the oscillations. Remarkably, with each [Ca2+]i rise, a small increase in [IP3]i is induced, reflecting the high Ca2+ sensitivity of PLCζ. Nevertheless, the fact that the peak in [IP3]i occurs slightly after the initiation of the [Ca2+]i rise, suggests the periodic [Ca2+]i rises during the fertilization-induced oscillations are primarily governed by changes in the sensitivity of IP3R1 (Iino 1990), which has the major role in triggering the rising phase [Ca2+]i rise (Matsu-Ura et al. 2019), and the refilling of the stores by influx from the extracellular media (Wakai et al. 2013).
PLCζ and Fertility
The advent of ICSI represented a major advance in the treatment of severe male factor infertility cases. However, ICSI is not successful in all couples, and in cases of repeated ICSI failure ∼80% of the eggs fail to exit the MII stage, suggesting a defect in egg activation (Flaherty et al. 1995). Yoon et al. (2008) first showed that sperm from certain patients that had failed ICSI were also unable to trigger Ca2+ oscillations and egg activation upon injection into mouse eggs. Analysis of PLCζ expression in these sperm revealed absence of PLCζ protein, although analysis of the Plcz1 gene failed to identify the molecular defects. Heytens et al. (2009) and subsequently Kashir et al. (2012) provided the first link between human male infertility and PLCζ mutations with debilitating functional effects on the PLCζ protein. Most recently, Escoffier et al. (2016) used whole exomic sequencing to trace back the infertility of two brothers with complete fertilization failure after ICSI despite normal sperm morphology to a homozygous mutation in the Plcz1 gene. The mutation, Ile489Phe (I489F), which is in the C2 domain of PLCζ, significantly reduced its expression, and using I489F PLCζ mRNA, it was shown that the construct had a reduced ability to generate Ca2+ oscillations and displayed abnormal distribution in oocytes and eggs (Escoffier et al. 2016). In addition, analysis of I489F PLCζ by Nomikos et al. (2017) showed diminished binding to PI3P- and PI5P-containing liposomes, confirming the role of the C2 domain in binding to such lipids. It is also consistent with modeling predictions in which the I489F mutation creates an unusually large hydrophobic area in the C2 domain that might compromise the targeting and/or binding of PLCζ to specific substrate(s) (Escoffier et al. 2016). These human studies, besides identifying the molecular basis of certain types of male infertility, revealed important clues about the structure–function relationships of the PLCζ protein. Thus far, PLCζ remains the only protein whose presence is required for the initiation of Ca2+ oscillations during human fertilization.
To show the role of PLCζ in the fertility of other mammals, it was necessary to prevent its expression by genetic approaches. The initial studies only accomplished partial down-regulation of PLCζ expression (Knott et al. 2005) and/or caused off-target effects that resulted in the arrest of spermatogenesis (Ito and Ikawa 2010), which prevented gaining insight into the mechanism and/or role of PLCζ in initiating Ca2+ oscillations and fertility. A full PLCζ gene KO was recently accomplished using clustered regularly interspaced palindromic repeats/CRISPR-associated protein 9 (CRISPR/Cas9) genome-editing technology (Hachem et al. 2017). These animals displayed normal spermatogenesis but lacked expression of WT Plcz1 in the testis and full-length PLCζ protein in the sperm. When used in fertilization studies, both by ICSI or IVF methods, Plcz1 KO sperm failed to trigger Ca2+ oscillations, which provided the first definitive evidence that PLCζ is the physiological trigger of the Ca2+ oscillations in mammals. Remarkably, when the outcome of fertilization was assessed soon after sperm entry, increased rates of polyspermy were observed both after IVF or in vivo mating. These results suggest that the lack of Ca2+ oscillations noted above compromised the ability of the fertilized eggs to engage the Ca2+-sensitive mechanisms responsible for blocking polyspermy (Avella et al. 2013). Notably, a few of these zygotes generated using KO sperm and/or KO males developed to the blastocyst stage, and after naturally mating, male PLCζ KO mice were subfertile, generating a few pups per litter. This constituted the first demonstration that in vivo fertilization without the normal physiological trigger of egg activation can result in offspring, at least in mice.
The study of Hachem et al. (2017) relied heavily on the use of cryopreserved sperm, which could have further compromised the ability of the KO sperm to initiate a [Ca2+]i response, even an atypical one. This might have been the case, as in a recent study using a similar CRISPR/Cas9 genome-editing technology to generate Plcz1 KO mice, the KO sperm initiated a [Ca2+]i response, albeit highly abnormal (Nozawa et al. 2018) with oscillations starting after a delay of ∼1 h after sperm entry and consisting of only a few Ca2+ oscillations. Remarkably, fresh Plcz1 KO sperm also failed to induce Ca2+ oscillations following fertilization by ICSI, and Plcz1 KO males were subfertile with embryos displaying high rates of polyspermy, just as noted by the previous study (Nozawa et al. 2018). Together, these studies show the importance of PLCζ in the initiation of Ca2+ oscillations and normal embryo development in mammals. Whereas there appears to be residual Ca2+-releasing activity in mouse sperm that does not express functional PLCζ, it seems highly unlikely this activity is associated with PAWP (gene name Wbp2nl), PAWP has been proposed for some time to represent an alternative egg-activating mechanism in mammals capable of initiating Ca2+ oscillations (Aarabi et al. 2014). Nevertheless, this does not seem to be the case, as a Wbp2nl KO mouse showed no obvious defects in spermatogenesis and ability to initiate Ca2+ oscillations (Satouh et al. 2015).
CONCLUSIONS
The study of the mechanisms that underlie Ca2+ homeostasis in oocytes and eggs during maturation and fertilization, respectively, in mammals has resulted in important contributions to the Ca2+-signaling field in general and for the field of gamete biology by providing important insights into the molecules and parameters required to initiate normal embryo development. For example, the indispensable role of IP3R1-mediated Ca2+ release in regulating cellular functions was first unequivocally shown in mouse fertilization (Miyazaki et al. 1992). Also, the initiation of Ca2+ oscillations by the sperm's cargo PLCζ (Sanders et al. 2018) represents a unique, intimate, and highly controlled method of activation of embryo development hardly seen in other animal models. The precise source of PIP2 as a substrate for PLCζ in mammalian oocytes remains to be elucidated, and may also constitute a novel adaptation of Ca2+ homeostasis and wave propagation. Last, significant progress has been made regarding identification of the channels that make Ca2+ influx possible in mouse oocytes and eggs. Nevertheless, we are still unaware of the full repertoire of Ca2+ channels in mammalian oocytes/eggs, knowing even less about their regulation, and we remain largely ignorant of what channel(s) are present in human eggs. Furthermore, the fact that these channels’ function may be differentially regulated during maturation and fertilization has the potential to uncover specific and novel mechanisms of Ca2+ influx regulation. Identification of all the components of the Ca2+ toolkit in these cells as well as elucidation of their regulatory mechanism(s) will deepen our understanding of maturation and fertilization, information that could then be used for more precise diagnosis of infertility, to improve the development of embryos generated by assisted reproductive technology procedures, and to develop novel and nonhormonal methods of contraception.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health/National Institute of Child Health and Human Development (NIH/NICHD HD092499) and National Institute of Food and Agriculture/Hatch (NIFA/Hatch) to R.A.F. We thank all members of the Fissore laboratory for their generous contributions and suggestions. We apologize to those whose work was not cited owing to space limitations.
Footnotes
Editors: Geert Bultynck, Martin D. Bootman, Michael J. Berridge, and Grace E. Stutzmann
Additional Perspectives on Calcium Signaling available at www.cshperspectives.org
REFERENCES
- Aarabi M, Balakier H, Bashar S, Moskovtsev SI, Sutovsky P, Librach CL, Oko R. 2014. Sperm-derived WW domain-binding protein, PAWP, elicits calcium oscillations and oocyte activation in humans and mice. FASEB J 28: 4434–4440. 10.1096/fj.14-256495 [DOI] [PubMed] [Google Scholar]
- Ajduk A, Małagocki A, Maleszewski M. 2008. Cytoplasmic maturation of mammalian oocytes: Development of a mechanism responsible for sperm-induced Ca2+ oscillations. Reprod Biol 8: 3–22. 10.1016/S1642-431X(12)60001-1 [DOI] [PubMed] [Google Scholar]
- Avella MA, Xiong B, Dean J. 2013. The molecular basis of gamete recognition in mice and humans. Mol Hum Reprod 19: 279–289. 10.1093/molehr/gat004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagur R, Hajnóczky G. 2017. Intracellular Ca2+ sensing: Its role in calcium homeostasis and signaling. Mol Cell 66: 780–788. 10.1016/j.molcel.2017.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, et al. 2011. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476: 341–345. 10.1038/nature10234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt ML, Zhang Y, Erxleben CF, Padilla-Banks E, McDonough CE, Miao YL, Armstrong DL, Williams CJ. 2015. CaV3.2 T-type channels mediate Ca2+ entry during oocyte maturation and following fertilization. J Cell Sci 128: 4442–4452. 10.1242/jcs.180026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt ML, Padilla-Banks E, Stein P, Zhang Y, Williams CJ. 2017. Store-operated Ca2+ entry is not required for fertilization-induced Ca2+ signaling in mouse eggs. Cell Calcium 65: 63–72. 10.1016/j.ceca.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt ML, Stein P, Carvacho I, Krapp C, Ardestani G, Mehregan A, Umbach DM, Bartolomei MS, Fissore RA, Williams CJ. 2018. TRPM7 and CaV3.2 channels mediate Ca2+ influx required for egg activation at fertilization. Proc Natl Acad Sci 115: E10370–E10378. 10.1073/pnas.1810422115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. 2009. Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta 1793: 933–940. 10.1016/j.bbamcr.2008.10.005 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. 2000. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21. 10.1038/35036035 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. 2003. Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4: 517–529. 10.1038/nrm1155 [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I. 2005. The inositol 1,4,5-trisphosphate receptors. Cell Calcium 38: 261–272. 10.1016/j.ceca.2005.06.030 [DOI] [PubMed] [Google Scholar]
- Blachly-Dyson E, Zambronicz EB, Yu WH, Adams V, McCabe ER, Adelman J, Colombini M, Forte M. 1993. Cloning and functional expression in yeast of two human isoforms of the outer mitochondrial membrane channel, the voltage-dependent anion channel. J Biol Chem 268: 1835–1841. 10.1007/978-1-4899-1775-1_5 [DOI] [PubMed] [Google Scholar]
- Bootman MD, Collins TJ, Peppiatt CM, Prothero LS, MacKenzie L, De Smet P, Travers M, Tovey SC, Seo JT, Berridge MJ, et al. 2001. Calcium signalling—An overview. Semin Cell Dev Biol 12: 3–10. 10.1006/scdb.2000.0211 [DOI] [PubMed] [Google Scholar]
- Brini M, Carafoli E. 2009. Calcium pumps in health and disease. Physiol Rev 89: 1341–1378. 10.1152/physrev.00032.2008 [DOI] [PubMed] [Google Scholar]
- Cai X, Clapham DE. 2012. Ancestral Ca2+ signaling machinery in early animal and fungal evolution. Mol Biol Evol 29: 91–100. 10.1093/molbev/msr149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, Vais H, Cheung KH, Yang J, Parker I, et al. 2010. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell 142: 270–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J. 2000. Na+-Ca2+ exchange in mouse oocytes: Modifications in the regulation of intracellular free Ca2+ during oocyte maturation. J Reprod Fertil 118: 337–342. 10.1530/reprod/118.2.337 [DOI] [PubMed] [Google Scholar]
- Carroll J, Swann K. 1992. Spontaneous cytosolic calcium oscillations driven by inositol trisphosphate occur during in vitro maturation of mouse oocytes. J Biol Chem 267: 11196–11201. [PubMed] [Google Scholar]
- Carroll J, Swann K, Whittingham D, Whitaker M. 1994. Spatiotemporal dynamics of intracellular [Ca2+]i oscillations during the growth and meiotic maturation of mouse oocytes. Development 120: 3507–3517. [DOI] [PubMed] [Google Scholar]
- Carvacho I, Lee HC, Fissore RA, Clapham DE. 2013. TRPV3 channels mediate strontium-induced mouse-egg activation. Cell Rep 5: 1375–1386. 10.1016/j.celrep.2013.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvacho I, Ardestani G, Lee HC, McGarvey K, Fissore RA, Lykke-Hartmann K. 2016. TRPM7-like channels are functionally expressed in oocytes and modulate post-fertilization embryo development in mouse. Sci Rep 6: 34236 10.1038/srep34236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvacho I, Piesche M, Maier TJ, Machaca K. 2018. Ion channel function during oocyte maturation and fertilization. Front Cell Dev Biol 6: 63 10.3389/fcell.2018.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Lamping KG, Nuno DW, Barresi R, Prouty SJ, Lavoie JL, Cribbs LL, England SK, Sigmund CD, Weiss RM, et al. 2003. Abnormal coronary function in mice deficient in α1H T-type Ca2+ channels. Science 302: 1416–1418. 10.1126/science.1089268 [DOI] [PubMed] [Google Scholar]
- Cheon B, Lee HC, Wakai T, Fissore RA. 2013. Ca2+ influx and the store-operated Ca2+ entry pathway undergo regulation during mouse oocyte maturation. Mol Biol Cell 24: 1396–1410. 10.1091/mbc.e13-01-0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. 2007. Calcium signaling. Cell 131: 1047–1058. 10.1016/j.cell.2007.11.028 [DOI] [PubMed] [Google Scholar]
- Clapper DL, Lee HC. 1985. Inositol trisphosphate induces calcium release from nonmitochondrial stores in sea urchin egg homogenates. J Biol Chem 260: 13947–13954. [PubMed] [Google Scholar]
- Clark JD, Lin LL, Kriz RW, Ramesha CS, Sultzman LA, Lin AY, Milona N, Knopf JL. 1991. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca2+-dependent translocation domain with homology to PKC and GAP. Cell 65: 1043–1051. 10.1016/0092-8674(91)90556-E [DOI] [PubMed] [Google Scholar]
- Cox LJ, Larman MG, Saunders CM, Hashimoto K, Swann K, Lai FA. 2002. Sperm phospholipase Cζ from humans and cynomolgus monkeys triggers Ca2+ oscillations, activation and development of mouse oocytes. Reproduction 124: 611–623. 10.1530/rep.0.1240611 [DOI] [PubMed] [Google Scholar]
- Cross NL, Elinson RP. 1980. A fast block to polyspermy in frogs mediated by changes in the membrane potential. Dev Biol 75: 187–198. 10.1016/0012-1606(80)90154-2 [DOI] [PubMed] [Google Scholar]
- Cui Y, Wen J, Hung Sze K, Man D, Lin D, Liu M, Zhu G. 2003. Interaction between calcium-free calmodulin and IQ motif of neurogranin studied by nuclear magnetic resonance spectroscopy. Anal Biochem 315: 175–182. 10.1016/S0003-2697(03)00007-1 [DOI] [PubMed] [Google Scholar]
- Day ML, Pickering SJ, Johnson MH, Cook DI. 1993. Cell-cycle control of a large-conductance K+ channel in mouse early embryos. Nature 365: 560–562. 10.1038/365560a0 [DOI] [PubMed] [Google Scholar]
- Deguchi R, Shirakawa H, Oda S, Mohri T, Miyazaki S. 2000. Spatiotemporal analysis of Ca2+ waves in relation to the sperm entry site and animal-vegetal axis during Ca2+ oscillations in fertilized mouse eggs. Dev Biol 218: 299–313. 10.1006/dbio.1999.9573 [DOI] [PubMed] [Google Scholar]
- Deguchi R, Kondoh E, Itoh J. 2005. Spatiotemporal characteristics and mechanisms of intracellular Ca2+ increases at fertilization in eggs of jellyfish (Phylum Cnidaria, Class Hydrozoa). Dev Biol 279: 291–307. 10.1016/j.ydbio.2004.11.036 [DOI] [PubMed] [Google Scholar]
- De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R. 2011. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476: 336–340. 10.1038/nature10230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson GD, Ellefsen KL, Dawson SP, Pearson JE, Parker I. 2016. Hindered cytoplasmic diffusion of inositol trisphosphate restricts its cellular range of action. Sci Signal 9: ra108 10.1126/scisignal.aag1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR. 2000. Mitochondria and calcium: From cell signalling to cell death. J Physiol 529: 57–68. 10.1111/j.1469-7793.2000.00057.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducibella T, Fissore R. 2008. The roles of Ca2+, downstream protein kinases, and oscillatory signaling in regulating fertilization and the activation of development. Dev Biol 315: 257–279. 10.1016/j.ydbio.2007.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumollard R, Marangos P, Fitzharris G, Swann K, Duchen M, Carroll J. 2004. Sperm-triggered [Ca2+] oscillations and Ca2+ homeostasis in the mouse egg have an absolute requirement for mitochondrial ATP production. Development 131: 3057–3067. 10.1242/dev.01181 [DOI] [PubMed] [Google Scholar]
- Dumollard R, Campbell K, Halet G, Carroll J, Swann K. 2008. Regulation of cytosolic and mitochondrial ATP levels in mouse eggs and zygotes. Dev Biol 316: 431–440. 10.1016/j.ydbio.2008.02.004 [DOI] [PubMed] [Google Scholar]
- Escoffier J, Yassine S, Lee HC, Martinez G, Delaroche J, Coutton C, Karaouzène T, Zouari R, Metzler-Guillemain C, Pernet-Gallay K, et al. 2015. Subcellular localization of phospholipase Cζ in human sperm and its absence in DPY19L2-deficient sperm are consistent with its role in oocyte activation. Mol Hum Reprod 21: 157–168. 10.1093/molehr/gau098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoffier J, Lee HC, Yassine S, Zouari R, Martinez G, Karaouzène T, Coutton C, Kherraf ZE, Halouani L, Triki C, et al. 2016. Homozygous mutation of PLCZ1 leads to defective human oocyte activation and infertility that is not rescued by the WW-binding protein PAWP. Hum Mol Genet 25: 878–891. 10.1093/hmg/ddv617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fissore RA, Longo FJ, Anderson E, Parys JB, Ducibella T. 1999. Differential distribution of inositol trisphosphate receptor isoforms in mouse oocytes. Biol Reprod 60: 49–57. 10.1095/biolreprod60.1.49 [DOI] [PubMed] [Google Scholar]
- FitzHarris G, Marangos P, Carroll J. 2003. Cell cycle–dependent regulation of structure of endoplasmic reticulum and inositol 1,4,5-trisphosphate-induced Ca2+ release in mouse oocytes and embryos. Mol Biol Cell 14: 288–301. 10.1091/mbc.e02-07-0431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty SP, Payne D, Swann NJ, Matthews CD. 1995. Aetiology of failed and abnormal fertilization after intracytoplasmic sperm injection. Hum Reprod 10: 2623–2629. 10.1093/oxfordjournals.humrep.a135757 [DOI] [PubMed] [Google Scholar]
- Fleig A, Chubanov V. 2014. TRPM7. In Mammalian transient receptor potential (TRP) cation channels, Handbook of experimental pharmacology (ed. Nilius B, Flockerzi V), Vol. 222, pp. 521–546. Springer, Berlin: 10.1007/978-3-642-54215-2_21 [DOI] [PubMed] [Google Scholar]
- Fukami K, Nakao K, Inoue T, Kataoka Y, Kurokawa M, Fissore RA, Nakamura K, Katsuki M, Mikoshiba K, Yoshida N, et al. 2001. Requirement of phospholipase Cδ4 for the zona pellucida–induced acrosome reaction. Science 292: 920–923. 10.1126/science.1059042 [DOI] [PubMed] [Google Scholar]
- Georgiou P, Bountra C, Bland KP, House CR. 1983. Calcium-evoked opening of potassium channels in hamster eggs. Q J Exp Physiol 68: 687–700. 10.1113/expphysiol.1983.sp002758 [DOI] [PubMed] [Google Scholar]
- Goud PT, Goud AP, Leybaert L, Van Oostveldt P, Mikoshiba K, Diamond MP, Dhont M. 2002. Inositol 1,4,5-trisphosphate receptor function in human oocytes: Calcium responses and oocyte activation-related phenomena induced by photolytic release of InsP3 are blocked by a specific antibody to the type I receptor. Mol Hum Reprod 8: 912–918. 10.1093/molehr/8.10.912 [DOI] [PubMed] [Google Scholar]
- Grasa P, Coward K, Young C, Parrington J. 2008. The pattern of localization of the putative oocyte activation factor, phospholipase Cζ, in uncapacitated, capacitated, and ionophore-treated human spermatozoa. Hum Reprod 23: 2513–2522. 10.1093/humrep/den280 [DOI] [PubMed] [Google Scholar]
- Hachem A, Godwin J, Ruas M, Lee HC, Ferrer Buitrago M, Ardestani G, Bassett A, Fox S, Navarrete F, de Sutter P, et al. 2017. PLCζ is the physiological trigger of the Ca2+ oscillations that induce embryogenesis in mammals but conception can occur in its absence. Development 144: 2914–2924. 10.1242/dev.150227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnóczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. 1995. Decoding of cytosolic calcium oscillations in the mitochondria. Cell 82: 415–424. 10.1016/0092-8674(95)90430-1 [DOI] [PubMed] [Google Scholar]
- Halet G. 2004. PKC signaling at fertilization in mammalian eggs. Biochim Biophys Acta 1742: 185–189. 10.1016/j.bbamcr.2004.09.012 [DOI] [PubMed] [Google Scholar]
- Halet G, Tunwell R, Balla T, Swann K, Carroll J. 2002. The dynamics of plasma membrane PtdIns(4,5)P2 at fertilization of mouse eggs. J Cell Sci 115: 2139–2149. [DOI] [PubMed] [Google Scholar]
- Herrick JR, Strauss KJ, Schneiderman A, Rawlins M, Stevens J, Schoolcraft WB, Krisher RL. 2015. The beneficial effects of reduced magnesium during the oocyte-to-embryo transition are conserved in mice, domestic cats and humans. Reprod Fertil Dev 27: 323–331. 10.1071/RD13268 [DOI] [PubMed] [Google Scholar]
- Heytens E, Parrington J, Coward K, Young C, Lambrecht S, Yoon SY, Fissore RA, Hamer R, Deane CM, Ruas M, et al. 2009. Reduced amounts and abnormal forms of phospholipase C ζ (PLCζ) in spermatozoa from infertile men. Hum Reprod 24: 2417–2428. 10.1093/humrep/dep207 [DOI] [PubMed] [Google Scholar]
- Homa ST, Swann K. 1994. Fertilization and early embryology: A cytosolic sperm factor triggers calcium oscillations and membrane hyperpolarizations in human oocytes. Hum Reprod 9: 2356–2361. 10.1093/oxfordjournals.humrep.a138452 [DOI] [PubMed] [Google Scholar]
- Horner VL, Wolfner MF. 2008. Transitioning from egg to embryo: Triggers and mechanisms of egg activation. Dev Dyn 237: 527–544. 10.1002/dvdy.21454 [DOI] [PubMed] [Google Scholar]
- Igusa Y, Miyazaki S. 1983. Effects of altered extracellular and intracellular calcium concentration on hyperpolarizing responses of the hamster egg. J Physiol 340: 611–632. 10.1113/jphysiol.1983.sp014783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igusa Y, Miyazaki S, Yamashita N. 1983. Periodic hyperpolarizing responses in hamster and mouse eggs fertilized with mouse sperm. J Physiol 340: 633–647. 10.1113/jphysiol.1983.sp014784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M. 1990. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol 95: 1103–1122. 10.1085/jgp.95.6.1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Ikawa M. 2010. Program and Abstracts of the 11th International Symposium on Spermatology. Presented at 11 International Symposium on Spermatology, Okinawa, Japan. [Google Scholar]
- Ito M, Shikano T, Oda S, Horiguchi T, Tanimoto S, Awaji T, Mitani H, Miyazaki S. 2008. Difference in Ca2+ oscillation-inducing activity and nuclear translocation ability of PLCZ1, an egg-activating sperm factor candidate, between mouse, rat, human, and medaka fish. Biol Reprod 78: 1081–1090. 10.1095/biolreprod.108.067801 [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Chiba K, Uchiyama T, Yoshikawa F, Suzuki F, Ikeda M, Furuichi T, Mikoshiba K. 2002. Molecular characterization of the starfish inositol 1,4,5-trisphosphate receptor and its role during oocyte maturation and fertilization. J Biol Chem 277: 2763–2772. 10.1074/jbc.M108839200 [DOI] [PubMed] [Google Scholar]
- Jaffe LA, Cross NL. 1984. Electrical properties of vertebrate oocyte membranes. Biol Reprod 30: 50–54. 10.1095/biolreprod30.1.50 [DOI] [PubMed] [Google Scholar]
- Jaffe LA, Cross NL, Picheral B. 1983. Studies of the voltage-dependent polyspermy block using cross-species fertilization of amphibians. Dev Biol 98: 319–326. 10.1016/0012-1606(83)90362-7 [DOI] [PubMed] [Google Scholar]
- Jellerette T, He CL, Wu H, Parys JB, Fissore RA. 2000. Down-regulation of the inositol 1,4,5-trisphosphate receptor in mouse eggs following fertilization or parthenogenetic activation. Dev Biol 223: 238–250. 10.1006/dbio.2000.9675 [DOI] [PubMed] [Google Scholar]
- Jellerette T, Kurokawa M, Lee B, Malcuit C, Yoon SY, Smyth J, Vermassen E, De Smedt H, Parys JB, Fissore RA. 2004. Cell cycle-coupled [Ca2+]i oscillations in mouse zygotes and function of the inositol 1,4,5-trisphosphate receptor-1. Dev Biol 274: 94–109. 10.1016/j.ydbio.2004.06.020 [DOI] [PubMed] [Google Scholar]
- Jin J, Desai BN, Navarro B, Donovan A, Andrews NC, Clapham DE. 2008. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science 322: 756–760. 10.1126/science.1163493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KT, Carroll J, Whittingham DG. 1995. Ionomycin, thapsigargin, ryanodine, and sperm induced Ca2+ release increase during meiotic maturation of mouse oocytes. J Biol Chem 270: 6671–6677. 10.1074/jbc.270.12.6671 [DOI] [PubMed] [Google Scholar]
- Jones KT, Cruttwell C, Parrington J, Swann K. 1998. A mammalian sperm cytosolic phospholipase C activity generates inositol trisphosphate and causes Ca2+ release in sea urchin egg homogenates. FEBS Lett 437: 297–300. 10.1016/S0014-5793(98)01254-X [DOI] [PubMed] [Google Scholar]
- Joughin BA, Naegle KM, Huang PH, Yaffe MB, Lauffenburger DA, White FM. 2009. An integrated comparative phosphoproteomic and bioinformatic approach reveals a novel class of MPM-2 motifs upregulated in EGFRvIII-expressing glioblastoma cells. Mol Biosyst 5: 59–67. 10.1039/B815075C [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D, Hur CG, Park JY, Han J, Hong SG. 2007. Acetylcholine increases Ca2+ influx by activation of CaMKII in mouse oocytes. Biochem Biophys Res Commun 360: 476–482. 10.1016/j.bbrc.2007.06.083 [DOI] [PubMed] [Google Scholar]
- Kashir J, Konstantinidis M, Jones C, Lemmon B, Lee HC, Hamer R, Heindryckx B, Deane CM, De Sutter P, Fissore RA, et al. 2012. A maternally inherited autosomal point mutation in human phospholipase C ζ (PLCζ) leads to male infertility. Hum Reprod 27: 222–231. 10.1093/humrep/der384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline D, Kline JT. 1992. Thapsigargin activates a calcium influx pathway in the unfertilized mouse egg and suppresses repetitive calcium transients in the fertilized egg. J Biol Chem 267: 17624–17630. 10.1016/0012-1606(92)90265-i [DOI] [PubMed] [Google Scholar]
- Kline JT, Kline D. 1994. Regulation of intracellular calcium in the mouse egg: Evidence for inositol trisphosphate-induced calcium release, but not calcium-induced calcium release. Biol Reprod 50: 193–203. 10.1095/biolreprod50.1.193 [DOI] [PubMed] [Google Scholar]
- Knott JG, Kurokawa M, Fissore RA, Schultz RM, Williams CJ. 2005. Transgenic RNA interference reveals role for mouse sperm phospholipase Cζ in triggering Ca2+ oscillations during fertilization. Biol Reprod 72: 992–996. 10.1095/biolreprod.104.036244 [DOI] [PubMed] [Google Scholar]
- Kono T, Jones KT, Bos-Mikich A, Whittingham DG, Carroll J. 1996. A cell cycle-associated change in Ca2+ releasing activity leads to the generation of Ca2+ transients in mouse embryos during the first mitotic division. J Cell Biol 132: 915–923. 10.1083/jcb.132.5.915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouchi Z, Fukami K, Shikano T, Oda S, Nakamura Y, Takenawa T, Miyazaki S. 2004. Recombinant phospholipase Cζ has high Ca2+ sensitivity and induces Ca2+ oscillations in mouse eggs. J Biol Chem 279: 10408–10412. 10.1074/jbc.M313801200 [DOI] [PubMed] [Google Scholar]
- Kouchi Z, Shikano T, Nakamura Y, Shirakawa H, Fukami K, Miyazaki S. 2005. The role of EF-hand domains and C2 domain in regulation of enzymatic activity of phospholipase Cζ. J Biol Chem 280: 21015–21021. 10.1074/jbc.M412123200 [DOI] [PubMed] [Google Scholar]
- Kuroda K, Ito M, Shikano T, Awaji T, Yoda A, Takeuchi H, Kinoshita K, Miyazaki S. 2006. The role of X/Y linker region and N-terminal EF-hand domain in nuclear translocation and Ca2+ oscillation-inducing activities of phospholipase Cζ, a mammalian egg-activating factor. J Biol Chem 281: 27794–27805. 10.1074/jbc.M603473200 [DOI] [PubMed] [Google Scholar]
- Lawrence YM, Cuthbertson KSR. 1995. Thapsigargin induces cytoplasmic free Ca2+ oscillations in mouse oocytes. Cell Calcium 17: 154–164. 10.1016/0143-4160(95)90084-5 [DOI] [PubMed] [Google Scholar]
- Lee B, Vermassen E, Yoon SY, Vanderheyden V, Ito J, Alfandari D, De Smedt H, Parys JB, Fissore RA. 2006. Phosphorylation of IP3R1 and the regulation of [Ca2+]i responses at fertilization: A role for the MAP kinase pathway. Development 133: 4355–4365. 10.1242/dev.02624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Wang C, Machaty Z. 2012. STIM1 is required for Ca2+ signaling during mammalian fertilization. Dev Biol 367: 154–162. 10.1016/j.ydbio.2012.04.028 [DOI] [PubMed] [Google Scholar]
- Li Y, Day ML, O'Neill C. 2007. Autocrine activation of ion currents in the two-cell mouse embryo. Exp Cell Res 313: 2786–2794. 10.1016/j.yexcr.2007.05.022 [DOI] [PubMed] [Google Scholar]
- Li Y, O'Neill C, Day ML. 2009. Activation of a chloride channel by a trophic ligand is required for development of the mouse preimplantation embryo in vitro. Biol Reprod 81: 759–767. 10.1095/biolreprod.108.074567 [DOI] [PubMed] [Google Scholar]
- Liu L, Hammar K, Smith PJS, Inoue S, Keefe DL. 2001. Mitochondrial modulation of calcium signaling at the initiation of development. Cell Calcium 30: 423–433. 10.1054/ceca.2001.0251 [DOI] [PubMed] [Google Scholar]
- Liu W, Su LT, Khadka DK, Mezzacappa C, Komiya Y, Sato A, Habas R, Runnels LW. 2011. TRPM7 regulates gastrulation during vertebrate embryogenesis. Dev Biol 350: 348–357. 10.1016/j.ydbio.2010.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb J. 1913. Reversibility in artificial parthenogenesis. Science 38: 749–751. 10.1126/science.38.986.749 [DOI] [PubMed] [Google Scholar]
- Machaca K, Haun S. 2002. Induction of maturation-promoting factor during Xenopus oocyte maturation uncouples Ca2+ store depletion from store-operated Ca2+ entry. J Cell Biol 156: 75–86. 10.1083/jcb.200110059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machaty Z. 2016. Signal transduction in mammalian oocytes during fertilization. Cell Tissue Res 363: 169–183. 10.1007/s00441-015-2291-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machaty Z, Ramsoondar JJ, Bonk AJ, Prather RS, Bondioli KR. 2002. Na+/Ca2+ exchanger in porcine oocytes. Biol Reprod 67: 1133–1139. 10.1095/biolreprod67.4.1133 [DOI] [PubMed] [Google Scholar]
- Malathi K, Li X, Krizanova O, Ondrias K, Sperber K, Ablamunits V, Jayaraman T. 2005. Cdc2/cyclin B1 interacts with and modulates inositol 1,4,5-trisphosphate receptor (type 1) functions. J Immunol 175: 6205–6210. 10.4049/jimmunol.175.9.6205 [DOI] [PubMed] [Google Scholar]
- Matsu-ura T, Shirakawa H, Suzuki KGN, Miyamoto A, Sugiura K, Michikawa T, Kusumi A, Mikoshiba K. 2019. Dual-FRET imaging of IP3 and Ca2+ revealed Ca2+-induced IP3 production maintains long lasting Ca2+ oscillations in fertilized mouse eggs. Sci Rep 9: 4829 10.1038/s41598-019-40931-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM, Carpenter G, Rhee SG, Jaffe LA. 1998. SH2 domain-mediated activation of phospholipase Cγ is not required to initiate Ca2+ release at fertilization of mouse eggs. Dev Biol 203: 221–232. 10.1006/dbio.1998.9051 [DOI] [PubMed] [Google Scholar]
- Miao YL, Stein P, Jefferson WN, Padilla-Banks E, Williams CJ. 2012. Calcium influx-mediated signaling is required for complete mouse egg activation. Proc Natl Acad Sci 109: 4169–4174. 10.1073/pnas.1112333109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S. 1988. Inositol 1,4,5-trisphosphate-induced calcium release and guanine nucleotide-binding protein-mediated periodic calcium rises in golden hamster eggs. J Cell Biol 106: 345–353. 10.1083/jcb.106.2.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S. 2006. Thirty years of calcium signals at fertilization. Semin Cell Dev Biol 17: 233–243. 10.1016/j.semcdb.2006.02.007 [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Igusa Y. 1981. Fertilization potential in golden hamster eggs consists of recurring hyperpolarizations. Nature 290: 702–704. 10.1038/290702a0 [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Igusa Y. 1982. Ca-mediated activation of a K current at fertilization of golden hamster eggs. Proc Natl Acad Sci 79: 931–935. 10.1073/pnas.79.3.931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S, Yuzaki M, Nakada K, Shirakawa H, Nakanishi S, Nakade S, Mikoshiba K. 1992. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science 257: 251–255. 10.1126/science.1321497 [DOI] [PubMed] [Google Scholar]
- Nakano Y, Shirakawa H, Mitsuhashi N, Kuwabara Y, Miyazaki S. 1997. Spatiotemporal dynamics of intracellular calcium in the mouse egg injected with a spermatozoon. Mol Hum Reprod 3: 1087–1093. 10.1093/molehr/3.12.1087 [DOI] [PubMed] [Google Scholar]
- Nomikos M, Blayney LM, Larman MG, Campbell K, Rossbach A, Saunders CM, Swann K, Lai FA. 2005. Role of phospholipase C-ζ domains in Ca2+-dependent phosphatidylinositol 4,5-bisphosphate hydrolysis and cytoplasmic Ca2+ oscillations. J Biol Chem 280: 31011–31018. 10.1074/jbc.M500629200 [DOI] [PubMed] [Google Scholar]
- Nomikos M, Elgmati K, Theodoridou M, Calver BL, Nounesis G, Swann K, Lai FA. 2011. Phospholipase Cζ binding to PtdIns(4,5)P2 requires the XY-linker region. J Cell Sci 124: 2582–2590. 10.1242/jcs.083485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomikos M, Kashir J, Lai FA. 2017. The role and mechanism of action of sperm PLC-ζ in mammalian fertilisation. Biochem J 474: 3659–3673. 10.1042/BCJ20160521 [DOI] [PubMed] [Google Scholar]
- Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, Wang H, Ke H, Nikolaev VO, Jaffe LA. 2009. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development 136: 1869–1878. 10.1242/dev.035238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa K, Satouh Y, Fujimoto T, Oji A, Ikawa M. 2018. Sperm-borne phospholipase C ζ-1 ensures monospermic fertilization in mice. Sci Rep 8: 1315 10.1038/s41598-018-19497-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozil JP, Sainte-Beuve T, Banrezes B. 2017. [Mg2+]o/[Ca2+]o determines Ca2+ response at fertilization: Tuning of adult phenotype? Reproduction 154: 675–693. 10.1530/REP-16-0057 [DOI] [PubMed] [Google Scholar]
- Palermo G, Joris H, Devroey P, Van Steirteghem AC. 1992. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 340: 17–18. 10.1016/0140-6736(92)92425-F [DOI] [PubMed] [Google Scholar]
- Parrington J, Swann K, Shevchenko VI, Sesay AK, Lai FA. 1996. Calcium oscillations in mammalian eggs triggered by a soluble sperm protein. Nature 379: 364–368. 10.1038/379364a0 [DOI] [PubMed] [Google Scholar]
- Parrington J, Brind S, De Smedt H, Gangeswaran R, Lai FA, Wojcikiewicz R, Carroll J. 1998. Expression of inositol 1,4,5-trisphosphate receptors in mouse oocytes and early embryos: The type I isoform is upregulated in oocytes and downregulated after fertilization. Dev Biol 203: 451–461. 10.1006/dbio.1998.9071 [DOI] [PubMed] [Google Scholar]
- Parrington J, Jones KT, Lai FA, Swann K. 1999. The soluble sperm factor that causes Ca2+ release from sea-urchin (Lytechinus pictus) egg homogenates also triggers Ca2+ oscillations after injection into mouse eggs. Biochem J 341: 1–4. 10.1042/bj3410001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrington J, Jones ML, Tunwell R, Devader C, Katan M, Swann K. 2002. Phospholipase C isoforms in mammalian spermatozoa: Potential components of the sperm factor that causes Ca2+ release in eggs. Reproduction 123: 31–39. 10.1530/rep.0.1230031 [DOI] [PubMed] [Google Scholar]
- Parrington J, Arnoult C, Fissore RA. 2019. The eggstraordinary story of how life begins. Mol Reprod Dev 86: 4–19. 10.1002/mrd.23083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parys JB, McPherson SM, Mathews L, Campbell KP, Longo FJ. 1994. Presence of inositol 1,4,5-trisphosphate receptor, calreticulin, and calsequestrin in eggs of sea urchins and Xenopus laevis. Dev Biol 161: 466–476. 10.1006/dbio.1994.1045 [DOI] [PubMed] [Google Scholar]
- Peres A. 1986. Calcium current in mouse eggs recorded with the tight-seal, whole-cell voltage-clamp technique. Cell Biol Int Rep 10: 117–119. 10.1016/0309-1651(86)90095-0 [DOI] [PubMed] [Google Scholar]
- Putney JW. 2011. Origins of the concept of store-operated calcium entry. Front Biosci (Schol Ed) 3: 980–984. 10.2741/s202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW. 2017. Store-operated calcium entry: An historical overview. In Membrane dynamics and calcium signaling, Advances in experimental medicine and biology (ed. Krebs J), Vol. 981, pp. 205–214. Springer, Cham, Switzerland: 10.1007/978-3-319-55858-5_9 [DOI] [PubMed] [Google Scholar]
- Putney JW, Steinckwich-Besançon N, Numaga-Tomita T, Davis FM, Desai PN, D'Agostin DM, Wu S, Bird GS. 2017. The functions of store-operated calcium channels. Biochim Biophys Acta Mol Cell Res 1864: 900–906. 10.1016/j.bbamcr.2016.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice A, Parrington J, Jones KT, Swann K. 2000. Mammalian sperm contain a Ca2+-sensitive phospholipase C activity that can generate InsP3 from PIP2 associated with intracellular organelles. Dev Biol 228: 125–135. 10.1006/dbio.2000.9929 [DOI] [PubMed] [Google Scholar]
- Ridgway EB, Gilkey JC, Jaffe LF. 1977. Free calcium increases explosively in activating medaka eggs. Proc Natl Acad Sci 74: 623–627. 10.1073/pnas.74.2.623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Bernardi P, Pozzan T. 2000. Mitochondria as all-round players of the calcium game. J Physiol 529(Pt 1): 37–47. 10.1111/j.1469-7793.2000.00037.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier MF. 2016. T-type calcium channel: A privileged gate for calcium entry and control of adrenal steroidogenesis. Front Endocrinol (Lausanne) 7: 43 10.3389/fendo.2016.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runft LL, Watras J, Jaffe LA. 1999. Calcium release at fertilization of Xenopus eggs requires type I IP3 receptors, but not SH2 domain-mediated activation of PLCγ or Gq-mediated activation of PLCβ. Dev Biol 214: 399–411. 10.1006/dbio.1999.9415 [DOI] [PubMed] [Google Scholar]
- Ryazanova LV, Rondon LJ, Zierler S, Hu Z, Galli J, Yamaguchi TP, Mazur A, Fleig A, Ryazanov AG. 2010. TRPM7 is essential for Mg2+ homeostasis in mammals. Nat Commun 1: 109 10.1038/ncomms1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JR, Ashley B, Moon A, Woolley TE, Swann K. 2018. PLCζ induced Ca2+ oscillations in mouse eggs involve a positive feedback cycle of Ca2+ induced InsP3 formation from cytoplasmic PIP2. Front Cell Dev Biol 6: 36 10.3389/fcell.2018.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satouh Y, Nozawa K, Ikawa M. 2015. Sperm postacrosomal WW domain-binding protein is not required for mouse egg activation. Biol Reprod 93: 94 10.1095/biolreprod.115.131441 [DOI] [PubMed] [Google Scholar]
- Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. 2002. PLCζ: A sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development 129: 3533–3544. [DOI] [PubMed] [Google Scholar]
- Schappe MS, Szteyn K, Stremska ME, Mendu SK, Downs TK, Seegren PV, Mahoney MA, Dixit S, Krupa JK, Stipes EJ, et al. 2018. Chanzyme TRPM7 mediates the Ca2+ influx essential for lipopolysaccharide-induced toll-like receptor 4 endocytosis and macrophage activation. Immunity 48: 59–74.e5. 10.1016/j.immuni.2017.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM. 2003. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 114: 191–200. 10.1016/S0092-8674(03)00556-7 [DOI] [PubMed] [Google Scholar]
- Sette C, Bevilacqua A, Bianchini A, Mangia F, Geremia R, Rossi P. 1997. Parthenogenetic activation of mouse eggs by microinjection of a truncated c-kit tyrosine kinase present in spermatozoa. Development 124: 2267–2274. [DOI] [PubMed] [Google Scholar]
- Stice SL, Robl JM. 1990. Activation of mammalian oocytes by a factor obtained from rabbit sperm. Mol Reprod Dev 25: 272–280. 10.1002/mrd.1080250309 [DOI] [PubMed] [Google Scholar]
- Stricker SA. 1999. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol 211: 157–176. 10.1006/dbio.1999.9340 [DOI] [PubMed] [Google Scholar]
- Sun L, Haun S, Jones RC, Edmondson RD, Machaca K. 2009. Kinase-dependent regulation of inositol 1,4,5-trisphosphate-dependent Ca2+ release during oocyte maturation. J Biol Chem 284: 20184–20196. 10.1074/jbc.M109.004515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann K. 1990. A cytosolic sperm factor stimulates repetitive calcium increases and mimics fertilization in hamster eggs. Development 110: 1295–1302. [DOI] [PubMed] [Google Scholar]
- Swann K, Lai FA. 2016. Egg activation at fertilization by a soluble sperm protein. Physiol Rev 96: 127–149. 10.1152/physrev.00012.2015 [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kikuchi T, Kidokoro Y, Shirakawa H. 2013. Ca2+ influx-dependent refilling of intracellular Ca2+ stores determines the frequency of Ca2+ oscillations in fertilized mouse eggs. Biochem Biophys Res Commun 430: 60–65. 10.1016/j.bbrc.2012.11.024 [DOI] [PubMed] [Google Scholar]
- Tesarik J, Sousa M, Testart J. 1994. Human oocyte activation after intracytoplasmic sperm injection. Hum Reprod 9: 511–518. 10.1093/oxfordjournals.humrep.a138537 [DOI] [PubMed] [Google Scholar]
- Thomas TW, Eckberg WR, Dubé F, Galione A. 1998. Mechanisms of calcium release and sequestration in eggs of Chaetopterus pergamentaceus. Cell Calcium 24: 285–292. 10.1016/S0143-4160(98)90052-5 [DOI] [PubMed] [Google Scholar]
- Turner PR, Sheetz MP, Jaffe LA. 1984. Fertilization increases the polyphosphoinositide content of sea urchin eggs. Nature 310: 414–415. 10.1038/310414a0 [DOI] [PubMed] [Google Scholar]
- Uehara T, Yanagimachi R. 1976. Microsurgical injection of spermatozoa into hamster eggs with subsequent transformation of sperm nuclei into male pronuclei. Biol Reprod 15: 467–470. 10.1095/biolreprod15.4.467 [DOI] [PubMed] [Google Scholar]
- Ullah A, Jung P, Ullah G, Machaca K. 2014. The role of IP3 receptor channel clustering in Ca2+ wave propagation during oocyte maturation. In Progress in molecular biology and translational science (ed. Blackwell KT), Vol. 123, pp. 83–101. Elsevier, Amsterdam: 10.1016/B978-0-12-397897-4.00006-1 [DOI] [PubMed] [Google Scholar]
- Wakai T, Fissore RA. 2013. Ca2+ homeostasis and regulation of ER Ca2+ in mammalian oocytes/eggs. Cell Calcium 53: 63–67. 10.1016/j.ceca.2012.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakai T, Fissore RA. 2019. Constitutive IP3R1-mediated Ca2+ release reduces Ca2+ store content and stimulates mitochondrial metabolism in mouse GV oocytes. J Cell Sci 132: jcs225441 10.1242/jcs.225441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakai T, Vanderheyden V, Fissore RA. 2011. Ca2+ signaling during mammalian fertilization: Requirements, players, and adaptations. Cold Spring Harb Perspect Biol 3: a006767 10.1101/cshperspect.a006767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakai T, Vanderheyden V, Yoon SY, Cheon B, Zhang N, Parys JB, Fissore RA. 2012. Regulation of inositol 1,4,5-trisphosphate receptor function during mouse oocyte maturation. J Cell Physiol 227: 705–717. 10.1002/jcp.22778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakai T, Zhang N, Vangheluwe P, Fissore RA. 2013. Regulation of endoplasmic reticulum Ca2+ oscillations in mammalian eggs. J Cell Sci 126: 5714–5724. 10.1242/jcs.136549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker M. 2006. Calcium at fertilization and in early development. Physiol Rev 86: 25–88. 10.1152/physrev.00023.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolny YM, Fissore RA, Wu H, Reis MM, Colombero LT, Ergün B, Rosenwaks Z, Palermo GD. 1999. Human glucosamine-6-phosphate isomerase, a homologue of hamster oscillin, does not appear to be involved in Ca2+ release in mammalian oocytes. Mol Reprod Dev 52: 277–287. [DOI] [PubMed] [Google Scholar]
- Wolosker H, Kline D, Bian Y, Blackshaw S, Cameron AM, Fralich TJ, Schnaar RL, Snyder SH. 1998. Molecularly cloned mammalian glucosamine-6-phosphate deaminase localizes to transporting epithelium and lacks oscillin activity. FASEB J 12: 91–99. 10.1096/fasebj.12.1.91 [DOI] [PubMed] [Google Scholar]
- Wortzman-Show GB, Kurokawa M, Fissore RA, Evans JP. 2007. Calcium and sperm components in the establishment of the membrane block to polyspermy: Studies of ICSI and activation with sperm factor. Mol Hum Reprod 13: 557–565. 10.1093/molehr/gam042 [DOI] [PubMed] [Google Scholar]
- Wozniak KL, Phelps WA, Tembo M, Lee MT, Carlson AE. 2018. The TMEM16A channel mediates the fast polyspermy block in Xenopus laevis. J Gen Physiol 150: 1249–1259. 10.1085/jgp.201812071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJ, Sweet TB, Clapham DE. 2010. International Union of Basic and Clinical Pharmacology. LXXVI: Current progress in the mammalian TRP ion channel family. Pharmacol Rev 62: 381–404. 10.1124/pr.110.002725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Kopf GS, Schultz RM. 1994. Involvement of inositol 1,4,5-trisphosphate-mediated Ca2+ release in early and late events of mouse egg activation. Development 120: 1851–1859. [DOI] [PubMed] [Google Scholar]
- Yamashita N. 1982. Enhancement of ionic currents through voltage-gated channels in the mouse oocyte after fertilization. J Physiol 329: 263–280. 10.1113/jphysiol.1982.sp014302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida K, Bedford JM, Yanagimachi R. 1991. Cleavage of rabbit eggs after microsurgical injection of testicular spermatozoa. Hum Reprod 6: 277–279. 10.1093/oxfordjournals.humrep.a137321 [DOI] [PubMed] [Google Scholar]
- Yoon SY, Jellerette T, Salicioni AM, Lee HC, Yoo MS, Coward K, Parrington J, Grow D, Cibelli JB, Visconti PE, et al. 2008. Human sperm devoid of PLC, ζ1 fail to induce Ca2+ release and are unable to initiate the first step of embryo development. J Clin Invest 118: 3671–3681. 10.1172/JCI36942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Sensui N, Inoue T, Morisawa M, Mikoshiba K. 1998. Role of two series of Ca2+ oscillations in activation of ascidian eggs. Dev Biol 203: 122–133. 10.1006/dbio.1998.9037 [DOI] [PubMed] [Google Scholar]
- Zhang N, Yoon SY, Parys JB, Fissore RA. 2015. Effect of M-phase kinase phosphorylations on type 1 inositol 1,4,5-trisphosphate receptor-mediated Ca2+ responses in mouse eggs. Cell Calcium 58: 476–488. 10.1016/j.ceca.2015.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chao CH, Jaeger LA, Papp AB, Machaty Z. 2018. Calcium oscillations in fertilized pig oocytes are associated with repetitive interactions between STIM1 and ORAI1. Biol Reprod 98: 510–519. 10.1093/biolre/ioy016 [DOI] [PMC free article] [PubMed] [Google Scholar]