Abstract
Ca2+ is an important intracellular messenger affecting diverse cellular processes. In eukaryotic cells, Ca2+ is handled by a myriad of Ca2+-binding proteins found in organelles that are organized into the cellular reticular network (CRN). The network is comprised of the endoplasmic reticulum, Golgi apparatus, lysosomes, membranous components of the endocytic and exocytic pathways, peroxisomes, and the nuclear envelope. Membrane contact sites between the different components of the CRN enable the rapid movement of Ca2+, and communication of Ca2+ status, within the network. Ca2+-handling proteins that reside in the CRN facilitate Ca2+ sensing, buffering, and cellular signaling to coordinate the many processes that operate within the cell.
INTRACELLULAR Ca2+ DYNAMICS AND THE CELLULAR RETICULAR NETWORK
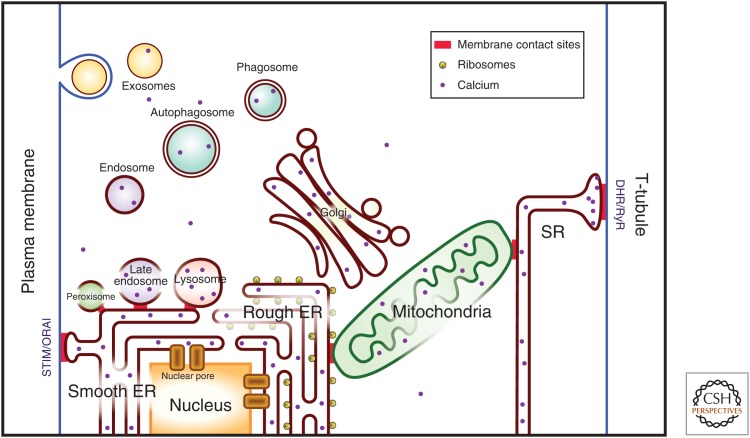
Ca2+ is a universal signaling ion involved in the regulation of numerous processes throughout the lifetime of the cell and the organism (Krebs et al. 2015; Berridge 2016). Inside the cell, the resting free Ca2+ concentration and Ca2+-signaling pathways must be tightly regulated because of the integral role of Ca2+ in nearly all aspects of cell physiology (Baumann and Walz 2001; Groenendyk et al. 2013; Krebs et al. 2015; Nunes-Hasler and Demaurex 2017; Prudent and McBride 2017). Cytoplasmic Ca2+ is maintained at a resting concentration that is two to three orders of magnitude lower than the extracellular free Ca2+ concentration (Krebs et al. 2015; Berridge 2016). This markedly lower cytoplasmic free Ca2+ concentration is maintained by action of Ca2+-handling proteins and the reticular network of intracellular membrane systems (Fig. 1) that comprise the endoplasmic reticulum (ER), sarcoplasmic reticulum (SR), Golgi apparatus, lysosomes, membranous components of the endocytic pathway, peroxisomes, and nuclear envelope (Prins and Michalak 2011; Krebs et al. 2015; Berridge 2016). This intracellular network of specialized membrane systems, the cellular reticular network (CRN), is linked together to facilitate and coordinate cellular function. The organelles of the CRN accumulate and store Ca2+ and maintain the Ca2+ stores at a higher concentration (mm) than that of cytoplasmic Ca2+. The ER is the dominating component of the CRN and is the major Ca2+ store of the cell. Importantly, Ca2+ movement between components of the CRN plays an important role in regulation of cellular functions such as gene transcription (Zhang et al. 2009), cellular stress-coping strategies (Groenendyk et al. 2013; Dicks et al. 2015; Jung et al. 2017), and mitochondrial oxidative metabolism (Griffiths and Rutter 2009; De Stefani et al. 2016). This is achieved, at least in part, through membrane contact sites (MCSs) (Fig. 1), which are formed between closely apposed organellar membranes including the plasma membrane (Prinz 2014; Filadi and Pozzan 2015; Penny et al. 2015; Phillips and Voeltz 2016; Agellon and Michalak 2017; Barneda and Christian 2017; Joshi et al. 2017; Nunes-Hasler and Demaurex 2017; Prudent and McBride 2017). The organelles that make up the CRN contain specialized proteins responsible for the sensing, transport, and storage of intracellular Ca2+, and in orchestrating Ca2+ dynamics within the lumen of the network that are necessary to exert this cation's regulatory role (Corbett and Michalak 2000). For example, ER luminal Ca2+ dictates many functions of the ER, including protein synthesis and modification, protein folding and quality control, activation of unfolded protein responses (UPRs), interchaperone interactions, lipid synthesis, and cholesterol metabolism (Sambrook 1990; Corbett et al. 2000; Groenendyk et al. 2013; Wang et al. 2017).
Figure 1.
The cellular reticular network. Intracellular organelles are organized into the cellular reticular network that is linked by membrane contact sites ([MCSs], in red) allowing for rapid exchange of molecules between component of the network. DHR, Plasma membrane dihydropyridine Ca2+ channel receptor; RyR, ryanodine receptor/Ca2+ channel; InsP3R, inositol 1,4,5-trisphosphate receptor; STIM, ER Ca2+ sensor; ORAI, plasma membrane Ca2+ channel; ER, endoplasmic reticulum.
ENDOPLASMIC RETICULUM
The ER is a major component of the CRN and a main Ca2+ storage organelle with a tightly regulated total (i.e., free and bound Ca2+) intraluminal Ca2+ concentration in excess of 2 mm. The free Ca2+ concentration in the lumen of ER is maintained at the 10–500 µm range (Corbett and Michalak 2000; Yu and Hinkle 2000; Solovyova et al. 2002). Ca2+ is released from the ER via the inositol 1,4,5-trisphosphate receptor/Ca2+ channel (InsP3R) or ryanodine receptor/Ca2+ channel (RyR) (Krebs et al. 2015; Berridge 2016). The resulting increase in cytoplasmic Ca2+ level regulates many cellular processes, including cell proliferation, metabolism, and apoptosis (Berridge et al. 2000). The depletion of ER Ca2+ triggers Ca2+ entry from the extracellular space via store-operated Ca2+ entry (SOCE), which further sustains cytoplasmic Ca2+ signal and, most importantly, supplies Ca2+ for refilling of the ER Ca2+ store (Berridge et al. 2000; Roos et al. 2005). Stromal interaction molecule 1 (STIM1) is an ER membrane type I transmembrane protein that acts as an ER Ca2+ sensor (Liou et al. 2005; Roos et al. 2005; Zhang et al. 2005). The EF-hand of STIM1 binds Ca2+ (Kd = ∼0.2–0.6 mm) within the lumen of the ER (Stathopulos et al. 2006; Zheng et al. 2008) and on ER Ca2+ depletion, STIM1 extends its conformation, clusters, moves toward the plasma membrane, and binds to the cytosolic domain of Orai1, a plasma membrane Ca2+ channel, to form MCS between the ER and the plasma membrane (Feske et al. 2006; Peinelt et al. 2006; Stathopulos et al. 2006; Muik et al. 2008; Park et al. 2009; Bhardwaj et al. 2016; Phillips and Voeltz 2016; Nwokonko et al. 2017). The interaction between STIM1 and Orai1 at the MCS causes oligomerization of Orai1 Ca2+ channel and the consequence of influx of Ca2+ (Peinelt et al. 2006; Prakriya et al. 2006; Mignen et al. 2008; Penna et al. 2008; Hou et al. 2012). The sarco-ER Ca2+ ATPase (SERCA) pumps Ca2+ from the cytosol into the ER to refill Ca2+ stores (Krebs et al. 2015; Berridge 2016).
The ER also plays a central role in managing cellular stress via mobilization of ER stress-coping responses, such as the UPR. The UPR involves three unique ER transmembrane signaling proteins: the inositol-requiring 1 (IRE1), ER kinase dsRNA-activated protein kinase-like ER kinase (PERK), and activating transcription factor 6 (ATF6) (Groenendyk et al. 2013; Wang and Kaufman 2016; Hetz and Papa 2018). Activation of ER stress-induced UPR signaling pathways result in translational attenuation and in transcriptional activation of genes encoding proteins involved in protein folding as well as genes for components of the ER-associated degradation pathway (Groenendyk et al. 2013; Wang and Kaufman 2016; Hetz and Papa 2018). Prolonged ER Ca2+ depletion is a potent activator of UPR (Mekahli et al. 2011; Groenendyk et al. 2013). ER luminal Ca2+-binding proteins, notably BiP/GRP78 and PDIA6, regulate UPR signaling and illustrate the important link between ER Ca2+ homeostasis and ER stress responses (Higo et al. 2010; Groenendyk et al. 2013, 2014; Hetz and Papa 2018).
There are two categories of Ca2+-handling proteins as defined by their Ca2+-binding properties. The first binds Ca2+ with low affinity but high capacity. These proteins contribute to the Ca2+ storage and buffering capacity within organelles. The second binds Ca2+ with high affinity but low capacity. These proteins act as Ca2+ sensors as well as conveyors of Ca2+-dependent events into downstream components of intracellular signaling pathways (Krebs et al. 2015). Additionally, there are a variety of Ca2+-binding protein chaperones and folding enzymes localized within the lumen and membrane of the ER.
Calreticulin
Calreticulin is an ER luminal protein chaperone and a major Ca2+-binding protein that is important for maintaining 50% of the total Ca2+ within the ER Ca2+ (Gelebart et al. 2005). Calreticulin is 400 amino acid and 46 kDa protein and is composed of three structural and functional domains. The highly conserved N-globular domain contains a disulfide-linkage and binding sites for polypeptides, carbohydrates, and Zn2+ (Baksh et al. 1995; Schrag et al. 2001; Leach et al. 2002; Kapoor et al. 2004). The P-domain is the proline-rich extended arm of calreticulin that contains a binding site for the thiol oxidoreductase ERp57/PDIA3 and a high-affinity (Kd = 1 µm) but low-capacity (1 mol of Ca2+/mol of protein) Ca2+-binding site (Baksh and Michalak 1991; Tjoelker et al. 1994; Ellgaard et al. 2001, 2002; Frickel et al. 2002). Together, the N- and P-domains comprise the protein chaperone unit of calreticulin (Nakamura et al. 2001). The C-domain contains many acidic residues and is the Ca2+-binding unit of calreticulin, binding Ca2+ with low affinity (Kd = 2 mm) but at high capacity (20–30 mol of Ca2+/mol of protein) (Tjoelker et al. 1994; Nakamura et al. 2001).
The role of calreticulin in maintaining ER homeostasis is illustrated in the effects of its loss- or gain-of-function on ER Ca2+-signaling dynamics. Loss of calreticulin in mice is embryonically lethal owing to inadequate ER Ca2+ signaling. This is attributed to both the chaperone and Ca2+ storage function of calreticulin (Mesaeli et al. 1999). In the absence of calreticulin, ER Ca2+ stores, as measured by thapsigargin-induced total ER Ca2+ release, is reduced by 50% (Nakamura et al. 2001). Furthermore, calreticulin-deficient cells were no longer responsive to bradykinin-induced ER Ca2+ release, but permeabilized calreticulin-deficient cells remained responsive to InsP3-induced ER Ca2+ release (Nakamura et al. 2001). The lack of response to bradykinin in calreticulin-deficient cells was caused by the absence of the chaperone function of calreticulin, which resulted in the misfolding of the plasma membrane bradykinin receptor and thus the loss of ability of cells to interact with bradykinin (Nakamura et al. 2001). This disrupted Ca2+ signaling from the ER caused by the absence of calreticulin led to insufficient activation of calcineurin and subsequent failure to stimulate transcription factors that induce the expression of responsive genes (Mesaeli et al. 1999; Guo et al. 2002; Lynch et al. 2005). In mice, calreticulin deficiency results in embryonic lethality owing to failure in cardiac development (Mesaeli et al. 1999). Remarkably, the lethality caused by calreticulin deficiency could be prevented by targeted expression of a constitutively active calcineurin solely in cardiac cells of mice with whole-body deficiency of calreticulin (Guo et al. 2002). On the other hand, overexpression of calreticulin increased total and free ER Ca2+ concentration (Bastianutto et al. 1995; Mery et al. 1996; Arnaudeau et al. 2002). This also led to a reduction in mitochondria Ca2+ causing mitochondrial damage and increasing cellular sensitivity to apoptosis (Arnaudeau et al. 2002). The increase in ER luminal Ca2+ concentration caused by calreticulin overexpression reduced STIM1 sensitivity to ER Ca2+, thereby decreasing and delaying SOCE activity (Mery et al. 1996).
The expected discovery of hyperlipidemia in calreticulin-deficient mice rescued from embryonic lethality by cardiac-specific expression of constitutively active calcineurin suggested a link between Ca2+ and lipid homeostasis (Guo et al. 2002). It is now evident that the decrease in ER luminal Ca2+ caused by the loss of calreticulin (Mesaeli et al. 1999; Nakamura et al. 2001) shifts the distribution of intracellular unesterified cholesterol, resulting in the depletion of the regulatory pool of cholesterol that controls cholesterol biosynthesis (Wang et al. 2017).
Immunoglobulin-Binding Protein/Glucose-Regulated Protein 78 (BiP/GRP78)
The molecular chaperone BiP/GRP78 binds hydrophobic regions of nascent proteins through the carboxy-terminal domain (C chain) and facilitates proper protein folding at the expense of ATP, as it is also an ATPase with an ATP/ADP-binding site in the N-domain (A chain) (Gaut and Hendershot 1993; Awad et al. 2008). BiP/GRP78 is a key player in sensing ER stress through binding to accumulating misfolded proteins. However, it is also important for buffering 25% of total ER Ca2+ stores (Lievremont et al. 1997). The protein has four Ca2+ ion-binding acidic amino acid residues; two Ca2+ ions are accommodated in opposite chains by the coordination of His-252, Asp-257, and Gly-315; two other Ca2+ ions are held at the base of α-helix-6 by the hydroxyl group of His-252 and the carboxylate group of Glu-256 and Asp-257 (Wisniewska et al. 2010). Although BiP/GRP78 binds Ca2+ with low capacity (1–2 mol Ca2+/mol of protein), it contributes to ER Ca2+ buffering owing to its high abundance in the lumen of the ER (∼5-fold higher abundance than calreticulin) (Lievremont et al. 1997).
There is an intrinsic relationship between the chaperone and the Ca2+-binding functions of BiP/GRP78. Stoichiometrically, the binding affinity of BiP/GRP78 to Ca2+ changes in the presence of ATP or ADP. It binds Ca2+ with a Kd of ∼0.7 mm in the absence of ATP and ADP; a Kd of ∼18 µm in the presence of ATP; and a Kd of ∼0.8 µm in the presence of ADP (Lamb et al. 2006). The reverse is also true; in the presence of Ca2+, the binding affinity of ATP to BiP/GRP78 increases ∼11-fold and the binding of ADP increases ∼930-fold (Lamb et al. 2006). Functionally, the ER Ca2+ level regulates the chaperone activity of BiP/GRP78 as reduction in ER Ca2+ levels causes dissociation of BiP/GRP78 from client proteins, and increases in ER Ca2+ level inhibits BiP/GRP78-associated ATPase activity (Kassenbrock and Kelly 1989; Suzuki et al. 1991). This interconnected relationship between ER Ca2+ store and signaling is a dynamic contributor to Ca2+ binding and chaperone activity of BiP/GRP78. Therefore, the abundance of BiP/GRP78 modulates ER–mitochondria Ca2+ flux and may play a protective role against mitochondria-induced apoptosis, depending on the level or duration of stress (Lievremont et al. 1997; Liu et al. 1997; Deniaud et al. 2008; Ouyang et al. 2011). Furthermore, BiP/GRP78 is responsible for selectively gating and closing of ribosome-associated Sec61 translocon to prevent Ca2+ leakage at ER–mitochondria MCS and induction of apoptosis from ER stress (Haigh and Johnson 2002; Van Coppenolle et al. 2004; Alder et al. 2005; Schauble et al. 2012; Hammadi et al. 2013; Gutiérrez and Simmen 2018).
Glucose-Regulated Protein
The glucose-regulated protein (GRP94) is an ER protein chaperone that is involved in the folding and secretion of membrane proteins (Marzec et al. 2012). GRP94 is a heat shock protein 90 (HSP90)-like protein that contains an ATP-binding domain within the amino-terminal region, a client-binding region, and a dimerization domain within the carboxyl terminus (Marzec et al. 2012). In addition, GRP94 has a peptide-binding region in its amino-terminal region (Biswas et al. 2007). This major chaperone is involved in the folding of immunoglobulins (Melnick et al. 1994) and Toll-like receptors (Randow and Seed 2001). The peptide-binding activity of GRP94 is important for the immune response during T-cell activation (Berwin et al. 2002; Li et al. 2005). GRP94 possesses four Ca2+-binding sites with high affinity (Kd = ∼2 µm) and 11 Ca2+-binding sites with low affinity (Kd = ∼600 µm) (Van et al. 1989), an EF-hand-like structure (Csermely et al. 1995), and is able to accommodate 16–28 mol of Ca2+/mol of protein (Macer and Koch 1988; Van et al. 1989). In addition, the ER Ca2+ level affects the peptide-binding activity of GRP94; specifically, Ca2+ binding within the amino terminus (amino acids 266–355) induces a conformational change that favors peptide interaction (Van et al. 1989; Biswas et al. 2007).
The expression of GRP94 proteins is stimulated by induction of the UPR during ER stress, resulting from misfolding of proteins or perturbed ER Ca2+ homeostasis (Lee 1992). Depletion of GRP94 does not cause ER stress nor loss of ER Ca2+ homeostasis (Poirier et al. 2015); however, cells lacking GRP94 were more susceptible to thapsigargin-induced ER Ca2+ depletion and cell death (Biswas et al. 2007). Expression of GRP94, similar to BiP/GRP78, showed antiapoptotic properties and protected cells from Ca2+ depletion-induced stress and apoptosis (Little and Lee 1995). Furthermore, the cleavage or degradation of GRP94 is mediated through the activity of Ca2+-dependent calpains during DNA damage–induced apoptosis (Reddy et al. 1999). Overexpression of GRP94 protected cardiomyocytes from intracellular Ca2+ overload and ischemia (Bando et al. 2003; Vitadello et al. 2003).
Protein Disulfide Isomerase
The protein disulfide isomerase (PDI) family of proteins were discovered in 1963 as ER proteins involved in co- and posttranslational modification of nascent synthesized proteins. Specifically, these proteins are important in the formation and cleavage of disulfide bonds (Goldberger et al. 1963), which is necessary for the proper folding of cysteine-containing proteins (Creighton et al. 1980). Subsequently, PDIs were identified as calsequestrin-like proteins of the ER (Oberdorf et al. 1988) and later glycoproteins with Ca2+-binding properties (Lebeche and Kaminer 1992). Mammalian PDIs have been shown to bind Ca2+ at high capacity (averaging at 19 mol of Ca2+/mol of protein) but with low affinity (Kd = 4.7 mm) (Lebeche et al. 1994). The Ca2+-binding region of PDIs consists of a number of paired acidic residues within the carboxyl terminus of the protein (Freedman et al. 1994; Lucero et al. 1998). As PDIs contribute to the Ca2+ store within the ER, the level of Ca2+ in the ER (1–5 mm) in turn affects their overall enzymatic activity (Lucero and Kaminer 1999).
Recent reports indicate that PDI family of proteins influence ER stress responses and impact on the ER and cellular Ca2+ homeostasis. For example, PDIA6 plays a role in regulation of IRE1α activity in response to ER Ca2+ depletion (Groenendyk et al. 2014). PDIA3 (ERp57) interacts with, and regulates, STIM1 activity, thereby impacting on SOCE and cytoplasmic Ca2+ signaling (Prins et al. 2011), in addition to its key role in the quality control of newly synthesized glycoproteins (Coe and Michalak 2010). Furthermore, PDIA3 modulates the redox state of ER-facing thiols in SERCA2b in a Ca2+-dependent manner (Li and Camacho 2004) and PDIA10 (ERp44) modulates Ca2+ release by InsP3R (Higo et al. 2005).
SARCOPLASMIC RETICULUM
In some cells, the ER is further subspecialized into rough ER, such as in secretory cell types, and smooth ER, such as in cells that actively synthesize and metabolize lipids. In muscle cells, in addition to perinuclear rough and smooth ER, the ER is subspecialized into the SR (Fig. 1), which is responsible for the regulation of excitation–contraction–coupling to facilitate muscle contraction and relaxation (Bers 2014). The presence of highly specialized SR membrane networks in muscle cells supports mechanical functions requiring large fluxes of Ca2+, without compromising other important Ca2+-requiring cellular processes that are normally associated with ER (Michalak and Opas 2009). There are two well-defined structural and functional regions of the SR. The longitudinal SR, a membrane network around contractile myofibrils extending into the junctional SR, is a membrane network with multiple MCS with T-tubules formed from invaginations in the plasma membranes of muscle cells such as cardiomyocytes (Wray and Burdyga 2010; Bers 2014). The longitudinal SR is enriched with SERCA, and is responsible for rapid removal of Ca2+ from the cytoplasm to initiate muscle relaxation (Wray and Burdyga 2010; Bers 2014). The junctional SR contains the RyR, responsible for Ca2+ release to the cytoplasm to initiate muscle contraction, and calsequestrin, a major Ca2+-binding protein of muscle (Rossi and Dirksen 2006; Wray and Burdyga 2010; Bers 2014). The SR luminal Ca2+-binding proteins calsequestrin, histidine-rich calcium-binding protein, junctate, and sarcalumenin are responsible for Ca2+ storage, whereas RyR is responsible for Ca2+ release to trigger muscle contraction.
Calsequestrin
Calsequestrin is the major Ca2+-binding protein within the SR, binding Ca2+ with high capacity (40–50 mol of Ca2+/mol of protein) but low affinity (Kd = ∼1 mm) (MacLennan and Wong 1971). Two isoforms of calsequestrin exist and are encoded by two distinct genes: skeletal muscle calsequestrin (CASQ1) and cardiac calsequestrin (CASQ2) (Györke et al. 2009; Knollmann 2009). Structurally, calsequestrin is composed of three thioredoxin-like domains linked together to form a monomer with a hydrophilic core and a carboxyl terminus that contains the majority of paired acidic residues for Ca2+ binding (Wang et al. 1998). These Ca2+-binding sites have been termed the consecutive aspartate stretch at the carboxy-terminal domain (Kumar et al. 2013). The structure and oligomerization state of calsequestrin is dependent on the concentration of Ca2+ within the SR lumen (Ikemoto et al. 1974). Calsequestrin can dimerize and polymerize, and can exist as stable polymers at a Ca2+ concentration of 1 mm (the physiological stable level of Ca2+ within the SR lumen) (Franzini-Armstrong et al. 1987; Wang et al. 1998). The binding of calsequestrin monomers to Ca2+ mediates the oligomeric state of calsequestrin, as shown through crystallization studies (Sanchez et al. 2012). High Ca2+ concentration in the junctional SR supports calsequestrin polymerization (Wang et al. 1998; Beard et al. 2005), whereas Ca2+ depletion in the junctional SR causes calsequestrin depolymerization (Manno et al. 2017). The carboxyl terminus portion of calsequestrin contains a disordered region that is involved in the Ca2+-dependent polymerization properties of these proteins and accounts for the unique polymerization differences between calsequestrin 1 and 2 isoforms (Bal et al. 2015). These conformational changes are important for the role of calsequestrin in regulating Ca2+ storage and release.
Mice deficient in skeletal muscle calsequestrin (CASQ1) are viable but they show reduced Ca2+ release and Ca2+ transients (Paolini et al. 2007). Deficiency in cardiac calsequestrin (CASQ2) leads to an increased diastolic SR Ca2+ leak and development of catecholaminergic ventricular arrhythmias (Knollmann et al. 2006). Mutations in the CASQ2 gene cause a severe form of catecholaminergic polymorphic ventricular tachycardia (CPVT) (Faggioni et al. 2012).
Histidine-Rich Ca2+-Binding Protein
The histidine-rich Ca2+-binding protein (HRC) was first discovered in 1989 as a 165 kDa Ca2+-binding protein within the lumen of the SR (Campbell et al. 1983; Hofmann et al. 1989). HRC binds Ca2+ with high capacity (reported at 200 nmol of Ca2+/mg of protein) but low affinity (Kd = 1.9 mm) (Picello et al. 1992). Overexpression of HRC in rat cardiomyocytes (Fan et al. 2004) and rat neonatal cardiomyocytes increased SR Ca2+ store content and prevented SR Ca2+ depletion induced by cyclopiazonic acid (Kim et al. 2003).
HRC has also been found to be important for Ca2+ cycling within the SR lumen. The conformation of HRC, and its interaction with other proteins, are Ca2+-dependent. HRC can exist as a multimeric complex, which dissociates on Ca2+ binding (conditions of high Ca2+ concentration) caused by changes in conformation (Suk et al. 1999). Similar to calsequestrin, the HRC protein can bind to triadin, a junctional SR protein, through its carboxy-terminal region in a Ca2+-dependent fashion (Lee et al. 2001; Sacchetto et al. 2001). Furthermore, HRC forms a complex with SERCA and triadin to modulate Ca2+ uptake and release from the SR (Gregory et al. 2006; Arvanitis et al. 2007). Mice deficient for HRC showed increases in Ca2+ signaling, cardiomyocyte contractility, and rate of SR Ca2+ uptake (Park et al. 2013). Therefore, HRC is integral to the maintenance of Ca2+ homeostasis within the SR.
Junctate
Junctate is a newly described protein that is expressed within the junctional SR. It is a 33-kDa single-pass integral SR membrane protein with the amino-terminal portion facing the cytoplasm and the carboxy-terminal portion within the SR lumen, and acts as a high-capacity/low-affinity Ca2+-binding protein (Treves et al. 2000). The carboxy-terminal luminal portion of junctate is enriched in negatively charged amino acid residues and the protein is able to bind 21 mol of Ca2+/mol of protein with an affinity of Kd = 217 µm (Treves et al. 2000). Junctate has a significant influence on SR Ca2+ store capacity. Specifically, overexpression of junctate in mouse skeletal muscle increased both the SR Ca2+ store content and Ca2+ release on induction (Divet et al. 2007). However, in cardiomyocytes isolated from transgenic mice, overexpression of junctate resulted in a reduced SR function and reduced abundance of SERCA and calsequestrin. This resulted in disrupted cardiac Ca2+ transients, inducing cardiac hypertrophy (Hong et al. 2008). On the other hand, the same investigators showed that overexpression of junctate in rat cardiomyocytes infected with junctate adenovirus system led to an increase in SERCA2a activity and Ca2+ uptake (Kwon and Kim 2009).
Additionally, junctate appears to play a major role in maintaining Ca2+ homeostasis in eukaryotic cells through the regulation of Ca2+ uptake/release and SOCE. Junctate was found to interact with SERCA2a, the cardiac SERCA isoform, in the SR of cardiomyocytes via its carboxy-terminal domain, and the luminal domain of SERCA2a (Kwon and Kim 2009). Junctate also regulates Ca2+ entry and stabilizes ER–plasma membrane junctions through complex interaction with InsP3R and transient receptor potential protein 3 (TRPC3) cation channels (Treves et al. 2004). Moreover, studies have implicated junctate as a component of the STIM1 and Orai1 complex that mediates SOCE (Srikanth et al. 2012; Guido et al. 2015). This interaction was shown to be important in Ca2+ signaling within immune cells, specifically affecting Ca2+ dynamics at both ER–plasma membrane junctions (Srikanth et al. 2012) and ER-phagosome junctions (Guido et al. 2015).
Sarcalumenin
Sarcalumenin was discovered as the 160-kDa protein variant generated by alternative splicing of a messenger RNA (mRNA) transcript of a gene that encodes a 53-kDa SR glycoprotein (Leberer et al. 1989). The protein binds Ca2+ at high capacity (30–35 mol of Ca2+/mol of protein) but with low affinity (Kd = 300 µm) (Leberer et al. 1990). The skeletal muscle of sarcalumenin-deficient mice showed delayed relaxation phase following contraction and reduced Ca2+ uptake into the SR (Yoshida et al. 2005). Similarly, the cardiac performance of sarcalumenin-deficient mice was impaired. Further analysis of sarcalumenin-deficient cardiomyocytes revealed slow contraction and relaxation along with disrupted Ca2+ signaling (Yoshida et al. 2005). These mice, however, showed an increase in SOCE and a resistance to muscle fatigue in response to treadmill exercise (Zhao et al. 2005). Furthermore, sarcalumenin-deficient mice displayed reduced cardiac function in the presence of biomechanical stress. This was attributed to the absence of sarcalumenin function in regulating SR Ca2+ handling through its interaction with SERCA (Shimura et al. 2008; Jiao et al. 2009, 2012).
MITOCHONDRIA
Mitochondrial Ca2+ uptake and accumulation are important components of cellular Ca2+ homeostasis and signaling. This process does not depend on any mitochondrial Ca2+-buffering proteins, but rather on the membrane potential difference resulting from the electrochemical gradient generated by the H+ ions pumped into the intermembrane space by the electron transport chain. This membrane potential drives Ca2+ from the cytoplasm through the outer and inner mitochondrial membranes. The outer mitochondrial membrane is ion permeable owing to the presence of large-conductance voltage-dependent anion channels, which are also permeable to Ca2+ ions (Rapizzi et al. 2002). Ca2+ transport across the ion impermeable inner mitochondrial membrane occurs through the mitochondrial Ca2+ uniporter complex (Kirichok et al. 2004), which allows rapid accumulation of Ca2+ into the mitochondrial matrix at a Vmax > 1400 nmol of Ca2+/mg of protein/min (Bragadin et al. 1979). Mitochondrial Ca2+ uptake occurs at ER–mitochondria MCS in proximity to ER membrane Ca2+channels such as the InsP3R and the RyR, and depends on the high Ca2+ concentration hotspots at these microdomains (Rizzuto et al. 1993, 1998; Szalai et al. 2000).
In this sense, the generation of mitochondrial Ca2+ hotspots (Csordas et al. 2010; Giacomello et al. 2010), together with the rapid and large accumulation of Ca2+ within mitochondria on stimulation, suggests a role for mitochondria as a Ca2+-buffer within the cell. This might also enable modulation of Ca2+ levels and thus Ca2+ signaling in the immediate proximity of mitochondria. The InsP3R activity is regulated by cytoplasmic Ca2+ concentrations (Bezprozvanny et al. 1991) and mitochondrial uptake or sequestering of Ca2+ during ER Ca2+ release suppresses Ca2+ feedback activation of InsP3R (Hajnoczky et al. 1999). Moreover, Ca2+ uptake by mitochondria can occur near Orai Ca2+ channels on the plasma membrane and thus modulate channel activity during SOCE (Hoth et al. 1997; Gilabert and Parekh 2000). Furthermore, Ca2+ uptake by mitochondria stimulates the tricarboxylic acid (TCA) cycle and oxidative phosphorylation and prevents induction of autophagy (Cardenas et al. 2016; Singh et al. 2017; Bootman et al. 2018; Morciano et al. 2018).
GOLGI APPARATUS
The Golgi apparatus accounts for up to 5% of the total cellular Ca2+ store (Chandra et al. 1991). The Golgi Ca2+ store is important for the optimal function of certain enzymes within the organelle (Oda 1992; Carnell and Moore 1994), retrograde transport (Ivessa et al. 1995; Micaroni et al. 2010), and secretory protein sorting (Chanat and Huttner 1991; Micaroni et al. 2010). The Golgi Ca2+ store is sensitive to InsP3 production as there are InsP3R in Golgi structures (Pinton et al. 1998). However, the Ca2+ released from InsP3R on Golgi membranes is functionally distinct from that originating from ER Ca2+ stores (Vanoevelen et al. 2004). The Golgi Ca2+ store is maintained via the activity of SERCA and the secretory pathway Ca2+ ATPase1 (SPCA1), which is able to pump Ca2+ from the cytoplasm into the Golgi (Wuytack et al. 2003; Micaroni et al. 2010; Vandecaetsbeek et al. 2011). Although Ca2+ is present within the lumen of the entire Golgi network (Pezzati et al. 1997), the handling of Ca2+ across the Golgi network is heterogeneous (Wong et al. 2013; Aulestia et al. 2015). There is a Ca2+ gradient within the Golgi apparatus whereby the Ca2+ concentration within the lumen of the Golgi is higher than that of trans-Golgi (but lower than that of the ER) (Wong et al. 2013). Furthermore, the cis-Golgi accumulates Ca2+ mostly from SERCA activity, whereas the trans-Golgi accumulates Ca2+ through the action of SPCA1 (Aulestia et al. 2015). In addition, Ca2+ release via InsP3R occurs only from the cis- and the second trans-Golgi portion, whereas Ca2+ release from all of Golgi can be induced by caffeine (Aulestia et al. 2015). The heterogeneity of Ca2+ concentration in the Golgi may reflect differing regulatory functions for Ca2+ within the different Golgi compartments.
CALNUC
CALNUC is a major Ca2+-binding protein within the Golgi apparatus and is highly homologous to calreticulin (Lin et al. 1998). Structurally, it consists of a signal peptide, followed by a basic amino acid region, an acidic amino acid region that makes up two EF-hand motifs (de Alba and Tjandra 2004), and a leucine zipper motif (Miura et al. 1992; Lin et al. 1998). CALNUC binds Ca2+ with high affinity (Kd = 6.6 µm) but at low capacity (1.1 mol of Ca2+/mol of protein) (Lin et al. 1999), and accounts for the majority of Golgi Ca2+ stores by virtue of its high abundance in this organelle (∼3.8 µg of CALNUC/mg of total Golgi protein) (Lin et al. 1999). CALNUC has been recently implicated in the endosome-to-trans-Golgi retrograde transport of lysosomal receptors the recruitment of retromers to endosomes (Larkin et al. 2016).
Calumenin
Calumenin is also a part of the CREC (acronym derived from the four main family members: Cab45, reticulocalbin1, ERC-55, and calumenin; Yabe et al. 1998) family of EF-hand-containing proteins and is localized to both the ER and the Golgi (Vorum et al. 1998). The protein contains an amino-terminal signal sequence, an HDEF ER retrieval signal, and seven EF-hand motifs, each with low Ca2+-affinity (Kd = 1.6 mm) (Vorum et al. 1998). Calumenin can be further processed and secreted by cultured cells (Vorum et al. 1999). Furthermore, it is highly expressed within the brain during development and may play a role in neuronal Ca2+-signaling (Vasiljevic et al. 2012).
Cab45 was the first Ca2+-binding luminal-resident protein of the Golgi apparatus to be discovered, and is a 45-kDa protein belonging to the superfamily of CREC proteins (Scherer et al. 1996; Honore and Vorum 2000). Cab45 is a soluble protein and contains an amino-terminal signal sequence and six EF-hand motifs (Scherer et al. 1996). The function of Cab45 includes sorting of secretory proteins within the trans-Golgi and occurs in a SPCA-1- and Ca2+-dependent manner (von Blume et al. 2012). The binding of Ca2+ evokes an oligomerized state that enables Cab45 activity (Crevenna et al. 2016). Oligomerized Cab45 binds a variety of proteins destined for cellular secretion. Cab45 plays an important Ca2+-dependent role in sorting cargo export at the trans-Golgi membrane through its association with SPCA-1 and actin (Blank and von Blume 2017).
p54/NEFA
The DNA-binding, EF-hand, acidic region (p54/NEFA) protein contains a basic region, two EF-hand motifs and a leucine zipper repeat (Karabinos et al. 1996). p54/NEFA is a resident of medial Golgi and is retained within the ER through its amino-terminal Leu/Ile rich region (Nesselhut et al. 2001; Morel-Huaux et al. 2002).
ENDOSOME/LYSOSOME/PEROXISOME/PHAGOSOME
Endosomes and lysosomes have emerging roles in Ca2+ storage and signaling. As endosomes mature into late endosomes and subsequently fuse with lysosomes, the gradual acidification of the vesicles is accompanied by a concomitant increase in Ca2+ concentration (Gerasimenko et al. 1998; Pryor et al. 2000). The concentration of Ca2+ within the lumen of lysosomes is estimated to range from 400 µm to 600 µm (Christensen et al. 2002; Lloyd-Evans et al. 2008). Ca2+ release from the endosome/s/lysosomes shown to be sensitive to nicotinic acid adenine dinucleotide phosphate (NAADP) treatment is mediated by the RyR and TPC2 channels (Mojzisova et al. 2001; Hohenegger et al. 2002; Gerasimenko et al. 2003; Brailoiu et al. 2010; Lin-Moshier et al. 2014; Davis et al. 2015; Galione 2015; Hockey et al. 2015; Ruas et al. 2015) and is distinct from Ca2+ released from InsP3-sensitive ER stores (Lee and Aarhus 1995; Genazzani and Galione 1996; Lopez-Sanjurjo et al. 2013). The transient receptor potential (TRPML) channels belonging to the mucolipin family and the two-pore channels are also involved in the release of Ca2+ from the acidic stores in these organelles (Patel and Muallem 2011; Kilpatrick et al. 2013, 2016; Penny et al. 2015; Atakpa et al. 2018).
Currently, the existence of endosomal/lysosomal Ca2+-buffering proteins are unknown. The uptake and accumulation of Ca2+ within these acidic stores seem to be highly dependent on pH (Christensen et al. 2002; Morgan et al. 2013). Uptake of Ca2+ into lysosomes occurs through the H+/Ca2+ exchanger, vacuolar (V)-type H+-ATPase (Pryor et al. 2000), and Ca2+/H+ exchanger (CAX) (Melchionda et al. 2016). Furthermore, the Ca2+ storage and signaling capacity of the endosomal/lysosomal pathway is highly dependent on Ca2+ signaling and exchange with the ER at ER-endosomal/-lysosomal MCS (Penny et al. 2015; Ronco et al. 2015). Movement of Ca2+ between the ER and the endosomal/lysosomal pathway is important for the progression, maturation, fusion events of the endosomes/lysosomes, and autophagy (Huotari and Helenius 2011; Coen et al. 2012; Bootman et al. 2018).
Peroxisomes are capable of storing Ca2+ within their lumen at concentrations higher than that in cytoplasm (Lasorsa et al. 2008; Costa et al. 2013). However, it is not known how Ca2+ is stored or buffered in this organelle. It is thought that Ca2+ is involved in the regulation of autophagy, a process that delivers proteins and damaged organelles to the lysosome for breakdown to promote cell survival under conditions of nutrient deprivation (Decuypere et al. 2011; Tong and Song 2015). The fate of Ca2+ trapped during phagosomal formation and how it is stored in phagosome is not known.
SUMMARY
It is evident that there is rich variety of Ca2+-handling proteins that bind the ion with differing affinities and capacities distributed throughout the cell, and especially within the organellar components that make up the CRN. Ca2+-handling proteins that bind Ca2+ with high affinity but low capacity likely function as sensors or conveyors of Ca2+-dependent signaling events, whereas those that bind Ca2+ with low affinity but high capacity are likely involved in storage or buffering of Ca2+ in the local milieu. The rapid movement of Ca2+ and communication of luminal Ca2+ status, among the different compartments of the CRN may be partly facilitated through MCS, which themselves are characterized by a unique set of accessory proteins, including specific Ca2+-handling proteins, depending on the membranes involved in the formation of the MCS. A recent study illustrated that luminal ER Ca2+ status has a dramatic impact on the intracellular distribution of unesterified cholesterol, and thus alters the set point of the basal-sensing mechanism responsible for cholesterol homeostasis. In the years to come, it will be interesting to uncover how the diverse collection of Ca2+-handling proteins within the cell are networked to coordinate the manifold and simultaneous cellular processes that enable the cell to exist and carry out its functions.
ACKNOWLEDGMENTS
Work in our laboratories is supported by the Canadian Institutes of Health Research (CIHR) Grants MOP-15291, MOP-15415, and PS-153325 to M.M.; a generous donation from the Kenneth and Sheelagh McCourt family; CIHR Grant MOP-15291 and PS-153325 to L.B.A. W.-A.W. was supported by a CIHR studentship.
Footnotes
Editors: Geert Bultynck, Martin D. Bootman, Michael J. Berridge, and Grace E. Stutzmann
Additional Perspectives on Calcium Signaling available at www.cshperspectives.org
REFERENCES
- Agellon LB, Michalak M. 2017. The endoplasmic reticulum and the cellular reticular network. Adv Exp Med Biol 981: 61–76. 10.1007/978-3-319-55858-5_4 [DOI] [PubMed] [Google Scholar]
- Alder NN, Shen Y, Brodsky JL, Hendershot LM, Johnson AE. 2005. The molecular mechanisms underlying BiP-mediated gating of the Sec61 translocon of the endoplasmic reticulum. J Cell Biol 168: 389–399. 10.1083/jcb.200409174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaudeau S, Frieden M, Nakamura K, Castelbou C, Michalak M, Demaurex N. 2002. Calreticulin differentially modulates calcium uptake and release in the endoplasmic reticulum and mitochondria. J Biol Chem 277: 46696–46705. 10.1074/jbc.M202395200 [DOI] [PubMed] [Google Scholar]
- Arvanitis DA, Vafiadaki E, Fan GC, Mitton BA, Gregory KN, Del Monte F, Kontrogianni-Konstantopoulos A, Sanoudou D, Kranias EG. 2007. Histidine-rich Ca-binding protein interacts with sarcoplasmic reticulum Ca-ATPase. Am J Physiol Heart Circ Physiol 293: H1581–H1589. 10.1152/ajpheart.00278.2007 [DOI] [PubMed] [Google Scholar]
- Atakpa P, Thillaiappan NB, Mataragka S, Prole DL, Taylor CW. 2018. IP3 receptors preferentially associate with ER-lysosome contact sites and selectively deliver Ca2+ to lysosomes. Cell Rep 25: 3180–3193.e7. 10.1016/j.celrep.2018.11.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulestia FJ, Alonso MT, Garcia-Sancho J. 2015. Differential calcium handling by the cis and trans regions of the Golgi apparatus. Biochem J 466: 455–465. 10.1042/BJ20141358 [DOI] [PubMed] [Google Scholar]
- Awad W, Estrada I, Shen Y, Hendershot LM. 2008. BiP mutants that are unable to interact with endoplasmic reticulum DnaJ proteins provide insights into interdomain interactions in BiP. Proc Natl Acad Sci 105: 1164–1169. 10.1073/pnas.0702132105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baksh S, Michalak M. 1991. Expression of calreticulin in Escherichia coli and identification of its Ca2+ binding domains. J Biol Chem 266: 21458–21465. [PubMed] [Google Scholar]
- Baksh S, Spamer C, Heilmann C, Michalak M. 1995. Identification of the Zn2+ binding region in calreticulin. FEBS Lett 376: 53–57. 10.1016/0014-5793(95)01246-4 [DOI] [PubMed] [Google Scholar]
- Bal NC, Jena N, Chakravarty H, Kumar A, Chi M, Balaraju T, Rawale SV, Rawale JS, Sharon A, Periasamy M. 2015. The C-terminal calcium-sensitive disordered motifs regulate isoform-specific polymerization characteristics of calsequestrin. Biopolymers 103: 15–22. 10.1002/bip.22534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bando Y, Katayama T, Kasai K, Taniguchi M, Tamatani M, Tohyama M. 2003. GRP94 (94 kDa glucose-regulated protein) suppresses ischemic neuronal cell death against ischemia/reperfusion injury. Eur J Neurosci 18: 829–40. 10.1046/j.1460-9568.2003.02818.x [DOI] [PubMed] [Google Scholar]
- Barneda D, Christian M. 2017. Lipid droplet growth: Regulation of a dynamic organelle. Curr Opin Cell Biol 47: 9–15. 10.1016/j.ceb.2017.02.002 [DOI] [PubMed] [Google Scholar]
- Bastianutto C, Clementi E, Codazzi F, Podini P, De Giorgi F, Rizzuto R, Meldolesi J, Pozzan T. 1995. Overexpression of calreticulin increases the Ca2+ capacity of rapidly exchanging Ca2+ stores and reveals aspects of their lumenal microenvironment and function. J Cell Biol 130: 847–855. 10.1083/jcb.130.4.847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann O, Walz B. 2001. Endoplasmic reticulum of animal cells and its organization into structural and functional domains. Int Rev Cytol 205: 149–214. 10.1016/S0074-7696(01)05004-5 [DOI] [PubMed] [Google Scholar]
- Beard NA, Casarotto MG, Wei L, Varsányi M, Laver DR, Dulhunty AF. 2005. Regulation of ryanodine receptors by calsequestrin: Effect of high luminal Ca2+ and phosphorylation. Biophys J 88: 3444–3454. 10.1529/biophysj.104.051441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. 2016. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol Rev 96: 1261–1296. 10.1152/physrev.00006.2016 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. 2000. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21. 10.1038/35036035 [DOI] [PubMed] [Google Scholar]
- Bers DM. 2014. Cardiac sarcoplasmic reticulum calcium leak: Basis and roles in cardiac dysfunction. Annu Rev Physiol 76: 107–127. 10.1146/annurev-physiol-020911-153308 [DOI] [PubMed] [Google Scholar]
- Berwin B, Rosser MF, Brinker KG, Nicchitta CV. 2002. Transfer of GRP94(Gp96)-associated peptides onto endosomal MHC class I molecules. Traffic 3: 358–366. 10.1034/j.1600-0854.2002.30505.x [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Watras J, Ehrlich BE. 1991. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature 351: 751–754. 10.1038/351751a0 [DOI] [PubMed] [Google Scholar]
- Bhardwaj R, Hediger MA, Demaurex N. 2016. Redox modulation of STIM-ORAI signalling. Cell Calcium 60: 142–152. 10.1016/j.ceca.2016.03.006 [DOI] [PubMed] [Google Scholar]
- Biswas C, Ostrovsky O, Makarewich CA, Wanderling S, Gidalevitz T, Argon Y. 2007. The peptide-binding activity of GRP94 is regulated by calcium. Biochem J 405: 233–241. 10.1042/BJ20061867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank B, von Blume J. 2017. Cab45—Unraveling key features of a novel secretory cargo sorter at the trans-Golgi network. Eur J Cell Biol 96: 383–390. 10.1016/j.ejcb.2017.03.001 [DOI] [PubMed] [Google Scholar]
- Bootman MD, Chehab T, Bultynck G, Parys JB, Rietdorf K. 2018. The regulation of autophagy by calcium signals: Do we have a consensus? Cell Calcium 70: 32–46. 10.1016/j.ceca.2017.08.005 [DOI] [PubMed] [Google Scholar]
- Bragadin M, Pozzan T, Azzone GF. 1979. Activation energies and enthalpies during Ca2+ transport in rat liver mitochondria. FEBS Lett 104: 347–351. 10.1016/0014-5793(79)80849-2 [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Rahman T, Churamani D, Prole DL, Brailoiu GC, Hooper R, Taylor CW, Patel S. 2010. An NAADP-gated two-pore channel targeted to the plasma membrane uncouples triggering from amplifying Ca2+ signals. J Biol Chem 285: 38511–38516. 10.1074/jbc.M110.162073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KP, MacLennan DH, Jorgensen AO. 1983. Staining of the Ca2+-binding proteins, calsequestrin, calmodulin, troponin C, and S-100, with the cationic carbocyanine dye “Stains-all.” J Biol Chem 258: 11267–11273. [PubMed] [Google Scholar]
- Cardenas C, Muller M, McNeal A, Lovy A, Jana F, Bustos G, Urra F, Smith N, Molgo J, Diehl JA, et al. 2016. Selective vulnerability of cancer cells by inhibition of Ca2+ transfer from endoplasmic reticulum to mitochondria. Cell Rep 15: 219–220. 10.1016/j.celrep.2016.03.045 [DOI] [PubMed] [Google Scholar]
- Carnell L, Moore HP. 1994. Transport via the regulated secretory pathway in semi-intact PC12 cells: Role of intra-cisternal calcium and pH in the transport and sorting of secretogranin II. J Cell Biol 127: 693–705. 10.1083/jcb.127.3.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanat E, Huttner WB. 1991. Milieu-induced, selective aggregation of regulated secretory proteins in the trans-Golgi network. J Cell Biol 115: 1505–1519. 10.1083/jcb.115.6.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Kable EP, Morrison GH, Webb WW. 1991. Calcium sequestration in the Golgi apparatus of cultured mammalian cells revealed by laser scanning confocal microscopy and ion microscopy. J Cell Sci 100: 747–752. [DOI] [PubMed] [Google Scholar]
- Christensen KA, Myers JT, Swanson JA. 2002. pH-dependent regulation of lysosomal calcium in macrophages. J Cell Sci 115: 599–607. [DOI] [PubMed] [Google Scholar]
- Coe H, Michalak M. 2010. ERp57, a multifunctional endoplasmic reticulum resident oxidoreductase. Int J Biochem Cell Biol 42: 796–799. 10.1016/j.biocel.2010.01.009 [DOI] [PubMed] [Google Scholar]
- Coen K, Flannagan RS, Baron S, Carraro-Lacroix LR, Wang D, Vermeire W, Michiels C, Munck S, Baert V, Sugita S, et al. 2012. Lysosomal calcium homeostasis defects, not proton pump defects, cause endo-lysosomal dysfunction in PSEN-deficient cells. J Cell Biol 198: 23–35. 10.1083/jcb.201201076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett EF, Michalak M. 2000. Calcium, a signaling molecule in the endoplasmic reticulum? Trends Biochem Sci 25: 307–311. 10.1016/S0968-0004(00)01588-7 [DOI] [PubMed] [Google Scholar]
- Corbett EF, Michalak KM, Oikawa K, Johnson S, Campbell ID, Eggleton P, Kay C, Michalak M. 2000. The conformation of calreticulin is influenced by the endoplasmic reticulum luminal environment. J Biol Chem 275: 27177–27185. [DOI] [PubMed] [Google Scholar]
- Costa A, Drago I, Zottini M, Pizzo P, Pozzan T. 2013. Peroxisome Ca2+ homeostasis in animal and plant cells. Subcell Biochem 69: 111–133. 10.1007/978-94-007-6889-5_7 [DOI] [PubMed] [Google Scholar]
- Creighton TE, Hillson DA, Freedman RB. 1980. Catalysis by protein-disulphide isomerase of the unfolding and refolding of proteins with disulphide bonds. J Mol Biol 142: 43–62. 10.1016/0022-2836(80)90205-3 [DOI] [PubMed] [Google Scholar]
- Crevenna AH, Blank B, Maiser A, Emin D, Prescher J, Beck G, Kienzle C, Bartnik K, Habermann B, Pakdel M, et al. 2016. Secretory cargo sorting by Ca2+-dependent Cab45 oligomerization at the trans-Golgi network. J Cell Biol 213: 305–314. 10.1083/jcb.201601089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csermely P, Miyata Y, Schnaider T, Yahara I. 1995. Autophosphorylation of grp94 (endoplasmin). J Biol Chem 270: 6381–6388. 10.1074/jbc.270.11.6381 [DOI] [PubMed] [Google Scholar]
- Csordas G, Varnai P, Golenar T, Roy S, Purkins G, Schneider TG, Balla T, Hajnoczky G. 2010. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell 39: 121–132. 10.1016/j.molcel.2010.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LC, Platt FM, Galione A. 2015. Preferential coupling of the NAADP pathway to exocytosis in T-cells. Messenger (Los Angel) 4: 53–66. 10.1166/msr.2015.1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Alba E, Tjandra N. 2004. Structural studies on the Ca2+-binding domain of human nucleobindin (calnuc). Biochemistry 43: 10039–10049. 10.1021/bi049310a [DOI] [PubMed] [Google Scholar]
- Decuypere JP, Bultynck G, Parys JB. 2011. A dual role for Ca2+ in autophagy regulation. Cell Calcium 50: 242–250. 10.1016/j.ceca.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Deniaud A, Sharaf el dein O, Maillier E, Poncet D, Kroemer G, Lemaire L, Brenner C. 2008. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene 27: 285–299. 10.1038/sj.onc.1210638 [DOI] [PubMed] [Google Scholar]
- De Stefani D, Rizzuto R, Pozzan T. 2016. Enjoy the trip: Calcium in mitochondria back and forth. Annu Rev Biochem 85: 161–192. 10.1146/annurev-biochem-060614-034216 [DOI] [PubMed] [Google Scholar]
- Dicks N, Gutierrez K, Michalak M, Bordignon V, Agellon LB. 2015. Endoplasmic reticulum stress, genome damage, and cancer. Front Oncol 5: 11 10.3389/fonc.2015.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divet A, Paesante S, Grasso C, Cavagna D, Tiveron C, Paolini C, Protasi F, Huchet-Cadiou C, Treves S, Zorzato F. 2007. Increased Ca2+ storage capacity of the skeletal muscle sarcoplasmic reticulum of transgenic mice over-expressing membrane bound calcium binding protein junctate. J Cell Physiol 213: 464–474. 10.1002/jcp.21121 [DOI] [PubMed] [Google Scholar]
- Ellgaard L, Riek R, Braun D, Herrmann T, Helenius A, Wüthrich K. 2001. Three-dimensional structure topology of the calreticulin P-domain based on NMR assignment. FEBS Lett 488: 69–73. 10.1016/S0014-5793(00)02382-6 [DOI] [PubMed] [Google Scholar]
- Ellgaard L, Bettendorff P, Braun D, Herrmann T, Fiorito F, Jelesarov I, Guntert P, Helenius A, Wuthrich K. 2002. NMR structures of 36 and 73-residue fragments of the calreticulin P-domain. J Mol Biol 322: 773–784. 10.1016/S0022-2836(02)00812-4 [DOI] [PubMed] [Google Scholar]
- Faggioni M, Kryshtal DO, Knollmann BC. 2012. Calsequestrin mutations and catecholaminergic polymorphic ventricular tachycardia. Pediatr Cardiol 33: 959–967. 10.1007/s00246-012-0256-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan GC, Gregory KN, Zhao W, Park WJ, Kranias EG. 2004. Regulation of myocardial function by histidine-rich, calcium-binding protein. Am J Physiol Heart Circ Physiol 287: H1705–H1711. 10.1152/ajpheart.01211.2003 [DOI] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. 2006. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441: 179–185. 10.1038/nature04702 [DOI] [PubMed] [Google Scholar]
- Filadi R, Pozzan T. 2015. Generation and functions of second messengers microdomains. Cell Calcium 58: 405–414. 10.1016/j.ceca.2015.03.007 [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Kenney LJ, Varriano-Marston E. 1987. The structure of calsequestrin in triads of vertebrate skeletal muscle: A deep-etch study. J Cell Biol 105: 49–56. 10.1083/jcb.105.1.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman RB, Hirst TR, Tuite MF. 1994. Protein disulphide isomerase: Building bridges in protein folding. Trends Biochem Sci 19: 331–336. 10.1016/0968-0004(94)90072-8 [DOI] [PubMed] [Google Scholar]
- Frickel EM, Riek R, Jelesarov I, Helenius A, Wuthrich K, Ellgaard L. 2002. TROSY-NMR reveals interaction between ERp57 and the tip of the calreticulin P-domain. Proc Natl Acad Sci 99: 1954–1959. 10.1073/pnas.042699099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galione A. 2015. A primer of NAADP-mediated Ca2+ signalling: From sea urchin eggs to mammalian cells. Cell Calcium 58: 27–47. 10.1016/j.ceca.2014.09.010 [DOI] [PubMed] [Google Scholar]
- Gaut JR, Hendershot LM. 1993. Mutations within the nucleotide binding site of immunoglobulin-binding protein inhibit ATPase activity and interfere with release of immunoglobulin heavy chain. J Biol Chem 268: 7248–7255. [PubMed] [Google Scholar]
- Gelebart P, Opas M, Michalak M. 2005. Calreticulin, a Ca2+-binding chaperone of the endoplasmic reticulum. Int J Biochem Cell Biol 37: 260–266. 10.1016/j.biocel.2004.02.030 [DOI] [PubMed] [Google Scholar]
- Genazzani AA, Galione A. 1996. Nicotinic acid-adenine dinucleotide phosphate mobilizes Ca2+ from a thapsigargin-insensitive pool. Biochem J 315: 721–725. 10.1042/bj3150721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko JV, Tepikin AV, Petersen OH, Gerasimenko OV. 1998. Calcium uptake via endocytosis with rapid release from acidifying endosomes. Curr Biol 8: 1335–1338. 10.1016/S0960-9822(07)00565-9 [DOI] [PubMed] [Google Scholar]
- Gerasimenko JV, Maruyama Y, Yano K, Dolman NJ, Tepikin AV, Petersen OH, Gerasimenko OV. 2003. NAADP mobilizes Ca2+ from a thapsigargin-sensitive store in the nuclear envelope by activating ryanodine receptors. J Cell Biol 163: 271–282. 10.1083/jcb.200306134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomello M, Drago I, Bortolozzi M, Scorzeto M, Gianelle A, Pizzo P, Pozzan T. 2010. Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol Cell 38: 280–290. 10.1016/j.molcel.2010.04.003 [DOI] [PubMed] [Google Scholar]
- Gilabert JA, Parekh AB. 2000. Respiring mitochondria determine the pattern of activation and inactivation of the store-operated Ca2+ current ICRAC. EMBO J 19: 6401–6407. 10.1093/emboj/19.23.6401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger RF, Epstein CJ, Anfinsen CB. 1963. Acceleration of reactivation of reduced bovine pancreatic ribonuclease by a microsomal system from rat liver. J Biol Chem 238: 628–635. [PubMed] [Google Scholar]
- Gregory KN, Ginsburg KS, Bodi I, Hahn H, Marreez YM, Song Q, Padmanabhan PA, Mitton BA, Waggoner JR, Del Monte F, et al. 2006. Histidine-rich Ca binding protein: A regulator of sarcoplasmic reticulum calcium sequestration and cardiac function. J Mol Cell Cardiol 40: 653–665. 10.1016/j.yjmcc.2006.02.003 [DOI] [PubMed] [Google Scholar]
- Griffiths EJ, Rutter GA. 2009. Mitochondrial calcium as a key regulator of mitochondrial ATP production in mammalian cells. Biochim Biophys Acta 1787: 1324–1333. 10.1016/j.bbabio.2009.01.019 [DOI] [PubMed] [Google Scholar]
- Groenendyk J, Agellon LB, Michalak M. 2013. Coping with endoplasmic reticulum stress in the cardiovascular system. Annu Rev Physiol 75: 49–67. 10.1146/annurev-physiol-030212-183707 [DOI] [PubMed] [Google Scholar]
- Groenendyk J, Peng Z, Dudek E, Fan X, Mizianty MJ, Dufey E, Urra H, Sepulveda D, Rojas-Rivera D, Lim Y, et al. 2014. Interplay between the oxidoreductase PDIA6 and microRNA-322 controls the response to disrupted endoplasmic reticulum calcium homeostasis. Sci Signal 7: ra54 10.1126/scisignal.2004983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido D, Demaurex N, Nunes P. 2015. Junctate boosts phagocytosis by recruiting endoplasmic reticulum Ca2+ stores near phagosomes. J Cell Sci 128: 4074–4082. 10.1242/jcs.172510 [DOI] [PubMed] [Google Scholar]
- Guo L, Nakamura K, Lynch J, Opas M, Olson EN, Agellon LB, Michalak M. 2002. Cardiac-specific expression of calcineurin reverses embryonic lethality in calreticulin-deficient mouse. J Biol Chem 277: 50776–50779. 10.1074/jbc.M209900200 [DOI] [PubMed] [Google Scholar]
- Gutiérrez T, Simmen T. 2018. Endoplasmic reticulum chaperones tweak the mitochondrial calcium rheostat to control metabolism and cell death. Cell Calcium 70: 64–75. 10.1016/j.ceca.2017.05.015 [DOI] [PubMed] [Google Scholar]
- Györke S, Stevens SC, Terentyev D. 2009. Cardiac calsequestrin: Quest inside the SR. J Physiol 587: 3091–3094. 10.1113/jphysiol.2009.172049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigh NG, Johnson AE. 2002. A new role for BiP: Closing the aqueous translocon pore during protein integration into the ER membrane. J Cell Biol 156: 261–270. 10.1083/jcb.200110074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnoczky G, Hager R, Thomas AP. 1999. Mitochondria suppress local feedback activation of inositol 1,4,5-trisphosphate receptors by Ca2+. J Biol Chem 274: 14157–14162. 10.1074/jbc.274.20.14157 [DOI] [PubMed] [Google Scholar]
- Hammadi M, Oulidi A, Gackiere F, Katsogiannou M, Slomianny C, Roudbaraki M, Dewailly E, Delcourt P, Lepage G, Lotteau S, et al. 2013. Modulation of ER stress and apoptosis by endoplasmic reticulum calcium leak via translocon during unfolded protein response: Involvement of GRP78. FASEB J 27: 1600–1609. 10.1096/fj.12-218875 [DOI] [PubMed] [Google Scholar]
- Hetz C, Papa FR. 2018. The unfolded protein response and cell fate control. Mol Cell 69: 169–181. 10.1016/j.molcel.2017.06.017 [DOI] [PubMed] [Google Scholar]
- Higo T, Hattori M, Nakamura T, Natsume T, Michikawa T, Mikoshiba K. 2005. Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell 120: 85–98. 10.1016/j.cell.2004.11.048 [DOI] [PubMed] [Google Scholar]
- Higo T, Hamada K, Hisatsune C, Nukina N, Hashikawa T, Hattori M, Nakamura T, Mikoshiba K. 2010. Mechanism of ER stress-induced brain damage by IP3 receptor. Neuron 68: 865–878. 10.1016/j.neuron.2010.11.010 [DOI] [PubMed] [Google Scholar]
- Hockey LN, Kilpatrick BS, Eden ER, Lin-Moshier Y, Brailoiu GC, Brailoiu E, Futter CE, Schapira AH, Marchant JS, Patel S. 2015. Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by TPC2 inhibition. J Cell Sci 128: 232–238. 10.1242/jcs.164152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SL, Goldstein JL, Orth K, Moomaw CR, Slaughter CA, Brown MS. 1989. Molecular cloning of a histidine-rich Ca2+-binding protein of sarcoplasmic reticulum that contains highly conserved repeated elements. J Biol Chem 264: 18083–18090. [PubMed] [Google Scholar]
- Hohenegger M, Suko J, Gscheidlinger R, Drobny H, Zidar A. 2002. Nicotinic acid-adenine dinucleotide phosphate activates the skeletal muscle ryanodine receptor. Biochem J 367: 423–431. 10.1042/bj20020584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong CS, Kwon SJ, Cho MC, Kwak YG, Ha KC, Hong B, Li H, Chae SW, Chai OH, Song CH, et al. 2008. Overexpression of junctate induces cardiac hypertrophy and arrhythmia via altered calcium handling. J Mol Cell Cardiol 44: 672–682. 10.1016/j.yjmcc.2008.01.012 [DOI] [PubMed] [Google Scholar]
- Honore B, Vorum H. 2000. The CREC family, a novel family of multiple EF-hand, low-affinity Ca2+-binding proteins localised to the secretory pathway of mammalian cells. FEBS Lett 466: 11–18. 10.1016/S0014-5793(99)01780-9 [DOI] [PubMed] [Google Scholar]
- Hoth M, Fanger CM, Lewis RS. 1997. Mitochondrial regulation of store-operated calcium signaling in T lymphocytes. J Cell Biol 137: 633–648. 10.1083/jcb.137.3.633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Pedi L, Diver MM, Long SB. 2012. Crystal structure of the calcium release-activated calcium channel Orai. Science 338: 1308–1313. 10.1126/science.1228757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huotari J, Helenius A. 2011. Endosome maturation. EMBO J 30: 3481–3500. 10.1038/emboj.2011.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto N, Nagy B, Bhatnagar GM, Gergely J. 1974. Studies on a metal-binding protein of the sarcoplasmic reticulum. J Biol Chem 249: 2357–2365. [PubMed] [Google Scholar]
- Ivessa NE, De Lemos-Chiarandini C, Gravotta D, Sabatini DD, Kreibich G. 1995. The brefeldin A-induced retrograde transport from the Golgi apparatus to the endoplasmic reticulum depends on calcium sequestered to intracellular stores. J Biol Chem 270: 25960–25967. 10.1074/jbc.270.43.25960 [DOI] [PubMed] [Google Scholar]
- Jiao Q, Bai Y, Akaike T, Takeshima H, Ishikawa Y, Minamisawa S. 2009. Sarcalumenin is essential for maintaining cardiac function during endurance exercise training. Am J Physiol Heart Circ Physiol 297: H576–H582. 10.1152/ajpheart.00946.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Q, Takeshima H, Ishikawa Y, Minamisawa S. 2012. Sarcalumenin plays a critical role in age-related cardiac dysfunction due to decreases in SERCA2a expression and activity. Cell Calcium 51: 31–39. 10.1016/j.ceca.2011.10.003 [DOI] [PubMed] [Google Scholar]
- Joshi AS, Zhang H, Prinz WA. 2017. Organelle biogenesis in the endoplasmic reticulum. Nat Cell Biol 19: 876–882. 10.1038/ncb3579 [DOI] [PubMed] [Google Scholar]
- Jung J, Michalak M, Agellon LB. 2017. Endoplasmic reticulum malfunction in the nervous system. Front Neurosci 11: 220 10.3389/fnins.2017.00220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor M, Ellgaard L, Gopalakrishnapai J, Schirra C, Gemma E, Oscarson S, Helenius A, Surolia A. 2004. Mutational analysis provides molecular insight into the carbohydrate-binding region of calreticulin: Pivotal roles of tyrosine-109 and aspartate-135 in carbohydrate recognition. Biochemistry 43: 97–106. 10.1021/bi0355286 [DOI] [PubMed] [Google Scholar]
- Karabinos A, Bhattacharya D, Morys-Wortmann C, Kroll K, Hirschfeld G, Kratzin HD, Barnikol-Watanabe S, Hilschmann N. 1996. The divergent domains of the NEFA and nucleobindin proteins are derived from an EF-hand ancestor. Mol Biol Evol 13: 990–998. 10.1093/oxfordjournals.molbev.a025667 [DOI] [PubMed] [Google Scholar]
- Kassenbrock CK, Kelly RB. 1989. Interaction of heavy chain binding protein (BiP/GRP78) with adenine nucleotides. EMBO J 8: 1461–1467. 10.1002/j.1460-2075.1989.tb03529.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick BS, Eden ER, Schapira AH, Futter CE, Patel S. 2013. Direct mobilisation of lysosomal Ca2+ triggers complex Ca2+ signals. J Cell Sci 126: 60–66. 10.1242/jcs.118836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick BS, Yates E, Grimm C, Schapira AH, Patel S. 2016. Endo-lysosomal TRP mucolipin-1 channels trigger global ER Ca2+ release and Ca2+ influx. J Cell Sci 129: 3859–3867. 10.1242/jcs.190322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Shin DW, Hong CS, Jeong D, Kim DH, Park WJ. 2003. Increased Ca2+ storage capacity in the sarcoplasmic reticulum by overexpression of HRC (histidine-rich Ca2+ binding protein). Biochem Biophys Res Commun 300: 192–196. 10.1016/S0006-291X(02)02829-2 [DOI] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham DE. 2004. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427: 360–364. 10.1038/nature02246 [DOI] [PubMed] [Google Scholar]
- Knollmann BC. 2009. New roles of calsequestrin and triadin in cardiac muscle. J Physiol 587: 3081–3087. 10.1113/jphysiol.2009.172098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, Knollmann BE, Horton KD, Weissman NJ, Holinstat I, et al. 2006. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest 116: 2510–2520. 10.1172/jci29128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs J, Agellon LB, Michalak M. 2015. Ca2+ homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem Biophys Res Commun 460: 114–121. 10.1016/j.bbrc.2015.02.004 [DOI] [PubMed] [Google Scholar]
- Kumar A, Chakravarty H, Bal NC, Balaraju T, Jena N, Misra G, Bal C, Pieroni E, Periasamy M, Sharon A. 2013. Identification of calcium binding sites on calsequestrin 1 and their implications for polymerization. Mol Biosyst 9: 1949–1957. 10.1039/c3mb25588c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SJ, Kim DH. 2009. Characterization of junctate-SERCA2a interaction in murine cardiomyocyte. Biochem Biophys Res Commun 390: 1389–1394. 10.1016/j.bbrc.2009.10.165 [DOI] [PubMed] [Google Scholar]
- Lamb HK, Mee C, Xu W, Liu L, Blond S, Cooper A, Charles IG, Hawkins AR. 2006. The affinity of a major Ca2+ binding site on GRP78 is differentially enhanced by ADP and ATP. J Biol Chem 281: 8796–8805. 10.1074/jbc.M503964200 [DOI] [PubMed] [Google Scholar]
- Larkin H, Costantino S, Seaman MN, Lavoie C. 2016. Calnuc function in endosomal sorting of lysosomal receptors. Traffic 17: 416–432. 10.1111/tra.12374 [DOI] [PubMed] [Google Scholar]
- Lasorsa FM, Pinton P, Palmieri L, Scarcia P, Rottensteiner H, Rizzuto R, Palmieri F. 2008. Peroxisomes as novel players in cell calcium homeostasis. J Biol Chem 283: 15300–15308. 10.1074/jbc.M800648200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach MR, Cohen-Doyle MF, Thomas DY, Williams DB. 2002. Localization of the lectin, ERp57 binding, and polypeptide binding sites of calnexin and calreticulin. J Biol Chem 277: 29686–29697. 10.1074/jbc.M202405200 [DOI] [PubMed] [Google Scholar]
- Lebeche D, Kaminer B. 1992. Characterization of a calsequestrin-like protein from sea-urchin eggs. Biochem J 287: 741–747. 10.1042/bj2870741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeche D, Lucero HA, Kaminer B. 1994. Calcium binding properties of rabbit liver protein disulfide isomerase. Biochem Biophys Res Commun 202: 556–561. 10.1006/bbrc.1994.1964 [DOI] [PubMed] [Google Scholar]
- Leberer E, Charuk JH, Green NM, MacLennan DH. 1989. Molecular cloning and expression of cDNA encoding a lumenal calcium binding glycoprotein from sarcoplasmic reticulum. Proc Natl Acad Sci 86: 6047–6051. 10.1073/pnas.86.16.6047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer E, Timms BG, Campbell KP, MacLennan DH. 1990. Purification, calcium binding properties, and ultrastructural localization of the 53,000- and 160,000 (sarcalumenin)-Dalton glycoproteins of the sarcoplasmic reticulum. J Biol Chem 265: 10118–10124. [PubMed] [Google Scholar]
- Lee AS. 1992. Mammalian stress response: Induction of the glucose-regulated protein family. Curr Opin Cell Biol 4: 267–273. 10.1016/0955-0674(92)90042-B [DOI] [PubMed] [Google Scholar]
- Lee HC, Aarhus R. 1995. A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J Biol Chem 270: 2152–2157. 10.1074/jbc.270.5.2152 [DOI] [PubMed] [Google Scholar]
- Lee HG, Kang H, Kim DH, Park WJ. 2001. Interaction of HRC (histidine-rich Ca2+-binding protein) and triadin in the lumen of sarcoplasmic reticulum. J Biol Chem 276: 39533–39538. 10.1074/jbc.M010664200 [DOI] [PubMed] [Google Scholar]
- Li Y, Camacho P. 2004. Ca2+-dependent redox modulation of SERCA2b by ERp57. J Cell Biol 164: 35–46. 10.1083/jcb.200307010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HT, Yan JB, Li J, Zhou MH, Zhu XD, Zhang YX, Tien P. 2005. Enhancement of humoral immune responses to HBsAg by heat shock protein gp96 and its N-terminal fragment in mice. World J Gastroenterol 11: 2858–2863. 10.3748/wjg.v11.i19.2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievremont JP, Rizzuto R, Hendershot L, Meldolesi J. 1997. BiP, a major chaperone protein of the endoplasmic reticulum lumen, plays a direct and important role in the storage of the rapidly exchanging pool of Ca2+. J Biol Chem 272: 30873–30879. 10.1074/jbc.272.49.30873 [DOI] [PubMed] [Google Scholar]
- Lin P, Le-Niculescu H, Hofmeister R, McCaffery JM, Jin M, Hennemann H, McQuistan T, De Vries L, Farquhar MG. 1998. The mammalian calcium-binding protein, nucleobindin (CALNUC), is a Golgi resident protein. J Cell Biol 141: 1515–1527. 10.1083/jcb.141.7.1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P, Yao Y, Hofmeister R, Tsien RY, Farquhar MG. 1999. Overexpression of CALNUC (nucleobindin) increases agonist and thapsigargin releasable Ca2+ storage in the Golgi. J Cell Biol 145: 279–289. 10.1083/jcb.145.2.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin-Moshier Y, Keebler MV, Hooper R, Boulware MJ, Liu X, Churamani D, Abood ME, Walseth TF, Brailoiu E, Patel S, et al. 2014. The two-pore channel (TPC) interactome unmasks isoform-specific roles for TPCs in endolysosomal morphology and cell pigmentation. Proc Natl Acad Sci 111: 13087–13092. 10.1073/pnas.1407004111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr, Meyer T. 2005. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 15: 1235–1241. 10.1016/j.cub.2005.05.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little E, Lee AS. 1995. Generation of a mammalian cell line deficient in glucose-regulated protein stress induction through targeted ribozyme driven by a stress-inducible promoter. J Biol Chem 270: 9526–9534. 10.1074/jbc.270.16.9526 [DOI] [PubMed] [Google Scholar]
- Liu H, Bowes RC III, van de Water B, Sillence C, Nagelkerke JF, Stevens JL. 1997. Endoplasmic reticulum chaperones GRP78 and calreticulin prevent oxidative stress, Ca2+ disturbances, and cell death in renal epithelial cells. J Biol Chem 272: 21751–21759. 10.1074/jbc.272.35.21751 [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E, Morgan AJ, He X, Smith DA, Elliot-Smith E, Sillence DJ, Churchill GC, Schuchman EH, Galione A, Platt FM. 2008. Niemann–Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat Med 14: 1247–1255. 10.1038/nm.1876 [DOI] [PubMed] [Google Scholar]
- Lopez-Sanjurjo CI, Tovey SC, Prole DL, Taylor CW. 2013. Lysosomes shape Ins(1,4,5)P3-evoked Ca2+ signals by selectively sequestering Ca2+ released from the endoplasmic reticulum. J Cell Sci 126: 289–300. 10.1242/jcs.116103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucero HA, Kaminer B. 1999. The role of calcium on the activity of ERcalcistorin/protein-disulfide isomerase and the significance of the C-terminal and its calcium binding. A comparison with mammalian protein-disulfide isomerase. J Biol Chem 274: 3243–3251. 10.1074/jbc.274.5.3243 [DOI] [PubMed] [Google Scholar]
- Lucero HA, Lebeche D, Kaminer B. 1998. ERcalcistorin/protein-disulfide isomerase acts as a calcium storage protein in the endoplasmic reticulum of a living cell. Comparison with calreticulin and calsequestrin. J Biol Chem 273: 9857–9863. 10.1074/jbc.273.16.9857 [DOI] [PubMed] [Google Scholar]
- Lynch J, Guo L, Gelebart P, Chilibeck K, Xu J, Molkentin JD, Agellon LB, Michalak M. 2005. Calreticulin signals upstream of calcineurin and MEF2C in a critical Ca2+-dependent signaling cascade. J Cell Biol 170: 37–47. 10.1083/jcb.200412156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macer DR, Koch GL. 1988. Identification of a set of calcium-binding proteins in reticuloplasm, the luminal content of the endoplasmic reticulum. J Cell Sci 91: 61–70. [DOI] [PubMed] [Google Scholar]
- MacLennan DH, Wong PT. 1971. Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc Natl Acad Sci 68: 1231–1235. 10.1073/pnas.68.6.1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno C, Figueroa LC, Gillespie D, Fitts R, Kang C, Franzini-Armstrong C, Rios E. 2017. Calsequestrin depolymerizes when calcium is depleted in the sarcoplasmic reticulum of working muscle. Proc Natl Acad Sci 114: E638–E647. 10.1073/pnas.1620265114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzec M, Eletto D, Argon Y. 2012. GRP94: An HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum. Biochim Biophys Acta 1823: 774–787. 10.1016/j.bbamcr.2011.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekahli D, Bultynck G, Parys JB, De Smedt H, Missiaen L. 2011. Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb Perspect Biol 3: a004317 10.1101/cshperspect.a004317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchionda M, Pittman JK, Mayor R, Patel S. 2016. Ca2+/H+ exchange by acidic organelles regulates cell migration in vivo. J Cell Biol 212: 803–813. 10.1083/jcb.201510019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick J, Dul JL, Argon Y. 1994. Sequential interaction of the chaperones BiP and GRP94 with immunoglobulin chains in the endoplasmic reticulum. Nature 370: 373–375. 10.1038/370373a0 [DOI] [PubMed] [Google Scholar]
- Mery L, Mesaeli N, Michalak M, Opas M, Lew DP, Krause KH. 1996. Overexpression of calreticulin increases intracellular Ca2+ storage and decreases store-operated Ca2+ influx. J Biol Chem 271: 9332–9339. 10.1074/jbc.271.16.9332 [DOI] [PubMed] [Google Scholar]
- Mesaeli N, Nakamura K, Zvaritch E, Dickie P, Dziak E, Krause KH, Opas M, MacLennan DH, Michalak M. 1999. Calreticulin is essential for cardiac development. J Cell Biol 144: 857–868. 10.1083/jcb.144.5.857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micaroni M, Perinetti G, Berrie CP, Mironov AA. 2010. The SPCA1 Ca2+ pump and intracellular membrane trafficking. Traffic 11: 1315–1333. 10.1111/j.1600-0854.2010.01096.x [DOI] [PubMed] [Google Scholar]
- Michalak M, Opas M. 2009. Endoplasmic and sarcoplasmic reticulum in the heart. Trends Cell Biol 19: 253–259. 10.1016/j.tcb.2009.03.006 [DOI] [PubMed] [Google Scholar]
- Mignen O, Thompson JL, Shuttleworth TJ. 2008. Orai1 subunit stoichiometry of the mammalian CRAC channel pore. J Physiol 586: 419–425. 10.1113/jphysiol.2007.147249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Titani K, Kurosawa Y, Kanai Y. 1992. Molecular cloning of nucleobindin, a novel DNA-binding protein that contains both a signal peptide and a leucine zipper structure. Biochem Biophys Res Commun 187: 375–380. 10.1016/S0006-291X(05)81503-7 [DOI] [PubMed] [Google Scholar]
- Mojzisova A, Krizanova O, Zacikova L, Kominkova V, Ondrias K. 2001. Effect of nicotinic acid adenine dinucleotide phosphate on ryanodine calcium release channel in heart. Pflugers Arch 441: 674–677. 10.1007/s004240000465 [DOI] [PubMed] [Google Scholar]
- Morciano G, Marchi S, Morganti C, Sbano L, Bittremieux M, Kerkhofs M, Corricelli M, Danese A, Karkucinska-Wieckowska A, Wieckowski MR, et al. 2018. Role of mitochondria-associated ER membranes in calcium regulation in cancer-specific settings. Neoplasia 20: 510–523. 10.1016/j.neo.2018.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel-Huaux VM, Pypaert M, Wouters S, Tartakoff AM, Jurgan U, Gevaert K, Courtoy PJ. 2002. The calcium-binding protein p54/NEFA is a novel luminal resident of medial Golgi cisternae that traffics independently of mannosidase II. Eur J Cell Biol 81: 87–100. 10.1078/0171-9335-00224 [DOI] [PubMed] [Google Scholar]
- Morgan AJ, Davis LC, Wagner SK, Lewis AM, Parrington J, Churchill GC, Galione A. 2013. Bidirectional Ca2+ signaling occurs between the endoplasmic reticulum and acidic organelles. J Cell Biol 200: 789–805. 10.1083/jcb.201204078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muik M, Frischauf I, Derler I, Fahrner M, Bergsmann J, Eder P, Schindl R, Hesch C, Polzinger B, Fritsch R, et al. 2008. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J Biol Chem 283: 8014–8022. 10.1074/jbc.M708898200 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Zuppini A, Arnaudeau S, Lynch J, Ahsan I, Krause R, Papp S, De Smedt H, Parys JB, Muller-Esterl W, et al. 2001. Functional specialization of calreticulin domains. J Cell Biol 154: 961–972. 10.1083/jcb.200102073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesselhut J, Jurgan U, Onken E, Götz H, Barnikol HU, Hirschfeld G, Barnikol-Watanabe S, Hilschmann N. 2001. Golgi retention of human protein NEFA is mediated by its N-terminal Leu/Ile-rich region. FEBS Lett 509: 469–475. 10.1016/S0014-5793(01)03187-8 [DOI] [PubMed] [Google Scholar]
- Nunes-Hasler P, Demaurex N. 2017. The ER phagosome connection in the era of membrane contact sites. Biochim Biophys Acta 1864: 1513–1524. 10.1016/j.bbamcr.2017.04.007 [DOI] [PubMed] [Google Scholar]
- Nwokonko RM, Cai X, Loktionova NA, Wang Y, Zhou Y, Gill DL. 2017. The STIM-Orai pathway: Conformational coupling between STIM and Orai in the activation of store-operated Ca2+ entry. Adv Exp Med Biol 993: 83–98. 10.1007/978-3-319-57732-6_5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorf JA, Lebeche D, Head JF, Kaminer B. 1988. Identification of a calsequestrin-like protein from sea urchin eggs. J Biol Chem 263: 6806–6809. 10.1042/bj2870741 [DOI] [PubMed] [Google Scholar]
- Oda K. 1992. Calcium depletion blocks proteolytic cleavages of plasma protein precursors which occur at the Golgi and/or trans-Golgi network. Possible involvement of Ca2+-dependent Golgi endoproteases. J Biol Chem 267: 17465–17471. [PubMed] [Google Scholar]
- Ouyang YG, Xu LJ, Emery JF, Lee AS, Giffard RG. 2011. Overexpressing GRP78 influences Ca2+ handling and function of mitochondria in astrocytes after ischemia-like stress. Mitochondrion 11: 279–286. 10.1016/j.mito.2010.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini C, Quarta M, Nori A, Boncompagni S, Canato M, Volpe P, Allen PD, Reggiani C, Protasi F. 2007. Reorganized stores and impaired calcium handling in skeletal muscle of mice lacking calsequestrin-1. J Physiol 583: 767–784. 10.1113/jphysiol.2007.138024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. 2009. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136: 876–890. 10.1016/j.cell.2009.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CS, Chen S, Lee H, Cha H, Oh JG, Hong S, Han P, Ginsburg KS, Jin S, Park I, et al. 2013. Targeted ablation of the histidine-rich Ca2+-binding protein (HRC) gene is associated with abnormal SR Ca2+-cycling and severe pathology under pressure-overload stress. Basic Res Cardiol 108: 344 10.1007/s00395-013-0344-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Muallem S. 2011. Acidic Ca2+ stores come to the fore. Cell Calcium 50: 109–112. 10.1016/j.ceca.2011.03.009 [DOI] [PubMed] [Google Scholar]
- Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. 2006. Amplification of CRAC current by STIM1 and CRACM1 (Orai1). Nat Cell Biol 8: 771–773. 10.1038/ncb1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna A, Demuro A, Yeromin AV, Zhang SL, Safrina O, Parker I, Cahalan MD. 2008. The CRAC channel consists of a tetramer formed by Stim-induced dimerization of Orai dimers. Nature 456: 116–120. 10.1038/nature07338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny CJ, Kilpatrick BS, Eden ER, Patel S. 2015. Coupling acidic organelles with the ER through Ca2+ microdomains at membrane contact sites. Cell Calcium 58: 387–396. 10.1016/j.ceca.2015.03.006 [DOI] [PubMed] [Google Scholar]
- Pezzati R, Bossi M, Podini P, Meldolesi J, Grohovaz F. 1997. High-resolution calcium mapping of the endoplasmic reticulum-Golgi-exocytic membrane system. Electron energy loss imaging analysis of quick frozen-freeze dried PC12 cells. Mol Biol Cell 8: 1501–1512. 10.1091/mbc.8.8.1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MJ, Voeltz GK. 2016. Structure and function of ER membrane contact sites with other organelles. Nat Rev Mol Cell Biol 17: 69–82. 10.1038/nrm.2015.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picello E, Damiani E, Margreth A. 1992. Low-affinity Ca2+-binding sites versus Zn2+-binding sites in histidine-rich Ca2+-binding protein of skeletal muscle sarcoplasmic reticulum. Biochem Biophys Res Commun 186: 659–667. 10.1016/0006-291X(92)90797-O [DOI] [PubMed] [Google Scholar]
- Pinton P, Pozzan T, Rizzuto R. 1998. The Golgi apparatus is an inositol 1,4,5-trisphosphate-sensitive Ca2+ store, with functional properties distinct from those of the endoplasmic reticulum. EMBO J 17: 5298–5308. 10.1093/emboj/17.18.5298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier S, Mamarbachi M, Chen WT, Lee AS, Mayer G. 2015. GRP94 regulates circulating cholesterol levels through blockade of PCSK9-induced LDLR degradation. Cell Rep 13: 2064–2071. 10.1016/j.celrep.2015.11.006 [DOI] [PubMed] [Google Scholar]
- Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. 2006. Orai1 is an essential pore subunit of the CRAC channel. Nature 443: 230–233. 10.1038/nature05122 [DOI] [PubMed] [Google Scholar]
- Prins D, Michalak M. 2011. Organellar calcium buffers. Cold Spring Harb Perspect Biol 3: a004069 10.1101/cshperspect.a004069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins D, Groenendyk J, Touret N, Michalak M. 2011. Modulation of STIM1 and capacitative Ca2+ entry by the endoplasmic reticulum luminal oxidoreductase ERp57. EMBO Rep 12: 1182–1188. 10.1038/embor.2011.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz WA. 2014. Bridging the gap: Membrane contact sites in signaling, metabolism, and organelle dynamics. J Cell Biol 205: 759–769. 10.1083/jcb.201401126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudent J, McBride HM. 2017. The mitochondria-endoplasmic reticulum contact sites: A signalling platform for cell death. Curr Opin Cell Biol 47: 52–63. 10.1016/j.ceb.2017.03.007 [DOI] [PubMed] [Google Scholar]
- Pryor PR, Mullock BM, Bright NA, Gray SR, Luzio JP. 2000. The role of intraorganellar Ca2+ in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J Cell Biol 149: 1053–1062. 10.1083/jcb.149.5.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randow F, Seed B. 2001. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat Cell Biol 3: 891–896. 10.1038/ncb1001-891 [DOI] [PubMed] [Google Scholar]
- Rapizzi E, Pinton P, Szabadkai G, Wieckowski MR, Vandecasteele G, Baird G, Tuft RA, Fogarty KE, Rizzuto R. 2002. Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J Cell Biol 159: 613–624. 10.1083/jcb.200205091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy RK, Lu J, Lee AS. 1999. The endoplasmic reticulum chaperone glycoprotein GRP94 with Ca2+-binding and antiapoptotic properties is a novel proteolytic target of calpain during etoposide-induced apoptosis. J Biol Chem 274: 28476–28483. 10.1074/jbc.274.40.28476 [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Brini M, Murgia M, Pozzan T. 1993. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science 262: 744–747. 10.1126/science.8235595 [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. 1998. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280: 1763–1766. 10.1126/science.280.5370.1763 [DOI] [PubMed] [Google Scholar]
- Ronco V, Potenza DM, Denti F, Vullo S, Gagliano G, Tognolina M, Guerra G, Pinton P, Genazzani AA, Mapelli L, et al. 2015. A novel Ca2+-mediated cross-talk between endoplasmic reticulum and acidic organelles: Implications for NAADP-dependent Ca2+ signalling. Cell Calcium 57: 89–100. 10.1016/j.ceca.2015.01.001 [DOI] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, et al. 2005. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 169: 435–445. 10.1083/jcb.200502019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi AE, Dirksen RT. 2006. Sarcoplasmic reticulum: The dynamic calcium governor of muscle. Muscle Nerve 33: 715–731. 10.1002/mus.20512 [DOI] [PubMed] [Google Scholar]
- Ruas M, Davis LC, Chen CC, Morgan AJ, Chuang KT, Walseth TF, Grimm C, Garnham C, Powell T, Platt N, et al. 2015. Expression of Ca2+-permeable two-pore channels rescues NAADP signalling in TPC-deficient cells. EMBO J 34: 1743–1758. 10.15252/embj.201490009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetto R, Damiani E, Turcato F, Nori A, Margreth A. 2001. Ca2+-dependent interaction of triadin with histidine-rich Ca2+-binding protein carboxyl-terminal region. Biochem Biophys Res Commun 289: 1125–1134. 10.1006/bbrc.2001.6126 [DOI] [PubMed] [Google Scholar]
- Sambrook JF. 1990. The involvement of calcium in transport of secretory proteins from the endoplasmic reticulum. Cell 61: 197–199. 10.1016/0092-8674(90)90798-J [DOI] [PubMed] [Google Scholar]
- Sanchez EJ, Lewis KM, Danna BR, Kang C. 2012. High-capacity Ca2+ binding of human skeletal calsequestrin. J Biol Chem 287: 11592–11601. 10.1074/jbc.M111.335075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauble N, Lang S, Jung M, Cappel S, Schorr S, Ulucan O, Linxweiler J, Dudek J, Blum R, Helms V, et al. 2012. BiP-mediated closing of the Sec61 channel limits Ca2+ leakage from the ER. EMBO J 31: 3282–3296. 10.1038/emboj.2012.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer PE, Lederkremer GZ, Williams S, Fogliano M, Baldini G, Lodish HF. 1996. Cab45, a novel Ca2+-binding protein localized to the Golgi lumen. J Cell Biol 133: 257–268. 10.1083/jcb.133.2.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag JD, Bergeron JJ, Li Y, Borisova S, Hahn M, Thomas DY, Cygler M. 2001. The structure of calnexin, an ER chaperone involved in quality control of protein folding. Mol Cell 8: 633–644. 10.1016/S1097-2765(01)00318-5 [DOI] [PubMed] [Google Scholar]
- Shimura M, Minamisawa S, Takeshima H, Jiao Q, Bai Y, Umemura S, Ishikawa Y. 2008. Sarcalumenin alleviates stress-induced cardiac dysfunction by improving Ca2+ handling of the sarcoplasmic reticulum. Cardiovasc Res 77: 362–370. 10.1093/cvr/cvm019 [DOI] [PubMed] [Google Scholar]
- Singh A, Chagtoo M, Tiwari S, George N, Chakravarti B, Khan S, Lakshmi S, Godbole MM. 2017. Inhibition of inositol 1, 4, 5-trisphosphate receptor induce breast cancer cell death through deregulated autophagy and cellular bioenergetics. J Cell Biochem 118: 2333–2346. 10.1002/jcb.25891 [DOI] [PubMed] [Google Scholar]
- Solovyova N, Fernyhough P, Glazner G, Verkhratsky A. 2002. Xestospongin C empties the ER calcium store but does not inhibit InsP3-induced Ca2+ release in cultured dorsal root ganglia neurones. Cell Calcium 32: 49–52. 10.1016/S0143-4160(02)00094-5 [DOI] [PubMed] [Google Scholar]
- Srikanth S, Jew M, Kim KD, Yee MK, Abramson J, Gwack Y. 2012. Junctate is a Ca2+-sensing structural component of Orai1 and stromal interaction molecule 1 (STIM1). Proc Natl Acad Sci 109: 8682–8687. 10.1073/pnas.1200667109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. 2006. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: An initiation mechanism for capacitive Ca2+ entry. J Biol Chem 281: 35855–35862. 10.1074/jbc.M608247200 [DOI] [PubMed] [Google Scholar]
- Suk JY, Kim YS, Park WJ. 1999. HRC (histidine-rich Ca2+ binding protein) resides in the lumen of sarcoplasmic reticulum as a multimer. Biochem Biophys Res Commun 263: 667–671. 10.1006/bbrc.1999.1432 [DOI] [PubMed] [Google Scholar]
- Suzuki CK, Bonifacino JS, Lin AY, Davis MM, Klausner RD. 1991. Regulating the retention of T-cell receptor α chain variants within the endoplasmic reticulum: Ca2+-dependent association with BiP. J Cell Biol 114: 189–205. 10.1083/jcb.114.2.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalai G, Csordas G, Hantash BM, Thomas AP, Hajnoczky G. 2000. Calcium signal transmission between ryanodine receptors and mitochondria. J Biol Chem 275: 15305–15313. 10.1074/jbc.275.20.15305 [DOI] [PubMed] [Google Scholar]
- Tjoelker LW, Seyfried CE, Eddy RL Jr, Byers MG, Shows TB, Calderon J, Schreiber RB, Gray PW. 1994. Human, mouse, and rat calnexin cDNA cloning: Identification of potential calcium binding motifs and gene localization to human chromosome 5. Biochemistry 33: 3229–3236. 10.1021/bi00177a013 [DOI] [PubMed] [Google Scholar]
- Tong Y, Song F. 2015. Intracellular calcium signaling regulates autophagy via calcineurin-mediated TFEB dephosphorylation. Autophagy 11: 1192–1195. 10.1080/15548627.2015.1054594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves S, Feriotto G, Moccagatta L, Gambari R, Zorzato F. 2000. Molecular cloning, expression, functional characterization, chromosomal localization, and gene structure of junctate, a novel integral calcium binding protein of sarco(endo)plasmic reticulum membrane. J Biol Chem 275: 39555–39568. 10.1074/jbc.M005473200 [DOI] [PubMed] [Google Scholar]
- Treves S, Franzini-Armstrong C, Moccagatta L, Arnoult C, Grasso C, Schrum A, Ducreux S, Zhu MX, Mikoshiba K, Girard T, et al. 2004. Junctate is a key element in calcium entry induced by activation of InsP3 receptors and/or calcium store depletion. J Cell Biol 166: 537–548. 10.1083/jcb.200404079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van PN, Peter F, Soling HD. 1989. Four intracisternal calcium-binding glycoproteins from rat liver microsomes with high affinity for calcium. No indication for calsequestrin-like proteins in inositol 1,4,5-trisphosphate-sensitive calcium sequestering rat liver vesicles. J Biol Chem 264: 17494–17501. [PubMed] [Google Scholar]
- Van Coppenolle F, Vanden Abeele F, Slomianny C, Flourakis M, Hesketh J, Dewailly E, Prevarskaya N. 2004. Ribosome-translocon complex mediates calcium leakage from endoplasmic reticulum stores. J Cell Sci 117: 4135–4142. 10.1242/jcs.01274 [DOI] [PubMed] [Google Scholar]
- Vandecaetsbeek I, Vangheluwe P, Raeymaekers L, Wuytack F, Vanoevelen J. 2011. The Ca2+ pumps of the endoplasmic reticulum and Golgi apparatus. Cold Spring Harb Perspect Biol 3: a004184 10.1101/cshperspect.a004184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoevelen J, Raeymaekers L, Parys JB, De Smedt H, Van Baelen K, Callewaert G, Wuytack F, Missiaen L. 2004. Inositol trisphosphate producing agonists do not mobilize the thapsigargin-insensitive part of the endoplasmic-reticulum and Golgi Ca2+ store. Cell Calcium 35: 115–121. 10.1016/j.ceca.2003.08.003 [DOI] [PubMed] [Google Scholar]
- Vasiljevic M, Heisler FF, Hausrat TJ, Fehr S, Milenkovic I, Kneussel M, Sieghart W. 2012. Spatio-temporal expression analysis of the calcium-binding protein calumenin in the rodent brain. Neuroscience 202: 29–41. 10.1016/j.neuroscience.2011.11.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitadello M, Penzo D, Petronilli V, Michieli G, Gomirato S, Menabo R, Di Lisa F, Gorza L. 2003. Overexpression of the stress protein Grp94 reduces cardiomyocyte necrosis due to calcium overload and simulated ischemia. FASEB J 17: 923–925. 10.1096/fj.02-0644fje [DOI] [PubMed] [Google Scholar]
- von Blume J, Alleaume AM, Kienzle C, Carreras-Sureda A, Valverde M, Malhotra V. 2012. Cab45 is required for Ca2+-dependent secretory cargo sorting at the trans-Golgi network. J Cell Biol 199: 1057–1066. 10.1083/jcb.201207180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorum H, Liu X, Madsen P, Rasmussen HH, Honoré B. 1998. Molecular cloning of a cDNA encoding human calumenin, expression in Escherichia coli and analysis of its Ca2+-binding activity. Biochim Biophys Acta 1386: 121–131. 10.1016/S0167-4838(98)00089-2 [DOI] [PubMed] [Google Scholar]
- Vorum H, Hager H, Christensen BM, Nielsen S, Honoré B. 1999. Human calumenin localizes to the secretory pathway and is secreted to the medium. Exp Cell Res 248: 473–481. 10.1006/excr.1999.4431 [DOI] [PubMed] [Google Scholar]
- Wang M, Kaufman RJ. 2016. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 529: 326–335. 10.1038/nature17041 [DOI] [PubMed] [Google Scholar]
- Wang S, Trumble WR, Liao H, Wesson CR, Dunker AK, Kang CH. 1998. Crystal structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum. Nat Struct Biol 5: 476–483. 10.1038/nsb0698-476 [DOI] [PubMed] [Google Scholar]
- Wang WA, Liu WX, Durnaoglu S, Lee SK, Lian J, Lehner R, Ahnn J, Agellon LB, Michalak M. 2017. Loss of calreticulin uncovers a critical role for calcium in regulating cellular lipid homeostasis. Sci Rep 7: 5941 10.1038/s41598-017-05734-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewska M, Karlberg T, Lehtio L, Johansson I, Kotenyova T, Moche M, Schuler H. 2010. Crystal structures of the ATPase domains of four human Hsp70 isoforms: HSPA1L/Hsp70-hom, HSPA2/Hsp70-2, HSPA6/Hsp70B′, and HSPA5/BiP/GRP78. PLoS ONE 5: e8625 10.1371/journal.pone.0008625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AK, Capitanio P, Lissandron V, Bortolozzi M, Pozzan T, Pizzo P. 2013. Heterogeneity of Ca2+ handling among and within Golgi compartments. J Mol Cell Biol 5: 266–276. 10.1093/jmcb/mjt024 [DOI] [PubMed] [Google Scholar]
- Wray S, Burdyga T. 2010. Sarcoplasmic reticulum function in smooth muscle. Physiol Rev 90: 113–178. 10.1152/physrev.00018.2008 [DOI] [PubMed] [Google Scholar]
- Wuytack F, Raeymaekers L, Missiaen L. 2003. PMR1/SPCA Ca2+ pumps and the role of the Golgi apparatus as a Ca2+ store. Pflugers Arch 446: 148–153. 10.1007/s00424-003-1011-5 [DOI] [PubMed] [Google Scholar]
- Yabe D, Taniwaki M, Nakamura T, Kanazawa N, Tashiro K, Honjo T. 1998. Human calumenin gene (CALU): cDNA isolation and chromosomal mapping to 7q32. Genomics 49: 331–333. 10.1006/geno.1998.5245 [DOI] [PubMed] [Google Scholar]
- Yoshida M, Minamisawa S, Shimura M, Komazaki S, Kume H, Zhang M, Matsumura K, Nishi M, Saito M, Saeki Y, et al. 2005. Impaired Ca2+ store functions in skeletal and cardiac muscle cells from sarcalumenin-deficient mice. J Biol Chem 280: 3500–3506. 10.1074/jbc.M406618200 [DOI] [PubMed] [Google Scholar]
- Yu R, Hinkle PM. 2000. Rapid turnover of calcium in the endoplasmic reticulum during signaling. Studies with cameleon calcium indicators. J Biol Chem 275: 23648–23653. 10.1074/jbc.M002684200 [DOI] [PubMed] [Google Scholar]
- Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. 2005. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437: 902–905. 10.1038/nature04147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SJ, Zou M, Lu L, Lau D, Ditzel DA, Delucinge-Vivier C, Aso Y, Descombes P, Bading H. 2009. Nuclear calcium signaling controls expression of a large gene pool: Identification of a gene program for acquired neuroprotection induced by synaptic activity. PLoS Genet 5: e1000604 10.1371/journal.pgen.1000604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Yoshida M, Brotto L, Takeshima H, Weisleder N, Hirata Y, Nosek TM, Ma J, Brotto M. 2005. Enhanced resistance to fatigue and altered calcium handling properties of sarcalumenin knockout mice. Physiol Genomics 23: 72–78. 10.1152/physiolgenomics.00020.2005 [DOI] [PubMed] [Google Scholar]
- Zheng L, Stathopulos PB, Li GY, Ikura M. 2008. Biophysical characterization of the EF-hand and SAM domain containing Ca2+ sensory region of STIM1 and STIM2. Biochem Biophys Res Commun 369: 240–246. 10.1016/j.bbrc.2007.12.129 [DOI] [PubMed] [Google Scholar]