The Japan Clinical Oncology Group established a policy for geriatric oncology research suggesting several solutions for various issues which commonly occur in geriatric research.
Keywords: JCOG, geriatric, endpoint, study designs, geriatric assessment
Abstract
Due to the rapid aging of Japan’s population, clinical research focusing on older patients with cancer is urgently needed. The Japan Clinical Oncology Group (JCOG) has conducted several such clinical trials, but there has been no formal policy for geriatric research. We have therefore established a JCOG policy for geriatric cancer research.
We defined the patient selection policy based on treatment tolerance and chronological age. Older patients are categorized into three conceptual groups: ‘fit patients’ who can undergo the same standard treatment given to younger patients, ‘frail patients’ for whom best supportive or palliative care is indicated and ‘vulnerable patients’ who fall between the fit and frail categories. Unmet needs often exist for vulnerable patients.
The policy recommends that study endpoints include not only survival but also other endpoints such as physical and cognitive function because the objective of therapy in older patients is not only extended life expectancy but also maintenance of the patient’s general condition. In this viewpoint, co-primary or composite endpoints that incorporate geriatric assessment in the study design are often applicable.
Study design will differ depending on the study population, clinical question, and treatment. Even for older patients, a randomized clinical trial is still the gold standard when the clinical question asks which treatment is better. An observational study of a broader population is applicable for investigating actual conditions of older patients.
This JCOG Geriatric Research Policy includes several practical solutions for various issues in geriatric research. We plan to revise this policy periodically to guide future geriatric research.
Introduction
As Japan’s population is the oldest in the world, the number of older patients with cancer is notably increasing (1–4). However, there is a lack of reliable evidence to support therapeutic decision making for this population. This lack is attributable to older individuals with cancer being excluded from clinical trials because, in this population, there is a high incidence of organ dysfunction and comorbidities, a high risk of death from other diseases, and a high risk of severe adverse events, all of which are common study exclusion criteria (5–9). However, given the burden of malignant disease among the elderly, clinical research focusing on older patients with cancer is urgently needed.
The Japan Clinical Oncology Group (JCOG) is the largest cancer cooperative group in Japan (10). The goal of JCOG is to establish better treatment standards for various cancer types, which has motivated the conduct of several pivotal randomized controlled trials. Several of these trials have specifically focused on older patients (11–16), but there has been no formal JCOG policy addressing principles or methodologies for geriatric research. A Geriatric Study Committee was therefore established to promulgate JCOG policy for geriatric oncology research (17).
The Geriatric Study Committee has held quarterly meetings beginning in 2013 with members from each disease- or treatment modality-oriented JCOG subgroup. The committee constituted members representing each disease or modality-oriented JCOG subgroup, including a psycho-oncologist, an epidemiologist, and a specialist in quality of life assessment. The committee held six meetings from December 2013 to December 2015 to develop this Geriatric Research Policy. The draft policy was developed by the members of Geriatric Study Committee and was reviewed by the JCOG Executive Committee. The finalized version was approved by the JCOG Executive Committee and was implemented in May 2016.
The policy in Japanese developed by the Geriatric Study Committee had been extensively reviewed by JCOG Executive Committee members and was formally adopted in May 2016 (18). This article is the English version.
There have been several consensus reports regarding the clinical trial methodology in older patients with cancer (19–23), but no policy exists that concretely and comprehensively describes the principles and methodologies for geriatric oncology research. This JCOG Geriatric Research Policy includes several solutions for issues common to geriatric research and we expect that this policy will serve as a practical framework in planning future geriatric oncology studies.
The European Organization for Research and Treatment of Cancer (EORTC) and the American Society of Clinical Oncology (ASCO) have published position papers describing clinical trial methodology for the patients with cancer. Additionally, the International Society for Geriatric Oncology (SIOG) has published consensus reports addressing geriatric assessment and endpoints for geriatric cancer research. However, these consensus reports only describe a basic outline or specific issues. Thus, we have established a policy that concretely and comprehensively describes the principles and methodologies for performing geriatric cancer research.
In accordance with other consensus reports, we categorized older patients into three conceptual groups: (i) fit patients, (ii) vulnerable patients and (iii) frail patients. However, at present, there are no clear criteria distinguishing between fit and vulnerable elderly patients or between vulnerable and frail elderly patients, and a more practical approach for categorization is needed. Therefore, we categorized older patients into five practical groups. As study designs will differ depending on the study population, this new categorization method may serve as a practical framework in planning future geriatric research.
In addition, we recommend that study endpoints not only include survival but also other endpoints (e.g., physical and cognitive function). Although study endpoints that incorporate geriatric assessment in the study design are important, it is not always easy to use them as primary endpoints, as problems arise such as missing data or difficulties in determining clinically significant differences in the endpoints based on the geriatric assessment—the most appropriate endpoints, and which study design elements are most relevant for older patients are actively debated.
We believe geriatric assessment is useful in geriatric cancer research, but it is not practical to evaluate all these items as part of routine care. Thus, we determined the recommended GA tools with simplicity and discriminative power in mind. Our recommended GA tools were similar to those recommended by the EORTC, which is beneficial as the use of standardized tools enables the possibility of cross-sectional investigations in the future.
Nevertheless, some challenges not covered in this policy remain, such as how to conduct cancer research involving older patients with cognitive dysfunction (24,25) and how to determine clinically significant differences in endpoints based on geriatric assessment (26). We plan to revise this policy periodically to guide future geriatric research.
Japan Clinical Oncology Group
Geriatric research
1. Objectives
The objective of this policy is to provide guidelines for JCOG clinical research that enrolls geriatric patients with cancer.
The policy aims:
To define the policy for selection of subjects for JCOG geriatric research
To establish standard endpoints and methodologic schemes for geriatric research
To establish standard tools for geriatric assessment (GA)
This policy provides basic considerations relating to geriatric cancer research without discussion of disease-specific aspects because detailed considerations and physiology of this population differ among various types of cancer.
2. Current situation
2.1. Characteristics of geriatric patients with cancer
Geriatric patients with cancer differ from younger patients in many ways, as biological and physiologic changes and new social issues emerge with age (27,28). For example, (i) geriatric patients have a high incidence of cancer, particularly including some types specific to the elderly due to age-related DNA damage (29,30); (ii) adverse drug reactions are more likely to occur because of impaired organ function;(31) (iii) multiple comorbidities are common (32); (iv) polypharmacy increases the likelihood of drug interactions and adverse drug reactions (33); (v) geriatric syndromes, such as those involving delirium or urinary incontinence, are likely to occur; (vi) nutritional status and drug compliance may vary depending on the need for caregivers; and (vii) some retirees may have economic hardship (21,34,35).
2.2. Current status of geriatric research
According to the 2015 Annual Report on the Aging Society, elderly individuals aged, 65 years or older accounted for less than 5% of Japan’s total population in 1950. However, this had risen to 7% by 1970, over 14% by 1994, and it reached an all-time high of 26.1% in 2014 (1–4). The number of geriatric patients with cancer has risen in line with the aging of society, but there is a lack of reliable evidence to support decision making in oncology treatment for this patient population (5–9). Elderly patients are usually excluded from clinical trials for several reasons. The high incidence of organ dysfunction and comorbidities may disqualify them. The high risk of death from other diseases in this population may decrease the power of a study to detect a true difference in survival benefit between treatment arms. The elderly also have a greater likelihood of severe adverse events, which may lead to overestimation of toxicity of the treatment being tested.
Treatment strategies may differ between geriatric and nongeriatric patients with cancer. Today, there is a growing need to develop evidence to support the provision of appropriate medical care for geriatric patients with cancer, but perspectives and methodologies have yet to be established for clinical research targeting this population. JCOG studies including geriatric patients with cancer have been increasing, but there have been no guidelines to plan and conduct geriatric oncology research. Therefore, the JCOG Executive Committee decided to develop this policy in 2013 (17).
2.3. Geriatric research ad-hoc committee
The JCOG Geriatric Research Ad-hoc Committee was organized to establish a policy for geriatric research in December 2013. The committee was constituted with members representing each disease or modality-oriented JCOG subgroup, a psycho-oncologist, an epidemiologist, and a specialist in assessing quality of life. The committee held six meetings from December 2013 to December 2015 to develop this Geriatric Research Policy. This policy was then reviewed and approved by the JCOG Executive Committee and was implemented in May 2016.
2.4. Geriatric study committee
The Ad-hoc committee, having accomplished it mission to establish Geriatric Research Policy, was superseded by the standing Geriatric Study Committee (GSC) in June 2016.
Mission of the geriatric study committee
To collect information on ongoing geriatric research and methodologic advances in geriatric cancer research and to optimize guidelines for geriatric cancer research. GSC members share collected information with JCOG investigators through presentations in JCOG Executive Committee or subgroup meetings on an as-needed basis. In addition, GSC members are consulted about study design and adequate use of GA tools.
To evaluate GA tools used in JCOG studies, seeking to improve them or provide guidance on how to use them. For example, GSC decides which GA tools to recommend and will explore the association between GA scores and efficacy and safety endpoints in JCOG studies which apply the GA tools. The GSC will consider whether the score of a GA tool can be incorporated into eligibility criteria in clinical trials targeting geriatric patients with cancer.
To establish methodology to define eligibility criteria for geriatric cancer research beyond simply using chronological age.
3. Subjects of geriatric research
3.1. Definitions of geriatric individuals and geriatric research
One way to define the elderly is as a population in which age-related biological and physiologic changes and socioeconomic issues emerge. Ideally, age-related biological changes can be evaluated by telomere length or p16INK4A expression, physiologic changes can be evaluated with laboratory tests or GA (see below), and socioeconomic issues can be measured through interviews. However, there is no established method to define the elderly based on the aforementioned issues. Definitions of the elderly based on telomere length or GA are unlikely to be widely accepted at present. As a result, this policy provisionally defines geriatric individuals according to chronological age.
The World Health Organization (WHO) defines the elderly as individuals aged 65 years or older (36). In Japan, the Act on Assurance of Medical Care for Elderly People (Act No. 80 of 1982) and related ordinances define individuals aged 65 to 74 years as young-old and those aged 75 years or older as old-old. Some guidelines define the elderly as ≥70 years and ≥75 years, but such guidelines are not widely accepted. One proposal is to define the elderly as a population for whom there is no reliable evidence to support therapeutic decision making, but this is not acceptable because such a criterion may vary considerably depending on the type of cancer, leading to differing age boundaries depending on the malignancy being addressed. In the current policy, therefore, an individual is defined as geriatric if they are aged 65 years or older, in line with the stipulations of the WHO and Japanese law. Similarly, those aged below 65 years are defined as nongeriatric.
Therefore, JCOG geriatric research is defined as studies with an eligibility criterion of minimum age of ≥65 years. Furthermore, research with an eligibility criterion of a maximum age of ≥65 years is defined as research that includes geriatric patients (this will apply to many JCOG studies), and research with an eligibility criterion of a maximum age of <65 years is considered research that does not include geriatric patients. However, the minimum age as an eligibility criterion should be decided as appropriate for each individual study, because eligibility criteria specifying a minimum age may differ in depending on the disease characteristics or treatment toxicity (for example, research among individuals aged 70 years or above).
3.2. Subject selection policy for geriatric research
This policy defines the following conceptual categories to enable the differentiation of patient populations enrolled in geriatric research.
Fit: Patients able to receive the same standard therapies as fit young individuals
Unfit: Patients unable to receive the same standard therapies as fit young individuals
Vulnerable: Patients able to receive some but not all of the standard therapies given to fit young individuals
Frail: Patients unable to receive aggressive therapy but who can receive best supportive care or palliative care
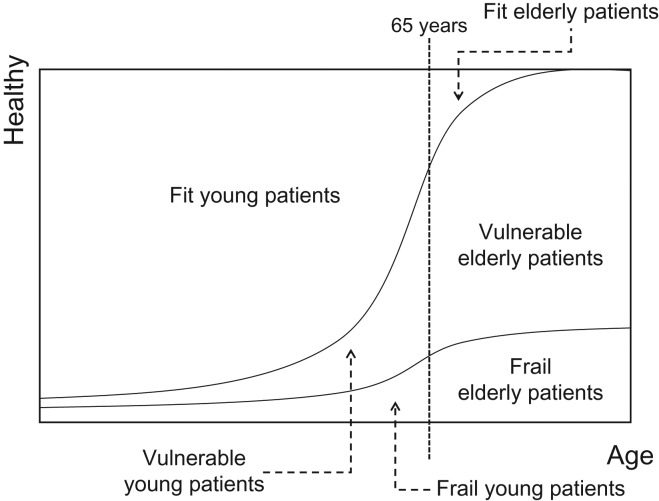
This policy is applicable to research that includes elderly patients aged ≥65 years (fit, vulnerable, and frail elderly), but most of the geriatric research performed by JCOG will likely target vulnerable elderly patient populations (Fig. 1).
Figure 1.
Conceptual classification to define patient populations for geriatric research.
Patients who are geriatric but can clearly be defined as fit elderly do not necessarily need to be treated separately from young patients but can be included in the same study population as young patients. Much geriatric research thus targets vulnerable elderly populations. These patients cannot be given the standard therapies given fit young patients, but they can be given some active treatment. However, there is a lack of evidence on therapies for vulnerable elderly populations, so research is specifically needed for this population to determine the best treatments. If a study is conducted with vulnerable patients regardless of age, the vulnerable elderly and vulnerable young patients can be included in the same treatment development group (for example, a study to confirm the efficacy of radiotherapy in patients with lung cancer not suitable for lobectomy). In this case, it is useful to refer to the considerations on vulnerable elderly patients in this policy to inform the definitions of vulnerable patients being enrolled in the research and the research study design.
3.3. Practical categories for subject selection policy for geriatric research
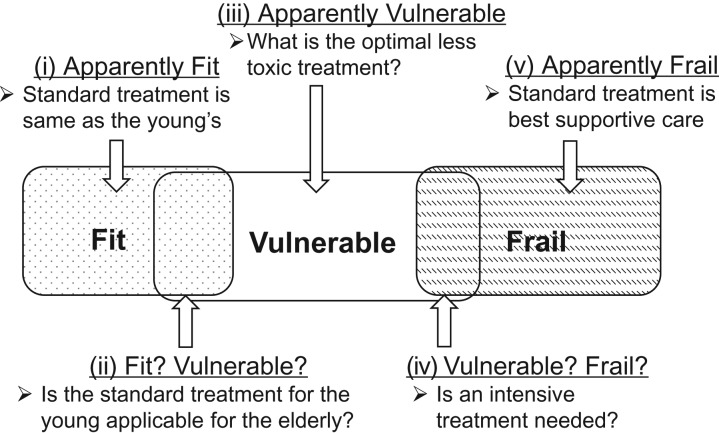
As described above, most of the target populations of JCOG geriatric research are vulnerable elderly. However, at present there are no clear criteria distinguishing between fit and vulnerable elderly or between vulnerable and frail elderly. We can define individuals conceptually as fit, vulnerable, or frail, but a more practical approach may be to categorize individuals as (i) clearly fit (elderly individuals who would be considered fit by most healthcare professionals), (ii) elderly individuals not clearly fit or vulnerable (healthcare professionals would find it difficult to decide if they were fit or vulnerable), (iii) clearly vulnerable elderly (elderly individuals who would be considered vulnerable by most healthcare professionals), (iv) elderly individuals not clearly vulnerable or frail (healthcare professionals would find it difficult to decide if they were vulnerable or frail) and (v) clearly frail elderly (elderly individuals who would be considered frail by most healthcare professionals).
Of these categories, patients in category five are not suitable for JCOG geriatric research. Those in categories (i) and (iv) are also basically not suitable for JCOG geriatric research, but patients in these categories may be included under certain conditions.
Therefore, categories (ii) and (iii) are the main target populations for JCOG geriatric research (Fig. 2).
Figure 2.
Practical categories of patient populations for geriatric research
3.4. Defining vulnerable elderly
When conducting clinical research with vulnerable elderly patients, we need to define the appropriate eligibility and exclusion criteria for each individual trial commensurate with the toxicity of the therapy and the disease characteristics. In some cases, the vulnerable elderly can be defined based on existing clinical research data, but in many cases they are defined based on speculation or assumptions due to the lack of existing data. Considerations for each individual trial should be based on laboratory values, chronological age, and performance status, as well as GA and other information. We recommend the following methods prioritized in the order listed.
Observational study: Set conditions that define the vulnerable elderly by conducting observational studies and investigating prognostic factors for vulnerable elderly who cannot receive standard therapies.
Questionnaire to investigators: Set conditions that define the vulnerable elderly by surveying investigators about clinical decision making about treatment choices in elderly patients.
Consensus: Set conditions that define the vulnerable elderly through group discussions to derive a consensus.
3.5. Considerations for eligibility and exclusion criteria in research with vulnerable elderly populations
3.5.1. Generalizability
Geriatric individuals have a higher incidence of complications such as organ dysfunction, comorbidities, and multiple cancers than do young individuals. Therefore, if eligibility and exclusion criteria are the same as those used in clinical trials for young individuals, only fit elderly will be enrolled in the study, with the study results potentially unsuitable for extrapolation to general geriatric practice. In geriatric practice, it is assumed that a certain proportion of patients are vulnerable. Therefore, broader eligibility and exclusion criteria must be set than for research in young individuals in order to include the vulnerably elderly and thus increase the generalizability of the trial results.
We recommend careful establishment of exclusion criteria. Specific examples include shorter disease-free periods after treatment for multiple cancers (e.g., eligible if disease-free for at least one year or if active therapy has ended) or limiting comorbidities or restricting exclusion criteria to only more severe disease (e.g., only exclude angina pectoris that is currently being treated).
3.5.2. Safety perspective
Research for the vulnerable elderly tends to have a higher incidence of serious adverse events, with those that occur being more likely to become severe when compared with research in young individuals. Therefore, careful consideration is needed to ensure the safety of study participants.
For example, when assessing organ function relevant to adverse events expected to occur frequently or adverse events that are likely to become severe, the eligibility criteria for laboratory values may be set at more restrictive levels, safety evaluations are required more frequently after the start of the protocol treatment, hospitalization may be required during the first course of treatment, or treatment modification criteria are set more conservatively (e.g., dose reduction or treatment interruption).
3.5.3. Minimum age
The patient populations typically targeted in geriatric research are vulnerable elderly who cannot be given the same standard therapies as young individuals but are not so vulnerable that they cannot receive any treatment at all. Few elderly individuals can tolerate the standard therapy given to young individuals if it is highly toxic, but many can be treated with the standard therapy if it is weakly toxic. Therefore, the target population for geriatric research is determined not simply based on the intensity and toxicity of the protocol treatment for elderly patients but also for that seen in young individuals. If the standard therapy for young individuals is highly toxic, the minimum age in the eligibility criteria for geriatric research needs to be lower, whereas if the standard therapy for young individuals is weakly toxic, the minimum age in the eligibility criteria for geriatric research may be higher.
On this basis, we think it is not appropriate to determine a uniform minimum age for eligibility in geriatric research, nor does the policy stipulate one. The minimum age for eligibility should be set for each individual study taking into consideration the degree of toxicity and intensity of the standard therapy for young individuals.
3.5.4. Maximum age
With regard to a maximum age for eligibility in geriatric research, considerations should be given to the toxicity and intensity of the protocol treatment. Where the protocol treatment is relatively toxic, it may be better to define a maximum age to ensure patient safety. In contrast, where the protocol treatment is not toxic and the patient satisfies eligibility criteria based on laboratory tests that demonstrate adequate organ function, it may not be necessary to define a maximum age. When a maximum age is established in the eligibility criteria, the age boundary should be determined with reference to average life expectancy.
4. Endpoints in geriatric research
4.1. Endpoints
In geriatric cancer research, we recommend that study endpoints include items such as physical and cognitive function in addition to common endpoints like overall survival (OS). For example, the objective of treatment in elderly patients is not simply to provide extended life expectancy but is also to benefit patients in terms of maintaining physical function (avoiding become bedridden) and cognitive function (avoiding progression of cognitive deficiency). Therefore, items measuring these potential benefits could be used as endpoints for geriatric research.
Note that where OS is used as the endpoint, it may be possible to obtain meaningful data by calculating average life expectancy of the enrolled patient population from data on the sex and age of patients and from the abridged life tables produced by the Ministry of Health, Labor and Welfare. The addition of such analysis is appropriate.
4.2. Co-primary endpoints and composite endpoints
Co-primary endpoints, which evaluate two endpoints separately but lead to one decision, and composite endpoints, which combine two or more outcomes into one endpoint, can be used when researchers intend to evaluate multiple endpoints, such as OS and physical or cognitive function. Which method is suitable should be determined on a study-by-study basis.
-
Co-primary endpoints.
It can be concluded that the study treatment is useful only if all or any of the endpoints meet prespecified criteria. As an example of the former, where OS and cognitive function (evaluated with the Mini-Mental State examination [MMSE]) are used as co-primary endpoints, if the study demonstrates superior OS and also that MMSE does not decline by more than X points (rejecting the null hypothesis that MMSE declines by more than X points), it is concluded that the study treatment is useful as standard therapy.
-
Composite endpoints.
This method includes different factors combined within one endpoint. An example is an endpoint of death or event-free survival with event defined as a 0.2 fall in EQ-5D. It can be concluded that the study treatment is useful if the stratified log-rank test is significant in a superiority trial, as with progression-free survival. For an inferiority trial, if the results of stratified Cox regression analysis are significant, it can be concluded that the study treatment is useful.
5. Study design for geriatric research
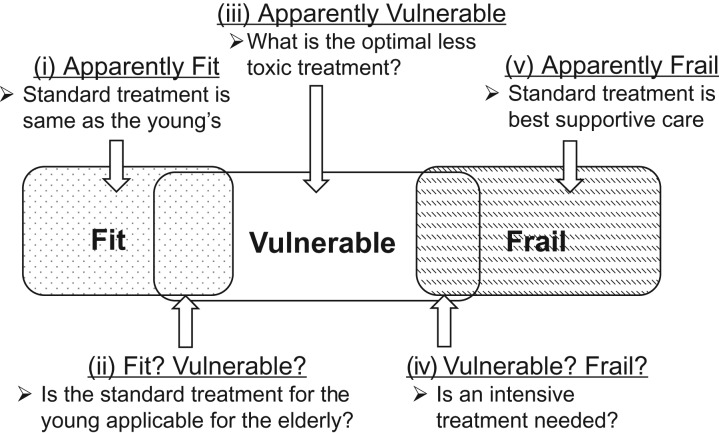
As discussed in Section 3.3, most JCOG geriatric research involves elderly individuals not clearly fit or vulnerable or those who are clearly vulnerable (Fig. 3). Appropriate study designs differ depending on the study population, clinical question, and treatment being evaluated. Although different studies will have different study designs, we provide some considerations on study design that will be suitable for typical research hypotheses in geriatric research.
Figure 3.
Practical categories of patient populations for geriatric research (repeated from section 3.3.)
5.1. Randomized controlled trial design when the objective is to compare treatments
When the research is intended to confirm the superiority or inferiority of a treatment in a particular study population, we recommend a randomized controlled trial (RCT) based on the same standards as for research in young individuals.
Example 1: Subjects not clearly fit or vulnerable.
These subjects correspond to category (ii) in Fig. 3. The clinical question is to determine whether the standard treatment used in young individuals is also appropriate for geriatric patients or whether a less intensive treatment is more appropriate. Therefore, an RCT should be conducted to compare two treatment methods.
Example 2: Subjects clearly vulnerable.
These subjects correspond to category (iii) in Fig. 3. The subjects are not suited to the standard treatment used in young individuals. The clinical question is to compare two less intensive treatments to determine if one is superior or if a less intense treatment is superior to no treatment. Therefore, an RCT should be conducted to compare two treatment methods.
5.2. Single-arm trial with an interventional study design when the objective is to investigate whether standard treatment in younger patients can be used for elderly patients.
An example is for subjects not clearly fit or vulnerable corresponding to category (ii) in Fig. 3. The enrolled elderly patients receive the standard treatment used for younger patients in a single-arm trial design, and key endpoints such as compliance and toxicity are evaluated as to whether they differ significantly from younger patient populations.
5.3. Observational study design when the objective is to identify conditions defining vulnerable elderly
Today, there is no clear demarcation criterion for distinguishing between fit and vulnerable elderly or between vulnerable and frail elderly.
When elderly patients are treated, some may experience a marked decline in physical function after treatment. In cases where a posttreatment decline in physical function was not expected before treatment began, the clinical question is whether the decline could have been predicted from pretreatment baseline factors. In this situation, an interventional study using common eligibility and exclusion criteria should enroll only patients with good general condition, and it may result in few events (such as marked decline in physical function) and is less likely to provide answers to the clinical question. An observational rather than an interventional study in this situation is recommended. It will collect comprehensive data on treatment provided as routine medical care and may investigate baseline factors which are predictive of adverse events of a certain level of severity, such as decline in physical function or impairment of cognitive function.
An observational study design is also the first choice where the main objective is to elucidate how a treatment is used in routine medical care.
5.4. Allowance for adverse events
Geriatric research needs to take a cautious approach in allowing for serious adverse events including treatment-related deaths. The elderly tend to have comorbidities or organ dysfunction, making adverse events more likely to rapidly increase in severity compared with what is seen in younger individuals. Proactive testing may not be implemented when adverse events become more severe simply because the patient is elderly. It is difficult to determine whether an adverse event is due to progression of the primary disease or if there is a causal relationship between a serious adverse event and the protocol treatment. In these situations, when stopping rules are established based on treatment-related deaths but a definite causal relationship between the treatment and death is unclear, the proportion of treatment-related deaths may be overestimated and the study may be inappropriately terminated. Therefore, it may be preferable to define a stopping rule such as the proportion of patients who died within 30 days after the start of the protocol treatment rather than simply treatment-related deaths, particularly in studies enrolling patients with highly advanced disease. On the other hand, when a causal relationship is based on the concept of reasonable possibility, the proportion of treatment-related deaths can be underestimated. Therefore, the appropriateness of setting stopping rules needs to be considered in light of the conditions in each individual study.
5.5. Defining clinically meaningful differences
Care needs to be taken in geriatric research to define clinically meaningful differences, such as the size of the difference in primary endpoints in superiority trials or the size of the noninferiority margin in noninferiority trials.
For example, in a phase III superiority trial for younger patients, a clinically meaningful difference may be determined based on considering the risk-benefit balance between the study treatment and treatments in previous studies. Defining the risk-benefit balance includes the frequency of adverse events by grade, the frequency of treatment-related deaths, and Grade four nonhematologic toxicity as serious adverse events according to the Common Terminology Criteria for Adverse Events or Clavien–Dindo classification for surgical complications.
A clinically meaningful difference is determined so as to correspond to the increased risk of the test treatment compared with standard treatment. However, adverse events are often difficult to evaluate by the usual criteria in elderly patients (e.g., reduced motivation or decline in activity). It has also been shown that adverse events that are reversible in younger patients may become irreversible in elderly patients. Therefore, when defining clinically meaningful differences in geriatric research, the true risk difference or risk ratio can be underestimated if risk is estimated based on the frequency or grade of adverse events in previous research, particularly in studies in young patients. This point should be taken into consideration for geriatric research, and the study should be designed such that a toxic new treatment will have a larger difference than that in trials for nonelderly patients (for example, for a hazard ratio of 0.8 in trial among young patients, the hazard ratio would be set at 0.7 for geriatric research).
6. Geriatric assessment
6.1. Geriatric assessment
GA is the general term describing methods to evaluate the physical, mental, and social functioning of elderly patient from a comprehensive perspective (37).
The term comprehensive geriatric assessment (CGA) commonly includes both multiple functional domains and assessment over time. However, in oncology, assessment of various functions at one point in time has mainly been studied rather than the effect of interventions over time. Therefore, in this policy, we refer only to GA rather than CGA to describe the evaluation of geriatric patients with cancer (38,39).
6.2. Geriatric assessment tools
GA tools include evaluation forms and other tools used to perform GA. The main GA domains and typical tools used are shown below.
Table 6.2.a.
GA domains and typical GA tools.
| Domain | Typical GA tools |
|---|---|
| Physical function | Activities of daily living (ADL) (40), Instrumental activities of daily living (IADL) (19,41,42)ECOG performance status (ECOG PS) |
| Comorbidities | Charlson Comorbidity index (CCI) (43) Cumulative Illness Rating Scale (CIRS) (44) |
| Medications | Medication Appropriateness Index (MAI) (45) |
| Nutrition | Body-mass index (BMI)Mini Nutritional Assessment (MNA) (46) |
| Cognitive function | Mini-Mental State Examination (MMSE) (47) Clock-drawing test (48) |
| Mood | Geriatric Depression Scale (GDS) (49) Center for Epidemiologic Studies Depression Scale (50) |
| Social support | MOS Social Support Survey (51) |
| Geriatric syndrome | Confusion Assessment Method (delirium) (52) Timed Up & Go Test (fall) (53) |
The table below shows a summary of the GA tools widely used in Japan to assess geriatric patients with cancer and the functions that each tool evaluates. There are no Japanese-language GA tools for medication, social support, or geriatric syndromes.
Table 6.2.b.
Summary of each domain.
| ADL | IADL | CCI | MNA | MMSE | GDS | |
|---|---|---|---|---|---|---|
| Physical function | Excellent | Excellent | - | - | - | - |
| Comorbidities | - | - | Excellent | - | - | - |
| Medication | - | - | - | - | - | - |
| Nutrition | - | - | - | Excellent | - | - |
| Cognitive function | - | - | - | - | Excellent | - |
| Mood | - | - | - | - | - | Excellent |
| Social support | - | - | - | - | - | - |
| Geriatric syndrome | - | - | - | - | - | - |
| Time required (minutes) | 5 | 5 | 5 | <5 | 15 | <5 |
Key: Evaluation not possible.
6.3. Screening tools
As it takes 90–120 minutes to comprehensively evaluate the above domains with each GA tool, it impractical to assess all these items as part of a busy routine practice. Physicians are therefore advised to use a few questions first to screen geriatric patients for any dysfunction and then use formal GA tools only for those deemed to have functional impairment (19). A number of different screening tools have been developed for this purpose. The table below shows the most frequently used screening tools and the domains they evaluate.
Table 6.3.
Screening tools.
| G8 | VES-13 | fTRST | MINI-COG | |
|---|---|---|---|---|
| Physical function | Average | Good | Good | - |
| Comorbidities | - | - | - | - |
| Medication | Average | - | Average | - |
| Nutrition | Good | - | Average | - |
| Cognitive function | - | - | Good | Good |
| Mood | Average | - | Average | - |
| Social support | - | - | - | - |
| Geriatric syndrome | - | - | - | - |
| Time required (minutes) | 3 | 3 | 3 | 5 |
6.4. GA tools used in JCOG study
Objectives of using GA tools in JCOG studies are to:
Collect information on background factors associated with geriatric function
Use as eligibility criteria to select subjects for the study (e.g., to select those who are vulnerable elderly)
Use as adjustment factors for randomization
Use as outcome measures (e.g., to investigate if cognitive function has declined after treatment)
Use as factors for treatment modification (e.g., dose reduction if ADL falls by X points)
Different GA tools will be appropriate for different studies, depending on disease characteristics or study endpoints, so the tools should be chosen on a study-by-study basis (38,39).
There are benefits, however, in the use of common sets of standardized tools, as this enables future cross-sectional investigations. We have defined our recommendations for the GA tools to be used in geriatric research below. The GSC should be consulted if necessary to discuss which GA tool is appropriate. The strength of the recommendation may differ according to the purpose for which a tool is being used (e.g., as an endpoint or for eligibility or exclusion criteria).
LEVEL 1: Indispensable in JCOG geriatric research
|
-
LEVEL 1: G8 is an indispensable tool that is, in principle, used in all JCOG geriatric research.
G8 is one of the mostly widely used screening tools worldwide (19,54). It can be performed in about 3 minutes with the healthcare professional completing the form. Using CGA as the gold standard, Decoster et al. compared the sensitivity and specificity the G8 with 16 other screening tools, including fTRST and VES-13. They concluded that G8 was the most useful screening tool because (i) it is highly sensitive (77–92%), (ii) it has a permissible specificity (52–75%), (iii) it gathers robust data and (iv) it is predictive of function in many different types of cancer (55).
-
LEVEL 2: Recommended tools are IADL, CCI, social situation and MINI-COG.
We recommend using all four tools rather choosing from among them so as to allow for future cross-sectional analysis. Note that G8, IADL, CCI, and social situation are used as standard tools in geriatric research by the European Organization for Research and Treatment of Cancer (EORTC) Minimum Dataset (MinDS) (38,39).
IADL (Instrumental Activities of Daily Living)
IADL is the most commonly used tool to evaluate physical function and is completed by the patients themselves (men: 0–5 points; women: 0–8 points). It takes about 5 minutes to complete (19,41,42).
CCI (Charlson Comorbidity Index)
CCI provides a risk category scale (low, medium, high, very high) based on the type of comorbidity and degree of severity. The healthcare professional completes the CCI form while discussing comorbidities with the patient. It takes about 5 minutes to complete (43).
Social situation
The social situation (lives alone, lives with someone else, resident of an institution) is an indicator reflecting connections with society, equating to the social support domain. It takes about 1 minute to complete.
MINI-COG
MINI-COG is a screening tool for cognitive function (55).
Although MINI-COG is not included in EORTC MinDS, JCOG recommends that it be performed, based on the consensus of the GSC that it is an important tool for the evaluation of cognitive function in geriatric research.
The healthcare professional completes the form in discussion with the patient. It takes about 5 minutes to complete. Based on the Mini-Mental State Exam (MMSE) as the gold standard, the MINI-COG has a sensitivity of 99% and a specificity of 93%. MMSE is also a widely used GA tool, but conducting it places a psychological and time burden on both the healthcare professional and the patient. Therefore, we decided to recommend that all JCOG geriatric research use MINI-COG as a simple screening tool for cognitive function.
LEVEL 3: Additional GA tools should be chosen according to the study characteristics.
Acknowledgments
We would like to express our sincere thanks to all Geriatric Study Committee and JCOG Data Center/Operations Office members.
Funding
This project was supported by the National Cancer Center Research and Development Fund (26-A-4) and by the Japan Agency for Medical Research and Development (AMED) under Grant Number JP 15ck0106075.
Conflict of interest statement
None declared.
References
- 1. Muramatsu N, Akiyama H. Japan: super-aging society preparing for the future. The Gerontologist 2011;51:425–32. [DOI] [PubMed] [Google Scholar]
- 2.CANCER STATISTICS IN JAPAN ‘17. https://ganjoho.jp/en/professional/statistics/brochure/2017_en.html.
- 3.World Population Ageing 2017. https://www.un.org/en/development/desa/population/theme/ageing/WPA2017.asp.
- 4.CANCER RESEARCH UK.
- 5. Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr., Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 1999;341:2061–7. [DOI] [PubMed] [Google Scholar]
- 6. Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol 2004;22:4626–31. [DOI] [PubMed] [Google Scholar]
- 7. Aapro MS, Kohne CH, Cohen HJ, Extermann M. Never too old? Age should not be a barrier to enrollment in cancer clinical trials. Oncologist 2005;10:198–204. [DOI] [PubMed] [Google Scholar]
- 8. Unger JM, Coltman CA Jr., Crowley JJ, et al. Impact of the year 2000 Medicare policy change on older patient enrollment to cancer clinical trials. J Clin Oncol 2006;24:141–4. [DOI] [PubMed] [Google Scholar]
- 9. Bleyer A. In and out, good and bad news, of generalizability of SWOG treatment trial results. J Natl Cancer Inst 2014;106:dju027. [DOI] [PubMed] [Google Scholar]
- 10.JCOG-Japan Clinical Oncology Group. http://www.jcog.jp/en/.
- 11. Mizoroki F, Hirose Y, Sano M, et al. A phase II study of VEPA/FEPP chemotherapy for aggressive lymphoma in elderly patients: Japan Clinical Oncology Group Study JCOG9203. Int J Hematol 2006;83:55–62. [DOI] [PubMed] [Google Scholar]
- 12. Okamoto H, Watanabe K, Kunikane H, et al. Randomised phase III trial of carboplatin plus etoposide vs split doses of cisplatin plus etoposide in elderly or poor-risk patients with extensive disease small-cell lung cancer: JCOG 9702. Br J Cancer 2007;97:162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsukada H, Yokoyama A, Goto K, et al. Randomized controlled trial comparing docetaxel-cisplatin combination with weekly docetaxel alone in elderly patients with advanced non-small-cell lung cancer: Japan Clinical Oncology Group (JCOG) 0207dagger. Jpn J Clin Oncol 2015;45:88–95. [DOI] [PubMed] [Google Scholar]
- 14. Atagi S, Kawahara M, Yokoyama A, et al. Thoracic radiotherapy with or without daily low-dose carboplatin in elderly patients with non-small-cell lung cancer: a randomised, controlled, phase 3 trial by the Japan Clinical Oncology Group (JCOG0301). Lancet Oncol 2012;13:671–8. [DOI] [PubMed] [Google Scholar]
- 15. Abe T, Takeda K, Ohe Y, et al. Randomized phase III trial comparing weekly docetaxel plus cisplatin versus docetaxel monotherapy every 3 weeks in elderly patients with advanced non-small-cell lung cancer: the intergroup trial JCOG0803/WJOG4307L. J Clin Oncol 2015;33:575–81. [DOI] [PubMed] [Google Scholar]
- 16. Mizutani T, Ando M, Mizusawa J, et al. Prognostic value of Lung Cancer Subscale in older patients with advanced non-small cell lung cancer: An integrated analysis of JCOG0207 and JCOG0803/WJOG4307L (JCOG1414A). J Geriatr Oncol 2018;9:583–8. [DOI] [PubMed] [Google Scholar]
- 17.Geriatric Study Committee. http://www.jcog.jp/en/committee/index.html.
- 18.Japan Clinical Oncology Group (JCOG). Geriatric research. http://www.jcog.jp/basic/policy/A_020_0010_39.pdf 2016.
- 19. Kenis C, Bron D, Libert Y, et al. Relevance of a systematic geriatric screening and assessment in older patients with cancer: results of a prospective multicentric study. Ann Oncol 2013;24:1306–12. [DOI] [PubMed] [Google Scholar]
- 20. Hurria A, Dale W, Mooney M, et al. Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J Clin Oncol 2014;32:2587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hurria A, Levit LA, Dale W, et al. Improving the Evidence Base for Treating Older Adults With Cancer: American Society of Clinical Oncology Statement. J Clin Oncol 2015;33:3826–33. [DOI] [PubMed] [Google Scholar]
- 22. Visvanathan K, Levit LA, Raghavan D, et al. Untapped Potential of Observational Research to Inform Clinical Decision Making: American Society of Clinical Oncology Research Statement. J Clin Oncol 2017;35:1845–54. [DOI] [PubMed] [Google Scholar]
- 23. Nipp RD, Yao NA, Lowenstein LM, et al. Pragmatic study designs for older adults with cancer: report from the U13 conference. J Geriatr Oncol 2016;7:234–41. [DOI] [PubMed] [Google Scholar]
- 24. Sessums LL, Zembrzuska H, Jackson JL. Does this patient have medical decision-making capacity? JAMA 2011;306:420–7. [DOI] [PubMed] [Google Scholar]
- 25. McKoy JM, Burhenn PS, Browner IS, et al. Assessing cognitive function and capacity in older adults with cancer. J Natl Compr Canc Netw 2014;12:138–44. [DOI] [PubMed] [Google Scholar]
- 26. Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol 2008;61:102–9. [DOI] [PubMed] [Google Scholar]
- 27. Balducci L. Geriatric oncology. Crit Rev Oncol Hematol 2003;46:211–20. [DOI] [PubMed] [Google Scholar]
- 28. Dale W, Mohile SG, Eldadah BA, et al. Biological, clinical, and psychosocial correlates at the interface of cancer and aging research. J Natl Cancer Inst 2012;104:581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Falandry C, Gilson E, Rudolph KL. Are aging biomarkers clinically relevant in oncogeriatrics? Crit Rev Oncol Hematol 2013;85:257–65. [DOI] [PubMed] [Google Scholar]
- 30. Pallis AG, Hatse S, Brouwers B, et al. Evaluating the physiological reserves of older patients with cancer: the value of potential biomarkers of aging? J Geriatr Oncol 2014;5:204–18. [DOI] [PubMed] [Google Scholar]
- 31.Committee tIS. STUDIES IN SUPPORT OF SPECIAL POPULATIONS: GERIATRICS.
- 32.(NCCN) TNCCN. NCCN GUIDELINES FOR SPECIFIC POPULATIONS: Older Adult Oncology. https://www.nccn.org/professionals/physician_gls/default.aspx.
- 33. Lichtman SM. Polypharmacy: geriatric oncology evaluation should become mainstream. J Clin Oncol 2015;33:1422–3. [DOI] [PubMed] [Google Scholar]
- 34. Mohile SG, Dale W, Somerfield MR, et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol 2018;36:2326–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Biganzoli L, Lichtman S, Michel JP, et al. Oral single-agent chemotherapy in older patients with solid tumours: a position paper from the International Society of Geriatric Oncology (SIOG). European journal of cancer (Oxford, England: 1990) 2015;51:2491–500. [DOI] [PubMed] [Google Scholar]
- 36. Organization WH. Proposed working definition of an older person in Africa for the MDS Project. https://www.who.int/healthinfo/survey/ageingdefnolder/en/.
- 37. Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014;32:2595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pallis AG, Fortpied C, Wedding U, et al. EORTC elderly task force position paper: approach to the older cancer patient. European journal of cancer (Oxford, England: 1990) 2010;46:1502–13. [DOI] [PubMed] [Google Scholar]
- 39. Pallis AG, Ring A, Fortpied C, et al. EORTC workshop on clinical trial methodology in older individuals with a diagnosis of solid tumors. Ann Oncol 2011;22:1922–6. [DOI] [PubMed] [Google Scholar]
- 40. Delva F, Marien E, Fonck M, et al. Factors influencing general practitioners in the referral of elderly cancer patients. BMC Cancer 2011;11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kenis C, Decoster L, Bastin J, et al. Functional decline in older patients with cancer receiving chemotherapy: a multicenter prospective study. J Geriatr Oncol 2017;8:196–205. [DOI] [PubMed] [Google Scholar]
- 42. Decoster L, Kenis C, Schallier D, et al. Geriatric assessment and functional decline in older patients with lung cancer. Lung 2017;195:619–26. [DOI] [PubMed] [Google Scholar]
- 43. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011;173:676–82. [DOI] [PubMed] [Google Scholar]
- 44. Kos FT, Yazici O, Civelek B, et al. Evaluation of the effect of comorbidity on survival in pancreatic cancer by using "Charlson Comorbidity Index" and "Cumulative Illness Rating Scale". Wien Klin Wochenschr 2014;126:36–41. [DOI] [PubMed] [Google Scholar]
- 45. Rakesh KB, Chowta MN, Shenoy AK, Shastry R, Pai SB. Evaluation of polypharmacy and appropriateness of prescription in geriatric patients: a cross-sectional study at a tertiary care hospital. Indian J Pharmacol 2017;49:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vellas B, Guigoz Y, Garry PJ, et al. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999;15:116–22. [DOI] [PubMed] [Google Scholar]
- 47. Trivedi D. Cochrane Review Summary: Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Prim Health Care Res Dev 2017;18:527–8. [DOI] [PubMed] [Google Scholar]
- 48. Spenciere B, Alves H, Charchat-Fichman H. Scoring systems for the Clock Drawing Test: a historical review. Dement Neuropsychol 2017;11:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lin X, Haralambous B, Pachana NA, et al. Screening for depression and anxiety among older Chinese immigrants living in Western countries: the use of the Geriatric Depression Scale (GDS) and the Geriatric Anxiety Inventory (GAI). Asia Pac Psychiatry 2016;8:32–43. [DOI] [PubMed] [Google Scholar]
- 50. Schure M, Goins RT. Psychometric examination of the Center for Epidemiologic Studies Depression scale with older American Indians: the Native Elder Care Study. Am Indian Alsk Native Ment Health Res 2017;24:1–17. [DOI] [PubMed] [Google Scholar]
- 51. Dafaalla M, Farah A, Bashir S, et al. Validity and reliability of Arabic MOS social support survey. Springerplus 2016;5:1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in older persons: advances in diagnosis and treatment. JAMA 2017;318:1161–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Barry E, Galvin R, Keogh C, Horgan F, Fahey T. Is the Timed Up and Go test a useful predictor of risk of falls in community dwelling older adults: a systematic review and meta-analysis. BMC Geriatr 2014;14:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Martinez-Tapia C, Paillaud E, Liuu E, et al. Prognostic value of the G8 and modified-G8 screening tools for multidimensional health problems in older patients with cancer. Eur J Cancer 2017;83:211–9. [DOI] [PubMed] [Google Scholar]
- 55. Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol 2015;26:288–300. [DOI] [PubMed] [Google Scholar]