Interim analysis from the first large-scale post-marketing surveillance study of eribulin treatment for soft tissue sarcomas (STSs) showed antitumor activity in both common and rare subtypes of STS (NCT03058406).
Keywords: Eribulin, soft tissue sarcoma (STS), post-marketing product surveillance
Abstract
Background
Although eribulin is used to treat soft tissue sarcomas (STSs), treatment data for rare subtypes are limited. We conducted a post-marketing surveillance study to assess safety and efficacy of eribulin in STS patients stratified by subtype.
Methods
Japanese patients (n = 256) with advanced or metastatic STS receiving eribulin treatment were monitored for treatment status, adverse events, diagnostic imaging, and clinical outcomes at 3 months and 1 year. Interim analysis was performed. Patients will be monitored up to 2 years.
Results
Interim analysis included 3-month (n = 255), imaging (n = 226), and 1-year (n = 105) data. STS subtype distribution was normal. Median number of eribulin cycles was 3.0 (range: 1–17 cycles). Among patients with imaging data, best overall tumor response (12 weeks) was partial response, 7.5% (n = 17); stable disease, 34.5% (n = 78); and stable disease ≥11 weeks, 10.2% (n = 23). Overall response rate (ORR), disease control rate (DCR), and clinical benefit rate (CBR) for all patients were 7.5%, 42.0% and 17.7%, respectively. ORR, DCR, and CBR were 10.3%, 32.0% and 16.5%, respectively, for patients with STS subtypes other than liposarcoma and leiomyosarcoma and included responses from patients with rare STS subtypes. Adverse drug reactions (ADRs) occurred in 211 (82.7%) patients (42 [16.5%] patients had serious ADRs), and none led to death. ADRs leading to drug withdrawal and dose reduction occurred in 27 (10.6%) and 55 (21.6%) patients, respectively.
Conclusion
Eribulin was generally well tolerated and showed antitumor activity against STSs, including rare subtypes that currently have few treatment options.
Clinical trial number
NCT03058406 (ClinicalTrials.gov)
Introduction
Soft tissue sarcoma (STS) describes a rare and heterogeneous group of cancers (<1% of all cancers) with more than 50 histologic subtypes, each with its own different treatment response and prognosis (1). Liposarcoma and leiomyosarcoma (L-type sarcomas) are types of STSs that occur more frequently than other subtypes, and have higher prevalence of approximately 15% and 5–10%, respectively. Therefore, they are often the focus of STS clinical studies.
Surgery, radiation, or a combination of both, are the mainstay for localized STSs. However, in patients with metastatic or recurrent diseases, standard treatment is systemic chemotherapy. Standard a first-line systemic therapy for advanced or metastatic STS involves treatment with doxorubicin either as a monotherapy or in combination with other cytotoxic drugs such as ifosfamide (2,3). Olaratumab, an anti-platelet-derived growth factor receptor-α monoclonal antibody, received accelerated approval in the US as a first-line treatment in combination with doxorubicin for patients with locally advanced or metastatic STS (2) based on the randomized phase 2 trial. However, the recently reported results of ANNOUNCE, the phase 3 study of olaratumab in combination with doxorubicin, did not meet the primary endpoints of overall survival (OS) (4,5). Second-line or later treatment options include gemcitabine, docetaxel, trabectedin, high-dose ifosfamide, pazopanib, and eribulin (3). Several rare STS histological types are reported to benefit from treatment with pazopanib, a multi-targeted receptor tyrosine kinase inhibitor (2). Overall, there are few effective treatments, especially for non-L-type sarcoma.
Eribulin mesylate (eribulin) is a non-taxane microtubule dynamics inhibitor for chemotherapy. Eribulin works by disrupting mitotic spindle formation to cause prolonged mitotic blockage, and it has shown antitumor activity in patients with STS in a phase 2 study (6,7). A phase 3 randomized, multicenter, clinical study reported that the OS of patients with advanced or metastatic liposarcoma, or leiomyosarcoma, was significantly improved in patients treated with eribulin compared with patients receiving dacarbazine treatment (8). Subgroup analysis revealed that eribulin treatment resulted in numerically longer OS in all three histological subgroups of liposarcoma included in the study (8,9). Based on the results of the phase 3 study, eribulin was approved for treatment of liposarcoma in 2016 in both the United States and Europe and is used as a second- or third-line treatment option for these patients (2,3,10–13). Results from a phase 2 study conducted in Japanese patients (14) along with those of the larger phase 3 study (8,9) earned eribulin approval in Japan for treatment of STS in 2016 (15).
In the phase 2 European Organisation for Research and Treatment of Cancer (EORTC) 62052 study, eribulin was reported to have some efficacy for synovial sarcoma as well as STS subtypes classified as ‘other’, with responses to eribulin observed in nine (47%) and 12 (46%) patients, respectively. However, these outcomes did not meet the prespecified efficacy criteria (6). Exploratory analysis from a Japanese phase 2 study showed some efficacy of eribulin in patients with several subtypes of rare STS including synovial sarcoma, endometrial stromal sarcoma, solitary fibrous tumor and fibrosarcoma (14). Aside from these studies, little data exists regarding eribulin efficacy in rare histologic subtypes of STS (non-L-type).
Overall, eribulin is considered a promising second-line treatment after doxorubicin for patients with STS (16). In elderly patients and patients with a history of cardiac dysfunction, it may be an earlier option as anthracyclines such as doxorubicin are accumulatively cardiotoxic (17).
Since its approval in Japan, eribulin has been available for patients with all types of STS, including non-L-type sarcomas such as undifferentiated pleomorphic sarcoma (UPS), synovial sarcoma, and angiosarcoma. In Japan, post-marketing surveillance studies of eribulin treatment for STS have included all types of STS (L-type and non-L-type). Here, we report the interim analysis of a post-marketing surveillance study conducted nationwide in Japan, including 3-month results for all registered patients and 1-year results for some registered patients. The purpose of this post-marketing surveillance study was to evaluate the safety and efficacy of eribulin for each type of STS.
Patients and methods
Study patients and design
A nationwide, multicenter, prospective, observational post-marketing surveillance study was conducted in 102 institutions throughout Japan to evaluate the safety and efficacy of eribulin (ClinicalTrials.gov: NCT03058406). Patients with STS were enrolled in this observational study at the registered institute responsible for administering eribulin to the patient during the study period. Key exclusion criteria were patients with severe myelosuppression, a history of hypersensitivity to eribulin, and pregnancy or childbearing potential.
Eribulin was infused intravenously at 1.4 mg/m2 on days 1 and 8 of each 3-week dosing cycle. Dose reductions to 1.1 and 0.7 mg/m2 were permitted at the investigator’s discretion and in accordance with the eribulin package insert (18), which instructs a dose reduction in the following cases: grade 4 neutropenia (<500/mm3) lasting more than 7 days, ≥grade 3 febrile neutropenia, ≥grade 3 neutropenia (<1000/mm3) requiring antibiotic treatment, ≥grade 3 thrombocytopenia (<25 000/mm3), thrombocytopenia (<50 000/mm3) requiring blood transfusion, ≥grade 3 nonhematologic toxicity, or discontinuation at day 8 due to adverse events (AEs). Dosing was adjusted or discontinued depending on the condition of each individual patient. Patients with STS will be followed up for a maximum of 2 years.
The study was conducted in accordance with the Declaration of Helsinki and the Japanese regulatory requirements stipulated in Good Post-Marketing Study Practice. Approval from the institutional ethics committee or institutional review board of each participating institution was obtained prior to initiation of the study. This post-marketing surveillance study did not require written formal consent but every patient gave consent to the treatment with eribulin.
Assessments
Patient characteristics, clinicopathological data and treatment history at baseline were recorded. Eribulin administration status, concomitant drugs, diagnostic imaging, AEs, adverse drug reactions (ADRs), and clinical outcome were assessed at 3 months and 1 year after the start of eribulin treatment. Clinical outcome will be evaluated 2 years after treatment initiation. The primary outcome was the frequency and severity of ADRs. Safety was assessed by AEs regardless of the causal relationship with eribulin. Severity and causality were assessed for each AE. When a causal relationship could not be ruled out based on physicians’ assessment, the AE was considered an ADR. AEs and ADRs were graded using the Common Terminology Criteria for Adverse Events (Japanese version 4.0). Safety information was collected for AEs causing drug withdrawal of any grade; all AEs ≥grade 3; and AEs related to myelosuppression, infection, peripheral neuropathy, hepatic impairment, interstitial pneumonia, and QT prolongation.
Secondary outcomes included overall response rate (ORR), disease control rate (DCR), and clinical benefit rate (CBR). ORR was assessed using the best imaging data obtained during the study (complete response [CR], partial response [PR], stable disease [SD], progressive disease [PD], and not evaluable) and was determined by individual physicians at each institution using the Response Evaluation Criteria in Solid Tumors guideline version 1.1. All evaluations were scheduled according to the clinical practice standards of each institution. In this study, ORR was defined as CR + PR, DCR was defined as CR + PR + SD and CBR was defined as CR + PR + SD (≥11 weeks).
Statistical analysis
We focused on ≥grade 3 infections because they are associated with a high frequency of myelosuppression, which may lead to a severe outcome. In a phase 2 study of Japanese patients with STS, ≥grade 3 infections with the lowest frequency were infected pleural fluid and infection, each with a frequency of 2.0% (1/51 patients) (13). A sample size of 160 patients was estimated to be large enough to detect at least one case of severe infection, with a known frequency of 2.0%, at a probability of 95% (13). The safety analysis set comprised all patients who received at least one dose of eribulin. Statistical analysis was performed using either Fisher’s exact test or chi-square test with FREQ procedure using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics
Patients with STS and receiving eribulin were enrolled in this study from 29 February 2016 through the end of March 2017. The total number of patients enrolled in the study was 256, which exceeded the target number of 160 patients. Interim analysis was performed using data acquired during the first 3 months of eribulin treatment. The data cutoff date for analysis was 14 November 2017. The median duration of treatment for the study was 10.3 weeks (range: 3.0–58.9 weeks). All 256 Japanese patients remained enrolled through to 13 May 2017; 255 patients were included in the safety analysis set as one was excluded due to an AE. The efficacy analysis set included 253 patients: two patients, one with malignant mesothelioma and one with bone UPS, were excluded as they did not meet the criteria for STS according to 2013 World Health Organization classification (19). Of the patients in the efficacy analysis set, 1-year assessment data were available for 105 patients at the time of the interim analysis and 226 patients with imaging data at 3 months were evaluated for efficacy (objective response).
Baseline patient characteristics are listed in Table 1. The study population had a median age of 62 years (range: 17–87 years), and 135 (52.9%) were female. Four patients were ≥85 years of age. The median duration from the initial diagnosis to initiation of eribulin treatment was 2.4 years (range: 0.2–29.2 years). Most patients had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 (39.2%) or 1 (44.7%). Target lesions were retroperitoneal or intraperitoneal in most patients (40.4%). The study included 173 (67.8%) patients with recurrent disease. Patients had received a median of two previous chemotherapies; previous chemotherapy regimens included doxorubicin monotherapy (36.9%), pazopanib (32.2%), gemcitabine + docetaxel (26.7%), doxorubicin + ifosfamide (22.7%), and trabectedin (18.8%).
Table 1.
Baseline patient characteristics
| Total patients | N = 255 |
|---|---|
| Sex, female | 135 (52.9) |
| Age, years, mean ± standard deviation | 59.4 ± 13.6 |
| Median (range) | 62 (17–87) |
| ECOG PS | |
| 0 | 100 (39.2) |
| 1 | 114 (44.7) |
| 2 | 28 (11.0) |
| 3 | 12 (4.7) |
| 4 | 1 (0.4) |
| Time from diagnosis to initiation of eribulin treatment (years), (n = 239) | |
| Mean ± standard deviation | 4.15 ± 4.73 |
| Median (range) | 2.43 (0.2–29.2) |
| Target lesiona | |
| Head and neck | 16 (6.3) |
| Truncus | 31 (12.2) |
| Inside thoracic cavity | 57 (22.4) |
| Inside retroperitoneum and abdominal cavity | 103 (40.4) |
| Upper extremities | 7 (2.7) |
| Lower extremities | 32 (12.5) |
| Viscera | 71 (27.8) |
| Genitourinary | 6 (2.4) |
| Digestive | 18 (7.1) |
| Gynecologic | 26 (10.2) |
| Breast | 4 (1.6) |
| Others | 22 (8.6) |
| Others | 24 (9.4) |
| Median number of previous chemotherapies (range) | 2.0 (1–11) |
| Number of previous chemotherapies | |
| 0 | 18 (7.1) |
| 1 | 81 (31.8) |
| 2 | 74 (29.0) |
| 3 | 45 (17.6) |
| 4 | 22 (8.6) |
| ≥5 | 12 (4.7) |
| Unknown | 3 (1.2) |
| Major previous regimens | |
| Doxorubicin monotherapy | 94 (36.9) |
| Pazopanib | 82 (32.2) |
| Gemcitabine + docetaxel | 68 (26.7) |
| Doxorubicin + ifosfamide | 58 (22.7) |
| Trabectedin | 48 (18.8) |
Safety analysis set. Values are n (%) unless stated otherwise.
aDuplicate count.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status.
The histological subtypes of STS were L-type sarcomas (56.1%; leiomyosarcoma 28.6%, liposarcoma 27.5%), UPS (7.5%), synovial sarcoma (5.1%), angiosarcoma (5.5%), and rhabdomyosarcoma (4.7%). The remaining patients had rare subtypes that illustrated the spectrum of histological subtypes in STS (Table 2).
Table 2.
Best overall tumor response and status after 12 weeks of eribulin treatment
| Soft tissue sarcoma subtype | Patient analysis set | CR | PR | SD | SD (≥11 W)b | PD | NE | ORRc (%) | DCRc (%) | CBRc (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SASa | (%) | Imaginga | ||||||||||
| All | 255 | 226 | 0 | 17 | 78 | 23 | 125 | 6 | 7.5 | 42.0 | 17.7 | |
| Leiomyosarcoma | 73 | (28.6) | 69 | 0 | 5 | 29 | 8 | 35 | 0 | 7.2 | 49.3 | 18.8 |
| Liposarcoma | 70 | (27.5) | 60 | 0 | 2 | 28 | 9 | 28 | 2 | 3.3 | 50.0 | 18.3 |
| Dedifferentiated | 41 | 36 | 0 | 0 | 19 | 7 | 16 | 1 | 0.0 | 52.8 | 19.4 | |
| Myxoid | 12 | 11 | 0 | 2 | 5 | 2 | 3 | 1 | 18.2 | 63.6 | 36.4 | |
| Well-differentiated | 7 | 4 | 0 | 0 | 2 | 0 | 2 | 0 | 0.0 | 50.0 | 0.0 | |
| Pleomorphic | 3 | 3 | 0 | 0 | 0 | 0 | 3 | 0 | 0.0 | 0.0 | 0.0 | |
| Unknown | 7 | 6 | 0 | 0 | 2 | 0 | 4 | 0 | 0.0 | 33.3 | 0.0 | |
| Undifferentiated pleomorphic sarcoma | 19 | (7.5) | 17 | 0 | 2 | 2 | 1 | 12 | 1 | 11.8 | 23.5 | 17.6 |
| Angiosarcoma | 14 | (5.5) | 12 | 0 | 1 | 2 | 1 | 9 | 0 | 8.3 | 25.0 | 16.7 |
| Synovial sarcoma | 13 | (5.1) | 13 | 0 | 3 | 3 | 1 | 7 | 0 | 23.1 | 46.2 | 30.8 |
| Rhabdomyosarcoma | 12 | (4.7) | 11 | 0 | 2 | 0 | 0 | 8 | 1 | 18.2 | 18.2 | 18.2 |
| Malignant peripheral nerve sheath tumor | 6 | (2.4) | 5 | 0 | 0 | 0 | 0 | 5 | 0 | |||
| Myxofibrosarcoma | 5 | (2.0) | 4 | 0 | 1 | 1 | 0 | 1 | 1 | |||
| Spindle cell sarcoma | 4 | (1.6) | 4 | 0 | 0 | 0 | 0 | 4 | 0 | |||
| Epithelioid sarcoma | 3 | (1.2) | 3 | 0 | 0 | 2 | 0 | 1 | 0 | |||
| Malignant Solitary fibrous tumor | 3 | (1.2) | 3 | 0 | 0 | 0 | 0 | 3 | 0 | |||
| Desmoplastic small round cell tumor | 2 | (0.8) | 2 | 0 | 0 | 1 | 0 | 1 | 0 | |||
| Phyllodes tumor | 2 | (0.8) | 2 | 0 | 0 | 1 | 0 | 1 | 0 | |||
| Undifferentiated sarcoma | 2 | (0.8) | 2 | 0 | 0 | 1 | 0 | 1 | 0 | |||
| Othersd | 27 | (10.6) | 19 | 0 | 1 | 8 | 3 | 9 | 1 | |||
| L-typee | 143 | 129 | 0 | 7 | 57 | 17 | 63 | 2 | 5.4 | 49.6 | 18.6 | |
| Non-L-typee | 112 | 97 | 0 | 10 | 21 | 6 | 62 | 4 | 10.3 | 32.0 | 16.5 | |
aPatients with imaging data.
bSD ≥11 W represents patients with a treatment period longer than 11 weeks from treatment initiation to when SD was confirmed by imaging assessment.
cORR, DCR, and CBR were not calculated for subtypes with < 10 patients.
dEfficacy in each subtype was as follows: PR, undifferentiated round-cell sarcoma; SD (≥11 W), extra-skeletal myxochondrosarcoma, paraganglioma, and uterine tumor resembling ovarian sex-cord tumor; SD, adenocarcinoma, alveolar soft-part sarcoma, intimal sarcoma, pleomorphic spindle epithelioid sarcoma, and sclerosing epithelioid fibrosarcoma.
eL-type, leiomyosarcoma, liposarcoma; Non-L-type, all other subtypes.
Abbreviations: SAS, safety analysis set; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluable; ORR, objective response rate; DCR, disease control rate; CBR, clinical benefit rate.
Dose exposure
The majority of patients in our study received eribulin as a second-line or later treatment (92.9%); eribulin treatment was third-line or later for 60.0% of patients. The starting dose of eribulin was 1.4 mg/m2, which is the standard recommended dose for eribulin (18,20) for the majority of patients (82.7%). The remaining 14.1% of patients received a 1.1 mg/m2 as starting dose, including older patients and patients with pronounced bone marrow suppression. The mean relative dose intensity (RDI) of all patients was 0.76 (range: 0.27–1.02). The median number of eribulin cycles was 3.0 (range: 1–17 cycles), and 104 patients (60.8%) completed eribulin treatment within 3 months.
Efficacy
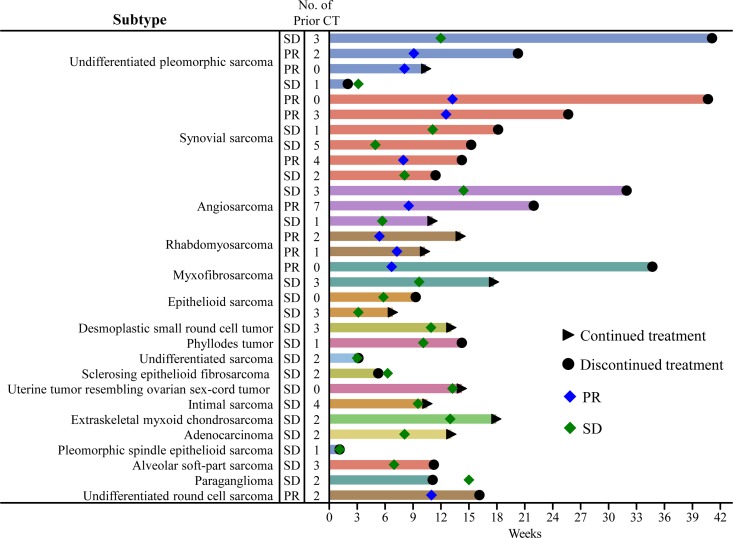
Best overall response at week 12 among the patients with imaging data (n = 226) was PR in 7.5% (n = 17); SD, 34.5% (n = 78); and SD ≥11 weeks, 10.2% (n = 23) (Table 2). ORR was 7.5%, DCR was 42.0%, and CBR was 17.7%, respectively. PD was observed in 125 patients (55.3%). Histologies of non-L-type STS patients who achieved PR or SD and having a treatment duration of more than three cycles were UPS (n = 3), synovial sarcoma (n = 6), myxofibrosarcoma (n = 2), rhabdomyosarcoma (n = 2), angiosarcoma (n = 3), desmoplastic small round cell tumor (n = 1), phyllodes tumor (n = 1), and epithelioid sarcoma (n = 1) (Figure 1). There was no association between the number of chemotherapy regimens and the response rate (Chi-square test, P = 0.4698). In addition, no statistically significant differences were observed in response rates between patients with and without chemotherapy (Fisher’s exact test, P = 0.1124).
Figure 1.
Treatment duration of eribulin in non-L-type soft tissue sarcoma patients. Response type and duration of eribulin treatment from start of treatment was plotted for patients with non-L-type soft tissue sarcoma for whom imaging data were available. Day 0 represents the start of treatment. Triangles indicate eribulin treatment was continued. Circles indicate eribulin treatment was discontinued. The number in the Y-axis column represents the number of prior CT. Abbreviations: SD, stable disease; PR, partial response; CT, chemotherapy.
Liposarcoma and leiomyosarcoma (L-type)
Among the patients diagnosed with leiomyosarcoma, 69 had imaging data. PR was achieved in five patients and SD ≥11 weeks was achieved in eight patients. The ORR was 7.2% and CBR was 18.8% (Table 2). Longer responses in leiomyosarcoma were observed in PR (median: 9.7 weeks, range: 7.6–12.7 weeks) and SD patients (median: 8.1 weeks, range: 2.1–33.0 weeks).
There were 60 liposarcoma patients with imaging data. Of these, PR was achieved in two patients and SD ≥11 weeks was achieved in nine patients. The ORR was 3.3% and CBR was 18.3% (Table 2). The majority of liposarcoma patients presented with dedifferentiated liposarcoma; in this subgroup, the CBR was 19.4%. Patients presenting with myxoid liposarcoma had both a high DCR and CBR (63.6% and 34.6%, respectively; Table 2). Longer responses in liposarcoma were observed in PR (median: 11.4 weeks, range: 10.6–12.1 weeks) and SD patients (median: 9.2 weeks, range: 2.1–18.1 weeks).
Other subtypes of sarcoma (non-L-type)
In this study, 43% (n = 97) of STS were a non-L-type STS (Table 2). PR was achieved in 10 of these patients. ORR, DCR and CBR were 10.3%, 32.0% and 16.5%, respectively. There were 19 UPS patients enrolled in the study; among them, PR was achieved in two patients and SD was achieved in two patients. CBR for the UPS patients was 17.6%. Among the patients with synovial sarcoma (n = 13), PR and SD was achieved in three patients each. CBR was 30.8% for these patients. One of 14 patients with angiosarcoma and two of 12 patients with rhabdomyosarcoma achieved PR. CBR was 16.7% and 18.2%, respectively. Among the other rare STS patients, one patient with myxofibrosarcoma achieved PR and several classified as ‘Others’ in Table 2 achieved PR or SD ≥11 weeks (one PR, undifferentiated round-cell sarcoma; one SD ≥11 weeks, extra-skeletal myxochondrosarcoma; one SD ≥11 weeks, paraganglioma; one SD ≥11 weeks, uterine tumor resembling ovarian sex-cord tumor) (Table 2).
Details of the response duration according to subtype (non-L-type) are shown in Figure 1. The longest duration of response/SD was approximately 41 weeks observed in one patient each with UPS and synovial sarcoma. Antitumor activity was not observed in patients with malignant peripheral nerve sheath tumor, spindle cell sarcoma, or malignant solitary fibrous tumor, but this may be explained by the small number of patients with these subtypes.
Safety
A total of 211 patients (82.7%) reported ADRs, which are summarized in Table 3. The most common ADRs (those occurring in >10% of patients) were neutropenia, 58.4%; leukopenia, 57.7%; lymphopenia, 14.9%; alanine aminotransferase (ALT) increase, 12.6%; and aspartate aminotransferase increase, 12.2%. Granulocyte colony stimulating factor was used in five patients. ADRs ≥grade 3 and having an incidence of >5% were neutropenia, 52.6%; leukopenia, 46.3%; lymphopenia, 14.5%; and anemia, 6.7%.
Table 3.
Adverse drug reactions occurring in ≥3% of patients
| Adverse drug reactions (N = 255) | ||
|---|---|---|
| All grades | ≥Grade 3 | |
| Any | 211 (82.7) | 174 (68.2) |
| Hematological toxicity | ||
| Neutropenia | 149 (58.4) | 134 (52.5) |
| Leukopenia | 147 (57.7) | 118 (46.3) |
| Lymphopenia | 38 (14.9) | 37 (14.5) |
| Anemia | 25 (9.8) | 17 (6.7) |
| Thrombocytopenia | 9 (3.5) | 5 (2.0) |
| Febrile neutropenia | 8 (3.1) | 8 (3.1) |
| Non-hematological toxicity | ||
| Neuropathy peripheral | 24 (9.4) | 4 (1.6) |
| Malaise | 11 (4.3) | 1 (0.4) |
| Alopecia | 11 (4.3) | |
| Pyrexia | 8 (3.1) | |
| Laboratory test abnormalities | ||
| ALT increase | 32 (12.6) | 3 (1.2) |
| AST increase | 31 (12.2) | 2 (0.8) |
| CRP increase | 17 (6.7) | 7 (2.7) |
| γ-GT increase | 12 (4.7) | 8 (3.1) |
| Hemoglobin decrease | 12 (4.7) | 6 (2.4) |
Safety analysis set, n (%).
Safety information was collected for any grade for priority AEs and AEs resulting in drug withdrawal and for ≥grade 3 for all other AEs.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, c-reactive protein; γ-GT, γ-glutamyl transferase.
Serious ADRs were reported in 42 patients (16.5%) and included neutropenia (7.8%), leukopenia (7.5%), febrile neutropenia (2.0%), and anemia (1.2%). There were no ADRs leading to death. Twenty-seven patients (10.6%) experienced ADRs that led to the discontinuation of eribulin. Fifty-five patients (21.6%) had ADRs that required at least one dose reduction of eribulin. The most common ADR leading to treatment discontinuation or dose reduction was myelosuppression. Among the patients with grade 3 or 4 neutropenia, five patients discontinued eribulin treatment; 21 and 26 patients with grade 3 or grade 4 neutropenia, respectively, successfully continued treatment after dose reduction. While all the eight patients who experienced febrile neutropenia recovered, two discontinued eribulin administration due to febrile neutropenia.
The incidence of peripheral neuropathy was relatively low (9.4% overall; 1.6% ≥grade 3). Grade 1 QT prolongation was observed in three patients; all three continued eribulin treatment, one with a dose reduction. None of the three patients had a history of cardiac disease; however, one patient had a complication of atrial fibrillation and two patients had previously been treated with an anthracycline.
There were no significant differences in the number of chemotherapy regimens reported at baseline (Table 1) and the incidence of serious ADRs (Chi-square test, P = 0.5327). No treatment-related deaths were observed.
Discussion
This is the largest study evaluating the efficacy and tolerability in STS patients with rare histology subtypes such as UPS, angiosarcoma and synovial sarcoma. Eribulin demonstrated antitumor activity and tolerability in patients with a range of advanced or metastatic tumors including STS. Although treatment options for rare subtypes of STS are limited, over 20 rare histological subtypes of STS were included in the current study, reflecting the real-world clinical setting in Japan. Eribulin was used as a first-line treatment in 7.1% of patients, second-line in 31.8% of patients, and third-line or later in 59.9% of patients in this study. Mean RDI in this study (0.76, range: 0.27–1.02) was comparable with that in a previous observational study for breast cancer (0.75, range: 0.21–1.25) (21), indicating that dose intensity was not affected by tumor types.
In pre-clinical studies, eribulin showed broad-spectrum cytotoxicity and had low IC50 values when tested using a large panel of cancer cell lines (22). Using in vivo xenograft models, the antitumor activity of eribulin against five histological subtypes of STS—leiomyosarcoma, liposarcoma, synovial sarcoma, Ewing’s sarcoma, and fibrosarcoma—was demonstrated (23). In another study, eribulin treatment of primary UPS cells demonstrated that eribulin had antitumor activity similar to that of standard treatments (24). Subsequent clinical studies have also shown antitumor effects of eribulin. Eribulin improved OS for liposarcoma compared with dacarbazine in a phase 3 study (8) and showed potential efficacy for other subtypes of sarcoma in two phase 2 studies (6,14). Taken together, these preclinical and clinical studies indicate that eribulin has the potential to exert antitumor effects on a wide range of STSs.
Efficacy data for previous clinical trials using eribulin are summarized in Table 4. Efficacy of eribulin for L-type STS have been established with 12-week progression-free survival (PFS) rate of 31% and improved OS compared with dacarbazine treatment. It is noted that the benefit of eribulin in terms of OS was pronounced in liposarcoma subgroups (Table 4) (8). A phase 2 EORTC 062052 study reported that the 12-week PFS rate for patients treated with eribulin was 46.9% in liposarcoma and 31.6% in leiomyosarcoma (6). Another phase 2 study conducted in Japan reported a 12-week PFS rate of 60% for liposarcoma/leiomyosarcoma patients treated with eribulin (14). In the current study, the DCRs for liposarcoma and leiomyosarcoma were 50.0% and 49.3%, respectively, and the CBR with ≥11-week SD was 18.3% and 18.8%, respectively. Patient status at 12 weeks was not calculated in our observational study, but the CBR with ≥11-week SD appears to be lower than what has been reported for previous studies. One possible reason for this may be the differences in patient background characteristics between the studies, especially in ECOG PS scores. In both phase 2 studies, only patients with an ECOG PS of 0–1 were included, whereas 16.1% of patients in the current study had an ECOG PS of 2–4. Similar to the previous studies, the majority of patients in our study had received doxorubicin as prior chemotherapy, thus confirming eribulin as a potential treatment option in patients resistant to doxorubicin. Among the patients with liposarcoma, the majority had dedifferentiated liposarcoma (n = 41). Patients with the myxoid liposarcoma had the highest DCR and CBR compared to all other liposarcoma subtypes.
Table 4.
Summary of objective response to eribulin by soft tissue sarcoma subtype reported in previous clinical studies
| Study | Soft tissue sarcoma subtype | n | CR | PR | SD | PD | NE | ORR (%) | DCR (%) | 12-week PFS (%) | Median PFS (months) | Median OS (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Schöffski 2011 (Phase 2), Ref 6 | Adipocytic sarcoma | 32 | 1 | 0 | 18 | 11 | 2 | 3 | 59 | 46.9 | 2.6 | - |
| Leiomyosarcoma | 38 | 0 | 2 | 20 | 14 | 2 | 5 | 58 | 31.6 | 2.9 | - | |
| Synovial sarcoma | 19 | 0 | 1 | 8 | 9 | 1 | 5 | 47 | 21.1 | 2.6 | - | |
| Othersa | 26 | 0 | 1 | 11 | 13 | 1 | 4 | 46 | 19.2 | 2.1 | - | |
| Kawai 2017 (Phase 2), Ref 14 | L-sarcoma | 35 | 0 | 0 | 28 | 7 | 0 | 0 | 80 | 60 | 5.5 | - |
| Non-L-typeb | 16 | 0 | 0 | 8 | 8 | 0 | 0 | 50 | 31 | 2.0 | - | |
| Schöffski 2016 (Phase 3), Ref 8 | L-sarcoma | 228 | 4 | 56 | 33 | 2.6 | 13.5 | |||||
| Liposarcoma | 71 | - | - | - | - | 15.6 | ||||||
| Leiomyosarcoma | 157 | - | - | - | - | 12.7 | ||||||
| Demetri 2017 (Phase 3 sub-analysis), Ref 9 | Liposarcoma | 71 | 1.4 | 66.2 | 41 | 2.9 | 15.6 | |||||
| Myxoid/round cell | 29 | - | - | - | 2.8 | 13.5 | ||||||
| Pleomorphic | 11 | - | - | - | 4.4 | 22.2 | ||||||
| Dedifferentiated | 31 | - | - | - | 2.0 | 18.0 |
aAchieved 12-week PFS and included fibroblastic sarcoma (n = 2), solitary fibrous tumor (n = 1), and epithelioid sarcoma (n = 2).
bAchieved 12-week PFS and included synovial sarcoma (n = 1), endometrial stromal sarcoma (n = 2), fibrosarcoma (n = 1), and solitary fibrous tumor (n = 1).
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluable; ORR, overall response rate; DCR, disease control rate; PFS, progression-free survival; OS, overall survival.L-sarcoma consists of liposarcoma and leiomyosarcoma.
Twelve-week PFS was achieved in 19.2% and 31% of non-L-type STS patients in the phase 2 EORTC 062052 study (6) and the phase 2 study conducted in Japan (14), respectively (Table 4). In this study, CBR for non-L-type STS was 16.5%, which appears to be low compared with the two previous phase 2 studies. The present study included heterogeneous non-L-type STS patients and, as mentioned previously, a number of patients with higher ECOG PS scores than those allowed in the phase 2 studies. Assessment of the results for each individual subtype may improve comparisons among the studies.
Chemotherapy, most commonly consisting of treatment with anthracyclines, is used to treat advanced and metastatic UPS. However, the outcome is inadequate, with a response rate of 16–27% when used as a monotherapy, although some patients do well on doxorubicin monotherapy (25,26). It has been reported that anthracyclines are frequently used in combination with ifosfamide (27–29). Eribulin had similar antitumor effects to commonly used STS chemotherapy drugs when used in vitro with primary UPS cells (24), indicating that eribulin should be further investigated as a potential treatment option for UPS, particularly in patients with tumors resistant to standard chemotherapy drugs. UPS was not evaluated in the previously reported phase 2 studies (6,14). We report that four UPS patients achieved PR/SD; of those, three patients received >3 treatment cycles, including one patient who achieved the longest treatment duration in the study, over 40 weeks.
In the EORTC 062052 study, 12-week PFS was achieved in 21% of synovial sarcoma patients (Table 4) (6). In this study, the DCR was 46.2%, with three patients achieving PR and three patients achieving SD. The six patients achieving PR or SD had received 0–5 prior chemotherapies; one patient received eribulin treatment for over 40 weeks (Figure 1). The CBR was 30.8% for patients with synovial sarcoma, which is higher than what we reported for other non-L-type STSs. The interim results of our study are consistent with the EORTC 062052 study (6).
Paclitaxel, a microtubule-targeting agent, is recommended as systemic therapy for patients with angiosarcoma (30,31). Paclitaxel binds to the inner surface of the microtubule lattice along its entire length, leading to net microtubule polymerization (32,33). Eribulin, also a microtubule-targeting agent, binds selectively to the plus ends of microtubules as well as to soluble tubulin subunits, leading to net microtubule depolymerization (33,34). Although these two microtubule-targeting agents exhibit different molecular mechanisms of action, both alter the normal patterns of microtubule dynamics and compromise normal microtubule activities, leading to cell death. Eribulin is expected to be a potential alternative for the treatment of angiosarcomas. Patients with angiosarcomas were not included in previous clinical studies. We included these patients in our study and report that eribulin demonstrated antitumor activity in angiosarcoma patients, with a CBR of 16.7%. One case of scalp angiosarcoma was previously reported as being successfully controlled by eribulin (35). In a second case involving a patient with heavily pretreated metastatic cardiac angiosarcoma, it was reported that PR was achieved and maintained for 4 months with eribulin used as the eighth-line treatment (36).
There is evidence in the literature of successful treatment of rare STSs with eribulin. Details of one such case in which eribulin was successfully used to treat epithelioid sarcoma—one patient, who achieved SD for >3 months—was reported recently (37). In addition, the EORTC 062052 study reported that five patients with ‘other subtypes’ achieved 12-week PFS; the subtypes were fibroblastic sarcoma (n = 2), solitary fibrous tumor (n = 1), and epithelioid sarcoma (n = 2) (6). In this study, eribulin showed antitumor activity for treatment of rare STS, such as rhabdomyosarcoma (two patients, PR), myxofibrosarcoma (one patient, PR), and undifferentiated round-cell sarcoma (one patient, PR) (Table 2). Eribulin is reported to improve blood perfusion in mouse xenograft models of STS and to induce expression of genes involved in cell differentiation in STS cell lines in vitro (23). Such mechanisms likely play an important role in the observed efficacy of eribulin treatment in STS patients. Our study evaluated the antitumor activity of eribulin in rare STSs with few effective treatment options. Importantly, antitumor activity in these rare STSs was confirmed by the DCR, suggesting that eribulin may be a promising therapeutic option for both non-L-type and L-type STSs. A clinical trial using eribulin to treat patients with angiosarcoma and epithelioid hemangioendothelioma is ongoing (NCT03331250) as is a trial evaluating eribulin in combination with irinotecan in pediatric patients with refractory rhabdomyosarcoma and non-rhabdomyosarcoma STS (NCT03245450).
There have been efforts to develop tumor-specific treatments by surveying genetic mutations in tumors from a variety of STS subtypes (38). Other therapies being developed to treat non-L-type STSs include T cell receptor-engineered T cells targeting the New York esophageal squamous cell carcinoma 1 (NY-ESO-1) tumor antigen on synovial cells (39,40) and a cancer vaccine targeting NY-ESO-1 (41) in patients with refractory Ewing’s sarcoma and sarcomas expressing the tumor antigen. This vaccine is currently being tested in combination with atezolizumab, an immune checkpoint inhibitor (NCT02387125).
AEs reported in our study were manageable and consistent with the known safety profile for eribulin; there were no treatment-related deaths. The incidence of ADRs was 82.7% and the safety profile was similar to that reported in the phase 2 study conducted in Japan, with the majority of grade 3–4 AEs being related to hematotoxicity. In the phase 3 study (8) (L-type STS), the most common AEs (≥25% for all grades) were fatigue, nausea, alopecia, constipation, pyrexia, anemia and neutropenia. The most common grade 3–4 AEs (≥5%) were neutropenia, leukopenia, and anemia. The EORTC 062052 study reported the most common grade 3–4 AEs (≥5%) as neutropenia (52%), white blood cell decreased (35%), fatigue (7%), anemia (7%), and ALT increased (5%) (6).
The frequency of reported AEs is generally lower in post-marketing surveillance studies than in clinical studies because the recorded incidence of AEs relies on reports from treating physicians. Over 50% of patients experienced neutropenia and leukopenia in our study, and this is a lower incidence than that reported in the previous phase 2 study conducted in Japan (14). The most common grade 3–4 AEs in the Japanese phase 2 study were neutropenia (86%), white blood cell decreased (75%), anemia (14%), ALT increased (6%), and cancer pain (6%) (14). A higher frequency of hematologic events was also observed in Japanese patients with breast cancer receiving eribulin treatment (42,43). Febrile neutropenia occurred in 3.1% of patients in this study and 7.8% in the Japanese phase 2 study (14). All patients who experienced febrile neutropenia in this study recovered; six of eight instances did not lead to withdrawal, suggesting that it could be manageable in actual clinical settings. Although neutropenia is commonly reported with eribulin treatment, it is reversible and not cumulative (44). In addition, bi-weekly administration of eribulin is a potential therapeutic option in cases where the patient is unable to tolerate side effects such as severe neutropenia (45). In this study, the majority of patients with neutropenia were able to continue eribulin treatment with a dose reduction. While grade 3–4 peripheral neuropathy was reported in 1.8% of patients in this study, slightly higher than reported in the Japanese phase 2 study, it can be improved as shown by a study in breast cancer patients treated with eribulin, which reported that the time to improvement of peripheral neuropathy was 2.1 weeks (46). In addition, two patients continued eribulin treatment more than 40 weeks (Figure 1), suggesting that eribulin could have a manageable safety profile, even in patients with rare types of STS.
One advantage of eribulin is that it has a lower drug-induced cardiotoxicity compared with other chemotherapy drugs. Doxorubicin has notable accumulative cardiotoxicity, and pazopanib is difficult to use in patients with primary cardiac angiosarcoma (16). In our study, we reported prolonged QT intervals in 1.2% of patients, but all of these patients were able to continue eribulin treatment. The occurrence of heart failure has not been reported in previous clinical studies for eribulin, including a study in elderly patients with breast cancer (47). However, an uncontrolled open-label electrocardiogram study observed QT prolongation in some patients with atrial fibrillation (48), indicating that patients should be monitored for prolonged QT if they have a history of cardiac disease.
Patients in our study have received a substantial number of pretreatments (up to 11 prior chemotherapy regimens); however, eribulin was tolerable regardless of the clinical setting and number of previous chemotherapies.
Here we reported short-term interim analysis results, limiting our study in that the analysis of long-term response and SD are not reported. The planned final analysis at the completion of this study will report results from a longer observation period (up to 2 years).
In conclusion, eribulin is a unique inhibitor of microtubule dynamics that has demonstrated antitumor activity and tolerability in patients with a range of advanced or metastatic tumors including STS. Preclinical and clinical data have shown that eribulin has broad antitumor activity in many subtypes of STS. However, there is an urgent need to better understand the histological basis of each STS subtype, and to improve disease management options. Interim analysis of our post-marketing surveillance study demonstrates the potential antitumor activity of eribulin against STS subtypes that currently have few effective treatment options.
Acknowledgments
The authors wish to thank Sarah Bubeck, PhD, of Edanz Medical Writing for providing medical writing services during the development of this manuscript, which was funded by Eisai Co., Ltd.
Funding
This work was supported by Eisai Co., Ltd.
Conflict of interest statement
EK reports personal fees from Novartis Pharma K.K. and Eisai Co., Ltd., outside the submitted work. YN reports speakers’ bureaus from Eisai Co., Ltd., Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical, Novartis Pharma K.K., Eli Lilly Japan K.K., Roche Diagnostics, Meiji Seika, and Bayer, and research funding from Merck Serono, AstraZeneca, Eli Lilly Japan K.K., Nippon Kayaku, Pfizer, and Taiho Pharmaceutical, outside the submitted work. NA reports personal fees from Eisai Co., Ltd., outside the submitted work. AM reports personal fees from Eisai Co., Ltd., outside the submitted work. ME reports personal fees from Eli Lilly Japan K.K., Eisai Co., Ltd., Takara Bio Inc., and Taiho Pharmaceutical Co., Ltd., and grants and personal fees from Novartis Pharma K.K., outside the submitted work. ST has received research funding and speakers’ honoraria from Taiho Pharmaceutical Co., Ltd., Eisai Co., Ltd., and Novartis Pharma K.K., outside the submitted work. YM is an employee of Eisai Co., Ltd. AK reports personal fees from Novartis Pharma K.K., Taiho Pharmaceutical Co. Ltd., Eisai Co., Ltd., and Eli Lilly Japan K.K., outside the submitted work.
References
- 1. Blay JY, Sleijfer S, Schöffski P, et al. . International expert opinion on patient-tailored management of soft tissue sarcomas. Eur J Cancer 2014;50:679–89. [DOI] [PubMed] [Google Scholar]
- 2. Hatcher H, Benson C, Ajithkumar T. Systemic treatments in soft tissue sarcomas. Clin Oncol 2017;29:507–15. [DOI] [PubMed] [Google Scholar]
- 3. In GK, Hu JS, Tseng WW. Treatment of advanced, metastatic soft tissue sarcoma: latest evidence and clinical considerations. Ther Adv Med Oncol 2017;9:533–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lilly [Internet] Lilly reports results of phase 3 soft tissue sarcoma study of LARTRUVO®. 2019. [cited 2019 May 16]. Available at: https://investor.lilly.com/node/40206/pdf
- 5. Tap WD, Wagner AJ, Papai Z, et al. ANNOUNCE: a randomized, placebo (PBO)-controlled, double-blind, phase (Ph) III trial of doxorubicin (dox) + olaratumab versus dox + PBO in patients (pts) with advanced soft tissue sarcomas (STS) [Abstract]. In: 2019 American Society of Clinical Oncology Annual Meeting; 2019 May 31–June 4; Chicago, IL. ASCO; 2019. Abstract LBA3.
- 6. Schöffski P, Ray-Coquard IL, Cioffi A, et al. . Activity of eribulin mesylate in patients with soft-tissue sarcoma: a phase 2 study in four independent histological subtypes. Lancet Oncol 2011;12:1045–52. [DOI] [PubMed] [Google Scholar]
- 7. Asano M, Matsui J, Towle MJ, et al. . Broad-spectrum preclinical antitumor activity of eribulin (Halaven®): combination with anticancer agents of differing mechanisms. Anticancer Res 2018;38:3375–85. [DOI] [PubMed] [Google Scholar]
- 8. Schöffski P, Chawla S, Maki RG, et al. . Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet 2016;387:1629–37. [DOI] [PubMed] [Google Scholar]
- 9. Demetri GD, Schöffski P, Grignani G, et al. . Activity of eribulin in patients with advanced liposarcoma demonstrated in a subgroup analysis from a randomized phase III study of eribulin versus dacarbazine. J Clin Oncol 2017;35:3433–9. [DOI] [PubMed] [Google Scholar]
- 10. Setola E, Noujaim J, Benson C, et al. . Eribulin in advanced liposarcoma and leiomyosarcoma. Expert Rev Anticancer Ther 2017;17:717–23. [DOI] [PubMed] [Google Scholar]
- 11. Emambux S, Italiano A. Clinical efficacy of eribulin mesylate for the treatment of metastatic soft tissue sarcoma. Expert Opin Pharmacother 2017;18:819–24. [DOI] [PubMed] [Google Scholar]
- 12. Swami U, Shah U, Goel S. Eribulin in cancer treatment. Mar Drugs 2015;13:5016–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schöffski P, van Cann T, Cornilie J. Treatment options for anthracycline-resistant, advanced soft-tissue sarcoma: the role of eribulin. Expert Opin Orphan. Drugs 2017;5:445–53. [Google Scholar]
- 14. Kawai A, Araki N, Naito Y, et al. . Phase 2 study of eribulin in patients with previously treated advanced or metastatic soft tissue sarcoma. Jpn J Clin Oncol 2017;47:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. The Pharmaceuticals and Medical Devices Agency , Japan [Internet]. Annual Report FY 2015 (April 2015–March 2016). 2015 [cited 2019 Feb 25]. Available from: https://www.pmda.go.jp/files/000214925.pdf.
- 16. Kawai A, Yonemori K, Takahashi S, et al. . Systemic therapy for soft tissue sarcoma: proposals for the optimal use of pazopanib, trabectedin, and eribulin. Adv Ther 2017;34:1556–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther 2017;31:63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Federal Drug Administration [Internet] Halaven (eribulin mesylate) Injection [cited 2019. Feb 25]. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/201532lbl.pdf.
- 19. Doyle LA. Sarcoma classification: an update based on the 2013 World Health Organization Classification of tumors of soft tissue and bone. Cancer 2014;120:1763–74. [DOI] [PubMed] [Google Scholar]
- 20. European Medicines Agency [Internet] HALAVIN, INN-eribulin [cited 2019. Feb 25]. Available at: http://ec.europa.eu/health/documents/community-register/2011/2011031798493/anx_98493_en.pdf.
- 21. Watanabe J. Eribulin monotherapy improved survivals in patients with ER-positive HER2-negative metastatic breast cancer in the real world: a single institutional review. Springerplus 2015;4:625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kolb EA, Gorlick R, Reynolds CP, et al. . Initial testing (stage 1) of eribulin, a novel tubulin binding agent, by the pediatric preclinical testing program. Pediatr Blood Cancer 2013;60:1325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kawano S, Asano M, Adachi Y, et al. . Antimitotic and non-mitotic effects of eribulin mesilate in soft tissue sarcoma. Anticancer Res 2016;36:1553–61. [PubMed] [Google Scholar]
- 24. De Vita A, Recine F, Mercatali L, et al. . Primary culture of undifferentiated pleomorphic sarcoma: molecular characterization and response to anticancer agents. Int J Mol Sci 2017;18:pii: E2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schoenfeld DA, Rosenbaum C, Horton J, Wolter JM, Falkson G, DeConti RC. A comparison of adriamycin versus vincristine and adriamycin, and cyclophosphamide versus vincristine, actinomycin-D, and cyclophosphamide for advanced sarcoma. Cancer 1982;50:2757–62. [DOI] [PubMed] [Google Scholar]
- 26. Bramwell VHC, Anderson D, Charette ML. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft-tissue sarcoma: a meta-analysis and clinical practice guideline. Sarcoma 2000;4:103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gronchi A, Ferrari S, Quagliuolo V, et al. . Full-dose neoadjuvant anthracycline + ifosfamide chemotherapy is associated with a relapse free survival (RFS) and overall survival (OS) benefit in localized high-risk adult soft tissue sarcomas (STS) of the extremities and trunk wall: Interim analysis of a prospective randomized trial. Ann Oncol 2016;27:Suppl_6. [Google Scholar]
- 28. Frezza AM, Stacchiotti S, Gronchi A. Systemic treatment in advanced soft tissue sarcoma: what is standard, what is new. BMC Med 2017;15:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. In GK, Hu JS, Tseng WW. Treatment of advanced, metastatic soft tissue sarcoma: latest evidence and clinical considerations. Ther Adv Med Oncol 2017;8:533–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. von Mehren M, Randall RL, Benjamin RS, et al. . Soft tissue sarcoma version 2.2018, Clinical practice guidelines in oncology. J Natl Compr Canc Netw 2018;16:536–63. [DOI] [PubMed] [Google Scholar]
- 31. Penel N, Bui BN, Bay JO, et al. . Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX study. J Clin Oncol 2008;26:5269–74. [DOI] [PubMed] [Google Scholar]
- 32. Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer 2004;4:253–65. [DOI] [PubMed] [Google Scholar]
- 33. Jordan MA, Kamath K, Manna T, et al. . The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol Cancer Ther 2005;4:1086–95. [DOI] [PubMed] [Google Scholar]
- 34. Smith JA, Wilson L, Azarenko O, et al. . Eribulin binds at microtubule ends to a single site on tubulin to suppress dynamic instability. Biochemistry 2010;49:1331–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wada N, Uchi H, Furue M. Case of angiosarcoma of the scalp successfully controlled by eribulin. J Dermato 2018;45:116–7. [DOI] [PubMed] [Google Scholar]
- 36. Inagaki C, Shimoi T, Okuma H, et al. . A case of heavily pretreated metastatic cardiac angiosarcoma treated successfully using eribulin. Anticancer Drugs 2018;29:97–101. [DOI] [PubMed] [Google Scholar]
- 37. Iwai T, Hoshi M, Oebisu N, Shimatani A, Takada N, Nakamura H. Promising effects of eribulin for cystic lung metastases of epithelioid sarcoma: a case report. Anticancer Drugs 2018;29:806–9. [DOI] [PubMed] [Google Scholar]
- 38. Grounder MM, Ali S, Robinson V, et al. . Impact of next-generation sequencing (NGS) on diagnostic and therapeutic options in soft-tissue and bone sarcoma. J Clin Oncol 2017;35:11001–11001. [Google Scholar]
- 39. Endo M, de Graff MA, Ingram DR, et al. . NY-ESO-1 (CTAG1B) expression in mesenchymal tumors. Mod Pathol 2015;28:587–95. [DOI] [PubMed] [Google Scholar]
- 40. Iura K, Maekawa A, Kohashi K, et al. . Cancer-tested antigen expression in synovial sarcoma: NY-ESO-1, PRAME, MAGEA4, and MAGEA1. Hum Pathol 2017;61:130–9. [DOI] [PubMed] [Google Scholar]
- 41. Thomas R, Al-Khadairi G, Roelands J, et al. . NY-ESO-1 based immunotherapy of cancer: Current perspectives. Front Immunol 2018;9:947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aogi K, Iwata H, Masuda N, et al. . A phase II study of eribulin in Japanese patients with heavily pretreated metastatic breast cancer. Ann Oncol 2012;23:1441–8. [DOI] [PubMed] [Google Scholar]
- 43. Cortes J, O’Shaughnessy J, Loesch D, et al. . Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet 2011;377:914–23. [DOI] [PubMed] [Google Scholar]
- 44. Ro J, Cheng FT, Sriuranpong V, et al. . Patient management with eribulin in metastatic breast cancer: a clinical practice guide. J Breast Cancer 2016;19:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ohtani S, Nakayama T, Yoshinami T, et al. . Bi-weekly eribulin therapy for metastatic breast cancer: a multicenter phase II prospective study (JUST-STUDY). Breast Cancer 2018;25:438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kaufman P, Olivo M, He Y, McCutcheon S, Vahdat L. Peripheral neuropathy (PN) in patients (pts) with metastatic breast cancer treated with eribulin: Resolution and association with efficacy. J Clin Oncol 2014;32:147. [Google Scholar]
- 47. Muss H, Cortes J, Vahdat LT, et al. . Eribulin monotherapy in patients aged 70 years and older with metastatic breast cancer. Oncologist 2014;19:318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lesimple T, Edeline J, Carrothers TJ, et al. . A phase I, open-label, single-arm study for QT assessment of eribulin mesylate in patients with advanced solid tumors. Invest New Drugs 2013;31:900–9. [DOI] [PubMed] [Google Scholar]