Abstract
Biological molecules and intracellular structures operate at the nanoscale; therefore, development of nanomedicines shows great promise for the treatment of disease by using targeted drug delivery and gene therapies. PAMAM dendrimers, which are highly branched polymers with low polydispersity and high functionality, provide an ideal architecture for construction of effective drug carriers, gene transfer devices and imaging of biological systems. For example, dendrimers bioconjugated with selective ligands such as Arg-Gly-Asp (RGD) would theoretically target cells that contain integrin-receptors, and show potential for use as drug-delivery devices. While RGD-conjugated dendrimers are generally considered not to be cytotoxic, there currently exists little information on the risks that such materials pose to human health. In an effort to compliment and extend the knowledge gleaned from cell culture assays, we have used the zebrafish embryo as a rapid, medium-throughput, cost-effective whole-animal model to provide a more comprehensive and predictive developmental toxicity screen for nanomaterials such as PAMAM dendrimers. Using the zebrafish embryo, we have assessed the developmental toxicity of low generation (G3.5 and G4) PAMAM dendrimers, as well as RGD-conjugated forms for comparison. Our results demonstrate that G4 dendrimers, which have amino functional groups, are toxic and attenuate growth and development of zebrafish embryos at sublethal concentrations; however, G3.5 dendrimers, with carboxylic acid terminal functional groups, are not toxic to zebrafish embryos. Furthermore, RGD-conjugated G4 dendrimers are less potent in causing embryo toxicity than G4 dendrimers. RGD-conjugated G3.5 dendrimers do not elicit toxicity at the highest concentrations tested and warrant further study for use as a drug-delivery device.
Keywords: dendrimer, RGD, zebrafish embryo, developmental toxicity, nanotherapeutics, nanotoxicology
Introduction
Ever-more sophisticated technologies are being developed to identify novel therapeutic strategies (e.g., diagnostics, targeted drug delivery, and gene therapy) and to improve treatments for life-threatening and debilitating diseases. This need for more effective drug therapies has given rise to the development of nano-sized (5–100 nm) polymer-based pharmaceuticals. For example, several peptides show great potential for development as drugs because they are efficacious and selective, however, their use is limited due to obstacles in drug delivery, as they are readily metabolized by proteases and peptidases. The synthesis of peptides in dendrimeric form retains the biological activity of the peptides while rendering them resistant to metabolism (Spetzler et al., 1995; Kim et al., 1998; Sadler et al., 2002; Bracci et al., 2003). Such characteristics of these peptide dendrimers make them particularly useful for development in drug delivery that may, for example, avoid triggering an immune response and have less intense side effects (Bracci et al., 2003).
Starburst™ polyamidoamine (PAMAM) dendrimers have a well-defined, mono-dispersive and stable molecular architecture that is advantageous for targeted drug delivery. Dendrimers are composed of an initiator ammonia core with layers of radially repeating units attached to the core, and an outer surface of terminal functional units; full generation dendrimers terminate with amine groups, while half-generation dendrimers terminate in carboxylic acid groups. Succeeding generations (referred to as G0–G10) have increased diameter and twice the number of terminal functional groups as their predecessor (Table 1) (Tomalia et al., 1990; Boas et al., 2004; Svenson et al., 2005; Tomalia et al., 2007). The highly branched, multivalent, and multifunctional surface of dendrimers allows for manipulation of their surface chemistry, and the relatively solvent-filled interior core renders them useful for drug delivery (Duncan et al., 2005; Svenson et al., 2005; Yang et al., 2006; Tomalia et al., 2007). While development of dendrimer based technologies has tremendous potential, these materials, particularly cationic, higher-generation (≥ G7), amino-terminated dendrimers, have been shown to be toxic in vitro. Few systematic in vivo toxicity studies have been performed on PAMAM dendrimers, but available information suggests that low generation PAMAM dendrimers (below G5.0) are more biocompatible. Roberts et al. (1996) have shown that a single or repeated i.p. dose of G3, 4, and 5 dendrimers were not toxic to male Swiss-Webster mice (i.e., did not induce behavioral abnormalities, mortality, altered body weight, or macroscopic or histopathologic tissue abnormalities). However, G7 dendrimers may pose potential biological complications as 20% of the rats treated with G7 dendrimers died and all surviving rats showed some liver vacuolorization. In addition, these investigators found no evidence of immunogenicity using immunoprecipitation or the Ouchterlong double diffusion assay for any of the dendrimers tested. Exposure to G3.5 PAMAM dendrimers caused no adverse weight changes or signs of toxicity in C57 mice administered a daily i.p. dose of 95 mg/kg for three days; however, animals were observed for only a brief amount of time (Malik et al., 1999). These in vivo studies support cell culture studies which suggest that the toxicity of dendrimers is generation (size)-dependent, and that cationic dendrimers are more cytotoxic than anionic counterparts (Roberts et al., 1996; Malik et al., 2000; Jevprasesphant et al., 2003; Duncan et al., 2005; Tomalia et al., 2007). Taken together, these findings along with the ability to conjugate PAMAM dendrimers to certain biologically relevant molecules, makes low generation dendrimers promising agents for targeted drug delivery. Bioconjugation of PAMAM dendrimers with bioactive or adhesive peptides that interact with the protein/carbohydrate network of the extracellular matrix (ECM) may allow for the development of targeted drug delivery to specific cell types. The Arg-Gly-Asp (RGD) peptide serves as a recognition motif for the integrin receptor located within the extracellular matrix of endothelial cells (Pasqualini et al., 1997) and can be conjugated to PAMAM dendrimers to target this cell type.
Table 1.
Physical Characteristics of PAMAM Dendrimers
| Generation | Molecular Weight | Diameter (Å) | # Surface Groups |
|---|---|---|---|
| 0 | 517 | 15 | 4 |
| 1 | 1430 | 22 | 8 |
| 2 | 3256 | 29 | 16 |
| 3 | 6909 | 36 | 32 |
| 3.5 | 12931 | 64 | |
| 4 | 14215 | 45 | 64 |
| 5 | 28826 | 54 | 128 |
| 6 | 58048 | 67 | 256 |
| 7 | 116493 | 81 | 512 |
| 8 | 233383 | 97 | 1024 |
| 9 | 467162 | 114 | 2048 |
| 10 | 934720 | 135 | 4096 |
Note: those in bold were used in these studies
Yang et al (2007) demonstrated that G3.5 PAMAM dendrimers do not reduce fibroblast viability in vitro, while G4 PAMAM dendrimers cause concentration- and time-dependent cytotoxicity in fibroblasts. Furthermore, conjugation of G4 PAMAM dendrimers with RGD reduced its cytotoxic potency. Among the PAMAM dendrimers tested, RGD conjugated 3.5 PAMAM dendrimers showed the greatest promise for use in dendrimer-based drug delivery systems that target cells containing integrin-receptors because it was not cytotoxic to fibroblasts. However, information regarding the in vivo toxicity of such compounds is necessary if they are to be used in drug delivery. The zebrafish has become a prominent vertebrate model for assessing the toxicity of drugs and chemicals (Dooley et al., 2000; Peterson et al., 2000; Spitsbergen et al., 2003; Teraoka et al., 2003; Carvan et al., 2005; Hill et al., 2005; Lieschke et al., 2007). Despite the obvious differences in physiology between fish and humans, the zebrafish offers an ideal platform for an in vivo, whole-animal, medium to high throughput screen for the field of drug discovery, as well as a cost-effective compliment to mouse models of human disease (Lieschke et al., 2007; Kari et al., 2007). The same attributes that make the zebrafish ideal for the field of developmental biology (e.g., low husbandry cost, small size, optical clarity of embryos, conservation of gene programming and early development, and the availability of genetic resources and tools), have prompted its use as a model for human disease such as DiGeorge syndrome (Piotrowski et al., 2003), hepatoerythropoietic porphyria (Wang et al., 1998), and erythropoietic protoporphyria (Childs et al., 2000). In the area of drug discovery, the zebrafish has emerged as the premier whole-animal vertebrate model for screening chemical libraries to identify compounds that suppress a particular disease phenotype associated with a known human disease (Peterson et al., 2000; Margolis et al., 2004; Burns et al., 2005). However, as zebrafish are evolutionarily more distant from humans, findings from such experiments will need to be repeated in other systems before they can be directly correlated to humans (Guyon et al., 2007). Nevertheless, the zebrafish model is ideal for screening nanomaterials for use as therapeutics, as well as obtaining reliable information regarding the toxicity of different nanomaterials at the whole animal level which can then be used to extrapolate adverse effects of nanomaterials exposure to humans and other vertebrates.
To determine whether RGD-modified dendrimers cause toxicity in the whole animal, we have used the zebrafish embryo to assess the developmental toxicity of RGD-conjugated G3.5 and G4 PAMAM dendrimers. Furthermore, we have assessed the developmental toxicity of G3.5 and G4 PAMAM dendrimers for comparison to the RGD-conjugated forms.
Methods
Dendrimers
Starburst™ G3.5 and G4 polyamidoamine (PAMAM) dendrimers were purchased from Sigma-Aldrich and used without further purification (>98% pure as determine by manufacturer). The average molecular weight range for G3.5 is 12,931, and it contains approximately 64 surface groups (with COO−Na+ termini). The average molecular weight range for G4 is 14,215, and it also contains approximately 64 surface groups (with –NH2 termini). Comparison of G3.5 and G4 dendrimers to other generations are listed in Table 1. Materials were evaporated and lyophilized to remove methanol. RGD-conjugated G3.5 and G4 dendrimer synthesis was performed as previously described (Yang et al., 2007). Before being coupled with RGD, the carboxylate surface groups of G3.5 were converted to active NHS esters. Briefly, desiccated G3.5 was then dissolved in deionized water and acidified to pH 3 or below with 1 N HCl. The acidified G3.5 was evaporated to dryness under vacuum, and then redissolved in a mixture solution containing DMF and deionized water. Carboxylate surface groups were converted to active NHS esters following incubation with NHS and EDC for14 h. Following a vacuum drying step, the NHS-activated G3.5 continued to react with RGD in 2 mL of a pH 8.5 sodium bicarbonate solution for two h with a feeding molar ratio of RGD/G3.5 of 64:1. The primary amine surface groups of G4 were converted to active NHS esters first in a similar manner for the synthesis of RGD-G3.5 dendrimers. G4 dendrimer was incubated in a DMF solution containing DSC. Afterwards, TEA was slowly added to the solution followed by an overnight reaction with stirring. The supernatant containing NHS-activated G4 was collected after centrifugation, precipitated out of cold ether and vacuum dried. A two-hour coupling reaction between NHS-G4 and RGD was carried out in a pH 8.5 bicarbonate buffer solution where the feeding molar ratio of RGD/G4 was 64:1. The resulting RGD-G3.5 and RGD-G4 dendrimers were purified by using extensive dialysis and then characterized with GPC and 1H-NMR. For embryo exposures, dendrimers were re-dissolved in double distilled water and stock solutions were diluted to appropriate concentrations in 1X Danieau solution + Tris, pH 7 (58 mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4(7H2O), 0.6 mM Ca(NO3)2 0.5 mM HEPES with 10mM Tris HCl).
Exposure of zebrafish embryos to dendrimers
Fertilized eggs were collected from AB strain zebrafish as described by Westerfield et al. (1997). Eggs were collected within 2 h post fertilization (hpf) and distributed in 96-well cell culture plates (1 embryo/well) for exposure to the PAMAM dendrimers. Initially, three replicate experiments (n=16 embryos/treatment) were conducted using various concentrations of G3.5 and G4 dendrimers (0 – 220 μM dendrimer) to observe effects on mortality, sublethal toxicity and hatching rate, and to determine the concentration that caused 100% mortality by 24 hpf. Once the dose range was established, three replicate experiments (n = 16 embryos/treatment/replicate) were conducted to determine dose-dependent effects on zebrafish embryo mortality and endpoints of toxicity for the 4 types of dendrimers. Fertilized eggs were exposed to 0, 0.2, 0.5, 1, 2 or 20 μM G3.5, G4, RGD-G3.5 or RGD-G4 dendrimers by daily static renewal beginning at 6 hpf (shield stage) through 120 hpf (i.e., 6–24, 24–48, 48–72, 72–96 and 96–120 hpf).
Developmental toxicity of dendrimers in zebrafish
For all experiments, mortality data were collected after the first 2 h of exposure (8 hpf) and then at 24, 48, 72, 96 and 120 hpf. Live embryos/larvae were observed to assess developmental progression (i.e., completion of gastrulation, formation of somites, proper heart beat and spontaneous movement), as well as alterations in morphology and signs of toxicity (i.e., altered body axis, malformations of the eye, jaw, heart or fins, failure to inflate the swimbladder, yolk sac deformity, growth retardation and edema). Embryos were scored for severity of morphological defects and signs of toxicity [0 = normal, 1 = minor (one morphological anomaly), 2 = moderate (two morphological anomalies), 3 = severe (three morphological anomalies) or 4 = dead] at 24, 48, 72, 96 and 120 hpf. Morphological anomalies included: chorion with attached debris, delayed development, lack of spontaneous movement at 24 hpf, pericardial edema, yolk sac edema, bent trunk, tail malformation and uninflated swim bladder. The cumulative score for each embryo was used to determine the mean toxicity score for each treatment group at each 24 h timepoint.
To quantify sublethal toxicity relative to control, embryos were exposed to vehicle (control) or 1 μM G4 dendrimer by daily static renewal from 6–96 hpf as described above, and the magnitude of sublethal adverse responses were determined in surviving control embryos (n=10) and surviving 1 μM G4-treated embryos (n=10) at 96 hpf. Embryos were immobilized in 3% methylcellulose and photographed live in the lateral orientation at 2.5× and 8× magnification (n = 10 embryos/treatment). The photomicrographs of the embryos were analyzed using Scion Image software to determine body length (mm), degree of axial curvature (bent trunk) and lateral area (mm2) of the head, eye, pericardial sac and caudal fin.
Influence of chorion on G4 developmental toxicity
To determine whether the chorion contributes to G4 dendrimer toxicity, embryos manually removed from their chorions at 24 hpf, were exposed to graded concentrations of G4 dendrimer by daily static renewal for 96 h from 24–120 hpf. Also embryos with chorions either chemically removed at 5 hpf or manually removed at 24 hpf were exposed to graded concentrations of the G3.5 dendrimer by daily static renewal for either 114 h from 6–120 hpf or 96 h from 24–120 hpf, respectively. To chemically remove the chorion, 5 hpf embryos were incubated in 0.1 mg/mL pronase for 10 min and then gently rinsed with swirling two times for 10 min. Embryos were allowed 0.5 h to recover before starting the exposure to the G4 or G3.5 dendrimer. In this and all other experiments involving dendrimer exposure
Duration of exposure - time-concentration-mortality relationships
In addition to assessing G4 dendrimer toxicity using a daily static renewal exposure protocol where embryos were continuously exposed for 114 h (6–120 hpf), the effect of a very short duration of exposure to the G4 dendrimer that should result in less bioaccumulation of the dendrimer was investigated. Accordingly, embryos were exposed to the G4 dendrimer for only 1.5 h (6–7.5 hpf) and then maintained in G4 dendrimer-free media until 120 hpf, and dose-dependent effects of the G4 dendrimer exposure on mortality and signs of early life-stage toxicity were assessed as described above.
LC50 values calculated at 120 hpf, following exposure to G4 dendrimers for 1.5 h and 114 h, were used to calculate Kenga’s “Index of Chronicity” (Kenga, 1973). Linear regression of mortality data from continuous exposure and pulse exposure (1.5 h) experiments were used to calculate the exposure time required at each dose to induce 50% mortality. From this, a concentration versus time plot was analyzed using a simple power law function (XαY=k) to determine whether exposure to G4 dendrimers follows Haber’s law (C × T = k) (Miller et al., 2000; Gaylor, 2000; Rozman, 2000).
Degree of embryonic development
To determine if G4 dendrimers are less potent in causing embryo toxicity when exposed to the G4 dendrimer beginning at 24 hpf after much of early developmental process and events have been completed, another exposure protocol was used. Embryos were exposed by daily static renewal from 24–120 hpf and endpoints of toxicity were assessed at 48, 72, 96 and 120 hpf.
Statistical analysis
Mortality data were used to calculate the median lethal concentration (LC50 and 95% confidence interval) at 24, 72 and 120 hpf using Probit method analysis (U.S. EPA Probit Analysis Program, Ver 1.5). Statistical analyses of ELS toxicity data was performed using Sigma-Stat software 2.0 and results are presented as the mean ± SE. Data were evaluated for homoscedasticity (Levene Median test) and one-way analysis of variance (ANOVA) was used to detect treatment-related effects. Where significant differences were indicated between treatment groups and the data were homogeneous, pair-wise multiple comparisons were conducted using Tukey’s test. When tests for homogeneous variance failed, the Kruskal-Wallis one way ANOVA on ranks was used and significant differences were evaluated using Dunn’s test. Two-way ANOVA was used to evaluate the effect of G4 exposure on mortality with respect to dose and time (mean % mortality of the three replicates were compared) and multiple comparisons were evaluated using Tukey’s test. The Student’s t-test was used to evaluate effects on body length, spine angle and areas of the pericardial sac, head, eye and caudal fin, respectively. The level of significance for all analyses was p ≤ 0.05.
Results
Developmental toxicity of G3.5 and G4 PAMAM dendrimers in zebrafish
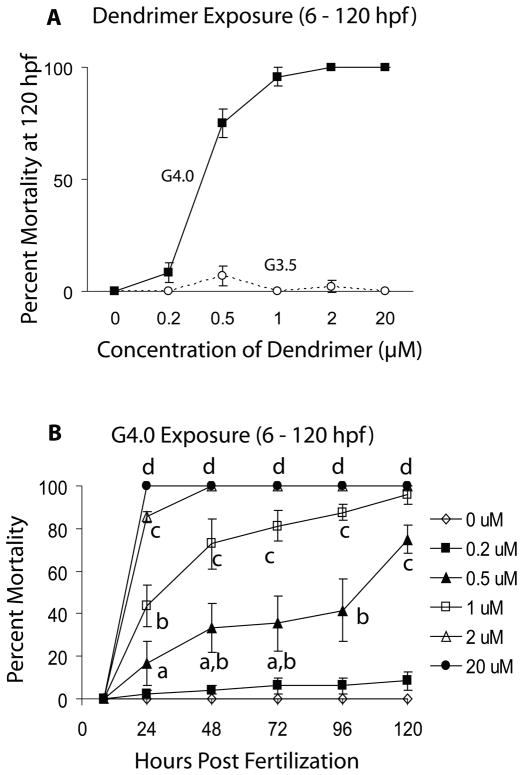
Exposure to ≥ 20 μM G4 dendrimer caused 100% mortality by 24 hpf, and hatching rate was not affected at sublethal concentrations (data not shown). Since hatching rate was not affected, hatching rate was not evaluated in the subsequent dose-response experiments. However, for each type of dendrimer investigated, it was noted whether the exposed embryos failed to hatch by 96 hpf.
Embryos treated with anionic G3.5 PAMAM dendrimers showed no increase in mortality (Table 2 and Fig. 1A) and demonstrated no sublethal signs of toxicity or malformations (results not shown).
Table 2.
Influence of PAMAM Dendrimer Type and Exposure Conditions on Lethality of Zebrafish Embryos
| Dendrimer | Time of Exposure (hpf) | LC50 (μM) - 24 hpf | LC50 (μM) - 72 hpf | LC50 (μM) - 120 hpf |
|---|---|---|---|---|
| G3.5 | 6–120 | --- | --- | --- |
| 24–120 (+) chorion | --- | --- | --- | |
| 24–120 (−) chorion | --- | --- | --- | |
| 6–120 (−) chorion | --- | --- | --- | |
| G4.0 | 6–120 | 1.0 (0.9–1.2) | 0.6 (0.5–0.6) | 0.4 (0.3–0.4) |
| 6–7.5 | 3.1 (1.5–18.9) | 1.6 (0.7–33.4) | 1.2 (0.7–3.0) | |
| 24–120 (+) chorion | n/a | 1.1 (0.8–1.3) | 0.7 (0.6–0.8) | |
| 24–120 (−) chorion | n/a | 4.4 (3.5–5.7) | 0.7 (0.6–0.8) | |
| G3.5-RGD | 6–120 | --- | --- | --- |
| G4.0-RGD | 6–120 | --- | --- | 4.1* |
slope not significantly different from zero, thus 95% confidence intervals could not be calculated.
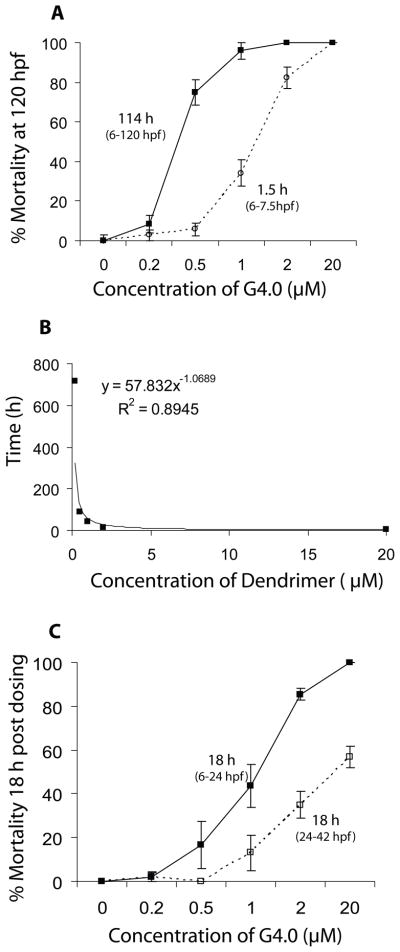
Figure 1.
A. Mortality of zebrafish embryos at 120 hpf following exposure to G4 and G3.5 PAMAM dendrimers from 6 – 120 hours post fertilization (hpf). B. Dose-response and time course of mortality of zebrafish embryos exposed to G4 PAMAM dendrimers from 6 – 120 hpf. Letters denote dose-and time-dependent differences in mortality (Two-Way ANOVA, p < 0.05).
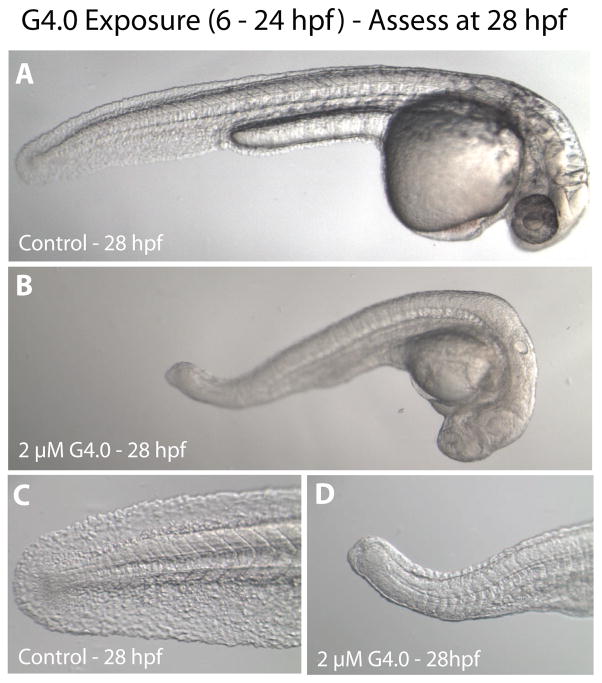
Cationic G4 PAMAM dendrimers, however, were toxic to zebrafish embryos (Table 2 and Fig. 1A). Mortality was both dose- and time-dependent (Fig. 1B) and sublethal signs of toxicity were seen following exposure to as little as 0.2 μM G4 (Table 2). Surviving embryos in the 2 μM dosage group were severely underdeveloped and showed signs of overt toxicity (Fig. 2); these embryos died within 48 h of exposure.
Figure 2.
Representative micrographs of overt toxicity seen in zebrafish embryos exposed to control or 2 μM G4 dendrimers beginning at 6 hpf. All micrographs were taken at 28 hpf. A. Control embryo manually removed from chorion (magnification 3.2×). B. G4 dendrimer-treated embryo manually removed from chorion (3.2×). C. Higher magnification of tail of control embryo (10×). D. Higher magnification of tail of G4 dendrimer-treated embryo (10×).
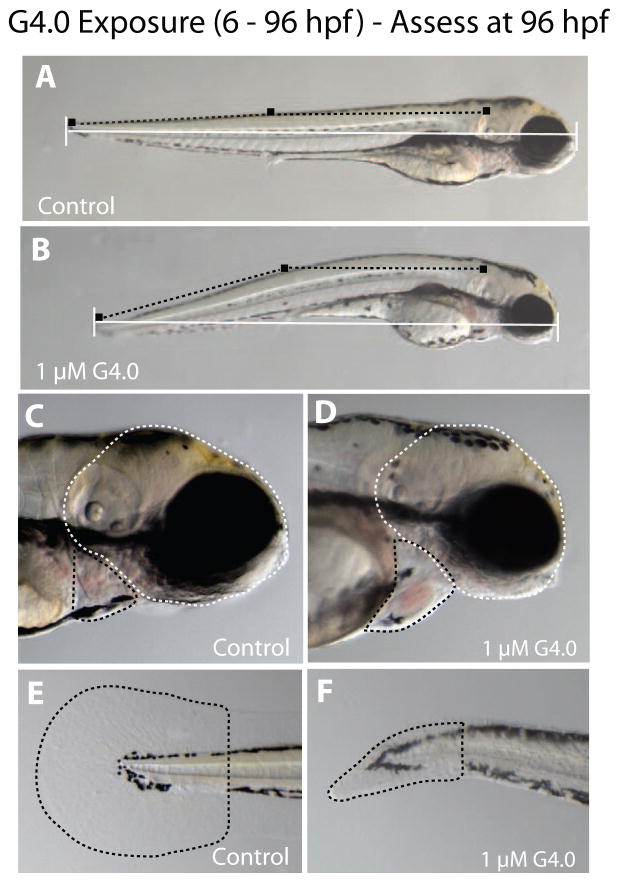
Sublethal toxicity was observed following exposure to 1 μM G4 or less and the endpoints of toxicity included: reduced body growth, bent trunk and smaller head and eyes (Fig. 3). All embryos successfully hatched from their chorions by 96 hpf. Embryos exposed to 1 μM of the G4 dendrimer from 6–96 hpf were 14% shorter with bent spines (13° angle) and had 27% smaller head and eyes (lateral areas) than control (p < 0.05). There was a tendency for G4 dendrimer exposed embryos to have mild pericardial edema and smaller caudal fin area but neither effect was statistically significant.
Figure 3.
Sublethal endpoints of toxicity in zebrafish embryos exposed to 1 μM G4 dendrimers from 6–96 hpf and assessed at 96 hpf. In A and B, the horizontal white line is the measure of total body length, and black squares connected by dotted lines represent the measure of axial curvature (i.e., bent trunk with the trunk angle measured in degrees). Panel A is a representative control zebrafish larva and Panel B is a representative G4 dendrimer-exposed zebrafish larva. In C and D, the white dotted line outlines the area of the head and the black dotted line outlines the pericardial sac. In E and F, the black dotted line outlines the area of the caudal fin. Panels C and E are representative control zebrafish larva, and D and F are representative G4 dendrimer-exposed larva.
Influence of chorion on G4 developmental toxicity
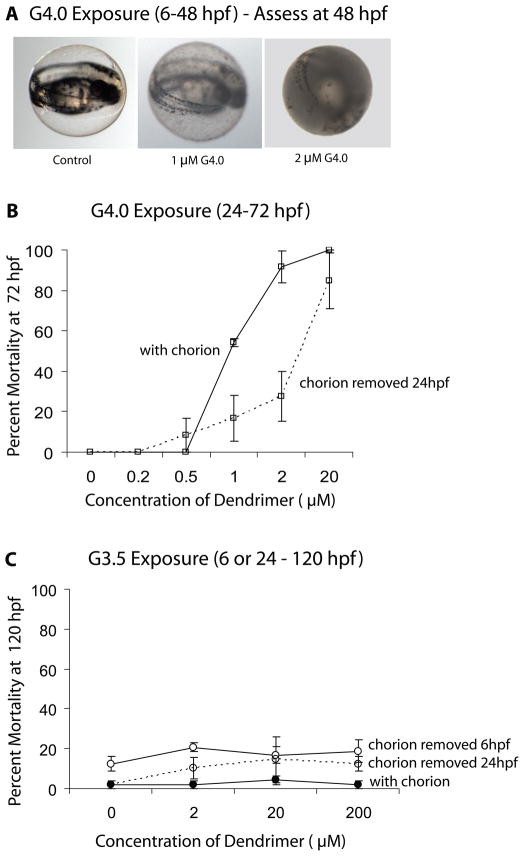
Following 24 h of exposure to greater than 1 μM of the G4 dendrimer, the chorion had debris attached to it, and a gelatinous material appeared inside the chorion in a dose-dependent manner (Fig. 4A). Therefore, we examined whether presence of a chorion would affect survival in embryos exposed to G4 dendrimer. Dechorionated embryos exposed to G4 dendrimers survive longer than those with an intact chorion (Fig. 4B). Furthermore, the LC50 of the G4 dendrimer calculated at 72 hpf is significantly less for those embryos with an intact chorion than for dechorionated embryos (Table 2; Fig. 4B). Thus, embryos with an intact chorion are more susceptible to G4 dendrimer-induced mortality than those that are dechorionated. This effect, however, is transient. When the LC50 of the G4 dendrimer is assessed at 120 hpf, the LC50 for intact and dechorionated embryos is not significantly different (Table 2).
Figure 4.
Effect of chorion removal on G4 dendrimer toxicity. A. Representative control and representative G4 dendrimer-treated embryo within the chorion with debris attached (1 μM G4) and debris attached to the chorion and gelatinous material within the chorion (2μM G4). All micrographs were taken at 48 hpf and at 3.2× magnification. B. Percent mortality of embryos with or without an intact chorion assessed at 72 hpf following exposure to G4 dendrimers from 24 – 72 hpf. C. Percent mortality of embryos with or without an intact chorion assessed at 120 hpf.
The absence of a chorion also initially protects embryos from the sublethal effects caused by G4 dendrimer exposure. That is, embryos lacking a chorion do not demonstrate sublethal signs of toxicity until 72 hpf (results not shown), and following exposure to 0.2 – 1 μM, show slightly reduced cumulative sublethal toxicity through 120 hpf (Table 4) compared to embryos with an intact chorion. In contrast to the G4 dendrimer, dechorionated embryos exposed to the G3.5 dendrimer show no significant change in either mortality (Table 2; Figure 4C) or sublethal toxicity (results not shown).
Table 4.
Influence of G4.0 PAMAM Dendrimer Exposure Conditions on Toxicity of Zebrafish Embryos at 120 hpf
| Exposure (hpf) | Toxicity Score (mean +SE) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| G4.0 Dendrimer Concentration (μM) | ||||||
| 0 | 0.2 | 0.5 | 1.0 | 2.0 | 20.0 | |
| 6–120 | 0.1 ± 0.1 | 0.4 ± 0.1a | 3.1 ± 0.2b | 3.9 ±0.1c | 4.0 ± 0.0c | 4.0 ± 0.0c |
| 6–7.5 | 0.4 ± 0.3 | 0.7 ± 0.4a | 0.7 ± 0.3a | 1.6 ± 0.4b | 3.4 ± 0.2c | 4.0 ± 0.0c |
| 24–120 + chorion | 0.1 ± 0.1 | 0.1 ± 0.1 | 1.9 ± 0.3a | 4.0 ± 0.0b | 4.0 ± 0.0b | 4.0 ± 0.0b |
| 24–120 − chorion | 0.1 ± 0.1 | 0.2 ± 0.1 | 1.0 ± 0.3a | 3.3 ±0.7b | 3.9 ± 0.1b | 4.0 ± 0.0c |
Note: Letters denote dose-related effects within each experimental paradigm.
Duration of exposure – concentration vs. time effects
The effect of exposure duration on dendrimer toxicity was also examined. As expected, toxic responses were more severe following longer exposures. Embryos exposed to G4 for 1.5 h (6–7.5 hpf) showed reduced toxicity compared to those exposed for 114 h (6–120 hpf) (Table 2, Fig. 5A). Kenga’s “Index of Chronicity” is commonly used to examine whether a compound is cumulatively toxic. If the LC50 following acute exposure divided by the LC50 following extended exposure is ≤1, then the compound is not cumulatively toxic. In the case of G4 dendrimer, the LC50 at 1.5 h divided by the LC50 at 114 h (1.2 μM/0.4 μM) = 3, indicating cumulative toxicity. Haber’s C × T law is often used to assess the potential risk of compounds in relation to concentration and duration of exposure to a fixed level of response for a given endpoint, and can be used to provide insight into potential contributions of toxicokinetics and toxicodynamics to the toxicity of the compound. When data for 50% lethality following continuous exposure are fit to a simple power function, α = 1.07 and R2 = 0.89; the data were found to fit the curve well (Fig. 5B). C × T values ranged from 36 – 80, with a mean of 49 ± 11. Similar results were found following pulse exposure to G4 dendrimer (α = 1.18 and R2 = 0.93, results not shown). Taken together this suggests that exposure to G4 dendrimers follow Haber’s law.
Figure 5.
Dose and time effects of G4 dendrimer-induced mortality in the zebrafish embryo. A. Influence of duration of exposure to G4 dendrimers: 1.5 h duration (6–7.5 hpf) versus 114 h duration (6–120 hpf) on dose-related mortality assessed at 120 hpf. B. The 50% lethal concentration of G4 in zebrafish embryos: influence of concentration and duration of exposure. The result of fitting the sample to a simple power function (XαY=k) is depicted. C. Influence of embryonic lifestage (degree of organization and differentiation): exposure from 6–24 hpf versus exposure from 24–42 hpf on dose-related mortality assessed at 18 h post dosing.
Degree of embryonic development
The timing of G4 dendrimer exposure (embryonic lifestage at which embryos were exposed) during zebrafish embryonic development was found to affect the susceptibility of embryos to toxicity. More explicitly, embryos exposed to graded concentrations of G4 dendrimers for 18 h late in development (24–42 hpf) were less susceptible to toxicity than those exposed for 18 h early in development (6–24 hpf, Fig. 5C). Consistent with this observation, LC50s determined for the G4 dendrimer at 72 and 120 hpf were higher for embryos exposed from 24–42 hpf than from 6–24 hpf (Table 1). Finally embryos exposed later in development (24–42 hpf) demonstrated qualitatively similar sublethal responses as embryos exposed earlier (6–24 hpf) but the magnitude of the responses at a particular G4 dendrimer concentration tended to be less in the embryos that were older at the time of the exposure (Table 3).
Table 3.
Influence of Duration of G4.0 PAMAM Dendrimer Exposure on Toxicity of Zebrafish Embryos
| Exposure (hpf) | Toxicity Score (mean +SE) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| G4.0 Dendrimer Concentration (μM) | ||||||
| 0 | 0.2 | 0.5 | 1.0 | 2.0 | 20.0 | |
| 24 | 0.0 ± 0.0 | 0.1 ± 0.1 | 1.3 ± 0.3a | 2.6 ± 0.3b | 3.6 ± 0.3c | 4.0 ± 0.0d |
| 48 | 0.0 ± 0.0 | 0.2 ± 0.1 | 1.4 ± 0.4a | 3.4 ± 0.3c | 4.0 ± 0.0d | 4.0 ± 0.0d |
| 72 | 0.0 ± 0.0 | 0.3 ± 0.1 | 1.5 ± 0.5a | 3.6 ± 0.2c | 4.0 ± 0.0d | 4.0 ± 0.0d |
| 96 | 0.0 ± 0.0 | 0.3 ± 0.1 | 2.0 ± 0.4a | 3.7 ± 0.1c | 4.0 ± 0.0d | 4.0 ± 0.0d |
| 120 | 0.1 ± 0.1 | 0.4 ± 0.1a | 3.1 ± 0.2b | 3.9 ± 0.1d | 4.0 ± 0.0d | 4.0 ± 0.0d |
Note: Letters denote dose- and time-dependent differences in toxicity (Two-way ANOVA, p < 0.05).
Developmental toxicity of RGD-conjugated dendrimers in zebrafish
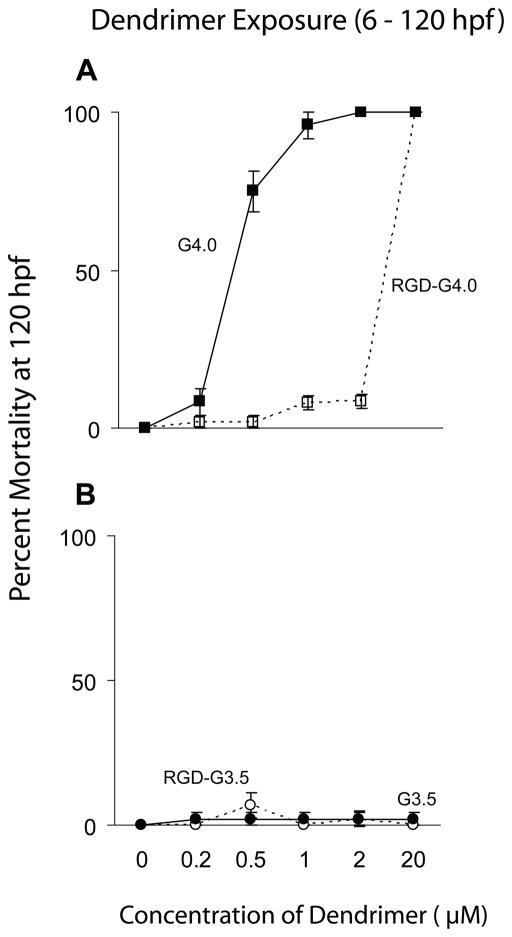
Bioconjugation of RGD to G4 dendrimers significantly reduced toxicity when compared with unconjugated G4 dendrimers. No mortality or sublethal toxicity was seen in any of the embryos exposed for 114 h (6 – 120 hpf) to concentrations of the RGD-G4 dendrimer up to and including 2 μM (Fig. 6A; Table 2). However, embryos exposed to 20 μM RGD-G4 show increased mortality compared with control embryos at 96 (results not shown) and 120 hpf (Fig. 6A). These embryos did not demonstrate signs of sublethal toxicity or impaired growth and development prior to their death at 96–120 hpf (results not shown). Zebrafish embryos exposed to G3.5-RGD dendrimers did not show increased mortality or any signs of sublethal toxicity (Fig. 6B; Table 2).
Figure 6.
Effect of net charge on G4 and G3.5 dendrimer-induced mortality in the zebrafish embryo. A. Mortality assessed at 120 hpf for embryos exposed to G4 or RGD-G4 dendrimers from 6 – 120 hpf. B. Mortality evaluated at 120 hpf for embryos exposed to G3.5 or RGD-G3.5 dendrimers from 6 – 120 hpf.
Discussion
Developmental toxicity of G3.5 and G4 PAMAM dendrimers in zebrafish
Toxicity of PAMAM dendrimers is generally considered to be species-, dose-, exposure duration-, and generation (size)-dependent and to be influenced by the nature of the terminal groups, that is, cationic amine terminal groups for full generation dendrimers, and anionic carboxylic acid terminal groups for half generation dendrimers (Roberts et al., 1996; Malik et al., 2000). In our experiments, zebrafish embryos treated with G3.5 dendrimers showed no sublethal signs of toxicity or increased mortality, even when exposed to concentrations as great as 200 μM. This is consistent with cell culture and in vivo results of others and further supports the safe use of anionic dendrimers as drug delivery devices (Malik et al., 2000; Jevprasesphant et al., 2003; Nigavekar et al., 2004; Yang et al., 2007). Even though dechorionated embryos exposed to G3.5 dendrimers at 8hpf did not show increased mortality, it is still possible that the lack of toxicity occurs because the G3.5 dendrimers are not taken up by the embryos following waterborne exposure. Anionic dendrimers show longer circulation time compared to their cationic counterparts; however, anionic dendrimers are still cleared relatively rapidly from the circulation (Malik et al., 2000; Nigavekar et al., 2004). Therefore, understanding the tissue distribution and metabolic fate of G3.5 dendrimers in a mammalian system is important in developing this type of dendrimer as a drug delivery device.
While low generation dendrimers (below G5.0) have been shown to be non-toxic to adult mammals (Roberts et al., 1996), our experiments demonstrate that the cationic G4 PAMAM dendrimers are toxic to zebrafish embryos in a dose- and time-dependent manner. At higher exposure concentrations, arrested development is associated with mortality. Since cationic molecules in general can destabilize cell membranes, resulting in cell lysis (Rittner et al., 2002), the cationic nature of the G4 dendrimers may similarly induce cytotoxicity in blastomeres, resulting in death of the embryo. Dendrimers could also interfere with critical signaling cascades during these early stages of development. Since organisms are generally more sensitive to chemical insult during embryonic development, exposure at this stage in zebrafish likely explains our finding of G4 dendrimer toxicity. However, it is possible that G4 toxicity is species-specific.
Static waterborne exposure to the G4 dendrimer also causes sublethal toxicity in zebrafish that is characterized by effects such as reduced body growth, bent trunk and small head and eyes. At the gross microscopic level, reduced growth was not correlated with obvious brain, notochord or muscular degeneration suggesting that at low concentrations, G4 dendrimers do not arrest embryonic development, but rather impair growth of the embryos. Low concentrations of dendrimers may also have a subtle impact on the regulation of embryonic development. For example, the small head and eyes in G4-exposed larvae could result from altered neural cell proliferation or differentiation. Further studies would be required to address these hypotheses. While it remains to be determined if G4 dendrimers cause developmental toxicity or antisomatic action in mammals, it would be prudent, particularly in view of our results in zebrafish, to evaluate this possibility.
Influence of the chorion on G4 developmental toxicity?
The chorion is generally considered a protective barrier for developing fish, and has been shown to prevent the uptake of contaminants by fish embryos (Rombough et al., 1982; Cameron et al., 1984; Gellert et al., 2001). Surprisingly, rather than serving as a protective barrier, the presence of the chorion surrounding the zebrafish embryo is associated with a transient increase in the rate of lethality following exposure to G4 dendrimers. Since hatching rate was not altered and embryos did hatch from their chorions by 96 hpf, it is unlikely that mortality is the result of secondary effects that often occur when embryos are not able to hatch from their chorions. Perhaps the net positive charge of the G4 dendrimer interacts with the negative charge of the chorion, thus concentrating the dendrimer around the embryo and increasing the effective dose of G4 dendrimer to the embryo. Following exposure to G4 dendrimers, debris collected on the chorion and a gelatinous material was observed within the chorion. Therefore, we cannot exclude a possible interaction between the dendrimer and chorion contributing to the sublethal effects observed between 48 and 96 hpf. Nonetheless, at 120 hpf, the LC50s were the same, regardless of whether or not the chorion was present. Together these findings suggest that the chorion has little effect on the toxicity of G4 dendrimers.
Duration of exposure – concentration vs. time effects
The longer duration of exposure to the G4 dendrimer resulting in greater uptake of G4 by the embryo, is the most likely explanation for the progressive decrease in LC50 compared to 1.5 h of exposure. Application of Kenga’s “Index of Chronicity” also suggests that G4 dendrimers are cumulatively toxic to zebrafish embryos. While embryos were more resistant to the toxic effects induced by G4-dendrimers following shorter periods of exposure, it is important to note that even a brief exposure to 2 and 20 uM G4 was sufficient to induce 50–94% mortality at 24 hpf. This supports the idea that the cationic nature of G4 dendrimers contributes to its toxicity, but may also reflect an impact of G4 dendrimers on early developmental events such as gastrulation. Furthermore, since exposure to G4 follows Haber’s law, this suggests that it is not the rate of exposure, but rather the total dose that results in G4 mortality and toxicity (Gaylor, 2000; Rozman, 2000), and that G4 dendrimers exert rapid, direct, systemic and irreversible toxicity in zebrafish embryos (Pieters et al., 1994).
Degree of embryonic development
When embryos were exposed beginning at 24 hpf, rather than at 6 hpf, the G4 dendrimer was slightly less potent in causing toxicity. This may be related to the increased organization and differentiation of the embryo at 24 hpf. However, as embryos develop, changes in the plasma membrane also occur beginning at the 6-somite stage (~12 hpf) decreasing their permeability (Hagedorn et al., 1997). Therefore, differences in toxicity might also reflect differences in uptake of the G4 dendrimer. Finally, it is possible that the observed developmental toxicity of G4 dendrimers might result in part from residual impurities (primarily minor structural deviations) that can be present in practical grade PAMAM dendrimers (see Peterson et al., (2003) and references therein).
Toxicity of RGD-conjugated dendrimers
RGD-conjugation of G4 dendrimers eliminates their sublethal toxicity and mortality when compared to unconjugated G4 dendrimers. This is probably caused by a reduction in the net positive charge of the surface groups of the RGD conjugated G4 dendrimer compared to its unconjugated counterpart. Partial derivatization of PAMAM dendrimers with chemically inert groups (e.g., PEG, fatty acids, or peptides) also is known to lessen the cytotoxicity of dendrimers in vitro also by either reducing the overall positive charge or encapsulating the cationic interior of the dendrimers (Haensler et al., 1993; Malik et al., 2000; Yoo et al., 2000; El Sayed et al., 2001; Rittner et al., 2002; Jevprasesphant et al., 2003; Fischer et al., 2003; Hong et al., 2004). This work further supports the hypothesis that G4-induced mortality results from cationic nature of the dendrimer. Furthermore, our results are similar to what has been demonstrated in cell culture (Yang et al., 2007) in that conjugation of RGD to G4 reduced cytotoxicity, but did not abolish it completely. This is because the RGD-G4 dendrimer is toxic at high doses to both fibroblasts and zebrafish embryos. Therefore, while RGD-conjugation affords some protection from G4 dendrimer-induced toxicity at low levels of exposure, higher levels of exposure to the RGD conjugated G4 dendrimer are still toxic. The physical events responsible for such a steep dose-response curve are not understood, and whether this is a function of dose (threshold) or stability of the RGD-conjugation has yet to be determined. Additionally, while in vitro studies demonstrate that RGD-conjugation does not affect uptake of G4 dendrimers in cell culture (Yang et al., 2007), it is possible that the RGD-conjugation restricts uptake of the dendrimer by zebrafish embryos, resulting in the observed reduction in toxicity. In contrast to RGD-G4 dendrimers, RGD-G3.5 dendrimers were not toxic to zebrafish embryos.
Conclusions
Here, we demonstrate that the zebrafish is an ideal system for assessing the initial toxicity of novel nano-therapeutic agents. Overall, our results agree with what has been published in mammalian systems and in cell culture regarding the toxicity of low generation dendrimers. Additionally, by assessing the toxicity of such materials in embryos, we were able to identify areas of research that require further investigation in mammalian systems. RGD-conjugated anionic G3.5 dendrimers have been shown to have no negative impact on cell viability (Yang et al., 2007) and were found in the present study to be non-toxic to zebrafish embryos at the highest concentrations tested. Information on persistence, bioaccumulation and tissue distribution of RGD conjugated G3.5 dendrimers in zebrafish embryos is lacking. While obtaining this information was beyond the scope of the present study, it is needed to fully interpret the toxicity results. This point not withstanding, RGD-3.5 dendrimers show great promise as efficient vectors for use as targeting drug delivery systems to recognize cells that contain integrin-receptors (e.g., for tumor treatment and gene therapy).
Acknowledgments
We thank Amy Gustafson for synthesizing the RGD-G4 and RGD-G3.5 dendrimers used in this study. We also acknowledge the outstanding assistance of Dr. Bing Liu, Chung Shan Medical University, Taichung, Taiwan and the technical assistance of Erick Sokn, Michael Baumann and Dorothy Nesbit, University of Wisconsin. This work was supported by the University of Wisconsin NanoScale Engineering Center (NSF grant DMR-0425880). Tisha King Heiden and Emelyne Dengler are both supported by the Molecular and Environmental Toxicology Postdoctoral and Predoctoral Training Grant number T32 ES007015 from the NIEHS, NIH (Contribution #369). The contents of this publication are solely the responsibility of the authors, and do not necessarily represent official views of the NIEHS, NIH.
Abbreviations used in text
- PAMAM
polyamidoamine
- RGD
Arg-Gly-Asp
- G
generation
Footnotes
Conflict of Interest Statement: The authors have no professional or financial affiliations that may be perceived as having biased the presentation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boas U, Heegaard PM. Dendrimers in drug research. Chem Soc Rev. 2004;33:43–63. doi: 10.1039/b309043b. [DOI] [PubMed] [Google Scholar]
- Bracci L, Falciani C, Lelli B, Lozzi L, Runci Y, Pini A, De Montis MG, Tagliamonte A, Neri P. Synthetic peptides in the form of dendrimers become resistant to protease activity. J Biol Chem. 2003;278:46590–46595. doi: 10.1074/jbc.M308615200. [DOI] [PubMed] [Google Scholar]
- Burns CG, Milan DJ, Grande EJ, Rottbauer W, MacRae CA, Fishman MC. High-throughput assay for small molecules that modulate zebrafish embryonic heart rate. Nat Chem Biol. 2005;1:263–264. doi: 10.1038/nchembio732. [DOI] [PubMed] [Google Scholar]
- Cameron IL, Hunter KE. Regulation of the permeability of the medaka fish embryo chorion by exogeneous sodium and calcium ions. J Exp Zool. 1984;231:447–454. doi: 10.1002/jez.1402310320. [DOI] [PubMed] [Google Scholar]
- Carvan MJ, King Heiden T, Tomasiawicz H. The Utility of Zebrafish as a Model for Toxicological Research. In: Mommsen TP, Moon TW, editors. Biochemistry and Molecular Biology of Fishes. Vol. 6. Elsevier BV; 2005. pp. 3–41. [Google Scholar]
- Childs S, Weinstein BM, Mohideen MA, Donohue S, Bonkovsky H, Fishman MC. Zebrafish dracula encodes ferrochelatase and its mutation provides a model for erythropoietic protoporphyria. Curr Biol. 2000;10:1001–1004. doi: 10.1016/s0960-9822(00)00653-9. [DOI] [PubMed] [Google Scholar]
- Dooley K, Zon LI. Zebrafish: a model system for the study of human disease. Curr Opin Genet Dev. 2000;10:252–256. doi: 10.1016/s0959-437x(00)00074-5. [DOI] [PubMed] [Google Scholar]
- Duncan R, Izzo L. Dendrimer biocompatibility and toxicity. Adv Drug Deliv Rev. 2005;57:2215–2237. doi: 10.1016/j.addr.2005.09.019. [DOI] [PubMed] [Google Scholar]
- El Sayed M, Kiani MF, Naimark MD, Hikal AH, Ghandehari H. Extravasation of poly(amidoamine) (PAMAM) dendrimers across microvascular network endothelium. Pharm Res. 2001;18:23–28. doi: 10.1023/a:1011066408283. [DOI] [PubMed] [Google Scholar]
- Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kissel T. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003;24:1121–1131. doi: 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- Gaylor DW. The use of Haber’s law in standard setting and risk assessment. Toxicology. 2000;149:17–19. doi: 10.1016/s0300-483x(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Gellert G, Heinrichsdorff J. Effect of age on the susceptibility of zebrafish eggs to industrial wastewater. Water Res. 2001;35:3754–3757. doi: 10.1016/s0043-1354(01)00084-7. [DOI] [PubMed] [Google Scholar]
- Guyon JR, Steffen LS, Howell MH, Pusack TJ, Lawrence C, Kunkel LM. Modeling human muscle disease in zebrafish. Biochim Biophys Acta. 2007;1772:205–215. doi: 10.1016/j.bbadis.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Haensler J, Szoka FC., Jr Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjug Chem. 1993;4:372–379. doi: 10.1021/bc00023a012. [DOI] [PubMed] [Google Scholar]
- Hagedorn M, Kleinhans FW, Freitas R, Liu J, Hsu EW, Wildt DE, Rall WF. Water distribution and permeability of zebrafish embryos, Brachydanio rerio. J Exp Zool. 1997;278:356–371. doi: 10.1002/(sici)1097-010x(19970815)278:6<356::aid-jez3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- Hong S, Bielinska AU, Mecke A, Keszler B, Beals JL, Shi X, Balogh L, Orr BG, Baker JR, Jr, Banaszak Holl MM. Interaction of poly(amidoamine) dendrimers with supported lipid bilayers and cells: hole formation and the relation to transport. Bioconjug Chem. 2004;15:774–782. doi: 10.1021/bc049962b. [DOI] [PubMed] [Google Scholar]
- Jevprasesphant R, Penny J, Jalal R, Attwood D, McKeown NB, D’Emanuele A. The influence of surface modification on the cytotoxicity of PAMAM dendrimers. Int J Pharm. 2003;252:263–266. doi: 10.1016/s0378-5173(02)00623-3. [DOI] [PubMed] [Google Scholar]
- Kari G, Rodeck U, Dicker AP. Zebrafish: an emerging model system for human disease and drug discovery. Clin Pharmacol Ther. 2007;82:70–80. doi: 10.1038/sj.clpt.6100223. [DOI] [PubMed] [Google Scholar]
- Kenga EE. Factors to be considered in the evaluation of the toxicity of pesticides to birds in their environment. In: Coulston F, Korte F, editors. Environmental Quality and Safety. Academic Press; New York, NY: 1973. [Google Scholar]
- Kim Y, Zimmerman SC. Applications of dendrimers in bio-organic chemistry. Curr Opin Chem Biol. 1998;2:733–742. doi: 10.1016/s1367-5931(98)80111-7. [DOI] [PubMed] [Google Scholar]
- Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- Malik N, Evagorou EG, Duncan R. Dendrimer-platinate: a novel approach to cancer chemotherapy. Anticancer Drugs. 1999;10:767–776. [PubMed] [Google Scholar]
- Malik N, Wiwattanapatapee R, Klopsch R, Lorenz K, Frey H, Weener JW, Meijer EW, Paulus W, Duncan R. Dendrimers: relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J Control Release. 2000;65:133–148. doi: 10.1016/s0168-3659(99)00246-1. [DOI] [PubMed] [Google Scholar]
- Margolis J, Plowman GD. Overcoming the gridlock in discovery research. Nat Biotechnol. 2004;22:522–524. doi: 10.1038/nbt0504-522. [DOI] [PubMed] [Google Scholar]
- Miller FJ, Schlosser PM, Janszen DB. Haber’s rule: a special case in a family of curves relating concentration and duration of exposure to a fixed level of response for a given endpoint. Toxicology. 2000;149:21–34. doi: 10.1016/s0300-483x(00)00229-8. [DOI] [PubMed] [Google Scholar]
- Nigavekar SS, Sung LY, Llanes M, El Jawahri A, Lawrence TS, Becker CW, Balogh L, Khan MK. 3H dendrimer nanoparticle organ/tumor distribution. Pharm Res. 2004;21:476–483. doi: 10.1023/B:PHAM.0000019302.26097.cc. [DOI] [PubMed] [Google Scholar]
- Pasqualini R, Koivunen E, Ruoslahti E. Alpha v integrins as receptors for tumor targeting by circulating ligands. Nat Biotechnol. 1997;15:542–546. doi: 10.1038/nbt0697-542. [DOI] [PubMed] [Google Scholar]
- Peterson J, Allikmaa V, Subbi J, Pehk T, Lopp M. Structural deviations in poly(amidoamine) dendrimers: a MALDI-TOF MS analysis. European Polymer J. 2003;39:33–42. [Google Scholar]
- Peterson RT, Link BA, Dowling JE, Schreiber SL. Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc Natl Acad Sci USA. 2000;97:12965–12969. doi: 10.1073/pnas.97.24.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters MN, Kramer HJ. Bilthoven, RIVM Report #659101002. 1994. Concentration * Time = constant? The validity of Haber’s Law in the extrapolation of discontinuous to continuous exposition. [Google Scholar]
- Piotrowski T, Ahn DG, Schilling TF, Nair S, Ruvinsky I, Geisler R, Rauch GJ, Haffter P, Zon LI, Zhou Y, Foott H, Dawid IB, Ho RK. The zebrafish van gogh mutation disrupts tbx1, which is involved in the DiGeorge deletion syndrome in humans. Development. 2003;130:5043–5052. doi: 10.1242/dev.00704. [DOI] [PubMed] [Google Scholar]
- Rittner K, Benavente A, Bompard-Sorlet A, Heitz F, Divita G, Brasseur R, Jacobs E. New basic membrane-destabilizing peptides for plasmid-based gene delivery in vitro and in vivo. Mol Ther. 2002;5:104–114. doi: 10.1006/mthe.2002.0523. [DOI] [PubMed] [Google Scholar]
- Roberts JC, Bhalgat MK, Zera RT. Preliminary biological evaluation of polyamidoamine (PAMAM) Starburst TM dendrimers. J Biomedical Materials Res. 1996;30:53–65. doi: 10.1002/(SICI)1097-4636(199601)30:1<53::AID-JBM8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Rombough PJ, Garside ET. Cadmium toxicity and accumulation in eggs and alevins of Atlantic salmon Salmo salar. Can J Zool. 1982;60:2006–2014. [Google Scholar]
- Rozman KK. The role of time in toxicology or Haber’s c x t product. Toxicology. 2000;149:35–42. doi: 10.1016/s0300-483x(00)00230-4. [DOI] [PubMed] [Google Scholar]
- Sadler K, Tam JP. Peptide dendrimers: applications and synthesis. J Biotechnol. 2002;90:195–229. doi: 10.1016/s1389-0352(01)00061-7. [DOI] [PubMed] [Google Scholar]
- Spetzler JC, Tam JP. Unprotected peptides as building blocks for branched peptides and peptide dendrimers. Int J Pept Protein Res. 1995;45:78–85. doi: 10.1111/j.1399-3011.1995.tb01570.x. [DOI] [PubMed] [Google Scholar]
- Spitsbergen JM, Kent ML. The state of the art of the zebrafish model for toxicology and toxicologic pathology research--advantages and current limitations. Toxicol Pathol. 2003;31(Suppl):62–87. doi: 10.1080/01926230390174959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson S, Tomalia DA. Dendrimers in biomedical applications--reflections on the field. Adv Drug Deliv Rev. 2005;57:2106–2129. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Dong W, Hiraga T. Zebrafish as a novel experimental model for developmental toxicology. Congenit Anom(Kyoto) 2003;43:123–132. doi: 10.1111/j.1741-4520.2003.tb01036.x. [DOI] [PubMed] [Google Scholar]
- Tomalia DA, Naylor AM, Goddard WA., III Starburst dendrimers: Molecular-level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Agnew Chem Int Ed Engl. 1990;29:138–175. [Google Scholar]
- Tomalia DA, Reyna LA, Svenson S. Dendrimers as multi-purpose nanodevices for oncology drug delivery and diagnostic imaging. Biochem Soc Trans. 2007;35:61–67. doi: 10.1042/BST0350061. [DOI] [PubMed] [Google Scholar]
- Wang H, Long Q, Marty SD, Sassa S, Lin S. A zebrafish model for hepatoerythropoietic porphyria. Nat Genet. 1998;20:239–243. doi: 10.1038/3041. [DOI] [PubMed] [Google Scholar]
- Westerfield M, Doerry E, Kirkpatrick AE, Driever W, Douglas SA. An on-line database for zebrafish development and genetics research. SEMINARS IN CELL AND DEVELOPMENTAL BIOLOGY. 1997;8:477–488. doi: 10.1006/scdb.1997.0173. [DOI] [PubMed] [Google Scholar]
- Yang H, Kao WJ. Dendrimers for pharmaceutical and biomedical applications. J Biomater Sci Polym Ed. 2006;17:3–19. doi: 10.1163/156856206774879171. [DOI] [PubMed] [Google Scholar]
- Yang H, Kao WJ. Synthesis and characterization of nanoscale dendritic RGD clusters for potential applications in tissue engineering and drug delivery. Internat J Nanomedicine. 2007;2:89–99. doi: 10.2147/nano.2007.2.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo H, Juliano RL. Enhanced delivery of antisense oligonucleotides with fluorophore-conjugated PAMAM dendrimers. Nucleic Acids Res. 2000;28:4225–4231. doi: 10.1093/nar/28.21.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]