Abstract
Depression is a mental disorder characterized by low mood and anhedonia that involves abnormalities in multiple brain regions and networks. Epidemiological studies demonstrated that depression has become one of the most important diseases affecting human health and longevity. The pathogenesis of the disease has not been fully elucidated. The clinical effect of treatment is not satisfactory in many cases. Neuroimaging studies have provided rich and valuable evidence that psychological symptoms and behavioral deficits in patients with depression are closely related to structural and functional abnormalities in specific areas of the brain. There were morphological differences in several brain regions, including the frontal lobe, temporal lobe, and limbic system, in people with depression compared to healthy people. In addition, people with depression also had abnormal functional connectivity to the default mode network, the central executive network, and the salience network. These findings provide an opportunity to re-understand the biological mechanisms of depression. In the future, magnetic resonance imaging (MRI) may serve as an important auxiliary tool for psychiatrists in the process of early and accurate diagnosis of depression and finding the appropriate treatment target for each patient to optimize clinical response.
Keywords: Depression, Magnetic resonance imaging, Central execution network, Salience network, Brain network, Neuroimaging, Default network, fMRI, Functional connectivity, Functional magnetic resonance imaging
Introduction
Major depressive disorder (MDD) is a mental illness characterized by significant persistent low mood and emotional changes. Its clinical manifestations include depression, sorrow, anhedonia, rumination. Patients with severe depression may have suicidal will or behavior. According to statistics, depression has become the most widely distributed mental disorder in the globe (Smith, 2014), and it is also one of the diseases with the highest disability-adjusted life year (Global Burden of Disease Study, 2015). Risk factors for depression include adverse life events, external environmental stress, cognitive impairment, depressed parents, social dysfunction, and being female (Hammen, 2018). Patients with malignant tumors, diabetes, chronic physical pain, or cardiovascular and cerebrovascular diseases also had higher rates of depression than healthy controls (Bortolato et al., 2017; Hare et al., 2014; Kales, Maixner & Mellow, 2005; Réus et al., 2019; Sheng et al., 2017). Thus, both structural and functional disorders, especially in the brain, may contribute to depression. Complex structural connectivity supports various physiological and social functions of the brain, which can process a variety of information efficiently and accurately (Park & Friston, 2013). At present, electroencephalogram (EEG) is often used by clinicians in the diagnosis of depression. However, due to the inability of EEG to provide spatial information and relatively low specificity, the diagnostic value provided by this method is limited.
On the other hand, neuroimaging can compensate for the defect of EEG, providing more spatial information and locating abnormal brain areas in patients with depression (Keren et al., 2018). For now, neuroimaging studies have confirmed that major depressive disorder is closely related to brain structural and functional abnormalities (De Kwaasteniet et al., 2013; Korgaonkar et al., 2014). Magnetic resonance imaging (MRI) is a noninvasive, reproducible, and acceptable technique that can provide more biological information than EEG with higher spatial resolution.
Gray matter is a significant component of the central nervous system, and the volume of gray matter in the brain is associated with many physiological senses and higher functions, including muscle control, vision and hearing, memory, emotion, language, decision-making and self-control (Rogers & De Brito, 2016; Zatorre, Fields & Johansen-Berg, 2012). Volume changes of gray matter can be detected by processing structural MRI information with Voxel-based morphometry (VBM).
The primary function of white matter is to transmit information efficiently and accurately between different gray matter areas of the central nervous system. Reduced white matter connectivity and volume can lead to impaired information delivery, which may cause deficits in attention, declarative memory, executive function, and intelligence (Fields, 2008; Reddick et al., 2006). Diffusion tensor imaging (DTI) can contribute to the assessment of the structural connectivity of nerve fiber bundles by displaying structural connections (Basser, Mattiello & LeBihan, 1994)
On the other hand, the resting and active status of brain regions can be observed by detecting fluctuations in blood oxygen levels. Blood oxygenation level-dependent (BOLD) functional MRI (fMRI) can contribute to the assessment of brain abnormalities by showing changes in brain activity of subjects in resting-state or task-state. Especially, resting-state functional magnetic resonance has become an essential basis for brain functional analysis (Biswal et al., 1995). The comprehensive application of structural and functional imaging provides a possible way to elucidate the etiology and pathogenesis of depression.
This article reviews recent advances in neuroimaging studies related to depression and summarizes the imaging changes of the disease from the structural and functional aspects.
Survey Methodology
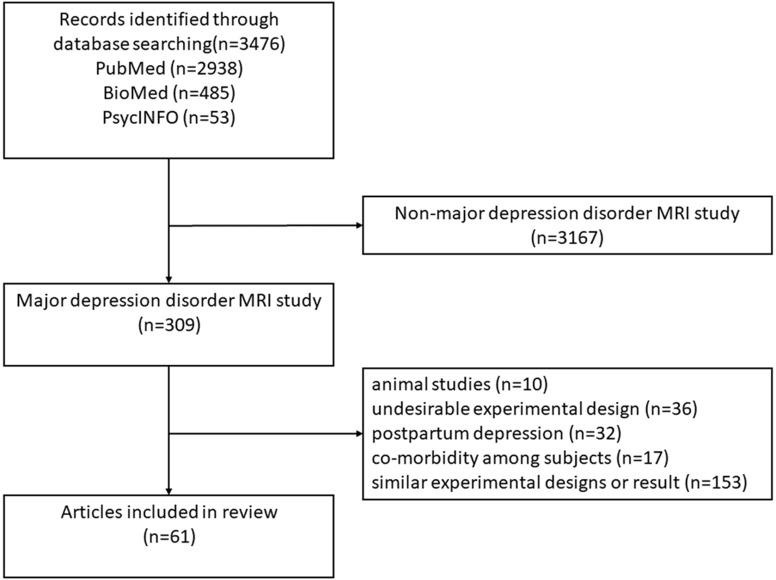
Article searching was performed in PubMed , BioMed and PsycINFO between earliest record and September 1, 2019, using (“major depressive disorder” OR “unipolar depression” OR “depressive disorder, treatment-resistant”) AND (MRI OR “magnetic resonance imaging” OR VBM OR “Voxel-based morphometry” OR DTI OR “diffusion tensor imaging” OR fMRI OR “functional magnetic resonance imaging” OR BOLD or “blood oxygen level-dependent” OR “resting-state fMRI” OR “functional connectivity” OR rsfMRI OR “resting-state functional connectivity”) as search terms in title and abstract. Two authors jointly established inclusion and exclusion criteria and applied them to literature screening and quality assessment. These criteria were: (1) DSM-III, DSM-IV, DSM-V, ICD-10 or ICD-11 was used as criteria for diagnosing depression; (2) The experimental group and the control group matched at the age and gender level; (3) Clearly grouped medicated and unmedicated patients instead of mixing them into one group; (4) Subjects in the experimental group were not accompanied by bipolar disorder or other mental or organic diseases; (5) Studies with fewer than 10 people in the experimental group were excluded; (6) Studies on postpartum depression was excluded; (7) Animal experiments were excluded. The process of literature selection is shown in the flowchart (Fig. 1). Participant information and results from MRI studies are showed in the Supplemental Information.
Figure 1. Flowchart of the decision tree.
Brain Structural Abnormality in Depression
Gray matter changes
VBM is widely used in the study of abnormal brain anatomy. This technique uses the statistical parameter map method to measure the volume and density of each voxel corresponding to gray matter and white matter. Changes were quantitatively calculated to assess changes in gray matter and white matter.
Meta-analyses indicate that hippocampal volume reduction is the most common brain anatomical change in patients with depression (Arnone et al., 2012; Cole et al., 2011), and, especially, the atrophy is most pronounced in the cornu ammonis, dentate gyrus, and subiculum (Roddy et al., 2019). As an essential part of the limbic system, the hippocampus plays a vital role in memory processing and emotional management. Moreover, studies (Buddeke et al., 2017; Den Heijer et al., 2011) found that depressive symptoms and hippocampal atrophy are mutually reinforcing and aggravating. Also, the volume of the cingulate cortex, another part of the limbic system associated with memory and mood formation, was smaller in depressed patients than in healthy controls (Rodriguez-Cano et al., 2014; Wise et al., 2017).
Frontal atrophy is also one of the critical changes in depression (Grieve et al., 2013). Studies have shown that the medial prefrontal cortex, frontal cortex, dorsolateral prefrontal cortex atrophy is particularly significant (Bludau et al., 2016; Van Eijndhoven et al., 2013; Zhao et al., 2014). The frontal cortex plays an important role in emotional cognition and working memory (Bludau et al., 2014).
In addition to the frontal lobe, the volume of the bilateral putamen and left thalamus in patients with depression is also smaller in contrast to healthy controls (Lu et al., 2016). These gray matter nuclei are related to memory, information transmission, and emotional management. Also, the degree of atrophy of the amygdala is positively correlated with the severity of depressive symptoms in patients (Zhang et al., 2016). And meta-analysis showed that patients with depression who had a larger gray matter before treatment were also better treated with medication (Fonseka, MacQueen & Kennedy, 2018).
White matter changes
Abnormal white matter is also widespread in patients with depression (Liao et al., 2013). DTI shows the location and direction of the white matter bundle. Tract-based spatial statistics (TBSS) can quantitatively measure the fraction anisotropy (FA) of the nerve white matter fibers, and compare the white matter bundle skeletons of different subjects to locate the microstructure abnormalities of the brain white matter accurately (Smith et al., 2006).
Meta-analysis (Jiang et al., 2017a) indicated that the FA values in the corpus callosum, white matter in the right cerebellar hemisphere and bilateral superior longitudinal plasma of depressed patients were significantly lower than those in the healthy control group, and it was indicated that the abnormality of the corpus callosum was particularly prominent (Han et al., 2014). And Studies (Cole et al., 2012; De Diego-Adelino et al., 2014) showed that the extent of the FA decline in the corpus callosum, and bilateral upper longitudinal was positively correlated with the severity of depressive symptoms and duration of onset. And patients with suicide attempt history had lower FA in the dorsomedial prefrontal cortex than those without suicide attempt history and healthy controls (Olvet et al., 2014). Not only that, but the lower FA value in the ventral medial prefrontal area is more pronounced in patients with refractory depression (De Diego-Adelino et al., 2014). The decrease in the FA value of the superior frontal gyrus, superior longitudinal fasciculus, and corpus callosum can even predict the depression in the elderly (Reppermund et al., 2014). A study (Henderson et al., 2013) of adolescents with depression found that patients with more severe depressive symptoms had a greater FA reduction in sagittal stratum, anterior thalamic radiation, genu of the corpus callosum and anterior cingulate near the precuneus. At present, there are still many inconsistencies in the study on the abnormal white matter fiber bundles in depression. In the future, multi-site large sample studies can be carried out to verify the above research results.
Cerebrovascular changes
On the other hand, compared with the healthy control group, elderly patients with depression have more severe cerebral vascular lesions such as white matter hyperintensities (WMH), subcortical lacunar, microinfarction, and microangiopathy (Wang et al., 2014). As early as 1997, Alexopoulos (Alexopoulos et al., 1997) proposed the “vascular depression hypothesis”, which believes that cerebrovascular disease and its subsequent white matter changes are an essential part of the pathogenesis of late-onset depression. A recent meta-analysis (Van Agtmaal et al., 2017) indicates that white matter hyperintensities are significantly associated with the incidence of depression. These white matter lesions are considered to be significant predictors of late-onset depression (Park et al., 2015), of which subcortical white matter lesions are strictly related to the severity of depressive symptoms and cognitive impairment (Taylor, Aizenstein & Alexopoulos, 2013). Besides, patients with depression with severe changes in leukoencephalopathy have worse symptoms and cognitive function after antidepressant treatment (Sheline et al., 2010).
Brain Functional Abnormality in Depression
fMRI is widely used in the study of abnormal brain activity. When the neuronal activity is enhanced, the local blood flow in the cortex of the functional brain area is significantly increased, and the oxygen consumption is relatively insignificant, resulting in the proportion of deoxygenated hemoglobin/oxyhemoglobin is reduced. Due to deoxygenated hemoglobin is a paramagnetic substance, the functional region shows a different BOLD signal compared with the inactive brain region. Neuronal activity in a resting state or giving emotional stimuli and cognitive tasks can be indirectly reflected by a BOLD signal representing the local neuron activity of the brain.
Regional brain activity changes
Depending on the purpose and the experimental design of the study, the fMRI study can be divided into two types: resting-state and task-based. The former mainly examines the spontaneous nerve activity of the subject in a calm and awake state, while the latter mainly explores the activity state of the brain when the individual is subjected to emotional stimulation or completing specific tasks.
A study (Hamilton et al., 2012) indicated, compared to the control group, MDD patients showed increased resting activity in the pulvinar, which is an essential nucleus in the thalamus and is thought to be functionally synchronized with nodes in the salient network, such as the amygdala, the insular lobes, and the anterior cingulate gyrus. Thus, it may enhance the response to negative emotional information in the salience network. Also, a meta-analysis (Kuhn & Gallinat, 2013) showed, at resting state, increased activity in the ventral medial prefrontal cortex, the left ventral striatum, and left thalamus and decreased activity in the left postcentral gyrus, left fusiform gyrus and left insula relative to controls in patients with depression.
On the other hand, due to the differences in experimental task design and sample selection methods, although many task-based fMRI experiments have studied the abnormalities of brain activity patterns in patients with depression during cognitive and emotional processing, consistent conclusions are still lacking (Muller et al., 2017). A meta-analysis (Miller et al., 2015) of adolescent depression found that hyperactivity of the anterior cingulate gyrus and thalamus may lead to depression patients being highly sensitive to emotional stimuli, while anhedonia may be caused by hypoactivation of the cuneus and posterior insula during reward processing. Interestingly, in depressed patients, the amygdala showed a “dual-separation” pattern of hyperactivity in response to negative stimuli (Tao et al., 2012) and decreased response to positive stimuli (Suslow et al., 2010). Likewise, in a small sample reward study (Takamura et al., 2017), MDD patients did not experience increased striatal activity in response to reward stimuli as healthy controls did.
Young et al.’s small sample studies (Young et al., 2017a; Young et al., 2017b) found that after real-time functional magnetic resonance imaging neurofeedback training, the amygdala activity of depressed patients could be relatively restored to normal, and patients’ depressive symptoms were reduced and their ability to recall positive memories was improved. A study (Holmes & Pizzagalli, 2008) indicates that the decline in cognitive ability in patients with depression is caused by the distribution of excessive neural pathway resources in negative consciousness and rumination. A small sample study by Liao et al. (2012) showed that patients with depression had a bias in their perception of pleasure and neutral stimuli, which was associated with abnormal activity in the bilateral amygdala and the right dorsolateral prefrontal cortex.
Brain network functional connectivity changes
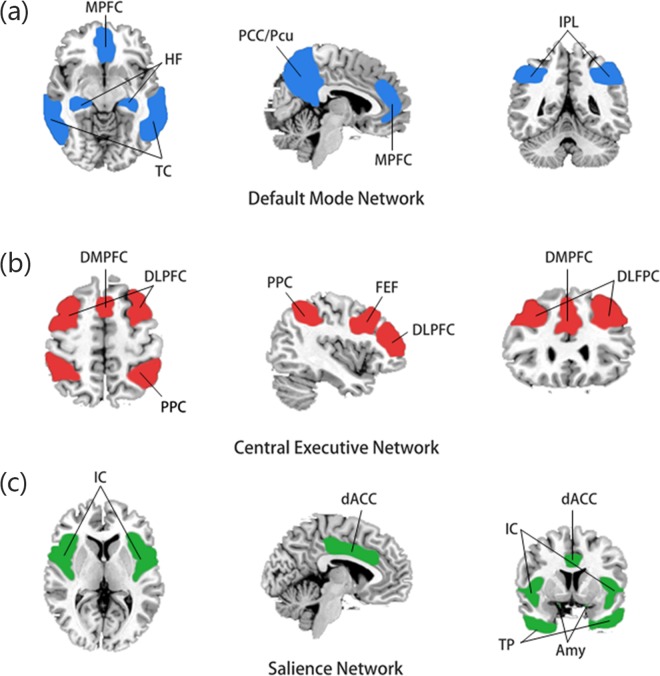
Functional connectivity is defined as the temporal correlation of multiple brain regions. Each brain region, defined as a node, is connected, eventually forming a brain network with highly complex and concentration. These brain networks play an important role in cognitive and emotional processing (Park & Friston, 2013). In 2011, Menon (Menon, 2011) proposed the “triple network model” theory, which concluded that abnormal functional connectivity of the default mode network (DMN), the central execution network (CEN), and the salience network (SN) (Fig. 2) is closely related to various mental illnesses, including depression. Not only that, by examining abnormalities in brain network functional connectivity in 711 depressed patients, Drysdale et al. (2017) defined four neurophysiological subtypes of depression and to some extent, successfully predicted their rTMS treatment effect.
Figure 2. Components of the triple network model.
(A) The default mode network is mainly composed of the medial prefrontal cortex (MPFC) and posterior cingulate cortex/precuneus (PCC/PCu), and the temporal cortex (TC), hippocampus formation (HF) and inferior parietal lobule (IPL) are also closely related to this network. (B) The central executive network (CEN) is mainly composed of the dorsolateral prefrontal cortex (DLPFC) and posterior parietal cortex (PPC), dorsolateral prefrontal cortex (DMPFC) and frontal eye field (FEF). (C) The salience network is composed of the insular cortex (IC), dorsal anterior cingulate cortex (dACC), temporal pole (TP) and amygdala (Amy).
The most common methods for studying brain function connectivity include seed-based correlation analysis (SCA) and independent component analysis (ICA). SCA predetermines “seed” (a region of interest) based on previous assumptions, and calculates the correlation with the other voxels or specific other regions of the brain by their BOLD signal fluctuations. In contrast, ICA uses all available data in the fMRI image and decomposes it several independent components. An increase in functional connectivity represents increased synchronization between the two regions.
Default mode network
The DMN consists mainly of the medial prefrontal cortex, posterior cingulate cortex/precuneus, and inferior parietal lobule (Raichle, 2015). Also, the hippocampal formation and the temporal cortex are thought to be closely related to the DMN (Raichle, 2015). The default network is usually active when a person is at rest, immersed in self-reflection, memory, and imagining the future. In some studies, the default mode network can be subdivided into an anterior sub-network and a posterior sub-network.
The default mode network is currently the most commonly studied brain network for depression. So far, one of the most consistent conclusions is that the connectivity of multiple nodes in the default mode network of depression patients is abnormally increased (Posner et al., 2013), and is considered to be closely related to the patient’s rumination symptom (Hamilton et al., 2015). Moreover, after treatment with antidepressants, the functional connectivity abnormality in the posterior network was restored, but the anterior connectivity abnormalities persisted (Li et al., 2013), and it is believed that the latter may be an important cause of higher recurrence rate of depression. And this “dissociative pattern” is also found in a study by Guo et al. (2014), in which it was suggested increased network homogeneity in the anterior DMN but decreased in the posterior one. In addition, many studies (Connolly et al., 2013; Greicius et al., 2007) found that though anterior cingulate cortex is not the central node of default mode network in healthy controls, at resting state, functional connectivity between anterior cingulate cortex and other nodes within default mode network is significantly enhanced in depressed patients. And this “over-recruitment” feature indicates that the anterior cingulate cortex of the depressed patients may be abnormally involved in the default mode network, which is considered to be unique to major depressive disorder (Menon, 2011). Moreover, children at familial risk for depression also exhibited greater functional connectivity between the default mode network and subgenual anterior cingulate cortex (Chai et al., 2016), suggesting that the abnormal default mode network connectivity may have occurred early in the onset of illness.
Central executive network
The CEN, also known as the executive control network, consists mainly of the dorsolateral prefrontal cortex, the dorsal anterior cingulate cortex, the posterior parietal cortex, and the frontal eye field and plays a role in working memory, problem-solving, goal-oriented behavior and decision-making.
Compared with the control group, the functional connectivity between the central executive network and the default mode network of MDD patients is decreased, while the functional connectivity with the salience network is increased, which might be related to the rumination (Jiang et al., 2017b). In addition, the internal connectivity within the central executive network of MDD patients is also decreased than that of the control group, and, in particular, the dorsolateral prefrontal cortex showed the most significant decline in functional connectivity with other nodes in the network (Liston et al., 2014), which is considered to be closely related to the patients’ depression symptoms and maladaptive mood regulation (Alexopoulos et al., 2012).
Salience network
The SN consists of the insular cortex, dorsal anterior cingulate cortex, temporal pole and amygdala and is responsible for detecting and filtering stimuli, as well as in recruiting relevant functional networks (Menon & Uddin, 2010). Completing a variety of complex functions, including communication, social behavior, and self-awareness.
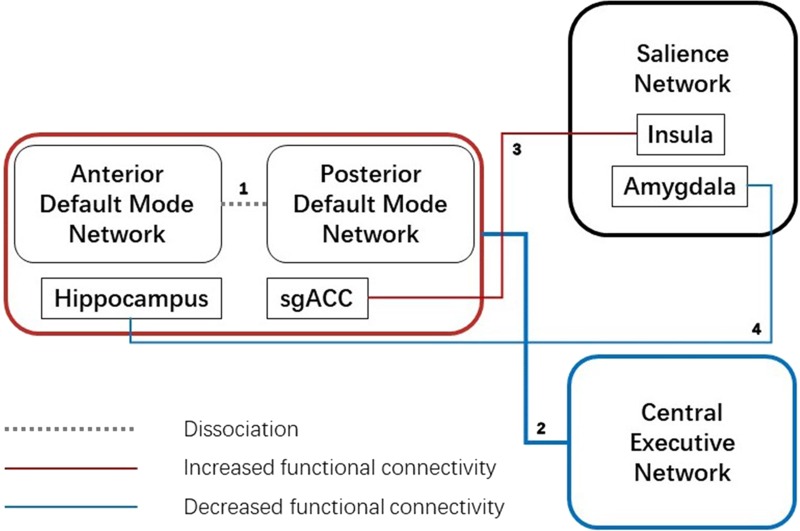
Abnormal salience network connectivity is considered to be one of the crucial links in the pathogenesis of depression, especially in the insula and amygdala. Salience network, especially the right anterior insula, is thought to be critical in the transition from the central execution network’s dominant “execution state”. to the default state-preferred “default state” (Goulden et al., 2014). A study (Connolly et al., 2013) found elevated connectivity between the subgenual anterior cingulate cortex and insula, which may result in enhanced functional connectivity between the default mode network and the salience network, thus hindering the above transition (Fig. 3).
Figure 3. Aberrant functional connectivity between three networks.
1. Dissociation between anterior and posterior default mode network; 2. Decreased functional connectivity between the default mode network and central executive network; 3. Increased functional connectivity between sgACC (subgenual anterior cingulate cortex), which is “over-recruited” in default mode network, and insula, a key node in the salience network; 4. Decreased functional connectivity between the hippocampus, which is functionally closely related to the default mode network, and amygdala, another vital node in the salience network.
Another important anomaly node in the salience network is the amygdala. In adults and adolescents with depression and children at high risk of depression, the functional connectivity between the amygdala and the hippocampus is found to be decreased (Cullen et al., 2014; Luking et al., 2011; Zeng et al., 2012), while hyperactivity in the amygdala, as found in brain activity studies (Liao et al., 2012; Tao et al., 2012), is considered to be a compensation mechanism for this weak functional connectivity. Also, increased functional connectivity between the amygdala and subgenual anterior cingulate cortex is thought to be associated with long-term negative emotions in patients (Davey et al., 2015). Moreover, functional connectivity between the amygdala and the brainstem and precuneus are reduced in depressed patients compared with controls (Zhang & Li, 2012).
Conclusion
In summary, neuroimaging studies have shown that depression involves multiple brain regions with structural and functional abnormalities, most of which are related to the limbic system, the default mode network, the central execution network, and the salience network. Together, they caused a variety of clinical symptoms of depression. Among them, atrophy and abnormal activity of parahippocampal gyrus and hippocampus led to patients’ positive memory recall disorder, which may further lead to anhedonia. Negative emotions and exaggerated responses to negative stimuli and degrading life events were mainly related to amygdala activity abnormality. While the decrease in the performance of cognitive processing and working memory is mainly related to the decrease in CEN functional connection, the abnormality of SN is mainly due to the abnormal adjustment of functional balance between DMN and CEN. However, due to the low consistency and reproducibility of the study results and the lack of clinical specificity at the individual level, the above examination methods have not been widely used in clinical diagnosis. Future research needs to enhance the homogeneity of the sample and obtain more data from patients of different age groups, different symptoms and related diseases to obtain highly specific results. It is also worthwhile to look for the commonality of the brain structure and/or brain function of patients in various subgroups and to find the best treatment.
Supplemental Information
* prospective study
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Lisong Dai conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Hongmei Zhou performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Xiangyang Xu conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Zhentao Zuo performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
Subject information and study results of depression-related MRI studies included in this paper are available as a Supplemental File.
References
- Alexopoulos et al. (2012).Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. Journal of Affective Disorders. 2012;139:56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos et al. (1997).Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Archives of General Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- Arnone et al. (2012).Arnone D, McIntosh AM, Ebmeier KP, Munafo MR, Anderson IM. Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. European Neuropsychopharmacology. 2012;22:1–16. doi: 10.1016/j.euroneuro.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Basser, Mattiello & LeBihan (1994).Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal et al. (1995).Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bludau et al. (2016).Bludau S, Bzdok D, Gruber O, Kohn N, Riedl V, Sorg C, Palomero-Gallagher N, Muller VI, Hoffstaedter F, Amunts K, Eickhoff SB. Medial prefrontal aberrations in major depressive disorder revealed by cytoarchitectonically informed voxel-based morphometry. American Journal of Psychiatry. 2016;173:291–298. doi: 10.1176/appi.ajp.2015.15030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bludau et al. (2014).Bludau S, Eickhoff SB, Mohlberg H, Caspers S, Laird AR, Fox PT, Schleicher A, Zilles K, Amunts K. Cytoarchitecture, probability maps and functions of the human frontal pole. NeuroImage. 2014;93(Pt 2):260–275. doi: 10.1016/j.neuroimage.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato et al. (2017).Bortolato B, Hyphantis TN, Valpione S, Perini G, Maes M, Morris G, Kubera M, Kohler CA, Fernandes BS, Stubbs B, Pavlidis N, Carvalho AF. Depression in cancer: the many biobehavioral pathways driving tumor progression. Cancer Treatment Reviews. 2017;52:58–70. doi: 10.1016/j.ctrv.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Buddeke et al. (2017).Buddeke J, Kooistra M, Zuithoff NP, Gerritsen L, Biessels GJ, Van der Graaf Y, Geerlings MI, Group SS. Hippocampal volume and the course of depressive symptoms over eight years of follow-up. Acta Psychiatrica Scandinavica. 2017;135:78–86. doi: 10.1111/acps.12662. [DOI] [PubMed] [Google Scholar]
- Chai et al. (2016).Chai XJ, Hirshfeld-Becker D, Biederman J, Uchida M, Doehrmann O, Leonard JA, Salvatore J, Kenworthy T, Brown A, Kagan E, De Los Angeles C, Gabrieli JDE, Whitfield-Gabrieli S. Altered intrinsic functional brain architecture in children at familial risk of major depression. Biological Psychiatry. 2016;80:849–858. doi: 10.1016/j.biopsych.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole et al. (2012).Cole J, Chaddock CA, Farmer AE, Aitchison KJ, Simmons A, McGuffin P, Fu CH. White matter abnormalities and illness severity in major depressive disorder. British Journal of Psychiatry. 2012;201:33–39. doi: 10.1192/bjp.bp.111.100594. [DOI] [PubMed] [Google Scholar]
- Cole et al. (2011).Cole J, Costafreda SG, McGuffin P, Fu CH. Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. Journal of Affective Disorders. 2011;134:483–487. doi: 10.1016/j.jad.2011.05.057. [DOI] [PubMed] [Google Scholar]
- Connolly et al. (2013).Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S, Frank G, Hendren R, Max JE, Paulus MP, Tapert SF, Banerjee D, Simmons AN, Yang TT. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biological Psychiatry. 2013;74:898–907. doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen et al. (2014).Cullen KR, Westlund MK, Klimes-Dougan B, Mueller BA, Houri A, Eberly LE, Lim KO. Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry. 2014;71:1138–1147. doi: 10.1001/jamapsychiatry.2014.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey et al. (2015).Davey CG, Whittle S, Harrison BJ, Simmons JG, Byrne ML, Schwartz OS, Allen NB. Functional brain-imaging correlates of negative affectivity and the onset of first-episode depression. Psychological Medicine. 2015;45:1001–1009. doi: 10.1017/S0033291714002001. [DOI] [PubMed] [Google Scholar]
- De Diego-Adelino et al. (2014).De Diego-Adelino J, Pires P, Gomez-Anson B, Serra-Blasco M, Vives-Gilabert Y, Puigdemont D, Martin-Blanco A, Alvarez E, Perez V, Portella MJ. Microstructural white-matter abnormalities associated with treatment resistance, severity and duration of illness in major depression. Psychological Medicine. 2014;44:1171–1182. doi: 10.1017/S003329171300158X. [DOI] [PubMed] [Google Scholar]
- De Kwaasteniet et al. (2013).De Kwaasteniet B, Ruhe E, Caan M, Rive M, Olabarriaga S, Groefsema M, Heesink L, Van Wingen G, Denys D. Relation between structural and functional connectivity in major depressive disorder. Biological Psychiatry. 2013;74:40–47. doi: 10.1016/j.biopsych.2012.12.024. [DOI] [PubMed] [Google Scholar]
- Den Heijer et al. (2011).Den Heijer T, Tiemeier H, Luijendijk HJ, Van der Lijn F, Koudstaal PJ, Hofman A, Breteler MM. A study of the bidirectional association between hippocampal volume on magnetic resonance imaging and depression in the elderly. Biological Psychiatry. 2011;70:191–197. doi: 10.1016/j.biopsych.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Drysdale et al. (2017).Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, Fetcho RN, Zebley B, Oathes DJ, Etkin A, Schatzberg AF, Sudheimer K, Keller J, Mayberg HS, Gunning FM, Alexopoulos GS, Fox MD, Pascual-Leone A, Voss HU, Casey BJ, Dubin MJ, Liston C. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nature Medicine. 2017;23:28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields (2008).Fields RD. White matter in learning, cognition and psychiatric disorders. Trends in Neurosciences. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseka, MacQueen & Kennedy (2018).Fonseka TM, MacQueen GM, Kennedy SH. Neuroimaging biomarkers as predictors of treatment outcome in major depressive disorder. Journal of Affective Disorders. 2018;233:21–35. doi: 10.1016/j.jad.2017.10.049. [DOI] [PubMed] [Google Scholar]
- Global Burden of Disease Study (2015).Global Burden of Disease Study C Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulden et al. (2014).Goulden N, Khusnulina A, Davis NJ, Bracewell RM, Bokde AL, McNulty JP, Mullins PG. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. NeuroImage. 2014;99:180–190. doi: 10.1016/j.neuroimage.2014.05.052. [DOI] [PubMed] [Google Scholar]
- Greicius et al. (2007).Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve et al. (2013).Grieve SM, Korgaonkar MS, Koslow SH, Gordon E, Williams LM. Widespread reductions in gray matter volume in depression. NeuroImage: Clinical. 2013;3:332–339. doi: 10.1016/j.nicl.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo et al. (2014).Guo W, Liu F, Zhang J, Zhang Z, Yu L, Liu J, Chen H, Xiao C. Abnormal default-mode network homogeneity in first-episode, drug-naive major depressive disorder. PLOS ONE. 2014;9:e91102. doi: 10.1371/journal.pone.0091102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton et al. (2012).Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. American Journal of Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton et al. (2015).Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biological Psychiatry. 2015;78:224–230. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen (2018).Hammen C. Risk factors for depression: an autobiographical review. Annual Review of Clinical Psychology. 2018;14:1–28. doi: 10.1146/annurev-clinpsy-050817-084811. [DOI] [PubMed] [Google Scholar]
- Han et al. (2014).Han KM, Choi S, Jung J, Na KS, Yoon HK, Lee MS, Ham BJ. Cortical thickness, cortical and subcortical volume, and white matter integrity in patients with their first episode of major depression. Journal of Affective Disorders. 2014;155:42–48. doi: 10.1016/j.jad.2013.10.021. [DOI] [PubMed] [Google Scholar]
- Hare et al. (2014).Hare DL, Toukhsati SR, Johansson P, Jaarsma T. Depression and cardiovascular disease: a clinical review. European Heart Journal. 2014;35:1365–1372. doi: 10.1093/eurheartj/eht462. [DOI] [PubMed] [Google Scholar]
- Henderson et al. (2013).Henderson SE, Johnson AR, Vallejo AI, Katz L, Wong E, Gabbay V. A preliminary study of white matter in adolescent depression: relationships with illness severity, anhedonia, and irritability. Frontiers in Psychiatry. 2013;4:152. doi: 10.3389/fpsyt.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes & Pizzagalli (2008).Holmes AJ, Pizzagalli DA. Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Archives of General Psychiatry. 2008;65:179–188. doi: 10.1001/archgenpsychiatry.2007.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang et al. (2017b).Jiang Y, Duan M, Chen X, Chang X, He H, Li Y, Luo C, Yao D. Common and distinct dysfunctional patterns contribute to triple network model in schizophrenia and depression: a preliminary study. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2017b;79:302–310. doi: 10.1016/j.pnpbp.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Jiang et al. (2017a).Jiang J, Zhao YJ, Hu XY, Du MY, Chen ZQ, Wu M, Li KM, Zhu HY, Kumar P, Gong QY. Microstructural brain abnormalities in medication-free patients with major depressive disorder: a systematic review and meta-analysis of diffusion tensor imaging. Journal of Psychiatry and Neuroscience. 2017a;42:150–163. doi: 10.1503/jpn.150341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kales, Maixner & Mellow (2005).Kales HC, Maixner DF, Mellow AM. Cerebrovascular disease and late-life depression. The American Journal of Geriatric Psychiatry. 2005;13:88–98. doi: 10.1176/appi.ajgp.13.2.88. [DOI] [PubMed] [Google Scholar]
- Keren et al. (2018).Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, Pan PM, Meffert L, Kaiser A, Wolke S, Pine DS, Stringaris A. Reward processing in depression: a conceptual and meta-analytic review across fMRI and EEG studies. American Journal of Psychiatry. 2018;175:1111–1120. doi: 10.1176/appi.ajp.2018.17101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgaonkar et al. (2014).Korgaonkar MS, Fornito A, Williams LM, Grieve SM. Abnormal structural networks characterize major depressive disorder: a connectome analysis. Biological Psychiatry. 2014;76:567–574. doi: 10.1016/j.biopsych.2014.02.018. [DOI] [PubMed] [Google Scholar]
- Kuhn & Gallinat (2013).Kuhn S, Gallinat J. Resting-state brain activity in schizophrenia and major depression: a quantitative meta-analysis. Schizophrenia Bulletin. 2013;39:358–365. doi: 10.1093/schbul/sbr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2013).Li B, Liu L, Friston KJ, Shen H, Wang L, Zeng LL, Hu D. A treatment-resistant default mode subnetwork in major depression. Biological Psychiatry. 2013;74:48–54. doi: 10.1016/j.biopsych.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Liao et al. (2012).Liao C, Feng Z, Zhou D, Dai Q, Xie B, Ji B, Wang X, Wang X. Dysfunction of fronto-limbic brain circuitry in depression. Neuroscience. 2012;201:231–238. doi: 10.1016/j.neuroscience.2011.10.053. [DOI] [PubMed] [Google Scholar]
- Liao et al. (2013).Liao Y, Huang X, Wu Q, Yang C, Kuang W, Du M, Lui S, Yue Q, Chan RC, Kemp GJ, Gong Q. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. Journal of Psychiatry and Neuroscience. 2013;38:49–56. doi: 10.1503/jpn.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston et al. (2014).Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, Voss HU, Casey BJ, Etkin A, Dubin MJ. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biological Psychiatry. 2014;76:517–526. doi: 10.1016/j.biopsych.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu et al. (2016).Lu Y, Liang H, Han D, Mo Y, Li Z, Cheng Y, Xu X, Shen Z, Tan C, Zhao W, Zhu Y, Sun X. The volumetric and shape changes of the putamen and thalamus in first episode, untreated major depressive disorder. NeuroImage: Clinical. 2016;11:658–666. doi: 10.1016/j.nicl.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking et al. (2011).Luking KR, Repovs G, Belden AC, Gaffrey MS, Botteron KN, Luby JL, Barch DM. Functional connectivity of the amygdala in early-childhood-onset depression. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:1027–1041. doi: 10.1016/j.jaac.2011.07.019. e1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon (2011).Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon & Uddin (2010).Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller et al. (2015).Miller CH, Hamilton JP, Sacchet MD, Gotlib IH. Meta-analysis of functional neuroimaging of major depressive disorder in youth. JAMA Psychiatry. 2015;72:1045–1053. doi: 10.1001/jamapsychiatry.2015.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller et al. (2017).Muller VI, Cieslik EC, Serbanescu I, Laird AR, Fox PT, Eickhoff SB. Altered brain activity in unipolar depression revisited: meta-analyses of neuroimaging studies. JAMA Psychiatry. 2017;74:47–55. doi: 10.1001/jamapsychiatry.2016.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet et al. (2014).Olvet DM, Peruzzo D, Thapa-Chhetry B, Sublette ME, Sullivan GM, Oquendo MA, Mann JJ, Parsey RV. A diffusion tensor imaging study of suicide attempters. Journal of Psychiatric Research. 2014;51:60–67. doi: 10.1016/j.jpsychires.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park & Friston (2013).Park HJ, Friston K. Structural and functional brain networks: from connections to cognition. Science. 2013;342(6158):1238411. doi: 10.1126/science.1238411. [DOI] [PubMed] [Google Scholar]
- Park et al. (2015).Park JH, Lee SB, Lee JJ, Yoon JC, Han JW, Kim TH, Jeong HG, Newhouse PA, Taylor WD, Kim JH, Woo JI, Kim KW. Epidemiology of MRI-defined vascular depression: a longitudinal, community-based study in Korean elders. Journal of Affective Disorders. 2015;180:200–206. doi: 10.1016/j.jad.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Posner et al. (2013).Posner J, Hellerstein DJ, Gat I, Mechling A, Klahr K, Wang Z, McGrath PJ, Stewart JW, Peterson BS. Antidepressants normalize the default mode network in patients with dysthymia. JAMA Psychiatry. 2013;70:373–382. doi: 10.1001/jamapsychiatry.2013.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle (2015).Raichle ME. The brain’s default mode network. Annual Review of Neuroscience. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Reddick et al. (2006).Reddick WE, Shan ZY, Glass JO, Helton S, Xiong X, Wu S, Bonner MJ, Howard SC, Christensen R, Khan RB, Pui CH, Mulhern RK. Smaller white-matter volumes are associated with larger deficits in attention and learning among long-term survivors of acute lymphoblastic leukemia. Cancer. 2006;106:941–949. doi: 10.1002/cncr.21679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppermund et al. (2014).Reppermund S, Zhuang L, Wen W, Slavin MJ, Trollor JN, Brodaty H, Sachdev PS. White matter integrity and late-life depression in community-dwelling individuals: diffusion tensor imaging study using tract-based spatial statistics. British Journal of Psychiatry. 2014;205:315–320. doi: 10.1192/bjp.bp.113.142109. [DOI] [PubMed] [Google Scholar]
- Réus et al. (2019).Réus GZ, Carlessi AS, Silva RH, Ceretta LB, Quevedo J. Relationship of oxidative stress as a link between diabetes mellitus and major depressive disorder. Oxidative Medicine and Cellular Longevity. 2019;2019:8637970. doi: 10.1155/2019/8637970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roddy et al. (2019).Roddy DW, Farrell C, Doolin K, Roman E, Tozzi L, Frodl T, O’Keane V, O’Hanlon E. The hippocampus in depression: more than the sum of its parts? Advanced hippocampal substructure segmentation in depression. Biological Psychiatry. 2019;85:487–497. doi: 10.1016/j.biopsych.2018.08.021. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Cano et al. (2014).Rodriguez-Cano E, Sarro S, Monte GC, Maristany T, Salvador R, McKenna PJ, Pomarol-Clotet E. Evidence for structural and functional abnormality in the subgenual anterior cingulate cortex in major depressive disorder. Psychological Medicine. 2014;44:3263–3273. doi: 10.1017/S0033291714000841. [DOI] [PubMed] [Google Scholar]
- Rogers & De Brito (2016).Rogers JC, De Brito SA. Cortical and subcortical gray matter volume in youths with conduct problems: a meta-analysis. JAMA Psychiatry. 2016;73:64–72. doi: 10.1001/jamapsychiatry.2015.2423. [DOI] [PubMed] [Google Scholar]
- Sheline et al. (2010).Sheline YI, Pieper CF, Barch DM, Welsh-Bohmer K, McKinstry RC, MacFall JR, D’Angelo G, Garcia KS, Gersing K, Wilkins C, Taylor W, Steffens DC, Krishnan RR, Doraiswamy PM. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Archives of General Psychiatry. 2010;67:277–285. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng et al. (2017).Sheng J, Liu S, Wang Y, Cui R, Zhang X. The link between depression and chronic pain: neural mechanisms in the brain. Neural Plasticity. 2017;2017:9724371. doi: 10.1155/2017/9724371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith (2014).Smith K. Mental health: a world of depression. Nature. 2014;515:180–181. doi: 10.1038/515180. [DOI] [PubMed] [Google Scholar]
- Smith et al. (2006).Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Suslow et al. (2010).Suslow T, Konrad C, Kugel H, Rumstadt D, Zwitserlood P, Schoning S, Ohrmann P, Bauer J, Pyka M, Kersting A, Arolt V, Heindel W, Dannlowski U. Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biological Psychiatry. 2010;67:155–160. doi: 10.1016/j.biopsych.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Takamura et al. (2017).Takamura M, Okamoto Y, Okada G, Toki S, Yamamoto T, Ichikawa N, Mori A, Minagawa H, Takaishi Y, Fujii Y, Kaichi Y, Akiyama Y, Awai K, Yamawaki S. Patients with major depressive disorder exhibit reduced reward size coding in the striatum. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2017;79:317–323. doi: 10.1016/j.pnpbp.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Tao et al. (2012).Tao R, Calley CS, Hart J, Mayes TL, Nakonezny PA, Lu H, Kennard BD, Tamminga CA, Emslie GJ. Brain activity in adolescent major depressive disorder before and after fluoxetine treatment. American Journal of Psychiatry. 2012;169:381–388. doi: 10.1176/appi.ajp.2011.11040615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, Aizenstein & Alexopoulos (2013).Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Molecular Psychiatry. 2013;18:963–974. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Agtmaal et al. (2017).Van Agtmaal MJM, Houben A, Pouwer F, Stehouwer CDA, Schram MT. Association of microvascular dysfunction with late-life depression: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74:729–739. doi: 10.1001/jamapsychiatry.2017.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eijndhoven et al. (2013).Van Eijndhoven P, Van Wingen G, Katzenbauer M, Groen W, Tepest R, Fernandez G, Buitelaar J, Tendolkar I. Paralimbic cortical thickness in first-episode depression: evidence for trait-related differences in mood regulation. American Journal of Psychiatry. 2013;170:1477–1486. doi: 10.1176/appi.ajp.2013.12121504. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2014).Wang L, Leonards CO, Sterzer P, Ebinger M. White matter lesions and depression: a systematic review and meta-analysis. Journal of Psychiatric Research. 2014;56:56–64. doi: 10.1016/j.jpsychires.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Wise et al. (2017).Wise T, Radua J, Via E, Cardoner N, Abe O, Adams TM, Amico F, Cheng Y, Cole JH, De Azevedo Marques Perico C, Dickstein DP, Farrow TFD, Frodl T, Wagner G, Gotlib IH, Gruber O, Ham BJ, Job DE, Kempton MJ, Kim MJ, Koolschijn P, Malhi GS, Mataix-Cols D, McIntosh AM, Nugent AC, O’Brien JT, Pezzoli S, Phillips ML, Sachdev PS, Salvadore G, Selvaraj S, Stanfield AC, Thomas AJ, Van Tol MJ, Van der Wee NJA, Veltman DJ, Young AH, Fu CH, Cleare AJ, Arnone D. Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Molecular Psychiatry. 2017;22:1455–1463. doi: 10.1038/mp.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young et al. (2017a).Young KD, Misaki M, Harmer CJ, Victor T, Zotev V, Phillips R, Siegle GJ, Drevets WC, Bodurka J. Real-time functional magnetic resonance imaging amygdala neurofeedback changes positive information processing in major depressive disorder. Biological Psychiatry. 2017a;82:578–586. doi: 10.1016/j.biopsych.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young et al. (2017b).Young KD, Siegle GJ, Zotev V, Phillips R, Misaki M, Yuan H, Drevets WC, Bodurka J. Randomized clinical trial of real-time fMRI amygdala neurofeedback for major depressive disorder: effects on symptoms and autobiographical memory recall. American Journal of Psychiatry. 2017b;174:748–755. doi: 10.1176/appi.ajp.2017.16060637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre, Fields & Johansen-Berg (2012).Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nature Neuroscience. 2012;15:528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng et al. (2012).Zeng LL, Shen H, Liu L, Wang L, Li B, Fang P, Zhou Z, Li Y, Hu D. Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain. 2012;135:1498–1507. doi: 10.1093/brain/aws059. [DOI] [PubMed] [Google Scholar]
- Zhang & Li (2012).Zhang S, Li CS. Functional connectivity mapping of the human precuneus by resting state fMRI. NeuroImage. 2012;59:3548–3562. doi: 10.1016/j.neuroimage.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2016).Zhang H, Li L, Wu M, Chen Z, Hu X, Chen Y, Zhu H, Jia Z, Gong Q. Brain gray matter alterations in first episodes of depression: a meta-analysis of whole-brain studies. Neuroscience & Biobehavioral Reviews. 2016;60:43–50. doi: 10.1016/j.neubiorev.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Zhao et al. (2014).Zhao YJ, Du MY, Huang XQ, Lui S, Chen ZQ, Liu J, Luo Y, Wang XL, Kemp GJ, Gong QY. Brain grey matter abnormalities in medication-free patients with major depressive disorder: a meta-analysis. Psychological Medicine. 2014;44:2927–2937. doi: 10.1017/S0033291714000518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
* prospective study
Data Availability Statement
The following information was supplied regarding data availability:
Subject information and study results of depression-related MRI studies included in this paper are available as a Supplemental File.