Abstract
The immune monocyte/phagocyte system (MPS) includes numerous subsets of cell in the myeloid lineage including monocyte, macrophage, and dendritic cell (DC) populations that are heterogeneous both phenotypically and functionally. Previously, we characterized these diverse MPS phenotypes with multi-parametric mass cytometry (CyTOF). In order to expansively characterize monocytes, macrophages, and dendritic cells, a CyTOF panel was designed to measure 35 identity-, activation-, and polarization- markers. Here we provide a protocol to define a reference map for the myeloid compartment, including sample preparation, to produce reference cell subsets from the monocyte/phagocyte system, in particular monocyte-derived macrophages further polarized in vitro with cytokine stimulation (i.e. M-CSF, GM-CSF, IL-4, IL-10, IFNγ, and LPS), monocyte-derived DCs, and myeloid-derived suppressor cells (MDSCs), generated in vitro from human bone marrow and/or peripheral blood.
Keywords: Mass cytometry, macrophage polarization, dendritic cells, myeloid-derived suppressor cells, myeloid regulatory cells
1. Introduction
The monocyte/phagocyte system (MPS) is a complex cellular compartment that includes phenotypically and functionally heterogeneous populations of cells, including monocytes, macrophages (MΦ), and dendritic cells (DC) [1]. A distinct phenotypic definition of myeloid cells remains contentious due to a number of factors. For instance, a lack of consistency between cellular expression of markers was first identified in mice and correlates on human myeloid cells (e.g., while murine macrophages are defined as F4/80high, the human F4/80 homolog is expressed on eosinophils). Further, many of the markers of interest expressed on human myeloid cells (e.g., CD14, CD11b, CD33, HLA-DR, CD64) are shared between various myeloid cell subsets and none are lineage specific. Moreover, myeloid cells are highly plastic with respect to phenotype and function and depend upon various environmental signals for differentiation and/or polarization. This complexity of phenotypic definition is highlighted by the growing literature on monocyte, DC, and macrophage nomenclature [1, 2, 3, 4, 5].
At the protein level, characterization of these heterogeneous cell types has been largely accomplished with “low resolution” approaches (e.g., morphological evaluation and immunohistochemistry), wherein only one or a few proteins were used to identify populations. As an example, CD68 and CD163 are frequently proposed to characterize macrophage subtypes. High-resolution approaches such as mass cytometry (also known as cytometry by time-of-flight, or CyTOF) are invaluable tools that will advance our understanding of cellular diversity and function, and identify potential targets for novel therapies [6, 7, 8]. CyTOF combined with high-dimensional analysis, in particular visualization of t-distributed stochastic neighbor embedding (viSNE), spanning-tree progression analysis of density-normalized events (SPADE), and marker enrichment modeling (MEM), are robust methods to identify numerous and novel subsets from heterogeneous populations [9, 10, 11, 12, 13, 14, 15].
During the last months, myeloid cell subsets were defined in humans within various healthy and tumor tissues. Beside definition of DC- [16, 17] or monocyte-subsets [18, 19], large numbers of tumor-associated macrophage (TAM) phenotypes were revealed in renal cell carcinoma (17 TAM phenotypes were distinguished) and lung adenocarcinoma [20, 21]. Notably, mass cytometry definition of myeloid cells subsets was also linked to patient outcomes, in particular, in stage IV melanoma monocytes (CD14posCD16negHLA-DRhigh) were predictive in response to anti-PD-1 immunotherapy [22]. Ex vivo preparation of myeloid counterparts might help to guide the high-dimensional analysis and to define a reference cartography (Fig. 1) of myeloid heterogeneity [23].
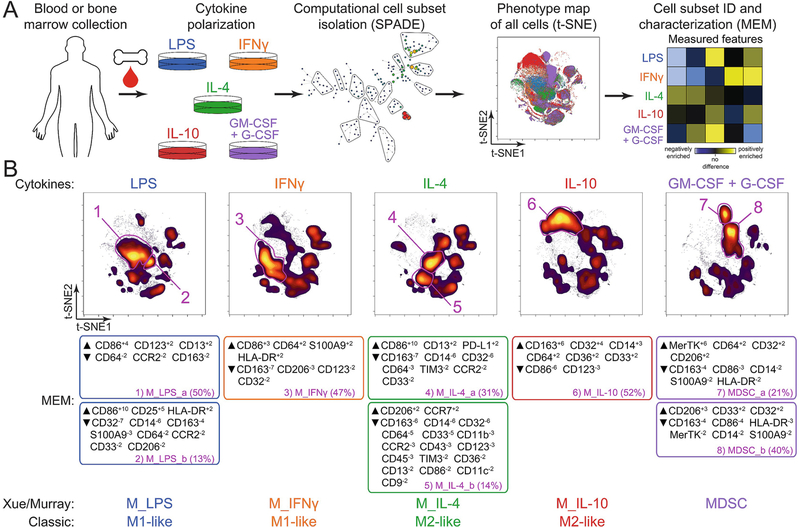
Figure 1.
Single-cell analysis platform for interrogation of human myeloid cell polarization phenotypes. (a) Patient-derived tissue (human blood or bone marrow) can be single-cell dissociated and cultured in a variety of cytokine stimulation conditions (LPS, IFNγ, IL-4, IL-10, GM-CSF/G-CSF) for various lengths of time in order to polarize myeloid populations into distinct phenotypes. Polarized cell populations may then be computationally isolated using minimum spanning tree algorithms (SPADE) and phenotypically identified using t-SNE and Marker Enrichment Modeling analysis. (b) viSNE maps (top) of polarized myeloid populations reveal distinct subsets of myeloid populations after cytokine stimulation, correlating with enrichment of phenotypic markers (MEM labels; middle) correlating to classically defined populations of macrophages or myeloid-derived suppressor cells (bottom)
2. Materials
2.1. In Vitro Polarization of Monocyte-Derived DC, MΦ, and MDSC
A source of human monocytes (see Note 1 ).
Six-well tissue-culture-treated plates.
Tissue culture media (RPMI-1640) with 10% FBS.
Percoll or Ficoll leukocyte isolation media.
Cytokines, growth factors, and stimulants: M-CSF, GM-CSF, IL-4, IL-10, LPS, IFNγ, TNFα, PGE2, Pam3CSK4 (a Toll-like receptor 2 agonist).
2.2. Antibody Staining and Mass Cytometry
Metal isotope-conjugated antibodies (see Note 2 ).
Staining buffer (PBS + BSA 0.5%).
Phosphate buffered saline (PBS).
Viability marker: e.g., cisplatin Cell-ID™ (Fluidigm).
Paraformaldehyde (PFA): 16%.
Ice-cold methanol at −20 °C.
Double-distilled water (ddH2O).
Cell intercalator dye (e.g., Iridium; Cell-ID™ Intercalator-Ir, Fluidigm).
CyTOF Calibration Beads (Fluidigm).
Mass cytometer (Fluidigm).
2.3. Data Analysis
Software for gating.
A pipeline for high-dimensional analysis (e.g., Cytobank, Cytofkit, or “R” algorithms).
3. Methods
The purpose of the protocol is to derive DC, MDSC, and MΦ populations from peripheral human monocytes and then to polarize these baseline MΦ. These polarization schema were inspired by Xue et al. [24].
3.1. Prepare Blood-Derived Monocytes
Prepare a Buffy coat by pooling at least 40 mL of human peripheral blood (see Note 3 ).
Centrifuge for 15 min at 600 g with no brake.
Decant plasma (see Note 4 ) and resuspend the buffy coat by 4× with RPMI + 10% FBS.
Add 1× Ficoll and overlay with diluted buffy coat.
Proceed with Ficoll gradient separation by centrifuging at 400 g for 30 min.
3.2. Monocyte Separation by Plastic Adherence (Optional, See Note 1 )
Use 6-well, tissue-culture-treated plates (surface 9.5 cm2).
After Ficoll separation, resuspend PBMC at 1 × 106 cells/mL in serum-free RPMI.
Add 2 mL of the suspension into 6-well plates; prepare wells for various conditions including Monocyte, DC, Macrophage baseline, Macrophage Unstimulated, and as many wells as the chosen stimulation conditions (in replicate if possible due to potential cell mortality).
Incubate at 37 °C, 5% CO2 for a minimum of 3 h.
Gently shake and discard non-adherent cells by washing twice with pre-warmed culture medium.
Collect only the cellular fraction of the wells labeled “Monocyte” after 5 min of Accutase treatment. For the other wells, start the differentiation as described in Subheading 3.3.
Discard the media (and freeze the supernatant as control).
Wash twice with 1 mL of PBS and decant.
Add 250 μL of Accutase pre-warmed at 37 °C.
Incubate and watch every 30 s (max 2 min).
Add 2 mL of media.
Pipette and transfer into a FACS tube, then begin Subheading 3.4 for the staining.
3.3. In Vitro Monocyte-Derived DC, MDSC, and MΦ Polarization
3.3.1. Polarization of Dendritic Cells
Add RPMI-1640 supplemented with 10% FBS and 1% PenStrep solution (1.5–2 × 106 cells/mL, 3 mL/well) to the “DC” well(s).
Add 800 IU/mL rhGM-CSF (40 ng/mL) and 500 IU/mL rhIL4 (40 ng/mL).
Incubate at 37 °C, 5% CO2 for 3 days.
At day 3: Change the medium (centrifuge any floating cells).
At day 6: For terminal moDC maturation, add rhTNF (800 IU/mL or 10 ng/mL) for 48 h.
At day 8: Collect the cellular fraction in the wells after Accutase treatment (after maturation of some adherent cells).
Discard the media (freeze supernatant if desired).
Wash with 1 mL of PBS and decant.
Add 250 μL of Accutase pre-warmed at 37 °C.
Place in 37 °C in incubator and watch every 30 s (max 2 min).
Add 2 mL of media.
Wash in PBS, and proceed to staining (Subheading 3.4).
3.3.2. Generation of MDSC (See Notes 5 and 6 )
Add RPMI-1640 supplemented with 10% FBS and 1% PenStrep solution (1.5–2 × 106 cells/mL, 3 mL/well) to the “MDSC” well(s).
Add rhGM-CSF (40 ng/mL) and rhG-CSF (40 ng/mL) and/or rhGM-CSF (40 ng/mL) and rhIL-6 (40 ng/mL).
Place in an incubator at 37 °C, 5% CO2 for 4 days.
At day 4: Collect the cellular fraction in the wells after Accutase treatment (after maturation of some adherent cells).
Discard the media and freeze the supernatant if desired.
Wash with 1 mL of PBS 1 mL and decant.
Add 250 μL of Accutase, pre-warmed at 37 °C.
Incubate at 37 °C and watch every 30 s (max 2 min).
Add 2 mL of RPMI culture media.
Wash in PBS, and proceed to staining (Subheading 3.4).
3.3.3. Generation of Macrophages
Add RPMI-1640 supplemented with 10% FBS and 1% PenStrep solution (1.5–2 × 106/mL, 2 mL/well) to the “macrophage” well(s).
Add 50 IU/mL rhM-CSF.
Incubate at 37 °C, 5% CO2 for 3 days.
At day 3: Follow the stimulation step (Subheading 3.3.3) for macrophages other than the “Macrophage baseline” wells.
For the Macrophage baseline well: Collect the cellular fraction in the well after Accutase treatment.
Discard the media and freeze the supernatant if so desired.
Wash once with 1 mL PBS.
Discard PBS.
Add 250 μL Accutase pre-warmed at 37 °C.
Incubate at 37 °C, 5% CO2, and watch every 30 s (max 2 min).
Add 2 mL of RMPI tissue culture media and collect the cells.
Wash wells one time in PBS and proceed to staining (Subheading 3.4).
3.3.4. Macrophage Polarization
Three days after initiating cytokine stimulation (Subheading 3.3.3), remove and freeze the supernatants.
During the following 3 days, stimulate cells with RPMI-1640 supplemented with 10% FBS and 1% PenStrep solution and Mock/Carrier, rhIFNγ (200 IU/mL), rhIL-4 (1000 IU/mL), TNF (800 IU/mL), Pam3CSK4 (P3C, 1 μg/mL), or prostaglandin E2 (PGE2, 1 μg/mL) (see Note 7 ).
At day 6 post initiation, collect the “Macrophage baseline” well after Accutase treatment.
Discard the media and freeze the supernatant if so desired.
Wash one time 1 mL PBS.
Discard PBS.
Add 250 μL Accutase pre-warmed to 37 °C.
Incubate at 37 °C, 5% CO2 and watch every 30 s (max 2 min).
Add 2 mL of media.
Wash in PBS, and proceed to staining (Subheading 3.4).
3.4. Antibody Staining and Mass Cytometry Analysis
3.4.1. Prepare Staining Cocktail (See Note 8 )
Make enough cocktail for the number of samples in PBS + BSA, plus add an extra 10% to account for pipette error. Samples can be stained in a final volume of 100 μL.
3.4.2. Live Cell Surface Stain
Transfer 30 μL of cells to a new FACS tube.
Add 80 μL of premade staining cocktail.
Vortex to mix.
Stain at room temperature for 30 min.
Wash 2× with 2 mL PBS + BSA.
Centrifuge at 200 g, for 5 min.
Repeat the staining if needed for secondary antibodies.
3.4.3. Cell Fixation
Decant the supernatant from Subheading 3.4.2.
Resuspend the cells in 200 μL PBS.
Fix by adding 25 μL of 16% PFA (for a final concentration of 1.8% PFA).
Vortex to mix.
Incubate for 10 min at room temperature.
Wash 2× with 2 mL PBS.
Centrifuge at 900 g for 5 min.
3.4.4. Cell Permeabilization
Decant supernatants from the last step in Subheading 3.4.3.
Resuspend the cells in the residual volume left after decanting by vortexing vigorously.
Add 1 mL of ice-cold methanol (−20 °C).
Vigorously vortex immediately.
Pipet as needed to break up clumps.
Incubate cells at −20 °C for at least 10 min. Cover the tube to avoid evaporation. Cells can be left overnight at −20 °C or for weeks at −80 °C.
Wash the cells 2× with 2 mL PBS.
Vortex to mix.
Centrifuge at 900 g for 5 min.
3.4.5. Intracellular Staining (If Required)
Resuspend the cells in staining media to a total volume of 40 μL.
Add 80 μL of premade staining cocktail.
Vortex to mix.
Stain for 30 min at room temperature.
Wash 2× with 2 mL PBS + BSA.
Centrifuge at 900 g for 5 min.
Repeat staining if needed for secondary antibodies.
3.4.6. Nucleic Acid Staining
Wash the samples from the previous step (Subheading 3.4.5) with 2 mL PBS + BSA.
Centrifuge at 900 g for 5 min. Decant.
Resuspend the cells in 200 μL PBS.
Add 4 μL 50× Iridium nucleic acid intercalator.
Vortex to mix.
Incubate for at least 15 min at room temperature. Cells can be left at 4 °C for several hours.
3.4.7. Running Samples on CyTOF (See Note 9 )
Wash the samples with 1 mL double deionized water.
Centrifuge at 900 g for 5 min.
Dilute the sample in 1× CyTOF calibration beads (400 μL to 1 mL according to the number of cells).
Run samples on a CyTOF cytometer according to the manufacturer’s protocol.
4 Notes
Monocytes can be obtained from buffy coats followed by cell sorting, plastic adherence, or elutriation.
Antibodies can be (1) bought from Fluidigm pre-conjugated to metal isotopes, (2) bought from another vendor and self-conjugated using the Fluidigm Maxpar conjugation kit, or (3) used in indirect staining with an anti-FITC, anti-PE, anti-APC, or anti-biotin metal-tagged antibodies.
The volume of blood or number of monocytes requested for the whole experiment depends on the number of experimental conditions and should be calculated before starting. Also take into account that a substantial number of monocytes and macrophages will adhere to the plastic dish and be lost in processing.
If molecule analyses are planned at different time points, spin the plasma at 1500 g for 10 min before aliquoting in 500 μL at −20 °C. These aliquots will constitute the reference point.
Peripheral blood or bone marrow may be used, but give rise to different suppressive myeloid cells both matching an MDSC phenotype [23, 25, 26].
Supernatant from a cell line or primary cells culture can also be used.
Make separate staining cocktails for surface and intracellular markers. Up to 4 staining cocktails might be necessary if secondary antibodies are employed in the panel. Transfer cells to new tubes for each staining cocktail so that volumes are precise (important for comparing between samples in particular for phosphoproteins).
Example data files available online: http://flowrepository.org/id/FR-FCM-Z2Z8
References
- 1.Guilliams M, Ginhoux F, Jakubzick C et al. (2014) Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol 14:571–578. 10.1038/nri3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ancuta P (2015) A slan-based nomenclature for monocytes? Blood 126:2536–2538. 10.1182/blood-2015-10-675470 [DOI] [PubMed] [Google Scholar]
- 3.Ziegler-Heitbrock L, Ancuta P, Crowe S et al. (2010) Nomenclature of monocytes and dendritic cells in blood. Blood 116:e74–e80. 10.1182/blood-2010-02-258558 [DOI] [PubMed] [Google Scholar]
- 4.Bronte V, Brandau S, Chen SH et al. (2016) Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 7:12150 10.1038/ncomms12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray PJ, Allen JE, Biswas SK et al. (2014) Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41:14–20. 10.1016/j.immuni.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engblom C, Pfirschke C, Pittet MJ (2016) The role of myeloid cells in cancer therapies. Nat Rev Cancer 16:447–462. 10.1038/nrc.2016.54 [DOI] [PubMed] [Google Scholar]
- 7.Ginhoux F, Schultze JL, Murray PJ et al. (2016) New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat Immunol 17:34–40. 10.1038/ni.3324 [DOI] [PubMed] [Google Scholar]
- 8.Greenplate AR, Johnson DB, Roussel M et al. (2016) Myelodysplastic syndrome revealed by systems immunology in a melanoma patient undergoing anti-PD-1 therapy. Cancer Immunol Res 4:474–480. 10.1158/2326-6066.CIR-15-0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bendall SC, Simonds EF, Qiu P et al. (2011) Single-cell mass Cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332:687–696. 10.1126/science.1198704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spitzer MH, Nolan GP (2016) Mass Cytometry: single cells, many features. Cell 165:780–791. 10.1016/j.cell.2016.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saeys Y, Van Gassen S, Lambrecht BN (2016) Computational flow cytometry: helping to make sense of high-dimensional immunology data. Nat Rev Immunol 16:449–462. 10.1038/nri.2016.56 [DOI] [PubMed] [Google Scholar]
- 12.Diggins KE, Greenplate AR, Leelatian N et al. (2017) Characterizing cell subsets using marker enrichment modeling. Nat Methods 14:275–278. 10.1038/nmeth.4149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amir EAD, Davis KL, Tadmor MD et al. (2013) viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat Biotechnol 31:545–552. 10.1038/nbt.2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu P, Simonds EF, Bendall SC et al. (2011) Extracting a cellular hierarchy from high-dimensional cytometry data with SPADE. Nat Biotechnol 29:886–891. 10.1038/nbt.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diggins KE, Gandelman JS, Roe CE et al. (2018) Generating quantitative cell identity labels with marker enrichment modeling (MEM). Curr Protoc Cytom 83(2018):10.21.1–10.21.28. 10.1002/cpcy.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alcántara-Hernández M, Leylek R, Wagar LE et al. (2017) High-dimensional phenotypic mapping of human dendritic cells reveals interindividual variation and tissue specialization. Immunity 47(6):1037–1050.e6. 10.1016/j.immuni.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.See P, Dutertre CA, Chen J et al. (2017) Mapping the human DC lineage through the integration of high-dimensional techniques. Science 356:eaag3009 10.1126/science.aag3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulz D, Severin Y, Zanotelli VRT et al. (2019) In-Depth Characterization of Monocyte-Derived Macrophages using a Mass Cytometry-Based Phagocytosis Assay. Scientific reports 9:1925 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6374473/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sander J, Schmidt SV, Cirovic B et al. (2017) Cellular differentiation of human monocytes is regulated by time-dependent interleukin-4 signaling and the transcriptional regulator NCOR2. Immunity 47:1051–1066.e12. 10.1016/j.immuni.2017.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chevrier S, Levine JH, Zanotelli VRT et al. (2017) An immune atlas of clear cell renal cell carcinoma. Cell 169:736–738.e18. 10.1016/j.cell.2017.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavin Y, Kobayashi S, Leader A et al. (2017) Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell 169:750–757.e15. 10.1016/j.cell.2017.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krieg C, Nowicka M, Guglietta S et al. (2018) High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat Med 9:2579–2514. 10.1038/nm.4466 [DOI] [PubMed] [Google Scholar]
- 23.Roussel M, Ferrell PB, Greenplate AR et al. (2017) Mass cytometry deep phenotyping of human mononuclear phagocytes and myeloid-derived suppressor cells from human blood and bone marrow. J Leukoc Biol 102:437–447. 10.1189/jlb.5MA1116-457R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue J, Schmidt SV, Sander J et al. (2014) Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40:274–288. 10.1016/j.immuni.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marigo I, Bosio E, Solito S et al. (2010) Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity 32:790–802. 10.1016/j.immuni.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 26.Lechner MG, Liebertz DJ, Epstein AL (2010) Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol 185:2273–2284. 10.4049/jimmunol.1000901 [DOI] [PMC free article] [PubMed] [Google Scholar]