1. Introduction
Retinal detachment (RD) is a prevalent cause of vision loss, affecting 1 in 300 individuals in the course of a lifetime. It is characterized by the separation of the outer retinal layer from the underlying retinal pigment epithelium (RPE) which compromises the photoreceptors’ nutritional and metabolic support and eventually leads to their death (Huckfeldt and Vavvas, 2013). RD can result from ocular trauma or as a complication of other retinal or systemic disease like diabetes (Ryan, 2006). Surgical repair of RD has a high rate of anatomical success; however, successful retinal reattachment is not always paralleled by good visual outcomes. Permanent visual disability still occurs despite prompt and successful surgical repair and up to 30%–40% of patients fail to obtain a final visual acuity of 20/40 or better (Hassan et al., 2002; Ross, 2002). This disparity between anatomical and functional outcomes highlights the need for understanding the mechanisms that lead to photoreceptor loss in RD and identifying molecular players that are involved in the process. Eventually this will help the goal to develop adjunctive neuroprotective strategies that reduce neurodegeneration and restore vision.
Crystallins were first identified as major structural proteins of the ocular lens and categorized into 3 distinct families: α, β, and γ (Clark et al., 1969). α-crystallin makes up more than 40% of protein in the human lens and consists of two subunits, αA- and αB, which possess nearly 55% sequence homology between them (Horwitz, 2003). Besides being structural proteins αA- and αB- crystallin belong to the family of small heat shock proteins (sHSPs) that serve as molecular chaperones (Nagaraj et al., 2016). αA- and αB- crystallin were found to be expressed in numerous non lenticular cells and tissues including the retina, brain, kidney, spleen, and skeletal muscles (Srinivasan et al., 1992). They function mainly in the cellular response to stress stimuli by modulating survival and apoptotic signals. However, more recent studies suggest other functions for α-crystallin in disease progression (Fort and Lampi, 2011).
In the retina, expression of α-crystallin has been shown to increase after retinal insult, such as light toxicity and retinal trauma, which coincides with increased levels of Bax, caspase-3, and other proteins involved in apoptosis (Vazquez-Chona et al., 2004). The functional consequence of this increase in α-crystallin is still under extensive research and might depend on the context of the disease. For example in age-related macular degeneration, upregulated α-crystallin was found to concentrate in drusen, a specific deposit that is associated with the disease and contributes to its progression (Nakata et al., 2005). In diabetes, α-crystallin upregulation is a hallmark of diabetic retinopathy and has been suggested to play a role in the pathogenesis of the disease (Kumar et al., 2005; Wang et al., 2007).
Several studies have suggested a neuroprotective function for αA-crystallin (Brownell et al., 2012). For example, overexpression of αA-crystallin has been shown to confer neuroprotection in the setting of autoimmune uveitis in mice (Rao et al., 2012). α-crystallin knock-out mice were shown to have higher rate of retinal degeneration during chemically induced hypoxia (Yaung et al., 2008). The expression of crystallins in the setting of other retinal disease like RD has not been studied yet.
In this study, we performed a proteomic analysis to identify proteins that are altered in the retina during the process of RD. We show that many crystallins are upregulated in a murine model of RD and that αA-crystallin shows the highest level of expression both on the transcriptional and translational levels. We further found a significant upregulation of αΑ-crystallin in vitreous samples of human pseudophakic RD patients.
2. Materials and methods
2.1. Animals and experimental retinal detachment surgery
This research adheres to the principles of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. It was conducted under a protocol approved by The Ohio States University Institutional Animal Care and Use Committee. Adult female C57BL/6 mice (age 16–31 weeks) were purchased from The Jackson Laboratory (Bar Harbor, ME). Better quality RDs are generated in mice aged 16 weeks or older (unpublished observation). Retinal detachments (RD) were induced in anesthetized mice by subretinal injection of undiluted hyaluronic acid (HA, 10 mg/ml, Abbott Medical Optics, Abbott Park, IL) into left eyes as previously described by our group (Kim et al., 2017). Eyes were enucleated for analysis at week 1 and week 2–5 post-RD.
2.2. Patient vitreous sample collection
This study was approved by the IRB at The Ohio State University (#2011H0399). The research followed the tenets of the Declaration of Helsinki. The study subjects were recruited from multiple referral-based subspecialty practices in the US as part of the OSU Vitreoretinal ERM–PVR study group (OVER-PVR) Study Group at The Ohio State University Wexner Medical Center in Columbus, Ohio; the Cincinnati Eye Institute in Cincinnati, Ohio; and Mary Lanning Health Care in Hastings, Nebraska. Once informed consent had been obtained, a brief medical history was obtained from each patient that included information regarding demographics, history of systemic disease, and ocular history, lens status, and demographic information including race, ethnicity, age, and sex. Vitreous humor samples were collected during surgery. Epiretinal membrane (ERM) and macular hole (MH) patients with similar ethnic background, predominantly Caucasian Americans, were used as controls. All patient and lab data were entered into REDCap Software for record collection and quality control. The vitreous of 17 RD and 20 control (15 ERM and 5 macular hole) pseudophakic patients was analyzed by ELISA for αΑ-crystallin. All patients were already pseudophakic at the time of the vitreous sample collection; none had cataract extraction at the time of vitreous sample collection. Demographic details of the study subjects are listed in Table 1.
Table 1.
Demographic details of study subjects.
| Controla | RD | |
|---|---|---|
| No. of subjects | 20 | 17 |
| Age [Median (Range)] | 69.5 (60–90) | 66 (48–85) |
| Sex | Male-14 (70%) | Male-10 (59%) |
| Female-6 (30%) | Female-7 (41%) | |
Epiretinal membrane n = 15.
Macular Hole n = 5.
2.3. Murine enucleation and mRNA and protein isolation
Anesthetized mice were euthanized and eyes enucleated. Retinas, with vitreous, were dissected under an operating microscope using 0.12 forceps and Vannas scissors to cut the sclera and retina posterior to the limbus and remove the anterior cap of the eye. In the remaining posterior globe, the retina was gently separated from the underlying RPE and choroid and was cut near the insertion into the optic nerve. Retinas that were processed for RNA were dissected with instruments wiped with RNase-Zap and retinas were placed in TRIzol. For protein isolation retinas were placed in ice-cold 1x cell lysis buffer (#9803 S, Cell Signaling) freshly prepared with protease inhibitor cocktail set (#539134, Calbiochem) and 1 mM of phenylmethylsulfonyl fluoride (#AC215740010, Acros Organics) as previously described (Kim et al., 2014).
2.4. Fixation and sectioning
Enucleated eyes were fixed with 4% paraformaldehyde and 3% sucrose in 0.1M phosphate buffer at pH 7.4 for 30min. Eyes were washed twice times in phosphate buffered saline (PBS) for 10min and cryopreserved in 30% sucrose in PBS overnight. The eyes were embedded in OCT (Optimal Cutting Temperature, Electron Microscopy Sciences) solution and snap frozen and cryosectioned into 12-μm serial sections of the area of the RD.
2.5. iTRAQ labeling and 2D–Liquid Chromatography and Mass Spectrometry
8-plex iTRAQ labeling of retina + vitreous protein lysates from each retina and 2D-LC/MS/MS was performed and previously reported by our group (Kim et al., 2017). In the present study, the Swissprot database results of crystallins in control (untreated and sham), 2wk and 4wk RD samples were tabulated and sorted from high expression to low expression based on ratio of RD/C in 2wk RD samples (Table 2).
Table 2.
iTRAQ proteomics data identified 11 crystallin proteins in murine retinal tissue with 2-wk or 4-wk old RDs, arranged in descending order according to the relative fold-change in expression of 2-wk old RD samples divided by Control samples. Undefined ratios are due to having no expression of the crystallin in the control samples and positive expression in the RD samples. Molecular weight values obtained from UniProt.
| Swiss Protein # | Protein Name | MW (kDa) | Total Area in 2wk Treatment | Total Area in 4wk Treatment | Total Area in Control | 2wk Ratio | 4wk Ratio | Coverage (%) |
|---|---|---|---|---|---|---|---|---|
| P24622.1 | αA-crystallin | 23 | 24181.09 | 0 | 0 | Undefined | Undefined | 78.03 |
| Q9WVJ5.3 | βB1-crystallin | 28 | 5042.3 | 0 | 0 | Undefined | Undefined | 20 |
| P04342.2 | γD-crystallin | 21 | 3924.47 | 810.78 | 0 | Undefined | Undefined | 47.7 |
| Q03740.0 | ||||||||
| Q9CXV3.3 | ||||||||
| P04344.3 | ||||||||
| P04345.2 | ||||||||
| Q61597.3 | ||||||||
| P62696.2 | βB2-crystallin | 23 | 56113.62 | 0 | 1700.19 | 33 | 0 | 80.49 |
| Q9JJU9.3 | βB3-crystallin | 24 | 12504.74 | 1626.71 | 1028.54 | 12.16 | 1.58 | 32.23 |
| P23927.2 | αB-crystallin | 20 | 56323.52 | 2261.71 | 7173.68 | 7.85 | 0.32 | 73.14 |
| Q9JJV1.3 | βA2-crystallin | 22 | 28616.83 | 2018.08 | 3726.7 | 7.68 | 0.54 | 20.81 |
| Q9JJV0.3 | βA4-crystallin | 23 | 30635.81 | 0 | 4182.34 | 7.33 | 0 | 38.78 |
| Q61597.3 | γC-crystallin | 21 | 212073.68 | 14072.34 | 40904.88 | 5.18 | 0.34 | 28.74 |
| O35486.3 | βS-crystallin | 21 | 23553.22 | 2388.94 | 4819.85 | 4.89 | 0.5 | 49.44 |
| P04344.3 | γB-crystallin | 21 | 102221.95 | 6393.76 | 22605.57 | 4.52 | 0.28 | 39.43 |
2.6. Western Blotting assay
Equal amounts of total protein from individual RD and fellow eye control retinas week 1 (n = 5) and week 2–5 (n = 6) were quantified by BCA analysis. Lysates were run on a precast 4–20% Tris-glycine gel (Novex Wedge well, Invitrogen). Proteins on the gel were electroblotted onto a polyvinylidene fluoride membrane (Bio-Rad Laboratories). After blocking with 5% nonfat dry milk, the membrane was immunoblotted for αΑ-crystallin levels using polyclonal anti-αΑ-crystallin antibody (Stressgen SPA-221). The expression of α-tubulin (11H10, rabbit monoclonal, Cell Signaling) was used as a loading control. Images were analyzed with densitometry using ImageJ (NIH). The experiment was repeated at least 3 times. Error bars represent SEM.
2.7. Immunohistochemistry
Immunofluorescence was performed as described (Kim et al., 2014) using αΑ-crystallin antibody (Rabbit polyclonal, Stressgen). Slides were blocked with PBS containing 5% bovine serum albumin, and 1% Triton-X 100. Alexa Fluor 488 conjugated secondary antibodies (Invitrogen) were applied after 30 min incubation with 100% normal goat serum. Cell nuclei were counterstained with DAPI (Invitrogen). Photomicrographs were obtained using a Leica DM5000B fluorescent microscope and Leica DC500 digital camera using the same illumination and settings. Images were optimized for color, brightness, and contrast.
2.8. RT-PCR
Total RNA was extracted from individual retinas at day 3, day 7 and day 14 post detachment and fellow eye control retinas (n = 4/group) using TRIzol (Invitrogen), followed by quantification by NanoDrop ONEc. cDNA was synthesized using cDNA Synthesis Kit (SuperScript VILO cDNA Synthesis Kit,Invitrogen). Gene expression of murine CRYΑA was quantified using previously published primers (Xi et al., 2003) and normalized to the average of two housekeeping genes, GAPDH and SDHA (ABI). Samples were run in triplicate. The ΔΔCT method was used to analyze expression differences. Individual retinas were evaluated and the normalized CRYAA expression in each RD retina was divided by expression in the fellow eye control retina to determine fold-change. Average fold-change for each time point was determined and error bars represent SEM of the biological replicates. Similar results were obtained from two independent experiments.
2.9. Enzyme linked immunosorbent assay (ELISA)
αA-crystallin protein levels were measured in human vitreous surgical samples from pseudophakic patients using colorimetric competitive Elisa kit (MBS7211785, MYBioSource) per the manufacturer’s instructions. Pseudophakic subjects were used to eliminate the lens as a significant source for crystallin. Thirty-seven subjects (17 RD and 20 control (15 ERM and 5MH patients)) were evaluated. Protein concentration was calculated from a standard curve of recombinant αA-crystallin. Samples were run in duplicate per assay. Readings outside the calibration curve were excluded.
2.10. Statistical analysis
Statistical analysis was performed with JMP software (Version 14). Western blots were analyzed with one-way ANOVA and Tukey post-hoc testing. Kruskal-Wallis two-sample testing was used to analyze the ELISA data. Two-tailed testing was performed. A P-value ≤0.05 was considered statistically significant. Standard error of the mean (SEM) was listed for the error bars of all graphs.
3. Results
3.1. Identification of αΑ-crystallin as an upregulated protein in murine model of retinal detachment
We initially became interested in the crystallins after performing a proteomic study in our model of experimental murine RD; the analysis included relative quantification data for 560 different proteins as previously reported by our group (Kim et al., 2017) and we noted that several crystallins were up-regulated after RD. We re-analyzed this previously published proteomic data to formally evaluate crystallin expression after murine RD.
The 11 crystallins identified and their relative expression in murine RD and control retina + vitreous are listed in Table 2; proteins are ordered from most to least abundant based on the ratio of 2 wk RD/Control. As the table shows, baseline levels of αΑ-crystallin were undetectable by iTRAQ labeling in control eyes. Two weeks post-RD αA-crystallin displayed the highest level of increase among all the other crystallins. This is why we decided to focus on αΑ-crystallin for the rest of our study.
3.2. αΑ-crystallin protein levels increase in experimental retinal detachment as detected by western blot analysis
In order to confirm our proteomic findings, αΑ-crystallin levels were measured using Western blot analysis. Murine retinal samples from the RD model were collected 1 or 2–5 weeks post-detachment. As shown in Fig. 1, RD induced a significant time-dependent increase in αA-crystallin levels one week after RD induction (n = 5; P = 0.0223). The increase was persistent at 2–5 weeks of RD induction (n = 6; P < 0.0001). These data confirm the iTRAQ findings that αΑ-crystallin is upregulated during RD.
Fig. 1.
Western Blot analysis of αΑ-crystallin in murine retinal detachment eyes. Retinal detachment (RD) was introduced in murine left eyes as described in methods section, right eyes were used as control (C). Retinas were harvested 1 week (n = 5) and 2–5 weeks (n = 6) after RD and equal amount of total protein was loaded for electrophoresis and subsequent Western Blotting with anti αΑ-crystallin antibody. Western blots representative of at least 3 different experiments are shown in A. The expression of α-tubulin was used as a control. Quantification of at least 3 different experiments is described in B.
3.3. Crystallin immunohistochemical staining shows diffuse upregulation of αΑ-crystallin in RD retina
To further investigate the upregulation of αΑ-crystallin and to localize αΑ-crystallin in murine retinal tissue, immunohistochemical staining using αΑ-crystallin antibody was performed. As Fig. 2 shows RD induces an increase in αΑ-crystallin level compared to control, with the immunoreactivity spread diffusely among several retinal layers.
Fig. 2.
Immunohistochemistry of αΑ-crystallin in murine retinal detachment eyes. Retinal detachment (RD) was introduced in murine left eyes and right eyes were used as control (C). Eyes were harvested 1 week after RD, fixed and sectioned as described in methods section. Sections were stained with αA-crystallin antibody (green) and counterstained with DAPI (blue) for nuclei. An increased diffuse αA-crystallin reactivity was observed diffusely in RD retina as compared to control (C). ONL: outer nuclear layer; INL: inner nuclear layer; GCL: ganglion cell layer.
3.4. RD-induced αΑ-crystallin upregulation occurs at the transcriptional level
In order to further investigate the transcriptional αΑ-crystallin upregulation in RD we decided to use quantitative RT-PCR analysis of retinal RNA. As Fig. 3 shows RT-PCR data revealed consistently increased expression of αΑ-crystallin in RD mice compared to fellow eye control at days 3, 7, and 14 (n = 12) αΑ-crystallin level showed approximately 100-fold increase and 250-fold increase at day 7 and 14 respectively.
Fig. 3.
Quantification of αΑ-crystallin mRNA using RT-PCR. RNA was collected from retinal samples 3 days, 1 week, or 2 weeks post RD (n = 4/group). RT-PCR shows a time dependent increase in αA-crystallin at the transcription level starting 3 days post RD as compared to control eyes. Error bars represent SEM of biological replicates.
3.5. αΑ-crystallin is upregulated in the vitreous of patients with RD
Our findings confirm αΑ-crystallin upregulation in murine RD model at the transcriptional and translational levels. We wanted to test whether these findings can be extended to human RD. To do so, we evaluated vitreous samples from RD patients and non-RD controls (patients undergoing surgery for macular hole and epiretinal membrane). Demographic details are listed in Table 1. Most subjects were of American Caucasian ethnic background (95%).
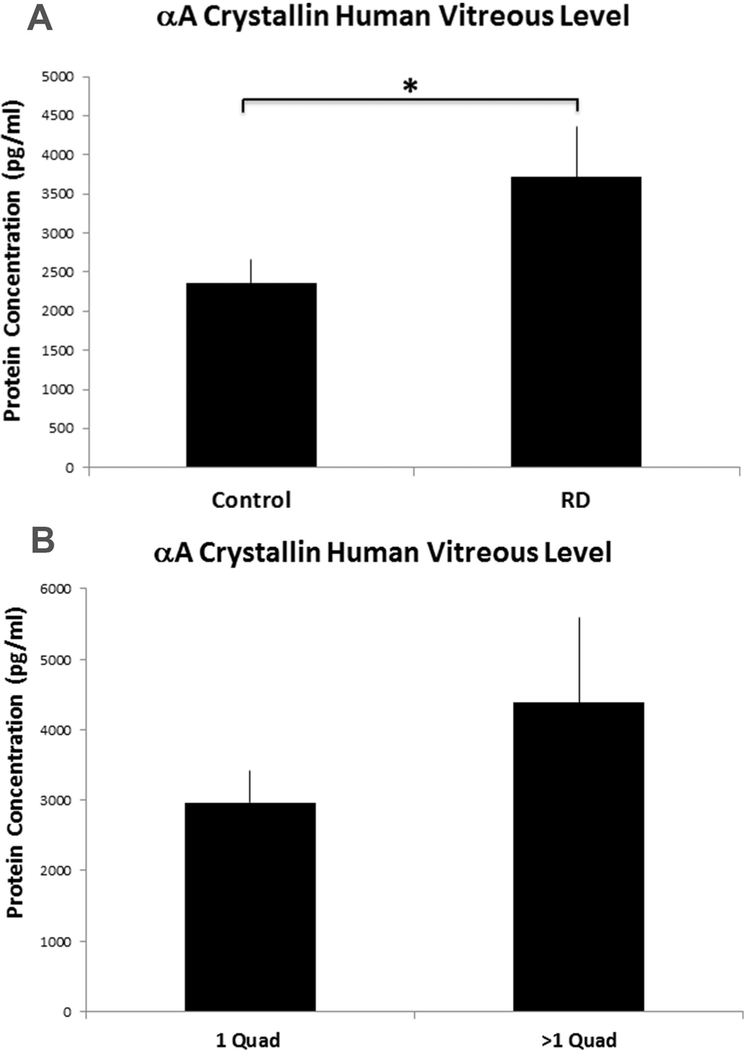
Samples from pseudophakic individuals with adequate sample were selectively analyzed in order to rule out the lens as a source of αA- crystallin. Enzyme linked Immunosorbent Assay (ELISA) was used to measure αΑ-crystallin level in the vitreous. Similar to the murine RD findings, RD increases the level of αΑ-crystallin in the vitreous of RD patients significantly compared to their control counterparts (RD = 3714 pg/mL ± 713 vs Control = 2356pg/ml ± 377, P = 0.0316).Fig. 4 summarizes the findings.
Fig. 4.
αA-crystallin measurement in control and RD patient vitreous samples using ELISA. (A) Vitreous samples were collected from RD patients (n = 17) and non RD controls (n = 20). The samples were selectively drawn from pseudophakic individuals to rule out the lens as a source of αA-crystallin. Colorimetric competitive ELISA was used to measure αA-crystallin level in the vitreous. αA-crystallin levels were significantly increased in the vitreous of RD patients compared to their control counterparts (P-value = 0.038). (B) αA-crystallin levels were evaluated in the RD patients (n = 17) divided into smaller vs larger RDs (1 quadrant involved vs. > 1 quadrant) (p = 0.27).
We decided to investigate this increase further by examining whether there was an association between the level of αΑ-crystallin in the vitreous of patients and the severity of their RD. To assess disease severity we used the extent of detachment across the four quadrants of the retina as a parameter. Subjects were divided into 2 groups; one consisting of patients whose RDs were localized to one quadrant and another whose RDs spread beyond one quadrant. Interestingly, αA-crystallin level in patients with more severe RDs (> 1 quadrant) showed a 50% increase compared to that of the localized RD group (4378.2 vs 2965.5 pg/ml respectively); however, the results were not statistically significant (p = 0.27).
4. Discussion
In the present study, we investigated the levels of αΑ-crystallin in mice subjected to retinal detachment and its correlation with human RD. Interestingly, αΑ-crystallin was significantly upregulated following murine experimental RD. Similarly, we found a significant increase of αΑ-crystallin levels in the vitreous of pseudophakic RD patients compared to pseudophakic ERM and MH control patients.
Our work adds to an already substantial body of literature showing increased αΑ-crystallin expression in response to multiple retinal diseases. In diabetes, αΑ-crystallin upregulation appears to be a hallmark of diabetic retinopathy. Increased levels of the protein have been found in the retinas of mice and rats with diabetic retinopathy (Fort et al., 2009; Kumar et al., 2005; Reddy et al., 2013) and in the eyes of humans with diabetes (Dong et al., 2012; Kase et al., 2011).
In addition, hypoxia, light damage, and neurodegenerative models have also shown up-regulation of αΑ-crystallin in the retina. α-crystallins have been found upregulated in rat retinas subjected to light damage as shown by 2D-PAGE (Sakaguchi et al., 2003) αΑ-crystallin was increased in the RPE cells of rats in a model of oxygen-induced retinopathy (Kim et al., 2012a). Yaung et al. found up-regulation of α-crystallins in a mouse model of retinal degeneration via chemically induced hypoxia and mice with genetic depletion of αA– and αB-crystallins showed greater retinal degeneration (Yaung et al., 2008). α-crystallins have also been found in the retinas of human donor eyes with age-related macular degeneration (Johnson et al., 2005; Nakata et al., 2005).
The functional impact of increased αΑ-crystallin in RD is unclear and requires further investigation. Several studies suggested a neuro-protective role for αΑ-crystallin. In murine experimental autoimmune uveitis, αΑ-crystallin was shown to preserve retinal architecture and protect photoreceptors (Rao et al., 2008, 2012). Using αΑ-crystallin knockout mice Ruebsam et al. have recently suggested a protective role for αΑ-crystallin in diabetic neuropathy (Ruebsam et al., 2018).
An adenovirus-mediated delivery of αΑ-crystallin reduced vascular leakage and pericyte apoptosis in experimental diabetes (Kim et al., 2012b). Systemic administration of αΑ-crystallin, but not αB-crystallin, has been shown to prevent photoreceptor degeneration induced by experimental autoimmune uveitis in mice (Rao et al., 2012). Similarly, neuroprotection of systemic or RGC-directed αΑ-crystallin was seen in optic nerve damage models (Munemasa et al., 2009; Ying et al., 2008). Importantly, genetic depletion of αΑ-crystallin was shown to enhance retinal cell death and was associated with enhanced diabetes-induced ER stress in the retina (Ruebsam et al., 2018). Whether αΑ-crystallin is playing the same neuroprotective role in RD is an intriguing hypothesis that would require further investigation.
Alternatively, other studies of α-crystallins suggest they may contribute to the pathology of some neurodegenerative diseases. For example, in Parkinson’s disease, αB-crystallin upregulation contributes to the formation of abnormal cytoplasmic aggregation in glial cells which exacerbates gliosis and ultimately leads to neurodegeneration (Liu et al., 2015). Additionally, αB-crystallin was found to co-precipitate with AP in Alzheimer’s brain and contributes to the progression of the disease. Similar findings for the involvement of α-crystallins in neurodegeneration were shown in tauopathies and prion disorders (Wang et al., 2013). So, whether the increase in αΑ-crystallin in RD is neuroprotective or contributing to the pathology of the disease has yet to be determined.
Interestingly, αB-crystallin has also been shown to be up-regulated in RD surgical vitreous samples compared to epiretinal membrane and macular hole controls (Baba et al., 2015). The study results were intriguing but confounded by cataract surgery being performed during the same surgery that the vitreous sample was collected in 28/32 RD eyes and in 14/32 controls. The vitreous crystallin levels could have been altered by the concurrent cataract surgery. In our study, we aimed to reduce the impact of lenticular sources of αΑ-crystallin. Only pseudophakic patients who had their cataract surgery performed well ahead of sample collection were compared. Both the control group and the RD group had their cataract surgery done in advance in a similar timeframe, to control for effects from prior cataract surgery. This would minimize the impact of lenticular sources of αΑ-crystallin and confirm that the increase of αΑ-crystallin levels in RD is not an artifact of the cataract surgery.
This study has some limitations. First, the sample size of the patients is limited. Despite this limitation, we have found a statistically significant increase in αΑ-crystallin levels in RD vs controls. Second, we did not evaluate the mechanistic impact of αΑ-crystallin in RD. Future studies, particularly with knockout mice, would be beneficial to assess this.
In conclusion, we found that αΑ-crystallin is significantly upregulated following rhegmatogenous RD in mouse and humans. These findings will open the door for future studies to explore the functional consequence and mechanistic pathways of this upregulation and the possibility of using it to develop neuroprotective strategies for retinal disease.
Acknowledgements
The authors thank Severin Pouly, Stephen Rasiah, and Jiaxi Ding for their technical assistance. The authors declare no competing financial interests. Supported by NEI Award Number K08EY022672, NCATS Award Number KL2TR001068, and NIH-CCC award P30 CA016058 (Nucleic Acids Shared Resource). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding institutions. Additional funds were provided by the Ohio Lions Eye Research Foundation, Fund #313310, and the Patti Blow Fund.
Abbreviations:
- RD
retinal detachment
- ERM
epiretinal membrane
- MH
macula hole
- RPE
retinal pigment epithelium
- sHSPs
small heat shock proteins
- ELISA
Enzyme linked Immunosorbent Assay
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.exer.2019.107811.
References
- Baba T, Oshitari T, Yamamoto S, 2015. Level of vitreous alpha-B crystallin in eyes with rhegmatogenous retinal detachment. Graefes Arch. Clin. Exp. Ophthalmol. 253, 1251–1254. [DOI] [PubMed] [Google Scholar]
- Brownell SE, Becker RA, Steinman L, 2012. The protective and therapeutic function of small heat shock proteins in neurological diseases. Front. Immunol. 3, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R, Zigman S, Lerman S, 1969. Studies on the structural proteins of the human lens. Exp. Eye Res. 8, 172–182. [DOI] [PubMed] [Google Scholar]
- Dong Z, Kase S, Ando R, Fukuhara J, Saito W, Kanda A, Murata M, Noda K, Ishida S, 2012. Alphab-crystallin Expression in Epiretinal Membrane of Human Proliferative Diabetic Retinopathy, vol. 32 Retina, Philadelphia, Pa, pp. 1190–1196. [DOI] [PubMed] [Google Scholar]
- Fort PE, Freeman WM, Losiewicz MK, Singh RS, Gardner TW, 2009. The retinal proteome in experimental diabetic retinopathy: up-regulation of crystallins and reversal by systemic and periocular insulin. Mol. Cell. Proteom. 8, 767–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort PE, Lampi KJ, 2011. New focus on alpha-crystallins in retinal neurodegenerative diseases. Exp. Eye Res. 92, 98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan TS, Sarrafizadeh R, Ruby AJ, Garretson BR, Kuczynski B, Williams GA, 2002. The effect of duration of macular detachment on results after the scleral buckle repair of primary, macula-off retinal detachments. Ophthalmology 109, 146–152. [DOI] [PubMed] [Google Scholar]
- Horwitz J, 2003. Alpha-crystallin. Exp. Eye Res. 76, 145–153. [DOI] [PubMed] [Google Scholar]
- Huckfeldt RM, Vavvas DG, 2013. Neuroprotection for retinal detachment. Int.Ophthalmol. Clin. 53, 105–117. [DOI] [PubMed] [Google Scholar]
- Johnson PT, Brown MN, Pulliam BC, Anderson DH, Johnson LV, 2005. Synaptic pathology, altered gene expression, and degeneration in photoreceptors impacted by drusen. Investig. Ophthalmol. Vis. Sci. 46, 4788–4795. [DOI] [PubMed] [Google Scholar]
- Kase S, Ishida S, Rao NA, 2011. Increased expression of αA-crystallin in human diabetic eye. Int. J. Mol. Med. 28, 505–511. [DOI] [PubMed] [Google Scholar]
- Kim B, Abdel-Rahman MH, Wang T, Pouly S, Mahmoud AM, Cebulla CM, 2014. Retinal MMP-12, MMP-13, TIMP-1, and TIMP-2 expression in murine experimental retinal detachment. Investig. Ophthalmol. Vis. Sci. 55, 2031–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Kusibati R, Heisler-Taylor T, Mantopoulos D, Ding JX, Abdel-Rahman MH, Satoskar AR, Godbout JP, Bhattacharya SK, Cebulla CM, 2017. MIF inhibitor ISO-1 protects photoreceptors and reduces gliosis in experimental retinal detachment. Sci. Rep. 7, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Jin J, Kim YJ, Kim Y, Yu HG, 2012a. Retinal proteome analysis in a mouse model of oxygen-induced retinopathy. J. Proteome Res. 11, 5186–5203. [DOI] [PubMed] [Google Scholar]
- Kim YH, Park SY, Park J, Kim YS, Hwang EM, Park JY, Roh GS, Kim HJ, Kang SS, Cho GJ, Choi WS, 2012b. Reduction of experimental diabetic vascular leakage and pericyte apoptosis in mice by delivery of αA-crystallin with a recombinant adenovirus. Diabetologia 55, 2835–2844. [DOI] [PubMed] [Google Scholar]
- Kumar PA, Haseeb A, Suryanarayana P, Ehtesham NZ, Reddy GB, 2005. Elevated expression of alphαA- and alphaB-crystallins in streptozotocin-induced diabetic rat. Arch. Biochem. Biophys. 444, 77–83. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhou Q, Tang M,Fu N, Shao W, Zhang S,Yin Y, Zeng R, Wang X, Hu G, Zhou J, 2015. Upregulation of alphaB-crystallin expression in the substantia nigra of patients with Parkinson’s disease. Neurobiol. Aging 36, 1686–1691. [DOI] [PubMed] [Google Scholar]
- Munemasa Y, Kwong JMK, Caprioli J, Piri N, 2009. The role of alpha A- and alpha B-crystallins in the survival of retinal ganglion cells after optic nerve axotomy. Investig. Ophthalmol. Vis. Sci. 50, 3869–3875. [DOI] [PubMed] [Google Scholar]
- Nagaraj RH, Nahomi RB, Mueller NH, Raghavan CT, Ammar DA, Petrash JM, 2016. Therapeutic potential of alpha-crystallin. Biochim. Biophys. Acta 1860, 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K, Crabb JW, Hollyfield JG, 2005. Crystallin distribution in Bruch’s mem- brane-choroid complex from AMD and age-matched donor eyes. Exp. Eye Res. 80, 821–826. [DOI] [PubMed] [Google Scholar]
- Rao NA, Saraswathy S, Pararajasegaram G, Bhat SP, 2012. Small heat shock protein αA-crystallin prevents photoreceptor degeneration in experimental autoimmune uveitis. PLoS One 7, e33582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao NA, Saraswathy S, Wu GS, Katselis GS, Wawrousek EF, Bhat S, 2008. Elevated retina-specific expression of the small heat shock protein, alphaA-crystallin, is associated with photoreceptor protection in experimental uveitis. Investig. Ophthalmol. Vis. Sci. 49, 1161–1171. [DOI] [PubMed] [Google Scholar]
- Reddy VS, Raghu G, Reddy SS, Pasupulati AK, Suryanarayana P, Reddy GB, 2013. Response of small heat shock proteins in diabetic rat retina. Investig. Ophthalmol. Vis. Sci. 54, 7674–7682. [DOI] [PubMed] [Google Scholar]
- Ross WH, 2002. Visual recovery after macula-off retinal detachment. Eye 16, 440–446. [DOI] [PubMed] [Google Scholar]
- Ruebsam A, Dulle JE, Myers AM, Sakrikar D, Green KM, Khan NW, Schey K, Fort PE, 2018. A specific phosphorylation regulates the protective role of alphaA-crystallin in diabetes. JCI Insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SJ, 2006. Retina, fourth ed Elsevier/Mosby, Philadelphia. [Google Scholar]
- Sakaguchi H, Miyagi M, Darrow RM, Crabb JS, Hollyfield JG, Organisciak DT, Crabb JW, 2003. Intense light exposure changes the crystallin content in retina. Exp. Eye Res. 76, 131–133. [DOI] [PubMed] [Google Scholar]
- Srinivasan AN, Nagineni CN, Bhat SP, 1992. Alpha A-crystallin is expressed in non-ocular tissues. J. Biol. Chem. 267, 23337–23341. [PubMed] [Google Scholar]
- Vazquez-Chona F, Song BK, Geisert EE Jr., 2004. Temporal changes in gene expression after injury in the rat retina. Investig. Ophthalmol. Vis. Sci. 45, 2737–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Zhang J, Xu Y, Ren K, Xie WL, Yan YE, Zhang BY, Shi Q, Liu Y, Dong XP, 2013. Abnormally upregulated alphaB-crystallin was highly coincidental with the astrogliosis in the brains of scrapie-infected hamsters and human patients with prion diseases. J. Mol. Neurosci. 51, 734–748. [DOI] [PubMed] [Google Scholar]
- Wang YD, Wu JD, Jiang ZL, Wang YB, Wang XH, Liu C, Tong MQ, 2007. Comparative proteome analysis of neural retinas from type 2 diabetic rats by two-dimensional electrophoresis. Curr. Eye Res. 32, 891–901. [DOI] [PubMed] [Google Scholar]
- Xi J, Farjo R, Yoshida S, Kern TS, Swaroop A, Andley UP, 2003. A comprehensive analysis of the expression of crystallins in mouse retina. Mol. Vis. 9, 410–419. [PubMed] [Google Scholar]
- Yaung J, Kannan R, Wawrousek EF, Spee C, Sreekumar PG, Hinton DR, 2008. Exacerbation of retinal degeneration in the absence of alpha crystallins in an in vivo model of chemically induced hypoxia. Exp. Eye Res. 86, 355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying X, Zhang J, Wang Y, Wu N, Wang Y, Yew DT, 2008. alpha-crystallin protected axons from optic nerve degeneration after crushing in rats. J. Mol. Neurosci. 35, 253–258. [DOI] [PubMed] [Google Scholar]