Abstract
Acute graft-versus-host disease (GVHD) is the major complication of allogeneic hematopoietic cell transplantation and is the primary cause of early non-relapse mortality (NRM) after transplant. GVHD of the gastrointestinal (GI) tract fuels the systemic inflammatory reaction and consequently is the principal driver of mortality. Recently, the MAGIC algorithm probability (MAP) that is computed from two biomarkers of GI GVHD has been validated to accurately predict risk of NRM throughout the course of early acute GVHD. This review focuses on the biology, clinical evidence, and practical application of the biomarkers in the measurement of acute GVHD.
Keywords: Allogeneic HCT, acute GVHD, biomarkers, GI tract, non-relapse mortality, NRM
Introduction
Hematopoietic cell transplant (HCT) can cure hematologic malignancies through the destruction of malignant cells known as the graft-versus-leukemia (GVL) effect. However, GVL also carries significant risk of graft-versus-host disease (GVHD) during which alloreactive T lymphocytes destroy healthy tissue primarily in the skin, liver, and gastrointestinal (GI) tract. GVHD is the leading cause of non-relapse mortality (NRM) after HCT and occurs in both acute and chronic forms [1]. Acute GVHD typically occurs in 40%-50% of HCT patients and requires systemic immunosuppression with glucocorticoids when severe [2,3]. The risks of such immunosuppression are significant and include life-threatening infections, osteoporosis and associated fractures, and myriad metabolic disturbances[4–6].
GVHD in the lower GI tract is the primary driver of mortality and patients with severe GI disease experience long-term overall survival of just 25% [7]. Clinical measures of GI dysfunction such as the volume and frequency of diarrheal episodes are routinely used in clinical practice to monitor disease burden. These are indirect and nonspecific measures, however, of the damage to intestinal crypts that is the root cause of GVHD pathophysiology in the GI tract. Improved metrics are therefore urgently needed to gauge accurately the severity of GI GVHD that would thereby allow clinicians to stratify patients for risk and more appropriately tailor immunosuppression in a medically fragile patient population.
The Mount Sinai Acute GVHD International Consortium (MAGIC) is a group of 25 international centers that monitors the clinical status of HCT patients using rigorous quality control procedures and collects serial blood samples for biomarker analysis [8]. In the past decade, studies of MAGIC patients have identified two serum biomarkers that predict long-term mortality in acute GVHD [9–11]. These biomarkers are suppressor of tumorigenesis 2 (ST2), the soluble receptor for the alarmin IL-33, which is shed from multiple cell types in the injured gastrointestinal crypt; and regenerating islet-derived 3-alpha (REG3α) a multifunctional protein that spills into the bloodstream from damaged Paneth cells [12,13]. The combination of the serum concentrations of these two biomarkers generates an estimated probability of 6-month NRM for each individual patient known as the MAGIC algorithm probability (MAP). When measured at GVHD diagnosis, the MAP separates patients into three distinct groups known as Ann Arbor scores that each carry significantly different risk of NRM. Thus, the MAP can be considered a “liquid biopsy” of the GI tract damaged by the inflammation of GVHD and represents a more accurate quantitation of disease burden than clinical symptoms. This review focuses on the biology of GI GVHD and its biomarkers, the ability of the MAP to predict long-term mortality throughout the course of acute GVHD, and the emerging application of repeated biomarker measurements to monitor the healing of intestinal crypts with the goal of individual tailoring of immunosuppressive medications.
Identification of GVHD biomarkers and biological processes
The intact-protein analysis system (IPAS) is a powerful strategy to isolate and characterize previously unidentified proteins in blood [14,15]. Both ST2 and REG3α were identified via IPAS because of their high concentrations in blood from patients with severe GVHD [13,16]. In a discovery study of 871 patients, REG3α concentrations were three-fold higher in patients with lower GI GVHD compared to patients with enteritis or patients with non-GI GVHD, and patients with high blood levels of REG3α had significantly greater long-term mortality than those with low levels [16]. In a separate study, IPAS analysis revealed ST2 to be elevated in the serum of patients with acute GVHD that was resistant to treatment with glucocorticoids. High serum concentrations of ST2 also predicted long-term mortality [13].
Both of these biomarkers derive primarily from distinct cell populations in the GI tract and play important roles in the homeostasis of intestinal crypts that continually regenerate GI epithelial tissue. Damage to the lower GI tract during GVHD triggers systemic inflammation and is the principal driver of mortality in both clinical and experimental GVHD [7,17]. ST2 is the receptor for IL-33, an alarmin shed from damaged epithelium, endothelium, and stroma of the lower gastrointestinal tract [18–20]. Once released, IL-33 acts through several pathways on multiple cell types to promote inflammation, including binding to two isoforms (membrane-bound and soluble) of ST2 [21]. Soluble ST2, the isoform measured as a biomarker, acts as a decoy receptor by sequestering IL-33 and thereby abrogating its downstream pro-inflammatory effects. In murine models, ST2-deficient transgenic mice develop less severe GVHD and the administration of exogenous ST2 reduces mortality [21].
REG3α, the second GVHD biomarker, is produced by and stored in Paneth cells, the “guardians of the crypt” that are critical to mucosal immunity and the proliferation and survival of adjacent intestinal stem cells via cell-to-cell contact and signaling. Paneth cells also secrete a variety of antimicrobial peptides such as defensins and REG3α, a C-type lectin which binds bacterial peptidoglycans [22], REG3α was recently discovered to have an additional key survival function for intestinal stem cells and inhibits their apoptosis [12]. Administration of IL-22 was able to reverse GVHD damage only when it induced expression of REG3α [12]. Paradoxically, REGα rises in serum as it decreases in GI epithelium during the course of GVHD as Paneth cells are destroyed; its presence in serum is thus a direct measurement of damage to intestinal crypts and serves as a “liquid biopsy” of the entire small bowel, which helps to account for the predictive power of elevated serum concentrations [12].
Development of the MAGIC algorithm probability
Both ST2 and REG3α independently predict NRM and their predictive accuracy is enhanced when they are combined into a competing risks regression model, considering relapse as a competing risk for death, that generates a single estimated probability of 6- month NRM or MAP [10]. An earlier model included the concentration of tumor necrosis factor receptor 1 (TNFR1) [9], and a critical role for TNFα in GVHD has been hypothesized in both experimental and clinical studies [23,24]. When a more sensitive ST2 capture antibody became commercially available, the MAP no longer required the inclusion of the concentration of TNFR1. This two biomarker model was validated using serum samples at the time GVHD onset [8,10].
The MAP assigns patients to one of three Ann Arbor GVHD scores at disease onset, each with a distinctly different risk of NRM. Ann Arbor 1 patients have 8% NRM after 6 months, Ann Arbor 2 patients have 24% NRM after 6 months, and Ann Arbor 3 patients have the highest risk of NRM, 46% after 6 months [10]. In that study, the proportions of patients in Ann Arbor 1, 2, and 3 groups were 45%, 28%, and 27%, respectively. After systemic treatment for GVHD, patients are classified as either high or low MAP using a single threshold of 0.290 [11]. The MAP predicts NRM for each Ann Arbor group within each clinical grade. An example of Ann Arbor groups within a single clinical grade (Glucksberg II) is shown in in Figure 1, together with the cumulative incidence of NRM and steroid resistance.
Figure 1. MAGIC biomarker probability predicts long-term outcomes in patients with Glucksberg Grade II GVHD at diagnosis.
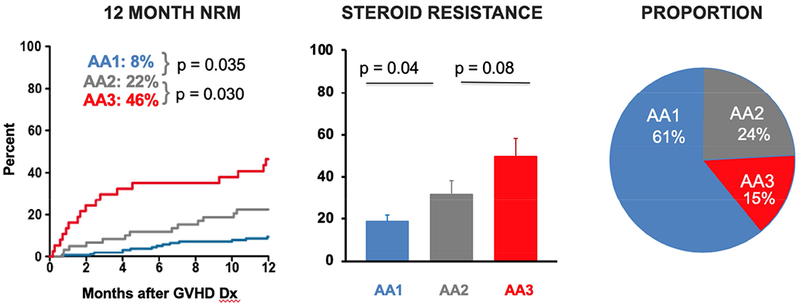
Patients with Glucksberg Grade II GVHD at diagnosis were stratified by their Ann Arbor score. — are Ann Arbor 1 (MAP < 0.141), — are Ann Arbor 2 (0.141 ≤ MAP ≤ 0.290), and — are Ann Arbor 3 (MAP > 0.290). (A) Cumulative incidence curves of NRM for 12 months after diagnosis. (B) Proportion of patients with steroid resistance after four weeks of treatment. (C) Proportion of patients in each Ann Arbor group.
Performance of the MAGIC algorithm probability at single timepoints
The MAP has now been tested at multiple time points throughout the course of acute GVHD and at each time point has predicted long-term outcomes better than key clinical variables. At 7 days following HCT, prior to the onset of acute GVHD symptoms in any patients, the MAP predicts NRM better than pre-transplant characteristics [10]. At the time of GVHD diagnosis, the MAP outperforms standard clinical measures of disease severity such as the CIBMTR severity and Glucksberg grade [9]. After 1 week of systemic glucocorticoid treatment for acute GVHD, the MAP also predicts long-term outcomes better than either the response of clinical symptoms or the Minnesota risk category [11]. At this same time point (7 days following systemic treatment), the MAP also predicts outcomes better than the volume of diarrhea, the clinical measure of lower GI GVHD [11]. In the patients without diarrhea, 17% had high MAPs and more NRM than the remaining 83% with low MAPs (42% vs 8%, P<0.001). Similarly, 34% of patients with ≥500cc of diarrhea daily had low MAPs and less NRM than those with high MAPs (20% vs 70%, P<0.001). These findings highlight the discordance between clinical symptomatology and the damage to the crypts, which is more accurately measured by the MAP.
We are currently investigating whether the MAP can predict long-term mortality after f4 weeks of systemic GVHD. The clinical response after 4 weeks of systemic treatment is currently the best validated measure of long-term NRM and as such it is used as the primary endpoint in the majority of treatment trials of acute GVHD. Our current analysis suggests MAPs after 4 weeks of treatment are superior to the clinical responses at this time point and may serve as a novel endpoint for GVHD treatment.
The MAP as a response biomarker
The accuracy of biomarker-based predictions at multiple timepoints throughout the course of early acute GVHD implies that the MAP may be a response biomarker, and a repeated measurement in an individual patient may provide a dynamic measurement of the crypt damage and further refine the prediction of NRM. The utility of real-time analysis of such dynamic measurements is an area of intensive ongoing research, and here we highlight the application of this approach in the following hypothetical patient case.
Case:
A 22-year-old male with acute lymphoblastic leukemia in second remission received an allogeneic HCT from an 8/8 HLA-matched unrelated donor following myeloablative conditioning with total body irradiation and cyclophosphamide. His GVHD prophylaxis consisted of tacrolimus and 4 doses of methotrexate on days +1, +3, +6, and +11. His early post-transplant course was uneventful, with engraftment on day +14 and hospital discharge on day +18. On day +28 post-transplant he presented to clinic with a maculopapular rash covering 60% of his body without bullae or desquamation, a bilirubin of 1.7 mg/dL, and fully formed stools. His GVHD staging was therefore skin stage 3, GI stage 0, liver stage 0, overall Grade II. No biopsy was obtained and he was started on 2 mg/kg methylprednisolone. His physician obtained serum for a MAP determination, which was 0.310 (Ann Arbor 3) or at high risk of NRM (Table 1). Treatment with oral prednisone was initiated. After one week of therapy he had not developed diarrhea, his bilirubin was stable at 1.8 mg/dL, but the rash still covered 55% of his body, indicating he had not achieved a clinical response (still overall Grade II). A repeated biomarker measurement revealed that although his ST2 remained stable at 35,935 pg/mL, his REG3α had decreased significantly to 369 and thus the MAP decreased to 0.222, well below the post-treatment high-risk threshold of 0.290. This decrease in MAP indicated that he was likely to achieve a complete response [11], He remained on 2 mg/kg of corticosteroids, then began a taper of 20% per week. His rash resolved by week 4of treatment.
Table 1.
Hypothetical clinical case summary.
| Pre-Treatment (At Onset) | Post-Treatment (1 Week) | |
|---|---|---|
| Skin Stage | 2 | 2 |
| GI Stage | 0 | 0 |
| Liver Stage | 0 | 0 |
| OVERALL GRADE | II | II |
| ST2 (pg/mL) | 38,590 | 35,935 |
| REG3α (ng/mL) | 1,402 | 369 |
| Biomarker Probability | 0.310 | 0.222 |
| Biomarker Risk | Ann Arbor 3 (46% 12-month NRM) | Low Risk (14% 12-month NRM) |
Discussion:
A GVHD rash without diarrhea or bilirubin elevation is a common presentation of GVHD. This patient’s initial biomarkers, however, indicated significant crypt damage even though he had not yet developed diarrhea. After 1 week of treatment, the skin disease remained unchanged and the same stage as prior to treatment; he would therefore be classified as a non-responder and considered high-risk, with many physicians prescribing additional immunosuppressive therapy such as ruxolitinib at that point. However, the decreasing MAP indicated that the clinically inapparent damage in the small bowel was healing. Indeed the MAP has a substantial improvement in specificity compared to clinical response after 1 week indicating that it is more useful in identifying patients who are truly at lower risk of NRM [11]. In this case, watchful waiting rather than additional immunosuppression would be a viable option.
Conclusions
Acute GVHD is a devastating complication of HCT, and accurate measures of risk are critical to individualizing therapeutic approaches. While clinical measures of GVHD severity have historically served this purpose, serum biomarkers of acute GVHD have emerged as an accurate method to predict NRM. The MAP specifically reflects intestinal crypt damage, the biological process that drives the most lethal form of the disease. The MAP can be used at multiple timepoints throughout the course of GVHD from prior to symptoms, through the onset of GVHD symptoms, to the weeks following systemic therapy. Studies are currently in progress to determine whether the MAP can serve as response biomarkers and track the disease burden throughout the lower GI tract. If validated, the MAP would enable tailored risk prediction for individual patients during the first month of acute GVHD treatment. In this regard, the MAP may prove similar to other laboratory measurements such as quantitative PCR of viral load and FACS analysis of leukemia burden that more accurately quantitate disease burden and have replaced clinical surrogate endpoints in HIV infections and B-cell acute lymphoblastic leukemia, respectively [25,26]. Such an application of the MAP could enable more finely tuned usage of systemic glucocorticoid therapy to treat acute GVHD.
Acknowledgments:
The authors thank the patients, their families, and the research staff for their participation. This work was supported by grants (P01CA03942 and P30CA196521) from the National Cancer Institute and (TL1 TR001434) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Ferrara is a co-inventor on a GVHD biomarker patent. Mr. Srinagesh has no financial relationships to disclose.
References
- [1].Ferrara JLM, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet 2009;373:1550–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zeiser R, Blazar BR. Acute graft-versus-host disease — biologic process, prevention, and therapy. N Engl J Med 2017;377:2167–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jagasia M, Arora M, Flowers MED, Chao NJ, McCarthy PL, Cutler CS, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood 2012;119:296–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mielcarek M, Storer BE, Boeckh M, Carpenter PA, McDonald GB, Deeg HJ, et al. Initial therapy of acute graft-versus-host disease with low-dose prednisone does not compromise patient outcomes. Blood 2009; 113:2888–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Staa TP van, Staa TP van, Staa TP van, Leufkens HGM, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 2002;13:777–787. [DOI] [PubMed] [Google Scholar]
- [6].Curtis JR, Westfall AO, Allison J, Bijlsma JW, Freeman A, George V, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum 2006;55:420–426. [DOI] [PubMed] [Google Scholar]
- [7].Castilla-Llorente C, Martin PJ, McDonald GB, Storer BE, Appelbaum FR, Deeg HJ, et al. Prognostic factors and outcomes of severe gastrointestinal GVHD after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2014;49:966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multi-center standardization of acute graft-versus-host disease clinical data collection: a report from the MAGIC consortium. Biol Blood Marrow Transplant 2016;22:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Levine JE, Braun TM, Harris AC, Holler E, Taylor A, Miller H, et al. A prognostic score for acute graft-versus-host disease based on biomarkers: a multicentre study. Lancet Haematol 2015;2:e21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hartwell MJ, Özbek U, Holler E, Renteria AS, Major-Monfried H, Reddy P, et al. An early-biomarker algorithm predicts lethal graft-versus-host disease and survival. JCI Insight 2018;2:e89798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Major-Monfried H, Renteria AS, Pawarode A, Reddy P, Ayuk F, Holler E, et al. MAGIC biomarkers predict long-term outcomes for steroid-resistant acute GVHD. Blood 2018;131:2846–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhao D, Kim Y-H, Jeong S, Greenson JK, Chaudhry MS, Hoepting M, et al. Survival signal REG3α prevents crypt apoptosis to control acute gastrointestinal graft-versus-host disease. J Clin Invest 2018;128:4970–4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vander Lugt MT, Braun TM, Hanash S, Ritz J, Ho VT, Antin JH, et al. ST2 as a marker for risk of therapy-resistant graft-versus-host disease and death. N Engl J Med 2013;369:529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Faca V, Coram M, Phanstiel D, Glukhova V, Zhang Q, Fitzgibbon M, et al. Quantitative analysis of acrylamide labeled serum proteins by LC-MS/MS. J Proteome Res 2006;5:2009–2018. [DOI] [PubMed] [Google Scholar]
- [15].Faca V, Pitteri SJ, Newcomb L, Glukhova V, Phanstiel D, Krasnoselsky A, et al. Contribution of protein fractionation to depth of analysis of the serum and plasma proteomes. J Proteome Res 2007;6:3558–3565. [DOI] [PubMed] [Google Scholar]
- [16].Ferrara JLM, Harris AC, Greenson JK, Braun TM, Holler E, Teshima T, et al. Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood 2011; 118:6702–6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hill GR, Ferrara JLM. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood 2000;95:2754–2759. [PubMed] [Google Scholar]
- [18].Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie ANJ, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest 2007;117:1538–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Moussion C, Ortega N, Girard J-P. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel “alarmin”? PLoS One 2008;3:e3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Carriere V, Roussel L, Ortega N, Lacorre D- A, Americh L, Aguilar L, et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A 2007;104:282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Reichenbach DK, Schwarze V, Matta BM, Tkachev V, Lieberknecht E, Liu Q, et al. The IL-33/ST2 axis augments effector T cell responses during acute GVHD. Blood 2015;125:3183–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 2006;313:1126–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Levine JE, Paczesny S, Mineishi S, Braun T, Choi SW, Hutchinson RJ, et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood 2008;111:2470–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JLM. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood 1997;90:3204–3213. [PubMed] [Google Scholar]
- [25].Murray JS, Elashoff MR, lacono-Connors LC, Cvetkovich TA, Struble KA. The use of plasma HIV RNA as a study endpoint in efficacy trials of antiretroviral drugs. AIDS 1999;13:797–804. [DOI] [PubMed] [Google Scholar]
- [26].Gökbuget N, Dombret H, Bonifacio M, Reichle A, Graux C, Faul C, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood 2018;131:1522–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]


