Abstract
The manifestation of risk versus resilience has been considered from varying perspectives including genetics, epigenetics, early life experiences, and type and intensity of the challenge with which the organism is faced. Although all of these factors are central to determining risk and resilience, the current review focuses on what may be a final common pathway: metabolism. When an organism is faced with a perturbation to the environment, whether internal or external, appropriate energy allocation is essential to resolving the divergence from equilibrium. This review examines the potential role of metabolism in the manifestation of stress-induced neural compromise. In addition, this review details the current state of knowledge on neuroendocrine factors which are poised to set the tone of the metabolic response to a systemic challenge. The goal is to provide an essential framework for understanding stress in a metabolic context and appreciation for key neuroendocrine signals.
Keywords: metabolism, neuroendocrine, hormones, sex differences, homeostasis, allostasis, mitochondria, stress, risk, resilience
1. Introduction
1.1. Defining Resilience in the Context of Metabolism and Neuroendocrinology
Resilience is broadly defined as an individual’s ability to endure and recover from major life adversity. In the context of neuroendocrine function, resilience can be understood as one’s ability to withstand stress exposure without the development of significant maladaptations. A common endpoint for defining resilience is the resistance to developing stress-induced neuropsychiatric syndromes, which can include mental health disorders such as major depressive disorder or post-traumatic stress disorder (PTSD). Although not widely linked to metabolism, there is an emerging body of evidence that many of these stress-induced neuropsychiatric syndromes are correlated with, and possibly driven by, disruptions in metabolic processing within key peripheral and brain tissues, suggesting that stress resilience may be linked to pro-adaptive metabolic processing within these tissues.
When considering the interaction between stress resilience and metabolism it is necessary to first establish how we should conceptualize metabolism. The historical (and most widely accepted) consensus on metabolism regards the most important metabolic function as the ability of an organism to produce sufficient energy to perform biological functions. However, there are other equally important functions of metabolism necessary for an organism, such as the production of intermediates for the biosynthesis of macromolecules and for the regulation of directionality of metabolic pathways. Therefore, it is necessary to not think about metabolic pathways within a vacuum, but rather in the context of integrated physiological functions, where metabolic pathways are constantly in a state of flux, responding to a diverse set of ever-changing stimuli and demands. For the purposes of this review, we will consider metabolism within the four essential functions of metabolism for cells, as laid out by Navdeep Chandel in his publication, Navigating Metabolism (Chandel, 2015). According to Chandel, metabolism provides energy through the generation of ATP in order to carry out cellular functions. In addition, metabolism converts nutrients into simpler structures (catabolism), which may contribute to energy production. In the other direction, metabolism serves to convert simpler structures, such as amino acids and fatty acids, into macromolecules through anabolism, a process which requires the input of energy. Finally, metabolism operates outside of the confines of energy production, catabolism, and anabolism to participate in cellular functions such as signaling and gene transcription.
Of the stress-induced neuropsychiatric syndromes, PTSD has a particularly robust link to a metabolic phenotype. The concept that PTSD encompasses metabolic aberrations that are similar to those present in individuals with metabolic disorders was discussed in a relatively recent review (Michopoulos et al., 2016). Of particular interest is the observation that, within populations suffering from PTSD, there is the recurrent theme of disrupted glucose metabolism and increased abdominal obesity (Cohen et al., 2009; Li et al., 2016; Rosenbaum et al., 2015; Thorp and Schlaich, 2015). Notably, both of these metabolic phenotypes are routinely correlated with insulin resistance. Additionally, even though peripheral glucocorticoid receptor (GR) expression has, at least in some reports, been documented to increase in both metabolic syndrome and PTSD (Gola et al., 2014; Kang et al., 2015; Moraitis et al., 2017), there are contradictory cortisol stress responses. Individuals with PTSD tend to demonstrate an increased cortisol response, while individuals with metabolic syndrome demonstrate a decreased cortisol response to stress (Epel et al., 2000; Kolassa et al., 2007). A meta-analysis establishing a correlation between physical exercise with the reduction in symptom severity for individuals with PTSD suggests a potential role for peripheral metabolism in influencing PTSD symptoms (Rosenbaum et al., 2015). In an earlier review, Michopoulos and colleagues highlighted metabolic factors that have been identified as potential biomarkers for the pathogenesis of PTSD (Michopoulos et al., 2015). These findings, in conjunction with the observed increase in pro-inflammatory cytokines (Michopoulos, 2017; Miller et al., 2017; Monteiro and Azevedo, 2010; Paoletti et al., 2006; Sharma, 2011; Speer et al., 2018), suggest that there is overlap in the metabolic features of PTSD and metabolic syndrome.
While the links between many mental health disorders and metabolism remain poorly understood, the correlation between depression and metabolism is becoming increasingly clear. Both patients diagnosed with depression and rats exposed to chronic stressors, a common technique for inducing depression-like phenotypes in rodents (Golden et al., 2011), demonstrate altered cerebral metabolic activity as quantified by positron emission tomography (Kumar et al., 1993; Su et al., 2014; Wang et al., 2014). The reduction in metabolic activity has been attributed to reduced glutamate release from neurons which thereby decreases local glucose transport (Popoli et al., 2012). Contrary to this traditional dogma, it is possible that a primary reduction in facilitated glucose transport subsequently suppresses neuronal activity. Facilitated glucose transport is mediated by a family of transmembrane glucose transporters (GLUT) expressed in endothelial cells that comprise the blood-brain barrier and mediate the transport of glucose to become accessible for the metabolic processing of astrocytes and neurons. Previous work has demonstrated that exposure to chronic stress in rats causes sex and age specific alterations in GLUT expression (Kelly, Harrell, and Neigh, 2013) and treatments that are currently effective in treating depression (i.e., pharmacological serotonin and norepinephrine selective reuptake inhibitors) have been shown to alter GLUT function (Hajduch et al., 1999; Shimizu et al., 1998).
Furthermore, the links between depression and metabolic syndrome have been discussed in a recent review (Chan et al., 2019). Potential commonalities between metabolic syndrome and depression may be explained, in part, due to the related underlying genetics among the disorders (Postolache et al., 2019). However, the role of diet as a means of altering peripheral metabolism and contributing to depression pathogenesis remains an active area of research. Indeed, dietary influences that shift metabolism increase the risk of manifestation of depressive-like behaviors in animal models. For example, maternal exposure to high fat diet in rats demonstrated increased depressive-like behavior in their offspring (Giriko et al., 2013). In addition, exposure to a high fructose diet starting in adolescence increases depressive-like behaviors in adult rats (Harrell et al., 2015a). In humans, a Mediterranean diet or vegetable-based diet were positively associated with improved resilience to psychological stress when compared to a Western-style diet consisting of higher fat and sugar intake (Appleton et al., 2015; Bonaccio et al., 2017; Freeman and Rapaport, 2011; Li et al., 2017). Collectively, many of the parallels drawn between PTSD and metabolic disorders by Michopoulos and colleagues, are also present with mood disorders (Gragnoli, 2014). A few of the main metabolic factors that are frequently mentioned in this context are glucocorticoids, leptin, and NPY, which are discussed in further detail later in this review along with less commonly considered neuroendocrine factors.
Collectively, this review aims to present a framework linking stress-induced disorders and the concepts of risk and resilience to the biological mediators of metabolism. We will recapitulate the foundations of stress and allostasis as developed by field founders Selye and McEwen. We will then analyze the connections between stressor exposure and metabolic responses both at the system level and at the level of cellular metabolism with a focus on mitochondria. Finally, we will examine the roles of neuroendocrine mediators of metabolism and their potential roles in risk and resilience. This review aims to provide the essential background and landscape of the current knowledge related to possible metabolic influences of risk and resilience through a neuroendocrine lens in order to provide a platform from which to design and implement critically needed research to explain the manifestation of stress-induced neural disruptions and improve the outcomes of individuals faced with such challenges.
1.2. Building a Context for Risk and Resilience: From Selye to McEwen
In 1926, Hans Selye, a second-year medical student at the University of Prague, remarked to his professor that all his patients, regardless of their diagnosis, appeared to have a similar syndrome. All the patients exhibited a loss of energy, reduced appetite, depressed mood, and general apathy. Selye described these collective observations as the “syndrome of being sick”. His ideas were dismissed as inconsequential at the time, but he went on to study this “syndrome” and in 1936 published his landmark paper on the topic in Nature (Selye, 1936). The degree to which a patient’s body changes in response to some other force, perhaps an infection or injury, and the phenotypic manifestation of that degree of change, which Selye first noted. What we now commonly refer to as stress, is actually ‘strain’. As Selye himself acknowledged, he originally chose the wrong word (Selye, 1956). Also central to this concept is the relationship between stress and strain. The fields of physics and materials science have established clear models for the relationship between stress and strain in elastic materials. Initially, as stress is applied to a material, the relationship between the applied stress and the resulting deformation (strain) is linear and reversible for a certain degree of interaction - the ‘elastic region’ (Figure 1). As stress is continuously applied, the relationship crosses the ‘elastic point’ the interactions are no longer linear yet remain reversible until the final, irreversible, breaking point is reached. Applying these concepts in a biological context provides a useful framework for understanding the principles of stress resilience. Changing the terms adopted by Selye, we replace ‘strain’ with ‘biological stress’, which has since been shortened to just ‘stress’. Given that Selye realized he had used ‘stress’ incorrectly, he had to generate a new term to replace it in order to describe the forces applied on the organism. For this, he chose ‘stressor’; which could include anything that applies biological stress to an organism. This could include physical insults like infection or injury, or physiological insults, such as grief, abuse, or being the subject of social stigma. When we think about resilience, it usually can be imagined in the ‘elastic region’. Biological adaptations, including alterations in metabolic function, will occur in response to stressors, commonly called an ‘acute stress response’. These changes are predictable and reversible. However, in the inelastic region of resilience, we begin to see permanent adaptations. These changes may help facilitate the survival of the organism, though they vary by individual, they may be associated with long-term maladaptations, and may be much harder to reverse. These long-term alterations in the response of an organism to stressors bring us to the concept promoted by Bruce McEwen: allostasis.
Figure 1.
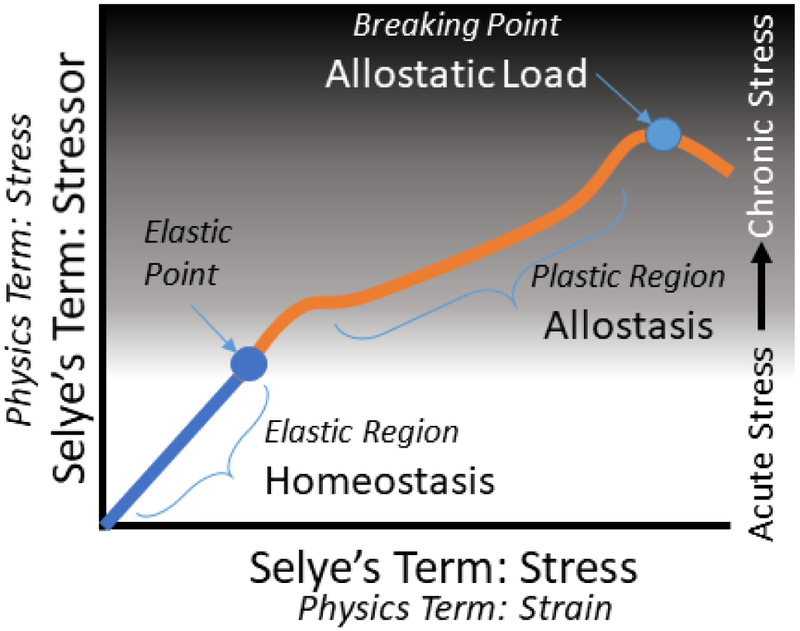
Selye’s ground-breaking concept of ‘stress’ was based on physics concepts. Selye initially confused the terms such that he labeled ‘strain’ as ‘stress’ and thereby had to rename the physics concept of ‘stress’ to ‘stressor’. The concepts of stressor, the external force applied to the organism, and stress, the internal response of the organism, exist in a dynamic relationship. Initially, there is a reversible and predictable relationship between the stressor applied and the stress on the system. This ‘elastic region’ is most akin to homeostasis and the changes that occur during an acute stress response. After repeated or extreme stressors, the relationship between stressors and the stress response passes the ‘elastic point’ and enters the plastic region where the relationship is less predictable, but the organism can compensate for the stressor(s) with systemic and cellular reorganization. This would be akin to allostasis. Finally, with repeated and cumulative stressor burden, the organism reaches the ‘breaking point’ and crosses the line to allostatic load such that significant negative adjustments begin to occur within the organism due to an inability to efficiently resolve the energetic demands placed by the cumulative stressor burden.
Allostasis is a modern expansion of the concept of physiological homeostasis. This concept, first introduced in 1988 by Sterling and Eyer, was proposed as a model to account for physiological and behavioral systems that exhibit variable regulatory responses in order to maintain homeostasis (McEwen; Figure 2). Under certain conditions, such as chronic stress, when the human body is no longer able to effectively maintain allostasis, a state of “allostatic load” arises to compensate for the abnormal performance of allostatic systems. Extensive allostatic load can contribute to disease, and overall dysregulation of the organism in question (Ghini et al., 2015). In one study, allostatic load was utilized as a measure of physiological frailty, and increased allostatic load correlated with increasing age (Crimmins et al., 2003). However, an organism’s ability to maintain allostasis is difficult to quantify, and biological measures must be used. Ghini and colleagues utilized metabolic phenotype, or metabotype, as a measure of an individual’s capacity to adapt to external stimuli or maintain allostasis (Ghini et al., 2015). Using long term tracking of the urine metabotype it was demonstrated that an individual has the ability to maintain a relatively narrow metabolic phenotype, within a much broader scale. In the same study, individuals that experienced a short-term stressor demonstrated metabotype drift but were able to return to their original metabotype range upon termination of the stressor. When the same individuals experienced longer term, chronic stressors, their metabotype was irreversibly changed. The ability to return to baseline metabotype confers a measure of resilience in the response to stress, and may dictate that metabolic parameters can be further used to quantify allostatic load in the face of events such as chronic stress.
Figure 2.
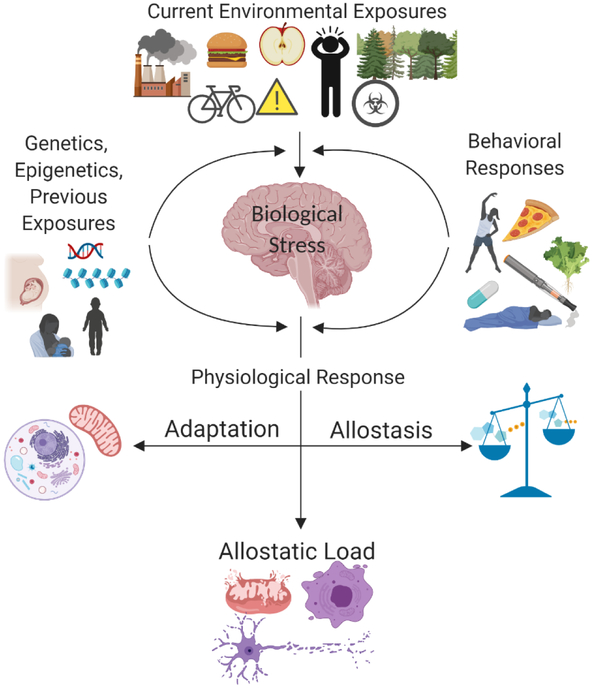
Adapted from McEwen et al., 1998. Allostatic load is the end result of the cumulative biological stress placed on an organism after attempts to adapt and regain allostasis. The biological stress experienced by the organism computational exposure influenced by the current environment (external and internal), previous exposures (genetics, epigenetics, early life experiences), and the behavioral responses to the stressor experienced. Collectively, these factors influence the physiological response and dictate the path to adaptation, allostasis, or cross-over to allostatic load.
An organism’s ability to maintain allostasis is in direct correlation with their capacity for resilience, and allostatic load can be utilized as an inverse measure of an organism’s resilience. Allostasis integrates the role of metabolism in the manifestation of stress-related repercussions and the concepts of stress originally introduced by Selye. As we build towards understanding the roles of neuroendocrine mediators of these phenomena, it is essential that we orient stress in a more complete metabolic context.
2. Defining ‘Stress’ in a Metabolic Context
2.1. Organism-level Responses to Acute and Chronic Stress
The sympathetic nervous system (SNS) and the hypothalamic pituitary adrenal (HPA) axis are both activated during the stress response; however, there are differences in the manner through which acute and chronic stressors activate these systems. These differences have both metabolic and neuroinflammatory implications. In general, the goal of a stress response is to mobilize energy in order to adequately remedy the impact of the stressor. Energy mobilization can occur through increased gluconeogenesis, lipolysis, ketosis or any other catabolic process that provides components that can be utilized as an energy source. In the context of acute stress, these energy mobilization demands are short-lived and generally do not have a lasting global effect on the system. In response to an acute stressor, activation of the SNS stimulates the release of catecholamines which have been shown to cause activation of brown adipose tissue (BAT) resulting in elevated thermogenesis through subsequent activation of mitochondrial UCP1 (uncoupling protein; Lowell and Spiegelman, 2000). As its name suggests, UCP1 is an uncoupling protein located in the mitochondria that works to effectively dissociate energy production from thermogenesis. This is achieved through bypassing the electron transport chain to allow for the oxidative energy to dissipate as heat. Unlike other proteins in the uncoupling protein family, UCP1 is specific to BAT (Klaus et al., 1991). Initially BAT was thought to be negligible in the context of adult human metabolism, with significance only in infants (Drubach et al., 2011; Gilsanz et al., 2011). This view has since changed with evidence that BAT can be generated in adults and exert metabolic activity under the appropriate conditions (Lee et al., 2011; Wang et al., 2015). In mice and rats, central activation of GLP-1R or activation of GLP-1R in the VMH respectively, led to increased UCP1 expression causing the “browning” of white adipose tissue (Beiroa et al., 2014). While we have talked about acute stress activation of BAT, it is important to note that chronic stress leads to an adaptive mechanism where brown adipose tissue is no longer activated as consistent thermogenesis through this mechanism would expend too much energy in a situation where the stressor is prolonged (Rabasa et al., 2019). This illustrates the flexible functionality of normal stress-induced metabolic responses which are programmed to address perturbations to homeostasis in the most efficient manner. Interestingly, an acute stressor of increased severity has been demonstrated to induce temporary anorexic effects, essentially reducing food intake and thereby limiting the supply of exogenous energy sources (Valles et al., 2000). A previous review examining the reciprocity between energy balance and stress response, elucidated the complex interactions between the HPA-axis, sympathetic nervous system and metabolic energy demands in the context of diet, calling for further research on the implications of sex differences on these associations (Harrell et al., 2016).
In the context of energy efficiency, it is important to consider diet as a factor, especially in the purview of chronic stress. Dietary intake provides a replenishing source of energetic fuel to be further broken down and consumed on a cellular level. The energetic makeup of an organism’s diet may have pertinent implications on the efficiency of energy usage and therefore impact the rate of energy exhaustion. Recently, a great deal of attention has turned to the ability of ketone bodies to act as an alternative energy source to glucose within the brain (Courchesne-Loyer et al., 2013). Due to this ability, a variety of studies have undertaken exploring the potential neuroprotective effects of a ketogenic diet (KD) in the face of neurological dysfunction (Choi et al., 2016; Kelley and Hartman, 2011; Prins, 2008). Brownlow and colleagues demonstrated that a KD could have both beneficial metabolic effects and provide cognitive protection in the face of chronic stress in rats (Brownlow et al., 2017) .
If chronic stress creates an energetic shortage in the organism, then in theory, resolution of inefficient metabolic patterns, or supplementation with additional metabolic fuel, should improve the maladaptations associated with chronic stress. Dallman and colleagues demonstrated ameliorative potential of acute sucrose supplementation proximate to stress (Dallman et al., 2003; Foster et al., 2009; Pecoraro et al., 2004); however, chronic increases in sugars within the diet can lead to adverse effects (Harrell et al., 2018, 2016, 2015a). Bypassing the metabolism of sugars and directly supplementing with glycolytic byproducts does appear to confer some benefit. Previous work demonstrates that supplementation with pyruvate, a product of glycolysis, has a protective effect against immunosuppression caused by chronic restraint stress (Neigh et al., 2004b).The idea of energy over-expenditure underlying stress effects (Giovambattista et al., 2000; Neigh et al., 2004a) is further supported by the finding that inhibiting or knocking down PARP-1, an enzyme linked to metabolic energy exhaustion when uncoupled from its cofactors, provides protection against immunosuppression induced by chronic exposure to stressors (Drazen et al., 2001; Neigh et al., 2005). These findings suggest that energy efficiency and balance play a critical role in regulating immune system resilience in conditions of chronic stress and the same principle could hold true for other biological systems.
Building on the idea of cellular metabolism as a critical variable in the manifestation of stress-induced consequences is evidence from the study of hypoxia induced factor-1 (HIF-1). HIF-1 is an oxygen responsive transcription factor that is constitutively expressed and regulated by enzymatic degradation (Sharp and Bernaudin, 2004). Because the brain is physiologically hypoxic, HIF-1 can play a larger role under systemically normoxic conditions as compared to other organ systems. HIF-1 also appears to be responsive to activation of the HPA axis via response to corticotropin releasing factor activation (Chen et al., 2007). Recent work has demonstrated that pharmacological stimulation of HIF-1α leads to an augmented corticosterone response to a brief air puff stressor in rats (Harrell et al., 2015b). This work not only highlighted interactions between the HIF-1 pathway and the HPA axis but also introduced the role of FKBP4 and FKPB5 (co-chaperones for the GR) as potential mediators. Earlier literature shows that hypoxia can lead to alterations in the gene expression of GR, and in turn allow for GR to regulate expression of other hypoxia related genes (Kodama et al., 2003). In a PTSD-like rat model, triple moderate hypobaric hypoxia (MH3) served as a substantial metabolic stressor and led to increased HIF-1α expression in the hippocampus; however, preconditioning with MH3 reduced HIF-1α overexpression upon re-exposure to stress (Baranova et al., 2017). Combined with the relationship between HIF-1α, GR activation, and increased glucocorticoids, this finding suggests that preconditioning to metabolic stressors may provide protection from dysfunctional stress response upon re-exposure. However, this protective effect is stressor specific and further investigation into preconditioning with other metabolic stressors is needed. Also, as the body and brain are comprised of many tissues and cell-types, each under differing metabolic strain in response to chronic stress, it can be appreciated that an increased resolution for understanding tissue-specific metabolic vulnerability to chronic stress is required for the development of appropriate pro-resilient therapies.
2.2. Cellular Effects of Stress: Mitochondria as Tone Setters
At the cellular level, the mitochondria are critical to consider when examining the bridge between organismal stress and long-lasting biological repercussions. Mitochondria are dynamic organelles within the cytoplasm of Mammalian cells that are well regarded as the primary producers of energy. Within the mitochondria oxidative phosphorylation occurs through a series of biochemical reactions, which utilize energetic substrates and oxygen to produce ATP for use in energy dependent reactions. Mitochondria are responsible for the synthesis of glucocorticoids and catecholamines (Picard et al., 2018). As previously discussed, these and other hormones are responsible for substrate mobilization when organismal stress increases energy demand. Mitochondria must then respond to this demand by transforming these liberated substrates into ATP and metabolic signals in order to facilitate allostatic compensation. Due to this reciprocal communication between stress hormones and the mitochondria, mitochondrial dysfunction has the potential to inhibit an adequate stress response or contribute to excessive allostatic load on the organism.
In a similar reciprocal connection to that of glucocorticoids and mitochondria, the metabolic machinery of mitochondria generates reactive oxygen species (ROS) when single electrons are passed down the electron transport chain to terminal oxygen during ATP production. At low levels, ROS are necessary for essential physiological functions such as redox signaling processes (Balaban et al., 2005; Lambert and Brand, 2009; Lambeth, 2004).While they are responsible for ROS production, mitochondria contain antioxidant capabilities and are responsible for elimination of excessive ROS. Mitochondrial dysfunction or excessive energetic availability can contribute to the production of more ROS than the organelle is capable of eliminating. This results in oxidative stress on the organelle and cell, which leads to oxidative damage of biomolecules and mitochondrial DNA (mtDNA) (Yakes and Van Houten, 1997).
The dynamic relationship that the mitochondria have with both glucocorticoids and ROS place mitochondria in the unique position of regulating stress pathophysiology at the subcellular level, while also being a particularly vulnerable target of stress (Picard et al., 2018). Oxidative stress brought on by ROS is akin to the adaptive stress response initiated in the hypothalamus as a result of a psychological or biological stressor. ROS production is a necessary part of mitochondrial function in order to make sure that biological processes continue in balance with one another just as the global stress response is critical to ensuring that an organism can overcome the perceived stressor and return to homeostasis (Rhee, 2006). As previously discussed, allostatic load arises when the stress response does not shut off properly or is not efficiently initiated in the organism. The same is true for the mitochondria. The mitochondria generate ROS and oxidative stress to regulate allostasis in the organism, but when the mitochondria are unable to efficiently control this process, a state of allostatic load arises, and the oxidative stress brought on by ROS has the potential for far reaching ramifications.
Recently, several groups have conducted studies elucidating the integrative connection between chronic stress and oxidative stress by way of the mitochondria. Chakravarty and colleagues found that subjecting zebrafish to chronic unpredictable stress (CUS) resulted in altered brain proteome profile, in particular proteins involved in the regulation of mitochondrial function, oxidative stress, and glycolysis (Chakravarty et al., 2013). An earlier study, exposed altered proteomic and metabolomic profiles in mice with high anxiety-related behavior in comparison to mice exhibiting normal and low anxiety-related behavior. These proteins and metabolites were indicative of alterations in mitochondrial structure and functions, revealing that the mitochondria play a substantial role in stress-related pathophysiology (Filiou et al., 2011). In mice subjected to chronic social defeat, it was observed that the stress-susceptible animals experienced elevated levels of the antioxidant glutathione within the anxiety-processing brain region of the ventral hippocampus, and aberrant antioxidant levels could be normalized upon antidepressant treatment (Hamilton et al., 2018). In another study, the neuroprotective effect of A68930, a selective D1 agonist that inhibits oxidative stress by modulating the antioxidant system was investigated in an acute stress and chronic unpredictable stress model in rodents. A68930 administration was shown to normalize stress induced modifications of the cell’s antioxidant machinery (Rasheed et al., 2011).
Genetic influences also appear to influence the mitochondrial response to challenges. The deletion of p66SHC, a gene linked to metabolic regulation, apoptosis and ROS, provided the benefit metabolic resilience in the context of both high fat diet and stress in mice, suggesting a genetic link to resilience (Bellisario et al., 2014). Further, investigation of p66shc revealed that it has dual functions, serving as a mediator of insulin signaling through generation of ROS and acting in a pro-apoptotic capacity (Bhat et al., 2015). Although the precise mechanisms for genetic alterations to central metabolism remain unclear, it is apparent that espousing mechanisms for resilience requires a better understanding of genetic influences on metabolic responses. Collectively, these studies shed light on the contribution of excess organismal stress on the mitochondria and subsequent oxidative stress, but further investigation is needed to determine if these mechanisms are correlative or causal in driving behavioral adaptations to stress.
3. Key Neuroendocrine Mediators of Metabolic Influences on Risk & Resilience
Neuroendocrine signals are well-positioned to influence metabolism broadly and mitochondria specifically. While it is already known that the HPA axis has reciprocal communication with metabolically active hormones including leptin and ghrelin; the primary messenger of the HPA axis, glucocorticoids, are themselves metabolically active. In addition, neuroendocrine signals that are less commonly associated with metabolism can play essential roles through interaction with classic metabolic regulators and through direct actions. In this section we will review existing knowledge of neuroendocrine regulators of metabolic responses at both the systemic and cellular levels. A brief overview of each candidate is also available in Table 1.
Table 1.
A snapshot view of the main candidate hormones and neurotransmitters that metabolically influence risk and resilience in the context of stress. Here, the normal physiological functions that allow for the maintenance of homeostasis are described. The categories depicted in Figure 2 (Current Environmental Exposures, Behavioral Responses, Genetics, Epigenetics, Previous Exposure, etc.) all have the ability to modify these physiological functions contributing to allostatic load and potential metabolic dysfunction. It is important to note that while the “Response to Stress” column reports either an increase or decrease in the corresponding factor, this relative quantity varies based on a multitude of factors ranging from type to intensity of stressor.
| Hormone/ Neurotransmitter |
Origin | Normal Metabolic/Physiological Function | Response to Stress |
|---|---|---|---|
| Cortisol | Zona fasciculata in cortex of adrenal gland |
|
Increase |
| Leptin | Adipose Tissue |
|
Increase |
| Ghrelin | Ghrelin cells in stomach and small intestine |
|
Decrease |
| Glucagon-like peptide-1 (GLP-1) | Gut; intestinal L cells |
|
Increase |
| Somatostatin | VMH; stomach, intestine, pancreatic δ cells |
|
Increase |
| Neuropeptide Y (NPY) | ARC, PVN, supraoptic nucleus, suprachiasmatic nucleus |
|
Varies |
| Oxytocin | PVN, supraoptic nucleus |
|
Increase |
| Estrogen | Ovaries, testis, adrenal cortex |
|
No Change (acute) Decrease (chronic) |
| Testosterone | Testis, ovaries, adrenal cortex |
|
Increase (acute) Varies (chronic) |
| Brain-derived neurotrophic factor (BDNF) | Brain, gut, and other tissues |
|
Increase (acute) Decrease (chronic) |
| Arginine Vasopressin (AVP) | Hypothalamus |
|
Increase |
| Insulin-like growth factor (IGF-1) | Liver |
|
Decrease |
3.1. HPA-axis: metabolic driver during the stress response
One of the main information centers for metabolic regulation is the hypothalamus, which is responsible for functions related to energy balance, thermoregulation, reproductive behaviors, and stress response. The hypothalamus receives input from the hippocampus and prefrontal cortex to assist in the modulation of the stress response. While many of these inputs are in the form of negative feedback, they help regulate the duration and intensity of responses to a stimulus within the context of stress (Heidbreder and Groenewegen, n.d.; Mizoguchi et al., 2003). The HPA-axis is activated when the paraventricular nucleus (PVN) of the hypothalamus releases corticotropin releasing hormone (CRH or CRF) which binds to its receptor (CRHR1) in the anterior pituitary gland. Binding of CRH to CRHR1 results in the release of adrenocorticotropic hormone (ACTH) from the pituitary. ACTH then acts on the adrenal glands to promote synthesis of glucocorticoids. One of the important glucocorticoids released by the adrenal gland is cortisol, corticosterone in rodents. Under normal circumstances, cortisol binds to the mineralocorticoid receptor and provides resting regulation of the HPA axis. When presented with a stressor, the glucocorticoid receptor binds cortisol and results in negative feedback to the hypothalamus and pituitary, causing a reduction in HPA axis activation. Chronic stress exposure causes desensitization to cortisol feedback and, over time, allows for inappropriate continuation of HPA axis activation. CRH has been described as the catalyst to HPA- axis cascade activation. It is important to note that CRH activity is modulated by the type and intensity of the stressor (Aguilera and Liu, 2012). Metabolic adaptations occur during stress which increase caloric efficiency (Rabasa and Dickson, 2016), and these adaptations may be linked to the actions of cortisol/corticosterone given that CRHR1/CRHR2 knockout mice do not exhibit the benefit of increased caloric efficiency (Preil et al., 2001)).
3.2. Cortisol: classic metabolic-acting stress hormone
Cortisol is a glucocorticoid produced by the zona fasciculata in the cortex of the adrenal gland. It is a steroid hormone that operates as the body’s main stress hormone, which affects a variety of organ systems, with a number of implications towards metabolism. Cortisol is released in response to a variety of stimuli, notably when the organism encounters a stressful situation, and this allostatic response is intended to help the organism overcome the stressor until it is able to return to physiologic homeostasis. When the stressful situation resolves negative feedback employed by cortisol acts on sections of the HPA axis to inhibit CRF, arginine vasopressin (AVP), and ACTH release. Once released, the primary role of cortisol is to increase blood glucose through promotion of gluconeogenesis, counteracting insulin. Cortisol also impacts protein and fat metabolism leading to further support of the CNS during an HPA axis response. Cortisol increases the levels of free amino acids in the bloodstream, inhibiting protein synthesis and muscle uptake of amino acids (Brillon et al., 1995). Furthermore, studies have shown that increased glucocorticoid levels promote lipolysis, increasing serum free fatty acid levels (Serr et al., 2011). Taken together, cortisol serves to mobilize components of macronutrients, shifting physiological systems away from anabolic efforts, in order to support the body during times of stress in an energy efficient manner. Cortisol levels also interact with other classical metabolic hormones, such as leptin and ghrelin, as discussed in further portions of this review. In conjunction with metabolic effects, cortisol plays a role in cardiovascular function, through the increase of blood pressure (Kelly et al., 1998) and suppression of the immune system. Furthermore, glucocorticoids are profoundly influential on the function of mitochondria as recently reviewed (Lapp et al., 2019; Picard et al., 2014).
3.3. Leptin: counteracting cortisol
Leptin, a peptide hormone primarily produced by adipose tissues, plays a key role in metabolic homeostasis via the inhibition of hunger. Plasma leptin levels have been shown to be higher in humans with higher BMI and higher body fat percentage (Schwartz et al., 1996). Following release by the adipose tissues, leptin signals to the brain regarding the energy available from said adipose tissue reserves. In both rodents and humans, this signaling results in an inhibition of hunger and an increase in energy expenditure in order to account for food intake and maintain metabolic homeostasis (Halaas et al., 1995; Jorgensen et al., 1998). In addition to the ability of leptin to modulate feeding behavior, leptin possesses the ability to inhibit glucocorticoid secretion from the adrenal gland (Pralong et al., 1998) and circulating leptin levels have been shown to be inversely related to HPA axis activity (Aschbacher et al., 2014; Licinio et al., 1997). In instances of chronic stress, HPA axis function is disrupted, and given leptin’s reciprocal relationship with glucocorticoids and the axis in general, this provides implications for metabolic energy balance.
Furthermore, adequate leptin signaling is essential for cell survival following injury, at least in part, through modulation of mitochondrial function (Hu et al., 2019). Mitochondrial modulation by leptin has also been demonstrated in hepatocytes, where peripheral injection of leptin stimulated mitochondrial fusion and attenuated high glucose-induced fatty acid accumulation (Hsu et al., 2015). Evidence of leptin’s direct impact on mitochondria and integral relationship with the HPA axis provides basis for the connection between metabolism and implications for risk and resilience.
3.4. Ghrelin: more than just appetite
Another classical metabolic hormone, ghrelin, is produced within cells in the gastrointestinal tract and acts on the hypothalamus to stimulate appetite, gastric acid secretion, and gastrointestinal motility. Ghrelin is also implicated in food reward associated behavior (Klok et al., 2007; Perello and Dickson, 2015). Considering ghrelin’s opposing action to that of leptin, it is unsurprising that the receptor for ghrelin is located in the same places within the brain as the leptin receptor. Together with leptin, ghrelin plays a key role in maintaining energy homeostasis. Aside from the adjustment of hunger levels, ghrelin provides insight into the connection between an organism’s response to stress and metabolism. Ghrelin plasma concentrations increase in parallel with cortisol plasma concentrations in humans following a psychological stress (Kristenssson et al., 2006); however, the physiological underpinnings for this relationship are presently unknown. Further studies have been conducted solidifying this relationship. Azzam and colleagues showed that increased cortisol serum levels are positively associated with serum ghrelin levels following ACTH stimulation and hydrocortisol administration, and subsequent blocking of HPA axis stimulated cortisol synthesis was associated with decreased ghrelin levels (Azzam et al., 2017). This suggests that circulating cortisol, and possibly central increases in ACTH and CRH, are necessary for elevations in ghrelin plasma concentration. In animal models, acute and chronic psychological stresses are associated with increased ghrelin secretion (Asakawa et al., 2001; Kristenssson et al., 2006; Ochi et al., 2008; Patterson et al., 2010). The mechanism behind stress induced ghrelin increases is not completely understood, but these studies suggest that ghrelin levels are not only based solely on energy availability and output but are affected by other neuroendocrine stimuli such as a chronic stress environment. Furthermore, additional studies have shown that ghrelin plays an important role in HPA axis regulation. Spencer and collaborators, demonstrated that following an acute stressor, endogenous ghrelin attenuates HPA axis activity and anxiety-like behavior utilizing ghrelin knockout mice (Spencer et al., 2015). Other studies have demonstrated an anxiolytic effect of ghrelin in the presence of chronic stress conditions, demonstrating an interesting duality to ghrelin’s regulation of the HPA axis in relation to experimental conditions (Lutter et al., 2008). The reciprocal nature of communication between the HPA axis and ghrelin in the context of stressful conditions denotes the need for further studies, especially in relation to the possibility of increasing resilience to stress through the manipulation of metabolic factors, such as ghrelin and leptin. Highlighting this point is the finding that, similar to leptin, ghrelin has neuroprotective functions through improved mitochondrial function in the face of neural challenge via modification of ROS (Ishii et al., 2018). Ghrelin has also demonstrated neuroprotective effects via suppression of apoptotic pathways within the mitochondria (Dong et al., 2009). Other neuroprotective effects of ghrelin have been shown to be dependent on UCP2, a mitochondrial protein that functions in mitochondrial respiration, mitochondrial biogenesis, and ROS production (Andrews et al., 2009, 2005). Although not directly connected to psychosocial stress, the well-documented neuroprotective effects of ghrelin via mitochondrial mechanisms provides additional backing to the prospect of regulating risk and resilience through modulation of metabolic components.
3.5. Oxytocin: exertion of metabolic control
Other hypothalamic hormones also have profound consequences on metabolism. Oxytocin is a neuropeptide produced in the paraventricular nucleus and supraoptic nucleus of the hypothalamus and secreted by the posterior pituitary. Oxytocin has well-defined roles in social bonding, parturition, reproduction, appetite regulation, and is a key modulator of HPA axis activity (Feldman et al., 2007; Kosfeld et al., 2005)With such diverse functions, all of which are associated with metabolic efficiency, it is not surprising that there is evidence that oxytocin exerts control over metabolic homeostasis. Injection of oxytocin both centrally and peripherally has been shown to induce anorexia (Herisson et al., 2016; Maejima et al., 2011). Rodents deficient in oxytocin have demonstrated obesity without changes in total food intake (Camerino, 2009; Takayanagi et al., 2008). In diet-induced obese mice, plasma oxytocin levels were shown to decrease and central oxytocin infusions have shown to induce weight loss in mice (Deblon et al., 2011). Other studies outline the importance of oxytocin in feeding behavior rhythm (Zhang and Cai, 2011) and in food preference. Oxytocin knockout mice exhibited enhanced intake of carbohydrates, but not fats, and these effects were dissociated from palatability (Miedlar et al., 2007; Sclafani et al., 2007). Collectively, these studies suggest a strong input of oxytocin on metabolic integrity.
The social function of oxytocin may also confer some aspects of resilience. Seeking and maintaining strong social support before, during, and after stress exposure encourages quicker recovery following a traumatic or stressful incident (Fitzsimmons and Bardone-Cone, 2010; Gunnar and Hostinar, 2015; Mahmoud et al., 2015; Perreault et al., 2017). Muroy and colleagues demonstrated that stress exposure in the “threatening” context of predator odor led to decreased social support seeking behavior, increased social withdrawal -akin to PTSD- and reduced oxytocin signaling (Muroy et al., 2016). In the same study, rats that were exposed to the same moderate stressor in a neutral context displayed the opposite, with increased social support seeking behaviors as well as increased oxytocin signaling. In a related study, rats that were exposed to an odor-shock stimulus while still in close proximity to their mother displayed a preference for that odor as well as suppression of corticosterone release compared to the counterparts who were exposed to the odor-shock in the absence of their mother (Moriceau et al., 2006).
Oxytocin also influences mitochondrial function through the reduction of damage from ROS and is protective of cardiomyocytes during injury (Gonzalez-Reyes et al., 2015). Although not yet extended to the context of psychosocial stress, it is possible that oxytocin provides some of its resilience-promoting features via mitochondrial influence.
3.6. Arginine Vasopressin: connections to metabolism
Another hypothalamic hormone connected to energy homeostasis is arginine vasopressin (AVP), also known as antidiuretic hormone (ADH). Akin to oxytocin, it is a peptide hormone produced in the hypothalamus that is subsequently released by the posterior pituitary. The primary role of AVP revolves around the maintenance of water and electrolyte balance, and its release is stimulated in instances of extracellular fluid tonicity. While connections to metabolic homeostasis are not as prevalent as other hormones previously discussed, studies have been done connecting copeptin to global energy maintenance. Copeptin is a peptide derived from the same pre-prohormone as AVP and is a useful measurement for studies because of AVP’s relatively short half-life (Katan et al., 2007). Increased levels of copeptin have been associated with insulin resistance and incidence of metabolic syndrome (Saleem et al., 2009)
AVP is also cardio-protective during ischemic injury through reduction of mitochondrial permeability and reduced ROS (Nazari et al., 2015). It is possible that in addition to global metabolic effects, AVP could influence resilience at the level of mitochondrial function. This could be either a direct action as proposed in the case of cardiac ischemia or through interactions with the HPA axis. Studies suggest that AVP serves as an activator of the HPA axis in instances of chronic psychosocial stress, subsequently amplifying the release of ACTH from the anterior pituitary (Katan et al., 2008). This mechanism is the proposed basis for the connection between insulin resistance and metabolic syndrome, as heightened ACTH and subsequent heightened cortisol levels often result in a variety of endocrine disruptions that negatively impact metabolic homeostasis. Studies conducted in humans and cows have additionally shown that AVP directly stimulates cortisol release through receptors present on adrenal cortex cells (Perraudin et al., 1993; Senn et al., 1995).
3.7. Somatostatin: stress and metabolism
Somatostatin is a peptide hormone synthesized both in the pancreatic islet in the digestive system and by neural sub-populations. Its actions are mediated through five G-protein coupled receptor subtypes (sst1-5). Traditionally, somatostatin is thought of in the context of diet and nutritional metabolism. However, somatostatin signaling has been implicated in the reduction of CRH release, and therefore, exerts metabolic regulation of stress response that is independent of mitochondrial involvement at this level. Engin and Treit found that intracerebroventricular administration of somatostatin was sufficient to provide antidepressant-like effects as modeled by elevated plus maze and forced swim behavioral testing (Engin et al., 2008; Engin and Treit, 2009). The actions of somatostatin on the release of CRH and overall HPA axis activation appear to be mediated by somatostatin receptor subtype sst2 in the pituitary (Engin and Treit, 2009; Prévôt et al., 2017). Additionally, somatostatin signaling led to reduced corticosterone elevation in hippocampus following a foot shock stressor (Prévôt et al., 2017). Although it remains unclear if somatostatin acts directly at the level of the PVN where CRH release occurs, it is evident that regulation of CRH via somatostatin signaling could play a role in the development of a resilience phenotype through interactions with the HPA axis.
Furthermore, somatostatin expression is a common molecular marker for local inhibitory GABAergic interneurons localized to key brain regions. Somatostatin positive neurons represent approximately one-third of the total interneuron population (Lee et al., 2010), and are increasingly implicated in regulating the pathogenesis of neuropsychiatric syndromes, like addiction and depression (Ribeiro et al., n.d.). Indeed, somatostatin positive interneurons are depleted in postmortem limbic and cortical tissues from humans diagnosed with depression (Guilloux et al., 2011; Sibille et al., 2011; Tripp et al., 2012, 2011), and their disinhibition within cortical and hippocampal regions of the rodent brain promote an antidepressant-like phenotype (Pryce and Fuchs, 2017). Specifically in the prefrontal cortex, a brain region central in stress processing (Duman, 2014), somatostatin positive interneurons regulate the balance of local excitatory and inhibitory neurotransmission, which has been shown to be dysregulated in stress and depression-related disorders (Ghosal et al., 2017a). Collectively, this emerging evidence suggests that the function of neuropeptide expressing GABAergic interneurons, like somatostatin positive interneurons, are required for normalizing the excitatory/inhibitory tone in a brain-region specific local micro-circuit, and their long-term dysregulation could be a cellular substrate of allostasis in response to chronic stress.
3.8. Neuropeptide Y: psychiatric resilience at a potential metabolic cost
Another signaling molecule of interest is Neuropeptide-Y (NPY), which is a hypothalamic orexigenic peptide that stimulates food intake (Beck, 2006). NPY, which is found in high concentrations in the hippocampus, hypothalamus, cortex, and amygdala, is released with norepinephrine under normal circumstances in response to sympathetic nervous system activity. NPY signaling leads to increased circulating corticosterone in rodents, suggesting an appropriate response to stressors (Cohen et al., 2012). This also demonstrates the integrated signaling that occurs between the SNS and HPA-axis, where SNS activation causes hypothalamic stimulation leading to both NPY release and HPA-axis activation, resulting in increased stress hormones. It is important to note that as a mediator of energy expenditure, mitochondria engage in a unique relationship with NPY signaling. In instances of food deprivation or reduced energy sources, the mitochondria found in NPY neurons undergo fusion to increase their size in an attempt to maximize energy efficiency and storage (Dietrich et al., 2012). Additionally, increased NPY leads to a reduction in UCP1, thereby decreasing the thermogenic potential of the mitochondria in instances where positive energy balance is favorable (Billington et al., 1994). Lack or reduction of NPY in the context of stress can produce a blunted stress response which has been linked to increased susceptibility to depression, and anxiety (Cohen et al., 2012; Hou et al., 2006; Rasmusson et al., 2000; Sah et al., 2009). A reduction in NPY signaling was discovered in rats displaying PTSD-like behaviors compared to their counterparts (Cohen et al., 2012). Reinforcing the idea that NPY plays a role in the resilience phenotype, Sabban and colleagues demonstrated that intranasal NPY prior to stress exposure reduced anxiety-like behavior in rats (Sabban et al., 2015). In humans, a study found higher levels of NPY in war veterans without PTSD in comparison to their counterparts with PTSD, again suggesting a potential role for NPY in resistance to PTSD manifestation (Yehuda et al., 2006). The benefits of NPY extend beyond PTSD and anxiety resilience as anti-depressant effects have also been demonstrated with increased NPY (Gelfo et al., 2012). NPY also interacts with brain derived neurotrophic factor (BDNF) to encourage neural growth in the hippocampus and may serve as a support for the resuming homeostasis following stress-induced disruptions (Cohen et al., 2012). Similarly to NPY, the actions of BDNF have also been linked to the resilience phenotype seen in the context of chronic stress with increased BDNF expression linked to reductions in anxiety-like and depressive-like behaviors in rodent models (Taliaz et al., 2011; Tyagi et al., 2015; Yao et al., 2016). However, these stress protective effects of BDNF are brain region specific; with positive effects linked to elevated BDNF in the hypothalamus and hippocampus, whereas elevations in the nucleus accumbens and ventral tegmental area (VTA) are associated with increased depressive-like behaviors; suggesting differential roles of BDNF (Berton et al., 2009; Eisch et al., 2003; Wook Koo et al., 2016). Taken together the actions of hippocampal or hypothalamic BDNF and NPY seem promising, however, NPY is potently stimulated by stress models that have been associated with increased adiposity (Rabasa and Dickson, 2016). While NPY is considered an element of the resilience phenotype for PTSD, and mood disorders, it appears to be linked to excess energy availability which is implicated in the development of metabolic dysregulation. This contradictory involvement of NPY suggests that like many components of the neuroendocrine and metabolic systems, NPY requires tight regulation to promote resilience from psychosocial impairments in the context of stress.
3.9. Insulin-like Growth Factor: from cells to cognitive behavior
Insulin-like growth factor-I (IGF-I) belongs to a family of hormones that primarily regulate cell growth and differentiation. The liver serves as the main site for IGF-I production in mammals (Yakar et al., 1999), but IGF-I has various roles throughout the body and is implicated in many functions of the brain, including astrocyte mediated neurogenesis (Aberg et al., 2000; Torres-Aleman, 2010). Although IGF-I has been linked to improved cognition (Trejo et al., 2007; Tronson and Collette, 2017; Vidal et al., 2016), it also plays a role in stress regulation through metabolic actions. One such action is its modulation of insulin sensitivity and carbohydrate metabolism, both of which are integral to energy availability and expenditure at baseline and in the activation of a stress response. IGF-I activity has been shown to increase BDNF activity, which may also contribute to its potential actions in conferring stress resilience (Carro et al., 2000; Landi et al., 2009). Depressive-like behaviors were observed in mice with prolonged IGF-I deficiency, and increased anxiety-like behaviors were seen in diabetes induced rats who exhibited an IGF-I deficiency (Aksu et al., 2012; Mitschelen et al., 2011). Similarly older adults exhibiting depressive symptoms who were otherwise healthy were found to have reduced levels of IGF-I (Lin et al., 2014). Exercise has been demonstrated to increase IGF-I levels in the hippocampus (Carro et al., 2000). However, it appears that IGF-I may exert sex-specific effects on exercise mediated stress resilience, though there seems to be conflicting evidence. In mice, the protective interaction between IGF-I and exercise was only evident in females with re-exposure to a stressor, although male mice exhibited a greater reduction in anxiety-like behavior following an initial stress exposure (Munive et al., 2016). In male mice given human IGF-I and exposed to exercise, a reduction in depressive-like behaviors was demonstrated, which was then blocked by administration of Anti-IGF-I (Duman et al., 2009). While Duman and colleagues did not re-expose their mice to the stressor, the results seen upon initial stress exposure with the male mice matches what Munive and colleagues reported. The actions of IGF-I in males suggest that a short-term benefit of reduced depressive-like behavior can be achieved by increasing IGF-I, potentially through exercise, whereas in females it is likely that a longer-term benefit is conferred with the resilience to stressor re-exposure. In a study with elderly men, an increase in serum IGF-I along with an improvement in anxiety and mood was observed after strength training exercise (Cassilhas et al., 2010). This reinforces the synergistic action of IGF-I and exercise on the promotion of anti-depressive-like behavior. the interaction between IGF-I and estrogen to promote anxiolytic effects is strengthened by physical activity (Munive et al., 2016). Given the higher prevalence of psychiatric disorders in females in comparison to males, the integrated impact of estrogen, IGF-I and exercise on mood regulation sound promising in terms of potential therapeutic regimens. However, it is important to note that aside from the animal models, there is a lack of studies that investigate the relationship between IGF-I and exercise in females within the context of stress and psychosocial outcomes. Additionally, given discrepancies in the intensity and duration of various exercise regimens, it has been difficult to pinpoint the combination that yields optimal IGF-I levels to promote resilience (Berg and Bang, 2004; Nindl et al., 2009). Because of these gaps in information, the question remains as to whether an equivalent intervention is just as efficacious in individuals with lower estrogen levels, or if hormone supplementation would boost the effects in males, hypogonadal individuals, and postmenopausal women.
3.10. GLP-1: estrogen specific promoter of energy balance
GLP-1 (Glucagon-like peptide-1) is an incretin peptide hormone expressed in both the gut and the brain. In addition to an emerging role for GLP-1 in regulating the addictive actions of drugs ranging from cocaine (Reddy et al., 2016)to nicotine (Tuesta et al., 2017), it plays a role in metabolism by acting in conjunction with estrogen to promote energy balance, and reduction in weight gain through modulating food intake and reward (Maske et al., 2017; Richard et al., 2016; Vogel et al., 2016). GLP-1 activity has been known to stimulate the HPA-axis leading to increases in ACTH, arginine vasopressin (AVP), and cortisol/corticosterone (Bojanowska and Stempniak, 2000; Gil-Lozano et al., 2010; Kinzig et al., 2003; Larsen et al., 1997). Given that its receptor; GLP-1r colocalizes with CRH neurons in the PVN, it follows that GLP-1 has the ability to act as an HPA axis modulator. Exposure to chronic stress has been linked to downregulation of the GLP-1 precursor, preproglucagon (PPG) and thus a reduction in GLP-1 (Zhang et al., 2010). This suggests an innate mechanism to reduce overstimulation of stress response mediators, especially given that chronic activation of the HPA axis often leads to diminished negative feedback from cortisol/corticosterone because of desensitization. However, deletion of GLP-1r resulted in a reduction of HPA response to stress, and a reduction in anxiety-like behaviors as modeled by elevated plus maze, further validating the effect of GLP-1 signaling on cortisol/corticosterone release via GLP-1r (Ghosal et al., 2017b). In contrast, chronic administration of the clinical analog and GLP-1r agonist, Exendin-4, reduced depression-like behaviors in rats (Anderberg et al., 2016), indicating that the brain-region specific actions of GLP-1 need to be further elucidated.
3.11. Traditional sex hormones: estrogen and testosterone
Previous work has demonstrated that the response to chronic stress is sex-dependent and heavily influenced by the developmental time period during which the initial stressor was encountered (Pyter et al., 2013). While females tend to be more susceptible to the development of affective disorders with exposure to early life stress, males display an increased risk of metabolic dysfunction (Bekhbat and Neigh, 2018; Bourke and Neigh, 2011; Neigh et al., 2009; Yang and Kozloski, 2011). There is also a distinction between symptomology with males expressing more externalization, marked by aggression; whereas, females demonstrate internalization through social avoidance. These distinctions result in dimorphic behavioral and metabolic phenotypes. Given the differences in susceptibility, many studies have investigated the role of sex-specific hormones in stress response outcomes and the resulting phenotypes.
Estrogen exerts powerful influence over both metabolic and stress-related outcomes. Assessment of the impact of estrogen therapy on the metabolic profile of post-menopausal women demonstrates significant alterations in pathways related to metabolism and stress responses, energy production, and inflammation (Stevens et al., 2018) In a purely metabolic context, estrogen encompasses antioxidant properties and promotes anti-apoptotic pathways in the mitochondria (Borrás et al., 2009; Engler-Chiurazzi et al., 2017; Simpkins et al., 2008). Given the previously described relationship between oxidative stress and negative outcomes, estrogen’s protective effects on the mitochondria serve as a mechanism to reduce metabolic stress. Furthermore, social stress interacts with estrogen and genotype to dictate metabolic and HPA axis outcomes for females (Michopoulos and Wilson, 2011) and this literature is thoroughly reviewed (Michopoulos, 2016; Novais et al., 2017). Therefore, we will focus here on estrogen influences on mechanisms and phenotypes related to resilience. Long-term ovarian hormone deprivation caused by ovariectomy in rats has been demonstrated to increase vulnerability to depressive-like and anxiety-like behaviors following stress exposure (Lagunas et al., 2010; Mahmoud et al., 2016). This aligns with increased incidence of depression among postmenopausal women (Ryan et al., 2011; Scott et al., 2012). In the context of estrogen, administration of estrogen receptor β (ERβ) agonist or estradiol replacement in ovariectomized female rats resulted in a reduction of anxiety-like and depressive-like behaviors (Bredemann and McMahon, 2014; Lund et al., 2005; Walf et al., 2004; Walf and Frye, 2005). Similar outcomes have been seen with estradiol supplementation in hypogonadal and postmenopausal women (Lee et al., 2012).
Postmenopausal women are more susceptible to anxiety and depression, which may be linked to the lower expression of circulating estradiol (Zanardi et al., 2007). While estrogen therapy seems like a promising option to combat anxiety/depression in both males and females, it remains unclear how efficacious it is in humans. Studies with postmenopausal women who naturally have severe reductions in circulating estrogen have either yielded very little or no change to anxiety behaviors with estrogen supplementation (Charney, 2004; Demetrio et al., 2011; Gleason et al., 2015). Additionally, a recent study on the use of hormone replacement therapy in postmenopausal women demonstrated an increased risk of Alzheimer’s disease, but concluded that the risk was also dependent upon the application of therapy, suggesting the need for further investigation (Savolainen-Peltonen et al., 2019). Animal studies, mainly using rodents have demonstrated both anxiolytic and anxiogenic effects of estradiol supplementation that appears to be dose-dependent and may also be reliant on the estrogen receptor subtype being activated (Spiteri et al., 2010). Several studies in ERβ knockdown rats have shown increased anxiety-like behaviors, suggesting that ERβ is critical to the anxiolytic functions of estradiol (Lund et al., 2005; Walf et al., 2008). Conversely, studies of ERα knockdown rats demonstrated decreased anxiety-like behavior(Spiteri et al., 2012, 2010). Together these reaffirm the idea that estrogen receptor subtypes are crucial in mediating the effects of estradiol on anxiety. Other research has shown the link between polymorphisms of the ESR1 and ESR2 genes that encodes for estrogen receptors ERα and ERβ respectively and susceptibility to anxiety(Ryan et al., 2011; Tiemeier et al., 2005). These studies focused primarily on elderly populations, so there is a possibility that age also plays a role in susceptibility. It is known that estradiol causes increased ACTH release via its interaction with the ERα, but this may also be part of its joint effect with GLP-1.
Testosterone is the sex steroid hormone associated with physiological male defining characteristics. However, testosterone is present in both sexes and plays a role in metabolic function as well as modulation of mood disorders and anxiety phenotypes. ADIOL (5-androsten-3β, 17β-diol) and 3β-diol(5α-androstane-3β,17β-diol), two androgen metabolites of dehydroepiandrosterone (DHEA) and dihydrotestosterone (DHT) respectively, have been shown to reduce anxiety-like behaviors through their actions on ERβ (Frye et al., 2008; Handa et al., 2008). Much like estrogen, they have also demonstrated anti-inflammatory properties, which are likely mediated through ERβ as well (Zuloaga et al., 2012). Given its status as a steroid hormone, testosterone is able to modulate downstream gene expression through its interactions with the androgen receptor (AR) and interactions of its metabolites with ERβ. While the mechanistic actions behind testosterone’s involvement with mitochondrial function still remain unclear, evidence suggests that testosterone plays a critical role in maintenance of mitochondrial integrity as testosterone levels have been linked to regulation of oxidative stress (Kang et al., 2018; Pintana et al., 2015; Rovira-Llopis et al., 2017; Tostes et al., 2016; Yan et al., 2017). Attesting to its significance, testosterone has positive implications for cellular outcomes following neural injury. Administration of testosterone reduces ROS, improving mitochondrial membrane potential (Toro-Urrego et al., 2016) and apoptosis and this is further highlighted following laboratory-induced traumatic brain injury such that neurodegeneration is reduced (Carteri et al., 2019; Kang et al., 2018).
The precise mechanisms by which testosterone interacts with both the HPA axis and cellular metabolism are not fully defined and the extent to which these effects replicate in humans is still being ascertained. Currently the missing link is more extensive investigation into whether effects seen in animal models are conserved in humans. Rubinow and colleagues demonstrated increased ACTH with testosterone replacement but found that cortisol modulation only occurred with hormone stimulation and not induced hormonal activity (Rubinow et al., 2005). This suggests that cortisol is more tightly regulated by endogenous hormones in comparison to ACTH. Interestingly, testosterone administration in gonadectomized male rats resulted in a reduction of depressive-like outcomes but was found to be mediated through its aromatization to estradiol (Carrier et al., 2015; Carrier and Kabbaj, 2012). Elevated testosterone expression has been documented in individuals who are engaged in a positive social environment and has been loosely linked to the concept of social inclusion/belonging (Edwards, 2006). In instances where a stressor is encountered, it has been shown that testosterone levels decrease. This decrease in testosterone allows for susceptibility to anxiety and depressive behaviors as demonstrated by outcomes of stress exposure in hypogonadism (DiBlasio et al., 2008; Jaime Herrera-Pérez et al., 2012; Shores et al., 2004; Wainwright et al., 2011; Zarrouf et al., 2009).
4. Harnessing Resilience: Interventions to Promote Positive Outcomes
Metabolic regulation is integral to the stress responses implicated in PTSD and mood disorders like depression and anxiety. For this reason, future research should focus on interventions that encourage pro-adaptive metabolic function. Given what is known about neuroendocrine signaling and its interdependent relationship with metabolic function, it seems imperative to explore the combined actions of both traditional neuroendocrine regulators of metabolism such as cortisol, leptin, and ghrelin, as well as less commonly associated neuroendocrine mediators including oxytocin and somatostatin. Evidence of synergism suggests that resilience is likely hinged upon some combination of neuroendocrine factors. For instance, Sadagurski and colleagues demonstrated that caloric restriction reduced hypothalamic inflammation in both male and female mice, while the use of anti-aging drugs acarbose (ACA), nordihydroguaiaretic acid (NDGA) and 17-α-estradiol (17αE2) only provided benefits to the male mice (Sadagurski et al., 2017). This highlights an important area of study which is the interaction of sex steroids and sex chromosomes with metabolic regulation and mitochondrial function. This area is a burgeoning point of focus and may lead to essential new findings related to the sex differences epidemiologically observed in stress-related disorders including depression and PTSD. Collectively, the information summarized in this review demonstrates that the neuroendocrine system is in a key regulatory position to contribute to the establishment of risk and resilience at the level of mitochondrial function and the level of systemic metabolism. Additional research efforts focused on improving energetic responses to stressors may confer resilience at both the cellular and organism levels and neuroendocrine substrates are likely key variables in the establishment of resilience.
Highlights.
Risk and resilience are important areas of study to improve human health.
The concepts of stress and allostatic load are metabolic concepts.
Metabolism and mitochondrial function may be an essential to risk vs. resilience.
Neuroendocrine signaling facilitates risk and resilience through multiple mechanisms.
5. Acknowledgements
GN, AK, and ST were supported in part by NIH: NR014886 and MH110364.
6. References
- Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS, 2000. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J. Neurosci 20, 2896–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera G, Liu Y, 2012. The molecular physiology of CRH neurons. Front. Neuroendocrinol 10.1016/j.yfrne.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksu I, Ates M, Baykara Basak, Kiray M, Riza Sisman A, Buyuk E, Baykara Burak, Cetinkaya C, Gumus H, Uysal N, 2012. Anxiety correlates to decreased blood and prefrontal cortex IGF-1 levels in streptozotocin induced diabetes. Neurosci. Lett 531, 176–181. 10.1016/j.neulet.2012.10.045 [DOI] [PubMed] [Google Scholar]
- Anderberg RH, Richard JE, Hansson C, Nissbrandt H, Bergquist F, Skibicka KP, 2016. GLP-1 is both anxiogenic and antidepressant; divergent effects of acute and chronic GLP-1 on emotionality. Psychoneuroendocrinology 65, 54–66. 10.1016/j.psyneuen.2015.11.021 [DOI] [PubMed] [Google Scholar]
- Andrews ZB, Diano S, Horvath TL, 2005. Mitochondrial uncoupling proteins in the cns: in support of function and survival. Nat. Rev. Neurosci 6, 829–840. 10.1038/nrn1767 [DOI] [PubMed] [Google Scholar]
- Andrews ZB, Erion D, Beiler R, Liu Z-W, Abizaid A, Zigman J, Elsworth JD, Savitt JM, DiMarchi R, Tschop M, Roth RH, Gao X-B, Horvath TL, 2009. Ghrelin Promotes and Protects Nigrostriatal Dopamine Function via a UCP2-Dependent Mitochondrial Mechanism. J. Neurosci 29, 14057–14065. 10.1523/JNEUROSCI.3890-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton KM, Sallis HM, Perry R, Ness AR, Churchill R, 2015. Omega-3 fatty acids for depression in adults. Cochrane Database Syst. Rev 10.1002/14651858.CD004692.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Fujimiya M, Katsuura G, Makino S, Fujino MA, Kasuga M, 2001. A role of ghrelin in neuroendocrine and behavioral responses to stress in mice. Neuroendocrinology 74, 143–147. 10.1159/000054680 [DOI] [PubMed] [Google Scholar]
- Aschbacher K, Rodriguez-Fernandez M, van Wietmarschen H, Janet Tomiyama A, Jain S, Epel E, Doyle FJ, van der Greef J, 2014. The hypothalamic- pituitary -adrenal -leptin axis and metabolic health: A systems approach to resilience, robustness and control. Interface Focus, 10.1098/rsfs.2014.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam I, Gilad S, Limor R, Stern N, Greenman Y, 2017. Ghrelin stimulation by hypothalamic–pituitary–adrenal axis activation depends on increasing cortisol levels. Endocr. Connect 6, 847–855. 10.1530/EC-17-0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T, 2005. Mitochondria, Oxidants, and Aging. Cell 120, 483–495. 10.1016/J.CELL.2005.02.001 [DOI] [PubMed] [Google Scholar]
- Baranova KA, Rybnikova EA, Samoilov MO, 2017. The dynamics of HIF-1α expression in the rat brain at different stages of experimental posttraumatic stress disorder and its correction with moderate hypoxia. Neurochem. J 11, 149–156. 10.1134/S1819712417020027 [DOI] [Google Scholar]
- Beck B, 2006. Neuropeptide Y in normal eating and in genetic and dietary-induced obesity. Philos. Trans. R. Soc. B Biol. Sci 361, 1159–1185. 10.1098/rstb.2006.1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F, Serrano M, Fernø J, Salvador J, Escalada J, Dieguez C, Lopez M, Frühbeck G, Nogueiras R, 2014. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 63, 3346–3358. 10.2337/db14-0302 [DOI] [PubMed] [Google Scholar]
- Bekhbat M, Neigh GN, 2018. Sex differences in the neuro-immune consequences of stress: Focus on depression and anxiety. Brain. Behav. Immun 10.1016/j.bbi.2017.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellisario V, Berry A, Capoccia S, Raggi C, Panetta P, Branchi I, Piccaro G, Giorgio M, Pelicci PG, Cirulli F, 2014. Gender-dependent resiliency to stressful and metabolic challenges following prenatal exposure to high-fat diet in the p66Shc−/− mouse. Front. Behav. Neurosci 10.3389/fnbeh.2014.00285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg U, Bang P, 2004. Exercise and Circulating Insulin-Like Growth Factor I. Horm. Res. Paediatr 62, 50–58. 10.1159/000080759 [DOI] [PubMed] [Google Scholar]
- Berton O, Mcclung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ, 2009. Essential Role of BDNF in the in Social Defeat Stress. Science (80-. ). 864, 864–869. 10.1126/science.1120972 [DOI] [PubMed] [Google Scholar]
- Bhat SS, Anand D, Khanday FA, 2015. p66Shc as a switch in bringing about contrasting responses in cell growth: implications on cell proliferation and apoptosis. Mol. Cancer 14, 76 10.1186/s12943-015-0354-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billington CJ, Briggs JE, Harker S, Grace M, Levine AS, 1994. Neuropeptide Y in hypothalamic paraventricular nucleus: a center coordinating energy metabolism. Am. J. Physiol. Integr. Comp. Physiol 266, R1765–R1770. 10.1152/ajpregu.1994.266.6.R1765 [DOI] [PubMed] [Google Scholar]
- Bojanowska E, Stempniak B, 2000. Effects of centrally or systemically injected glucagon-like peptide-1 (7-36) amide on release of neurohypophysial hormones and blood pressure in the rat, Regulatory Peptides. [DOI] [PubMed] [Google Scholar]
- Bonaccio M, Di Castelnuovo A, Costanzo S, Pounis G, Persichillo M, Cerletti C, Donati MB, De Gaetano G, Iacoviello L, 2017. Mediterranean-type diet is associated with higher psychological resilience in a general adult population: findings from the Moli-sani study. Eur. J. Clin. Nutr 72, 154–160. 10.1038/ejcn.2017.150 [DOI] [PubMed] [Google Scholar]
- Borrás C, Gambini J, López-Grueso R, Pallardó FV, Viña J, 2009. Direct antioxidant and protective effect of estradiol on isolated mitochondria. BBA - Mol. Basis Dis 1802, 205–211. 10.1016/j.bbadis.2009.09.007 [DOI] [PubMed] [Google Scholar]
- Bourke CH, Neigh GN, 2011. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm. Behav 60, 112–120. 10.1016/j.yhbeh.2011.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredemann TM, McMahon LL, 2014. 17beta Estradiol increases resilience and improves hippocampal synaptic function in helpless ovariectomized rats. Psychoneuroendocrinology 42, 77–88. 10.1016/j.psyneuen.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brillon DJ, Zheng B, Campbell RG, Matthews DE, 1995. Effect of cortisol on energy expenditure and amino acid metabolism in humans. Am. J. Physiol 268, E501–13. 10.1152/ajpendo.1995.268.3.E501 [DOI] [PubMed] [Google Scholar]
- Brownlow ML, Jung SH, Moore RJ, Bechmann N, Jankord R, 2017. Nutritional Ketosis Affects Metabolism and Behavior in Sprague-Dawley Rats in Both Control and Chronic Stress Environments. Front. Mol. Neurosci 10, 129 10.3389/fnmol.2017.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerino C, 2009. Low Sympathetic Tone and Obese Phenotype in Oxytocin-deficient Mice. Obesity 17, 980–984. 10.1038/oby.2009.12 [DOI] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M, 2012. Testosterone and imipramine have antidepressant effects in socially isolated male but not female rats. 10.1016/j.yhbeh.2012.03.001 [DOI] [PMC free article] [PubMed]
- Carrier N, Saland SK, Duclot F, He H, Mercer R, Kabbaj M, 2015. The anxiolytic and antidepressant-like effects of testosterone and estrogen in gonadectomized male rats. Biol. Psychiatry 78, 259–269. 10.1016/j.biopsych.2014.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E, Nunez A, Busiguina S, Torres-Aleman I, 2000. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J. Neurosci 20, 2926–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carteri RB, Kopczynski A, Rodolphi MS, Strogulski NR, Sartor M, Feldmann M, De Bastiani MA, Duval Wannmacher CM, Franceschini ID, Hansel G, Smith DHD, Portela LVC, 2019. Testosterone administration after traumatic brain injury reduces mitochondrial dysfunction and neurodegeneration. J. Neurotrauma 10.1089/neu.2018.6266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassilhas RC, Antunes HKM, Tufik S, de Mello MT, 2010. Mood, Anxiety, and Serum IGF-1 in Elderly Men Given 24 Weeks of High Resistance Exercise. Percept. Mot. Skills 110, 265–276. 10.2466/pms.110.1.265-276 [DOI] [PubMed] [Google Scholar]
- Chakravarty S, Reddy BR, Sudhakar SR, Saxena S, Das T, Meghah V, Brahmendra Swamy CV, Kumar A, Idris MM, 2013. Chronic unpredictable stress (CUS)-induced anxiety and related mood disorders in a zebrafish model: altered brain proteome profile implicates mitochondrial dysfunction. PLoS One 8, e63302 10.1371/journal.pone.0063302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KL, Cathomas F, Russo SJ, 2019. Central and Peripheral Inflammation Link Metabolic Syndrome and Major Depressive Disorder. Physiology. 10.1152/physiol.00047.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel NS, 2015. Navigating Metabolism. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York. [Google Scholar]
- Charney DS, 2004. Psychobiological Mechanism of Resilience and Vulnerability: Implications for Successful Adaptation to Extreme Stress. Am. J. Psychiatry. 10.1176/appi.ajp.161.2.195 [DOI] [PubMed] [Google Scholar]
- Chen X-Q, Dong J, Niu C-Y, Fan J-M, Du J-Z, 2007. Effects of Hypoxia on Glucose, Insulin, Glucagon, and Modulation by Corticotropin-Releasing Factor Receptor Type 1 in the Rat. 10.1210/en.2006-1224 [DOI] [PubMed]
- Choi IY, Piccio L, Childress P, Bollman B, Ghosh A, Brandhorst S, Suarez J, Michalsen A, Cross AH, Morgan TE, Wei M, Paul F, Bock M, Longo VD, 2016. A Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell Rep. 15, 2136–2146. 10.1016/j.celrep.2016.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Kozlovsky N, Savion N, Matar MA, Loewenthal U, Loewenthal N, Zohar J, Kaplan Z, 2009. An association between stress-induced disruption of the hypothalamic-pituitary-adrenal axis and disordered glucose metabolism in an animal model of post-traumatic stress disorder. J. Neuroendocrinol 21, 898–909. 10.1111/j.1365-2826.2009.01913.x [DOI] [PubMed] [Google Scholar]
- Cohen H, Liu T, Kozlovsky N, Kaplan Z, Zohar J, Mathé AA, 2012. The neuropeptide y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology 37, 350–363. 10.1038/npp.2011.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne-Loyer A, Fortier M, Tremblay-Mercier J, Chouinard-Watkins R, Roy M, Nugent S, Castellano C-A, Cunnane SC, 2013. Stimulation of mild, sustained ketonemia by medium-chain triacylglycerols in healthy humans: Estimated potential contribution to brain energy metabolism. Nutrition 29, 635–640. 10.1016/J.NUT.2012.09.009 [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Johnston M, Hayward M, Seeman T, 2003. Age differences in allostatic load: An index of physiological dysregulation, in: Experimental Gerontology. pp. 731–734. 10.1016/S0531-5565(03)00099-8 [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Laugero KD, Gomez F, Manalo S, Bell ME, Bhatnagar S, 2003. A spoonful of sugar: feedback signals of energy stores and corticosterone regulate responses to chronic stress. Physiol. Behav 79, 3–12. [DOI] [PubMed] [Google Scholar]
- Deblon N, Veyrat-Durebex C, Bourgoin L, Caillon A, Bussier A-L, Petrosino S, Piscitelli F, Legros J-J, Geenen V, Foti M, Wahli W, Di Marzo V, Rohner-Jeanrenaud F, 2011. Mechanisms of the Anti-Obesity Effects of Oxytocin in Diet-Induced Obese Rats. PLoS One 6, e25565 10.1371/journal.pone.0025565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetrio FN, Renno JJ, Gianfaldoni A, Goncalves M, Halbe HW, Filho AHGV, Gorenstein C, 2011. Effect of estrogen replacement therapy on symptoms of depression and anxiety in non-depressive menopausal women: a randomized double-blind, controlled study. Arch. Womens. Ment. Health 14, 479–486. 10.1007/s00737-011-0241-3 [DOI] [PubMed] [Google Scholar]
- DiBlasio CJ, Hammett J, Malcolm JB, Judge BA, Womack JH, Kincade MC, Ogles ML, Mancini JG, Patterson AL, Wake RW, Derweesh IH, 2008. Prevalence and predictive factors for the development of de novo psychiatric illness in patients receiving androgen deprivation therapy for prostate cancer. Can. J. Urol 15, 4249–56; discussion 4256. [PubMed] [Google Scholar]
- Dietrich MO, Bober J, Ferreira JG, Tellez LA, Mineur YS, Souza DO, Gao X-B, Picciotto MR, Araújo I, Liu Z-W, Horvath TL, 2012. AgRP neurons regulate development of dopamine neuronal plasticity and nonfood-associated behaviors. Nat. Neurosci advance on. 10.1038/nn.3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Song N, Xie J, Jiang H, 2009. Ghrelin Antagonized 1-Methyl-4-Phenylpyridinium (MPP+)-Induced Apoptosis in MES23.5 Cells. J. Mol. Neurosci 37, 182–189. 10.1007/s12031-008-9162-7 [DOI] [PubMed] [Google Scholar]
- Drazen DL, Bilu D, Edwards N, Nelson RJ, 2001. Disruption of poly (ADP-ribose) polymerase (PARP) protects against stress-evoked immunocompromise. Mol. Med 7, 761–766. [PMC free article] [PubMed] [Google Scholar]
- Drubach LA, Palmer EL, Connolly LP, Baker A, Zurakowski D, Cypess AM, 2011. Pediatric Brown Adipose Tissue: Detection, Epidemiology, and Differences from Adults. J. Pediatr 159, 939–944. 10.1016/j.jpeds.2011.06.028 [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Terwilliger R, Russell DS, Newton SS, Duman RS, 2009. Peripheral insulin-like growth factor-I produces antidepressant-like behavior and contributes to the effect of exercise. Behav. Brain Res 198, 366–371. 10.1016/j.bbr.2008.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, 2014. Neurobiology of stress, depression, and rapid acting antidepressants: Remodeling synaptic connections. Depress. Anxiety 31, 291–296. 10.1002/da.22227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DA, 2006. doi: 10.1016/j.yhbeh.2006.09.005. 10.1016/j.yhbeh.2006.09.005 [DOI]
- Eisch AJ, Bolaños CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, Verhaagen J, Nestler EJ, 2003. Brain-derived neurotrophic factor in the ventral midbrain–nucleus accumbens pathway: a role in depression. Biol. Psychiatry 54, 994–1005. 10.1016/j.biopsych.2003.08.003 [DOI] [PubMed] [Google Scholar]
- Engin E, Stellbrink J, Treit D, Dickson CT, 2008. Anxiolytic and antidepressant effects of intracerebroventricularly administered somatostatin: behavioral and neurophysiological evidence. NSC 157, 666–676. 10.1016/j.neuroscience.2008.09.037 [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D, 2009. Anxiolytic and antidepressant actions of somatostatin: the role of sst2 and sst3 receptors. Psychopharmacology (Berl). 206, 281–289. 10.1007/s00213-009-1605-5 [DOI] [PubMed] [Google Scholar]
- Engler-Chiurazzi EB, Covey DF, Simpkins JW, 2017. A novel mechanism of non-feminizing estrogens in neuroprotection. Exp. Gerontol 94, 99–102. 10.1016/j.exger.2016.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, McEwen B, Seeman T, Matthews K, Castellazzo G, Brownell KD, Bell J, Ickovics JR, 2000. Stress and body shape: stress-induced cortisol secretion is consistently greater among women with central fat. Psychosom. Med 62, 623–632. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A, 2007. Evidence for a Neuroendocrinological Foundation of Human Affiliation. Psychol. Sci 18, 965–970. 10.1111/j.1467-9280.2007.02010.x [DOI] [PubMed] [Google Scholar]
- Filiou MD, Zhang Y, Teplytska L, Reckow S, Gormanns P, Maccarrone G, Frank E, Kessler MS, Hambsch B, Nussbaumer M, Bunck M, Ludwig T, Yassouridis A, Holsboer F, Landgraf R, Turck CW, 2011. Proteomics and metabolomics analysis of a trait anxiety mouse model reveals divergent mitochondrial pathways. Biol. Psychiatry 70, 1074–1082. 10.1016/j.biopsych.2011.06.009 [DOI] [PubMed] [Google Scholar]
- Fitzsimmons EE, Bardone-Cone AM, 2010. Coping and Social Support as Potential Moderators of the Relation Between Anxiety and Eating Disorder Symptomatology. 10.1016/j.eatbeh.2010.09.002 [DOI] [PMC free article] [PubMed]
- Foster MT, Warne JP, Ginsberg AB, Horneman HF, Pecoraro NC, Akana SF, Dallman MF, 2009. Palatable Foods, Stress, and Energy Stores Sculpt Corticotropin-Releasing Factor, Adrenocorticotropin, and Corticosterone Concentrations after Restraint N E U R O E N D O C R I N O L O G Y. 10.1210/en.2008-1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MP, Rapaport MH, 2011. Omega-3 Fatty Acids and Depression: From Cellular Mechanisms to Clinical Care. J. Clin. Psychiatry 72, 258–259. 10.4088/JCP.11ac06830 [DOI] [PubMed] [Google Scholar]
- Frye CA, Koonce CJ, Edinger KL, Osborne DM, Walf AA, 2008. Androgens with activity at estrogen receptor beta have anxiolytic and cognitive-enhancing effects in male rats and mice. Horm. Behav 54, 726–734. 10.1016/j.yhbeh.2008.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfo F, Tirassa P, De Bartolo P, Croce N, Bernardini S, Caltagirone C, Petrosini L, Angelucci F, 2012. NPY Intraperitoneal Injections Produce Antidepressant-Like Effects and Downregulate BDNF in the Rat Hypothalamus. CNS Neurosci. Ther 18, 487–492. 10.1111/j.1755-5949.2012.00314.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghini V, Saccenti E, Tenori L, Assfalg M, Luchinat C, 2015. Allostasis and Resilience of the Human Individual Metabolic Phenotype. J. Proteome Res 14, 2951–2962. 10.1021/acs.jproteome.5b00275 [DOI] [PubMed] [Google Scholar]
- Ghosal S, Packard AEB, Mahbod P, McKlveen JM, Seeley RJ, Myers B, Ulrich-Lai Y, Smith EP, D’Alessio DA, Herman JP, 2017a. Disruption of Glucagon-Like Peptide 1 Signaling in Sim1 Neurons Reduces Physiological and Behavioral Reactivity to Acute and Chronic Stress. J. Neurosci 37, 184–193. 10.1523/JNEUROSCI.1104-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal S, Packard AEB, Mahbod P, Mcklveen JM, Seeley RJ, Myers B, Ulrich-Lai Y, Smith EP, David X, D’alessio A, Herman JP, 2017b. Disruption of Glucagon-Like Peptide 1 Signaling in Sim1 Neurons Reduces Physiological and Behavioral Reactivity to Acute and Chronic Stress. 10.1523/JNEUROSCI.1104-16.2016 [DOI] [PMC free article] [PubMed]
- Gil-Lozano M, Pérez-Tilve D, Alvarez-Crespo M, Martís A, Fernandez AM, Catalina PAF, Gonzalez-Matias LC, Mallo F, 2010. GLP-1(7-36)-amide and exendin-4 stimulate the HPA axis in rodents and humans. Endocrinology 151, 2629–2640. 10.1210/en.2009-0915 [DOI] [PubMed] [Google Scholar]
- Gilsanz V, Chung SA, Jackson H, Dorey FJ, Hu HH, 2011. Functional Brown Adipose Tissue is Related to Muscle Volume in Children and Adolescents. J. Pediatr 158, 722–726. 10.1016/j.jpeds.2010.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovambattista A, Chisari AN, Corró L, Gaillard RC, Spinedi E, 2000. Metabolic, Neuroendocrine and Immune Functions in Basal Conditions and during the Acute-Phase Response to Endotoxic Shock in Undernourished Rats. 10.1159/000026426 [DOI] [PubMed]
- Giriko CÁ, Andreoli CA, Mennitti V, Fazion Hosoume L, Dos T, Souto S, Valotta Da Silva A, Mendes-Da-Silva C, 2013. Delayed physical and neurobehavioral development and increased aggressive and depression-like behaviors in the rat offspring of dams fed a high-fat diet. Int. J. Devl Neurosci 31, 731–739. 10.1016/j.ijdevneu.2013.09.001 [DOI] [PubMed] [Google Scholar]
- Gleason CE, Dowling NM, Wharton W, Manson JE, Miller VM, Atwood CS, Brinton EA, Cedars MI, Lobo RA, Merriam GR, Neal-Perry G, Santoro NF, Taylor HS, Black DM, Budoff MJ, Hodis HN, Naftolin F, Harman SM, Asthana S, 2015. Effects of Hormone Therapy on Cognition and Mood in Recently Postmenopausal Women: Findings from the Randomized, Controlled KEEPS-Cognitive and Affective Study. PLoS Med. 12, e1001833; discussion e1001833. 10.1371/journal.pmed.1001833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gola H, Engler A, Morath J, Adenauer H, Elbert T, Kolassa I-T, Engler H, 2014. Reduced peripheral expression of the glucocorticoid receptor alpha isoform in individuals with posttraumatic stress disorder: a cumulative effect of trauma burden. PLoS One 9, e86333 10.1371/journal.pone.0086333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Covington Iii HE, Berton O, Russo SJ, 2011. A standardized protocol for repeated social defeat stress in mice. 10.1038/nprot.2011.361 [DOI] [PMC free article] [PubMed]
- Gonzalez-Reyes A, Menaouar A, Yip D, Danalache B, Plante E, Noiseux N, Gutkowska J, Jankowski M, 2015. Molecular mechanisms underlying oxytocin-induced cardiomyocyte protection from simulated ischemia–reperfusion. Mol. Cell. Endocrinol 412, 170–181. 10.1016/j.mce.2015.04.028 [DOI] [PubMed] [Google Scholar]
- Gragnoli C, 2014. Hypothesis of the neuroendocrine cortisol pathway gene role in the comorbidity of depression, type 2 diabetes, and metabolic syndrome. Appl. Clin. Genet 43 10.2147/TACG.S39993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloux J-P, Douillard-Guilloux G, Kota R, Wang X, Martinowich K, Tseng GC, Lewis DA, Sibille E, 2011. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol. Psychiatry 17, 1130–1142. 10.1038/mp.2011.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Hostinar CE, 2015. The social buffering of the hypothalamic–pituitary–adrenocortical axis in humans: Developmental and experiential determinants. Soc. Neurosci 10.1080/17470919.2015.1070747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajduch E, Rencurel F, Balendran A, Batty IH, Downes CP, Hundal HS, 1999. Serotonin (5-hydroxytryptamine), a novel regulator of glucose transport in rat skeletal muscle* Downloaded from. J. Biol. Chem 274, 13563–13568. [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM, 1995. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269, 543–6. 10.1126/SCIENCE.7624777 [DOI] [PubMed] [Google Scholar]
- Hamilton PJ, Chen EY, Tolstikov V, Pena CJ, Shah P, Panagopoulos K, Strat AN, Walker DM, Lorsch ZS, Mervosh NL, Kiraly DD, Sarangarajan R, Narain NR, Kiebish MA, Nestler EJ, 2018. Multi-OMIC analysis of brain and serum from chronically-stressed mice reveals network disruptions in purine metabolism, fatty acid beta-oxidation, and antioxidant activity that are reversed by antidepressant treatment. bioRxiv 490748. 10.1101/490748 [DOI] [Google Scholar]
- Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L, 2008. An alternate pathway for androgen regulation of brain function: Activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5α-androstane-3β,17β-diol. Horm. Behav 53, 741–752. 10.1016/j.yhbeh.2007.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell CS, Burgado J, Kelly SD, Johnson ZP, Neigh GN, 2015a. High-fructose diet during periadolescent development increases depressive-like behavior and remodels the hypothalamic transcriptome in male rats HHS Public Access. Psychoneuroendocrinology 62, 252–264. 10.1016/j.psyneuen.2015.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell CS, Gillespie CF, Neigh GN, 2016. Energetic stress: The reciprocal relationship between energy availability and the stress response. Physiol. Behav 166, 43–55. 10.1016/j.physbeh.2015.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell CS, Rowson SA, Neigh GN, 2015b. Pharmacological stimulation of hypoxia inducible factor-1α facilitates the corticosterone response to a mild acute stressor. Neurosci. Lett 600, 75–79. 10.1016/j.neulet.2015.05.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell CS, Zainaldin C, McFarlane D, Hyer MM, Stein D, Sayeed I, Neigh GN, 2018. High-fructose diet during adolescent development increases neuroinflammation and depressive-like behavior without exacerbating outcomes after stroke. Brain. Behav. Immun 73, 340–351. 10.1016/j.bbi.2018.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ, n.d. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. 10.1016/j.neubiorev.2003.09.003 [DOI] [PubMed]
- Herisson FM, Waas JR, Fredriksson R, Schiöth HB, Levine AS, Olszewski PK, 2016. Oxytocin Acting in the Nucleus Accumbens Core Decreases Food Intake. J. Neuroendocrinol 28 10.1111/jne.12381 [DOI] [PubMed] [Google Scholar]
- Hou C, Jia F, Liu Y, Li L, 2006. CSF serotonin, 5-hydroxyindolacetic acid and neuropeptide Y levels in severe major depressive disorder. 10.1016/j.brainres.2006.04.026 [DOI] [PubMed]
- Hsu W-H, Lee B-H, Pan T-M, 2015. Leptin-induced mitochondrial fusion mediates hepatic lipid accumulation. Int. J. Obes 39, 1750–1756. 10.1038/ijo.2015.120 [DOI] [PubMed] [Google Scholar]
- Hu S, Cheng D, Peng D, Tan J, Huang Y, Chen C, 2019. Leptin attenuates cerebral ischemic injury in rats by modulating the mitochondrial electron transport chain via the mitochondrial STAT3 pathway. Brain Behav. 9, e01200 10.1002/brb3.1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii N, Tsubouchi H, Miura A, Yanagi S, Ueno H, Shiomi K, Nakazato M, 2018. Ghrelin alleviates paclitaxel-induced peripheral neuropathy by reducing oxidative stress and enhancing mitochondrial anti-oxidant functions in mice. Eur. J. Pharmacol 819, 35–42. 10.1016/j.ejphar.2017.11.024 [DOI] [PubMed] [Google Scholar]
- Jaime Herrera-Pérez J, Martínez-Mota L, Chavira R, Fernández-Guasti A, 2012. Testosterone prevents but not reverses anhedonia in middle-aged males and lacks an effect on stress vulnerability in young adults. Horm. Behav 61, 623–630. 10.1016/j.yhbeh.2012.02.015 [DOI] [PubMed] [Google Scholar]
- Jorgensen JO, Vahl N, Dall R, Christiansen JS, 1998. Resting metabolic rate in healthy adults: relation to growth hormone status and leptin levels. Metabolism. 47, 1134–1139. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Yoon S, Lyoo IK, 2015. Peripheral Biomarker Candidates of Posttraumatic Stress Disorder. Exp. Neurobiol 24, 186–196. 10.5607/en.2015.24.3.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Jia Z, Ping Y, Liu Z, Yan X, Xing G, Yan W, 2018. Testosterone alleviates mitochondrial ROS accumulation and mitochondria-mediated apoptosis in the gastric mucosa of orchiectomized rats. Arch. Biochem. Biophys 649, 53–59. 10.1016/j.abb.2018.05.002 [DOI] [PubMed] [Google Scholar]
- Katan M, Morgenthaler NG, Dixit KCS, Rutishauser J, Brabant GE, Müller B, Christ-Crain M, 2007. Anterior and Posterior Pituitary Function Testing with Simultaneous Insulin Tolerance Test and a Novel Copeptin Assay. J. Clin. Endocrinol. Metab 92, 2640–2643. 10.1210/jc.2006-2046 [DOI] [PubMed] [Google Scholar]
- Katan Mira, Morgenthaler N, Widmer I, Puder JJ, König C, Christ-Crain M, Katan M, 2008. Copeptin, a stable peptide derived from the vasopressin precursor, correlates with the individual stress level, Neuroendocrinol Lett. [PubMed] [Google Scholar]
- Kelley SA, Hartman AL, 2011. Metabolic treatments for intractable epilepsy. Semin. Pediatr. Neurol 18, 179–185. 10.1016/j.spen.2011.06.004 [DOI] [PubMed] [Google Scholar]
- Kelly JJ, Mangos G, Williamson PM, Whitworth JA, 1998. Cortisol and hypertension. Clin. Exp. Pharmacol. Physiol. Suppl 25, S51–6. [DOI] [PubMed] [Google Scholar]
- Kinzig KP, D’alessio DA, Herman JP, Sakai RR, Vahl TP, Figueiredo HF, Murphy EK, Seeley RJ, 2003. Behavioral/Systems/Cognitive CNS Glucagon-Like Peptide-1 Receptors Mediate Endocrine and Anxiety Responses to Interoceptive and Psychogenic Stressors. [DOI] [PMC free article] [PubMed]
- Klaus S, Casteilla L, Bouillaud F, Ricquier D, 1991. The uncoupling protein ucp: A membraneous mitochondrial ion carrier exclusively expressed in brown adipose tissue. Int. J. Biochem 23, 791–801. 10.1016/0020-711X(91)90062-R [DOI] [PubMed] [Google Scholar]
- Klok MD, Jakobsdottir S, Drent ML, 2007. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes. Rev 8, 21–34. 10.1111/j.1467-789X.2006.00270.x [DOI] [PubMed] [Google Scholar]
- Kodama T, Shimizu N, Yoshikawa N, Makino Y, Ouchida R, Okamoto K, Hisada T, Nakamura H, Morimoto C, Tanaka H, 2003. Role of the Glucocorticoid Receptor for Regulation of Hypoxia-dependent Gene Expression*. 10.1074/jbc.M302581200 [DOI] [PubMed]
- Kolassa I-T, Eckart C, Ruf M, Neuner F, Jf De Quervain D, Elbert T, 2007. Lack of cortisol response in patients with posttraumatic stress disorder (PTSD) undergoing a diagnostic interview. 10.1186/1471-244X-7-54 [DOI] [PMC free article] [PubMed]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E, 2005. Oxytocin increases trust in humans. Nature 435, 673–676. 10.1038/nature03701 [DOI] [PubMed] [Google Scholar]
- Kristenssson E, Sundqvist M, Astin M, Kjerling M, Mattsson H, Dornonville de la Cour C, Håkanson R, Lindström E, 2006. Acute psychological stress raises plasma ghrelin in the rat. Regul. Pept 134, 114–117. 10.1016/j.regpep.2006.02.003 [DOI] [PubMed] [Google Scholar]
- Kumar A, Newberg A, Alavi A, Berlin J, Smith R, Reivich M, 1993. Regional cerebral glucose metabolism in late-life depression and Alzheimer disease: A preliminary positron emission tomography study, Proc. Natl. Acad. Sci. USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunas N, Calmarza-Font I, Diz-Chaves Y, Garcia-Segura LM, 2010. Long-term ovariectomy enhances anxiety and depressive-like behaviors in mice submitted to chronic unpredictable stress. Horm. Behav 58, 786–791. 10.1016/j.yhbeh.2010.07.014 [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Brand MD, 2009. Reactive oxygen species production by mitochondria. Methods Mol. Biol 554, 165–181. 10.1007/978-1-59745-521-3_11 [DOI] [PubMed] [Google Scholar]
- Lambeth JD, 2004. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol 4, 181–189. 10.1038/nri1312 [DOI] [PubMed] [Google Scholar]
- Landi S, Ciucci F, Maffei L, Berardi N, Cenni MC, 2009. Setting the Pace for Retinal Development: Environmental Enrichment Acts Through Insulin-Like Growth Factor 1 and Brain-Derived Neurotrophic Factor. J. Neurosci 29, 10809–10819. 10.1523/JNEUROSCI.1857-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapp HE, Bartlett AA, Hunter RG, 2019. Stress and glucocorticoid receptor regulation of mitochondrial gene expression. J. Mol. Endocrinol R121–R128. 10.1530/JME-18-0152 [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Tang-Christensen M, Jessop DS, 1997. Central Administration of Glucagon-Like Peptide-1 Activates Hypothalamic Neuroendocrine Neurons in the Rat*. [DOI] [PubMed]
- Lee P, Zhao JT, Swarbrick MM, Gracie G, Bova R, Greenfield JR, Freund J, Ho KKY, 2011. High Prevalence of Brown Adipose Tissue in Adult Humans. J. Clin. Endocrinol. Metab 96, 2450–2455. 10.1210/jc.2011-0487 [DOI] [PubMed] [Google Scholar]
- Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B, 2010. The Largest Group of Superficial Neocortical GABAergic Interneurons Expresses Ionotropic Serotonin Receptors. 10.1523/JNEUROSCI.1869-10.2010 [DOI] [PMC free article] [PubMed]
- Li B, Lang N, Cheng Z-F, 2016. Serum Levels of Brain-Derived Neurotrophic Factor Are Associated with Diabetes Risk, Complications, and Obesity: a Cohort Study from Chinese Patients with Type 2 Diabetes. Mol. Neurobiol 53, 5492–5499. 10.1007/s12035-015-9461-2 [DOI] [PubMed] [Google Scholar]
- Li Y, Lv M-R, Wei Y-J, Sun L, Zhang J-X, Zhang H-G, Li B, 2017. Dietary patterns and depression risk: A meta-analysis. 10.1016/j.psychres.2017.04.020 [DOI] [PubMed]
- Licinio J, Mantzoros C, Negrão AB, Cizza G, Wong ML, Bongiorno PB, Chrousos GP, Karp B, Allen C, Flier JS, Gold PW, 1997. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat. Med 3, 575–9. [DOI] [PubMed] [Google Scholar]
- Lin F, Suhr J, Diebold S, Heffner KL, 2014. Associations between depressive symptoms and memory deficits vary as a function of insulin-like growth factor (IGF-1) levels in healthy older adults. Psychoneuroendocrinology 42, 118–123. 10.1016/j.psyneuen.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB, Spiegelman BM, 2000. Towards a molecular understanding of adaptive thermogenesis. Nature 404, 652–660. 10.1038/35007527 [DOI] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WCJ, Handa RJ, 2005. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology 146, 797–807. 10.1210/en.2004-1158 [DOI] [PubMed] [Google Scholar]
- Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM, 2008. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat. Neurosci 11, 752–753. 10.1038/nn.2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima Y, Iwasaki Y, Yamahara Y, Kodaira M, Sedbazar U, Yada T, 2011. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging (Albany. NY). 3, 1169–77. 10.18632/aging.100408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud JSR, Staten RT, Lennie TA, Hall LA, 2015. The Relationships of Coping, Negative Thinking, Life Satisfaction, Social Support, and Selected Demographics With Anxiety of Young Adult College Students. J. Child Adolesc. Psychiatr. Nurs 28, 97–108. 10.1111/jcap.12109 [DOI] [PubMed] [Google Scholar]
- Mahmoud R, Wainwright SR, Chaiton JA, Lieblich SE, Galea LAM, 2016. Ovarian hormones, but not fluoxetine, impart resilience within a chronic unpredictable stress model in middle-aged female rats. Neuropharmacology. 10.1016/j.neuropharm.2016.01.033 [DOI] [PubMed] [Google Scholar]
- Maske CB, Jackson CM, Terrill SJ, Eckel LA, Williams DL, 2017. Estradiol modulates the anorexic response to central glucagon-like peptide 1. Horm. Behav 93, 109–117. 10.1016/j.yhbeh.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, 2017. Genomics are CReePing up on inflammation in PTSD. Sci. Transl. Med 9, eaao6887 10.1126/scitranslmed.aao6887 [DOI] [Google Scholar]
- Michopoulos V, 2016. Stress-induced alterations in estradiol sensitivity increase risk for obesity in women. Physiol. Behav 166, 56–64. 10.1016/j.physbeh.2016.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Norrholm SD, Jovanovic T, 2015. Diagnostic Biomarkers for Posttraumatic Stress Disorder: Promising Horizons from Translational Neuroscience Research. Biol. Psychiatry 78, 344–353. 10.1016/j.biopsych.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Vester A, Neigh G, 2016. Posttraumatic stress disorder: A metabolic disorder in disguise? Exp. Neurol 284, 220–229. 10.1016/j.expneurol.2016.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Wilson ME, 2011. Body weight decreases induced by estradiol in female rhesus monkeys are dependent upon social status. Physiol. Behav 102, 382–388. 10.1016/j.physbeh.2010.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedlar JA, Rinaman L, Vollmer RR, Amico JA, 2007. Oxytocin gene deletion mice overconsume palatable sucrose solution but not palatable lipid emulsions. Am. J. Physiol. Integr. Comp. Physiol 293, R1063–R1068. 10.1152/ajpregu.00228.2007 [DOI] [PubMed] [Google Scholar]
- Miller K, Driscoll D, Smith LM, Ramaswamy S, 2017. The Role of Inflammation in Late-Life Post-Traumatic Stress Disorder. Mil. Med 182, 1815 10.7205/MILMED-D-17-00073 [DOI] [PubMed] [Google Scholar]
- Mitschelen M, Yan H, Farley JA, Warrington JP, Han S, Hereñú CB, Csiszar A, Ungvari Z, Bailey-Downs LC, Bass CE, Sonntag WE, 2011. Long-term deficiency of circulating and hippocampal insulin-like growth factor I induces depressive behavior in adult mice: a potential model of geriatric depression. Neuroscience 185, 50–60. 10.1016/j.neuroscience.2011.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi K, Ishige A, Aburada M, Tabira T, 2003. Chronic stress attenuates glucocorticoid negative feedback: involvement of the prefrontal cortex and hippocampus. Neuroscience 119, 887–897. 10.1016/S0306-4522(03)00105-2 [DOI] [PubMed] [Google Scholar]
- Monteiro R, Azevedo I, 2010. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010, 289645 10.1155/2010/289645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraitis AG, Block T, Nguyen D, Belanoff JK, 2017. The role of glucocorticoid receptors in metabolic syndrome and psychiatric illness. J. Steroid Biochem. Mol. Biol 165, 114–120. 10.1016/j.jsbmb.2016.03.023 [DOI] [PubMed] [Google Scholar]
- Moriceau S, Wilson DA, Levine S, Sullivan RM, 2006. Dual Circuitry for Odor-Shock Conditioning during Infancy: Corticosterone Switches between Fear and Attraction via Amygdala, J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munive V, Santi A, Torres-Aleman I, 2016. A Concerted Action Of Estradiol And Insulin Like Growth Factor I Underlies Sex Differences In Mood Regulation By Exercise. Sci. Rep 6, 25969 10.1038/srep25969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroy SE, Long KLP, Kaufer D, Kirby ED, 2016. Moderate Stress-Induced Social Bonding and Oxytocin Signaling are Disrupted by Predator Odor in Male Rats. Neuropsychopharmacology. 10.1038/npp.2016.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazari A, Sadr SS, Faghihi M, Azizi Y, Hosseini M-J, Mobarra N, Tavakoli A, Imani A, 2015. Vasopressin attenuates ischemia-reperfusion injury via reduction of oxidative stress and inhibition of mitochondrial permeability transition pore opening in rat hearts. Eur. J. Pharmacol 760, 96–102. 10.1016/j.ejphar.2015.04.006 [DOI] [PubMed] [Google Scholar]
- Neigh GN, Bilbo SD, Hotchkiss AK, Nelson RJ, 2004a. Exogenous pyruvate prevents stress-evoked suppression of mitogen-stimulated proliferation. Brain. Behav. Immun 18, 425–433. 10.1016/j.bbi.2003.10.001 [DOI] [PubMed] [Google Scholar]
- Neigh GN, Bowers SL, Pyter LM, Gatien ML, Nelson RJ, 2004b. Pyruvate Prevents Restraint-Induced Immunosuppression via Alterations in Glucocorticoid Responses. Endocrinology 145, 4309–4319. 10.1210/en.2003-1748 [DOI] [PubMed] [Google Scholar]
- Neigh GN, Gillespie CF, Nemeroff CB, 2009. The neurobiological toll of child abuse and neglect. Trauma, Violence, Abus 10.1177/1524838009339758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neigh GN, Samuelsson AR, Bowers SL, Nelson RJ, 2005. 3-Aminobenzamide prevents restraint-evoked immunocompromise. Brain. Behav. Immun 19, 351–356. 10.1016/j.bbi.2004.11.001 [DOI] [PubMed] [Google Scholar]
- Nindl BC, Alemany JA, Tuckow AP, Kellogg MD, Sharp MA, Patton JF, 2009. Effects of exercise mode and duration on 24-h IGF-I system recovery responses. Med. Sci. Sports Exerc 41, 1261–1270. 10.1249/MSS.0b013e318197125c [DOI] [PubMed] [Google Scholar]
- Novais A, Monteiro S, Roque S, Correia-Neves M, Sousa N, 2017. How age, sex and genotype shape the stress response. 10.1016/j.ynstr.2016.11.004 [DOI] [PMC free article] [PubMed]
- Ochi M, Tominaga K, Tanaka F, Tanigawa T, Shiba M, Watanabe T, Fujiwara Y, Oshitani N, Higuchi K, Arakawa T, 2008. Effect of chronic stress on gastric emptying and plasma ghrelin levels in rats. Life Sci. 82, 862–868. 10.1016/J.LFS.2008.01.020 [DOI] [PubMed] [Google Scholar]
- Paoletti R, Bolego C, Poli A, Cignarella A, 2006. Metabolic syndrome, inflammation and atherosclerosis. Vasc. Health Risk Manag 2, 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson ZR, Ducharme R, Anisman H, Abizaid A, 2010. Altered metabolic and neurochemical responses to chronic unpredictable stressors in ghrelin receptor-deficient mice. Eur. J. Neurosci 32, 632–639. 10.1111/j.1460-9568.2010.07310.x [DOI] [PubMed] [Google Scholar]
- Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF, 2004. Chronic Stress Promotes Palatable Feeding, which Reduces Signs of Stress: Feedforward and Feedback Effects of Chronic Stress. 10.1210/en.2004-0305 [DOI] [PubMed]
- Perello M, Dickson SL, 2015. Ghrelin Signalling on Food Reward: A Salient Link Between the Gut and the Mesolimbic System. J. Neuroendocrinol 27, 424–434. 10.1111/jne.12236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perraudin V, Delarue C, Lefebvre H, Vaudry H, Kuhn JM, Contesse V, 1993. Vasopressin stimulates cortisol secretion from human adrenocortical tissue through activation of V1 receptors. J. Clin. Endocrinol. Metab 76, 1522–1528. 10.1210/jcem.76.6.7684742 [DOI] [PubMed] [Google Scholar]
- Perreault M, Touré El, •, Perreault Nicole, Caron J, 2017. Employment Status and Mental Health: Mediating Roles of Social Support and Coping Strategies. Psychiatr Q 88, 501–514. 10.1007/s11126-016-9460-0 [DOI] [PubMed] [Google Scholar]
- Picard M, Juster R-P, McEwen BS, 2014. Mitochondrial allostatic load puts the “gluc” back in glucocorticoids. Nat. Rev. Endocrinol 10, 303–310. 10.1038/nrendo.2014.22 [DOI] [PubMed] [Google Scholar]
- Picard M, McEwen BS, Epel ES, Sandi C, 2018. An energetic view of stress: Focus on mitochondria. Front. Neuroendocrinol 49, 72–85. 10.1016/j.yfrne.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintana H, Pongkan W, Pratchayasakul W, Chattipakorn N, Chattipakorn SC, 2015. Testosterone replacement attenuates cognitive decline in testosterone-deprived lean rats, but not in obese rats, by mitigating brain oxidative stress. Age (Omaha). 37, 84 10.1007/s11357-015-9827-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, Sanacora G, 2012. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci 13, 22–37. 10.1038/nrn3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postolache TT, Bosque-Plata L, Jabbour S, Vergare M, Wu R, Gragnoli C, 2019. Co-shared genetics and possible risk gene pathway partially explain the comorbidity of schizophrenia, major depressive disorder, type 2 diabetes, and metabolic syndrome. Am. J. Med. Genet. Part B Neuropsychiatr. Genet ajmg.b.32712. 10.1002/ajmg.b.32712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pralong FP, Roduit R, Waeber G, Castillo E, Mosimann F, Thorens B, Gaillard RC, 1998. Leptin Inhibits Directly Glucocorticoid Secretion by Normal Human and Rat Adrenal Gland 1. Endocrinology 139, 4264–4268. 10.1210/endo.139.10.6254 [DOI] [PubMed] [Google Scholar]
- Preil J, Müller MB, Gesing A, Reul JMHM, Sillaber I, van Gaalen MM, Landgrebe J, Holsboer F, Stenzel-Poore M, Wurst W, 2001. Regulation of the Hypothalamic-Pituitary-Adrenocortical System in Mice Deficient for CRH Receptors 1 and 2. Endocrinology 142, 4946–4955. 10.1210/endo.142.11.8507 [DOI] [PubMed] [Google Scholar]
- Prévôt TD, Gastambide F, Viollet C, Henkous N, Martel G, Epelbaum J, Béracochéa D, Guillou J-L, 2017. Roles of Hippocampal Somatostatin Receptor Subtypes in Stress Response and Emotionality. Neuropsychopharmacology 42, 1647–1656. 10.1038/npp.2016.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins M, 2008. Diet, ketones, and neurotrauma. Epilepsia 49 Suppl 8, 111–113. 10.1111/j.1528-1167.2008.01852.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR, Fuchs E, 2017. Chronic psychosocial stressors in adulthood: Studies in mice, rats and tree shrews. Neurobiol. Stress 6, 94–103. 10.1016/j.ynstr.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyter LM, Kelly SD, Harrell CS, Neigh GN, 2013. Sex differences in the effects of adolescent stress on adult brain inflammatory markers in rats. Brain Behav. Immun 30, 88–94. 10.1016/j.bbi.2013.01.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabasa C, Askevik K, Schéle E, Hu M, Vogel H, Dickson SL, 2019. Divergent Metabolic Effects of Acute Versus Chronic Repeated Forced Swim Stress in the Rat. Obesity 27, 427–433. 10.1002/oby.22390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabasa C, Dickson SL, 2016. Impact of stress on metabolism and energy balance. Curr. Opin. Behav. Sci 9, 71–77. 10.1016/j.cobeha.2016.01.011 [DOI] [Google Scholar]
- Rasheed N, Ahmad A, Al-Sheeha M, Alghasham A, Palit G, 2011. Neuroprotective and anti-stress effect of A68930 in acute and chronic unpredictable stress model in rats. Neurosci. Lett 504, 151–155. 10.1016/J.NEULET.2011.09.021 [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Hauger RL, Morgan CA, Bremner JD, Charney DS, Southwick SM, 2000. Low baseline and yohimbine-stimulated plasma neuropeptide Y (NPY) levels in combat-related PTSD. Biol. Psychiatry 47, 526–539. [DOI] [PubMed] [Google Scholar]
- Reddy IA, Pino JA, Weikop P, Osses N, Sørensen G, Bering T, Valle C, Bluett RJ, Erreger K, Wortwein G, Reyes JG, Graham D, Stanwood GD, Hackett TA, Patel S, Fink-Jensen A, Torres GE, Galli A, 2016. Glucagon-like peptide 1 receptor activation regulates cocaine actions and dopamine homeostasis in the lateral septum by decreasing arachidonic acid levels. Transl. Psychiatry 6, 809 10.1038/tp.2016.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG, 2006. Cell signaling. H2O2, a necessary evil for cell signaling. Science 312, 1882–1883. 10.1126/science.1130481 [DOI] [PubMed] [Google Scholar]
- Ribeiro EA, Salery M, Scarpa JR, Calipari ES, Hamilton PJ, Ku SM, Kronman H, Purushothaman I, Juarez B, Heshmati M, Doyle M, Lardner C, Burek D, Strat A, Pirpinias S, Mouzon E, Han M-H, Neve RL, Bagot RC, Kasarskis A, Koo JW, Nestler EJ, n.d. Transcriptional and physiological adaptations in nucleus accumbens somatostatin interneurons that regulate behavioral responses to cocaine. 10.1038/s41467-018-05657-9 [DOI] [PMC free article] [PubMed]
- Richard JE, Anderberg RH, López-Ferreras L, Olandersson K, Skibicka KP, 2016. Sex and estrogens alter the action of glucagon-like peptide-1 on reward. 10.1186/s13293-016-0059-9 [DOI] [PMC free article] [PubMed]
- Rosenbaum S, Stubbs B, Ward PB, Steel Z, Lederman O, Vancampfort D, 2015. The prevalence and risk of metabolic syndrome and its components among people with posttraumatic stress disorder: A systematic review and meta-analysis. Metabolism. 64, 926–933. 10.1016/j.metabol.2015.04.009 [DOI] [PubMed] [Google Scholar]
- Rovira-Llopis S, Bañuls C, de Marañon AM, Diaz-Morales N, Jover A, Garzon S, Rocha M, Victor VM, Hernandez-Mijares A, 2017. Low testosterone levels are related to oxidative stress, mitochondrial dysfunction and altered subclinical atherosclerotic markers in type 2 diabetic male patients. Free Radic. Biol. Med 108, 155–162. 10.1016/j.freeradbiomed.2017.03.029 [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Roca CA, Schmidt PJ, Danaceau MA, Putnam K, Cizza G, Chrousos G, Nieman L, 2005. Testosterone Suppression of CRH-Stimulated Cortisol in Men. Neuropsychopharmacology 30, 1906–1912. 10.1038/sj.npp.1300742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J, Scali J, Carrière I, Scarabin P-Y, Ritchie K, Ancelin M-L, 2011. Estrogen receptor gene variants are associated with anxiety disorders in older women. Psychoneuroendocrinology 36, 1582–1586. 10.1016/j.psyneuen.2011.04.011 [DOI] [PubMed] [Google Scholar]
- Sabban Esther L, Serova LI, Alaluf LG, Laukova M, Peddu C, Sabban EL, 2015. Comparative effects of intranasal neuropeptide Y and HS014 in preventing anxiety and depressive-like behavior elicited by single prolonged stress. Behav. Brain Res 295, 9–16. 10.1016/j.bbr.2014.12.038 [DOI] [PubMed] [Google Scholar]
- Sadagurski M, Cady G, Miller RA, 2017. Anti-aging drugs reduce hypothalamic inflammation in a sex-specific manner. Aging Cell 16, 652–660. 10.1111/acel.12590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah R, Ekhator NN, Strawn JR, Sallee FR, Baker DG, Horn PS, Geracioti TD, 2009. Low Cerebrospinal Fluid Neuropeptide Y Concentrations in Posttraumatic Stress Disorder. BPS 66, 705–707. 10.1016/j.biopsych.2009.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem U, Khaleghi M, Morgenthaler NG, Bergmann A, Struck J, Mosley TH, Kullo IJ, Kullo IJ, 2009. Plasma carboxy-terminal provasopressin (copeptin): a novel marker of insulin resistance and metabolic syndrome. J. Clin. Endocrinol. Metab 94, 2558–64. 10.1210/jc.2008-2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savolainen-Peltonen H, Rahkola-Soisalo P, Hoti F, Vattulainen P, Gissler M, Ylikorkala O, Mikkola TS, 2019. Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ 364, l665 10.1136/bmj.l665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG, 1996. Identification of targets of leptin action in rat hypothalamus. J. Clin. Invest 98, 1101–6. 10.1172/JCI118891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Rinaman L, Vollmer RR, Amico JA, 2007. Oxytocin knockout mice demonstrate enhanced intake of sweet and nonsweet carbohydrate solutions. Am. J. Physiol. Integr. Comp. Physiol 292, R1828–R1833. 10.1152/ajpregu.00826.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott E, Zhang Q, Wang R, Vadlamudi R, Brann D, 2012. Estrogen neuroprotection and the critical period hypothesis. Front. Neuroendocrinol 33, 85–104. 10.1016/j.yfrne.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye H, 1956. The Stress of Life. McGraw Hill, New York, New York, USA. [Google Scholar]
- Selye H, 1936. A Syndrome produced by Diverse Nocuous Agents. Nature 138, 32–32. 10.1038/138032a0 [DOI] [PubMed] [Google Scholar]
- Senn M, Maier PM, Langhans W, 1995. ACTH, cortisol and glucose responses after administration of vasopressin in cattle and sheep. J. Comp. Physiol. B 164, 570–578. 10.1007/BF00261398 [DOI] [PubMed] [Google Scholar]
- Serr J, Suh Y, Oh S-A, Shin S, Kim M, Latshaw JD, Lee K, 2011. Acute up-regulation of adipose triglyceride lipase and release of non-esterified fatty acids by dexamethasone in chicken adipose tissue. Lipids 46, 813–820. 10.1007/s11745-011-3583-8 [DOI] [PubMed] [Google Scholar]
- Sharma P, 2011. Inflammation and the metabolic syndrome. Indian J. Clin. Biochem 26, 317–318. 10.1007/s12291-011-0175-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp FR, Bernaudin M, 2004. HIF1 and Oxygen Sensing in the Brain. Nature. 10.1038/nrn1408 [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Satoh S, Yano H, Minokoshi Y, Cushman SW, Shimazu T, 1998. Effects of noradrenaline on the cell-surface glucose transporters in cultured brown adipocytes : novel mechanism for selective activation of GLUT1 glucose transporters, Biochem. J [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shores MM, Sloan KL, Matsumoto AM, Moceri VM, Felker B, Kivlahan DR, 2004. Increased incidence of diagnosed depressive illness in hypogonadal older men. Arch. Gen. Psychiatry 61, 162–167. 10.1001/archpsyc.61.2.162 [DOI] [PubMed] [Google Scholar]
- Sibille E, Morris HM, Kota RS, Lewis DA, 2011. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int. J. Neuropsychopharmacol 14, 721–734. 10.1017/S1461145710001616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins JW, Yang S-H, Sarkar SN, Pearce V, 2008. Estrogen actions on mitochondria—Physiological and pathological implications. Mol. Cell. Endocrinol 290, 51–59. 10.1016/j.mce.2008.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer K, Upton D, Semple S, McKune A, 2018. Systemic low-grade inflammation in posttraumatic stress disorder: a systematic review. J. Inflamm. Res 11, 111–121. 10.2147/JIR.S155903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, Emmerzaal TL, Kozicz T, Andrews ZB, 2015. Ghrelin’s Role in the Hypothalamic-Pituitary-Adrenal Axis Stress Response: Implications for Mood Disorders. Biol. Psychiatry 78, 19–27. 10.1016/J.BIOPSYCH.2014.10.021 [DOI] [PubMed] [Google Scholar]
- Spiteri T, Musatov S, Ogawa S, Ribeiro A, Pfaff DW, Ågmo A, 2010. The role of the estrogen receptor α in the medial amygdala and ventromedial nucleus of the hypothalamus in social recognition, anxiety and aggression. Behav. Brain Res 210, 211–220. 10.1016/j.bbr.2010.02.033 [DOI] [PubMed] [Google Scholar]
- Spiteri T, Ogawa S, Musatov S, Pfaff DW, Ågmo A, 2012. The role of the estrogen receptor α in the medial preoptic area in sexual incentive motivation, proceptivity and receptivity, anxiety, and wheel running in female rats. Behav. Brain Res 230, 11–20. 10.1016/j.bbr.2012.01.048 [DOI] [PubMed] [Google Scholar]
- Stevens VL, Wang Y, Carter BD, Gaudet MM, Gapstur SM, 2018. Serum metabolomic profiles associated with postmenopausal hormone use. Metabolomics 14, 97 10.1007/s11306-018-1393-1 [DOI] [PubMed] [Google Scholar]
- Su L, Cai Y, Xu Y, Dutt A, Shi S, Bramon E, 2014. Cerebral metabolism in major depressive disorder: a voxel-based meta-analysis of positron emission tomography studies. 10.1186/s12888-014-0321-9 [DOI] [PMC free article] [PubMed]
- Takayanagi Y, Kasahara Y, Onaka T, Takahashi N, Kawada T, Nishimori K, 2008. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport 19, 951–955. 10.1097/WNR.0b013e3283021ca9 [DOI] [PubMed] [Google Scholar]
- Taliaz D, Loya A, Gersner R, Haramati S, Chen A, Zangen A, 2011. Resilience to Chronic Stress Is Mediated by Hippocampal Brain-Derived Neurotrophic Factor. J. Neurosci 10.1523/JNEUROSCI.5725-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp AA, Schlaich MP, 2015. Relevance of Sympathetic Nervous System Activation in Obesity and Metabolic Syndrome. J. Diabetes Res 2015, 1–11. 10.1155/2015/341583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemeier H, Schuit SCE, den Heijer T, van Meurs JBJ, van Tuijl HR, Hofman A, Breteler MMB, Pols HAP, Uitterlinden AG, 2005. Estrogen receptor alpha gene polymorphisms and anxiety disorder in an elderly population. Mol. Psychiatry. 10.1038/sj.mp.4001697 [DOI] [PubMed] [Google Scholar]
- Toro-Urrego N, Garcia-Segura LM, Echeverria V, Barreto GE, 2016. Testosterone Protects Mitochondrial Function and Regulates Neuroglobin Expression in Astrocytic Cells Exposed to Glucose Deprivation. Front. Aging Neurosci 8, 152 10.3389/fnagi.2016.00152 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Torres-Aleman I, 2010. Toward a comprehensive neurobiology of IGF-I. Dev. Neurobiol 70, 384–396. 10.1002/dneu.20778 [DOI] [PubMed] [Google Scholar]
- Tostes RC, Carneiro FS, Carvalho, Reckelhoff JF, 2016. Reactive oxygen species: players in the cardiovascular effects of testosterone. Am. J. Physiol. Regul. Integr. Comp. Physiol 310, R1–R14. 10.1152/ajpregu.00392.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo J, Piriz J, Llorens-Martin MV, Fernandez AM, Bolós M, LeRoith D, Nuñez A, Torres-Aleman I, 2007. Central actions of liver-derived insulin-like growth factor I underlying its pro-cognitive effects. Mol. Psychiatry 12, 1118–1128. 10.1038/sj.mp.4002076 [DOI] [PubMed] [Google Scholar]
- Tripp A, Kota RS, Lewis DA, Sibille E, 2011. Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol. Dis 42, 116–124. 10.1016/j.nbd.2011.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp A, Oh H, Guilloux J-P, Martinowich K, Lewis DA, Sibille E, 2012. Brain-Derived Neurotrophic Factor Signaling and Subgenual Anterior Cingulate Cortex Dysfunction in Major Depressive Disorder. Am J Psychiatry 169, 1194–1202. 10.1176/appi.ajp.2012.12020248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Collette KM, 2017. (Putative) sex differences in neuroimmune modulation of memory. J. Neurosci. Res 95, 472–486. 10.1002/jnr.23921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuesta LM, Chen Z, Duncan A, Fowler CD, Ishikawa M, Lee BR, Liu X-A, Lu Q, Cameron M, Hayes MR, Kamenecka TM, Pletcher M, Kenny PJ, 2017. GLP-1 acts on habenular avoidance circuits to control nicotine intake 20 10.1038/nn.4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi E, Zhuang Y, Agrawal R, Ying Z, Gomez-Pinilla F, 2015. Interactive actions of Bdnf methylation and cell metabolism for building neural resilience under the influence of diet. Neurobiol. Dis 10.1016/j.nbd.2014.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles A, Marti O, Garcia A, Armario A, 2000. Single exposure to stressors causes long-lasting, stress-dependent reduction of food intake in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol 279, R1138–44. 10.1152/ajpregu.2000.279.3.R1138 [DOI] [PubMed] [Google Scholar]
- Vidal J-S, Hanon O, Funalot B, Brunel N, Viollet C, Rigaud A-S, Seux M-L, le-Bouc Y, Epelbaum J, Duron E, 2016. Low Serum Insulin-Like Growth Factor-I Predicts Cognitive Decline in Alzheimer’s Disease. J. Alzheimer’s Dis 52, 641–649. 10.3233/JAD-151162 [DOI] [PubMed] [Google Scholar]
- Vogel H, Wolf S, Rabasa C, Rodriguez-Pacheco F, Babaei CS, Stöber F, Goldschmidt J, DiMarchi RD, Finan B, Tschöp MH, Dickson SL, Schürmann A, Skibicka KP, 2016. GLP-1 and estrogen conjugate acts in the supramammillary nucleus to reduce food-reward and body weight. Neuropharmacology 110, 396–406. 10.1016/j.neuropharm.2016.07.039 [DOI] [PubMed] [Google Scholar]
- Wainwright SR, Lieblich SE, Galea LAM, 2011. Hypogonadism predisposes males to the development of behavioural and neuroplastic depressive phenotypes. Psychoneuroendocrinology 36, 1327–1341. 10.1016/j.psyneuen.2011.03.004 [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA, 2005. ERbeta-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology 30, 1598–1609. 10.1038/sj.npp.1300713 [DOI] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA, 2008. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout, mice enhances performance in the object recognition and object placement tasks. 10.1016/j.nlm.2008.01.008 [DOI] [PMC free article] [PubMed]
- Walf AA, Rhodes ME, Frye CA, 2004. Antidepressant effects of ERbeta-selective estrogen receptor modulators in the forced swim test. Pharmacol. Biochem. Behav 78, 523–529. 10.1016/j.pbb.2004.03.023 [DOI] [PubMed] [Google Scholar]
- Wang G-X, Zhao X-Y, Lin JD, 2015. The brown fat secretome: metabolic functions beyond thermogenesis. Trends Endocrinol. Metab 26, 231–237. 10.1016/j.tem.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang H, Tang S, Liu X, O’Neil A, Turner A, Chai F, Chen F, Berk M, 2014. Assessing Regional Cerebral Blood Flow in Depression Using 320-Slice Computed Tomography. PLoS One 9, e107735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wook Koo J, Labonté B, Engmann O, Calipari ES, Juarez B, Lorsch Z, Walsh JJ, Friedman AK, Yorgason JT, Han M-H, Nestler EJ, 2016. Essential Role of Mesolimbic Brain-Derived Neurotrophic Factor in Chronic Social Stress–Induced Depressive Behaviors. Biol. Psychiatry 80, 469–478. 10.1016/j.biopsych.2015.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D, 1999. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc. Natl. Acad. Sci. U. S. A. 96, 7324–7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakes FM, Van Houten B, 1997. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. U. S. A. 94, 514–9. 10.1073/PNAS.94.2.514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Kang Y, Ji X, Li S, Li Y, Zhang G, Cui H, Shi G, 2017. Testosterone Upregulates the Expression of Mitochondrial ND1 and ND4 and Alleviates the Oxidative Damage to the Nigrostriatal Dopaminergic System in Orchiectomized Rats. Oxid. Med. Cell. Longev 2017, 1–13. 10.1155/2017/1202459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Kozloski M, 2011. Sex differences in age trajectories of physiological dysregulation: inflammation, metabolic syndrome, and allostatic load. Journals Gerontol. Ser. A 66A, 493–500. 10.1093/gerona/glr003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W, Zhang JC, Ishima T, Dong C, Yang C, Ren Q, Ma M, Han M, Wu J, Suganuma H, Ushida Y, Yamamoto M, Hashimoto K, 2016. Role of Keap1-Nrf2 signaling in depression and dietary intake of glucoraphanin confers stress resilience in mice. Sci. Rep 6 10.1038/srep30659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Brand S, Yang R-K, 2006. Plasma Neuropeptide Y Concentrations in Combat Exposed Veterans: Relationship to Trauma Exposure, Recovery from PTSD, and Coping. Biol. Psychiatry 59, 660–663. 10.1016/j.biopsych.2005.08.027 [DOI] [PubMed] [Google Scholar]
- Zanardi R, Rossini D, Magri L, Malaguti A, Colombo C, Smeraldi E, 2007. Response to SSRIs and role of the hormonal therapy in post-menopausal depression. Eur. Neuropsychopharmacol 17, 400–405. 10.1016/j.euroneuro.2006.11.001 [DOI] [PubMed] [Google Scholar]
- Zarrouf FA, Artz S, Griffith J, Sirbu C, Kommor M, 2009. Testosterone and depression: systematic review and meta-analysis. J. Psychiatr. Pract 15, 289–305. 10.1097/01.pra.0000358315.88931.fc [DOI] [PubMed] [Google Scholar]
- Zhang G, Cai D, 2011. Circadian intervention of obesity development via resting-stage feeding manipulation or oxytocin treatment. Am. J. Physiol. Metab 301, E1004–E1012. 10.1152/ajpendo.00196.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Jankord R, Flak JN, Solomon MB, D’Alessio DA, Herman JP, 2010. Role of Glucocorticoids in Tuning Hindbrain Stress Integration. J. Neurosci 30, 14907–14914. 10.1523/JNEUROSCI.0522-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga KL, O’Connor DT, Handa RJ, Gonzales RJ, 2012. Estrogen receptor beta dependent attenuation of cytokine-induced cyclooxygenase-2 by androgens in human brain vascular smooth muscle cells and rat mesenteric arteries. Steroids 77, 835–844. 10.1016/j.steroids.2012.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]