Abstract
The purpose of this study was to determine sex differences in the contribution of sensory nerves to rapid cutaneous thermal hyperemia. Healthy young females (n = 15, tested during both the early follicular (EF) and the mid-luteal (ML) phase of the menstrual cycle) and males (n = 15) had a 4 cm2 area of skin on one forearm and one leg treated with a eutectic mixture of local anesthetic (EMLA). EMLA sites, along with corresponding control sites, were instrumented with laser Doppler flowmetry probes and local skin heaters. Baseline (33 °C), rapid and sustained vasodilation (42 °C), and maximal vasodilation (44 °C) skin blood flow data were obtained and expressed as a percentage of maximal cutaneous vascular conductance (%CVCmax). Contribution of sensory nerve involvement was determined by comparing the EMLA site to its matched control site utilizing the formula [(% CVCmax control - % CVCmax treatment) / % CVCmax control] × 100. The contribution of sensory nerves to rapid cutaneous thermal hyperemia in the forearm was 24 ± 18 %CVCmax in males, 41 ± 17 %CVCmax in ML females (p = 0.02 vs. males), and 35 ± 17 %CVCmax in EF females (p > 0.05 vs. males). In the leg, the contribution of sensory nerves was 16 ± 15 %CVCmax in males, 34 ± 17 %CVCmax for ML females (p = 0.02 vs. males), and 28 ± 21 %CVCmax in EF females (p > 0.05 vs. males). ML females exhibited a greater contribution of sensory nerves to rapid cutaneous thermal hyperemia in the forearm and leg, possibly attributed to elevated reproductive hormones during the ML phase.
Keywords: Adrenergic, thermal stimulus, vascular response
INTRODUCTION
Cutaneous sensory nerves can detect and respond to a variety of stimuli including touch (33), pressure (18), warmth (14), cold (4), and pruritus (34). When a localized warm stimulus is detected, unmyelinated warmth-sensing afferent C fibers release vasodilatory neuropeptides such as substance P and calcitonin gene-related peptide (12) to rapidly increase blood flow to the skin. If the localized warm stimulus persists, skin blood flow will transiently decrease to a nadir before slowly rising again to a nitric oxide-dependent sustained vasodilation (25). Although chemical (3) contributors respond to the rapid initial vasodilation that begins immediately after local warming have been identified, the response is largely dependent on sensory nerves (19).
Local warming of the skin can be utilized to assess microvascular reactivity, and mechanistic investigations into the various components of the cutaneous thermal hyperemic response can bring to light underlying vascular dysfunction (29). Previously it was determined that diabetic individuals exhibited a significantly higher (~58%) sensory thermal threshold and significantly lower (~58%) rapid initial cutaneous vasodilation in the dorsum of the hand during local heating compared to healthy controls (36). Thus, our understanding of the mechanisms that lead to microvascular dysfunction in aging, in different sexes, and in people with various clinical conditions is imperative (22) for developing appropriate preventive measures such as aerobic exercise training (37) and amino acid supplementation (27), or treatment therapies such as resistance exercise training (9) or pharmaceuticals (5).
Numerous vascular control mechanisms differ according to sex (21, 24, 35). Sex-specific factors that drive these differences include reproductive hormones, (17) sympathetic tone, (15) and baroreflex sensitivity (26). Thus, investigations into the integrated physiological response to day-to-day perturbations such as thermal stress, and long-term stressors like aging and disease need to be individualized so that the influence of sex does not confound research findings. As such, the mechanisms of cutaneous vascular control that differ between sexes should be investigated to further our understanding of how microvascular reactivity is impacted by sex-specific factors.
Although sensory nerve-mediated cutaneous vasodilation has been studied extensively in healthy (20, 37) and diseased populations (10), no study has exclusively attempted to discern if there are sex-related differences in the mechanisms of the initial rapid cutaneous vasodilation response to local heating. Therefore, in this study, we aimed to determine if there were sex-related differences in the contribution of sensory nerves to the initial rapid cutaneous thermal hyperemia via pharmacologically blocking sensory nerves and monitoring skin blood flow with laser-Doppler flowmetry (LDF) in females and males. Based on the known influence of female reproductive hormones on systemic hemodynamics (16), we hypothesized that there would be significant, sex-related differences in the contribution of sensory nerves to rapid cutaneous thermal hyperemia only when females were in the mid-luteal phase of the menstrual cycle, when estrogen and progesterone are elevated.
METHODS
Participants
Ethical approval for human subjects research was obtained from the Ball State University Institutional Review Board and conformed to the guidelines set forth by the Declaration of Helsinki. Additionally, this research was carried out in accordance to the ethical standards of the International Journal of Exercise Science (32). All participants were informed of the experimental procedures and risks of the study before participating. Verbal and written informed consent were obtained from each participant.
Participant characteristics are provided in Table 1. A minimum of 15 male and 15 female participants was determined by conducting a power analysis using nQuery (version 3.0) (Statistical Solutions, Cork, Ireland) and using standard deviation variations from our laboratory’s previous work in skin blood flow (8, 21). Thirty total participants were required to identify a statistical difference (power = 0.80, alpha = 0.05). All participants were required to be healthy, without signs or symptoms of disease determined via self-report on a health history questionnaire, and between the ages of 18 and 40 years. Since hormone phases in human females have been shown to affect skin blood flow (7, 21) female participants were tested once during the early follicular (EF) phase of the menstrual cycle, when reproductive hormones are at their lowest, and once during the mid-luteal (ML) phase of the menstrual cycle, when reproductive hormones are elevated. The EF was separated from the ML by collecting data within six days from the start of menstruation as reported by self-report (28).
Table 1.
Participant resting data and demographic and anthropometric characteristics.
| Characteristics | Male | Female |
|---|---|---|
| Age (yrs) | 23 ± 2 | 23± 4 |
| Resting HR (bpm) | 56 ± 11 | 64 ± 12 |
| Resting MAP (mm Hg) | 101 ± 9 | 97 ± 13 |
| Height (cm) | 178.7 ± 7.4 | 162.5 ± 7.3 * |
| Weight (kg) | 87 ± 14.1 | 67.3 ± 12.6 * |
| Body Composition (% Fat) | 19.5 ± 10.7 | 29.8 ± 9.5 * |
| VO2max (ml · kg · min−1) | 44.5 ± 8.9 | 39.3 ± 7.0 |
Values presented as mean ± SD. Yrs = years; HR = heart rate; bpm = beats per minute; MAP = mean arterial blood pressure; mm Hg = millimeter of mercury; cm = centimeter; kg = kilogram; % fat = percent fat; ml · kg · min−1 = milliliters per kilogram per minute.
p < 0.05
Protocol
Session One
Participants were instructed to arrive at the laboratory after a three hour fast, well hydrated, having not consumed alcohol or caffeine for 24 hours, and to not have used tobacco or performed vigorous exercise for 24 hours prior. After completing the health history questionnaire and providing informed consent, height and weight were measured using a standard stadiometer and scale (Health o meter; McCook, IL). Then, each participant completed a body composition analysis via air displacement plethysmography (BOD POD®, COSMED; Rome, Italy), according to manufacturer instructions. Finally, VO2max was measured using a ramped cycle ergometer protocol and indirect calorimeter (Parvo Medics; Sandy, UT). During the exercise test, participants were instrumented with a telemetric heart rate monitor (Polar Electro Inc.; Bethpage, NY), a nose clip and two-way valve breathing mouthpiece (Hans Rudolph, Inc.; Shawnee, KS). The exercise intensity started at 50 watts and increased by 23 watts per minute, in a continuous ramp of .38 watts per s, until volitional exhaustion was achieved, the participant requested to stop, or the test became unsafe for any reason.
Session Two
On a separate occasion occurring a minimum of 48 hours following session one, each participant underwent an experimental skin blood flow assessment. Female participants completed this trial twice – once during the EF phase and once during the ML phase of the menstrual cycle. Participants were instructed to arrive at the laboratory at 07:00 having fasted for a minimum of two hours (permitted to drink water ad libitum) and having refrained from caffeine, tobacco, alcohol, and vigorous exercise for at least 24 hours prior. All data collection took place in a 23 ± 0.5 °C climate-controlled room. Each participant was acclimated to the room temperature for 90 minutes prior to data collection.
Each participant rested in the supine position on an adjustable table for instrumentation, where they remained for the duration of the study. Four sites (two control; two treatment), at least five centimeters apart, two on one forearm and two on one leg were selected. These sites were free from major skin anomalies, such as moles or scars, and care was taken to prevent selecting sites that were located above superficial, prominent veins. Cutaneous sensory nerve function was blocked by applying 2.5 g of EMLA cream (2.5% lidocaine & 2.5% prilocaine; AstraZeneca; Wilmington, DE) over a 4 cm2 area in one of the two selected skin sites on one forearm and one leg (20, 37). The sites were covered with an occlusive dressing for one hour. The first application of EMLA cream was then wiped from the skin and a second application of the cream and a corresponding dressing were applied for an additional 30 minutes. After the second application of EMLA cream was wiped clear, sensory nerve blockade was verified by lightly pricking and brushing the sites with a hypodermic needle (37). Once confirmation of sensory nerve blockade was completed, with local heating discs (Perimed AB; Järfälla-Stockholm, Sweden) and LDF probes (Perimed AB; Järfälla-Stockholm, Sweden) were placed on the participant. The heating discs were placed over the four predetermined sites on the left side of the body and then taped in place. The LDF probes were then placed in the aperture in the center of the heating discs. Additionally, an oscillometric blood pressure cuff (BpTRU Medical Devices; Coquitlam, BC, Canada) was placed on the right arm opposite to the skin blood flow assessment.
Once local skin sensory nerve blockade was achieved in one forearm and one leg, resting HR was taken via automated blood pressure cuff, and skin blood flow data collection began at time zero. Blood pressure measurements were recorded every five minutes thereafter throughout the study. MAP was calculated for each five-minute section and used to determine CVC. Then, the local heating discs were set to 33 °C (thermoneutral) and held constant for 10 minutes following confirmation that local skin blood flow responses were stable (20). After baseline skin blood flow measurements were complete, the rapid initial vasodilation phase of the cutaneous hyperemic response to local heating was initiated by increasing local skin temperature from 33 °C to 42 °C at a rate of 1 °C • 20 s-1 (19). Once all sites were at 42 °C, this local skin temperature was maintained for 35 minutes, thereby producing the sustained vasodilation phase of the thermal hyperemic response (20). Upon completion of the sustained vasodilation phase, maximal cutaneous vasodilation was induced by increasing the local skin temperature from 42 to 44 °C at a rate of 1 °C • 5 s-1 (19). This temperature was then held for 20 minutes (20). After the maximal vasodilation phase, the experiment was complete and all instruments were removed from the participant.
The data from the local skin heating and LDF units were collected via PowerLab (ADInstruments – North America; Colorado Springs, CO) and displayed on a personal computer screen via the LabChart software (ADInstruments – North America; Colorado Springs, CO). The skin blood flow LDF data, in mV, were obtained for each site by selecting stable 30 s sections (the middle of the initial response to the beginning of the nadir) from the rapid thermal hyperemia skin blood flow tracing for each testing site; in addition, 5 min sections of skin blood flow data were captured from the thermoneutral baseline and sustained and maximal vasodilation phases. Cutaneous vascular conductance (CVC), was then determined by dividing the LDF data (in mV) by mean arterial blood pressure (mm Hg). Furthermore, since differences in the heterogeneity of cutaneous blood vessels are numerous between participants, all CVC data were expressed and analyzed as a percentage of maximum obtained through maximal cutaneous vasodilation (23), where %CVCmax = CVC (during the evaluated period) / CVC (at maximal vasodilation). The percentage contribution of sensory nerves to rapid initial cutaneous vasodilation from the heat stimulus was calculated by comparing the experimental site for each limb to its accompanying control site utilizing the formula [(% CVCmax control - % CVCmax treatment) / % CVCmax control] × 100 (20).
Statistical Analysis
All data were processed using the SPSS software v.22 (SPSS, Inc., Chicago, IL) and statistical significance was set at p < .05. CVC data were normally distributed as indicated by the Kolmogorov-Smirnov goodness of fit test. Text and illustrative results are reported as mean ± standard deviation. Paired t-statistics were used to compare data during phases of the menstrual cycle in women, and independent samples t-statistics were used to compare women to men. One-way analysis of variance (ANOVA) was utilized to detect differences in CVC among treatment sites for all groups during the thermoneutral, rapid vasodilation, and sustained vasodilation phases of the heating protocol. One-way ANOVA was also employed to analyze the CVC data for the contribution of sensory nerves to rapid cutaneous thermal hyperemia. A Bonferroni post hoc analysis was performed when indicated by the ANOVA significance.
RESULTS
While age and relative VO2max were similar between male and female participants, male participants were significantly taller and heavier than the female participants (see Table 1). Additionally, females exhibited higher body fat percentages than men.
Skin Blood Flow Responses
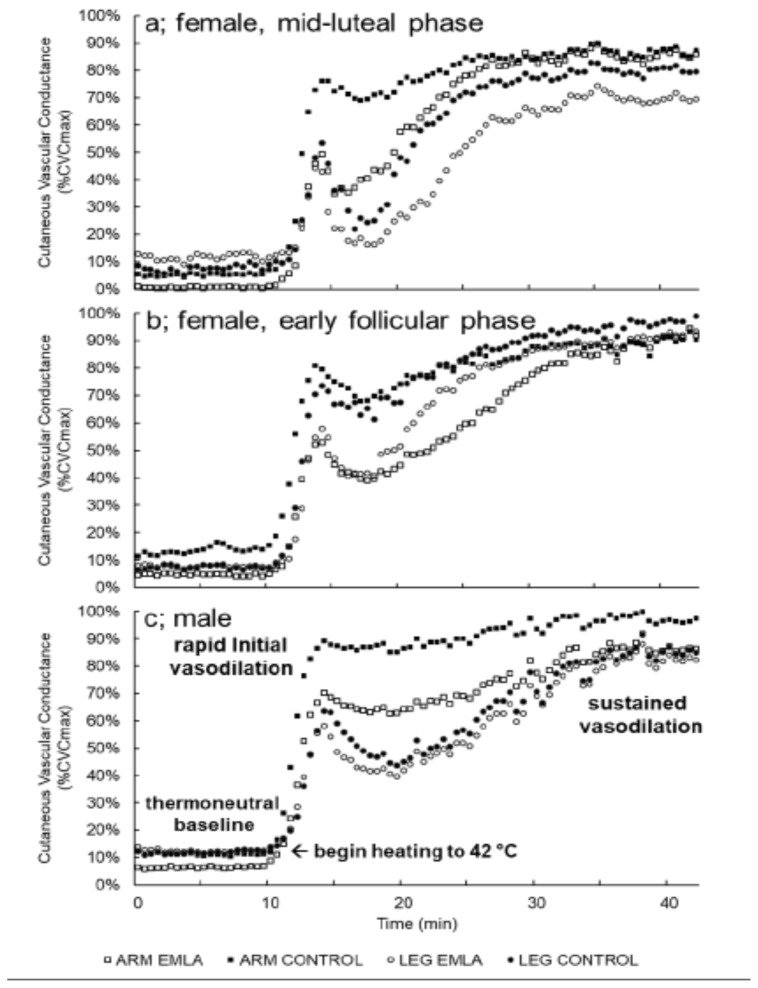
Forearm and leg %CVCmax data for each time point and group can be found in Table 2. Representative tracings of the skin blood flow response at baseline and during local heating, showing the rapid initial vasodilation, nadir, and sustained vasodilation phases of the thermal hyperemic response, from two female participants (one for each phase of the menstrual cycle studied) and one male participant, are provided within Figure 1.
Table 2.
Percent Maximal Cutaneous Vascular Conductance Values
| Limb and Time Point | Sex | Phase | Treatment | %CVC | SD |
|---|---|---|---|---|---|
|
| |||||
| Forearm Baseline |
FEMALE | MID-LUTEAL | CONTROL | 7.66% | 3.70% |
| EMLA | 2.18%* | 1.81% | |||
|
| |||||
| EARLY FOLLICULAR | CONTROL | 8.36% | 3.66% | ||
| EMLA | 2.68%* | 1.70% | |||
|
| |||||
| MALE | CONTROL | 9.98% | 3.77% | ||
| EMLA | 4.28%* | 3.15% | |||
|
| |||||
| Leg Baseline |
FEMALE | MID-LUTEAL | CONTROL | 6.72% | 2.28% |
| EMLA | 7.40% | 2.19% | |||
|
| |||||
| EARLY FOLLICULAR | CONTROL | 6.20% | 1.76% | ||
| EMLA | 7.93% | 3.06% | |||
|
| |||||
| MALE | CONTROL | 11.25% | 6.29% | ||
| EMLA | 11.45% | 4.21% | |||
|
| |||||
| Forearm Rapid Initial Response |
FEMALE | MID-LUTEAL | CONTROL | 74.32% | 9.09% |
| EMLA | 49.72%* | 18.59% | |||
|
| |||||
| EARLY FOLLICULAR | CONTROL | 78.16% | 5.85% | ||
| EMLA | 52.66%* | 16.35% | |||
|
| |||||
| MALE | CONTROL | 74.90% | 10.07% | ||
| EMLA | 58.36%* | 17.00% | |||
|
| |||||
| Leg Rapid Initial Response |
FEMALE | MID-LUTEAL | CONTROL | 66.33% | 14.53% |
| EMLA | 45.10%* | 14.73% | |||
|
| |||||
| EARLY FOLLICULAR | CONTROL | 64.50% | 10.97% | ||
| EMLA | 50.10% | 21.52% | |||
|
| |||||
| MALE | CONTROL | 63.65% | 10.06% | ||
| EMLA | 53.71% | 9.44% | |||
|
| |||||
| Forearm Sustained Response |
FEMALE | MID-LUTEAL | CONTROL | 87.89% | 8.82% |
| EMLA | 87.55% | 8.04% | |||
|
| |||||
| EARLY FOLLICULAR | CONTROL | 90.24% | 3.62% | ||
| EMLA | 88.32% | 5.73% | |||
|
| |||||
| MALE | CONTROL | 87.51% | 6.40% | ||
| EMLA | 84.24% | 5.90% | |||
Phase = phase of the menstrual cycle; %CVC = Percentage of maximal cutaneous vascular conductance;
indicates that p < 0.05 when compared to control sites.
Figure 1.
Representative skin blood flow tracings from one female in the mid-luteal phase (a), one female in the early follicular phase (b), and one male (c) showing the thermoneutral baseline, rapid cutaneous thermal hyperemia, and sustained vasodilation phases of local heating.
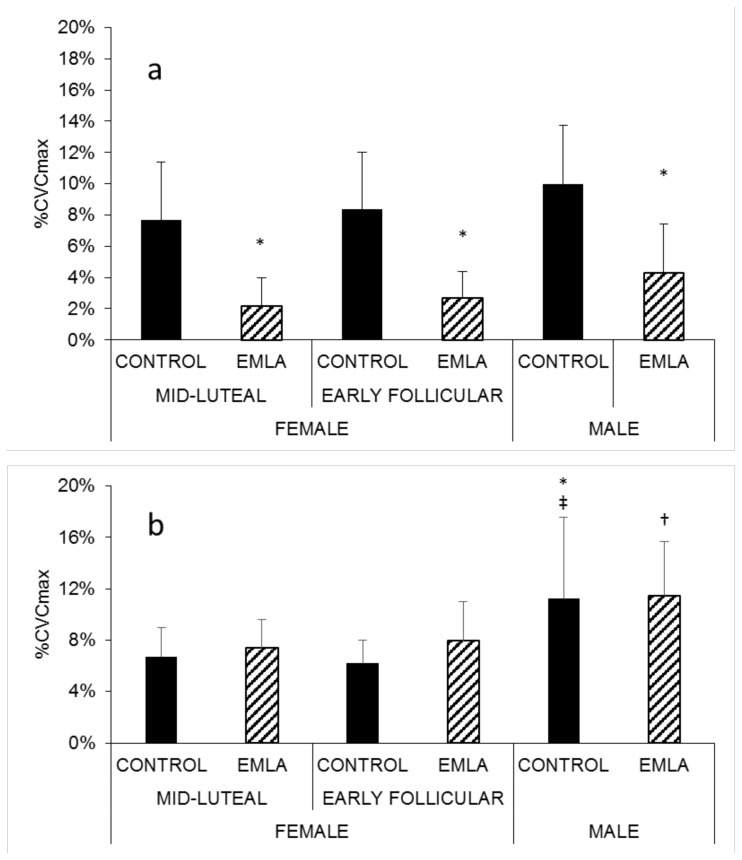
Baseline Skin Blood Flow
Baseline skin blood flow in the forearm EMLA sites was significantly lower than in the forearm control sites in all groups (Figure 2a); however, this effect was not observed between the EMLA and control sites of the leg in any group (Figure 2b). The EMLA-treated leg sites in males (11 ± 4 %CVCmax) exhibited significantly greater vasodilation at baseline than did the EMLA-treated legs sites of mid-luteal females (7 ± 2 %CVCmax; p = .036; Figure 2b). Additionally, baseline skin blood flow in the leg control sites of male participants (11 ± 6 %CVCmax) was significantly greater than in the leg control sites of the early follicular (6 ± 2 %CVCmax; p = .004) and mid-luteal (7 ± 2 %CVCmax; p = .013) female participants (Figure 2b).
Figure 2.
Baseline (thermoneutral, 33 °C) skin blood flow in the forearm (a) for EMLA and control sites for all participants. * p < 0.05 vs control. Baseline (thermoneutral, 33 °C) skin blood flow in the leg (b) for EMLA and control sites in all participants. † p < 0.05 vs control site in mid-luteal females. ‡ p < 0.05 vs control site in early follicular females. * p < 0.05 vs control site in mid-luteal females.
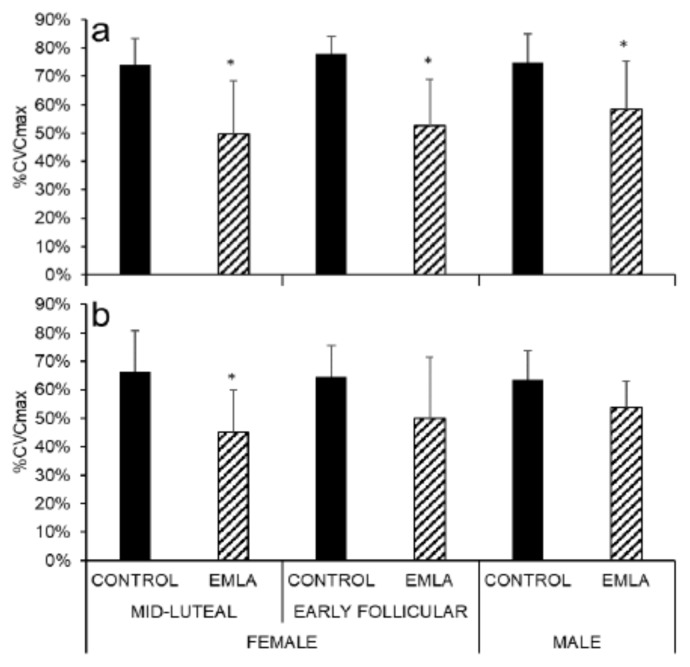
Rapid Initial Vasodilation
During the rapid initial vasodilation phase of the skin blood flow response to local heating, skin blood flow was significantly reduced in the EMLA-treated forearm sites in all groups when compared to the respective forearm control sites (p < .0001 for early follicular and mid-luteal phases; p = .017 for males). (Figure 3a). However, in the leg, only mid-luteal females exhibited a significant skin blood flow reduction in the EMLA-treated sites as compared to control (p = .001; Figure 3b).
Figure 3.
Rapid initial forearm (a) and leg (b) skin vasodilation during local heating for EMLA and control sites in all participants. * p < .05 vs control.
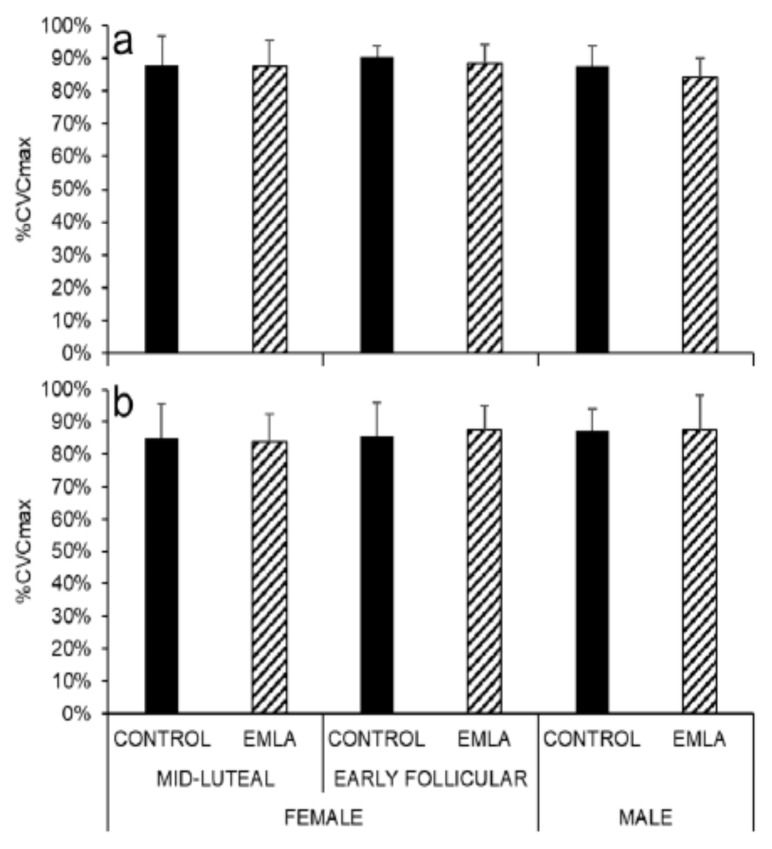
Sustained Vasodilation
The sustained vasodilation phase of the skin blood flow response to local heating elicited comparable responses in the EMLA-treated and control sites of the forearm (Figure 4a) and the leg (Figure 4b) for all groups.
Figure 4.
Sustained cutaneous vasodilation during local heating in the forearm (a) and leg (b) EMLA and control sites in all participants.
Sensory Nerves and Rapid Cutaneous Vasodilation
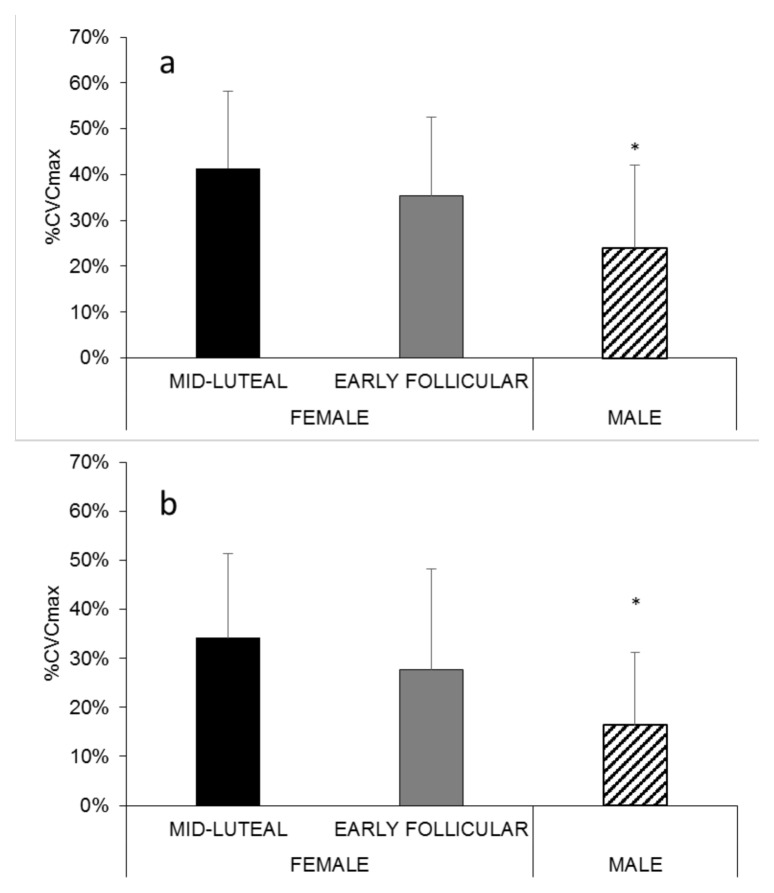
The contribution of sensory nerves to rapid cutaneous thermal hyperemia in the forearm (Figure 5a) of mid-luteal females (41 ± 17 %CVCmax), but not early follicular females (35 ± 17 %CVCmax), was significantly higher than males (24 ± 18 %CVCmax; p = .036 vs mid-luteal females). This trend was also observed in the leg (Figure 5b), where, mid-luteal females (34 ± 17 %CVCmax), but not early follicular females (28 ± 21 %CVCmax), exhibited a significantly greater contribution of sensory nerves than males (16 ± 15 %CVCmax) during the rapid initial vasodilation phase of the thermal hyperemic response (p = .026 for mid-luteal females vs males).
Figure 5.
The contribution of sensory nerves to rapid cutaneous thermal hyperemia in the forearm (a) and leg (b) of all participants. * p < .05 vs mid-luteal females.
DISCUSSION
The purpose of this study was to determine possible sex differences in the contribution of sensory nerves in response to a local heat stimulus. We observed greater contribution of sensory nerves to rapid cutaneous thermal hyperemia in female participants who were in the mid-luteal phase of the menstrual cycle as compared to men. However, these sex differences were not detected when female participants were assessed during the early follicular phase of the menstrual cycle. Furthermore, there were no detectable differences in the contribution of sensory nerves to the sustained hyperemic response between the two hormone phases of the female participants.
The results of this study indicate that the contribution of sensory nerves to rapid cutaneous thermal hyperemia is not uniform across the sexes. In order to control for the influence of sex hormones on cutaneous blood flow mechanisms, previous studies have tested female participants during the early follicular phase (within six days from the start of menstruation) of the menstrual cycle, when estrogen and progesterone are at their lowest levels. However, females are only in this phase for approximately 25% of their reproductive lives, and this method fails to properly represent physiological responses during the remainder of the menstrual cycle (6). Therefore, in this study, we also tested female participants during the mid-luteal phase to examine the potential influence of high levels of progesterone and relatively elevated levels of estrogen. Our findings did not support the notion that sensory nerve involvement during rapid cutaneous thermal hyperemia is modulated by reproductive hormone differences throughout the menstrual cycle.
The absolute magnitude of the initial (2) and sustained (7) cutaneous vasodilation provoked by a local heat stimulus has been shown to be significantly augmented by elevated estrogen and progesterone levels. However, no study until now has investigated the relative contribution of sensory nerves and the potential effect of sex hormones on rapid cutaneous thermal hyperemia. Moderately high levels of estrogen and near-maximal levels of progesterone have been observed during the mid-luteal phase of the menstrual cycle and may have had an influence on the sex differences in this study. Calcitonin gene-related peptide (1) and substance P (13) are potent vasodilating agents, thought to be released in an axon reflex fashion from local sensory nerves within the skin when subjected to a local thermal stimulus (38). Estrogen and/or progesterone administration has been shown to increase substance P and calcitonin gene-related peptide synthesis in sensory neurons (11, 30, 31). Therefore, it is possible that elevated sex hormones in the females who were in the mid-luteal phase of the menstrual cycle in our study stimulated increased substance P and calcitonin gene-related peptide production and release from sensory nerves, thereby explaining the augmented contribution of sensory nerves to the rapid thermal hyperemic response.
Local control of skin blood flow aids in the maintenance of a myriad of homeostatic mechanisms within the body (10). Due to the importance of sensory nerves in maintaining local cutaneous homeostasis (39) and the preponderance of evidence for sex- and reproductive hormone-related differences in local skin blood flow control (7, 21, 35), the findings of this study provide unique insight into sensory nerve-mediated skin blood flow responses between sexes and between two reproductive hormone phases in female participants. A possible explanation for the sex differences detected in this study could rest in the contribution of nitric oxide (NO) to the rapid thermal hyperemic response in the skin. Previous researchers have determined that females exhibited less dependence on nitric oxide for sustained cutaneous vasodilation during local heating (35). Thus, the male participants in our study may have achieved the same absolute level of rapid cutaneous vasodilation during local heating by relying more heavily on NO-dependent mechanisms rather than sensory nerves, but this remains to be directly tested.
Limitations of this study include a relatively small sample size, and the fact that serum concentrations of reproductive hormones were not measured. Although we tested participants during the self-reported early follicular and mid-luteal phases of the menstrual cycle, we cannot confirm circulating levels of estrogen or progesterone. Approximately half of the female participants were taking oral contraceptive medication, which artificially alters hormonal balances. We cannot exclude the possibility that this may have skewed some of the reported observations. Future research is recommended to more clearly elucidate the influence of various contraceptive methods on thermoregulation.
The present study suggests women who are in the mid-luteal phase of the menstrual cycle exhibit a greater contribution of sensory nerves to the thermal hyperemic response than males. However, there were no differences detected between females who were in the early follicular phase of the menstrual cycle and males. It is theorized that the mechanism underlying these observations are related to calcitonin gene-related peptide (11, 30) and substance P (31) synthesis in sensory neurons. Given that women have a higher prevalence rate of rare vascular skin conditions (e.g. Erythromelalgia and Raynaud’s phenomenon), where sensory dysfunction plays a role, these results may be valuable for improving our understanding of the pathophysiology of such conditions. Future mechanistic skin blood flow studies should consider sex differences in the contribution of sensory nerves during rapid cutaneous thermal hyperemia. The sensory nerve-mediated rapid increase in skin blood flow seen during a local thermal perturbation is part of the integrated response to maintain local tissue homeostasis. Females in the mid-luteal phase of the menstrual cycle, where estrogen and progesterone are elevated, exhibited augmented sensory nerve involvement during rapid cutaneous thermal hyperemia when compared to females in the early follicular phase of the menstrual cycle (low levels estrogen and progesterone) and males. Thus, our data indicate that the mechanisms by which males and females regulate local skin temperature differs, but only when females are in the mid-luteal phase of the menstrual cycle.
REFERENCES
- 1.Brain SD, Tippins JR, Morris HR, Maclntyre I, Williams TJ. Potent vasodilator activity of calcitonin gene-related peptide in human skin. J Investig Dermatol. 1986;87(4):533–536. doi: 10.1111/1523-1747.ep12455620. [DOI] [PubMed] [Google Scholar]
- 2.Brunt VE, Miner JA, Meendering JR, Kaplan PF, Minson CT. 17β-estradiol and progesterone independently augment cutaneous thermal hyperemia but not reactive hyperemia. Microcirculation. 2011;18(5):347–355. doi: 10.1111/j.1549-8719.2011.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunt VE, Minson CT. Kca channels and epoxyeicosatrienoic acids: Major contributors to thermal hyperaemia in human skin. J Physiol. 2012;590(15):3523–3534. doi: 10.1113/jphysiol.2012.236398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campero M, Serra J, Bostock H, Ochoa J. Slowly conducting afferents activated by innocuous low temperature in human skin. J Physiol. 2001;535(3):855–865. doi: 10.1111/j.1469-7793.2001.t01-1-00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casellini CM, Barlow PM, Rice AL, Casey M, Simmons K, Pittenger G, Bastyr EJ, Wolka AM, Vinik AI. A 6-month, randomized, double-masked, placebo-controlled study evaluating the effects of the protein kinase c-β inhibitor ruboxistaurin on skin microvascular blood flow and other measures of diabetic peripheral neuropathy. Diabetes Care. 2007;30(4):896–902. doi: 10.2337/dc06-1699. [DOI] [PubMed] [Google Scholar]
- 6.Charkoudian N, Stachenfeld NS. Reproductive hormone influences on thermoregulation in women. Compr Physiol. 2014;4(2):793–804. doi: 10.1002/cphy.c130029. [DOI] [PubMed] [Google Scholar]
- 7.Charkoudian N, Stephens DP, Pirkle KC, Kosiba WA, Johnson JM. Influence of female reproductive hormones on local thermal control of skin blood flow. J Appl Physiol. 1999;87(5):1719–1723. doi: 10.1152/jappl.1999.87.5.1719. [DOI] [PubMed] [Google Scholar]
- 8.Del Pozzi AT, Carter SJ, Collins AB, Hodges GJ. The regional differences in the contribution of nitric oxide synthase to skin blood flow at forearm and lower leg sites in response to local skin warming. Microvasc Res. 2013;90:106–111. [PubMed] [Google Scholar]
- 9.Dias I, Farinatti P, De Souza M, Manhanini DP, Balthazar E, Dantas D, de Andrade Pinto EH, Bouskela E, Kraemer-Aguiar LG. Effects of resistance training on obese adolescents. Med Sci Sports Exerc. 2015;47(12):2636–2644. doi: 10.1249/MSS.0000000000000705. [DOI] [PubMed] [Google Scholar]
- 10.Fromy B, Sigaudo-Roussel D, Gaubert-Dahan M-L, Rousseau P, Abraham P, Benzoni D, Berrut G, Saumet JL. Aging-associated sensory neuropathy alters pressure-induced vasodilation in humans. J Investig Dermatol. 2010;130(3):849–855. doi: 10.1038/jid.2009.279. [DOI] [PubMed] [Google Scholar]
- 11.Gangula P, Lanlua P, Wimalawansa S, Supowit S, DiPette D, Yallampalli C. Regulation of calcitonin gene-related peptide expression in dorsal root ganglia of rats by female sex steroid hormones. Biol Reprod. 2000;62(4):1033–1039. doi: 10.1095/biolreprod62.4.1033. [DOI] [PubMed] [Google Scholar]
- 12.Gibbins I, Wattchow D, Coventry B. Two immunohistochemically identified populations of calcitonin gene-related peptide (cgrp)-immunoreactive axons in human skin. Brain Res. 1987;414(1):143–148. doi: 10.1016/0006-8993(87)91335-7. [DOI] [PubMed] [Google Scholar]
- 13.Hägermark Ö, Hökfelt T, Pernow B. Flare and itch induced by substance p in human skin. J Investig Dermatol. 1978;71(4):233–235. doi: 10.1111/1523-1747.ep12515092. [DOI] [PubMed] [Google Scholar]
- 14.Hallin RG, Torebjörk HE, Wiesenfeld Z. Nociceptors and warm receptors innervated by c fibres in human skin. J Neurol Neurosurg Psychiatry. 1982;45(4):313–319. doi: 10.1136/jnnp.45.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: Implications for human blood pressure regulation. Hypertension. 2009;53(3):571–576. doi: 10.1161/HYPERTENSIONAHA.108.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harvey RE, Hart EC, Charkoudian N, Curry TB, Carter JR, Fu Q, Minson CT, Joyner MJ, Barnes JN. Oral contraceptive use, muscle sympathetic nerve activity, and systemic hemodynamics in young womennovelty and significance. Hypertension. 2015;66(3):590–597. doi: 10.1161/HYPERTENSIONAHA.115.05179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto M, Akishita M, Eto M, Ishikawa M, Kozaki K, Toba K, Sagara Y, Taketani Y, Orimo H, Ouchi Y. Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation. 1995;92(12):3431–3435. doi: 10.1161/01.cir.92.12.3431. [DOI] [PubMed] [Google Scholar]
- 18.Hensel H, Boman KK. Afferent impulses in cutaneous sensory nerves in human subjects. J Neurophysiol. 1960;23(5):564–578. doi: 10.1152/jn.1960.23.5.564. [DOI] [PubMed] [Google Scholar]
- 19.Hodges GJ, Del Pozzi AT, McGarr GW, Mallette MM, Cheung SS. The contribution of sensory nerves to cutaneous vasodilatation of the forearm and leg to local skin heating. Eur J Appl Physiol. 2015;115(10):2091–2098. doi: 10.1007/s00421-015-3188-7. [DOI] [PubMed] [Google Scholar]
- 20.Hodges GJ, Del Pozzi AT, McGarr GW, Mallette MM, Cheung SS. The contribution of sensory nerves to cutaneous vasodilatation of the forearm and leg to local skin heating. Eur J Appl Physiol. 2015;115(10):2091–2098. doi: 10.1007/s00421-015-3188-7. [DOI] [PubMed] [Google Scholar]
- 21.Hodges GJ, Martin ZT, Del Pozzi AT. Neuropeptide y not involved in cutaneous vascular control in young human females taking oral contraceptive hormones. Microvasc Res. 2017;113:9–15. doi: 10.1016/j.mvr.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol. 2008;105(1):370–372. doi: 10.1152/japplphysiol.00858.2007. [DOI] [PubMed] [Google Scholar]
- 23.Johnson JM, Taylor W, Shepherd A, Park MK. Laser-doppler measurement of skin blood flow: Comparison with plethysmography. J Appl Physiol. 1984;56(3):798–803. doi: 10.1152/jappl.1984.56.3.798. [DOI] [PubMed] [Google Scholar]
- 24.Joyner MJ, Barnes JN, Hart EC, Wallin BG, Charkoudian N. Neural control of the circulation: how sex and age differences interact in humans. Compr Physiol. 2015;5(1):193–215. doi: 10.1002/cphy.c140005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kellogg D, Liu Y, Kosiba I, O’Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol. 1999;86(4):1185–1190. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- 26.Kim A, Deo SH, Vianna LC, Balanos GM, Hartwich D, Fisher JP, Fadel PJ. Sex differences in carotid baroreflex control of arterial blood pressure in humans: Relative contribution of cardiac output and total vascular conductance. Am J Physiol Heart Circ Physiol. 2011;301(6):H2454–H2465. doi: 10.1152/ajpheart.00772.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim K, Hurr C, Patik JC, Brothers RM. Attenuated cutaneous microvascular function in healthy young african americans: Role of intradermal l-arginine supplementation. Microvasc Res. 2018;118:1–6. doi: 10.1016/j.mvr.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Lorenzo S, Minson CT. Human cutaneous reactive hyperaemia: Role of bkca channels and sensory nerves. J Physiol. 2007;585(Pt 1):295–303. doi: 10.1113/jphysiol.2007.143867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minson CT. Thermal provocation to evaluate microvascular reactivity in human skin. J Appl Physiol. 2010;109(4):1239–1246. doi: 10.1152/japplphysiol.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mowa C, Usip S, Collins J, Storey-Workley M, Hargreaves K, Papka R. The effects of pregnancy and estrogen on the expression of calcitonin gene-related peptide (cgrp) in the uterine cervix, dorsal root ganglia and spinal cord. Peptides. 2003;24(8):1163–1174. doi: 10.1016/j.peptides.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Mowa C, Usip S, Storey-Workley M, Amann R, Papka R. Substance p in the uterine cervix, dorsal root ganglia and spinal cord during pregnancy and the effect of estrogen on sp synthesis. Peptides. 2003;24(5):761–771. doi: 10.1016/s0196-9781(03)00120-7. [DOI] [PubMed] [Google Scholar]
- 32.Navalta JW, Stone WJ, Lyons TS. Ethical issues relating to scientific discovery in exercise science. Int J Exerc Sci. 2019;12(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 33.Reinisch CM, Tschachler E. The touch dome in human skin is supplied by different types of nerve fibers. Ann Neurol. 2005;58(1):88–95. doi: 10.1002/ana.20527. [DOI] [PubMed] [Google Scholar]
- 34.Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE. Specific c-receptors for itch in human skin. J Neurosci. 1997;17(20):8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanhewicz AE, Greaney JL, Kenney WL, Alexander LM. Sex- and limb-specific differences in the nitric oxide-dependent cutaneous vasodilation in response to local heating. Am J Physiol Regul Integr Comp Physiol. 2014;307(7):R914–919. doi: 10.1152/ajpregu.00269.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stansberry KB, Peppard HR, Babyak LM, Popp G, McNitt PM, Vinik AI. Primary nociceptive afferents mediate the blood flow dysfunction in non-glabrous (hairy) skin of type 2 diabetes: A new model for the pathogenesis of microvascular dysfunction. Diabetes Care. 1999;22(9):1549–1554. doi: 10.2337/diacare.22.9.1549. [DOI] [PubMed] [Google Scholar]
- 37.Tew GA, Klonizakis M, Moss J, Ruddock AD, Saxton JM, Hodges GJ. Role of sensory nerves in the rapid cutaneous vasodilator response to local heating in young and older endurance-trained and untrained men. Exp Physiol. 2011;96(2):163–170. doi: 10.1113/expphysiol.2010.055434. [DOI] [PubMed] [Google Scholar]
- 38.Wong BJ, Minson CT. Altered thermal hyperaemia in human skin by prior desensitization of neurokinin-1 receptors. Exp Physiol. 2011;96(6):599–609. doi: 10.1113/expphysiol.2011.057356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zegarska B, Leliñska A, Tyrakowski T. Clinical and experimental aspects of cutaneous neurogenic inflammation. Pharmacol Rep. 2006;58(1):13. [PubMed] [Google Scholar]