Abstract
The aim of this case report and literature review is to explore the rarely highlighted co-occurrence of malignant melanoma and Parkinson’s disease (PD) and to describe the devastating effects on those affected. We present a case of acute confusion and worsening shuffling gait in a patient with Parkinson’s disease who eventually succumbed to metastatic malignant melanoma, secondary to a primary malignant melanoma on the back that was treated 6 years earlier. More research is needed to better characterize the associations between these two conditions, and to evaluate the effectiveness of prevention strategies such as melanoma screening for adults with PD.
LEARNING POINTS
The underlying mechanism for the co-occurrence of Parkinson’s disease (PD) and malignant melanoma remains unclear but available evidence suggests genetic associations and environmental factors as potential causes.
Patients with PD may require closer dermatological surveillance, particularly those with other risk factors for melanoma such as prolonged sun exposure.
More research is needed to better characterize the associations between PD and skin cancers, and to evaluate the effectiveness of prevention strategies such as melanoma screening for adults with PD.
Keywords: Parkinson’s, malignant, melanoma
INTRODUCTION
The aim of this case report and literature review is to explore the rarely highlighted co-occurrence of malignant melanoma and Parkinson’s disease (PD). Several large epidemiological studies have established a link between the two conditions despite the fact that they seem to have nothing in common. PD is a neurodegenerative disease in which loss of dopaminergic neurons results in asymmetric motor symptoms, namely bradykinesia, rigidity and tremors, while melanoma is a malignant transformation of melanocytes in the skin and other organs. The lifetime risk of being diagnosed with PD in the United Kingdom is expected to increase by 23.9% (from 2.7% in 2015) by 2025. Malignant melanoma is the fifth most common cancer in the United Kingdom and accounts for 4% of all new cancer cases. Individually, both conditions can have a significant effect on those affected, but the co-occurrence of both diseases rapidly increases morbidity and mortality as evidenced by the case presented. Exploring this association allows us to consider potential implications such as the possibility of melanoma screening for patients with PD.
CASE DESCRIPTION
A 77-year-old man with a 12-year history of PD was admitted to the Geriatric Ward for new onset confusion over the last 2 days and worsening shuffling gait. He was compliant with his medications, namely co-beneldopa 125 mg tablets six times per day, ropinirole 16 mg tablets once daily and citalopram 20 mg once daily. His past medical history was significant for several dermatological malignancies with the following histopathological diagnoses:
2012: Nodular basal cell carcinoma on the right neck.
2013: Invasive nodular malignant melanoma on the right lower back. Clark’s level 4 with a Breslow thickness of 4.8 mm. No lymphovascular invasion was seen. It was surgically excised and there was no lymphadenopathy at presentation and no clinical evidence of persisting malignancy in the surgical scar.
2014: Basal cell carcinoma on the right lower back.
2015: Lentigo maligna on the right ear lobe, a benign squamous lesion on the right lower leg, and invasive squamous cell cancer of the left leg.
The patient did not have a significant smoking or alcohol history, but he was retired from a job with a great deal of sun exposure. Most of his examination findings were normal, but significant neurological examination findings included a left homonymous hemianopia and features of Parkinsonism more pronounced in the right upper and lower limbs in keeping with PD. There was no associated weakness or sensory loss. On presentation he scored 8/10 on the Abbreviated Mental Test but his confusion progressively worsened with fewer lucid intervals. By the time he was discharged he no longer had the capacity to make decisions regarding his care.
Investigations
The patient’s blood results were normal except for a normocytic normochromic anaemia.
Computed tomography (CT) of the head demonstrated multiple enhancing brain lesions, likely metastatic deposits with an acute right occipital intracranial-axial haematoma (Fig. 1). Magnetic resonance imaging (MRI) of the head revealed in greater detail multiple lesions in both cerebral hemispheres and in the cerebellar vermis in keeping with melanoma deposits (Figs. 2 and 3). CT of the thorax, abdomen and pelvis revealed lung metastases and extensive bilateral calcified pleural plaques (Fig. 4). No intra-abdominal malignancy or destructive bony lesions were identified. Given his history of malignant melanoma and the investigation findings, the patient was diagnosed as having metastatic malignant melanoma.
Figure 1.
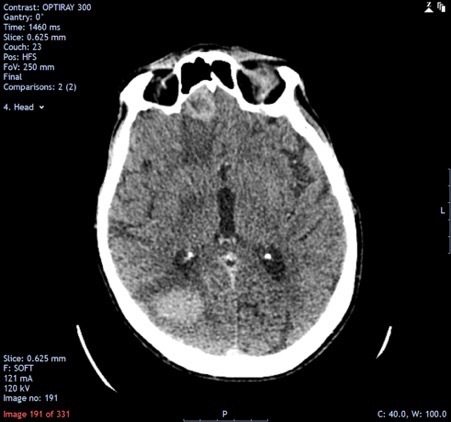
Non-contrast computed tomography (CT) of the head demonstrating multiple enhancing brain lesions, likely metastatic deposits with an acute right occipital intra-axial haematoma
Figure 2.
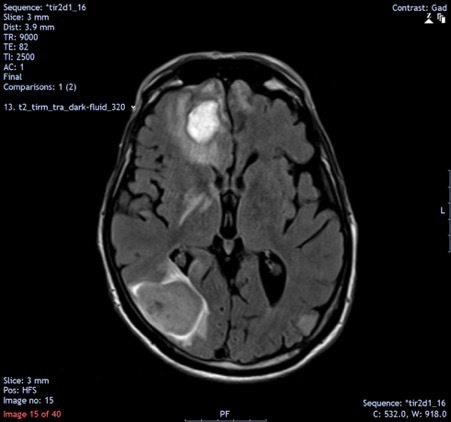
Magnetic resonance imaging of the head demonstrating high density lesions in keeping with melanoma deposits, with surrounding oedematous changes particularly in the right frontal and occipital lobes
Figure 3.
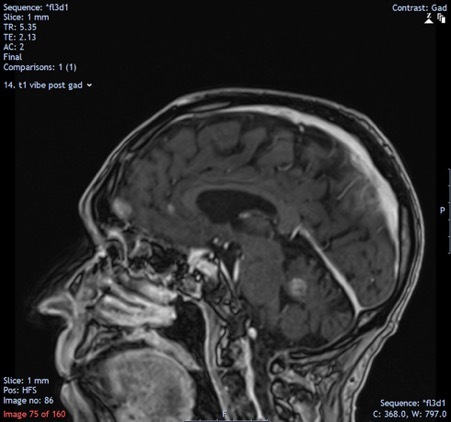
Magnetic resonance imaging of the head demonstrating high density lesions in keeping with melanoma deposits
Figure 4.
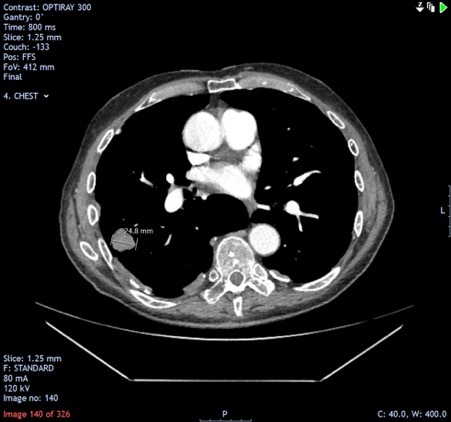
Computed Tomography (CT) scan of the thorax demonstrating a metastatic lesion in the right lung
Outcome
The Skin Multidisciplinary Team meeting concluded that best supportive care was the ideal management for him, and he was discharged to a care home for palliation. His neurological deterioration progressed, and he died a few months later.
DISCUSSION
PD and cancer in theory are polar opposites when it comes to their pathophysiology as one involves cellular degeneration and the other uncontrolled cellular proliferation, as one review article aptly puts it[1]. Therefore, it is not unreasonable to postulate that where one exists there should be a low risk for the other. Many epidemiological studies do in fact support this theory where PD patients have a decreased risk of developing lung, bladder and colorectal cancers. However, several studies have revealed a significant association between PD and malignant melanoma (Table 1). One study concluded that there was a seven-fold increase in the risk of developing any type of melanoma in patients with PD[1]. Additionally, a retrospective cohort study published by the Mayo Clinic in 2017 revealed that on average patients with PD were diagnosed with melanoma 7 years earlier than patients without PD[2]. Interestingly, the reverse is also true, where melanoma patients were found to have a 50% increased risk of developing PD[1]. The findings linking PD and melanoma are so consistent that one study commented that this association could not be easily explained away by improved medical surveillance of these patients[2].
Table 1.
Epidemiological studies demonstrating statistically significant associations between malignant melanoma and Parkinson’s disease using confidence intervals
| Study type | Authors | Location | Number of participants | 95% Confidence Interval |
|---|---|---|---|---|
| Malignant Melanoma before Parkinson’s Disease | ||||
| Case-control | Olsen et al. [4] | Denmark | 8,090 | 1.03–2.01 |
| Cohort | Wirdefeldt et al. [5] | Sweden | 11,786 | 1.23–1.91 |
| Retrospective cohort | Dalvin et al. [2] | USA | 1,544 | 2–8.8 |
| Malignant Melanoma after Parkinson’s Disease | ||||
| Cohort | Wirdefeldt et al. [5] | Sweden | 11,786 | 1.01–2.10 |
| Retrospective cohort | Dalvin et al. [2] | USA | 974 | 2.1–6.8 |
| Cohort | Rugbjerg et al. [6] | Denmark | 20,343 | 1.09–1.80 |
| Prospective cohort | Bertoni et al. [7] | USA | 1,692 | 1.24–4.17 |
While the root cause of this association remains unclear, several genes have been implicated in the pathogenesis of both melanomas and PD, namely:
Leucine-rich repeat kinase 2 (LRRK2), also known as dardarin: The LRRK2 mutation associated with PD is in the same structural position as the B-raf kinase mutation that is responsible for a significant portion of malignant melanomas[1].
DJ-1: This is an established oncogene which has been found in melanomas and its mutated form was linked to early-onset familial PD[1].
SNCA (Alpha synuclein): This gene has a pathological role in PD. Its accumulation within the central nervous system is associated with neurotoxicity, but it also has increased expression in melanoma cells. Its presence in both PD and melanoma provides a convincing argument that it may very well be a key instigator in the concurrence of both conditions[1].
Environmental factors such as exposure to pesticides that affect the mitochondrial electron transfer pathway (paraquat, maneb and rotenone) have also been linked to PD. The risk is found to be increased in those with more than 10–20 years of exposure[3].
CONCLUSION
The literature shows that there is a statistically significant association between PD and melanoma, although the root cause remains unclear. More research is needed to better characterize the associations between PD and melanoma, and to evaluate the effectiveness of prevention strategies such as melanoma screening for adults with PD.
Acknowledgements
I would like to thank Dr Brendan Moriarty, Dr Footitt (Consultant Neurologist), Alison Tonks and Dr John Young (Consultant Dermatologist) for their contributions towards the completion of this article.
Footnotes
Conflicts of Interests: The Authors declare that there are no competing interest
REFERENCES
- 1.Bose A, Petsko G, Eliezer D. Parkinson’s disease and melanoma: co-occurrence and mechanisms. J Parkinson’s Dis. 2018;8(3):385–398. doi: 10.3233/JPD-171263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalvin L, Damento G, Yawn B, Abbott B, Hodge D, Pulido J. Parkinson’s disease and melanoma: confirming and reexamining an association. Mayo Clin Proc. 2017;92(7):1070–1079. doi: 10.1016/j.mayocp.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown T, Rumsby P, Capleton A, Rushton L, Levy L. Pesticides and Parkinson’s disease—is there a link? Environ Health Perspect. 2006;114(2):156–164. doi: 10.1289/ehp.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olsen J, Friis S, Frederiksen K. Malignant melanoma and other types of cancer preceding Parkinson disease. Epidemiology. 2006;17(5):582–587. doi: 10.1097/01.ede.0000229445.90471.5e. [DOI] [PubMed] [Google Scholar]
- 5.Wirdefeldt K, Weibull C, Chen H, Kamel F, Lundholm C, Fang F, et al. Parkinson’s disease and cancer: a register-based family study. Am J Epidemiol. 2013;179(1):85–94. doi: 10.1093/aje/kwt232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rugbjerg K, Friis S, Lassen C, Ritz B, Olsen J. Malignant melanoma, breast cancer and other cancers in patients with Parkinson’s disease. Int J Cancer. 2012;131(8):1904–1911. doi: 10.1002/ijc.27443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertoni J, Arlette J, Fernandez H, Fitzer-Attas C, Frei K, Hassan M. Increased melanoma risk in Parkinson disease. Arch Neurol. 2010;67(3):347. doi: 10.1001/archneurol.2010.1. [DOI] [PubMed] [Google Scholar]


