Abstract
Antigen-specific immunotherapy (ASI) holds great promise for the treatment of autoimmune diseases. In mice, administration of major histocompatibility complex (MHC) binding synthetic peptides which modulate T cell receptor (TCR) signaling under sub-immunogenic conditions induces selective tolerance without suppressing the global immune responses. However, clinical translation has yielded limited success. It has become apparent that the TCR signaling pathway via synthetic peptide antigen alone is inadequate to induce an effective tolerogenic immunity in autoimmune diseases. Bioconjugate strategies combining additional immunomodulatory functions with TCR signaling can amplify the antigen-specific immune tolerance and possibly lead to the development of new treatments in autoimmune diseases. In this review, we provide a summary of recent advances in the development of bioconjugates to achieve antigen-specific immune tolerance in vivo, with the discussion focused on the underlying design principles and challenges that must be overcome to target these therapies to patients suffering from autoimmune diseases.
Graphical Abstract
INTRODUCTION
Autoimmune diseases are characterized by aberrant activation of T- and B-cells in vivo which results in the immune destruction of the host tissues or organs.1,2 These diseases represent a heterogeneous group of disorders with a combination of genetic factors and environmental triggers (virus, bacteria, and other infectious pathogens).1–4 According to the National Institute of Allergy and Infectious Diseases (NIAID), there are more than 80 autoimmune diseases affecting nearly 20 million people in the US alone and the prevalence is rising each year.5 Some of the most common autoimmune diseases include type 1 diabetes (T1D), rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), inflammatory bowel disease (IBD), and multiple sclerosis (MS).5 The precise causes of many autoimmune diseases remain unknown, and there is no effective screening to detect individuals at risk for most autoimmune diseases, making disease prevention almost impossible.1,2 Thus, most patients have significant disease progression and tissue destruction before clinical diagnosis and initiation of appropriate treatments.
Currently available treatments for autoimmune diseases include physical therapy, immunosuppressants, corticosteroids, anti-inflammatory drugs, and cell or tissue transplantation, among others.6,7 These treatments alleviate the symptoms but do not alter the overall chronic course of diseases. For example, immunosuppressants (i.e., cyclosporine A) inhibit the activity of the immune system by reducing the proliferation and function of cells associated with immune reactions and show partial efficacy in many autoimmune diseases.7 However, their therapeutic effects are dependent on chronic drug administration that can lead to systemic immune suppression, with the potential risk of development of cancer and opportunistic infections.7 Antigen-specific immunotherapy (ASI) is the treatment able to modify the outcome of the diseases by restoring self-tolerance toward autoantigens.8–10 Under sub-immunogenic conditions (i.e., in the presence of low levels of stimulatory molecules), ASI introduces autoantigens to antigen presenting cells (APCs) in order to correct the immune responses, acting directly through T cell receptor (TCR) on effector T cells (via clonal deletion or anergy) and/or via regulatory T cells (Tregs) that secrete anti-inflammatory cytokines.8–10 One of the main implications of this intervention is its specificity: compared with other immune therapies, ASI selectively targets disease-relevant T cells, while leaving the normal immune system intact. Initial experimental approaches have included oral antigen administration,11 particulate autoantigen delivery,12 altered peptide ligands,13 and dose escalating immunotherapy,14 among many others. Although these approaches have shown efficacy in preclinical models, results from human clinical trials have not shown the same level of efficacy as in mice.9,10 In spite of intensive research, to date, no FDA approved ASI is available for treating patients with autoimmune diseases. Induction of antigen-specific tolerance to dominant immune responses driving autoimmunity remains an unmet challenge.
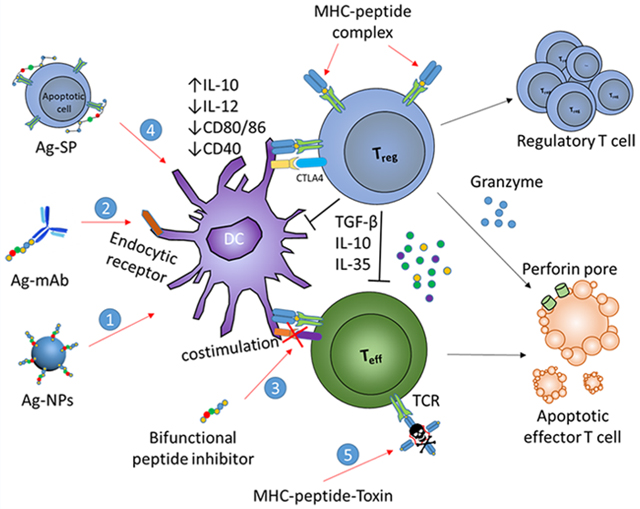
Though our current understanding of the enormously complex human immune system is far from complete, it is clear that modulation of the TCR signaling pathway via administration of autoantigen alone is insufficient and that alternative strategies which can amplify the antigen-specific immune tolerance in animal models are needed to increase the validity and predictive power in the development of new treatment in humans.13 In the past two decades, many technologies have been developed in the creation of a new generation of ASI in order to attenuate the inflammation in autoimmune diseases. Efforts have been devoted to designing delivery vehicles which target key antigen-presenting cells,15 or combination immunomodulation which codelivers immune-modulators with autoantigens.16 These strategies target TCR signaling pathways, cosignaling molecules and cytokines, or inhibit the formation of the immunological synapse, enabling the augmentation of TCR-mediated tolerance. Among these different approaches, one of the most attractive strategies is to incorporate additional immunomodulatory functions into autoantigens through bioconjugation (Figure 1). In fact, modification of autoantigen through functional conjugation to polymers, nanoparticles (NPs), antibodies, or small molecules have been extensively used in vaccines.16–18 In this review, we highlight the recent advances in experimental mouse models using various bioconjugate strategies aimed at the induction of autoantigen-specific tolerance for the potential treatment of autoimmune diseases. We focus our discussion on the design principles for each of the strategies, along with their limitations and potential for clinical translation. Some common autoimmune diseases and corresponding bioconjugate strategies reviewed in this paper are summarized in Table 1.
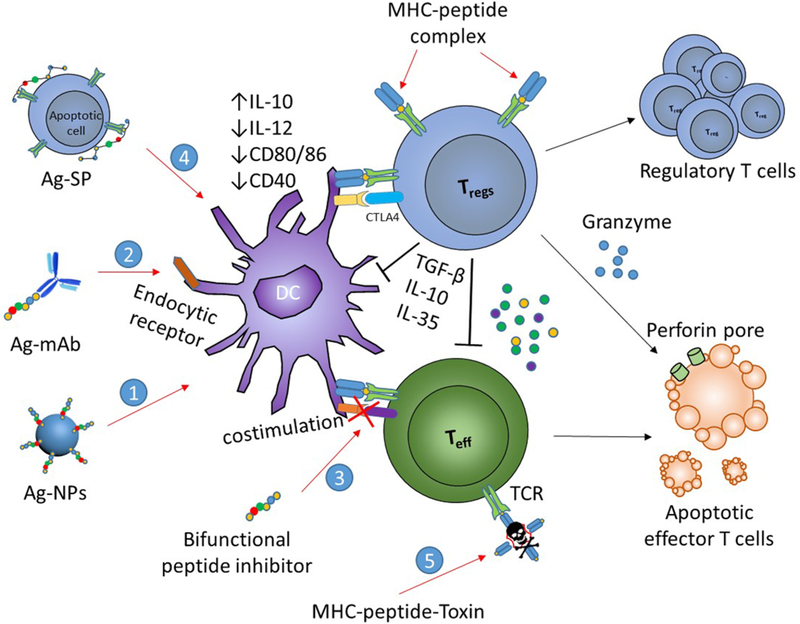
Figure 1.
Bioconjugate-based strategies for the induction of antigen-specific tolerance in autoimmune diseases. Bioconjugates have been engineered to target autoantigens and tolerogenic molecules to dendritic cells (DCs) (1); to facilitate antigen-processing via endocytic receptors (2); to inhibit costimulation (3); to link to apoptotic cells for tolerogenic presentation (4); and to deliver toxin to autoantigen-specific T cells (5). These strategies lead to peripheral tolerance as results of anergy and deletion of cognate T cells, or induction of Tregs.
Table 1.
Some Common Autoimmune Diseases Studied by Different Bioconjugate Strategies
| common autoimmune diseases | affected cells or tissues |
disease mechanism | proposed causes | bioconjugate strategies |
|---|---|---|---|---|
| Type 1 diabetes | Pancreatic β cells | CD4+, CD8+ T cell and B cell | Genetics, chemicals, environmental factors | Antibody–antigen; Cell-peptide; Soluble peptide-MHC II complex |
| Rheumatoid arthritis | Joints, skin | CD4+, CD8+ T cell and B cell | Genetics, environmental factors | Antigen-NPs; Small molecule-antigen; Soluble peptide-MHC II complex |
| Systemic lupus erythematosus | Mainly on skin | CD4+, CD8+ T cell and B cell | Genetics and drugs | Antibody–antigen; Soluble peptide-MHC II complex |
| Multiple sclerosis | Nerve cells | CD4+, CD8+ T cell | Genetics and infectious agents | Antigen-NPs; Cell-peptide; Soluble peptide-MHC II complex |
| Chronic inflammatory demyelinating polyneuropathy | Peripheral nervous system | CD4+ T cell | Dysfunction of immune cells | Albumin-antigen |
IMMUNOLOGICAL BASIS OF SELF-TOLERANCE AND TARGETING DC–T CELL INTERACTIONS FOR THE PROMOTION OF ANTIGEN-SPECIFIC TOLERANCE
The central role of the immune system is to discriminate between self-and non-self-antigens and respond specifically to remove non-self-pathogens invading the body while leaving the self-organs intact. The immune response is a complex process involving multiple coordinated actions of diverse immune cells and molecular signals (Figure 2). Specialized sentinel cells known as APCs phagocytose antigens and present fragments of them to T cells via major histocompatibility complex (MHC) molecules. In the presence of appropriate inflammatory cues, CD8+ and CD4+ T cells with matching receptors are activated by interacting with APCs presenting an antigen, and proliferate and differentiate into cytotoxic “killer” cells (CD8+ T-cells) or “helper” cells (CD4+ T-cells). The protective functions of T-cells are collectively known as cellular response. In addition, B-cells acquire and process antigen, and with the “help” from CD4+ T cells, differentiate into antibody-producing plasma cells. Immune protection mediated by secreted antibodies is referred to as humoral response.
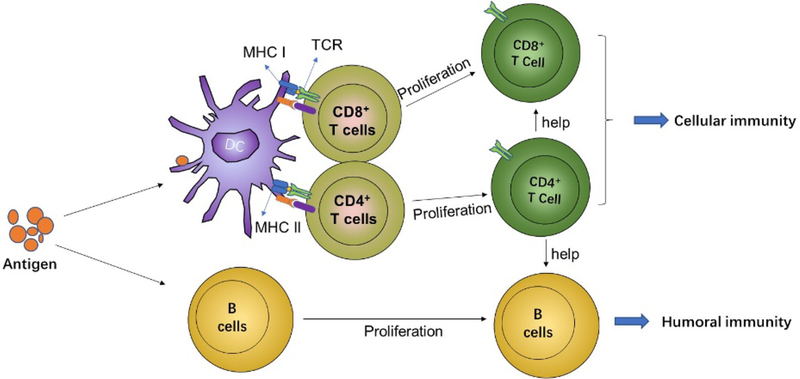
Figure 2.
Induction of adaptive immune responses. DCs and B cells acquire antigens and in the presence of inflammatory cues (costimulatory signals), activate T- and B-cells. Activated CD4+ T cells differentiate into T helper cell subtypes and CD8+ T cells differentiate into cytotoxic T effector cells. In parallel, activated B cells differentiate into antibody-producing plasma cells.
In addition to immune activation, the immune system is equipped with mechanisms to suppress immune responses. In healthy individuals, the immune system maintains unresponsiveness to antigens recognized as “self”, and thus does not react to antigens expressed in endogenous tissues. When such self-tolerance is lost, underlying self-recognition can result in tissue destruction mediated by humoral or cellular mechanisms, or both, leading to autoimmune diseases.
Self-tolerance is maintained by a coordinating operation of central and peripheral mechanisms (Figure 3). In central tolerance, most T- and B-lymphocytes with receptors specific for autoantigens are eliminated at an early stage in lymphoid cell development in the thymus and bone marrow, respectively. This process is also known as negative selection and it allows most self-reactive B and T cells to be removed before they enter the peripheral. However, it is becoming clear that the negative selection is not a perfect process as the peripheral repertoire of healthy individuals contains a high frequency of diverse, self-reactive lymphocytes.19 Fortunately, a healthy immune system can effectively prevent both the self-reactive B and T cells that escaped from central deletion from initiating potentially dangerous immune responses against the body’s own tissues. This is achieved by a number of regulatory mechanisms outside of the primary lymphoid tissues collectively known as peripheral tolerance (Figure 3). These mechanisms affect the survival, differentiation, and function of T and B cells and play an important role in the induction and maintenance of self-tolerance.
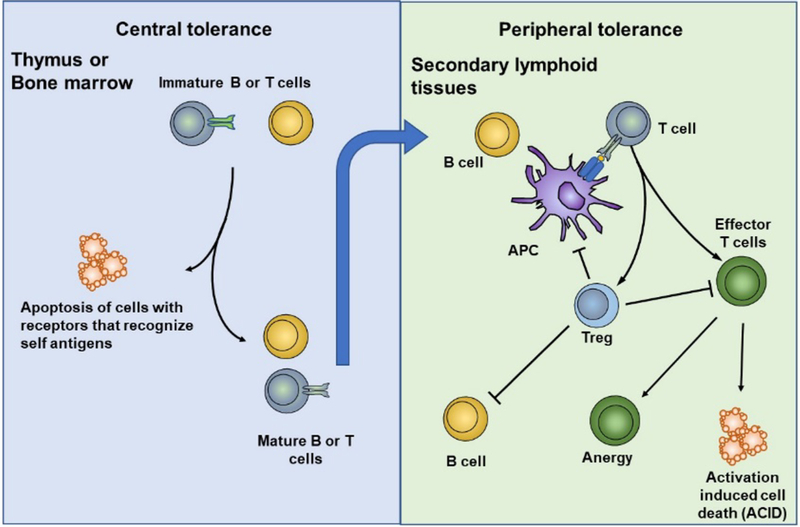
Figure 3.
Central and peripheral tolerance mechanisms in T cells and B cells. Central tolerance occurs in the primary lymphoid organs where high-affinity self-reactive T cells and B cells are deleted in the thymus and bone marrow (negative selection), respectively. Peripheral tolerance occurs as mature self-reactive T cells and B cells escape central tolerance and enter the periphery, where they are inactivated (anergy), deleted (activation induced cell death, ACID), or suppressed.
Full activation of T cells requires an antigen-specific signal delivered through the TCR and appropriate costimulatory signals. However, engagement of TCR alone produces intrinsically unresponsive T cells that remain alive for an extended period, a state known as anergy.20 These cells are associated with diminished proliferation and cytokine production, and compromised effector functions.20 Another peripheral tolerance mechanism is activation-induced cell death (AICD) caused by the interaction between Fas receptors (FasR) and Fas ligands (FasL).21 AICD in T cells by FasR/FasL-mediated apoptosis is an important regulatory mechanism to down-regulate inflammation and promote peripheral tolerance as mice with defective FasR or FasL develop a number of autoimmune diseases.21 Unlike anergy, activation-induced cell death requires stimulation of T cells by competent APCs and it is dependent on IL-2 (interleukin 2), which enhances AICD by up-regulating FasL expression.22
A third mechanism of peripheral tolerance which prevents autoimmune reactions is the suppression of self-reactive lymphocytes by Tregs. It is now known that various subsets of Tregs exist in the immune system. CD4+ Tregs are classified into thymus-derived natural Tregs, which are CD4+CD25+Foxp3+, and peripherally induced Tregs including type 1 Tregs (Tr1) which secret IL-10 (interleukin 10), Th3 (T helper 3) Tregs which secret IL-10 and TFG-β (transforming growth factor beta), and Foxp3+ Tregs. Besides, based on their regulatory function, other lymphocyte subsets are also recognized as Tregs: inducible CD8+ Tregs, double negative Tregs (CD3+CD4−CD8− Tregs), CD4+Vα14+ natural killer T cell, and γδ T cells.23 These Tregs have been shown to suppress T-cell responses by either induction of effector T cell death or by elaborating soluble immunosuppressive factors such as TGF-β and IL-10.23 The most important subset of Tregs expresses the transcription factor forkhead box P3 (Foxp3). Evidence has accumulated that Tregs, defined by the expression of CD4, CD25, and Foxp3 have an indispensable role in the maintenance of self-tolerance.24 In mice, depletion of Foxp3+CD25+CD4+ Tregs results in the spontaneous development of a variety of autoimmune diseases, including autoimmune thyroiditis, diabetes, and IBD, and that the disease could be reversed by adoptive transfer of Tregs.24,25 Immune suppression mediated by Tregs is antigen-dependent. In the secondary lymphoid organs, Tregs encounter specific antigens presented on antigen presenting cells and in the presence of IL-2, undergo clonal expansion.26 The immune suppression of effector T cells by Tregs is more complicated. Both antigen-specific and bystander suppression of effector cells have been reported.27,28 However, it is speculated that antigen-specific suppression is more effective than bystander suppression.29 Tregs can exert their suppressive functions directly on the effector T cells or indirectly on the function and maturation of DCs.29 It has been shown that inhibition by Tregs can be mediated by immunoregulatory cytokines such as TGF-β, IL-10, and IL-35.29 These cytokines inhibit the production of inflammatory cytokines such as IL-12, causing a decrease in the T helper type 1 (Th1) response and IFN-γ (interferon gamma) production, thereby inhibiting the self-reactive immune responses.29 Tregs were also found to be able to produce granzyme B, which induced apoptosis in effector T cells via cell-to-cell interaction. This mechanism decreases the number of effector T cells and promotes the induction of self-tolerance.29 In addition to the direct effect of Tregs on effector T cells, Tregs can inhibit the maturation and function of DCs by promoting the tolerogenic phenotype of DCs. Although the mechanism is unknown, it is believed that Tregs achieve this by cell surface molecules or cytokines such CTLA-4, IL-10, and TGF-β.30
B cell activation and plasma cell differentiation are critically dependent on T cell help.31 T helper cells (Th) recognize peptide/MHC II on B cells and provide costimulation via CD40L and CD40 interaction as well as cytokines such as IL-4 and IL-21, which in turn sustain B cell activation and differentiation into antibody-secreting cells. If helper T cells specific for the autoantigens are deleted, suppressed, or rendered anergic, self-reactive B cell responses do not occur. Thus, CD4+ T helper cells are ultimately crucial for B cell tolerance.31
Antigen presenting cells especially DCs play essential roles in the initiation and maintenance of peripheral tolerance.15,32 Both T- and B-cell-mediated immunity are tightly linked to DCs through antigen presentation, costimulatory interaction, and cytokine factors. As outlined above, induction of antigen-specific tolerance is a complex process that involves activation and/or inhibition of multiple immunological pathways. Therapeutic manipulation of these pathways by different strategies provides mechanisms to induce antigen-specific immune suppression without compromising the ability for global immune responses. One of the first targets in tolerance induction is the MHC/peptide/TCR interaction. T cell tolerance can be achieved by inducing a partial or altered signal through the MHC/peptide/TCR trimolecular interaction.8–10 Typically, TCR stimulation in the absence or low levels of costimulation leads to T cell anergy, depletion, or differentiation to Tregs.8,9 Targeting MHC/peptide/TCR based on administration of autoantigens is being pursued treating autoimmune diseases, such as MS, SLE, and T1D.8–10 Antigen dose, routes, and kinetics that promote tolerance have been explored to modulate disease progression but with limited success.11–14 These failures may be related to the choice of antigen, dose, frequency, route, and mode of delivery, but most probably suggest TCR signal alone is insufficient in reversing the disease-causing autoimmunity in vivo.
Exogenous targeting the costimulatory and/or coinhibitory pathways appears to be a logical approach for therapeutic manipulation to down-regulate the pathologic immunity.16,33–35 It is now clear that both costimulatory and coinhibitory molecules are essential for the initial induction and function of Tregs. Along with this line of research, a variety of costimulatory and coinhibitory pathways have been explored. CD28, B7 (CD80/CD86), CTLA-4, CD40, CD154, ICOS, OX40, and 4–1BB have been targeted with agonists or antagonists to modulate the APC–T cell interaction.16,36 These treatments alone or combined with autoantigen have sparked enormous research. Much work has been conducted on the B7-CD28/CTLA-4 costimulatory system which plays a critical role in effector T cells activation versus suppression. For example, Abatacept is an FDA approved B7-binding fusion protein used to treat RA.37 It has also been shown to be effective in treating T1D.38 Blockade of CD28 with anti-CD28 antagonists promotes the engagement of CTLA-4 with B7, delivering a negative signal into effector T cells and inhibiting their activation. Several antagonists against CD28 have been developed and inhibited disease progression in both rodents and humans.39 In line with this research, similar pathways have been targeted for autoimmune modulation. Efficacy in several murine autoimmune diseases has been demonstrated where 4–1BBL/4–1BB, PD-1/PD-L1 were targeted alone or in combination with TCR stimulation.16,36 These studies demonstrated the potential of targeting DC/T cell interaction in tolerance induction. However, immunomodulatory effects of targeting costimulatory or coinhibitory molecules are potentially more complex. Achieving a delicate balance appears to be important for an effective tolerance induction: while costimulation through 4–1BB, OX40 promotes Tregs proliferation, it also reduces Tregs suppressive capacity.40,41 Similarly, in an EAE (experimental autoimmune encephalomyelitis) mouse model of MS, mice were immunized with MOG35–55 (myelin oligodendrocyte glycoprotein) and then treated with CTLA-4Ig (anti-B7 monoclonal antibody) to achieve B7 blockade. Surprisingly, it was revealed that CTLA-4Ig treated mice had about two times higher disease scores compared with PBS treated control mice, implying that the B7 blockade exacerbated disease. This study suggested that a certain level of B7/CD28 engagement was required for Tregs proliferation and function.42
Another promising target is the CD40/CD154 interaction. Engaging CD40/CD154 delivers activating intracellular signals to ACPs and subsequently controls T-dependent B cell response and T cell priming. Thus, disrupting CD40/CD154 interaction is likely a viable strategy in treating autoimmune diseases in which activated T and B cells are involved.43,44 In fact, CD154-blocking antibodies (Ab) can prevent or ameliorate autoimmunity in several disease models, including T1D,45 RA,46 SLE.47 However, anti-CD154 Ab treatment led to thromboembolic complications.48 Alternative small molecules which target CD40 are emerging in autoimmunity. For example, a CD40 blocking peptide derived from human CD154 was discovered recently.49 Daily administration of CD40 blocking peptide successfully reversed T1D in NOD (nonobese diabetic) mice.49
BIOCONJUGATE STRATEGIES FOR THE INDUCTION OF ANTIGEN-SPECIFIC TOLERANCE
Antibody–Antigen Conjugates.
Monoclonal antibody (mAb)-based therapies have become a critical part of the treatment for patients with autoimmunity, cancer, and infectious disease. In autoimmune diseases, antigen–antibody conjugates are mainly used to target DCs, the most efficient antigen presenting cells, to enhance the antigen uptake, processing, and presentation.15,32,50 A direct delivery of antigens to DCs subset in the most appropriate tissue in the absence of costimulatory stimuli not only leads to the deletion of antigen-specific T cells, but also to the induction of peripheral tolerance mediated by Tregs, as demonstrated in murine models of autoimmune arthritis, EAE, and T1D.51–54 One of the early examples was demonstrated by Finkelman et al. in 1996.55 The authors showed a rat IgG2b anti-DC antibody induced rat IgG2b-specific T cell and B cell tolerance, unresponsive to the subsequent challenge. The tolerance induction appeared to be dependent on the specific DC surface receptor, as injection of a DC-specific hamster anti-CD11c mAb stimulated antibody responses rather than tolerance.55 Since then, a variety of different antibodies which deliver autoantigens via DC surface receptors have been explored for tolerance induction. DEC-205 is one of the well-studied DC surface receptors with tolerogenic potential.51,54–62 Autoantigens conjugated to anti-DEC-205 in the absence of activating stimuli are selectively delivered to DEC-205+ DCs, inducing profound peripheral T cell tolerance.51,54 Antigen targeted DEC-205 is internalized by receptor-mediated endocytosis, and traffics to late endosomal compartments where high concentrations of MHC II molecules are present (Figure 4).56 Low-dose of antigen delivered via DEC-205 exhibited 100-fold increase in antigen presentation (Figure 4),57 and favored the conversion of naïve CD4+CD25−Foxp3− T cells into functional CD25+Foxp3+ Tregs.51,54,58 These regulatory responses were very efficient as mice administrated with appropriate autoantigens fused with anti-DEC-205 were protected from autoimmune arthritis,59 and EAE,60 and T1D.61,62 In the T1D studies, linking either CD4+ or CD8+ epitope to anti-DEC-205 led to the deletion of autoreactive T cells and protected mice from disease in two different mouse T1D models, strongly suggesting this specific endocytic receptor is a valid target for tolerance induction.61,62
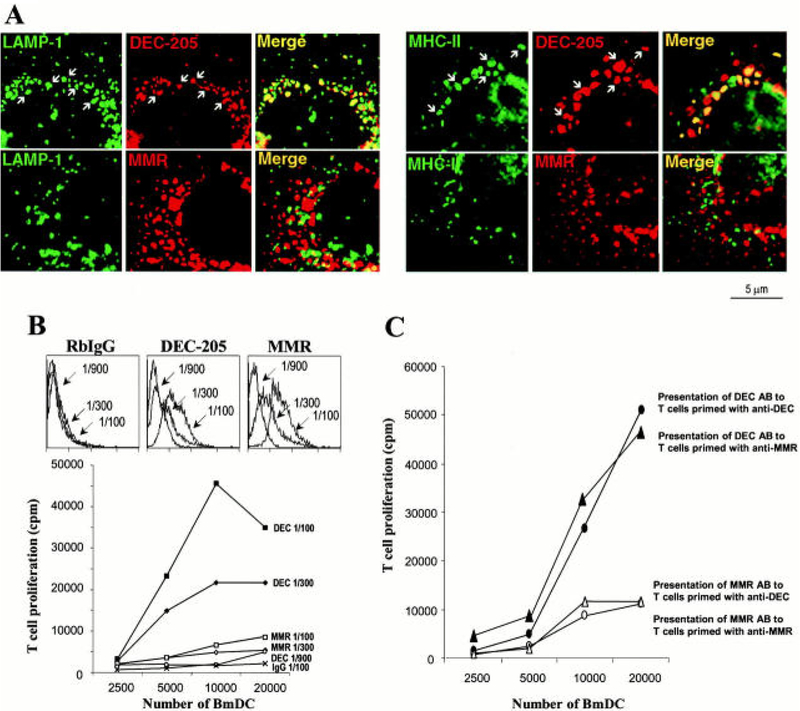
Figure 4.
Antigen delivered via DEC-205 enhances antigen presentation. (A) Anti-DEC-205, but not anti-MMR (Macrophage Mannose Receptor), is internalized by receptor-mediated endocytosis, and traffics to late endosomalcompartments where high concentrations of MHC II molecules are present. (B and C) Antigen delivered via anti-DEC-205 greatly enhances its presentation to T cells. Reproduced with permission from ref 57.
Antigen–antibody conjugates targeting plasmacytoid DCs (pDCs) via sialic acid binding Ig-like lectin H (Siglec-H) have been shown to inhibit Th cell-dependent autoimmunity in an antigen-specific manner in mice, even in the presence of strong immune stimulation.63 Siglec-H is an efficient endocytic receptor on plasmacytoid DC precursors and Siglec-mediated antigen delivery of myelin autoantigen induced a hyporesponsive state in CD4+ T cells without conversion to Foxp3+ Tregs, and effectively delayed the onset of EAE.63
Targeting several other receptors on DCs has been shown to be effective in Tregs and tolerance induction. Antigen–antibody conjugates targeting migratory DCs promoted peripheral tolerance induction as delivering to Langerin+ (CD207) DCs and pDCs induced Foxp3+ Tregs proliferation and EAE suppression.64 Furthermore, DCs expressing CD103 in the intestine primed Foxp3+ Tregs.65 These specialized CD103+ DCs in the gut lymphoid tissues produced both TGF-β and retinoic acid and efficiently promoted the differentiation of Tregs.65
Protein expression pattern differs between mice and humans. To test whether antigen targeting autoantigens to human DCs could suppress autoimmunity, the human IgE Fc domain was fused with autoantigen.66 In human FcεRIα-transgenic mice, such antigen-Fcε conjugate was efficiently delivered to DCs, enhanced antigen presentation by at least 1000-fold, and resulted in the deletion of antigen-specific T cells.66 These results warrant consideration of DC targeting for further clinical studies.
Antibody–antigen conjugate represents a simple and effect approach for the induction of antigen-specific immunosuppression. However, like all therapeutic proteins, antibody–antigen conjugates induce antibody responses in patients.67 Such antidrug antibodies are a common cause for the treatment failure and adverse hypersensitivity reaction. Nevertheless, management of the immunogenicity of therapeutic antibody by optimizing the antibody design67 or by tolerogenic strategies68 have been tested to overcome this limitation.
Albumin–Antigen Conjugate.
The effectiveness of antigen-specific immunotherapy is dependent on the accumulation of antigens in the antigen presenting cells. Subunit peptides and proteins have a short half-life in vivo due to rapid renal clearance, degradation, and nonspecific tissue accumulation. One effective approach is to tag antigens to albumin, a natural transporter protein with long circulating half-life.69 Recently, Tregitopes,70 MHC class II epitopes in the Fc fragment of IgG which were capable of specifically activating Tregs, were fused with albumin.71 Administration of Tregitope-albumin extended antigen half-life. Compared to antigen alone, mice that received a single dose of Tregitope-albumin fusion protein showed a significant reduction of antigen-specific T cell proliferation in OVA-specific T cell proliferation. Albumin fusion, administered either prophylactically or therapeutically ameliorate the disease progression in chronic inflammatory demyelinating polyneuropathy (CIDP) in a spontaneous mouse model.71 In addition to increased half-life, recent studies demonstrated that amphiphilic vaccines bind to albumin in situ efficiently accumulated in the antigen presenting cells in the lymph node (LN) following subcutaneous injections, leading to up to 30-fold increase over unmodified vaccines in antigen-specific CD8+ T cell proliferation.72,73 These studies provide evidence that autoantigen–albumin conjugate improves antigen presentation by prolonging antigen half-life and enhancing lymphatic accumulation.
As a natural transporter protein with long circulatory half-life, albumin has been considered as one of the most attractive carriers for delivery of therapeutic agents in immunomodulation.74,75 In addition, the successes of albumin-based conjugates such as long-acting insulin (Levemir) and glucagon-like peptide (Victoza), which are attached to an albumin-binding moiety, potentially promote the development of albumin-based biologicals in clinical studies.75 While an albumin-based conjugate platform demonstrated superior immunomodulation effects, how the modification impacts on antigen uptake, processing, and presentation have not been elucidated. In addition, it remains to be investigated whether antigen–albumin conjugates can be a general approach in other autoimmune diseases.
Soluble Peptide–MHC Complex.
Soluble peptide-MHC II complexes (pMHC II) have long been recognized for their ability to blunt autoimmune responses.76–79 This method directly targets cognate T cells without the need of APCs for antigen presentation. Early studies using pMHC monomers have shown promise in the preclinical study in EAE but with limited clinical beneficial effects in Phase I trial in MS patients.80,81 Similarly, a Phase I/II clinical trial for RA using an epitope of human cartilage glycoprotein (HCgp39) loaded pMHC II monomer yielded only short-term effects.82 Clinical responses were seen in patients treated with 7 infusions of 150 μg/kg peptide/MHC over 6 weeks. One possible limitation for monomeric pMHC is that, in general, pMHC/TCR interaction is very weak and classically lasts for no longer than a few seconds, especially for autoantigens.83 To improve the direct stimulation of antigen-specific T cells, multivalent pMHC complexes have been designed. Unlike the monovalent pMHC, the multivalent interaction between pMHC and T cells greatly enhances both the TCR binding affinity and stability.83 Soluble peptide-MHC multimers, in addition to being a powerful tool in the monitoring of T cell response, can mediate downstream signaling after T cell receptor engagement.84,85 In the absence of costimulation, peptide-MHC multimer leads to anergy, deletion of cognate T cells, or induction of Tregs.85 To avoid the transfer of peptides from multimers to cell surface MHC molecules which could possibly exacerbate autoimmune responses, peptide antigens are often covalently linked to MHC. Such pMHC class II multimer can inhibit autoreactive CD4+ T cells in T1D85–88 or MS.77,89 Masteller et al. developed soluble peptide/I-Ag7 dimer with a linked peptide specific for islet-reactive BDC2.5 transgenic CD4+ T cells in NOD mice.87 These dimers were shown to specifically bind BDC2.5 T cells. Treatment with BDC2.5 peptide I-Ag7 dimer protected mice from diabetes mediated by adoptive transfer of diabetogenic BDC2.5 CD4+ T cells.87 In a similar approach, Casares et al. demonstrated soluble dimeric peptide-MHC II chimera not only prevented the onset of T1D disease but also restored normoglycemia in diabetic animals.86 In a spontaneous T1D model, administration of soluble IAg7 dimers covalently linked to β-cell autoantigen-derived peptide GAD65 blocked the progression of insulitis and the development of diabetes.88 In addition to pMHC class II dimers, pMHC class I complexes have been reported to inhibit alloreactive CD8+ CTL (cytotoxic T lymphocyte) in vivo,90,91 and covalent linkage of MHC class I with long rigid linkers efficiently inhibited CTL target cells by interfering with TCR-mediated activation of lymphocyte function-associated antigen 1 (LFA-1).92 Another intriguing idea is to deplete the autoreactive T cells with a pMHC multimer immunotoxin.93–96 In this approach, cytotoxic drugs such as 225Actinium, saporin, and doxorubicin were conjugated to pMHC multimer, resulting in a rapid antigen-specific T cell deletion. Together, though long-term efficacy and toxicity data are lacking, these results suggested that multimeric peptide-MHC might be useful in the development of immunospecific therapies for autoimmune diseases.
Antigen–NPs Conjugates.
NPs delivery has recently been investigated to promote tolerance induction. The use of NPs for tolerogenic vaccine delivery improves the antigen stability, targets key immune cells, and enhances antigen presentation. For these purposes, autoantigens and/or suppressive signals are often encapsulated or conjugated to nanosized vehicles. Here we limit the scope of the discussion to techniques and approaches that can modulate the immune system via molecular conjugates.
Antigen-Linked NPs as APC Targeted Delivery.
The efficacy of NPs in immune modulation largely depends on their ability to target and alter the functions of antigen presentation cells in vivo.15,32,52 NP’s physical and chemical characteristics such as size, shape, geometry, and surface chemistry affect their biodistribution, uptake, and intracellular trafficking.97 Because their sizes are comparable to those of microorganisms, a particulate system is more efficient in antigen delivery than soluble antigens.97,98 In the absence of danger signals, the presentation of autoantigens on APCs can induce antigen-specific tolerance. Getts et al. showed that antigen-decorated 500 nm polystyrene NPs induced long-term T cell tolerance in mice with EAE.99 Intravenous injection of disease-relevant peptide such as PLP139–151 (proteolipid protein) conjugated polystyrene NPs enhanced the antigen uptake in splenic phagocytes via Macrophage Receptor with Collagenous Structure (MARCO), a scavenger receptor, and prevented the onset of EAE, a model of human MS.99 These beneficial effects are associated with the vaccine-elicited Tregs and inhibition of effector T cell activation.99 A more biocompatible poly(lactic-co-glycolic acid) (PLG) NPs have also been shown to function as a safe, cost-effective, and efficient carrier for the induction of antigen-specific T cell tolerance.100 Myelin antigen-coupled PLG NPs were able to ameliorate the ongoing diseases in an EAE model and, importantly, minimize epitope spreading when administered systemically.100 Autoantigen–polymer conjugate PLGA NPs with favorable release kinetics were developed in a recent study. It was found the Tregs induction was dependent on antigen density as well as nanoparticle concentration.101
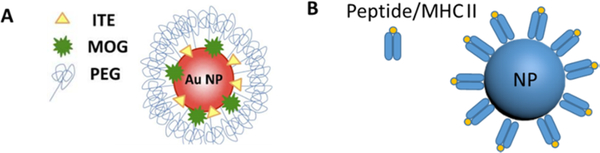
Using NPs to codeliver antigen and immunomodulators is an emerging trend in antigen-specific immunotherapy. Yeste et al. used small gold NPs (60 nm in diameter) for delivery of EAE-relevant peptide antigen MOG35–55 and 2-(1Hindole-3-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE), a small molecular agonist activating aryl hydrocarbon receptor transcription factor (AhR) (Figure 5A).102 The addition of ITE promoted the differentiation of CD4+ CD25+ Foxp3+ Tregs. Using gold nanoparticle incorporated with β-cell antigen proinsulin, the same group reported the induction of a tolerogenic phenotype in DCs and Tregs generation in vivo, which in turn suppressed autoimmune diabetes in a mouse model.103 Similarly, poly(lactic-co-glycolic) NPs combined autoantigen and rapamycin were shown to efficiently induce tolerogenic DCs, and suppress both T-and B-cell-mediated immunity in mice.104
Figure 5.
Tolerogenic NPs for antigen-specific immunotherapy. Schematic representation of antigen (MOG35–55) and AhR ligand (ITE) conjugated gold NPs (A) and NPs coated with peptides bound to MHC class II (B). Reproduced with permission from ref 102 (A).
Targeting antigen to APCs in the lymph nodes is emerging as an efficient strategy for tolerance induction.105,106 NPs with optimized parameters such as size, charge, and shape were able to drain to the lymph nodes and accumulated in the LN-resident APCs.97 Immunological tolerance can thus be elicited by conjugating autoantigens to size-optimized NPs (5–100 nm) such as quantum dots. Hess et al. in a recent study showed quantum dots (QDs) with uniform size (<20 nm) efficiently concentrated in the draining lymph nodes following subcutaneous injection, colocalizing with macrophages expressing the scavenge receptor MACRO.107 Treatment of autoantigen loaded QDs markedly reduced disease incidence when compared with soluble antigen group in an EAE mouse model of MS. Importantly, the antigen density has been correlated with the efficacy of tolerance induction, with low-density antigen displayed on high numbers of tolerogenic particles driving the most efficient tolerance.107 Though the underlying mechanism is still not clear, this finding suggests, in addition to efficient antigen delivery to APCs in the LN, an antigen display in which the spatial organization of the antigens on the surface of NPs regulates autoimmunity.
NPs Coated with Autoimmune Disease Relevant Peptide/MHC.
To improve the therapeutic outcomes of soluble peptide-MHC multimers in autoimmune diseases, pMHC conjugated NPs has been proposed.108–111 pMHC coated NPs can provide protection of the pMHC molecules from degradation, which would prolong the circulating half-life. In addition, the particle size, pMHC valency, spacing, and density can be fine-tuned to optimize the regulation of T cell responsiveness.109,112,113 Tsai et al. coated iron oxide NPs with T1D-relevant pMHC complexes and found such pMHC class I-coated NPs elicited TCR clustering and expanded memory-like autoregulatory T cells, which subsequently inhibited the activation of other autoantigen-specific autoreactive CD8+ T cell populations in the pancreas.108 Treatment of pMHC-NPs not only blunted T1D progression in prediabetic mice but also restored normoglycemia in newly diagnosed diabetic NOD mice.108 Similarly, NPs coated with autoimmune-disease relevant peptides bound to MHC II (Figure 5B) profoundly induced antigen-specific T cells with regulatory function, and led to the suppression of disease in mouse models of RA, T1D, and MS, without compromising the global immunity.110 These pMHC-NPs provide a “plug and play” vaccine platform to reprogram the immune responses, with the possibility to codeliver other tolerogenic signals, such as costimulatory inhibitors or suppressive cytokines.
However, the major barrier for pMHC-NPs approach lies in the complexity of the production of pMHC-NPs that match human leukocyte antigen of individuals.114 Although NPs-based approaches are promising in the treatment of autoimmune diseases, the clinical translation of these strategies remains in its infancy due to the concerns underlying immunogenicity and toxicity associated with synthetic NPs.
Small Molecule–Antigen Conjugates.
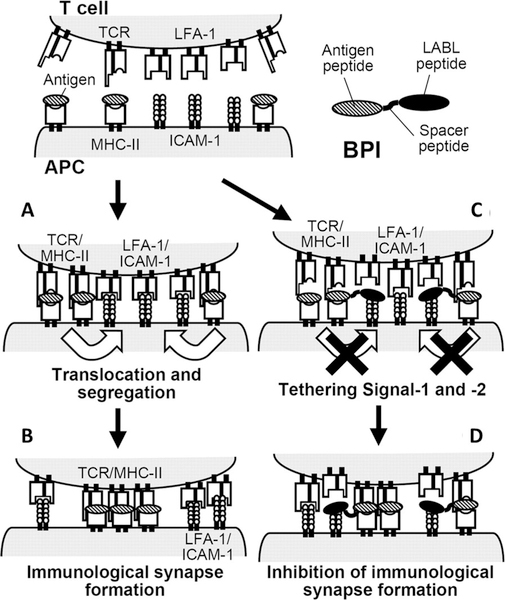
Compared with protein drugs, small molecular compounds have the advantages in manufacturability, cost, stability, and safety. Small molecular ligands that specifically block the engagement of costimulatory molecules or inhibit the formation of the immunological synapse are thus conjugated to autoantigens to amplify the tolerance induction. Such bispecific heterodimers are designed to selectively inhibit the maturation of T cells specific for autoantigens. Siahaan and colleagues have designed a bifunctional peptide inhibitor (BPI, Figure 6), which consists of an antigenic peptide epitope conjugated to an inhibitory peptide blocking DC-T cell interaction.115–119 Conjugating peptide autoantigens to LFA-α-blocker left (LABL) peptide inhibited the formation of the immunological synapse by binding to both MHC II and intercellular adhesion molecular-1 (ICAM-1), and skewed the differentiation of T cells from stimulatory to regulatory cells.107 Similarly, BPI composed of an antigenic peptide and a B7 binding peptide derived from CD28 receptor was designed to simultaneously target MHC II and B7 on the surface of antigen presenting cells.119 Administration of low concentrations of BPI consisting of appropriate disease-specific antigens was highly potent in T1D, and RA.115–119 To overcome the short in vivo half-lives of peptide, White et al. in a recent study covalently conjugated the bifunctional peptide to the fragment crystallizable (Fc) region of the human IgG1 antibody via a sortase-ligation strategy.120 The initial tests of the conjugate showed significantly reduced EAE symptoms.120
Figure 6.
Proposed mechanism of action of bifunctional peptide inhibitor (BPI). BPI prevents the formation of immunological synapse by binding to MHC-II and ICAM-1 on the surface of APC, which leads to inhibition of T cell activation. (A) Around the central zone, LFA-1/ICAM-1 and TCR/MHC-antigen complexes are first formed at the early stage of T cell activation; (B) both pairs are translocated to form complete immunological synapse. (C) In the presence of BPI, BPI binds to MHC-II and ICAM-1 on the surface of APC; (D) it inhibits their migration, which leads to inhibition of the immunological synapse formation. Reproduced with permission from ref 115.
The LEAPS (Ligand Epitope Antigen Presentation System) platform represents another technology to the development of antigen-specific immunomodulating peptide vaccines for autoimmune diseases.121 In this technology, disease-specific peptide epitopes were conjugated to an Immune Cell Binding Ligand (ICBL), a short peptide which activates precursors to initiate and redirect appropriate T cell responses. Depending upon the nature of the attached ICBL, the LEAPs technology can direct the antigen-specific T cell responses toward Th1, Th2 orTregs.121 Conceived in the late 1980s, LEAPS technology has since been demonstrated to be able to block the progression of several autoimmune diseases, including RA in the collagen-induced arthritis model122 and experimental autoimmune myocarditis in a mouse model.121
Small molecule immunomodulators, including vitamin D3,123,124 mycophenolic acid, dexamethasone,123 D-man-nose,125 and rapamycin104,123 have been shown to be effective in augmenting the tolerance induction through a variety of mechanisms which include the induction of Tregs, or by altering the profile of pathogenic immunity. However, the combination of most of these “tolerogenic” adjuvants with immunogens is achieved by coadministration or encapsulation in particulate carriers. Chemical conjugation of antigen and immunomodulators ensures delivery to the same antigen presenting cells and are widely used in the generation of immune responses.126 Due to the lack of direct head to head studies, it remains to be determined whether covalent linkage can be more effective in immune suppression. Nevertheless, small molecules have been conjugated to autoantigen for immune suppression. In a recent study, Perdicchio et al. conjugated sialic acid to autoantigens for the induction of Tregs. Sialic acids bind to Ig-like lectins (Siglecs), which are inhibitory receptors expressed on DCs.127 The authors suggested that modification of antigens with sialic acids targeted DCs via Siglecs and altered the immunogenicity of antigens.127 Administration of sialylated antigens led to inhibition of the proliferation and functions of effector T cells and induction of Tregs, even under inflammatory conditions.127 Modulation of T cell responses via myelin antigen sialylation was effective in mice with EAE, providing evidence that sialic acid–antigen conjugates could induce antigen-specific immune tolerance.127
Although cross-linking antigen to immunomodulatory is a straightforward design for immune suppression, one of the potential complications with using chemical conjugation is that it offers limited protection to the immunogen, resulting in premature degradation once exposed to the harsh physiological environments.
Cell–Peptide Conjugates.
Although the precise mechanisms have not been determined, physiological cell death (apoptosis) is intrinsically tolerogenic.128 Unlike pathological cell death (necrosis), under homeostatic conditions, apoptotic cells are engulfed and processed by antigen presenting cells and are presented to induce tolerance to autoantigens.128 Exploiting this intrinsic peripheral tolerance process, several groups have tried to target autoantigens to different types of cells.129–139 One of the most studied approaches to induce tolerance is intravenous treatment with antigen-coupled, ethylene carbodiimide (ECDI)-fixed splenocytes.129–135 In this approach, splenocytes were isolated from the donner mice and were chemically coupled with antigen via a small molecular linker ECDI. ECDI also induces apoptosis of splenocytes, creating a cell-based autoantigen processed by tolerogenic pathways.128 It has been shown that ECDI-coupled splenocytes induce tolerance via both direct and indirect mechanisms.130 In the direct pathway, peptide-coupled cells directly present auto-antigens to antigen-specific T cells via T cell receptor, leading to anergy through TCR stimulation in the absence of costimulation. In an indirect pathway, intravenous infusion of antigen-coupled cells accumulates in the marginal zone of the spleen where they are phagocytosed and presented on the surface of macrophages, which produce IL-10, upregulating the expression of the immunomodulatory costimulatory molecules PD-L1.131 Antigen-coupled cell infusion also induces Tregs that are essential for long-term tolerance maintenance.131 Notably, antigens chemically conjugated ex vivo to splenocytes have been shown to induce antigen-specific tolerance in T1D132,136 and EAE.133 Recently, autologous peripheral blood mononuclear cells chemically coupled with an array of myelin peptides were tested in MS patients.134 This first-in-man, phase 1 clinical trial demonstrated that the treatment was safe, and a high dose of treatment reduced antigen-specific T cell responses.134 This study established the safety and feasibility of using antigen-coupled cells in MS patients, providing evidence to justify further clinical investigations on the treatment of autoimmune diseases.
Immunological tolerance can also be induced by antigen coupled red blood cells (RBCs).137–139 Transfusion of RBCs covalently modified with disease-relevant autoantigen payload has been shown to blunt the contribution to immunity from B cells, CD4+, and CD8+ T cells in an antigen-specific manner.138 Mice treated with autoantigen-coupled RBCs demonstrated prophylactic and therapeutic efficacy in EAE, and this strategy also protected the majority of NOD mice from T1D.138
The strategies just described rely on the isolation and ex vivo manipulation of autologous cells. Such ex vivo cell approaches have in general been tedious, complex, and inefficient. Kontos and co-workers reported an innovative strategy by which an antigen was coupled to circulating RBCs via in situ binding.139 Autoantigens were fused to an erythrocyte binding domain and following intravenous infusion, bound to RBCs avidly, deleting both CD8+ and CD4+ T cells in an antigen-specific manner. Using mimetope peptides, the authors reported complete prevention of hyperglycemia was achieved by injection of RBC-targeted peptide conjugate in an adoptive T cell transfer-induced T1D model.139 As this strategy uses molecularly defined peptide conjugates for in situ erythrocyte targeting and avoids the ex vivo cell manipulation, it could be, at least in principle, readily translated to the clinic for human studies.
Polymeric Peptide Antigens.
The physicochemical properties, and thus the immunological fate of an antigen, can be tuned by conjugating to different types of polymers. For example, while antigen conjugated to oxidized mannan has been shown to induce cellular and humoral immune responses,140 reduced mannan conjugates skewed the predominant T-helper 1 responses to T-helper 2 responses.141 Protein antigen conjugated with polyethylene glycol (PEG), a non-immunogenic synthetic polymer, induces immune tolerance of antigen-specific Th cells.142 Interestingly, size, structure, and linker chemistry have been found to play an important role in the levels of tolerance induction in a peptide-polyglycerol conjugate.143 Polyglycerol with an ester linkage was the most tolerogenic, while the same polymer with an amide linkage induced strong effector T cell proliferation.143
An autoantigen delivery platform based on Soluble Antigen Arrays (SAgAs) has been developed recently.144–146 This delivery system has a polymeric backbone with grafted autoantigens and inhibitory signals. One of the advantages with this system is that it is highly tunable: by varying the polymer backbone and the type, density of peptide attachment, a wide variety of design parameters such as molecular size, solubility, flexibility, antigen valency, spacing, and binding avidity can be controlled.145 These parameters have been correlated with therapeutic efficacy in murine models of autoimmune diseases.145
CONCLUSION AND FUTURE PERSPECTIVE
The continued development of ASI depends highly upon our understanding of human immunology, as well as discovery and delivery of tolerogenic adjuvants which can effectively amplify the immune suppression across a wide range of autoimmune disease types. As outlined above, bioconjugates hold great promise in immunomodulation of autoimmune diseases, bridging synthetic molecular functions with immunological features. Bioconjugates can be tailored and functionalized to target DCs, to codeliver autoantigens and immunomodulators, or to target specific immunological pathways. Among all the strategies we reviewed here, myelin peptide-coupled blood mononuclear cells represent the most clinically advanced strategy to date, showing good tolerance and safety in MS patients. Despite the progress, it has become clear that current strategies for ASI are not sufficiently active for many diseases. There is clearly a need to improve therapeutic efficacy in humans. As the field of immunomodulation continues to evolve, new bioconjugate strategies will emerge as innovative treatment modalities. For example, bioconjugate strategies might be developed to control the localization, dose, and kinetics of tolerogenic vaccines. Bioconjugates might also be engineered to program immune cell differentiation and thus control immune cell fates. Future efforts will explore the biological functions of biomaterials, and combination therapy by which disease-modifying biologics and autoantigens are conjugated should provide a framework to improve efficacy and reduce side effects of antigen-specific immunotherapy. In the long term, these efforts will continue guiding the rational design of ASI to improve current treatment and ultimately lead us toward a cure.
ACKNOWLEDGMENTS
This work is supported in part by NIH (R56DK103651), American Cancer Society (11–053-01-IRG) and Wayne State University President’s Research Enhancement Program.
ABBREVIATIONS
- ASI
Antigen-specific immunotherapy
- MHC
Major histocompatibility complex
- TCR
T cell receptor
- NIAID
National Institute of Allergy and Infectious Diseases
- T1D
Type 1 diabetes
- RA
Rheumatoid arthritis
- SLE
Systemic lupus erythematosus
- EAE
Experimental autoimmune encephalomyelitis
- IBD
Inflammatory bowel disease
- MS
Multiple sclerosis
- APCs
Antigen presenting cells
- Tregs
regulatory T cells
- Th
T helper cell
- MMR
Macrophage mannose receptor
- NPs
nanoparticles
- AICD
Activation-induced cell death
- FasR
Fas receptors
- FasL
Fas ligand
- DCs
Dendritic cells
- Foxp3
Forkhead box P3
- CTLA-4
Cytotoxic T-lymphocytes-associated protein 4
- ICOS
Inducible T-cell costimulatory
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed death ligand 1
- NOD
Nonobese diabetic
- mAb
Monoclonal antibody
- pDCs
Plasmacytoid dendritic cells
- Siglec-H
Sialic acid binding Ig-like lectin H
- HSA
Human serum albumin
- CIDP
Chronic inflammatory demyelinating polyneuropathy
- LN
Lymph node
- HCgp39
Human cartilage glycoprotein 39
- CTL
Cytotoxic T lymphocyte
- LFA-1
Lymphocyte function-associated antigen 1
- PLGA
Poly(lactic-co-glycolic acid)
- MARCO
Macrophage receptor with collagenous structure
- AhR
Aryl hydrocarbon receptor
- QDs
Quantum dots
- PLP
Proteolipid protein
- MOG
Myelin oligodendrocyte glycoprotein
- ITE
2-(1Hindole-3-carbonyl)-thiazole-4-carboxylic acid methyl ester
- BPI
Bifunctional peptide inhibitor
- LABL
LFA-alpha blocker left
- ICAM-1
Intercellular adhesion molecular-1
- Fc
Fragment crystallizable
- LEAPS
Ligand epitope antigen presentation system
- ICBL
Immune cell binding ligand
- ECDI
Ethylene carbodiimide
- RBCs
Red blood cells
- PEG
Polyethylene glycol
- SAgAs
Soluble Antigen Arrays
- IL-2
Interleukin-2
- IL-4
Interleukin-4
- IL-10
Interleukin-10
- IL-12
Interleukin-12
- IL-21
Interleukin-21
- IL-35
Interleukin-35
- IFN-γ
Interferon-γ
- TGF-β
Transforming growth factor beta
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Wang L, Wang FS, and Gershwin ME (2015) Human autoimmune diseases: a comprehensive update. J. Intern. Med 278, 369–395. [DOI] [PubMed] [Google Scholar]
- (2).Goris A, and Liston A (2012) The immunogenetic architecture of autoimmune disease. Cold Spring Harbor Perspect. Biol 4, a007260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Zenewicz LA, Abraham C, Flavell RA, and Cho JH (2010) Unraveling the genetics of autoimmunity. Cell 140, 791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Marson A, Housley WJ, and Hafler DA (2015) Genetic basis of autoimmunity. J. Clin. Invest 125, 2234–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).https://www.niaid.nih.gov/diseases-conditions/autoimmune-diseases. Retrieved Nov. 18, 2017.
- (6).Rosenblum MD, Gratz IK, Paw JS, and Abbas AK (2012) Treating human autoimmunity: current practice and future prospects. Sci. Transl. Med 4, 125sr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Chandrashekara S (2012) The treatment strategies of autoimmune disease may need a different approach from conventional protocol: A review. Indian J. Pharmacol 44, 665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Peakman M, and Dayan CM (2001) Antigen-specific immunotherapy for autoimmune disease: fighting fire with fire? Immunology 104, 361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Hirsch DL, and Ponda P (2014) Antigen-based immunotherapy for autoimmune disease: current status. ImmunoTargets Ther 4, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).MacLeod MK, and Anderton SM (2015) Antigen-based immunotherapy (AIT) for autoimmune and allergic disease. Curr. Opin. Pharmacol 23, 11–16. [DOI] [PubMed] [Google Scholar]
- (11).Faria AM, and Weiner HL (2006) Oral tolerance: therapeutic implications for autoimmune diseases. Clin. Dev. Immunol 13, 143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Serra P, and Santamaria P (2015) Nanoparticle-based autoimmune disease therapy. Clin. Immunol 160, 3–13. [DOI] [PubMed] [Google Scholar]
- (13).Anderton SM (2001) Peptide-based immunotherapy of autoimmunity: a path of puzzles, paradoxes and possibilities. Immunology 104, 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Burton BR, Britton GJ, Fang H, Verhagen J, Smithers B, Sabatos-Peyton CA, Carney LJ, Gough J, Strobel S, and Wraith DC (2014) Sequential transcriptional changes dictate safe and effective antigen-specific immunotherapy. Nat. Commun 5, 4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Ganguly D, Haak S, Sisirak V, and Reizis B (2013) The role of dendritic cells in autoimmunity. Nat. Rev. Immunol 13, 566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Northrup L, Christopher MA, Sullivan BP, and Berkland C (2016) Combining antigen and immunomodulators: Emerging trends in antigen-specific immunotherapy for autoimmunity. Adv. Drug Delivery Rev 98, 86–98. [DOI] [PubMed] [Google Scholar]
- (17).Baekkeskov S, Hubbell JA, and Phelps EA (2017) Bioengineering strategies for inducing tolerance in autoimmune diabetes. Adv. Drug Delivery Rev 114, 256–265. [DOI] [PubMed] [Google Scholar]
- (18).Purwada A, Roy K, and Singh A (2014) Engineering vaccines and niches for immune modulation. Acta Biomater 10, 1728–1740. [DOI] [PubMed] [Google Scholar]
- (19).Miller SD, Turley DM, and Podojil JR (2007) Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat. Rev. Immunol 7, 665–677. [DOI] [PubMed] [Google Scholar]
- (20).Schwartz RH (2003) T cell anergy. Annu. Rev. Immunol 21, 305–334. [DOI] [PubMed] [Google Scholar]
- (21).Pender MP (1999) Activation-induced apoptosis of autoreactive and alloreactive T lymphocytes in the target organ as a major mechanism of tolerance. Immunol. Cell Biol 77, 216–223. [DOI] [PubMed] [Google Scholar]
- (22).Dai Z, Arakelov A, Wagener M, Konieczny BT, and Lakkis FG (1999) The Role of the Common Cytokine Receptor γ-Chain in Regulating IL-2-Dependent, Activation-Induced CD8+ T Cell Death. J. Immunol 163, 3131–3137. [PubMed] [Google Scholar]
- (23).Peterson RA (2012) Regulatory T-cells: diverse phenotypes integral to immune homeostasis and suppression. Toxicol. Pathol 40, 186–204. [DOI] [PubMed] [Google Scholar]
- (24).Sakaguchi S, Yamaguchi T, Nomura T, and Ono M (2008) Regulatory T cells and immune tolerance. Cell 133, 775–787. [DOI] [PubMed] [Google Scholar]
- (25).Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, and Nomura T (2006) Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev 212, 8–27. [DOI] [PubMed] [Google Scholar]
- (26).Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, and Steinman RM (2003) Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J. Exp. Med 198, 235–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Yu P, Gregg RK, Bell JJ, Ellis JS, Divekar R, Lee HH, Jain R, Waldner H, Hardaway JC, Collins M, et al. (2005) Specific T regulatory cells display broad suppressive functions against experimental allergic encephalomyelitis upon activation with cognate antigen. J. Immunol 174, 6772–6780. [DOI] [PubMed] [Google Scholar]
- (28).Homann D, Holz A, Bot A, Coon B, Wolfe T, Petersen J, Dyrberg TP, Grusby MJ, and von Herrath MG (1999) Autoreactive CD4+ T cells protect from autoimmune diabetes via bystander suppression using the IL-4/Stat6 pathway. Immunity 11, 463–472. [DOI] [PubMed] [Google Scholar]
- (29).Corthay A (2009) How do regulatory T cells work? Scand. J. Immunol 70, 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Sojka DK, Huang Y-H, and Fowell DJ (2008) Mechanisms of regulatory T-cell suppression – a diverse arsenal for a moving target. Immunology 124, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Gururajan M, Sindhava JV, and Bondada S (2014) B Cell Tolerance in Health and Disease. Antibodies 3, 116–129. [Google Scholar]
- (32).Iberg CA, Jones A, and Hawiger D (2017) Dendritic Cells As Inducers of Peripheral Tolerance. Trends Immunol 38, 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Lenschow DJ, Ho SC, Sattar H, Rhee L, Gray G, Nabavi N, Herold KC, and Bluestone JA (1995) Differential effects of anti-B7–1 and anti-B7–2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J. Exp. Med 181, 1145–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Finck BK, Linsley PS, and Wofsy D (1994) Treatment of murine lupus with CTLA4Ig. Science 265, 1225–1227. [DOI] [PubMed] [Google Scholar]
- (35).Vincenti F (2008) Costimulation blockade in autoimmunity and transplantation. J. Allergy Clin. Immunol 121, 299–306. [DOI] [PubMed] [Google Scholar]
- (36).Chittasupho C, Siahaan TJ, Vines CM, and Berkland C (2011) Autoimmune therapies targeting costimulation and emerging trends in multivalent therapeutics. Ther. Delivery 2, 873–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Sibilia J, and Westhovens R (2007) Safety of T-cell co-stimulation modulation with abatacept in patients with rheumatoid arthritis. Clin. Exp. Rheumatol 25, S46–56. [PubMed] [Google Scholar]
- (38).Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Marks JB, Monzavi R, et al. (2011) Co-Stimulation Modulation with Abatacept in Patients with Recent-Onset Type 1 Diabetes: A Randomised Double-Masked Controlled Trial. Lancet 378, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Poirier N, Blancho G, and Vanhove B (2012) CD28-specific immunomodulating antibodies: what can be learned from experimental models? Am. J. Transplant 12, 1682–1690. [DOI] [PubMed] [Google Scholar]
- (40).Xiao X, Gong W, Demirci G, Liu W, Spoerl S, Chu X, Bishop DK, Turka LA, and Li XC (2012) New insights on OX40 in the control of T cell immunity and immune tolerance in vivo. J. Immunol 188, 892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Wang C, Lin GH, McPherson AJ, and Watts TH (2009) Immune regulation by 4–1BB and 4–1BBL: complexities and challenges. Immunol. Rev 229, 192–215. [DOI] [PubMed] [Google Scholar]
- (42).Vogel I, Kasran A, Cremer J, Kim YJ, Boon L, Van Gool SW, and Ceuppens JL (2015) CD28/CTLA-4/B7 costimulatory pathway blockade affects regulatory T-cell function in autoimmunity. Eur. J. Immunol 45, 1832–1841. [DOI] [PubMed] [Google Scholar]
- (43).Durie FH, Fava RA, Foy TM, Aruffo A, Ledbetter JA, and Noelle RJ (1993) Prevention of collagen-induced arthritis with an antibody to gp39, the ligand for CD40. Science 261, 1328–1330. [DOI] [PubMed] [Google Scholar]
- (44).Howard LM, Miga AJ, Vanderlugt CL, Dal Canto MC, Laman JD, Noelle RJ, and Miller SD (1999) Mechanisms of immunotherapeutic intervention by anti-CD40L (CD154) antibody in an animal model of multiple sclerosis. J. Clin. Invest 103, 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).McGregor CM, Schoenberger SP, and Green EA (2004) CD154 is a negative regulator of autoaggressive CD8+ T cells in type 1 diabetes. Proc. Natl. Acad. Sci. U. S. A. 101, 9345–9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Boumpas DT, Furie R, Manzi S, Illei GG, Wallace DJ, Balow JE, and Vaishnaw A (2003) A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum 48, 719–727. [DOI] [PubMed] [Google Scholar]
- (47).Davis JC Jr., Totoritis MC, Rosenberg J, Sklenar TA, and Wofsy D (2001) Phase I clinical trial of a monoclonal antibody against CD40-ligand (IDEC-131) in patients with systemic lupus erythematosus. J. Rheumatol 28, 95–101. [PubMed] [Google Scholar]
- (48).Kawai T, Andrews D, Colvin RB, Sachs DH, and Cosimi AB (2000) Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat. Med 6, 114. [DOI] [PubMed] [Google Scholar]
- (49).Vaitaitis GM, Olmstead MH, Waid DM, Carter JR, and Wagner DH Jr. (2014) A CD40-targeted peptide controls and reverses type 1 diabetes in NOD mice. Diabetologia 57, 2366–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Stern JN, Keskin DB, Kato Z, Waldner H, Schallenberg S, Anderson A, von Boehmer H, Kretschmer K, and Strominger JL (2010) Promoting tolerance to proteolipid protein-induced experimental autoimmune encephalomyelitis through targeting dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 107, 17280–17285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, Nussenzweig MC, and Steinman RM (2008) CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J. Immunol 181, 6923–6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, and Nussenzweig MC (2001) Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med 194, 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Price JD, and Tarbell KV (2015) The Role of Dendritic Cell Subsets and Innate Immunity in the Pathogenesis of Type 1 Diabetes and Other Autoimmune Diseases. Front. Immunol 6, 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, and Steinman RM (2002) Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med 196, 1627–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Finkelman FD, Lees A, Birnbaum R, Gause WC, and Morris SC (1996) Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion. J. Immunol 157, 1406–1414. [PubMed] [Google Scholar]
- (56).Mahnke K, Qian Y, Knop J, and Enk AH (2003) Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood 101, 4862–4869. [DOI] [PubMed] [Google Scholar]
- (57).Mahnke K, Guo M, Lee S, Sepulveda H, Swain SL, Nussenzweig M, and Steinman RM (2000) The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J. Cell Biol 151, 673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Petzold C, Schallenberg S, Stern JN, and Kretschmer K (2012) Targeted antigen delivery to DEC-205(+) dendritic cells for tolerogenic vaccination. Rev. Diabet. Stud 9, 305–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Spiering R, Margry B, Keijzer C, Petzold C, Hoek A, Wagenaar-Hilbers J, van der Zee R, van Eden W, Kretschmer K, and Broere F (2015) DEC205+ Dendritic Cell-Targeted Tolerogenic Vaccination Promotes Immune Tolerance in Experimental Autoimmune Arthritis. J. Immunol 194, 4804–4813. [DOI] [PubMed] [Google Scholar]
- (60).Ring S, Maas M, Nettelbeck DM, Enk AH, and Mahnke K (2013) Targeting of autoantigens to DEC205(+) dendritic cells in vivo suppresses experimental allergic encephalomyelitis in mice. J. Immunol 191, 2938–2947. [DOI] [PubMed] [Google Scholar]
- (61).Mukhopadhaya A, Hanafusa T, Jarchum I, Chen YG, Iwai Y, Serreze DV, Steinman RM, Tarbell KV, and DiLorenzo TP (2008) Selective delivery of beta cell antigen to dendritic cells in vivo leads to deletion and tolerance of autoreactive CD8+ T cells in NOD mice. Proc. Natl. Acad. Sci. U. S. A. 105, 6374–6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Bruder D, Westendorf AM, Hansen W, Prettin S, Gruber AD, Qian Y, von Boehmer H, Mahnke K, and Buer J (2005) On the edge of autoimmunity: T-cell stimulation by steady-state dendritic cells prevents autoimmune diabetes. Diabetes 54, 3395–3401. [DOI] [PubMed] [Google Scholar]
- (63).Loschko J, Heink S, Hackl D, Dudziak D, Reindl W, Korn T, and Krug AB (2011) Antigen targeting to plasmacytoid dendritic cells via Siglec-H inhibits Th cell-dependent autoimmunity. J. Immunol 187, 6346–6356. [DOI] [PubMed] [Google Scholar]
- (64).Idoyaga J, Fiorese C, Zbytnuik L, Lubkin A, Miller J, Malissen B, Mucida D, Merad M, and Steinman RM (2013) Specialized role of migratory dendritic cells in peripheral tolerance induction. J. Clin. Invest 123, 844–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, and Powrie F (2007) A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med 204, 1757–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Baravalle G, Greer AM, LaFlam TN, and Shin J-S (2014) Antigen-Conjugated Human IgE Induces Antigen-Specific T Cell Tolerance in a Humanized Mouse Model. J. Immunol 192, 3280–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Schellekens H (2010) The immunogenicity of therapeutic proteins. Discovery Med 9, 560–564. [PubMed] [Google Scholar]
- (68).Kishimoto TK, Ferrari JD, LaMothe RA, Kolte PN, Griset AP, O’Neil C, Chan V, Browning E, Chalishazar A, Kuhlman W, et al. (2016) Improving the efficacy and safety of biologic drugs with tolerogenic nanoparticles. Nat. Nanotechnol 11, 890. [DOI] [PubMed] [Google Scholar]
- (69).Strohl WR (2015) Fusion Proteins for Half-Life Extension of Biologics as a Strategy to Make Biobetters. BioDrugs 29, 215–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Cousens LP, Su Y, McClaine E, Li X, Terry F, Smith R, Lee J, Martin W, Scott DW, and De Groot AS (2013) Application of IgG-Derived Natural Treg Epitopes (IgG Tregitopes) to Antigen-Specific Tolerance Induction in a Murine Model of Type 1 Diabetes. J. Diabetes Res 2013, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).http://abstracts.aaps.org/Verify/NBC14/PosterSubmissions/W3031.pdf. Retrieved Nov. 18, 2017.
- (72).Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, and Irvine DJ (2014) Structure-based programming of lymph-node targeting in molecular vaccines. Nature 507, 519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Yu C, An M, Li M, and Liu H (2017) Immunostimulatory Properties of Lipid Modified CpG Oligonucleotides. Mol. Pharmaceutics 14, 2815–2823. [DOI] [PubMed] [Google Scholar]
- (74).Larsen MT, Kuhlmann M, Hvam ML, and Howard KA (2016) Albumin-based drug delivery: harnessing nature to cure disease. Mol. Cell. Ther 4, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Elsadek B, and Kratz F (2012) Impact of albumin on drug delivery — New applications on the horizon. J. Controlled Release 157, 4–28. [DOI] [PubMed] [Google Scholar]
- (76).Casares S, Zong CS, Radu DL, Miller A, Bona CA, and Brumeanu TD (1999) Antigen-specific signaling by a soluble, dimeric peptide/major histocompatibility complex class II/Fc chimera leading to T helper cell type 2 differentiation. J. Exp. Med 190, 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Appel H, Seth NP, Gauthier L, and Wucherpfennig KW (2001) Anergy induction by dimeric TCR ligands. J. Immunol 166, 5279–5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Casares S, Bona CA, and Brumeanu TD (1997) Engineering and characterization of a murine MHC class II-immunoglobulin chimera expressing an immunodominant CD4 T viral epitope. Protein Eng., Des. Sel 10, 1295–1301. [DOI] [PubMed] [Google Scholar]
- (79).Zuo L, Cullen CM, DeLay ML, Thornton S, Myers LK, Rosloniec EF, Boivin GP, and Hirsch R (2002) A single-chain class II MHC-IgG3 fusion protein inhibits autoimmune arthritis by induction of antigen-specific hyporesponsiveness. J. Immunol 168, 2554–2559. [DOI] [PubMed] [Google Scholar]
- (80).Sharma SD, Nag B, Su XM, Green D, Spack E, Clark BR, and Sriram S (1991) Antigen-specific therapy of experimental allergic encephalomyelitis by soluble class II major histocompatibility complex-peptide complexes. Proc. Natl. Acad. Sci. U. S. A. 88, 11465–11469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Nag B., Arimilli S., Mukku PV., and Astafieva I. (1996) Functionally active recombinant alpha and beta chain-peptide complexes of human major histocompatibility class II molecules. J. Biol. Chem 271, 10413–10418. [DOI] [PubMed] [Google Scholar]
- (82).Kavanaugh A, Genovese M, Baughman J, Kivitz A, Bulpitt K, Olsen N, Weisman M, Matteson E, Furst D, van Vollenhoven R, et al. (2003) Allele and antigen-specific treatment of rheumatoid arthritis: a double blind, placebo controlled phase 1 trial. J. Rheumatol 30, 449–454. [PubMed] [Google Scholar]
- (83).Wooldridge L, Lissina A, Cole DK, van den Berg HA, Price DA, and Sewell AK (2009) Tricks with tetramers: how to get the most from multimeric peptide-MHC. Immunology 126, 147–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Kozono H, White J, Clements J, Marrack P, and Kappler J (1994) Production of soluble MHC class II proteins with covalently bound single peptides. Nature 369, 151–154. [DOI] [PubMed] [Google Scholar]
- (85).Mallone R, and Nepom GT (2005) Targeting T lymphocytes for immune monitoring and intervention in autoimmune diabetes. Am. J. Ther 12, 534–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Casares S, Hurtado A, McEvoy RC, Sarukhan A, von Boehmer H, and Brumeanu TD (2002) Down-regulation of diabetogenic CD4+ T cells by a soluble dimeric peptide-MHC class II chimera. Nat. Immunol 3, 383–391. [DOI] [PubMed] [Google Scholar]
- (87).Masteller EL, Warner MR, Ferlin W, Judkowski V, Wilson D, Glaichenhaus N, and Bluestone JA (2003) Peptide-MHC class II dimers as therapeutics to modulate antigen-specific T cell responses in autoimmune diabetes. J. Immunol 171, 5587–5595. [DOI] [PubMed] [Google Scholar]
- (88).Li L, Yi Z, Wang B, and Tisch R (2009) Suppression of ongoing T cell-mediated autoimmunity by peptide-MHC class II dimer vaccination. J. Immunol 183, 4809–4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Burrows GG, Adlard KL, Bebo BF Jr., Chang JW, Tenditnyy K, Vandenbark AA, and Offner H (2000) Regulation of encephalitogenic T cells with recombinant TCR ligands. J. Immunol 164, 6366–6371. [DOI] [PubMed] [Google Scholar]
- (90).Schneck J, Maloy WL, Coligan JE, and Margulies DH (1989) Inhibition of an allospecific T cell hybridoma by soluble class I proteins and peptides: estimation of the affinity of a T cell receptor for MHC. Cell 56, 47–55. [DOI] [PubMed] [Google Scholar]
- (91).Dal Porto J, Johansen TE, Catipović B, Parfiit DJ, Tuveson D, Gether U, Kozlowski S, Fearon DT, and Schneck JP (1993) A soluble divalent class I major histocompatibility complex molecule inhibits alloreactive T cells at nanomolar concentrations. Proc. Natl. Acad. Sci. U. S. A. 90, 6671–6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Angelov GS, Guillaume P, Cebecauer M, Bosshard G, Dojcinovic D, Baumgaertner P, and Luescher IF (2006) Soluble MHC-peptide complexes containing long rigid linkers abolish CTL-mediated cytotoxicity. J. Immunol 176, 3356–3365. [DOI] [PubMed] [Google Scholar]
- (93).Yuan RR, Wong P, McDevitt MR, Doubrovina E, Leiner I, Bornmann W, O’Reilly R, Pamer EG, and Scheinberg DA (2004) Targeted deletion of T-cell clones using alpha-emitting suicide MHC tetramers. Blood 104, 2397–2402. [DOI] [PubMed] [Google Scholar]
- (94).Hess PR, Barnes C, Woolard MD, Johnson MD, Cullen JM, Collins EJ, and Frelinger JA (2007) Selective deletion of antigen-specific CD8+ T cells by MHC class I tetramers coupled to the type I ribosome-inactivating protein saporin. Blood 109, 3300–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Clark BR, Deshpande SV, Sharma SD, and Nag B (1994) Antigen-specific deletion of cloned T cells using peptide-toxin conjugate complexed with purified class II major histocompatibility complex antigen. J. Biol. Chem 269, 94–99. [PubMed] [Google Scholar]
- (96).Casares S, Stan AC, Bona CA, and Brumeanu TD (2001) Antigen-specific downregulation of T cells by doxorubicin delivered through a recombinant MHC II–peptide chimera. Nat. Biotechnol 19, 142–147. [DOI] [PubMed] [Google Scholar]
- (97).Irvine DJ, Hanson MC, Rakhra K, and Tokatlian T (2015) Synthetic Nanoparticles for Vaccines and Immunotherapy. Chem. Rev 115, 11109–11146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Storni T, Kundig TM, Senti G, and Johansen P (2005) Immunity in response to particulate antigen-delivery systems. Adv. Drug Delivery Rev 57, 333–355. [DOI] [PubMed] [Google Scholar]
- (99).Getts DR, Martin AJ, McCarthy DP, Terry RL, Hunter ZN, Yap WT, Getts MT, Pleiss M, Luo X, King NJ, et al. (2012) Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat. Biotechnol 30, 1217–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Hunter Z, McCarthy DP, Yap WT, Harp CT, Getts DR, Shea LD, and Miller SD (2014) A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease. ACS Nano 8, 2148–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Pearson RM, Casey LM, Hughes KR, Wang LZ, North MG, Getts DR, Miller SD, and Shea LD (2017) Controlled Delivery of Single or Multiple Antigens in Tolerogenic Nanoparticles Using Peptide-Polymer Bioconjugates. Mol. Ther 25, 1655–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Yeste A, Nadeau M, Burns EJ, Weiner HL, and Quintana FJ (2012) Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U. S. A. 109, 11270–11275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Yeste A, Takenaka MC, Mascanfroni ID, Nadeau M, Kenison JE, Patel B, Tukpah AM, Babon JA, DeNicola M, Kent SC, et al. (2016) Tolerogenic nanoparticles inhibit T cell-mediated autoimmunity through SOCS2. Sci. Signaling 9, ra61. [DOI] [PubMed] [Google Scholar]
- (104).Maldonado RA, LaMothe RA, Ferrari JD, Zhang AH, Rossi RJ, Kolte PN, Griset AP, O’Neil C, Altreuter DH, Browning E, et al. (2015) Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc. Natl. Acad. Sci. U. S. A. 112, E156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Andorko JI, Hess KL, and Jewell CM (2015) Harnessing biomaterials to engineer the lymph node microenvironment for immunity or tolerance. AAPS J. 17, 323–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Ludvigsson J, Wahlberg J, and Casas R (2017) Intra-lymphatic Injection of Autoantigen in Type 1 Diabetes. N. Engl. J. Med 376, 697–699. [DOI] [PubMed] [Google Scholar]
- (107).Hess KL, Oh E, Tostanoski LH, Andorko JI, Susumu K, Deschamps JR, Medintz IL, and Jewell CM (2017) Engineering Immunological Tolerance Using Quantum Dots to Tune the Density of Self-Antigen Display. Adv. Funct. Mater 27, 1700290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Tsai S, Shameli A, Yamanouchi J, Clemente-Casares X, Wang J, Serra P, Yang Y, Medarova Z, Moore A, and Santamaria P (2010) Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity 32, 568–580. [DOI] [PubMed] [Google Scholar]
- (109).Clemente-Casares X, Tsai S, Yang Y, and Santamaria P (2011) Peptide-MHC-based nanovaccines for the treatment of autoimmunity: a ″one size fits all″ approach? J. Mol. Med. (Heidelberg, Ger.) 89, 733–742. [DOI] [PubMed] [Google Scholar]
- (110).Clemente-Casares X, Blanco J, Ambalavanan P, Yamanouchi J, Singha S, Fandos C, Tsai S, Wang J, Garabatos N, Izquierdo C, et al. (2016) Expanding antigen-specific regulatory networks to treat autoimmunity. Nature 530, 434–440. [DOI] [PubMed] [Google Scholar]
- (111).Singha S, Shao K, Yang Y, Clemente-Casares X, Sole P, Clemente A, Blanco J, Dai Q, Song F, Liu SW, et al. (2017) Peptide-MHC-based nanomedicines for autoimmunity function as T-cell receptor microclustering devices. Nat. Nanotechnol 12, 701–710. [DOI] [PubMed] [Google Scholar]
- (112).Hickey JW, Vicente FP, Howard GP, Mao H-Q, and Schneck JP (2017) Biologically Inspired Design of Nanoparticle Artificial Antigen-Presenting Cells for Immunomodulation. Nano Lett 17, 7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Anikeeva N, Gakamsky D, Schøller J, and Sykulev Y (2012) Evidence that the Density of Self Peptide-MHC Ligands Regulates T-Cell Receptor Signaling. PLoS One 7, e41466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Feldmann M, and Steinman L (2005) Design of effective immunotherapy for human autoimmunity. Nature 435, 612. [DOI] [PubMed] [Google Scholar]
- (115).Kobayashi N, Kobayashi H, Gu L, Malefyt T, and Siahaan TJ (2007) Antigen-specific suppression of experimental autoimmune encephalomyelitis by a novel bifunctional peptide inhibitor. J. Pharmacol. Exp. Ther 322, 879–886. [DOI] [PubMed] [Google Scholar]
- (116).Murray JS, Oney S, Page JE, Kratochvil-Stava A, Hu Y, Makagiansar IT, Brown JC, Kobayashi N, and Siahaan TJ (2007) Suppression of type 1 diabetes in NOD mice by bifunctional peptide inhibitor: modulation of the immunological synapse formation. Chem. Biol. Drug Des 70, 227–236. [DOI] [PubMed] [Google Scholar]
- (117).Badawi AH, Kiptoo P, Wang WT, Choi IY, Lee P, Vines CM, and Siahaan TJ (2012) Suppression of EAE and prevention of blood-brain barrier breakdown after vaccination with novel bifunctional peptide inhibitor. Neuropharmacology 62, 1874–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Buyuktimkin B, Kiptoo P, and Siahaan TJ (2014) Bifunctional Peptide Inhibitors Suppress Interleukin-6 Proliferation and Ameliorates Murine Collagen-Induced Arthritis. J. Clin. Cell. Immunol 5, 1 DOI: 10.4172/2155-9899.1000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Badawi AH, Kiptoo P, and Siahaan TJ (2015) Immune Tolerance Induction against Experimental Autoimmune Encephalomyelitis (EAE) Using A New PLP-B7AP Conjugate that Simultaneously Targets B7/CD28 Costimulatory Signal and TCR/MHC-II Signal. J. Mult. Scler. (Foster City) 2, 1 DOI: 10.4172/2376-0389.1000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).White DR, Khedri Z, Kiptoo P, Siahaan TJ, and Tolbert TJ (2017) Synthesis of a Bifunctional Peptide Inhibitor-IgG1 Fc Fusion That Suppresses Experimental Autoimmune Encephalomyelitis. Bioconjugate Chem 28, 1867–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Zimmerman DH, Steiner H, Carmabula R, Talor E, and Rosenthal KS (2012) LEAPS therapeutic vaccines as antigen specific suppressors of inflammation in infectious and autoimmune diseases. J. Vaccines Vaccination 3, 1 DOI: 10.4172/2157-7560.1000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).Mikecz K, Glant TT, Markovics A, Rosenthal KS, Kurko J, Carambula RE, Cress S, Steiner HLR, and Zimmerman DH (2017) An epitope-specific DerG-PG70 LEAPS vaccine modulates T cell responses and suppresses arthritis progression in two related murine models of rheumatoid arthritis. Vaccine 35, 4048–4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Keijzer C, van der Zee R, van Eden W, and Broere F (2013) Treg inducing adjuvants for therapeutic vaccination against chronic inflammatory diseases. Front. Immunol 4, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Agmon-Levin N, Theodor E, Segal RM, and Shoenfeld Y (2013) Vitamin D in systemic and organ-specific autoimmune diseases. Clin. Rev. Allergy Immunol 45, 256–266. [DOI] [PubMed] [Google Scholar]
- (125).Zhang D, Chia C, Jiao X, Jin W, Kasagi S, Wu R, Konkel JE, Nakatsukasa H, Zanvit P, Goldberg N, et al. (2017) Dmannose induces regulatory T cells and suppresses immunopathology. Nat. Med 23, 1036–1045. [DOI] [PubMed] [Google Scholar]
- (126).Yu H-Y, Yao J-M, Qin Z-Y, Liu L, and Yang X-G (2013) Comparison of covalent and noncovalent interactions of carbon nanotubes on the crystallization behavior and thermal properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). J. Appl. Polym. Sci 130, 4299–4307. [Google Scholar]
- (127).Perdicchio M, Ilarregui JM, Verstege MI, Cornelissen LA, Schetters ST, Engels S, Ambrosini M, Kalay H, Veninga H, den Haan JM, et al. (2016) Sialic acid-modified antigens impose tolerance via inhibition of T-cell proliferation and de novo induction of regulatory T cells. Proc. Natl. Acad. Sci. U. S. A. 113, 3329–3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Green DR, Ferguson T, Zitvogel L, and Kroemer G (2009) Immungenic and tolerogenic cell death. Nat. Rev. Immunol 9, 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Jenkins MK, and Schwartz RH (1987) Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J. Exp. Med 165, 302–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Turley DM, and Miller SD (2007) Peripheral tolerance induction using ethylenecarbodiimide-fixed APCs uses both direct and indirect mechanisms of antigen presentation for prevention of experimental autoimmune encephalomyelitis. J. Immunol 178, 2212–2220. [DOI] [PubMed] [Google Scholar]
- (131).Getts DR, Turley DM, Smith CE, Harp CT, McCarthy D, Feeney EM, Getts MT, Martin AJ, Luo X, Terry RL, et al. (2011) Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1+ and IL-10-producing splenic macrophages and maintained by T regulatory cells. J. Immunol 187, 2405–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Prasad S, Kohm AP, McMahon JS, Luo X, and Miller SD (2012) Pathogenesis of NOD Diabetes is Initiated by Reactivity to the Insulin B Chain 9–23 Epitope and Involves Functional Epitope Spreading. J. Autoimmun 39, 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Smith CE, Eagar TN, Strominger JL, and Miller SD (2005) Differential induction of IgE-mediated anaphylaxis after soluble vs. cell-bound tolerogenic peptide therapy of autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U. S. A. 102, 9595–9600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Lutterotti A, Yousef S, Sputtek A, Sturner KH, Stellmann JP, Breiden P, Reinhardt S, Schulze C, Bester M, Heesen C, et al. (2013) Antigen-specific tolerance by autologous myelin peptide-coupled cells: a phase 1 trial in multiple sclerosis. Sci. Transl. Med 5, 188ra175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Miller SD, Turley DM, and Podojil JR (2007) Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat. Rev. Immunol 7, 665–677. [DOI] [PubMed] [Google Scholar]
- (136).Fife BT, Guleria I, Gubbels Bupp M, Eagar TN, Tang Q, Bour-Jordan H, Yagita H, Azuma M, Sayegh MH, and Bluestone JA (2006) Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J. Exp. Med 203, 2737–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Cremel M, Guerin N, Horand F, Banz A, and Godfrin Y (2013) Red blood cells as innovative antigen carrier to induce specific immune tolerance. Int. J. Pharm 443, 39–49. [DOI] [PubMed] [Google Scholar]
- (138).Pishesha N, Bilate AM, Wibowo MC, Huang NJ, Li Z, Dhesycka R, Bousbaine D, Li H, Patterson HC, Dougan SK, et al. (2017) Engineered erythrocytes covalently linked to antigenic peptides can protect against autoimmune disease. Proc. Natl. Acad. Sci. U. S. A. 114, 3157–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Kontos S, Kourtis IC, Dane KY, and Hubbell JA (2013) Engineering antigens for in situ erythrocyte binding induces T-cell deletion. Proc. Natl. Acad. Sci. U. S. A. 110, E60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).McKenzie IF, Apostolopoulos V, Lees C, Xing PX, Lofthouse S, Osinski C, Popovski V, Acres B, and Pietersz G (1998) Oxidised mannan antigen conjugates preferentially stimulate T1 type immune responses. Vet. Immunol. Immunopathol 63, 185–190. [DOI] [PubMed] [Google Scholar]
- (141).Katsara M, Deraos G, Tselios T, Matsoukas J, and Apostolopoulos V (2008) Design of novel cyclic altered peptide ligands of myelin basic protein MBP83–99 that modulate immune responses in SJL/J mice. J. Med. Chem 51, 3971–3978. [DOI] [PubMed] [Google Scholar]
- (142).Saito T, Kumagai Y, Hiramatsu T, Kurosawa M, Sato T, Habu S, Mitsui K, Kodera Y, Hiroto M, Matsushima A, et al. (2000) Immune tolerance induced by polyethylene glycol-conjugate of protein antigen: clonal deletion of antigen-specific Th-cells in the thymus. J. Biomater. Sci., Polym. Ed. 11, 647–656. [DOI] [PubMed] [Google Scholar]
- (143).Gupta S, Pfeil J, Kumar S, Poulsen C, Lauer U, Hamann A, Hoffmann U, and Haag R (2015) Tolerogenic modulation of the immune response by oligoglycerol- and polyglycerol-peptide conjugates. Bioconjugate Chem 26, 669–679. [DOI] [PubMed] [Google Scholar]
- (144).Sestak JO, Sullivan BP, Thati S, Northrup L, Hartwell B, Antunez L, Forrest ML, Vines CM, Siahaan TJ, and Berkland C (2014) Codelivery of antigen and an immune cell adhesion inhibitor is necessary for efficacy of soluble antigen arrays in experimental autoimmune encephalomyelitis. Mol. Ther.–Methods Clin. Dev 1, 14008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Sestak JO, Fakhari A, Badawi AH, Siahaan TJ, and Berkland C (2014) Structure, size, and solubility of antigen arrays determines efficacy in experimental autoimmune encephalomyelitis. AAPS J. 16, 1185–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Chittasupho C, Sestak J, Shannon L, Siahaan TJ, Vines CM, and Berkland C (2014) Hyaluronic Acid Graft Polymers Displaying Peptide Antigen Modulate Dendritic Cell Response in Vitro. Mol. Pharmaceutics 11, 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]