Abstract
Introduction
During recent wars in Iraq and Afghanistan, improved survivability in severe trauma corresponded with a rise in the proportion of trauma-related infections, including those associated with multidrug-resistant organisms (MDROs). Significant morbidity was reported in association with the infections. There is also concern regarding potential long-term impacts of the trauma-related infectious complications. Therefore, to meet the critical need of prospective collection of standardized infection-related data to understand the disease burden and improve outcomes of wounded personnel, the Trauma Infectious Disease Outcomes Study (TIDOS) was developed. Herein, we review accomplishments and key peer-reviewed findings of TIDOS.
Methods
The TIDOS project is a multicenter observational study of short- and long-term infectious complications following deployment-related trauma. Wounded military personnel medevac’d to Landstuhl Regional Medical Center (LRMC; Germany) before transfer to a participating US military hospital between June 2009 and December 2014 were eligible for inclusion. An infectious disease module to supplement the Department of Defense Trauma Registry by collecting infection-related data from all trauma patients admitted to participating hospitals was developed. Specimens from trauma patients were also collected and retained in a microbiological isolate repository. During the initial hospitalization, patients were given the opportunity to enroll in a prospective follow-up cohort study. Patients who received Department of Veterans Affairs (VA) care were also given the opportunity to consent to ongoing VA follow-up
Results
A total of 2,699 patients transferred to participating military hospitals in the USA, of which 1,359 (50%) patients enrolled in the TIDOS follow-up cohort. In addition, 638 enrolled in the TIDOS-VA cohort (52% of TIDOS enrollees who entered VA healthcare). More than 8,000 isolates were collected from infection control surveillance and diagnostic evaluations and retained in the TIDOS Microbiological Repository. Approximately 34% of the 2,699 patients at US hospitals developed a trauma-related infection during their initial hospitalization with skin and soft-tissue infections being predominant. After discharge from the US hospitals, approximately one-third of TIDOS cohort enrollees developed a new trauma-related infection during follow-up and extremity wound infections (skin and soft-tissue infections and osteomyelitis) continued to be the majority. Among TIDOS cohort enrollees who received VA healthcare, 38% developed a new trauma-related infection with the incident infection being diagnosed a median of 88 days (interquartile range: 19–351 days) following hospital discharge. Data from TIDOS have been used to support the development of Joint Trauma System clinical practice guidelines for the prevention of combat-related infections, as well as the management of invasive fungal wound infections. Lastly, due to the increasing proportion of infections associated with MDROs, TIDOS investigators have collaborated with investigators across military laboratories as part of the Multidrug-Resistant and Virulent Organisms Trauma Infections Initiative with the objective of improving the understanding of the complex wound microbiology in order to develop novel infectious disease countermeasures.
Conclusions
The TIDOS project has focused research on four initiatives: (1) blast-related wound infection epidemiology and clinical management; (2) DoD-VA outcomes research; (3) Multidrug- Resistant and other Virulent Organisms Trauma Infections Initiative; and (4) Joint Trauma System clinical practice guidelines and antibiotic stewardship. There is a continuing need for longitudinal data platforms to support battlefield wound research and clinical practice guideline recommendation refinement, particularly to improve care for future conflicts. As such, maintaining a research platform, such as TIDOS, would negate the lengthy time needed to initiate data collection and analysis.
Keywords: trauma-related infections, combat-related infections, wound infections, military health
INTRODUCTION
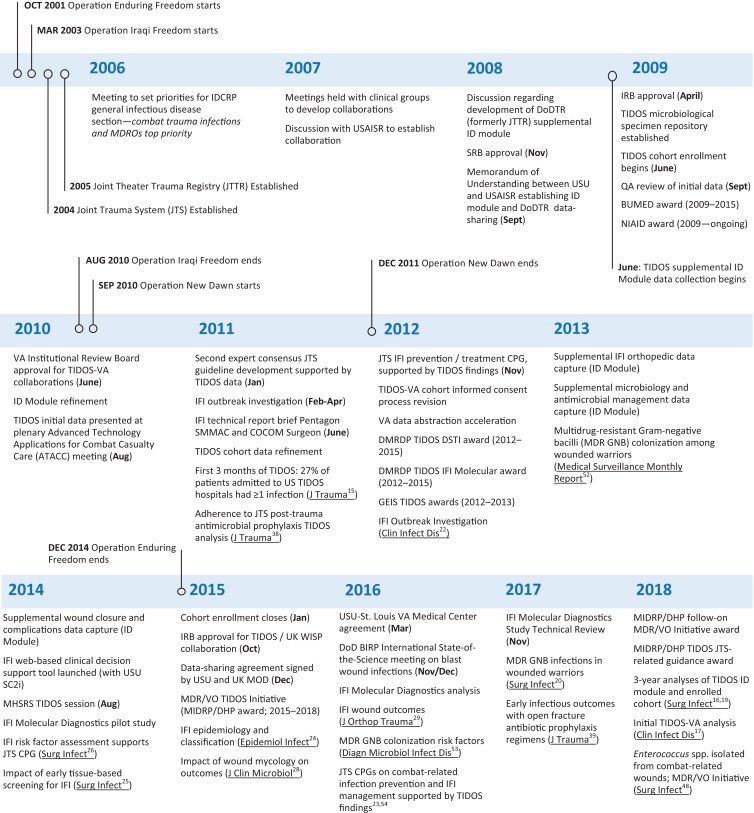
Following the initiation of Operations Enduring Freedom (OEF) and Iraqi Freedom (OIF) in 2001 and 2003, respectively, the joint military forces of the USA recognized a need for a formal system of distributed trauma care in order to improve battlefield casualty and follow-on care. Subsequently, the Joint Trauma System (JTS) was implemented in November 2004 (Fig. 1).1 To support the JTS mandate as a learning trauma care system, a Joint Theater Trauma Registry (later renamed the Department of Defense Trauma Registry; DoDTR) was established in 2005 to collect data related to injury patterns, treatment, and functional outcomes (e.g., return-to-duty, evacuated, or death).1,2 The DoDTR captured very little information on infectious complications.
figure 1.
Timeline of events associated with the Trauma Infectious Disease Outcomes Study (TIDOS) and related research. BIRP – Blast Injury Research Program; BUMED – Navy Bureau of Medicine and Surgery; COCOM – Combatant Command; CPG – clinical practice guidelines; DHP – Defense Health Program; DMRDP – Defense Medical Research and Development Program; DoDTR – Department of Defense Trauma Registry; DSTI – deep soft-tissue infection; ID – infectious disease; GEIS – Global Emerging Infections Surveillance; IDCRP – Infectious Disease Clinical Research Program; IFI – invasive fungal wound infection; IRB – institutional review board; MDROs – multidrug-resistant organisms; MDR/VO – Multidrug-resistant and Virulent Organisms; MHSRS – Military Health System Research Symposium; MIDRP – Military Infectious Diseases Research Program; NIAID – National Institute of Allergy and Infectious Diseases; QA – quality assessment; SC2i – Surgical Critical Care Initiative; SMMAC – Senior Military Medical Advisory Committee; SRB – scientific review board; VA – Veterans Affairs; UK MOD - United Kingdom Ministry of Defence; UK WISP - WISP – United Kingdom Wound Infection Surveillance Programme; USAISR; US Army Institute of Surgical Research; USU – Uniformed Services University of the Health Sciences.
Combined with enhanced preventive measures (e.g., body armor and use of tourniquets), the improvements in combat casualty care from the implementation of the JTS resulted in a marked decrease in service members killed in action and case fatality rates in OEF/OIF.1,3 Nevertheless, the proportion of personnel who died of wounds was higher than the Vietnam War.3 Among service members with severe trauma who survived, there was a corresponding rise in infectious complications.4–10 In addition, the growing proportion of multidrug-resistant organisms (MDROs) associated with trauma-related infections presented further challenges for military clinicians.4,8,9,11–14
Shortly after the Infectious Disease Clinical Research Program (IDCRP) was founded as a research center within the Department of Preventive Medicine and Biostatistics of the Uniformed Services University of the Health Sciences (USU) in late 2006, meetings held to set research priorities identified trauma-related infections and MDROs as the number one priority for the Military Health System (Fig. 1). In particular, the lack of routine capture of standardized infection-related data was recognized as a significant data gap. The culmination of these discussions led to development of the Department of Defense – Department of Veterans Affairs (VA) Multicenter Cohort Study Evaluating Infection-Associated Clinical Outcomes in Hospitalized Medical Evacuees following Traumatic Injury (i.e., the Trauma Infectious Disease Outcomes Study; TIDOS).15 A critical component of this was a joint effort between IDCRP/USU and the JTS (based at the US Army Institute of Surgical Research) to develop an Infectious Disease supplemental module to the DoDTR. Herein, we provide an overview of TIDOS, including a summarization of key peer-reviewed findings, impacts on clinical practice, and future initiatives.
TRAUMA INFECTIOUS DISEASE OUTCOMES STUDY
Study Design
On June 1, 2009, the TIDOS project was initiated to evaluate the short- and long-term infectious outcomes among military personnel with deployment-related trauma (Fig. 1).15 Service members were eligible for inclusion in TIDOS if they were over 18 years of age, active-duty personnel or DoD beneficiary, and sustained a deployment-related injuring requiring medevac to Landstuhl Regional Medical Center (LRMC) in Germany before transition to a participating military hospital in the United States between June 1, 2009 and December 31, 2014. The participating US hospitals were Walter Reed National Military Medical Center (Walter Reed Army Medical Center and National Naval Medical Center prior to September 2011) in the National Capital Region and Brook Army Medical Center at JBSA Fort Sam Houston in TX.
Information related to demographics, injury characteristics, trauma history, and early casualty care was obtained from the DoDTR for all patients admitted to LRMC. Infection-related data were prospectively and systematically collected through the Infectious Disease module for all patients admitted to one of the participating military hospitals in the USA. The type of information captured through the supplemental infectious disease module included infection diagnoses (i.e., skin and soft-tissue, bloodstream, central nervous system, sepsis, osteomyelitis, intra-thoracic/pulmonary, and intra-abdominal), antimicrobial treatment, microbiology findings from clinical work-ups and active surveillance cultures for asymptomatic colonization (i.e., admission surveillance swabs of the groin and axilla at LRMC and groin, axilla, and nares in the USA), and outcomes related to specific infection syndromes.15
Prior to discharge from a participating military hospital in the USA, patients were given the opportunity to enroll in the follow-up cohort of TIDOS (enrollment ended on January 31, 2015). If the patient consented, infection-related events that occurred after discharge from the US military hospital were collected at predetermined follow-up intervals through telephone interviews (concluded on January 31, 2015) and abstraction of DoD electronic medical records.15,16 Furthermore, patients enrolled in the TIDOS follow-up cohort who entered VA healthcare were given the opportunity to consent to additional follow-up through data abstraction of VA healthcare records.17 Follow-up data collection through the DoD electronic medical records concluded on June 8, 2016; however, periodic sweeps of the Military Health System Data Repository are ongoing.
TIDOS Population
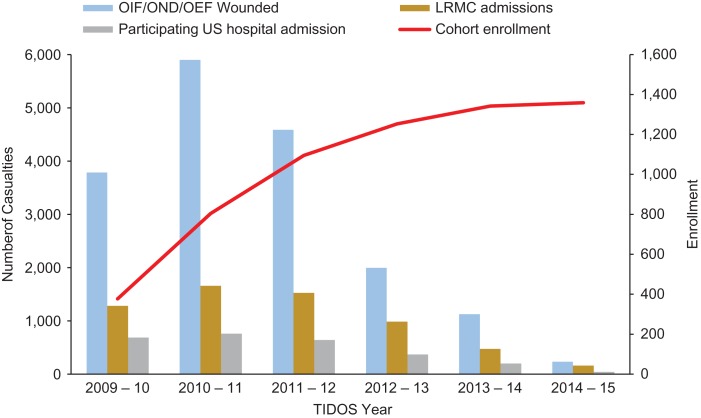
The TIDOS project was initiated in June 2009 as combat operations were decreasing in Iraq and increasing in Afghanistan. Between June 1, 2009 and December 31, 2014, 6,079 US military personnel sustaining deployment-related trauma were admitted to LRMC, of which 4,953 (81.5%) were wounded in support of OEF, 522 (8.6%) in support of OIF/OND, and 604 (9.9%) were injured outside of the Afghanistan and Iraq theaters. Following initial surgical care at LRMC (approximately 2–3 days), 2,699 (44.4% of 6,079 LRMC admissions) patients were transitioned to one of the participating hospitals in the USA, of which 1,359 (50.4%) enrolled in the TIDOS follow-up cohort (Fig. 2).
FIGURE 2.
US Wounded Military Personnel Population and TIDOS Cohort Enrollment. TIDOS year spans June 1 through May 30. Number of wounded in action from Defense Casualty Analysis System (https://dcas.dmdc.osd.mil/dcas/pages/casualties.xhtml). OEF – Operation Enduring Freedom; OIF – Operation Iraqi Freedom; OND – Operation New Dawn.
The majority of the overall LRMC trauma population were young (median age of 25 years) men (95%) injured in support of OEF (82%). Approximately 49% of the service members sustained blast injuries and 34% had an injury severity score (ISS)18 ≥16, indicating severe or life-threatening injuries. The 2,699 patients who transferred to a participating US hospital typically had more severe injuries19 with 65% sustaining a blast-related injury (largely the result of improvised explosive devices; IED), resulting in 58% with an ISS ≥15. Approximately 20% of the population had a traumatic or early surgical amputation, while 75% sustained at least one fracture (excluding digits).20
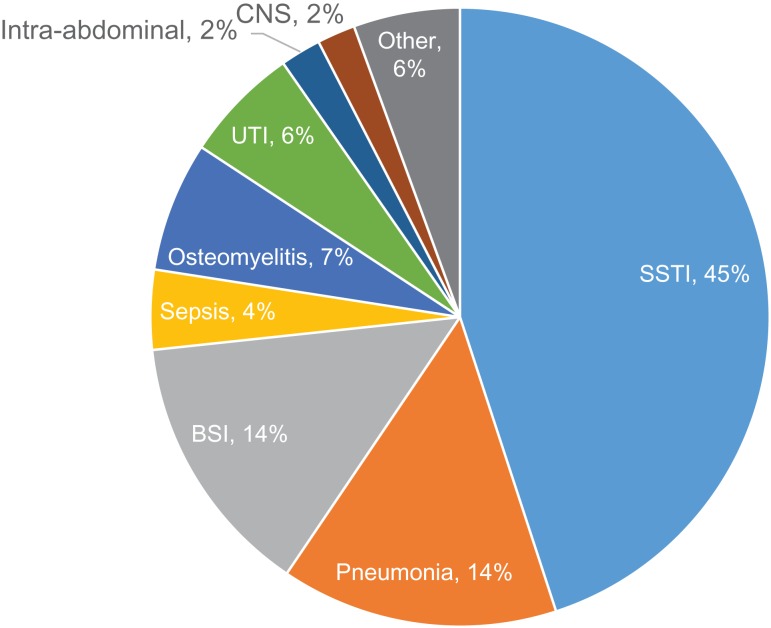
While receiving care at LRMC, 321 (5% of 6,079) patients developed an infection with skin and soft-tissue infections being predominant (49% of 321). Among the 2699 wounded personnel admitted to participating US hospitals, 913 (34%) patients were diagnosed with at least one infection for a total of 2,210 infection events. On an infection level, the majority were skin and soft-tissue (45%) followed by pneumonia (14%) and bloodstream infections (14%) (Fig. 3). In addition, 25% of the infections were associated with a multidrug-resistant Gram-negative bacilli, which were defined in accordance with standardized definitions from the Centers for Disease Control and Prevention.20 Injury mechanism (i.e., IED blast), sustaining a traumatic amputation, being classified with an ISS ≥16, receiving blood transfusions within 24 hours post-injury, admission to the intensive care unit, requiring mechanical ventilation at LRMC, and receipt of post-trauma antibiotic prophylaxis within 48 hours of injury were associated with risk of developing an infection.19,21
FIGURE 3.
Distribution of infection syndromes (N = 2,210) among wounded military personnel who transferred to participating US hospitals. BSI – bloodstream infection; CNS – central nervous system infection; SSTI – skin and soft-tissue infection; UTI – urinary tract infection.
Among the 1,359 TIDOS cohort enrollees, 22 withdrew, leaving 1,337 in the cohort. Presently, 1,221 (91.0%) TIDOS enrollees have entered VA healthcare and 638 (52%) consented to follow-up through the VA. Follow-up findings from both DoD and VA sources have demonstrated that infectious complications continue long after the initial period of trauma hospitalization for the patient.16 In particular, incident trauma-related infections (largely skin and soft-tissue and osteomyelitis) have been reported in approximately 38% of enrollees during follow-up with many requiring re-hospitalization and additional surgical procedures, demonstrating the continued impact of trauma-related infections.17
TIDOS – Major Impact
Analyses under TIDOS have focused on priority issues of the Military Health System, including invasive fungal wound infections (IFIs), clinical practice guidelines, and MDROs. Key peer-reviewed findings related to these priority issues are presented.
Invasive Fungal Wound Infections
Following the unexpected outbreak of IFIs in 2009/2010 among combat casualties with blast trauma in Afghanistan, TIDOS investigators led the DoD outbreak investigation of 36 patients with an IFI diagnosed between June 2009 and December 2010 and presented their findings to the Assistant Secretary of Defense (Health Affairs), Surgeons General, and COCOM Surgeons in 2011. The outbreak investigation was published in Clinical Infectious Diseases.22 Findings from the case investigation led to the standardized capture of fungal-related data, including wound necrosis, histopathology, and use of antifungal therapy. In addition, the initial results were utilized by the JTS to develop the first JTS-wide clinical practice guideline for the prevention and management of IFI wounds in 2012 (superseded by revised guideline).23
As part of the case investigation, definitions and classifications for trauma-related IFIs were derived from the 2008 Mycoses Study Group definitions for fungal infections in immunocompromised individuals with modification for the military-unique setting.24 Extending the time period to August 2011, 77 patients with an IFI were identified (6.8% of 1,133 LRMC trauma admissions) and assessed with regards to epidemiology, risk factors, clinical outcomes, diagnostics, and mycology.24–32 Independent predictors for the development of an IFI included sustaining a blast injury (odds ratio [OR]: 5.7; 95% Confidence Interval [CI]: 1.1–29.6) while dismounted (OR: 8.5: 95% CI: 1.2–59.8), having a traumatic above knee amputations (OR: 4.1; CI: 1.3–12.7), and requiring large-volume (≥20 units) of packed red blood cells plus whole blood within 24 hours of injury (OR: 7.0; CI: 2.5–19.7).26 The TIDOS analyses also demonstrated the increased morbidity of fungal infections on wound healing along with higher rates of surgical amputations and amputation revisions. In particular, the time to wound closure was a median of 16 days post-injury for wounds with fungal infections compared to 9 days in wounds without fungal infections (p < 0.001).29 The pathogenic nature of fungi from the order Mucorales was confirmed in another TIDOS investigation that found that wounds that grew Mucorales fungi had a significantly longer time to wound closure compared to wounds with non-Mucorales mold growth (e.g., Aspergillus spp. and Fusarium spp.).28
Early identification of an IFI and timely initiation of surgical and antifungal treatment are the cornerstones of IFI management. With the goal of supporting early IFI risk stratification by clinicians in theater or at LRMC, a web-based clinical decision support tool was developed in collaboration with the USU Surgical Critical Care Initiative (SC2i) (http://www.sc2i.org/tools/).33
Following extensive evaluation of IFI data and publication of numerous analyses, TIDOS investigators worked with the JTS leadership to further refine the existing IFI clinical practice guideline in August 2016.23 Overall, TIDOS investigations have greatly added to the knowledge base and practice guidance for combat-related IFI. While TIDOS assessed IFI in a military population, the combined efforts of TIDOS investigators and JTS clinicians have also had significant impact on the approach to IFI management in civilian trauma.
Joint Trauma System Clinical Practice Guidelines
As a way to standardize treatment of combat casualties, the JTS convened consensus panels comprised of civilian and military experts to review scientific literature and develop evidence-based recommendations for the prevention of combat-related trauma infections. The first guideline was published in 2008 and later updated in 2011.34,35 With the goal of supporting process improvement, data collected through TIDOS has been utilized to assess compliance with the JTS post-trauma antibiotic prophylaxis recommendations in three separate analyses.36–38 The findings demonstrate a significant improvement in compliance over the study years for all injury patterns evaluated (i.e., open fracture, skin and soft-tissue, closed, maxillofacial, and penetrating abdomen). In particular, the use of expanded Gram-negative coverage with open fractures, which is not recommended by the guidelines, decreased from 61% in 2009–2010 to 7% in 2013-2014.36
In addition to assessing adherence to JTS clinical practice guidelines, it is also important to measure effectiveness of those guidelines through outcomes research. In recent analyses, infectious outcomes were examined among wounded military personnel with regards to antibiotic prophylactic regimen (i.e., recommended antibiotics with narrow-spectrum activity or antibiotics with expanded Gram-negative coverage). With open fractures, there was a risk reduction in extremity skin and soft-tissue infections among patients who received the expanded Gram-negative coverage (22% versus 28% in patients who only received narrow-spectrum antibiotics; p = 0.029); however, there was no difference in rates of osteomyelitis between the two groups (8% in both). Furthermore, patients who received expanded Gram-negative coverage more frequently had isolation of Gram-negative organisms that were resistant to the fluoroquinolone, aminoglycosides, or both antibiotics (49% vs 40% with narrow-spectrum only use; p < 0.001).39 Among patients with open extremity soft-tissue injuries, there was no significant difference in proportion of skin and soft-tissue infections between the antibiotic prophylactic regimens groups; however, a higher proportion of resistant Gram-negative bacteria was recovered from patients who received expanded Gram-negative coverage (36% vs 19%; p = 0.001).40 Overall, based on the limited benefit and potential adverse effect with acquisition of resistant organisms, these findings support the JTS recommendations for use of narrow-spectrum antibiotics.
The Multidrug-Resistant and Virulent Organisms (MDR/VO) Trauma Infections Initiative
The rising proportion of infections associated with MDROs resulted in further complications, including delayed wound healing and high resource utilization.4,8,14,20,41–46 Adding to the challenge is that many wound infections are polymicrobial.28,44,47 Thus, the understanding of the complicated microbiology of war wounds became a research priority. Analyses are conducted using clinical data linked to specimens contained in the TIDOS Microbiological Specimen Repository, which includes more than 8,000 isolates from surveillance swabs and clinical work-ups, of which approximately 30% were multidrug-resistant.
To address this priority, TIDOS, in collaboration with partner military laboratories (i.e., Walter Reed Army Institute of Research, Naval Medical Research Center, and US Army Institute of Surgical Research), formed the MDR/VO Trauma Infections Initiative in 2015. The objective of this initiative was to conduct analyses aimed at better understanding complex wound microbiology in order to lead to novel infectious disease countermeasures. Research areas under the MDR/VO Initiative include the combat wound bacterial microbiome, biofilm production and clinical impact, emergence of antimicrobial resistance, wound bacteria interactions/antagonism, and MDR/VO wound infection clinical outcomes.
Due to frequent isolation from extremity wounds, Enterococcus spp. are the focus of many MDR/VO Initiative analyses. For example, Enterococcus faecium has been identified as the predominant species and was recovered from 74% of polymicrobial extremity wounds infection. Patients with Enterococcus extremity wound infection are also typically more severely injured, require more large-volume blood transfusion, and have longer hospitalization (median of 55 days vs 40 days; p = 0.004) than those who have infections caused by other organisms.48
Trauma-Related Research Area Initiatives and Future Endeavors
Within the IDCRP Trauma-Related Infections Research Area, four primary research initiatives have been identified based on relevance to the Military Health System: blast-related wound infections, DoD-VA outcomes research, the MDR/VO Trauma Infections Initiative, and JTS clinical practice guidelines and antibiotic stewardship.
Blast-Related Wound Infections
In 2007, the DoD Blast Injury Research Program Coordinating Office was established in response to the Congressional mandate to improve the coordination of blast-related research.49 Although the Blast Injury Research Program has primarily focused on traumatic brain injuries, wound infections are also a concern and the first International State-of-the-Science Meeting on Minimizing the Impact of Wound Infections Following Blast-related Injuries was held in 2016. Findings from TIDOS analyses related to extremity wound infections, IFIs, and wound microbiology were presented and carefully considered by the expert panel during their discussions of key research questions. The expert panel recognized a need for further data related to blast wound microbiology, risk factors, and wound definitions in order to develop evidence-based recommendations for wound care. Specifically, it was agreed that longitudinal data platforms (e.g., DoDTR, TIDOS, and the Military Orthopaedic Trauma Registry) are a necessity to support clinical practice refinement, research, and military medical readiness.
Ongoing TIDOS analyses currently focus on blast trauma and extremity wound infections, IFIs, and non-extremity wound infections (e.g., genitourinary, penetrating abdominal, and maxillofacial injuries). Analyses are also conducted under a separate protocol that focuses on trauma-associated osteomyelitis among combat casualties injured between 2003 and 2009. Specifically, characteristics of patients who develop osteomyelitis following open fractures of the tibia, femur, and arm long bones were assessed, including surgical and antibiotic management, microbiology, and outcomes (e.g., infection recurrence). Due to the high morbidity associated with osteomyelitis, the identification of risk factors is of crucial importance. Among patients with open tibia fractures, sustaining a blast injury, fracture severity, and muscle loss or damage were independent predictors of osteomyelitis risk.50 Ongoing analyses are investigating late-onset consequences through extended VA follow-up.
DoD-VA Outcomes Research
Information on short- and long-term infectious outcomes following trauma is critical for improving the health of combat casualties. Using resources through both the DoD and VA provides nearly complete capture of infection-related information and allows for a better understanding of the impact of trauma-related infections. Among the first 337 TIDOS cohort enrollees who entered VA healthcare, 38% had a new trauma-related infection diagnosed during the follow-up period with skin and soft-tissue infections and osteomyelitis being predominant.17 The collaboration between the St. Louis VA Health Care System with TIDOS investigators is cited as a successful case study providing lessons learned in the VA/DoD Collaboration Guidebook for Healthcare Research.51 Along with continued data abstraction of infection-related information from the VA electronic medical records, information on social, physical, and mental health metrics are also being collected and will be analyzed for their association with infectious outcomes.
MDR/VO Trauma Infections Initiative
As part of the MDR/VO Trauma Infections Initiative, antagonism of common bacteria (e.g., Enterococcus spp.) isolated from polymicrobial wounds is being evaluated as well as antimicrobial susceptibility patterns and clinical outcomes. In addition, there is ongoing evaluation of biofilm formation in wound bacterial isolates and concurrent assessment of biofilm dispersal agents. Furthermore, the interaction of critically important bacteria collectively known as ESKAPE pathogens (i.e., Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae [and Escherichia coli], Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.) are being examined.
JTS Clinical Practice Guidelines and Antibiotic Stewardship
Analyses intended to support or refine injury-specific antimicrobial prophylaxis recommendations from the JTS clinical practice guideline on infection prevention are ongoing. Moreover, analyses on the effectiveness of different antibiotic regimens are either underway or being planned with the goal of supporting the development of recommendations focused on the management of trauma-related infections. The first analyses are focused on developing recommendations for the treatment of skin and soft-tissue infections and osteomyelitis. A secondary aim is to develop recommendations for the management of non-extremity trauma-related infections (e.g., sepsis, bloodstream infections, intra-abdominal infections, and pneumonia). Best practices will be examined through collaboration with the United Kingdom Ministry of Defence Wound Infection Surveillance Programme by comparing treatment regimens and infectious outcomes in the two study populations. Similar analyses will be conducted with the goal of refining the JTS clinical practice guideline on IFIs.
CONCLUSION
Since inception, the TIDOS project has produced findings that have resulted in practice pattern changes and improving combat casualty care. Although combat operations ended in Afghanistan in December 2014, the analysis of infection-related data remains crucial in order to optimize current JTS clinical practice guidelines with the overall objective of improving outcomes of combat casualties involved in future military operations or wars. Overall, there is a need to sustain combat trauma-related infection research to improve care readiness (i.e., prevention and treatment of infections) for future conflicts.
Acknowledgments
We are indebted to the Infectious Disease Clinical Research Program Trauma Infectious Disease Outcomes Study (TIDOS) study team of nurses, investigators spanning multiple disciplines, clinical coordinators, microbiology technicians, data managers, clinical site managers, and administrative support personnel for their tireless hours to ensure the success of this project. Special thanks to Leigh Carson for her assistance in manuscript preparation.
The views expressed herein are those of the authors and do not reflect the official policy or position of Uniformed Services University of the Health Sciences, Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., National Institutes of Health or the Department of Health and Human Services, Brooke Army Medical Center, Walter Reed National Military Medical Center, Landstuhl Regional Medical Center, St. Louis Health Care System, the US Army Medical Department, the US Army Office of the Surgeon General, the Department of the Air Force, the Department of the Navy, the Department of the Army or the Department of Defense or the US Government.
Funding
This work was conducted by the Infectious Disease Clinical Research Program, a Department of Defense program executed through the Uniformed Services University of the Health Sciences, Department of Preventive Medicine and Biostatistics through a cooperative agreement with the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. This project has been funded by the National Institute of Allergy and Infectious Diseases, National Institute of Health [Inter-Agency Agreement Y1-AI-5072, the Department of the Navy under the Wounded, Ill, and Injured Program, the Defense Medical Research and Development Program, and the Military Infectious Diseases Research Program.
References
- 1. Eastridge BJ, Jenkins D, Flaherty S, Schiller H, Holcomb JB: Trauma system development in a theater of war: experiences from Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma 2006; 61(6): 1366–72. [DOI] [PubMed] [Google Scholar]
- 2. Blackbourne LH, Baer DG, Eastridge BJ, et al. : Military medical revolution: prehospital combat casualty care. J Trauma Acute Care Surg 2012; 73(6 Suppl 5): S372–7. [DOI] [PubMed] [Google Scholar]
- 3. Nessen SC, Gurney J, Rasmussen TE, et al. : Unrealized potential of the US military battlefield trauma system: DOW rate is higher in Iraq and Afghanistan than in Vietnam, but CFR and KIA rate are lower. J Trauma Acute Care Surg 2018; 85(1S): S4–12. [DOI] [PubMed] [Google Scholar]
- 4. O’Shea MK: Acinetobacter in modern warfare. Int J Antimicrob Agents 2012; 39(5): 363–75. [DOI] [PubMed] [Google Scholar]
- 5. Murray CK, Hinkle MK, Yun HC: History of infections associated with combat-related injuries. J Trauma 2008; 64(3 Suppl): S221–31. [DOI] [PubMed] [Google Scholar]
- 6. Murray CK, Hsu JR, Solomkin JS, et al. : Prevention and management of infections associated with combat-related extremity injuries. J Trauma 2008; 64(3 Suppl): S239–51. [DOI] [PubMed] [Google Scholar]
- 7. Murray CK, Wilkins K, Molter NC, et al. : Infections in combat casualties during Operations Iraqi and Enduring Freedom. J Trauma 2009; 66(4 Suppl): S138–44. [DOI] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention : Acinetobacter baumannii infections among patients at military medical facilities treating injured U.S. service members, 2002-2004. MMWR Morb Mortal Wkly Rep 2004; 53(45): 1063–6. [PubMed] [Google Scholar]
- 9. Davis KA, Moran KA, McAllister CK, Gray PJ: Multidrug-resistant Acinetobacter extremity infections in soldiers. Emerg Infect Dis 2005; 11(8): 1218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson EN, Burns TC, Hayda RA, Hospenthal DR, Murray CK: Infectious complications of open type III tibial fractures among combat casualties. Clin Infect Dis 2007; 45(4): 409–15. [DOI] [PubMed] [Google Scholar]
- 11. Kang G, Hartzell JD, Howard R, et al. : Mortality associated with Acinetobacter baumannii complex bacteremia among patients with war-related trauma. Infect Control Hosp Epidemiol 2010; 31(1): 92–4. [DOI] [PubMed] [Google Scholar]
- 12. Weintrob AC, Roediger MP, Barber M, et al. : Natural history of colonization with gram-negative multidrug-resistant organisms among hospitalized patients. Infect Control Hosp Epidemiol 2010; 31(4): 330–7. [DOI] [PubMed] [Google Scholar]
- 13. Ake J, Scott P, Wortmann G, et al. : Gram-negative multidrug-resistant organism colonization in a US military healthcare facility in Iraq. Infect Control Hosp Epidemiol 2011; 32(6): 545–52. [DOI] [PubMed] [Google Scholar]
- 14. Keen EF 3rd, Murray CK, Robinson BJ, Hospenthal DR, Co EM, Aldous WK: Changes in the incidences of multidrug-resistant and extensively drug-resistant organisms isolated in a military medical center. Infect Control Hosp Epidemiol 2010; 31(7): 728–32. [DOI] [PubMed] [Google Scholar]
- 15. Tribble DR, Conger NG, Fraser S, et al. : Infection-associated clinical outcomes in hospitalized medical evacuees after traumatic injury: Trauma Infectious Disease Outcome Study. J Trauma 2011; 71(1 Suppl): S33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tribble DR, Krauss M, Murray CK, et al. : Epidemiology of trauma-related infections among a combat casualty cohort after initial hospitalization: the Trauma Infectious Disease Outcomes Study. Surg Infect (Larchmt) 2018; 19(5): 494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McDonald JR, Liang SY, Li P, et al. : Infectious complications after deployment trauma: following wounded United States military personnel into Veterans Affairs care. Clin Infect Dis 2018; 67(8): 1205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Linn S: The injury severity score – importance and uses. Ann Epidemiol 1995; 5(6): 440–6. [DOI] [PubMed] [Google Scholar]
- 19. Weintrob AC, Murray CK, Xu J, et al. : Early infections complicating the care of combat casualties from Iraq and Afghanistan. Surg Infect (Larchmt) 2018; 19(3): 286–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Campbell WR, Li P, Whitman TJ, et al. : Multi-drug–resistant gram-negative infections in deployment-related trauma patients. Surg Infect (Larchmt) 2017; 18(3): 357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tribble DR, Li P, Warkentien TE, et al. : Impact of operational theater on combat and noncombat trauma-related infections. Mil Med 2016; 181(10): 1258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Warkentien T, Rodriguez C, Lloyd B, et al. : Invasive mold infections following combat-related injuries. Clin Infect Dis 2012; 55(11): 1441–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodriguez CJ, Tribble DR, Murray CK, et al. : Invasive fungal infection in war wounds (CPG: 28). Joint Trauma System. Available at https://jts.amedd.army.mil/assets/docs/cpgs/JTS_Clinical_Practice_Guidelines_(CPGs)/Invasive_Fungal_Infection_04_Aug_2016_ID28.pdf; accessed December 4, 2018.
- 24. Weintrob AC, Weisbrod AB, Dunne JR, et al. : Combat trauma-associated invasive fungal wound infections: epidemiology and clinical classification. Epidemiol Infect 2015; 143(1): 214–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lloyd B, Weintrob A, Rodriguez C, et al. : Effect of early screening for invasive fungal infections in U.S. service members with explosive blast injuries. Surg Infect (Larchmt) 2014; 15(5): 619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rodriguez C, Weintrob AC, Shah J, et al. : Risk factors associated with invasive fungal Infections in combat trauma. Surg Infect (Larchmt) 2014; 15(5): 521–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodriguez CJ, Weintrob AC, Dunne JR, et al. : Clinical relevance of mold culture positivity with and without recurrent wound necrosis following combat-related injuries. J Trauma Acute Care Surg 2014; 77(5): 769–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Warkentien TE, Shaikh F, Weintrob AC, et al. : Impact of Mucorales and other invasive molds on clinical outcomes of polymicrobial traumatic wound infections. J Clin Microbiol 2015; 53(7): 2262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lewandowski LR, Weintrob AC, Tribble DR, et al. : Early complications and outcomes in combat injury related invasive fungal wund infections: a case-control analysis. J Orthop Trauma 2016; 30(3): e93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heaton SM, Weintrob AC, Downing K, et al. : Histopathological techniques for the diagnosis of combat-related invasive fungal wound infections. BMC Clin Pathol 2016; 16(1): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tribble DR, Rodriguez CJ, Weintrob AC, et al. : Environmental factors related to fungal wound contamination after combat trauma in Afghanistan, 2009-2011. Emerg Infect Dis 2015; 21(10): 1759–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tribble DR, Rodriguez CJ: Combat-related invasive fungal wound infections. Curr Fungal Infect Rep 2014; 8(4): 277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Potter BK, Forsberg JA, Silvius E, et al. : Combat-related invasive fungal infections: development of a clinically applicable clinical decision support system for early risk stratification. Mil Med 2018. 10.1093/milmed/usy182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hospenthal DR, Murray CK, Andersen RC, et al. : Guidelines for the prevention of infections associated with combat-related injuries: 2011 update: endorsed by the Infectious Diseases Society of America and the Surgical Infection Society. J Trauma 2011; 71(2 Suppl 2): S210–34. [DOI] [PubMed] [Google Scholar]
- 35. Hospenthal DR, Murray CK, Andersen RC, et al. : Guidelines for the prevention of infection after combat-related injuries. J Trauma 2008; 64(3): S211–20. [DOI] [PubMed] [Google Scholar]
- 36. Lloyd BA, Murray CK, Bradley W, et al. : Variation in postinjury antibiotic prophylaxis patterns over five years in a combat zone. Mil Med 2017; 182(S1): 346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lloyd BA, Weintrob AC, Hinkle MK, et al. : Adherence to published antimicrobial prophylaxis guidelines for wounded service members in the ongoing conflicts in southwest Asia. Mil Med 2014; 179(3): 324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tribble DR, Lloyd B, Weintrob A, et al. : Antimicrobial prescribing practices following publication of guidelines for the prevention of infections associated with combat-related injuries. J Trauma 2011; 71(2 Suppl 2): S299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lloyd BA, Murray CK, Shaikh F, et al. : Early infectious outcomes following addition of fluoroquinolone or aminoglycoside to post-trauma antibiotic prophylaxis in combat-related open fracture injuries. J Trauma Acute Care Surg 2017; 83(5): 854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lloyd BA, Murray CK, Shaikh F, et al. : Antibiotic prophylaxis with combat-related open soft-tissue injuries. Mil Med 2018; 183(9–10): e260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Murray CK: Epidemiology of infections associated with combat-related injuries in Iraq and Afghanistan. J Trauma 2008; 64(3 Suppl): S232–8. [DOI] [PubMed] [Google Scholar]
- 42. Murray CK, Wilkins K, Molter NC, et al. : Infections complicating the care of combat casualties during Operations Iraqi Freedom and Enduring Freedom. J Trauma 2011; 71(1 Suppl): S62–73. [DOI] [PubMed] [Google Scholar]
- 43. Aronson NE, Sanders JW, Moran KA: In harm’s way: infections in deployed American military forces. Clin Infect Dis 2006; 43(8): 1045–51. [DOI] [PubMed] [Google Scholar]
- 44. Petersen K, Riddle MS, Danko JR, et al. : Trauma-related infections in battlefield casualties from Iraq. Ann Surg 2007; 245(5): 803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yun HC, Branstetter JG, Murray CK: Osteomyelitis in military personnel wounded in Iraq and Afghanistan. J Trauma 2008; 64(2 Suppl): S163–8. [DOI] [PubMed] [Google Scholar]
- 46. Eardley WG, Brown KV, Bonner TJ, Green AD, Clasper JC: Infection in conflict wounded. Philos Trans R Soc Lond B Biol Sci 2011; 366(1562): 204–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wallum TE, Yun HC, Rini EA, et al. : Pathogens present in acute mangled extremities from Afghanistan and subsequent pathogen recovery. Mil Med 2015; 180(1): 97–103. [DOI] [PubMed] [Google Scholar]
- 48. Heitkamp RA, Li P, Mende K, Demons ST, Tribble DR, Tyner SD: Association of Enterococcus spp. with severe combat extremity injury, intensive care, and polymicrobial wound infection. Surg Infect (Larchmt) 2018; 19(1): 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. U.S. Army Medical Research and Material Command: U.S. Department of Defense Blast Injury Research Program Coordinating Office. Available at https://blastinjuryresearch.amedd.army.mil/; accessed December 4, 2018.
- 50. Tribble DR, Lewandowski LR, Potter BK, et al. : Osteomyelitis risk factors related to combat trauma open tibia fractures: a case-control analysis. J Orthop Trauma 2018; 32(9): e344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Office of Research & Development : VA/DoD Collaboration Guidebook for Healthcare Research 2013. Available at https://www.med.navy.mil/sites/nmcp/Dept/Shared%20Documents/CID/VA-DoD-Guidebook-2013.pdf; accessed December 4, 2018.
- 52. Weintrob AC, Murray CK, Lloyd B, et al. : Active surveillance for asymptomatic colonization with multidrug-resistant Gram-negative bacilli among injured service members – a three year evaluation. MSMR 2013; 20(8): 17–22. [PMC free article] [PubMed] [Google Scholar]
- 53. Gilbert LJ, Li P, Murray CK, et al. : Multidrug-resistant gram-negative bacilli colonization risk factors among trauma patients. Diagn Microbiol Infect Dis 2016; 84(4): 358–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Saeed O, Tribble D, Biever K, Kavanaugh M, Crouch H: Infection prevention in combat-related injuries (CPG ID: 24). Joint Trauma System. Available at https://jts.amedd.army.mil/assets/docs/cpgs/JTS_Clinical_Practice_Guidelines_(CPGs)/Infection_Prevention_08_Aug_2016_ID24.pdf; accessed December 5, 2018. [DOI] [PubMed]