Abstract
In a cohort of approximately 200 Bangladeshi men, equally divided into smokers and nonsmokers and equally divided by exposure to high and low levels of drinking water arsenic, we examined ex vivo a series of immune markers and immune function tests in peripheral blood mononuclear cells (PBMC). These immune parameters included PBMC cell surface markers (CSM) for B, T, monocytes, and NK cells, activated T and B cell markers, cytokine production in vitro, and analysis of CD4 subsets (Th1, Th2, Treg, and Th17 cells). We found that the effects of cigarette smoke were quite different than those associated with arsenic or polycyclic aromatic hydrocarbon (PAH)-DNA adducts. Cigarette smoking was associated with a significant increase in the number of PAH-DNA adducts as well as an increase in urinary levels of 1-hydropxypyrene (1-OHP). After correcting for arsenic exposure and PAH-DNA adducts, we found that cigarette smoking was associated with an increase in the percentage of CD19+ B cells, as well as the percentage of activated B cells (CD19+, HLA-DRbright cells) found in PBMC. These findings demonstrate activation of the immune system during chronic exposure to cigarette smoke, which is a known risk factor for autoimmune diseases.
Keywords: human immunotoxicity, arsenic, PAHs, cigarette smoke, B cells, flow cytometry
INTRODUCTION
Despite the huge global health burden associated with arsenic exposure (Argos et al., 2010; Naujokas et al., 2013) and cigarette smoking (Doll et al., 2004), there have been few studies that have examined the effects of these exposures alone or in combination on immune function measured in human PBMC. Cigarette smoking exerts complex effects on the immune system, and is associated with many diseases, including cancer, chronic obstructive pulmonary disease (COPD), heart disease, and others (Qiu et al. 2017). The immune system is likely to play a role in the etiology of many of these diseases.
In our previous studies, arsenic and PAHs were found to exert complex effects on numerous immune parameters measured in PBMC (Lauer et al., 2019; Parvez et al., 2019). In brief, we found that urinary arsenic adjusted for creatinine (UAsCr) was associated with an increase in in vitro production of IL-1β in activated T cells (Parvez et al., 2019) and an increase in CD4+ T cells, particularly in the Th17 subset (Lauer et al., 2019). Using PAH-DNA adducts obtained from PBMC, as a biomarker for exposure to large PAHs, such as benzo(a)pyrene (BaP), we found associations with a decrease in T cell proliferation (TCP) and the in vitro production of numerous cytokines (γIFN, IL-1β, IL-2, IL-10 and IL17A). The overall correlation of TCP and cytokine productions with PAH-DNA adducts was non-monotonic and trended towards suppression, whereas arsenic exposures had more stimulatory effects. Unlike our previous studies of mouse bone marrow (Ezeh et al., 2015) and thymus cells (Xu et al., 2016), we did not find an interaction between PAHs and arsenic (As) in PBMC immune markers.
Using whole blood differential cell counts, a previous study reported an increase in total white blood cells in response to cigarette smoking (Schwartz and Weiss, 1994). However, we are not aware of previous studies that have performed immunophenotyping and immune function tests comparable to those reported in this study. We performed a multivariable analysis of PBMC from smokers and never-smokers for CSM, TCP, in vitro production of cytokines in activated T cell cultures, B and T cell activation markers, and Th cell subsets. Results show that cigarette smoking is associated with complex effects on the immune system that can be detected in human peripheral blood.
METHODS
Recruitment and consent
Participants for this study were recruited under an Institutional Research Board protocol developed and approved at Columbia University and the Bangladesh Medical Research Council. Participants were recruited from the Health Effects Arsenic Longitudinal Study (HEALS) in Araihazar, Bangladesh (Ahsan et al., 2006), and were consented as described by Parvez et al. (2019). Recruitment was based on a two-by-two design with participants being smokers and non-smokers exposed to low and high levels of well water arsenic (WAs) (<50 and > 50 μg/L) in wells located near their homes. For this study, only males, aged 18–75, were recruited due to very low rates of smoking in women. Excluded from the study were men with illnesses related to cardiovascular disease, diabetes, or immune dysfunction and/or taking medications that might impact immune function. There were 317 eligible participants identified, of whom 246 visited the study clinic and completed all study procedures. Of the 246 participants, urine and blood samples were successfully obtained and PBMC isolated from 200. Some samples did not have an adequate number of cells to conduct all of the study assays. Therefore, 197 samples were assayed for TCP and cytokine production and 179 were assayed for CSM and 180 for intracellular markers. Sample analysis for immune function and immunophenotyping was performed at University of New Mexico (UNM) under a protocol approved by the UNM Health Science Center’s Human Rights Protection Office (HRC HRPO).
Collection and cryopreservation of peripheral blood from consented donors
Blood samples were collected at the field clinic in Araihazar, Bangladesh by trained and proficient technicians. Great care was taken in the collection of blood, separation of PBMC, freezing, storage (liquid nitrogen), and shipment at −180°C in dry nitrogen shippers (Cryoport, Irvine, CA) according to detailed procedures (Lauer et al., 2017b). We have found that PBMC collected, shipped, and stored using our procedures are stable in liquid nitrogen for many months. Cell viability was measured on a Nexcelom Cellometer Auto 2000 Cell Viability Counter using acridine orange and propidium iodide (AO/PI; Nexcelom Bioscience, CS2–0106) to identify nucleated and dead cells, respectively. Cell thaws routinely had viabilities 85–90% and we only used cell thaws for immune function testing where viabilities exceeded 80%. We have previously compared fresh PBMC with cryopreserved PBMC and have obtained comparable results for immune function tests (Lauer et al., 2017b). Cytokine concentrations were reported in pg/ml supernatant. For analytical purposes, samples falling below the fit curve or detection range were considered non-detectable and were assigned a value of one-half the lower detection limit for the assay.
T cell proliferation assay
We assessed T cell proliferation utilizing a standard mitogenesis assay previously described (Parvez et al., 2019). Briefly, stimulation of HPBMC was carried out by immobilized anti-human CD3 [clone OKT3 functional grade eBiosciences; 16–0037-85], 0.5 μg/ml anti-hu CD3 (in DPBS), onto micowells in a 96 well plate and anti-human CD28 [clone CD28.2 functional grade eBiosciences, 16–0289-85] solubilized in media at 2 μg/ml per well. Cells were plated at 1×105 cells/well in replicates of six and were incubated for 72 hr in a humidified incubator at 37°C with 5% CO2. A “no mitogen” control was used to evaluate background stimulation using media in place of the mitogen (anti-CD3/anti-CD28). After 72 hours, cultures were pulsed with 1 μCi/well tritiated (3H) thymidine and returned to the incubator for overnight incubation (16–18 hr). Following the overnight incubation, cells were harvested onto angel hair filters using a Brandel 96 well harvester (Gaithersburg, MD). Filters were air-dried for at least 1.5 hr at RT, then placed into scintillation vials containing scintillation fluid. Vials were counted on a Beckman Coulter LS6500 Multipurpose Scintillation Counter for 1.5 min per sample, data is reported as counts per minute (CPM).
Detection of cytokines using a multi-spot assay system
We used the Meso Scale Discovery (MSD) multiplex electrochemiluminescence immunoassay for cytokine analyses as previously described (Parvez et al., 2019). Briefly, cells were plated and stimulated with anti-CD3, immobilized in micro wells of a 96 well plate, and anti-CD28 solubilized in media. Samples were placed in a humidified 37°C, 5% CO2 incubator for 24 hr or 72 hr after which time supernatant samples were collected and stored at −80°C until assayed. A MSD V-Plex Proinflammatory Panel 1 (Human) kit was used to analyze samples diluted 1:400 for IFNγ and IL-2 after 24 hr stimulation and samples diluted 1:100 for IL-1β, IL-4, IL-6, IL-10 and TNFα after 72 hr stimulation. IL-8, diluted 1:400 following a 24 hr stimulation, and IL-17A, diluted 1:100 following a 72 hr stimulation, were analyzed using IL-8 or IL-17A V-Plex, single spot (Human) kits, respectively. All of the dilutions for the cytokine supernatants were carried out with media. Calibrator dilutions (8-point standard curve) were prepared at 1:4 dilutions following kit instructions. Cytokine concentrations were reported in pg/ml supernatant. For analytical purposes, samples falling below the fit curve or detection range were considered non-detectable and were assigned a value of one-half the lower detection limit for the assay.
Detection of cell surface markers by flow cytometry
Fluorescent-labeled antibodies and fixable viability stains (FVS) were obtained from BD Biosciences and used according to the manufacturer’s recommendations to detect, by flow cytometry, phenotypic markers on the surface of previously cryopreserved PBMC. This procedure was previously detailed (Lauer et al., 2017b; Lauer et al., 2019). Briefly, 1×106 cells were washed using a buffer containing Dulbecco’s Phosphate Buffer, 0.2% heat inactivated FBS and 0.09% sodium azide. Cells were stained with a cocktail of CD16, HLA-DR, CD56, CD19, CD8, CD127 CD4, FVS 780, CD45RO, CD3 and CD14 and incubated on ice for 20 min in the dark. Following staining cells were fixed with Cytofix (BD Bioscience; Cat. No. 554655) according to the manufacturer’s directions, then held at 4°C until analyzed on a LSR Fortessa (BD Biosciences; San Jose, CA) flow cytometer with blue, yellow/green, red and violet lasers and BD FACSDiva software v6. Gating and discrimination of cell populations were conducted using FlowJo v10 software. The gating strategy described by Lauer et al. (2017b) was utilized to identifying immune cell subsets: T-cells (CD3+CD19-); Th cells (CD3+CD4+CD8-); cytotoxic T lymphocytes (CTL; CD3+CD4-CD8+); T-memory cells (CD3+CD45RO+); monocytes (classically defined as CD14+CD16-); monocytes (defined as CD14+CD16+); B-cells CD3-CD19+; activated B-cells CD19+HLA-DR+; natural killer cells (NK; CD3-CD56+); NKT cells (CD3+CD56+) and cells expressing the IL-7 receptor alpha (IL-7Rα; CD127+). Color compensation and instrument quality control were run at the onset of each assay as described in detail by Lauer et al. (2019).
Detection of Intracellular markers by flow cytometry
This method, previously described by Lauer et al. (2019), utilized fluorescent-labeled antibodies and fixable viability stains from BD Bioscience to detect intracellular markers to identify Th cell subsets. Briefly, cells were rested overnight, then plated into micro well flat bottom plates containing anti-CD3 (clone OKT3; eBiosciences Cat. No. 16–0037-85) antibody, anti-CD28 antibody was solubilized and added after plating, to stimulate antigen specific T cells to expand and differentiate. Following an overnight incubation, cells were collected, washed and stained for 20 min on ice in the dark with an antibody cocktail, for the surface markers CD127, CD4, CD69, CD45RO, CD3, CD25 and viability marker FVS620. Antibodies were used according to manufacturer’s recommendations, Cells were washed then permeabilized and fixed using a BD Pharmingen Transcription Factor Buffer set (TF; BD Biosciences Cat. No. 562574) following manufacturer’s directions. Following permeabilization and fixation cells were washed and stained on ice in the dark for 45 min for intracellular markers using a cocktail of antibodies for IFNγ, IL-17A, IL-4, and Foxp3. Samples were washed and resuspended in stain buffer and held at 4°C until they were run on the LSR Fortessa described above in our Flow Cytometry Core Facility. Antibody panels, gating strategies and analytical approaches used in this study are detailed in previous publications (Lauer et al., 2017a; Lauer et al., 2019).
Measurement of arsenic exposure
These HEALs cohort participants have detailed records of arsenic exposure over the past 10 years, assessed in blood (BAs) and urine (UAs). Water As (WAs) levels from households were used as an initial screening tool for recruitment of participants. Our analysis showed a high correlation between WAs, used for recruiting participants, and total UAsCr (r = 0.502, p<0.0001). In this study, UAs, measured in spot urine collected concurrently with blood, was compared with immune endpoints as UAs most likely reflected total arsenic exposure at the time of immune measurements. Total UAs was analyzed and quantified as previously described (Lauer et al., 2019; Parvez et al., 2019) using graphite furnace atomic-absorption spectrophotometry (GFAAS). Urinary creatinine was quantified by a colorimetric method based on the Jaffe reaction. All UAs were corrected for creatinine and the result is expressed as ng UAs per mg of creatinine (UAsCr).
Cigarette smoke exposure assessment
Never-smokers were recruited from homes who did not have smokers in the household. For smokers, data on smoking history and habit were collected by in-person interview using a detailed structured questionnaire. For smokers, both filtered and non-filtered cigarettes were counted and participants smoked an average of 19.8 (+ 4) cigarettes/day for an average of 20.0 (+ 3.6) pack years.
PAH-DNA adducts in peripheral blood mononuclear cells
As previously reported (Lauer et al., 2019; Parvez et al., 2019), PAH-DNA adducts were analyzed using a competitive ELISA method detailed by Divi et al. 2002. Briefly, wells in 96 microwell plates were coated with benzo[a]pyrene diol epoxide (BPDE)-I-DNA (5 adducts/103 nucleotides) and a diluted rabbit antiserum, characterized prior to the assay. DNA was isolated from frozen PBMCs following a standard phenol/chloroform/isoamyl alcohol procedure. DNA was isolated, sonicated, denatured, and then added to the microwells. A standard curve to detect DNA adducts (Poirier et al., 1980) consisting of diluted rabbit antiserum, BPDE-I-DNA in carrier nonmodified calf thymus was used to quantitate the adducts. Biotinylated goat anti-rabbit IgG-alkaline phosphatase and avidin-alkaline phosphatase were used to produce a chemiluminescence signal measured at 542 nm on a TR717 Microplate Luminometer (PE Applied Biosystems, Foster City, CA). For analytical purposes, those samples with <15% inhibition were deemed non-detectable and assigned a value of 1 adduct/108 nucleotides. A 5% blinded duplication was carried out using those subjects with the most DNA available and a DNA sample from an animal treated with BP was also assayed with each sample batch. Laboratory technicians were blinded to exposure status for this and all other assays.
15-F2t-isoprostane in urine
As described previously (Wu et al., 2008), urinary 15-F2t-isoprostane (15-F2t-IsoP, formerly called 8-iso-PGF2a or 8-isoprostane) levels were analyzed using competitive enzyme-linked immunosorbent assay kits form Oxford Biomedical Research (Oxford, MI) according to the manufacturer’s directions. According to the manufacturer, the lower limit of reliable detection is 0.2 ng/ml. A quality control sample consisting of urine pooled from five controls was analyzed with each batch of test samples. The coefficient of variation was 19% (n=10). Urinary creatinine was measured with a commercial kit from BioAssay System (Hayward, CA), as directed by the manufacturer.
1-Hydroxypyrene in urine
1-Hydroxypyrene was analyzed by HPLC with fluorescence detection as described previously (Santella et al., 1994) using a Pharmacia LKB (Pharmacia Fine Chemicals, Piscataway, NJ) instrument with a Spectrovision Model FD-200 fluorometer. Excitation was at 242 nm and emission was measured with a 360-nm blocking filter. A linear gradient from 50–100% methanol in water over 20 mm was used for elution of samples on a 5-pm Bondapak C,3 column (Waters). A standard curve was generated by measurement of peak height with serial dilutions (1 .5 pmol-5 nmol) of a 1-hydroxypyrene standard (Molecular Probes, Eugene, OR).
Statistical analysis
In preliminary statistical analyses we calculated summary statistics to describe the characteristics in smokers and non-smokers, and tested differences between the two groups using Wilcoxon rank sum test for quantitative variables and Chi-square test for categorical variables. We used linear models to examine the associations between smoking and three sets of immune parameters (T cell proliferation, immune cell phenotypes and T cell subsets) controlling for age, BMI and UAsCr, with and without further controlling for PAH-DNA adducts. The immune parameters with right skewed distributions were transformed by either logarithmic, square root, or cube root transformations, as in Parvez et al. (2019) and Lauer et al. (2019), to reduce the impact of extreme values and meet assumptions of the linear models. As we have done previously, the models included polynomials of UAsCr and PAH-DNA adducts in logarithmic scale to describe possible non-monotonic associations with the immune parameters. To consistently describe the covariates-adjusted effect of smoking on various immune parameter outcomes, we used change in R-square (ΔR2) or the percent of variation in outcomes attributed to smoking. To adjust for multiple tests on detecting group differences in each set of immune parameters, we used the Benjamini and Hochberg procedure to control for false discovery rate (FDR). All statistical analyses were performed using SAS 9.4.
RESULTS
Characteristics of the study population
A majority of the participants were over 50 years old (66%), and 75% had a BMI less than 25. By design, half of the study participants were active smokers (49%) and half (49%) were consuming water at their residence with As >50 μg/L, the maximum contaminant level for As in Bangladesh. The characteristics of the study population by smoking status are shown in Table 1. Smokers were older than never-smokers (p<.04). BMI was lower for smokers compared to never smokers (p<.0001). As shown in Fig 1, PAH-DNA adducts in PBMC were significantly associated with smoking (p<.001). Urinary levels of 1-hydroxypyrene (1-OHP) was also increased in smokers (Fig 2). 15-F2t-IsoP did not differ between smokers and never-smokers (Table 1). While smoking was not associated with WAs, UAsCr in smokers were higher compared to never-smokers (p <0.03). Also as shown in the previous studies, UAsCr and PAH-DNA adducts were associated with several immune parameters and the associations differed by smoking status; therefore, UAsCr and PAH-DNA adducts were included as covariates in the analyses.
Table 1.
Sample characteristics of smokers and non-smokers
| Non-smokers | Smokers | Difference | |||||
|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median (Range) | n | Mean (SD) | Median (Range) | p-value | |
| Age | 93 | 50.7 (6.3) | 51.0 (36– 65) | 88 | 52.7 (6.2) | 53 (41–65) | 0.04 |
| BMI | 93 | 23.5 (3.9) | 23.6 (14.7– 34.7) | 88 | 20.7 (3.3) | 20.3 (13.7– 29.8) | <.0001 |
| Water As (μg/L) | 93 | 90.4 (126.1) | 29.5 (0.1– 730) | 88 | 97.5 (115.7) | 53.5 (0.1– 476) | 0.34 |
| UAsCr ng/mg | 93 | 151 (187.0) | 76.9 (13.7– 1046) | 88 | 172.8 (172.8) | 122.2 (10– 1116) | 0.03 |
| Urinary 1-OHPμmol/Crmol | 89 | 19.3 (23.5) | 13.7 (0.09 – 190.42) | 82 | 24.5 (21.9) | 19.6 (1.0 – 122.6) | 0.03 |
| PAH-DNA adducts (per/108 nucleotides) | 93 | 1.8 (1.1) | 1.6 (0.5– 5) | 88 | 2.7 (1.6) | 2.1 (0.5– 8) | <.0001 |
| Urinary 15-F2t-isoprostane ng/Cr mg | 94 | 103.7 (145.6) | 33.0 (0.01– 637.3) | 89 | 116.1 (218.5) | 25.5 (0.6 −1310.9) | 0.93 |
| T cell Proliferation (TCP) and Cytokines | |||||||
| Anti-CD3/CD28 proliferation (/10,000) | 93 | 9.7 (3.3) | 9.1 (1.6– 19) | 88 | 10.7 (4.4) | 11.5 (1.8– 20.5) | 0.09 |
| TNF-α | 93 | 4.4 (2.0) | 4.4 (0.1– 11.7) | 88 | 4.9 (2.6) | 4.9 (0.01– 10.7) | 0.20 |
| γlFN | 93 | 10165 (8411) | 8166 (0.2– 37847) | 88 | 9862 (8818) | 8516 (0.2– 59727 | 0.85 |
| IL-1β | 93 | 158.9 (104.2) | 146.3 (9.5– 557) | 88 | 145.5 (105.1) | 145 (2.8– 725.6) | 0.58 |
| IL-2 | 93 | 2664 (1868) | 2095 (0.04– 8490) | 88 | 2964 (1868) | 2949 (0.04– 9241) | 0.14 |
| IL-4 | 93 | 21.5 (12.3) | 18.2 (0.6– 56.6) | 88 | 28.9 (20.1) | 23.5 (0.01– 123.8) | 0.02 |
| IL-6 | 93 | 410 (357) | 313 (26.4– 2106) | 88 | 462.3 (1069.5) | 291.7 (13.7– 9983.6) | 0.46 |
| IL-8 | 93 | 27447 (18218) | 24204 (2733–115200) | 88 | 26717 (16108) | 24136 (4272–110208) | 0.84 |
| IL-10 | 93 | 280 (170) | 255 (6.3– 997) | 88 | 269.8 (173.7) | 258.7 (0.07– 854.9) | 0.76 |
| IL-17A | 93 | 2166 (1962) | 1711 (62.6– 11885) | 88 | 1644 (1459) | 1284 (0.2– 6798) | 0.04 |
| Immune cell phenotypes (CSM) | |||||||
| T cell (CD3) | 92 | 59.8 (9.5) | 60.8 (37.5– 81.4) | 87 | 57.9 (9.3) | 58.5 (33.2– 76.9) | 0.23 |
| Th (CD3CD4) | 92 | 32.8 (8) | 32 (14.2– 58.2) | 87 | 32.7 (6.8) | 32.8 (13.4– 48.7) | 0.73 |
| CTL (CD3CD8CD4-) | 92 | 22.1 (8.4) | 21.5 (6.9– 49.3) | 87 | 20.8 (7) | 19.3 (9.9– 43.6) | 0.28 |
| B cell (CD19) | 92 | 9.9 (3.3) | 9.4 (4.9– 22) | 87 | 14.2 (5.1) | 13.1 (3– 29.5) | <.0001 |
| ActB cell (CD19HLA-DRbright) | 92 | 9.1 (3.4) | 8.4 (3.7– 21.7) | 87 | 13.2 (4.9) | 12.8 (2.9– 28.4) | <.0001 |
| Monocyte (CD14) | 92 | 8.9 (4.2) | 9 (1.1– 19.5) | 87 | 8.4 (4.7) | 7.7 (1.4– 20.7) | 0.28 |
| Monocyte (CD14CD16) | 92 | 1.4 (1) | 1.2 (0.2– 6.3) | 87 | 1.2 (1) | 0.9 (0.1– 5.6) | 0.20 |
| Tmem (CD3CD45RO) | 92 | 32.7 (8) | 32.6 (17– 55.1) | 87 | 34.4 (7.4) | 34.5 (16.1– 48.9) | 0.12 |
| NK (CD56CD3-) | 92 | 13.5 (7.2) | 11.4 (2.3– 34.9) | 87 | 12.1 (6.6) | 10.2 (2.4– 30.4) | 0.15 |
| NKT (CD56CD3) | 92 | 4 (4.1) | 3 (0.2– 33.9) | 87 | 3.2 (2.9) | 2.3 (0.4– 15) | 0.06 |
| IL7α Receptor (CD127) | 92 | 36.4 (7.4) | 35.3 (19.5– 54) | 86 | 37.2 (7.1) | 37.1 (18.9– 54) | 0.25 |
| T cell Subsets (ICS) | |||||||
| Th cell (CD3CD4) | 92 | 39.9 (8.8) | 39.8 (13.6– 59.2) | 88 | 39 (8) | 38.1 (10.9– 54.9) | 0.47 |
| Th1 cell (CD3CD4gIFN) | 92 | 4 (2.6) | 3.6 (0.1–12.2) | 88 | 4.8 (3.6) | 3.9 (0.02– 17.8) | 0.23 |
| Th2 cell (CD3CD4IL4) | 92 | 2.2 (2) | 1.2 (0.5– 9.8) | 88 | 2.3 (2.9) | 1.3 (0.4– 20.3) | 0.88 |
| Treg (CD3CD4Foxp3) | 92 | 14.5 (4.1) | 13.5(5.3– 27) | 88 | 14.7 (5.1) | 14.7 (3.2– 27) | 0.54 |
| Th17 (CD3CD4IL17A) | 92 | 4.1 (3.7) | 3.1 (0.1– 18.4) | 88 | 3.1 (3.4) | 2.4 (0.1– 23.3) | 0.06 |
| Activated T (CD3CD69CD25) | 92 | 45.7(10.5) | 46.6 (4.7– 69.7) | 88 | 42.6 (11) | 43.8 (1– 61.5) | 0.04 |
| Activated Th (CD3CD4CD69CD25) | 92 | 30.9 (8.6) | 30.9 (3.9– 49.1) | 88 | 29.5 (8.5) | 29.7 (0.7– 47.1) | 0.32 |
| Activated Live cells (CD69CD25) | 92 | 53.8 (12.3) | 53.9 (5.2– 75.4) | 88 | 51.2 (12.6) | 53.2 (2.1– 69.4) | 0.19 |
Fig 1.
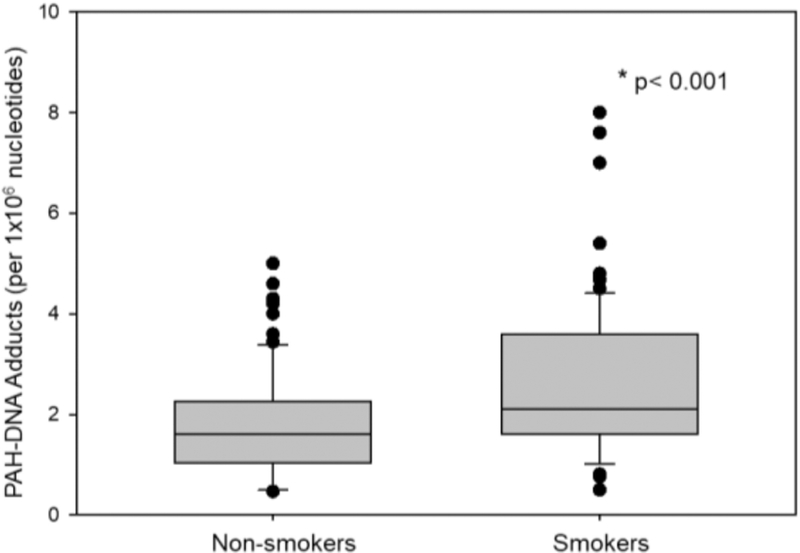
PAH-DNA adducts detected in peripheral blood mononuclear cells in non-smokers compared to that of smokers. Bar indicates median with 10th and 90th percentiles. *p<0.001 Mann-Whitney Rank Sum Test.
Fig 2.
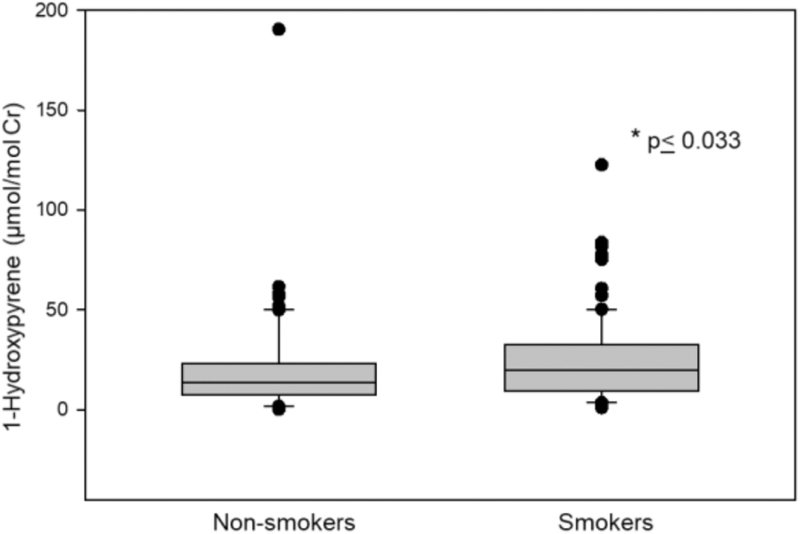
1-Hydroxypyrene adjusted for creatinine detected in the urine of non-smokers compared to that of smokers. Bar indicates median with 10th and 90th percentiles. * p<0.033 Mann-Whitney Rank Sum Test.
Three sets of immune parameters have been examined in our studies. One set, TCP and Cytokines, was obtained from cells stimulated with anti-CD3 and anti-CD28 antibodies for TCP and cytokine production (TNFα, γIFN, IL-1β, IL-2 IL-4, IL-6, IL-8, IL-10 and IL-17A). In Table 1, we found that IL-4 was increased (p<.02) in smokers, whereas IL-17A production was decreased in smokers (p<.04). The second set of immune parameters was obtained by flow cytometry for CSM in viable cells. In this group of CSM, we found that total B cells (CD19+%) were increased in PBMC obtained from smokers (Table 1). B cell activation measured in smokers using HLA-DR was also increased in CD19+ cells (referred to as ActB, CD19+HLA-DRbright). Finally the third set, T cell subsets (Th1, Th2, Th17, Treg, Tact, and Tmem), were measured using intracellular staining (ICS) following ex vivo activation of PBMC with anti-CD3/anti-CD28. We found that the percentage of Activated T cells (CD3+CD69+CD25+) were decreased in smokers (p<.04).
Effects of smoking on TCP and T-cell cytokine production
Summarized results of multivariable analyses for associations between smoking and the immune parameters are shown in Table 2. Separate regression models assessed the associations between smoking and TCP and cytokine production. In Model A, we estimated the effect of smoking on immune TCP and production of cytokines controlling for age, BMI and urinary As. In the extended Model B, we further controlled for PAH-DNA adducts. In Model A, we observed a strong negative association between smoking and IL-17A production (p<0.01). However, after multiple testing adjustment this association was no longer evident (FDR=0.14). When further controlling for PAH in Model B the association between cigarette smoking and IL-17A production was attenuated, suggesting that the association, in part, could be explained by the effect of PAH. We found no associations between smoking and TNFα, γIFN, IL-1β, IL-2, IL-4, IL-6, IL-8 or IL-10.
Table 2.
Covariates-adjusted difference in HPBMC variables between smokers and non-smokers.
| Model A (controlled for Age, BMI, UAsCr) | Model B (controlled for Age, BMI, UAsCr, PAH-DNA adduct) | |||||||
|---|---|---|---|---|---|---|---|---|
| TCP and cytokines | ΔR2 | B (se) | p-value | FDR | ΔR2 | B (se) | p-value | FDR |
| Anti-CD3/CD28 stimulated proliferation (/10,000) | 0.65 | 0.68 (0.62) | 0.28 | 0.55 | 0.85 | 0.81 (0.63) | 0.20 | 0.50 |
| TNF-α (/1000) | 0.25 | 0.25 (0.37) | 0.50 | 0.55 | 0.48 | 0.36 (0.38) | 0.35 | 0.69 |
| (ΓIFN)1/2 | 0.42 | −5.98 (6.8) | 0.38 | 0.55 | 0.04 | 1.96 (6.66) | 0.77 | 0.84 |
| (IL-1β)1/2 | 0.61 | −0.73 (0.67) | 0.27 | 0.55 | 0.08 | −0.29 (0.68) | 0.67 | 0.84 |
| (IL-2)1/2 | 0.33 | 2.97 (2.92) | 0.43 | 0.55 | 1.64 | 5.33 (2.80) | 0.06 | 0.34 |
| (IL-4)1 /2 | 1.27 | 0.39 (0.25) | 0.12 | 0.55 | 1.72 | 0.48 (0.26) | 0.07 | 0.34 |
| log(IL-6) | 0.11 | −0.06 (0.14) | 0.64 | 0.64 | 0.02 | −0.03 (0.14) | 0.84 | 0.84 |
| (IL-8)1/3 | 0.25 | −0.65 (0.94) | 0.49 | 0.55 | 0.12 | −0.48 (0.98) | 0.62 | 0.84 |
| (IL-10)1/2 | 0.95 | −1.16 (0.88) | 0.19 | 0.55 | 0.15 | −0.49 (0.87) | 0.58 | 0.84 |
| (IL-17A)1/3 | 3.35 | −1.48 (0.59) | 0.01 | 0.14 | 0.94 | −0.82 (0.58) | 0.16 | 0.50 |
| Immune cell phenotypes (CSM) | ||||||||
| T cell (CD3) | 1.33 | −2.36 (1.52) | 0.12 | 0.44 | 0.50 | −1.52 (1.51) | 0.32 | 0.58 |
| Th (CD3CD4) | 0.12 | −0.56 (1.17) | 0.63 | 0.63 | 0.0008 | −0.05 (1.13) | 0.97 | 0.97 |
| log(CTL) (CD3CD8CD4-) | 0.28 | −0.04 (0.06) | 0.48 | 0.63 | 0.35 | −0.05 (0.06) | 0.43 | 0.62 |
| log(B cell) (CD19) | 14.32 | 0.32 (0.06) | <.0001 | 0.0005 | 10.86 | 0.29 (0.06) | <.0001 | 0.0005 |
| log(Activated B) (CD19HLADR) | 14.55 | 0.35 (0.06) | <.0001 | 0.0005 | 11.45 | 0.32 (0.06) | <.0001 | 0.0005 |
| Monocyte (CD14) | 0.15 | 0.39 (0.71) | 0.58 | 0.63 | 0.04 | 0.22 (0.71) | 0.75 | 0.83 |
| Log(Monocyte CD14CD16) | 1.07 | −0.16 (0.12) | 0.16 | 0.44 | 1.23 | −0.18 (0.12) | 0.13 | 0.29 |
| Tmem (CD3CD45RO) | 0.43 | 1.10 (1.23) | 0.37 | 0.58 | 1.29 | 2.0 (1.26) | 0.11 | 0.29 |
| log(NK) (CD56CD3-) | 0.83 | −0.11 (0.09) | 0.22 | 0.49 | 1.47 | −0.15 (0.09) | 0.10 | 0.29 |
| log(NKT) (CD56CD3) | 0.52 | −0.14 (0.14) | 0.32 | 0.58 | 0.30 | −0.11 (0.14) | 0.45 | 0.62 |
| IL17α Receptor (CD127) | 0.23 | 0.76 (1.18) | 0.52 | 0.63 | 0.08 | 0.47 (1.23) | 0.70 | 0.83 |
| T cell Subsets | ||||||||
| Th cells (CD3CD4) | 0.26 | −0.94 (1.33) | 0.48 | 0.67 | 0.03 | −0.34 (1.34) | 0.80 | 0.80 |
| (Th1)1/2 (CD3CD4gIFN) | 0.99 | 0.16 (0.12) | 0.18 | 0.49 | 0.13 | 0.06 (0.12) | 0.61 | 0.80 |
| log(Th2) (CD3CD4IL4) | 0.10 | −0.06 (0.13) | 0.66 | 0.67 | 0.04 | −0.04 (0.13) | 0.76 | 0.80 |
| Treg (CD3CD4Foxp3) | 0.10 | 0.32 (0.75) | 0.67 | 0.67 | 0.33 | 0.60 (0.78) | 0.44 | 0.80 |
| (Th17)1/3 (CD3CD4IL17A) | 2.70 | −0.16 (0.07) | 0.02 | 0.19 | 1.19 | −0.11 (0.07) | 0.12 | 0.80 |
| Stimulated T (CD3CD69CD25) | 1.12 | −2.47 (1.72) | 0.15 | 0.49 | 0.70 | −2.05 (1.80) | 0.26 | 0.80 |
| Stimulated Th (CD3CD4CD69CD25) | 0.17 | −0.78 (1.36) | 0.57 | 0.67 | 0.19 | −0.84 (1.42) | 0.55 | 0.80 |
| Stimulated Live cells (CD69CD25) | 0.47 | −1.85 (1.99) | 0.35 | 0.67 | 0.36 | −1.68 (2.08) | 0.42 | 0.80 |
ΔR2 : Percent of variation in outcome attributed to smoking
Associations between smoking and Cell Surface Markers (CSM)
We found a strong positive association between smoking and ActB cells (CD19+HLA-DRbright) in models A and B (p<0.0001). The relationship remained highly significant with adjustment for multiple testing (FDR p=0.0005). Our analysis also revealed a similar association with CD19+ B cell percentage in PBMC (p<0.0001) in models A and B and with adjustment for multiple testing (FDR=0.0005). Importantly, we found a very high ΔR2 percent variation for ActB cells (14.6%) and B cells (14.3%) that are due to the effect of smoking adjusting for age, BMI and UAsCR. Although additional adjustment for PAH-DNA adducts reduced the association with smoking it remained significant. An example of the effects of smoking on CD19+ B cells and ActB (CD19+HLA-DRbright) cells in a representative donor PBMC sample is shown in Fig 3. The gated window for CD19+ B cell in a non-smoker had 9.62% positive cells, whereas a smoker had 15.7% positive cells. For HLA-DRbright cells, two non-smokers had 9.42 and 7.06% positive cells, whereas two smokers had 15.9 and 15.8% positive cells. Although we did not find a statistically significant effect of cigarette smoking on T cell subsets, we include an example of T cell subset analysis for a representative non-smoker and smoker (Supp Fig 1).
Fig 3.
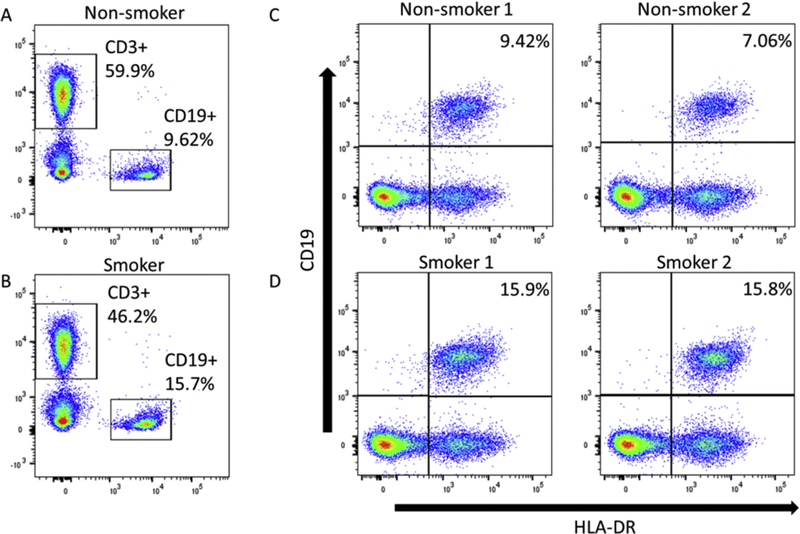
Analysis of B cells and activated B cells by flow cytometry. Top panels are non-smokers and bottom panels are smokers. T (CD3+) and B (CD19+) cell populations were identified for non-smokers (A) and smokers (B), and the CD19+ cells were analyzed for activated B cells (CD19+ HLA-DR+) in two non-smokers (C) and two smokers (D).
DISCUSSION
For many years, our laboratories have been interested in the effects of PAHs on the immune system of mice (Burchiel and Luster, 2001) and humans (Davila et al., 1996). Most PAHs are organic combustion products formed during the burning of fossil fuels (Zedeck, 1980) and cigarettes (USFDA). We recently conducted two epidemiologic studies in a cohort of smokers and never smokers in Bangladesh. We found that PAH-DNA adducts were associated with non-monotonic decreases in TCP and decreased production of cytokines, including γIFN, IL-1β, IL-2, IL-10 and IL17A (Parvez et al., 2019). We also found that UAsCr was associated with a non-monotonic increase in IL-1β in ex vivo activated T cells. In a study of CSM and T cell subsets (Lauer et al., 2019), PAH-DNA adducts were associated with changes in T cells, monocytes and T memory (T mem) cells as well as non-monotonic changes in Th, Th1, Th2 and Th17 (Lauer et al., 2019). Urinary As was associated with an increase in the percentage of Th cells and a dose-dependent change in monocytes, NKT cells and a monocyte subset.
The present study extended these results to focus on the effects of cigarette smoking on immune parameters obtained from PBMC. After controlling for age, BMI, and UAsCr, and PAH-DNA adducts we found that smoking was associated with an robust increase in the percentage of CD19+ B cells and activated B cells (ActB, CD19+HLA-DRbright). B cell activation by antigen receptors is associated with an increase in MHC II molecule expression, including HLA-DR, which are important in antigen presentation and T cell activation (Adler et al., 2017; Kwak et al., 2019). As is discussed below, increased HLA-DR expression is detected in several autoimmune diseases (Forestier et al., 2018). Interestingly, increased HLA-DR expression has been associated with activated memory B cells in systemic sclerosis (Forestier et al., 2018) and in memory B cells is smokers (Brandsma et al., 2009). Therefore, the association of smoking and memory B cells is a future opportunity for investigation.
Cigarette smoke is associated with an increased production of proinflammatory mediators (IL-1β, IL-6, and TNFα) in PBMC obtained from asymptomatic donors (Zeidel et al., 2002). However, McCrea et al. (1994) found that IL-1β and other cytokines detected in BALF macrophages were decreased by smoking, suggesting a suppression of innate immunity. An increase in IL-4 production was associated with PBMCs obtained from smokers (Byron et al., 1994). We did not see changes in other CSM or cytokines in PBMC, perhaps because we controlled for other covariates. Immunologically, cigarette smoking has been also associated with decreased host immunity to TB and impaired vaccine responses (Shaler et al., 2013), and second hand smoke also increases the risk for TB infection (Patra et al., 2015).
Cigarette smoke is a complex mixture of volatile chemicals, semi-volatiles, and non-volatiles that are present in gaseous and particulate phases, each component having the ability to modify the immune system (Stampfli and Anderson, 2009; IARC, 2010). The exposure from each component is determined by the type of cigarette smoked and the manner in which it is smoked. Main stream smoke components are different than side-stream (second-hand) components (USFDA; IARC, 2004). In the present study, we examined the effect of cigarette smoking on PBMC in long-term smokers of both filtered and non-filtered cigarettes. Internal biomarkers of exposure included PBMC PAH-DNA adducts, urinary 1-hydroxypyrene (1-OHP), and urinary 15-F2t-IsoP, the latter as a marker of oxidative stress. These markers are commonly used in cigarette smoking studies (Hecht, 2002; Hansen et al., 2008; Hakim et al., 2012; Li et al., 2016; Ma et al., 2019; Wang et al., 2019). The use of 15-F2t-IsoP as a urinary biomarker of oxidative stress in smokers is well established (Hakim et al., 2012). In our study, we did not find a significant difference between the urinary levels of 15-F2t-IsoP in smokers and nonsmokers.
It is interesting that we did find differences between smokers and non-smokers for 1-OHP and PAH-DNA adducts. The PAH-DNA adduct levels we detected were less than those reported for rickshaw drivers in Dhaka (Rahman et al., 2003), suggesting that urban environments have higher levels of BaP-like PAHs than the rural areas from which our cohort was recruited. However, the urinary 1-OHP levels in nonsmokers and smokers were quite high in our cohort and, in fact, were comparable to those detected in coke furnace foundry workers and coal tar treated psoriasis patients (Santella et al., 1993; Santella et al., 1994) and PAH-exposed workers (Hansen et al., 1995; Strickland and Kang, 1999; Hansen et al., 2008). These results suggest that the background environmental exposures to pyrene-like PAHs and other chemicals is quite high.
The key findings of our study relate to the increase in B cells and B cell activation in PBMC, which suggest that something in cigarette smoke is activating B cells in the blood. Large PAHs like BaP that are detected in our PAH-DNA adduct assay, are not likely responsible for this activation because B cell activation was detected after correction for these components. Stimulation of B cells is not likely explained by the presence of nicotine, since nicotine studies in PBMC in vitro and in smokers reveal immunosuppression, not activation (Sopori, 2002; Razani-Boroujerdi et al., 2007).
It is well known that smoking increases the risk of several autoimmune disease symptoms including Systemic Lupus Erythematous (Parks et al., 2017; Barbhaiya et al., 2018), rheumatoid arthritis (Wasen et al., 2017), and inflammatory bowel diseases (Piovani et al., 2019). Smoking has also been shown to increase CD38+ (a B cell co-receptor) HLA-DR expression, our marker of B cell activation, in HIV patients (Valiathan et al., 2014). In previous studies, we found that cigarette smoke exacerbated autoimmune symptoms in a genetically prone mouse model (Rubin et al., 2005). However, one previous study did not see an increase in CD19+ in PBMC that was associated with cigarette smoking in men and women (Schaberg et al., 1997). This was a smaller study (n=30) than ours with equal numbers of men and women. In addition, the average age of these smokers was younger than the men we examined in this study. Thus, it is important to continue to conduct PBMC studies in smoker cohorts to better understand the immunotoxicity of cigarette smoke in humans, as well as to develop models to better understand potential mechanisms of B cell activation in smokers.
In summary, our results show that men exposed to cigarette smoke have increased numbers of circulating B cells and an increase in their B cell activation markers. It is unclear what the consequences are of this increased B cell activation. It is also unclear whether this cohort is at increased risk for autoimmune diseases. Future studies will examine potential markers of autoimmunity is this population.
Supplementary Material
HIGHLIGHTS.
Cigarette smoking was evaluated for PBMC immunotoxicity in a cohort of 200 men in Bangladesh
Cigarette smoking increased PAH-DNA adducts in PBMC and urinary 1-hydroxypyrene
Cigarette smoking increased the CD19+ B cells in PBMC and activated B cells defined by HLA-DR
The increase in HLA-DR in smokers may be a risk factor for autoimmune diseases
ACKNOWLEDGEMENTS
For technical assistance with the15-F2t-isoprostane, 1-hydroxypyrene and creatinine assays we thank Qiao Wang, Vesna Ilievski, Vesna Slavkovich and Iryna Sirosh.
FUNDING
This work was supported by the National Institutes of Environmental Health Sciences (NIEHS) ViCTER Program R01 ES019968S1, R01-ES023888; UNM Comprehensive Cancer Center Support Grant NCI P30CA118100 and the Flow Cytometry shared resource; Columbia NIEHS Superfund Basic Research Program P42 ES010349, S10 0D016384, P01 ES009089 and P30 CA013696.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
REFERENCES
- Adler LN, Jiang W, Bhamidipati K, Millican M, Macaubas C, Hung SC, Mellins ED, 2017. The Other Function: Class II-Restricted Antigen Presentation by B Cells. Frontiers in immunology 8, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahsan H, Chen Y, Parvez F, Argos M, Hussain AI, Momotaj H, Levy D, van Geen A, Howe G, Graziano J, 2006. Health Effects of Arsenic Longitudinal Study (HEALS): description of a multidisciplinary epidemiologic investigation. J Expo Sci Environ Epidemiol 16, 191–205. [DOI] [PubMed] [Google Scholar]
- Argos M, Kalra T, Rathouz PJ, Chen Y, Pierce B, Parvez F, Islam T, Ahmed A, Rakibuz-Zaman M, Hasan R, Sarwar G, Slavkovich V, van Geen A, Graziano J, Ahsan H, 2010. Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): a prospective cohort study. Lancet 376, 252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbhaiya M, Tedeschi SK, Lu B, Malspeis S, Kreps D, Sparks JA, Karlson EW, Costenbader KH, 2018. Cigarette smoking and the risk of systemic lupus erythematosus, overall and by anti-double stranded DNA antibody subtype, in the Nurses’ Health Study cohorts. Annals of the rheumatic diseases 77, 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandsma CA, Hylkema MN, Geerlings M, van Geffen WH, Postma DS, Timens W, Kerstjens HA, 2009. Increased levels of (class switched) memory B cells in peripheral blood of current smokers. Respir Res 10, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchiel SW, Luster MI, 2001. Signaling by environmental polycyclic aromatic hydrocarbons in human lymphocytes. Clin Immunol 98, 2–10. [DOI] [PubMed] [Google Scholar]
- Byron KA, Varigos GA, Wootton AM, 1994. IL-4 production is increased in cigarette smokers. Clinical and experimental immunology 95, 333–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila DR, Romero DL, Burchiel SW, 1996. Human T cells are highly sensitive to suppression of mitogenesis by polycyclic aromatic hydrocarbons and this effect is differentially reversed by alpha-naphthoflavone. Toxicol Appl Pharmacol 139, 333–341. [DOI] [PubMed] [Google Scholar]
- Doll R, Peto R, Boreham J, Sutherland I, 2004. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ (Clinical research ed.) 328, 1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeh PC, Lauer FT, Liu KJ, Hudson LG, Burchiel SW, 2015. Arsenite Interacts with Dibenzo[def,p]chrysene (DBC) at Low Levels to Suppress Bone Marrow Lymphoid Progenitors in Mice. Biol Trace Elem Res 166, 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forestier A, Guerrier T, Jouvray M, Giovannelli J, Lefevre G, Sobanski V, Hauspie C, Hachulla E, Hatron PY, Zephir H, Vermersch P, Labalette M, Launay D, Dubucquoi S, 2018. Altered B lymphocyte homeostasis and functions in systemic sclerosis. Autoimmun Rev 17, 244–255. [DOI] [PubMed] [Google Scholar]
- Hakim IA, Harris R, Garland L, Cordova CA, Mikhael DM, Sherry Chow HH, 2012. Gender difference in systemic oxidative stress and antioxidant capacity in current and former heavy smokers. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 21, 2193–2200. [DOI] [PubMed] [Google Scholar]
- Hansen AM, Christensen JM, Sherson D, 1995. Estimation of reference values for urinary 1-hydroxypyrene and alpha-naphthol in Danish workers. The Science of the total environment 163, 211–219. [DOI] [PubMed] [Google Scholar]
- Hansen AM, Mathiesen L, Pedersen M, Knudsen LE, 2008. Urinary 1-hydroxypyrene (1-HP) in environmental and occupational studies--a review. International journal of hygiene and environmental health 211, 471–503. [DOI] [PubMed] [Google Scholar]
- Hecht SS, 2002. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis 23, 907–922. [DOI] [PubMed] [Google Scholar]
- IARC, 2004. International Agency for Research on Cancer (IARC). Tobacco smoke and involuntary smoking In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 83 Lyon, France: International Agency for Research on Cancer; 2004., IARC Monograph, pp. [PMC free article] [PubMed] [Google Scholar]
- IARC, 2010. International Agency for Research on Cancer (IARC). Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 92 Lyon, France: International Agency for Research on Cancer; 2010. IARC. [PMC free article] [PubMed] [Google Scholar]
- Kwak K, Akkaya M, Pierce SK, 2019. B cell signaling in context. Nat Immunol 20, 963–969. [DOI] [PubMed] [Google Scholar]
- Lauer FT, Denson JL, Beswick E, Burchiel SW, 2017a. Intracellular Cytokine Detection by Flow Cytometry in Surface Marker-Defined Human Peripheral Blood Mononuclear T Cells. Curr Protoc Toxicol 73, 18 19 11–18 19 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer FT, Denson JL, Burchiel SW, 2017b. Isolation, Cryopreservation, and Immunophenotyping of Human Peripheral Blood Mononuclear Cells. Curr Protoc Toxicol 74, 18 20 11–18 20 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer FT, Parvez F, Factor-Litvak P, Liu X, Santella RM, Islam T, Eunus M, Alam N, Hasan A, Rahman M, Ahsan H, Graziano J, Burchiel SW, 2019. Changes in human peripheral blood mononuclear cell (HPBMC) populations and T-cell subsets associated with arsenic and polycyclic aromatic hydrocarbon exposures in a Bangladesh cohort. PLoS One 14, e0220451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wilker EH, Dorans KS, Rice MB, Schwartz J, Coull BA, Koutrakis P, Gold DR, Keaney JF Jr., Lin H, Vasan RS, Benjamin EJ, Mittleman MA, 2016. Short-Term Exposure to Air Pollution and Biomarkers of Oxidative Stress: The Framingham Heart Study. Journal of the American Heart Association 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Stepanov I, Hecht SS, 2019. Recent Studies on DNA Adducts Resulting from Human Exposure to Tobacco Smoke. Toxics 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea KA, Ensor JE, Nall K, Bleecker ER, Hasday JD, 1994. Altered cytokine regulation in the lungs of cigarette smokers. American journal of respiratory and critical care medicine 150, 696–703. [DOI] [PubMed] [Google Scholar]
- Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, Suk WA, 2013. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect 121, 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks CG, de Souza Espindola Santos A, Barbhaiya M, Costenbader KH, 2017. Understanding the role of environmental factors in the development of systemic lupus erythematosus. Best practice & research. Clinical rheumatology 31, 306–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez F, Lauer FT, Factor-Litvak P, Liu X, Santella RM, Islam T, Eunus M, Alam N, Sarwar G, Rahman M, Ahsan H, Graziano J, Burchiel SW, 2019. Assessment of arsenic and polycyclic aromatic hydrocarbon (PAH) exposures on immune function among males in Bangladesh. PLoS One 14, e0216662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra J, Bhatia M, Suraweera W, Morris SK, Patra C, Gupta PC, Jha P, 2015. Exposure to second-hand smoke and the risk of tuberculosis in children and adults: a systematic review and meta-analysis of 18 observational studies. PLoS medicine 12, e1001835; discussion e1001835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piovani D, Danese S, Peyrin-Biroulet L, Nikolopoulos GK, Lytras T, Bonovas S, 2019. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-analyses. Gastroenterology. [DOI] [PubMed] [Google Scholar]
- Poirier MC, Santella R, Weinstein IB, Grunberger D, Yuspa SH, 1980. Quantitation of benzo(a)pyrene-deoxyguanosine adducts by radioimmunoassay. Cancer Res 40, 412–416. [PubMed] [Google Scholar]
- Rahman MH, Arslan MI, Chen Y, Ali S, Parvin T, Wang LW, Santella RM, Ahsan H, 2003. Polycyclic aromatic hydrocarbon-DNA adducts among rickshaw drivers in Dhaka City, Bangladesh. Int Arch Occup Environ Health 76, 533–538. [DOI] [PubMed] [Google Scholar]
- Razani-Boroujerdi S, Boyd RT, Davila-Garcia MI, Nandi JS, Mishra NC, Singh SP, Pena-Philippides JC, Langley R, Sopori ML, 2007. T cells express alpha7-nicotinic acetylcholine receptor subunits that require a functional TCR and leukocyte-specific protein tyrosine kinase for nicotine-induced Ca2+ response. Journal of immunology (Baltimore, Md. : 1950) 179, 2889–2898. [DOI] [PubMed] [Google Scholar]
- Rubin RL, Hermanson TM, Bedrick EJ, McDonald JD, Burchiel SW, Reed MD, Sibbitt WL Jr., 2005. Effect of cigarette smoke on autoimmunity in murine and human systemic lupus erythematosus. Toxicol Sci 87, 86–96. [DOI] [PubMed] [Google Scholar]
- Santella RM, Hemminki K, Tang DL, Paik M, Ottman R, Young TL, Savela K, Vodickova L, Dickey C, Whyatt R, et al. , 1993. Polycyclic aromatic hydrocarbon-DNA adducts in white blood cells and urinary 1-hydroxypyrene in foundry workers. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2, 59–62. [PubMed] [Google Scholar]
- Santella RM, Nunes MG, Blaskovic R, Perera FP, Tang D, Beachman A, Lin JH, DeLeo VA, 1994. Quantitation of polycyclic aromatic hydrocarbons, 1-hydroxypyrene, and mutagenicity in urine of coal tar-treated psoriasis patients and untreated volunteers. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 3, 137–140. [PubMed] [Google Scholar]
- Schaberg T, Theilacker C, Nitschke OT, Lode H, 1997. Lymphocyte subsets in peripheral blood and smoking habits. Lung 175, 387–394. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Weiss ST, 1994. Cigarette smoking and peripheral blood leukocyte differentials. Ann Epidemiol 4, 236–242. [DOI] [PubMed] [Google Scholar]
- Shaler CR, Horvath CN, McCormick S, Jeyanathan M, Khera A, Zganiacz A, Kasinska J, Stampfli MR, Xing Z, 2013. Continuous and discontinuous cigarette smoke exposure differentially affects protective Th1 immunity against pulmonary tuberculosis. PLoS One 8, e59185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopori M, 2002. Effects of cigarette smoke on the immune system. Nature reviews. Immunology 2, 372–377. [DOI] [PubMed] [Google Scholar]
- Stampfli MR, Anderson GP, 2009. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nature reviews. Immunology 9, 377–384. [DOI] [PubMed] [Google Scholar]
- Strickland P, Kang D, 1999. Urinary 1-hydroxypyrene and other PAH metabolites as biomarkers of exposure to environmental PAH in air particulate matter. Toxicology letters 108, 191–199. [DOI] [PubMed] [Google Scholar]
- USFDA, Chemicals in Cigarettes: From Plant to Product to Puff, https://www.fda.gov/tobacco-products/products-ingredients-components/chemicals-cigarettes-plant-product-puff, pp.
- Valiathan R, Miguez MJ, Patel B, Arheart KL, Asthana D, 2014. Tobacco smoking increases immune activation and impairs T-cell function in HIV infected patients on antiretrovirals: a cross-sectional pilot study. PLoS One 9, e97698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wong LY, Meng L, Pittman EN, Trinidad DA, Hubbard KL, Etheredge A, Del Valle-Pinero AY, Zamoiski R, van Bemmel DM, Borek N, Patel V, Kimmel HL, Conway KP, Lawrence C, Edwards KC, Hyland A, Goniewicz ML, Hatsukami D, Hecht SS, Calafat AM, 2019. Urinary concentrations of monohydroxylated polycyclic aromatic hydrocarbons in adults from the U.S. Population Assessment of Tobacco and Health (PATH) Study Wave 1 (2013–2014). Environment international 123, 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasen C, Turkkila M, Bossios A, Erlandsson M, Andersson KM, Ekerljung L, Malmhall C, Brisslert M, Toyra Silfversward S, Lundback B, Bokarewa MI, 2017. Smoking activates cytotoxic CD8(+) T cells and causes survivin release in rheumatoid arthritis. Journal of autoimmunity 78, 101–110. [DOI] [PubMed] [Google Scholar]
- Wu HC, Wang Q, Yang HI, Ahsan H, Tsai WY, Wang LY, Chen SY, Chen CJ, Santella RM, 2008. Urinary 15-F2t-isoprostane, aflatoxin B1 exposure and hepatitis B virus infection and hepatocellular carcinoma in Taiwan. Carcinogenesis 29, 971–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Lauer FT, Liu KJ, Hudson LG, Burchiel SW, 2016. Editor’s Highlight: Interactive Genotoxicity Induced by Environmentally Relevant Concentrations of Benzo(a)Pyrene Metabolites and Arsenite in Mouse Thymus Cells. Toxicol Sci 154, 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zedeck MS, 1980. Polycyclic aromatic hydrocarbons: a review. Journal of environmental pathology and toxicology 3, 537–567. [PubMed] [Google Scholar]
- Zeidel A, Beilin B, Yardeni I, Mayburd E, Smirnov G, Bessler H, 2002. Immune response in asymptomatic smokers. Acta anaesthesiologica Scandinavica 46, 959–964. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


