Abstract
Leptin is the current treatment for metabolic disorders associated with acquired and congenital generalized lipodystrophy (CGL). Although excess leptin levels have been associated with vascular inflammation and cardiovascular disease in the context of obesity, the effects of chronic leptin treatment on vascular function remain unknown in CGL. Here, we hypothesized that leptin treatment will improve endothelial function via direct vascular mechanisms. We investigated the cardiovascular consequences of leptin deficiency and supplementation in male gBscl2−/− (Berardinelli-Seip 2 gene–deficient) mice—a mouse model of CGL. CGL mice exhibited reduced adipose mass and leptin levels, as well as impaired endothelium-dependent relaxation. Blood vessels from CGL mice had increased NADPH Oxidase 1 (Nox1) expression and reactive oxygen species production, and selective Nox1 inhibition restored endothelial function. Remarkably, chronic and acute leptin supplementation restored endothelial function via a PPARγ-dependent mechanism that decreased Nox1 expression and reactive oxygen species production. Selective ablation of leptin receptors in endothelial cells promoted endothelial dysfunction, which was restored by Nox1 inhibition. Lastly, we confirmed in aortic tissue from older patients undergoing cardiac bypass surgery that acute leptin can promote signaling in human blood vessels. In conclusion, in gBscl2−/− mice, leptin restores endothelial function via peroxisome proliferator activated receptor gamma-dependent decreases in Nox1. Furthermore, we provide the first evidence that vessels from aged patients remain leptin sensitive. These data reveal a new direct role of leptin receptors in the control of vascular homeostasis and present leptin as a potential therapy for the treatment of vascular disease associated with low leptin levels.
Keywords: endothelium, humans, leptin, mice, oxidative stress
In 2014, the US Food and Drug Administration approved the use of the adipocyte-derived hormone, leptin for the treatment of eating and metabolic disorders associated with congenital and acquired generalized lipodystrophy (CGL)—a disease also known as Berardinelli-Seip syndrome (BSCL). Restoration of leptin levels in these patients, who have a near total lack of adipose tissue and, therefore, negligible leptin levels, has proven to be extremely efficient at decreasing appetite, blood glucose, triglycerides, and liver fat content and markedly improved insulin sensitivity, enabling these patients to discontinue or reduce diabetes mellitus medications.1–3
The adipokine leptin is best known as a key regulator of food intake, energy expenditure, insulin sensitivity, and lipid metabolism.4–7 However, it is also a pleiotropic hormone, and leptin receptors are more broadly expressed enabling many additional roles, notably in the regulation of cardiovascular function.8–12 Compelling evidence from our group13–15 and others16,17 has indicated that increased leptin sensitivity or levels in the context of obesity contributes to hypertension and cardiovascular disease via increased sympathetic activity, aldosterone production, and renal sodium retention. Leptin is also a potent modulator of vascular function.11,18–21 However, the effect of leptin on the vascular endothelium, which delicately balances counterregulatory pathways that control vasomotion, vascular cell proliferation, thrombosis, inflammation, and oxidation, remains controversial. Indeed, although a few studies have suggested that leptin promotes endothelial dysfunction via reactive oxygen species (ROS)–dependent mechanisms,22 others have shown that leptin stimulates NO production and protects the vascular endothelium from oxidative stress.18,23–25 An explanation for these seemingly disparate effects has remained elusive and a barrier to the field.
Despite a vast body of literature reporting the deleterious effects of excess leptin on the cardiovascular system, the cardiovascular consequences of a deficiency in leptin and the effects of chronic leptin supplementation on vascular function remain unknown in patients experiencing CGL. Mice deficient in the BSCL2 (Berardinelli-Seip 2) gene (also called Seipin) closely recapitulate the human CGL syndrome associated with BSCL2 mutation. As humans, gBscl2−/− (global Bscl2-deficient) mice present with a near total absence of adipose tissue, organomegaly, insulin resistance, and type 2 diabetes mellitus. The function of BSCL2 is not completely elucidated. However, this endoplasmic reticulum protein has been shown to regulate lipid droplet biogenesis and phospholipid metabolisms, as well as adipocyte differentiation and maintenance.26–30 Herein, we used gBscl2−/− mice to better understand the contribution of leptin to cardiovascular health and more specifically to test the hypothesis that leptin restores cardiovascular function via direct vascular mechanisms in CGL.
Materials and Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Detailed description of the methods used is available in the online-only Data Supplement.
Statistical Analysis
All data are presented as mean±SEM. P<0.05 was considered significant. Differences in means between 2 groups for nonrepeated variables were compared by t test. Differences in means among groups and treatments were compared by 2-way ANOVA with repeated measures, when appropriate. Tukey test was used as the post hoc test (GraphPad).
Results
Leptin Treatment Restores Blood Glucose Levels in Lipodystrophic Mice
The lipodystrophic phenotype in gBscl2−/− mice was confirmed by the reduction in fat mass (gBscl2+/+: 8.6±0.3 versus gBscl2−/−: *2.4±0.2% body fat; *P<0.05), reduced plasma leptin levels, and elevated blood glucose levels (Table S2 in the online-only Data Supplement) compared with gBscl2+/+ mice. Chronic leptin treatment restored blood glucose and circulating leptin levels by decreasing and increasing respective levels in gBscl2−/− mice. Neither plasma cholesterol nor triglyceride levels were affected by CGL or leptin treatment (Table S2).
Lipodystrophy Impairs Endothelial Function via Mechanisms Independent of Hyperglycemia
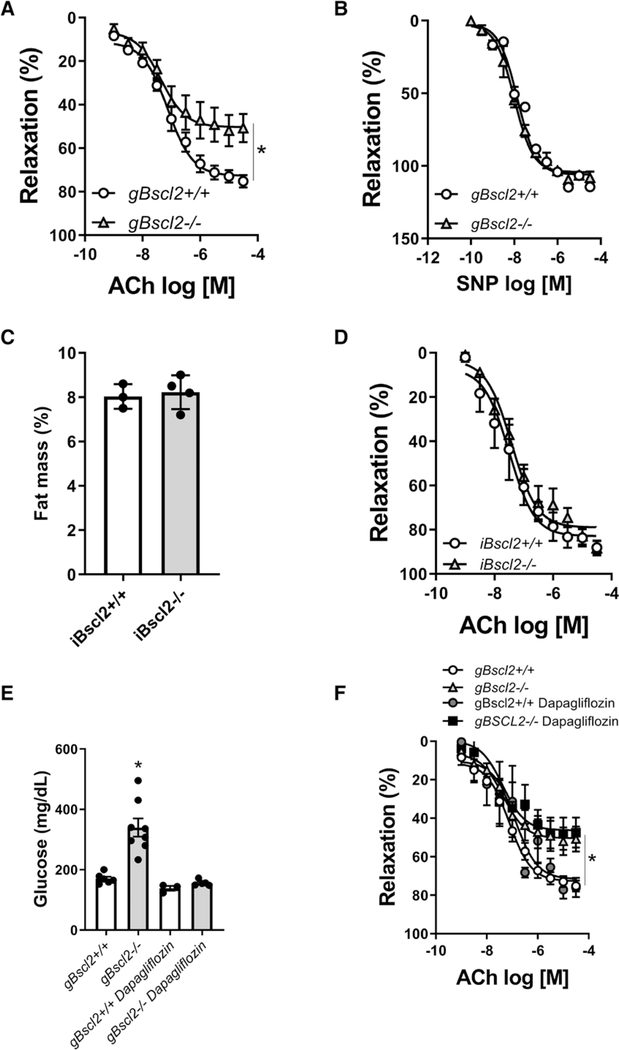
The effects of CGL on vascular function were first investigated by conducting concentration response curves to acetylcholine (ACh) and sodium nitroprusside in aortic rings from gBscl2−/− and gBscl2+/+ mice. gBscl2−/− mice had a marked reduction in ACh-mediated relaxation (Figure 1A) compared with control mice with no differences in sodium nitroprusside-induced vasodilatation (Figure 1B), which together reflect impaired endothelium-dependent relaxation. To determine whether Bscl2 deletion per se is responsible for endothelial dysfunction, concentration response curves to ACh were conducted in iBscl2−/− (inducible Bscl2 KO) mice a week after the last tamoxifen injection. iBscl2−/− mice exhibited significant decreases in Bscl2 transcript expression in aorta and adipose tissue (Figure S1A and S1B) but maintained fat mass (Figure 1C). Endothelium-dependent relaxation in iBscl2−/− mice (Figure 1D) was not impaired which suggests that 1 week of Bscl2 deficiency in aorta and adipose tissue is insufficient to cause endothelial dysfunction and strongly support a role for fat mass reduction in endothelial dysfunction. To investigate the potential role of hyperglycemia in lipodystrophy-associated endothelial dysfunction, gBscl2−/− mice were treated with the SGLT2 (sodium glucose cotransporter 2) inhibitor dapagliflozin. Dapagliflozin restored blood glucose levels in gBscl2−/− mice (Figure 1E) in a manner similar to leptin (Table S2); however, it failed to restore endothelial function (Figure 1F) excluding hyperglycemia as a cause of endothelial dysfunction in CGL mice.
Figure 1.
Congenital lipodystrophy induces endothelial dysfunction independent of hyperglycemia and acute loss of Bscl2 (Berardinelli-Seip 2). Concentration response curves (CRCs) to acetylcholine (ACh; A) and sodium nitroprusside (SNP; B) in aortic rings from gBscl2+/+ and gBscl2−/− (Berardinelli-Seip 2 gene–deficient) mice; body fat composition analyzed by nuclear magnetic resonance (C), CRC to ACh in aortic rings from Gt(ROSA)26Sortm1 (cre/tamoxifen inducible estrogen receptor)Tyj/J mice treated with corn oil (iBscl2+/+) or tamoxifen (iBscl2−/− [inducible Bscl2 KO]; D). Mice were treated for 5 consecutive days. Experiments were performed 1 wk after the last injection. Glucose levels (E) and CRC to ACh in aortic rings from gBscl2+/+ and gBscl2−/− mice treated or not with the SGLT2 (sodium glucose cotransporter 2; dapagliflozin, 1 mg/kg per d for 7 d; F). Data are presented as means±SEM. n=4 to 8. *P<0.05 vs gBscl2+/+. t test was used for comparison between 2 groups. Two-way ANOVA test followed by Tukey multiple comparison test was used for comparison between 4 groups.
Leptin Restores Endothelial Function by Attenuating Nox1-Derived ROS Production
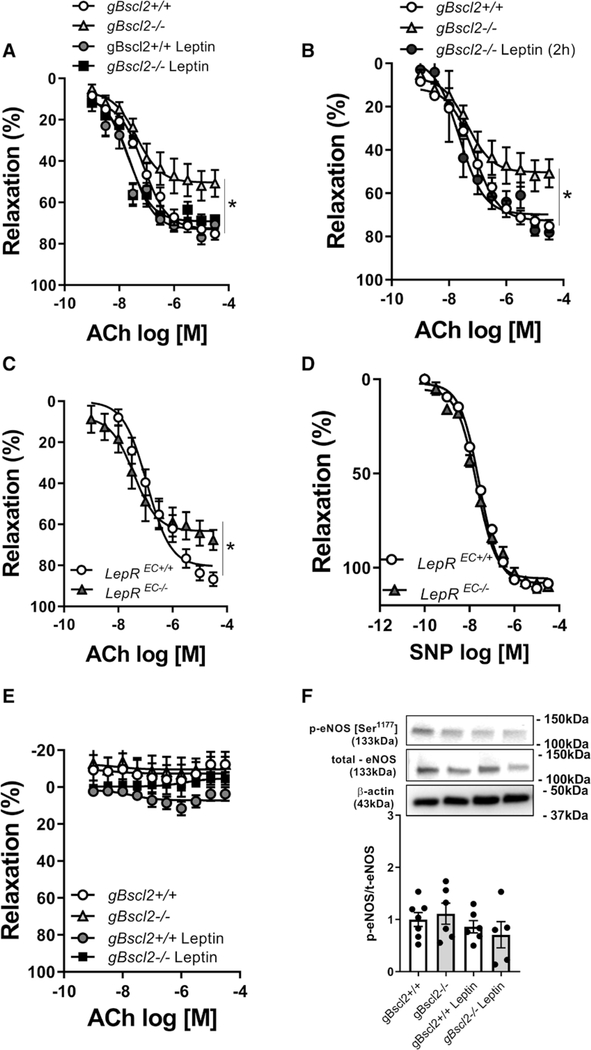
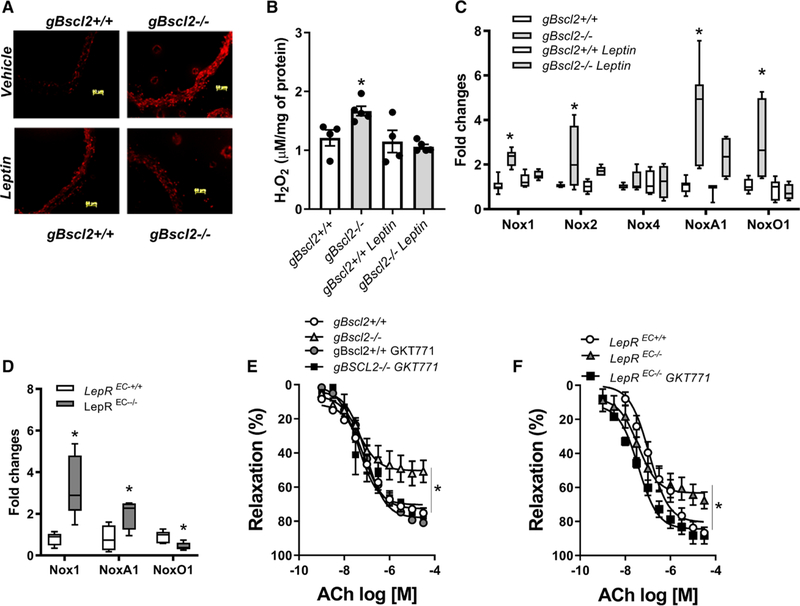
To determine whether leptin deficiency in gBscl2−/− mice is the cause of endothelial dysfunction, vascular studies were repeated 7 days after chronic leptin infusion, as well as in aortic rings exposed to leptin for 2 hours. As reported in Figure 2A and 2B, both chronic and acute leptin treatment fully restored endothelium-dependent relaxation, supporting a direct action of leptin on the vascular endothelium. This was further supported by data showing that endothelial cell–specific deletion of the leptin receptor (Figure S2) impaired ACh- (Figure 2C) but not sodium nitroprusside–mediated relaxation (Figure 2D). Inhibition of nitric oxide (NO) production with N(ω)-nitro-L-arginine methyl ester completely abolished ACh-mediated relaxation in both gBscl2−/− and gBscl2+/+ mice confirming the NO dependence of vasorelaxation in the aorta but also suggesting that the levels of bioavailable NO are compromised in gBscl2−/− (Figure 2E). Neither lipodystrophy nor leptin treatment altered eNOS (endothelial NO synthase) expression or phosphorylation levels (Figure 2F) suggesting that CGL-induced reductions in bioavailable NO are not mediated by decreases in eNOS expression or activity and that leptin restores endothelial function via mechanisms independent of eNOS. Therefore, we next investigated the potential contribution of ROS, which are potent inhibitors of NO signaling.31 CGL increased ROS levels and the expression of ROS-generating enzymes as reflected by increased aortic dihydroethidium staining (Figure 3A), H2O2 levels (Figure 3B), and aortic Nox1, NoxA1, NoxO1, and Nox2 gene expression levels (Figure 3C). No difference was observed for Nox4 (Figure 3C). Chronic leptin treatment (Figure 3C), but not blood glucose restoration with SGLT2 inhibition (Figure S3A), abolished ROS production and completely restored Nox expression levels. Endothelial leptin receptor deficiency led to an increase in NADPH Oxidase 1 (Nox1) and its coactivator NoxA1 in aorta (Figure 3D). The contribution of high ROS levels and specifically Nox1-derived ROS to endothelial dysfunction was demonstrated by the ability of the superoxide mimetic, tempol, catalase, the Nox1–4 inhibitor (GKT137831; Figure S3B through S3D), and the specific Nox1 (GKT771; Figure 3E) inhibitor to improve endothelial function in gBscl2−/− mice. Consistent with these data, selective Nox1 inhibition with GKT771 restored endothelial function in leptin receptorEC−/− mice (Figure 3F), suggesting that the lack of endothelial leptin signaling leads to endothelial dysfunction via Nox1-dependent mechanisms. Selectivity of the Nox1 inhibitor was confirmed by demonstrating that GKT77l inhibits Nox1-derived ROS in human umbilical vascular endothelial cells transduced with Nox1/NoxA1/NoxO1 but not Nox5 transduced human umbilical vascular endothelial cells (Figure S4A and S4B). Although chronic leptin treatment reduced Nox1, Nox1, NoxA1, and NoxO1 expression, acute leptin incubation (2 hours) was without effects on their level of expression (Figure S5).
Figure 2.
Leptin replacement restores endothelial function in congenital generalized lipodystrophy by direct vascular mechanisms. Concentration response curves (CRCs) to acetylcholine (ACh) in aortic rings from gBscl2+/+ and gBscl2−/− (Berardinelli-Seip 2 gene–deficient) mice treated with leptin (10 μg/d for 7 d, osmotic mini-pump; A) or in aortic rings from gBscl2+/+ and gBscl2−/− mice incubated with leptin (10 ug/mL for 2 h; B). CRC to ACh (C) or sodium nitroprusside (SNP; D) in aortic rings from endothelial leptin receptor (LepR) wild-type (LepREC+/+) or deficient (LepREC−/−) mice. CRC to Ach in the presence of N(ω)-nitro-L-arginine methyl ester (100 μmol/L) in aortic rings from gBscl2+/+ and gBscl2−/− mice (E). eNOS (endothelial NO synthase) phosphorylation at Ser1177 site, and total eNOS levels in thoracic aortas from gBscl2+/+ and gBscl2−/− mice treated chronically with leptin or not (F). n=4 to 8. *P<0.05 vs gBscl2+/+; *P<0.05 vs LepREC+/+. t test was used for comparison between 2 groups. One-way ANOVA followed by Tukey multiple comparison test was used for comparison between 3 different groups. Two-way ANOVA test followed by Tukey multiple comparison test was used for comparison between 4 groups.
Figure 3.
Lipodystrophy-associated endothelial dysfunction is mediated by NADPH Oxidase 1-derived reactive oxygen species. Dihydroethidium staining in aortic sections (10 μm; A), H2O2 quantification in aortic segments measured by Amplex Red (B), Nox1 NoxA1, NoxO1, Nox2, Nox4 gene expression in thoracic aorta from gBscl2+/+ and gBscl2−/− (Berardinelli-Seip 2 gene–deficient) mice (C) or from endothelial leptin receptor (LepR) wild-type (LepREC+/+) or deficient (LepREC−/−) mice (D). Concentration response curve (CRC) to acetylcholine (Ach) in the presence of GKT771 (10 μM; E) in aortic rings from gBscl2+/+ and gBscl2−/−. CRC to Ach in presence of GKT771 (10 μM) in aortic rings from LepREC+/+ or LepREC−/− mice (F). Data are presented as means±SEM. Real-time polymerase chain reaction graphs are presented as interleaved from minimal to maximal. n=4 to 6. *P<0.05 vs gBscl2+/+. *P<0.05 vs LepREC+/+. t test was used for comparison between 2 groups. One-way ANOVA followed by Tukey multiple comparison test was used for comparison between 3 different groups. Two-way ANOVA test followed by Tukey multiple comparison test was used for comparison between 4 groups.
Leptin Restores Endothelial Function via PPARγ-Dependent Mechanisms
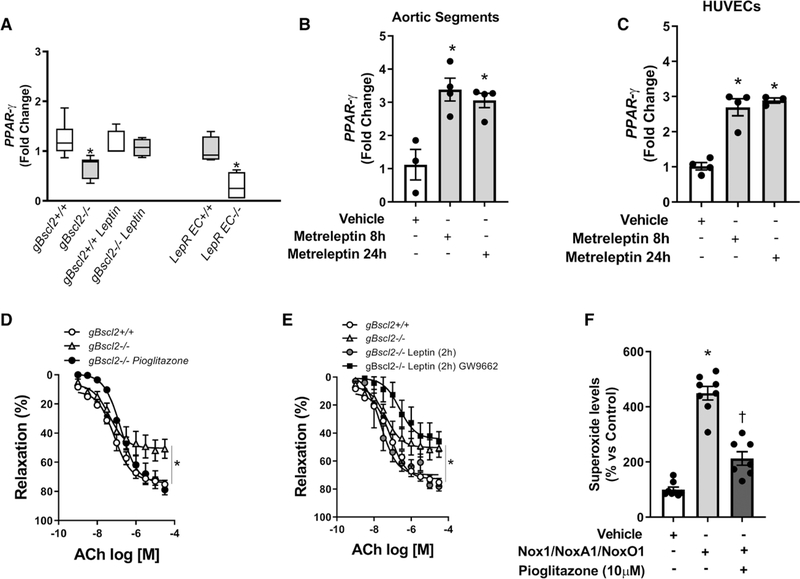
The expression of peroxisome proliferator activated receptor gamma PPARγ), which has been identified as a regulator of vascular oxidative stress, was reduced in aortic tissue (Figure 4A) from gBscl2−/− mice, similarly to its target genes CD36 and CYP27a1 (Figure S6). Reduced endothelial cell expression of PPARγ was also observed in mice with endothelial-specific deletion of leptin receptor (Figure 4A), while chronic leptin treatment restored aortic PPARγ expression in gBscl2−/− mice (Figure 4A) and increased CD36 and CYP27a1 expression (Figure S6). This pathway was conserved in humans as leptin increased PPARγ expression in discarded human aortic segments (Figure 4B) and isolated human umbilical vascular endothelial cells (Figure 4C). The functional importance of PPARγ was shown by the ability of the agonist pioglitazone to restore endothelial function in lipodystrophic mice (Figure 4D), while antagonism of PPARγ with GW9662 blunted the protective effects of leptin on endothelial function in gBscl2−/− mice (Figure 4E) without altering relaxation in gBscl2+/+ mice (Figure S7), suggesting that leptin confers vascular protection via PPARγ-dependent mechanisms. Lastly, PPARγ activation with pioglitazone reduced Nox1-derived ROS in endothelial cells transduced with Nox1/NoxA1/NoxO1 (Figure 4F).
Figure 4.
Leptin increases peroxisome proliferator-activated receptor gamma expression and restores endothelial function in congenital generalized lipodystrophy. PPARγ gene expression in thoracic aorta from gBscl2+/+ or gBscl2−/− (Berardinelli-Seip 2 gene–deficient) mice treated or not with leptin (10 μg/d for 7 d; osmotic mini-pump) and PPARγ gene expression in pulmonary endothelial cells from endothelial leptin receptor (LepR) wild-type (LepREC+/+) or deficient (LepREC−/−) mice (A). PPAR-γ gene expression after metreleptin stimulation (8 or 24 h) in human aortic segments (B) or human umbilical vascular endothelial cells (HUVECs; C); concentration response curves to acetylcholine (ACh) in presence or not of pioglitazone (10 μM; D) and in presence or not of leptin (10 μg/mL) +GW9662 (5 μM; E) in aortic rings from gBscl2+/+ or gBscl2−/− mice; pioglitazone preincubation effects (10 μM/2 h) on superoxide production in HUVEC transduced with Nox1/NoxA1/NoxO1 (F). Data are presented as means±SEM. For PPARγ gene expression in mouse samples, Real-time polymerase chain reaction graph is presented as interleaved from minimal to maximal. n=3 to 6. *P<0.05 vs gBscl2+/+; *P<0.05 vs LepREC+/+; †P<0.05 vs Nox1/NoxA1/NoxO1-transduced HUVEC. t test was used for comparison between 2 groups. One-way ANOVA followed by Tukey multiple comparison test was used for comparison between 3 different groups. Two-way ANOVA test followed by Tukey multiple comparison test was used for comparison between 4 groups.
Leptin Lowers CGL-Associated Vascular and Systemic Inflammation
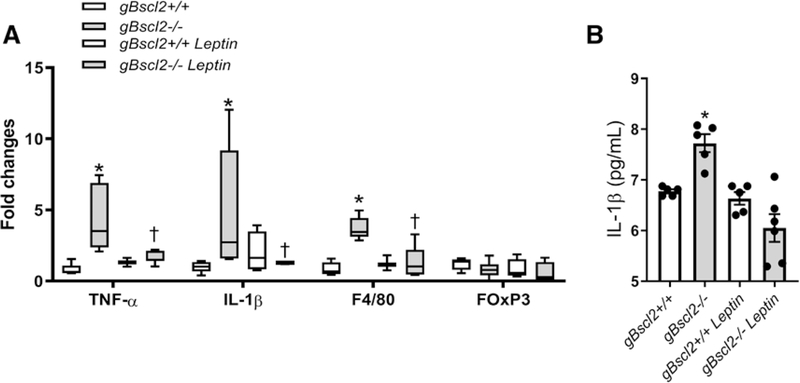
As leptin has been associated with inflammation,32,33 we quantified the expression of markers of macrophage and T-regulatory cell infiltration in aorta, as well as IL (interleukin)-1β plasma levels. These experiments revealed that CGL increased vascular TNF-α (tumor necrosis factor alpha), IL-1β, and F4/80 gene expression but did not alter the expression of the T-regulatory cell marker forkhead box P3 (FOxP3) (Figure 5A). In addition, CGL elevated circulating IL-1β levels (Figure 5B). Remarkably, chronic leptin treatment completely abolished vascular inflammation and reduced IL-1β levels in gBscl2−/− mice (Figure 5A and 5B).
Figure 5.
Leptin reduces inflammation in lipodystrophy. Inflammatory markers in thoracic aorta measured by real-time polymerase chain reaction (RT-PCR; A) and IL (interleukin)-1β plasma levels (B) from gBscl2+/+ or gBscl2−/− (Berardinelli-Seip 2 gene–deficient) mice treated or not with leptin (10 μg/d for 7 d; osmotic mini-pump). Data are presented as means±SEM. RT-PCR graph is presented as interleaved from minimal to maximal. *P<0.05 vs gBscl2+/+; †P<0.05 vs gBscl2−/−. n=4 to 6. Two-way ANOVA test followed by Tukey multiple comparison test was used for comparison between 4 groups. FOxP3 indicates forkhead box P3; IL-1 Interleukine 1B; and TNF-α, tumor necrosis factor-α.
Arteries From Patients Undergoing Cardiac Bypass Surgery Remain Sensitive to Leptin
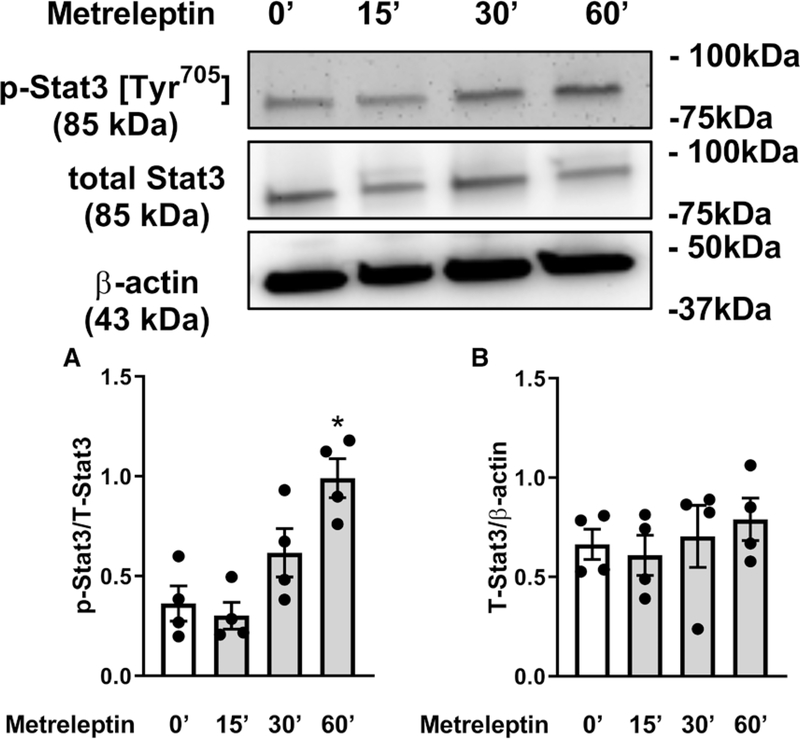
To investigate whether arteries develop leptin resistance, which would limit the beneficial effects of leptin therapy, aortic segments discarded from patients undergoing cardiac bypass surgery (patient information in Table S3) were incubated with leptin for 15 to 60 minutes. Leptin treatment elevated signal transducer and activator of transcription 3 phosphorylation levels in the 60-minute incubation time point (Figure 6) suggesting that arteries from older patients remained leptin sensitive.
Figure 6.
Leptin activates leptin signaling in aortic segments from older patients. Signal transducer and activator of transcription 3 phosphorylation levels at the Tyr705 site (A) and total Stat3 and β-actin ratio (B) in human aortic segments after short-term stimulation with leptin (10 ug/mL; 0′=baseline; 15′–60′ of leptin exposition). Data are presented as means±SEM. *P<0.05 vs 0′. One-way ANOVA followed by Tukey multiple comparison test was used.
Discussion
While leptin is the current therapy for the management of appetite and metabolic dysfunction associated with congenital and acquired generalized lipodystrophy, an underlying concern for the patients receiving leptin is the potential cardiovascular side effects of chronic administration of a hormone that is elevated in obesity and associated with the development of hypertension, vascular inflammation, and cardiovascular disease. Our study is the first to address this concern, and our results show that chronic leptin infusion, in addition to restoring metabolic function, fully restores endothelial function and significantly reduced vascular and systemic inflammation in a mouse model of CGL. These findings imply that in individuals with low leptin levels, chronic leptin treatment is not only safe but beneficial for the cardiovascular system. Moreover, our data indicate that blood vessels do not become leptin resistant and that endothelial leptin signaling has an important role in the maintenance of endothelial function likely via PPARγ-dependent regulation of vascular redox status.
Our studies were focused on the Bscl2-deficient mouse—a model established by Chen et al26 that generally recapitulates the metabolic phenotype of patients experiencing Berardinelli-Seip congenital lipodystrophy.34 Indeed, gBscl2−/− mice exhibit a near total absence of adipose tissue and develop hyperglycemia, insulin resistance, and hepatic steatosis, which are consistent with that observed in human patients.3,28,35,36 Therefore, the gBscl2−/− mouse is an appropriate model of the human disease and useful to investigate the impact of leptin on cardiovascular function.
Hyperglycemia, insulin resistance, and hepatic steatosis are all leading risk factors for cardiovascular disease. However, the cardiovascular phenotype of lipodystrophic patients remains unclear. Indeed, only a few isolated case reports have focused on congenital lipodystrophy and cardiovascular function and reported that atherosclerosis, coronary artery disease, hypertrophic cardiomyopathy, hypertension, and sudden cardiac death appear early in life in lipodystrophic patients.36–42 The present study utilized gBscl2−/− mice to determine the effects of CGL on endothelial function, which is a robust index of cardiovascular health, and also to provide pertinent information on whether leptin therapy provided to correct metabolic disorders associated with CGL has deleterious effects on the cardiovascular function of individuals with lipodystrophy.
We reported that mice with CGL have markedly reduced endothelial function, which is a direct consequence of reduced circulating leptin levels and impaired endothelial leptin signaling. Chronic leptin supplementation was capable of restoring endothelial function, and acute application of leptin to isolated blood vessels demonstrates a direct effect. Conceptually, this is further supported by the compromised endothelial function seen in mice with selective ablation of leptin receptors in endothelial cells. Further arguments in support of reduced endothelial leptin signaling mediating endothelial dysfunction in CGL were provided by data ruling out direct contributions of Bscl2 gene deletion and hyperglycemia on endothelial function. Taken together, these results suggest that endothelial leptin signaling is a key regulator of vascular integrity and that leptin, which has been widely regarded as both a positive20 and negative43 modulator of endothelial function, exerts vasculoprotective effects at physiological levels. Therefore, in CGL patients, leptin therapy is likely to improve cardiovascular function and reduce the risk of cardiovascular disease via direct regulation of endothelial function.
In the context of obesity, high leptin levels and sustained stimulation of hypothalamic leptin receptors lead to the development of a resistance to the metabolic effects of leptin.9 This key feature of obesity, which contributed to the failure of clinical trials testing leptin as a therapy for obesity,41 has raised the question of whether lipodystrophic patients receiving leptin supplementation for life also develop a progressive vascular leptin resistance. This concern is reduced by our data in aortic biopsies discarded from older overweight and obese patients undergoing coronary artery bypass surgery showing that blood vessels remain sensitive to the direct actions of leptin despite having been exposed to high circulating leptin for numerous years. These data suggest that the vascular endothelium and the neurons controlling leptin-mediated sympathoactivation and the adrenal glands12 do not desensitize in response to sustained leptin14 and support the concept of selective resistance previously proposed by Hall et al,9 Haynes et al,10 and Rahmouni et al.17
Previous reports investigating the contribution of leptin to endothelial function have reported conflicting results. While several studies have shown that leptin relaxes conduit and resistance arteries and increases NO production via Akt-dependent mechanisms,20,21,44 others have presented excess leptin as a major contributor to endothelial dysfunction in cardiovascular diseases.45–47 In contradiction with all these studies, we reported that leptin protects the vascular endothelium through mechanisms that are independent of eNOS levels or phosphorylation (indices of activity). Instead, we found that a reduction and lack of endothelial leptin signaling increase vascular Nox1 expression and conversely that chronic leptin supplementation reduces Nox1 expression, as well as that of its coactivators, NoxO1 and NoxA1. Interestingly, acute leptin supplementation restored endothelial function without reducing Nox1, NoxO1, and NoxA1 expression. These findings suggest that leptin can restore endothelial function via 2 different mechanisms: reduction in Nox1 expression while administered chronically and reduction in Nox1 activity in acute conditions. However, both mechanisms appear to involve PPARγ. Indeed, we showed that acute PPARγ agonism mimicked the effects of leptin and restored endothelial function, whereas acute PPARγ antagonism abolished the protective effects of leptin supplementation without altering endothelial function in control conditions, which may suggest that PPARγ-mediated regulation of endothelial function is leptin specific. In addition, while chronic leptin treatment elevated PPARγ expression back to control levels, endothelial cell–specific deletion of the leptin receptor triggered an Nox1-dependent endothelial dysfunction and reduced PPARγ similar to CGL. Therefore, leptin appears to control both Nox1 expression and activity via PPARγ-dependent mechanisms. These results are in line with previous studies reporting that PPARγ ligands decrease Nox1 and its coactivators subunits p22phox and p47phox expression in endothelial cells and showing that PPARγ activators diminish NADPH oxidase activity in endothelial cell membrane fraction.48,49 However, a limitation of our study is the lack of unambiguous evidence that leptin restores endothelial function through PPARγ-dependent mechanisms and the lack of experiments investigating the direct molecular links between leptin receptor and PPARγ, as well as between PPARγ and Nox1. Although additional studies are required, recent work may suggest the contribution of intermediary factors such as retinol-binding protein 7, an endothelium-specific PPARγ target, or even adiponectin, which has been shown to mediate the protective effects of PPARγ.50,51 Other studies may also involve the reduction in endothelial PPARγ in the metabolic alterations associated with CGL.52
Lastly, in addition to lowering vascular redox status and improving endothelium-dependent relaxation, leptin reduced vascular and systemic inflammation, likely macrophage infiltration, and macrophage-mediated cytokine production. Although in apparent contradiction with the many reports indicating that excess leptin leads to activation of monocytes, T cells, and neutrophils, these novel findings support the potential protective effects of leptin reported in certain severe inflammatory conditions.53 Additional studies are warranted to determine whether these protective effects of leptin on vascular inflammation are also PPARγ-Nox1 dependent.
In conclusion, this study provides the first evidence that lipodystrophy-associated reductions in leptin levels impair endothelial leptin signaling leading to increased Nox1 expression and ROS production, likely via a PPARγ-dependent mechanism that impairs endothelium-dependent relaxation. We have also demonstrated that leptin replacement therapy fully restores endothelial function. Taken together, these results indicate that the leptin replacement therapy provided to control the metabolic alterations associated with lipodystrophy may also be extremely beneficial to cardiovascular health and may limit the progression of cardiovascular disease associated with lipodystrophy.
Perspectives
Overall, our findings provide novel mechanistic insights into the underlying causes of endothelial dysfunction and cardiovascular disease in CGL. In addition, our data present leptin therapy as a possible new avenue for the treatment of vascular diseases developing in conditions associated with reduction in adiposity such as type 1 diabetes mellitus, cachexia, or even acquired lipodystrophy.
Supplementary Material
Novelty and Significance.
What Is New?
Endothelial leptin signaling regulates NO bioavailability via reducing endothelial Nox1 expression.
Endothelial leptin signaling regulates Nox1 expression, which appears to be via PPARγ-dependent mechanisms.
Human arteries remain leptin sensitive with aging and metabolic diseases.
Chronic leptin treatment decreases systemic and vascular inflammation in lipodystrophy.
What Is Relevant?
Leptin replacement therapy fully restores endothelial function in congenital generalized lipodystrophy via endothelium-dependent mechanisms.
Leptin may represent a new avenue for the treatment of vascular diseases in conditions associated with reduced adipose mass.
Summary
We found that leptin replacement therapy reverts endothelial dysfunction in a mouse model of congenital generalized lipodystrophy by upregulating endothelial PPARγ expression and reducing Nox1-derived reactive oxygen species.
Acknowledgments
T. Bruder-Nascimento and E.J. Belin de Chantemèle participated in the conception, design of the work, acquisition of the data, analysis and interpretation of the data, and redaction of the manuscript. W. Chen provided the 2 mouse models of lipodystrophy. S. Kennard, J.L. Faulkner, G. Antonova, S. Haigh, and V.S. Patel participated in the acquisition and the interpretation of the data. D.J.R. Fulton participated in the design of the work and redaction of the manuscript.
Sources of Funding
This work was supported by a postdoctoral fellowship (17POST33410363 to T. Bruder-Nascimento) and an innovation research grant (16IRG27770047) from the American Heart Association, a K99/R00 (1K99HL140139–01A1 to T. Bruder-Nascimento), a R01s (1R01HL130301–01 and 1R01HL147639–01A1 to E.J. Belin de Chantemèle), and an R01 (1R01HL132182–01 to W. Chen) from the National Heart, Lung, and Blood Institute, and start-up funds from Augusta University.
Footnotes
Disclosures
None.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.119.1339.
References
- 1.Mulligan K, Khatami H, Schwarz JM, Sakkas GK, DePaoli AM, Tai VW, Wen MJ, Lee GA, Grunfeld C, Schambelan M. The effects of recombinant human leptin on visceral fat, dyslipidemia, and insulin resistance in patients with human immunodeficiency virus-associated lipoatrophy and hypoleptinemia. J Clin Endocrinol Metab. 2009;94:1137–1144. doi: 10.1210/jc.2008-1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437 [DOI] [PubMed] [Google Scholar]
- 3.Javor ED, Cochran EK, Musso C, Young JR, Depaoli AM, Gorden P. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes. 2005;54:1994–2002. doi: 10.2337/diabetes.54.7.1994 [DOI] [PubMed] [Google Scholar]
- 4.Flier JS. Clinical review 94: what’s in a name? In search of leptin’s physiologic role. J Clin Endocrinol Metab. 1998;83:1407–1413. doi: 10.1210/jcem.83.5.4779 [DOI] [PubMed] [Google Scholar]
- 5.Flier JS, Maratos-Flier E. Leptin’s physiologic role: does the emperor of energy balance have no clothes? Cell Metab. 2017;26:24–26. doi: 10.1016/j.cmet.2017.05.013 [DOI] [PubMed] [Google Scholar]
- 6.Baskin DG, Blevins JE, Schwartz MW. How the brain regulates food intake and body weight: the role of leptin. J Pediatr Endocrinol Metab. 2001;14(suppl 6):1417–1429. [PubMed] [Google Scholar]
- 7.Schwartz MW, Baskin DG. Leptin and the brain: then and now. J Clin Invest. 2013;123:2344–2345. doi: 10.1172/JCI69346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010;285:17271–17276. doi: 10.1074/jbc.R110.113175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall JE, Hildebrandt DA, Kuo J. Obesity hypertension: role of leptin and sympathetic nervous system. Am J Hypertens. 2001;14(6 pt 2):103S–115S. doi: 10.1016/s0895-7061(01)02077-5 [DOI] [PubMed] [Google Scholar]
- 10.Haynes WG, Sivitz WI, Morgan DA, Walsh SA, Mark AL. Sympathetic and cardiorenal actions of leptin. Hypertension. 1997;30(3 pt 2):619–623. doi: 10.1161/01.hyp.30.3.619 [DOI] [PubMed] [Google Scholar]
- 11.Hubert A, Bochenek ML, Schütz E, Gogiraju R, Münzel T, Schäfer K. Selective deletion of leptin signaling in endothelial cells enhances neointima formation and phenocopies the vascular effects of diet-induced obesity in mice. Arterioscler Thromb Vasc Biol. 2017;37:1683–1697. doi: 10.1161/ATVBAHA.117.309798 [DOI] [PubMed] [Google Scholar]
- 12.Huby AC, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, Filosa JA, Belin de Chantemèle EJ. Adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation. 2015;132:2134–2145. doi: 10.1161/CIRCULATIONAHA.115.018226 [DOI] [PubMed] [Google Scholar]
- 13.Belin de Chantemele EJ, Mintz JD, Rainey WE, Stepp DW. Impact of leptin-mediated sympatho-activation on cardiovascular function in obese mice. Hypertension. 2011;58:271–279. doi: 10.1161/HYPERTENSIONAHA.110.168427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belin de Chantemele EJ, Muta K, Mintz J, Tremblay ML, Marrero MB, Fulton DJ, Stepp DW. Protein tyrosine phosphatase 1b, a major regulator of leptin-mediated control of cardiovascular function. Circulation. 2009;120:753–763. doi: 10.1161/CIRCULATIONAHA.109.853077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huby AC, Otvos L Jr, Belin de Chantemele EJ. Leptin induces hypertension and endothelial dysfunction via aldosterone-dependent mechanisms in obese female mice. Hypertension. 2016;67:1020–1028. doi: 10.1161/HYPERTENSIONAHA.115.06642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116:991–1006. doi: 10.1161/CIRCRESAHA.116.305697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahmouni K, Correia ML, Haynes WG, Mark AL. Obesity-associated hypertension: new insights into mechanisms. Hypertension. 2005;45:9–14. doi: 10.1161/01.HYP.0000151325.83008.b4 [DOI] [PubMed] [Google Scholar]
- 18.Artwohl M, Roden M, Hölzenbein T, Freudenthaler A, Waldhäusl W, Baumgartner-Parzer SM. Modulation by leptin of proliferation and apoptosis in vascular endothelial cells. Int J Obes Relat Metab Disord. 2002;26:577–580. [DOI] [PubMed] [Google Scholar]
- 19.Benkhoff S, Loot AE, Pierson I, Sturza A, Kohlstedt K, Fleming I, Shimokawa H, Grisk O, Brandes RP, Schröder K. Leptin potentiates endothelium-dependent relaxation by inducing endothelial expression of neuronal NO synthase. Arterioscler Thromb Vasc Biol. 2012;32:1605–1612. doi: 10.1161/ATVBAHA.112.251140 [DOI] [PubMed] [Google Scholar]
- 20.Lembo G, Vecchione C, Fratta L, Marino G, Trimarco V, d’Amati G, Trimarco B. Leptin induces direct vasodilation through distinct endothelial mechanisms. Diabetes. 2000;49:293–297. doi: 10.2337/diabetes.49.2.293 [DOI] [PubMed] [Google Scholar]
- 21.Vecchione C, Maffei A, Colella S, Aretini A, Poulet R, Frati G, Gentile MT, Fratta L, Trimarco V, Trimarco B, Lembo G. Leptin effect on endothelial nitric oxide is mediated through Akt-endothelial nitric oxide synthase phosphorylation pathway. Diabetes. 2002;51:168–173. doi: 10.2337/diabetes.51.1.168 [DOI] [PubMed] [Google Scholar]
- 22.Bouloumie A, Marumo T, Lafontan M, Busse R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999;13:1231–1238. [PubMed] [Google Scholar]
- 23.Rodríguez A, Fortuño A, Gómez-Ambrosi J, Zalba G, Díez J, Frühbeck G. The inhibitory effect of leptin on angiotensin II-induced vasoconstriction in vascular smooth muscle cells is mediated via a nitric oxide-dependent mechanism. Endocrinology. 2007;148:324–331. doi: 10.1210/en.2006-0940 [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez A, Gómez-Ambrosi J, Catalán V, Fortuño A, Frühbeck G. Leptin inhibits the proliferation of vascular smooth muscle cells induced by angiotensin II through nitric oxide-dependent mechanisms. Mediators Inflamm. 2010;2010:105489. doi: 10.1155/2010/105489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang R, Sikka G, Larson J, Watts VL, Niu X, Ellis CL, Miller KL, Camara A, Reinke C, Savransky V, Polotsky VY, O’Donnell CP, Berkowitz DE, Barouch LA. Restoring leptin signaling reduces hyperlipidemia and improves vascular stiffness induced by chronic intermittent hypoxia. Am J Physiol Heart Circ Physiol. 2011;300:H1467–H1476. doi: 10.1152/ajpheart.00604.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W, Chang B, Saha P, Hartig SM, Li L, Reddy VT, Yang Y, Yechoor V, Mancini MA, Chan L. Berardinelli-Seip congenital lipodystrophy 2/seipin is a cell-autonomous regulator of lipolysis essential for adipocyte differentiation. Mol Cell Biol. 2012;32:1099–1111. doi: 10.1128/MCB.06465-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen W, Yechoor VK, Chang BH, Li MV, March KL, Chan L. The human lipodystrophy gene product Berardinelli-Seip congenital lipodystrophy 2/seipin plays a key role in adipocyte differentiation. Endocrinology. 2009;150:4552–4561. doi: 10.1210/en.2009-0236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friguls B, Coroleu W, del Alcazar R, Hilbert P, Van Maldergem L, Pintos-Morell G. Severe cardiac phenotype of Berardinelli-Seip congenital lipodystrophy in an infant with homozygous E189X BSCL2 mutation. Eur J Med Genet. 2009;52:14–16. doi: 10.1016/j.ejmg.2008.10.006 [DOI] [PubMed] [Google Scholar]
- 29.Magré J, Delépine M, Khallouf E, et al. ; BSCL Working Group. Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat Genet. 2001;28:365–370. doi: 10.1038/ng585 [DOI] [PubMed] [Google Scholar]
- 30.Windpassinger C, Auer-Grumbach M, Irobi J, et al. Heterozygous missense mutations in BSCL2 are associated with distal hereditary motor neuropathy and Silver syndrome. Nat Genet. 2004;36:271–276. doi: 10.1038/ng1313 [DOI] [PubMed] [Google Scholar]
- 31.Zhang Q, Malik P, Pandey D, Gupta S, Jagnandan D, Belin de Chantemele E, Banfi B, Marrero MB, Rudic RD, Stepp DW, Fulton DJ. Paradoxical activation of endothelial nitric oxide synthase by NADPH oxidase. Arterioscler Thromb Vasc Biol. 2008;28:1627–1633. doi: 10.1161/ATVBAHA.108.168278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iikuni N, Lam QL, Lu L, Matarese G, La Cava A. Leptin and inflammation. Curr Immunol Rev. 2008;4:70–79. doi: 10.2174/157339508784325046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koh KK, Park SM, Quon MJ. Leptin and cardiovascular disease: response to therapeutic interventions. Circulation. 2008;117:3238–3249. doi: 10.1161/CIRCULATIONAHA.107.741645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joubert M, Jagu B, Montaigne D, Marechal X, Tesse A, Ayer A, Dollet L, Le May C, Toumaniantz G, Manrique A, Charpentier F, Staels B, Magre J, Cariou B, Prieur X. The sodium-glucose cotransporter 2 inhibitor dapagliflozin prevents cardiomyopathy in a diabetic lipodystrophic mouse model. Diabetes. 2017;66:1030–1040. doi: 10.2337/db16-0733 [DOI] [PubMed] [Google Scholar]
- 35.Brown RJ, Meehan CA, Cochran E, Rother KI, Kleiner DE, Walter M, Gorden P. Effects of metreleptin in pediatric patients with lipodystrophy. J Clin Endocrinol Metab. 2017;102:1511–1519. doi: 10.1210/jc.2016-3628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fiorenza CG, Chou SH, Mantzoros CS. Lipodystrophy: pathophysiology and advances in treatment. Nat Rev Endocrinol. 2011;7:137–150. doi: 10.1038/nrendo.2010.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viégas RF, Diniz RV, Viégas TM, Lira EB, Almeida DR. Cardiac involvement in total generalized lipodystrophy (Berardinelli- Seip syndrome). Arq Bras Cardiol. 2000;75:243–248. doi: 10.1590/s0066-782x2000000900006 [DOI] [PubMed] [Google Scholar]
- 38.Brown RJ, Oral EA, Cochran E, Araújo-Vilar D, Savage DB, Long A, Fine G, Salinardi T, Gorden P. Long-term effectiveness and safety of metreleptin in the treatment of patients with generalized lipodystrophy. Endocrine. 2018;60:479–489. doi: 10.1007/s12020-018-1589-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown RJ, Valencia A, Startzell M, et al. Metreleptin-mediated improvements in insulin sensitivity are independent of food intake in humans with lipodystrophy. J Clin Invest. 2018;128:3504–3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan JL, Lutz K, Cochran E, Huang W, Peters Y, Weyer C, Gorden P. Clinical effects of long-term metreleptin treatment in patients with lipodystrophy. Endocr Pract. 2011;17:922–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilsey J, Zolotukhin S, Prima V, Scarpace PJ. Central leptin gene therapy fails to overcome leptin resistance associated with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1011–1020. [DOI] [PubMed] [Google Scholar]
- 42.Sanon VP, Handelsman Y, Pham SV, Chilton R. Cardiac manifestations of congenital generalized lipodystrophy. Clin Diabetes. 2016;34:181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tune JD, Considine RV. Effects of leptin on cardiovascular physiology. J Am Soc Hypertens. 2007;1:231–241. doi: 10.1016/j.jash.2007.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohammed MM, Myers DS, Sofola OA, Hainsworth R, Drinkhill MJ. Vasodilator effects of leptin on canine isolated mesenteric arteries and veins. Clin Exp Pharmacol Physiol. 2007;34:771–774. doi: 10.1111/j.1440-1681.2007.04648.x [DOI] [PubMed] [Google Scholar]
- 45.Martens FM, Rabelink TJ, op ‘t Roodt J, de Koning EJ, Visseren FL. Tnf-alpha induces endothelial dysfunction in diabetic adults, an effect reversible by the ppar-gamma agonist pioglitazone. Eur Heart J. 2006;27:1605–1609. doi: 10.1093/eurheartj/ehl079 [DOI] [PubMed] [Google Scholar]
- 46.Campia U, Matuskey LA, Panza JA. Peroxisome proliferator-activated receptor-gamma activation with pioglitazone improves endothelium-dependent dilation in nondiabetic patients with major cardiovascular risk factors. Circulation. 2006;113:867–875. doi: 10.1161/CIRCULATIONAHA.105.549618 [DOI] [PubMed] [Google Scholar]
- 47.Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD. Ppar(gamma) agonist rosiglitazone improves vascular function and lowers blood pressure in hypertensive transgenic mice. Hypertension. 2004;43:661–666. doi: 10.1161/01.HYP.0000116303.71408.c2 [DOI] [PubMed] [Google Scholar]
- 48.Hwang J, Kleinhenz DJ, Lassegue B, Griendling KK, Dikalov S, Hart CM. Peroxisome proliferator-activated receptor-gamma ligands regulate endothelial membrane superoxide production. Am J Physiol Cell Physiol. 2005;288:C899–C905. doi: 10.1152/ajpcell.00474.2004 [DOI] [PubMed] [Google Scholar]
- 49.Inoue I, Goto S, Matsunaga T, Nakajima T, Awata T, Hokari S, Komoda T, Katayama S. The ligands/activators for peroxisome proliferator-activated receptor alpha (PPARalpha) and PPARgamma increase Cu2+,Zn2+-superoxide dismutase and decrease p22phox message expressions in primary endothelial cells. Metabolism. 2001;50:3–11. doi: 10.1053/meta.2001.19415 [DOI] [PubMed] [Google Scholar]
- 50.Wong WT, Tian XY, Xu A, Yu J, Lau CW, Hoo RL, Wang Y, Lee VW, Lam KS, Vanhoutte PM, Huang Y. Adiponectin is required for ppargamma-mediated improvement of endothelial function in diabetic mice. Cell Metab. 2011;14:104–115. doi: 10.1016/j.cmet.2011.05.009 [DOI] [PubMed] [Google Scholar]
- 51.Hu C, Keen HL, Lu KT, Liu X, Wu J, Davis DR, Ibeawuchi SC, Vogel S, Quelle FW, Sigmund CD. Retinol-binding protein 7 is an endothelium-specific ppargamma cofactor mediating an antioxidant response through adiponectin. JCI Insight. 2017;2:e91738. doi: 10.1172/jci.insight.91738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanda T, Brown JD, Orasanu G, Vogel S, Gonzalez FJ, Sartoretto J, Michel T, Plutzky J. PPARgamma in the endothelium regulates metabolic responses to high-fat diet in mice. J Clin Invest. 2009;119:110–124. doi: 10.1172/JCI36233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guzik TJ, Mangalat D, Korbut R. Adipocytokines - novel link between inflammation and vascular function? J Physiol Pharmacol. 2006;57:505–528. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.