Abstract
BACKGROUND.
Serotonin transporter (5-HTT) binding and polyunsaturated fatty acids (PUFAs) are implicated in major depressive disorder (MDD). Links between the two systems in animal models have not been investigated in humans.
METHODS.
Using positron emission tomography (PET) and [11C]DASB, we studied relationships between 5-HTT binding potential and plasma levels of PUFAs docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), and arachidonic acid (AA) in medication-free MDD patients (n=21). PUFAs were quantified using transesterification and gas chromatography. Binding potential BPP, and alternative outcome measures BPF and BPND, were determined for [11C]DASB in six a priori brain regions of interest (ROIs) using likelihood estimation in graphical analysis (LEGA) to calculate radioligand total distribution volume (VT), and a validated hybrid deconvolution approach (HYDECA) that estimates radioligand non-displaceable distribution volume (VND) without a reference region. Linear mixed models used PUFA levels as predictors and binding potential measures as outcomes across the specified ROIs; age and sex as fixed effects; and subject as random effect to account for across-region binding correlations. As nonlinear relationships were observed, a quadratic term was added to final models.
RESULTS.
AA predicted both 5-HTT BPP and depression severity nonlinearly, described by an inverted U-shaped curve. 5-HTT binding potential mediated the relationship between AA and depression severity.
LIMITATIONS.
Given the small sample and multiple comparisons, results require replication.
CONCLUSIONS.
Our findings suggest that AA status may impact depression pathophysiology through effects on serotonin transport. Future studies should examine whether these relationships explain therapeutic effects of PUFAs in the treatment of MDD.
Keywords: Depression, polyunsaturated fatty acids, arachidonic acid, PET, [11C]DASB, serotonin transporter
Introduction
Major depressive disorder (MDD) is a leading cause of disability worldwide, affecting 300 million people (World Health Organization, 2016). The exact pathogenesis of MDD is unknown, but both altered levels of polyunsaturated fatty acids (PUFAs) (Lin et al., 2010) and serotonergic system abnormalities (Selvaraj et al., 2011) are potential contributors.
PUFAs are long-chain lipids that are ubiquitous in the phospholipid bilayers of human cell membranes (Benatti et al., 2004), where they are important both structurally and functionally, affecting physicochemical cell membrane properties, serving as second messengers, and generating metabolites with key roles in inflammatory cascades (Liu et al., 2015). PUFAs are essential dietary components that play a critical role in brain development and health (Spector, 1999). Major PUFA species include n-3 (omega-3) PUFAs docosahexaenoic acid (DHA, 22:6n-3) and eicosapentaenoic acid (EPA, 20:5n-3), and the n-6 PUFA arachidonic acid (AA, 20:4n-6). DHA and EPA occupy somewhat different functional niches in many physiological contexts (Gorjao et al., 2009). In the brain, DHA and AA are found in large quantities (Lin et al., 2010) and are important for brain development and function. EPA’s brain functions are less well understood; EPA is much less abundant in the brain, but exhibits rapid turnover and metabolism through beta-oxidation (Chen and Bazinet, 2015). The relative peripheral levels of PUFA species are associated with neuropsychiatric illnesses, with elevated AA in proportion to DHA and EPA in MDD (Lin et al., 2010) and suicide risk (Huan et al., 2004, Lewis et al., 2011, Sublette et al., 2006). AA is particularly implicated in bipolar disorder, both postmortem (Kim et al., 2009) and in translational studies showing that multiple mood stabilizing medications downregulate AA metabolism (Rapoport, 2014).
We have previously reported lower 5-HTT binding (BPP), using positron emission tomography (PET) and [11C]McN5652, in unmedicated MDD (Parsey et al., 2006a) and bipolar depressed (Oquendo et al., 2007a) groups compared with healthy controls. [11C]McN5652, however, has high non-specific binding, dampening the signal-to-noise ratio, and lacks a measurable plasma free fraction (fP). The PET radioligand [11C]DASB does not have these shortcomings and thus is a superior alternative for imaging 5-HTT (Frankle et al., 2004). Using [11C]DASB, Selvaraj et al. (Selvaraj et al., 2011) also found lower 5-HTT binding (BPP) in MDD patients. We have reported lower [11C]DASB binding (VT/fP) in MDD patients with a history of suicide attempt (Miller et al., 2013), and lower binding potentials (BPF, BPP, and BPND, using a suboptimal reference region) in bipolar depression (Miller et al., 2016), considering a priori regions of interest (ROIs), although VT/fP did not differ in that study. (For further discussion about methodological advances in quantification and optimal outcome measures for [11C]DASB see the Methods section.) In studies by other groups, compared with 5-HTT binding in healthy controls, 5-HTT binding in depression has been variously reported as lower (Selvaraj et al., 2011, Parsey et al., 2006a, Newberg et al., 2005, Malison et al., 1998, Joensuu et al., 2007, Oquendo et al., 2007a, Reimold et al., 2008, Staley et al., 2006, Willeit et al., 2000, Lehto et al., 2006, Nye et al., 2013), higher (Boileau et al., 2008, Cannon et al., 2007, Cannon et al., 2006, Ichimiya et al., 2002, Reivich et al., 2004, Dahlstrom et al., 2000), or not different (Meyer et al., 2001, Meyer et al., 2004, Miller et al., 2013). However, the present study did not have a control group so is not directly comparable to these case-control studies.
Of note, while previous work has focused on either PUFAs or the serotonin system as being key factors in MDD, these systems are not independent of one another. Rodent studies indicate a relationship between AA and the serotonin system, including specific interactions with 5-HTT. One study reported that a high omega-6 PUFA diet reduces 5-HTT binding significantly in hippocampus and at a trend level in amygdala and dorsomedial and ventromedial hypothalamic nuclei, compared with high omega-3, low-fat, and saturated fat diets (du Bois et al., 2006). Higher AA brain uptake is also seen in 5-HTT knockout mouse models (Basselin et al., 2009). Together, these studies suggest a bidirectional, inverse AA – 5-HTT relationship. Yet, while we have previously demonstrated associations of plasma phospholipid DHA with the cerebrospinal (CSF) dopaminergic metabolite homovanillic acid in MDD, we did not identify a relationship of DHA with CSF serotonergic metabolite 5-hydroxyindoleacetic acid (Sublette et al., 2014). Nonetheless, relationships of PUFAs with human cerebral 5-HTT binding have never been studied. We therefore sought to investigate relationships between plasma PUFA levels and 5-HTT binding potential in six a priori brain ROIs associated with depression pathophysiology in MDD patients, and possible relationships with depression severity.
Methods and Materials
Sample
Adult MDD patients (n=21) were recruited through advertisement and clinician referrals. Each participant was evaluated by a Masters’-level psychologist and a Board-certified, licensed psychiatrist, and found to have capacity to give written informed consent to participate in the relevant New York State Psychiatric Institute IRB-approved studies, in conformity with US Federal Policy for the Protection of Human Subjects. This study was performed in a subset of MDD patients enrolled in [11C]DASB neuroimaging studies who also gave informed consent to have blood PUFA levels assayed. Other articles based on data from the larger [11C]DASB dataset have been published answering different research questions (Miller et al., 2013, Parsey et al., 2006a, Oquendo et al., 2016, Schneck et al., 2016).
All patients were evaluated diagnostically with the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1994) and underwent a comprehensive psychiatric and medical assessment, physical examination, and screening laboratory tests including urine toxicology. Depression severity was assessed using the 17-item Hamilton Depression Rating Scale (HDRS-17) (Hamilton, 1960).
Inclusion criteria for participants included: 1) current DSM-IV major depressive episode; 2) HDRS-17 ≥16 at screening; 3) age 18 to 65 years; 4) off all drugs or medications that interact with the arachidonic acid pathway or affect the 5-HT system for ≥2 weeks prior to scan, and ≥ 6 weeks for fluoxetine or antipsychotic medications; and 5) no active inflammatory or neurologic illness.
Plasma polyunsaturated fatty acid analysis
Most participants had non-fasting blood drawn for PUFA levels within one (n=13) or two (n=4) weeks preceding their PET scans; the remaining four participants’ blood samples were drawn at 22, 29, 38 and 50 days prior to the scan. Blood samples were collected in tubes containing ethylenediaminetetraacetic acid (EDTA), placed on ice, and centrifuged for ten minutes. Samples were kept in a freezer at −80°C until analyzed. The plasma PUFAs EPA, DHA, and AA were separated and quantified as previously described (Lepage et al., 1989). Briefly, plasma PUFAs were quantified using direct transesterification to produce fatty acid methyl esters (FAMEs). The FAMEs were then separated using gas chromatography. Peaks were identified using standardized retention times and quantified using an internal standard.
Radiochemistry and input function measurement
Preparation of [11C]DASB and measurement of the radioligand’s arterial input function, metabolites, and fP were performed as previously outlined (Belanger et al., 2004, Ogden et al., 2007). The chemical purity of [11C]DASB was ≥95%.
PET protocol
The PET scanning protocol has previously been described (Ogden et al., 2007). Briefly, each participant had a venous and an arterial catheter placed. Following a short transmission scan, the [11C]DASB radioligand was injected intravenously over 30 seconds, and 3-dimensional emission data were collected for 100 minutes with 19 frames of increasing duration using the ECAT HR+ scanner (Siemens/CTI, Knoxville, TN).
Magnetic resonance imaging (MRI)
As previously described (Parsey et al., 2000), MRI images were acquired on either a 1.5T Signa Advantage or a 3T Signa HDx system (General Electric Medical Systems, Milwaukee, WI) for co-registration with PET images and extraction of ROIs.
Image analysis
PET frames were motion-corrected using the FMRIB Software Library (FSL) linear image registration tool (FLIRT; FMRIB Image Analysis Group, Oxford, UK). A mean PET image was determined for each participant, and this was aligned to its corresponding MRI using FLIRT (DeLorenzo et al., 2009). An automated algorithm was used to create masks for each ROI; ROI masks were then used to extract regional time activity curves (TACs) for the PET analysis (DeLorenzo et al., 2011).
Outcome measure estimation
[11C]DASB binding potentials BPP, BPF, and BPND were calculated as BPP = (VT − VND), BPF = (VT − VND)/fP, and BPND = (VT − VND)/VND, respectively, where VT is the radioligand total volume of distribution in the ROI, and VND is the radioligand non-displaceable distribution volume, common to all ROIs within a subject (Innis et al., 2007). VT was obtained using likelihood estimation in graphical analysis (LEGA) (Ogden, 2003, Parsey et al., 2003), considered the quantification approach of choice for [11C]DASB, given its test-retest repeatability (Ogden et al., 2007). For LEGA, the initial time for the linear phase, t*, was set to 25 minutes post injection (Ogden et al., 2007). VND was estimated using a hybrid deconvolution approach (HYDECA) (Zanderigo et al., 2017) that combines model-free deconvolution (Zanderigo et al., 2015) and simultaneous search across regions (Todd Ogden et al., 2015) to estimate VND without relying on any reference region, which is appropriate for [11C]DASB, given the ubiquitous distribution of 5-HTT in the brain. HYDECA tuning parameters β and γ were set to 3.5 and 10, respectively, which are optimal values for [11C]DASB as determined via blocking studies (Zanderigo et al., 2017). Brain tissue TACs were corrected for the contribution of plasma activity, assuming a 5% blood volume in the ROIs (Mintun et al., 1984), before applying LEGA and HYDECA.
As a check on the consistency of our HYDECA results with historically used approaches, we performed regional scatterplots comparing HYDECA with the simplified reference tissue model (SRTM) (Lammertsma and Hume, 1996). Correlating BPND (the only metric possible with SRTM) estimated with the two methods yielded the following Pearson’s correlation coefficients: r=0.404 (amygdala, midbrain), r=0.476 (anterior cingulate), r=0.526 (hippocampus), r=0.710 (putamen), and r=0.806 (thalamus). This is consistent with our previous work comparing HYDECA and SRTM applied to [11C]DASB, in which BPND correlates fairly well but SRTM underestimates the BPND values with respect to those generated by HYDECA (Zanderigo et al., 2018). When the main statistical models were run with SRTM, however, the relationships seen with HYDECA were not detectable with SRTM (data not shown).
Prior to the development and validation of our HYDECA method, in some previous studies we had quantified [11C]DASB binding using VT/fp (Miller et al., 2016, Miller et al., 2013) due to uncertainty about the validity of the cerebellum as a reference region (Parsey et al., 2006b). Now that binding potentials are available as a preferable outcome measure, among the possible in vivo binding potentials, BPF theoretically would be the outcome measure of choice, since it is the closest to Bavail/Kd and thus to the density of available target. However, fP sometimes can add more noise than the variance it explains (Innis et al., 2007). To assess the reliability of fP for [11C]DASB, we performed assessments of this measure in a test-retest healthy volunteer sample (n=11) in which the characteristics of other measures have previously been studied (Ogden et al., 2007). This test-retest analysis returned an intraclass correlation coefficient of only 0.54 (mean percent difference=13.17%; SD=14.81%; CV=112.43%; range=[0 43]%). Therefore our primary outcome measure of choice was BPP; we also report here the analyses using both BPF and BPND, for the interested reader.
Regions of Interest
Consistent with previous studies by our group examining regional 5-HTT binding in depression (Miller et al., 2013, Parsey et al., 2006a), we selected six a priori brain ROIs that have measurable 5-HTT and are associated with depression pathophysiology: amygdala, anterior cingulate cortex, hippocampus, midbrain, putamen, and thalamus.
Statistical Analyses
All analyses were performed in R, version 3.3.0 (http://cran.r-project.org). To mitigate skew, to stabilize variance levels, and to allow for proportionality of fixed effects variables across brain regions, plasma PUFA levels and measures of binding potential were logarithmically (In)-transformed before modeling the data (Emerson and Stoto, 1983, Tukey, 1957). A bootstrap algorithm was used to calculate standard errors for each binding value while controlling for errors in metabolite, plasma and brain data; the errors were then used to weight the data (Ogden and Tarpey, 2006). One subject was found to have an unusually low BPP value in the hippocampal region; thus all subsequent analyses excluded this data point from this subject.
To test relationships between PUFAs and 5-HTT binding potential, linear mixed models were used initially. We examined in separate models each plasma PUFA species (DHA, EPA, and AA) as a predictor variable and BPP across the aggregated 6 specified ROIs as outcome. Region, PUFA levels, age, and sex were treated as fixed effects; random effect of subject was included to account for correlations in binding across regions for each subject. The same analysis procedure was applied to both BPND and BPF data. After finding that among PUFAs, only the model containing AA had a significant effect on 5-HTT binding potential, we also tested for a nonlinear relationship between AA levels and binding potential by adding a quadratic term, given that visual inspection of correlation plots suggested nonlinearity was present.
Since our ultimate interest lies in the clinical significance of relationships between PUFAs and 5-HTT, we next tested whether 5-HTT binding potential mediated a relationship between AA levels and depression severity, measured by the HDRS-17, using mediation analysis based on Baron and Kenny (Baron and Kenny, 1986). Our situation is somewhat different from the straightforward mediation model based on ordinary linear regression models, because we considered binding potential measures in six different ROIs and because a nonlinear relationship was potentially the best fit between the AA levels and binding potential. Therefore, we used a measure of binding potential aggregated across all considered ROIs for each subject as a univariate potential mediator. Specifically, this is the predicted subject-level random effect from the linear mixed model that contains both a linear and a quadratic term of AA level. Apart from these differences, our mediation analysis is the same as that used in (Baron and Kenny, 1986). To rule out the possibility that AA levels obtained more than two weeks distant from the PET scanning had a differential effect on the results, we repeated the significant AA - HDRS-17 and AA - binding potential correlations after removing those four data points. Similarly, we performed post-hoc testing of possible medication effects by removing the four patients who had been most recently taking medications (2 to 6 weeks prior to scanning) and repeating the analyses of AA effects on BPP and on HDRS-17.
Significance was defined as a p-value < 0.05 and all tests were two-sided. Results are presented throughout with no adjustment for multiple comparisons.
Results
Sample
Demographic and clinical characteristics are detailed in Table 1. With regard to history of medication use, all twenty-one patients were medication-free at the time of the scan. Nine patients were medication-naïve; six had no medication exposure within 1 year prior to the PET scan; and two had no medication exposure within 3 months. The remaining four patients had not taken antidepressants within 2 to 3 weeks of their scans: of these, two patients had previously been on selective serotonin reuptake inhibitors (escitalopram and sertraline, respectively) and two had been on other antidepressants (duloxetine plus bupropion, and venlafaxine, respectively).
Table 1.
Demographic and Clinical Characteristics of Study Participants (n=21).
| Characteristic | Number (%) |
|---|---|
| Sex (% male) | 10 (47.6) |
| History of suicide attempt | 3 (14.3) |
| Suicidal ideation present | 14 (66.7) |
| Prior exposure to anti-depressants | 11 (52.4) |
| Prior substance use disorder (alcohol or cannabis) | 3 (14.3) |
| Comorbid anxiety disorder | 10 (47.6) |
| Comorbid dysthymic disorder | 3 (14.3) |
| Ethnicity (%Hispanic) | 7 (33.3) |
| Race: | |
| Asian | 1 ( 4.8) |
| American Indian or Alaskan Native | 1 ( 4.8) |
| Black or African American | 4 (19.0) |
| White | 13 (61.9) |
| More than one race | 2 ( 9.5) |
| Mean ± SD | |
| Age (yrs) | 36.1 ± 11.9 |
| BMI (kg*m−2) | 26.6 ± 7.0 |
| Education (yrs) | 15.3 ± 2.7 |
| Income (US $1000/yr) | 31.1 ± 26.1 |
| Illness duration (yrs) | 14.6 ± 13.8 |
| Number of depressive episodes | 16.4 ± 35.9 |
| Age of onset (yrs) | 21.7 ± 9.0 |
| Length of current episode (wks) | 125.2 ±236.4 |
| HDRS-17 | 19.0 ± 4.6 |
| Plasma PUFAs (μg/ml) | |
| Docosahexaenoic acid (DHA) | 51.5 ± 31.3 |
| Eicosapentaenoic acid (EPA) | 21.9 ± 22.1 |
| Arachidonic acid (AA) | 234.5 ± 36.9 |
Abbreviations: BMI, body-mass index; HDRS-17, 17-item Hamilton Depression Rating Scale; PUFA, polyunsaturated fatty acids.
Relationships between PUFA levels and 5-HTT binding potential
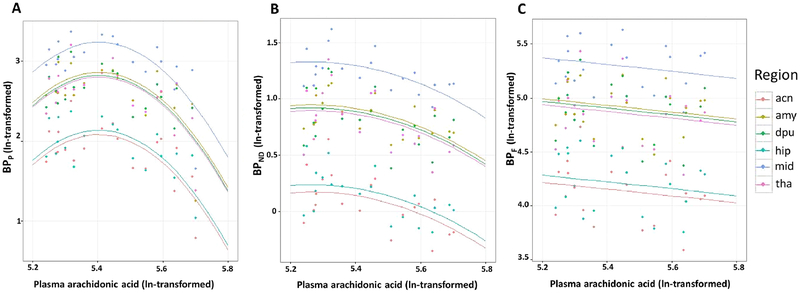
Separate models testing effects of DHA, EPA, and AA revealed no effects of DHA nor EPA on [11C]DASB binding potential (data not shown). However, AA had an effect on BPP (F=9.14; df=1,19; p=0.006) across regions, and given graphical evidence of a nonlinear relationship between AA level and BPP (Figure 1A), we found that an added quadratic term was significant (F=9.62; df=1,19; p=0.006; also see Figure 4, path a). The resulting inverted U-shaped relationship demonstrates that for most of the concentration range of AA, higher BPP correlated with lower AA levels. Exploratory analyses of PUFA effects on BPND and BPF (see Figures 1B and 1C) similarly found that AA, but not DHA or EPA, demonstrated a comparable effect on BPND (F=7.40; df=1, 19; p=0.014) while controlling for region, but had no statistically significant effect on BPF (F=0.51; df=1,19; p=0.484). Neither age nor sex showed a statistically significant effect on any measure of binding potential, so these covariates were removed from the models. When we removed four participants whose PUFA levels were obtained more than 2 weeks prior to scanning, the significance of correlations between AA and HDRS-17, and between AA and binding potential were essentially unchanged (data not shown).
Figure 1. Plasma arachidonic acid levels as a predictor of [11C]DASB binding potentials in six a priori regions of interest.
Arachidonic acid and estimated binding potentials are In-transformed. Data from separate brain regions and their corresponding regression lines are displayed in different colors as shown in the legend. Independent variables are estimated binding potentials: A) BPP = (VT − VND) B) BPND (VT − VND)/VND C) BPF (VT − VND)/fP. Abbreviations: acn, anterior cingulate; amy, amygdala; dpu, dorsal putamen; hip, hippocampus; mid, midbrain; tha, thalamus.
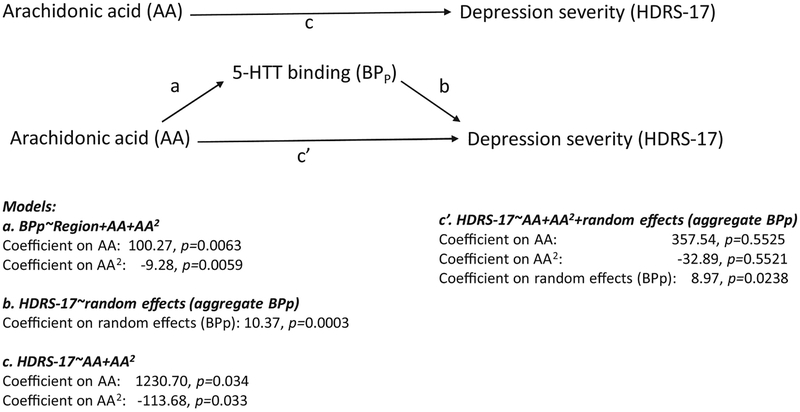
Figure 4. Mediation model for effects of arachidonic acid on depression severity via effects of serotonin transporter (5-HTT) binding potential (BPP).
Note: BPp, AA and AA2 are all In-transformed values.
Mediation analysis: 5-HTT binding potential as a mediator of AA effects on depression
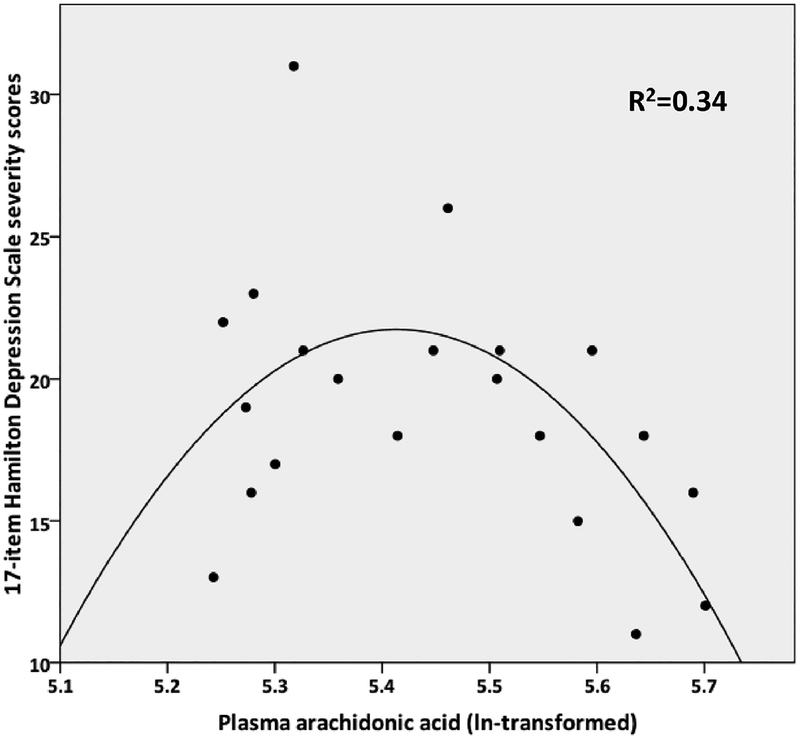
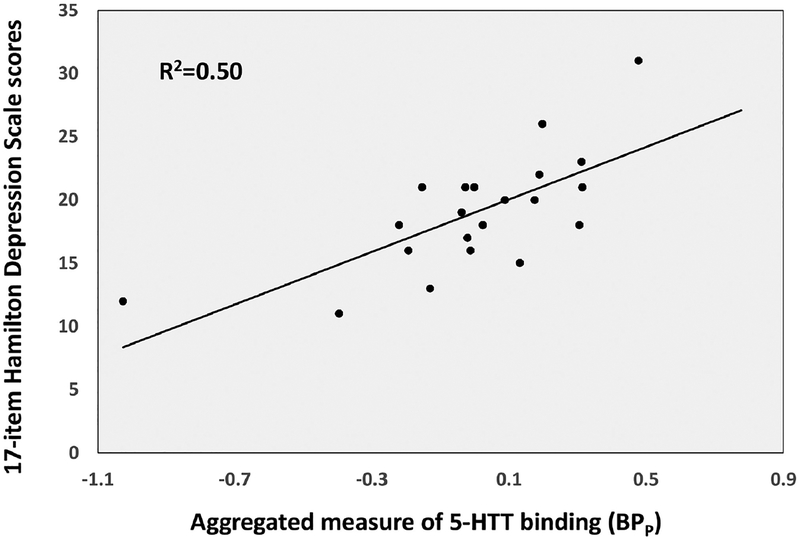
In a simple model for effects of AA and the corresponding quadratic term (AA2) on HDRS-17 severity (Figure 2 and Figure 4, path c), both the linear (F=5.25; df=1,18; p=0.034) and quadratic (F=5.35; df=1,18; p=0.033) term effects were significant. In a simple correlation between 5-HTT binding potential (aggregated across ROIs as described in Materials and Methods) and HDRS-17 severity, 5-HTT positively correlated with depression severity (F=19.16, df=1,19 p=0.0003; Figure 3 and Figure 4, path b). However, when AA and the corresponding quadratic term were included in the model as predictors of HDRS-17 severity, along with the aggregate measure of 5-HTT binding potential as another predictor (random effect), 5-HTT binding was significant as a positive predictor of HDRS-17 score (F=6.16; df=1,17; p=0.024; Figure 4, path c’), while the effects of AA and AA2 were no longer significant (linear: F=0.37; df=1,17; p=0.553; quadratic: F=0.37; df=1,17; p=0.552). These results are consistent with the classic definition of mediation, in which the inclusion of the mediator (in this case, 5-HTT binding potential) in the model partially or completely attenuates the effects of the predictor (AA) on the outcome (HDRS-17). Therefore, our results suggest that 5-HTT binding potential may mediate the effect of AA levels on severity of depression symptoms.
Figure 2.
Correlation between plasma arachidonic acid and depression severity fit to a quadratic function.
Figure 3.
Correlation between 5-HTT binding potential, aggregated across regions of interest, and depression severity.
Effects of medications
When we removed the four participants who had taken medications within 2 to 3 weeks prior to the PET scanning, the quadratic relationship between AA and BPP remained significant (AA p=0.019; AA2 p=0.018), as did the linear relationship between BPP and HDRS-17 (p=0.0004), while the relationship between AA and HDRS-17 remained significant in a linear model (p=0.028), but the quadratic model lost significance to a trend level (AA p=0.105; AA2 p=0.100).
Discussion
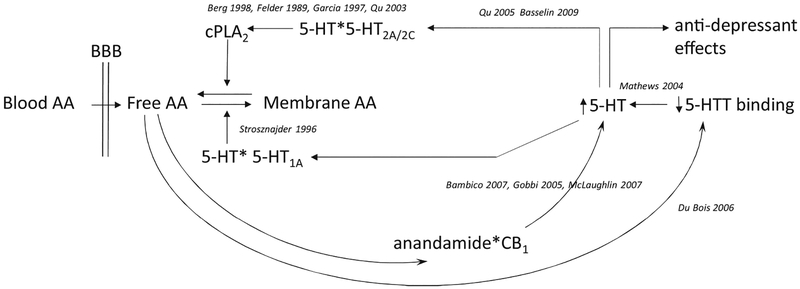
This is the first report that AA, but not DHA or EPA, levels are associated with 5-HTT BPP. Dietary intake is the primary determinant of AA levels, either directly or through intake of precursor shorter-chain molecules such as linoleic acid (18:2) that are converted at variable, generally low rates to AA (reviewed in (Liu et al., 2015)). Based on the mediation results, it appears that AA affects depression severity through effects on 5-HTT binding potential. For this to be true, AA would have to have a biological effect that involves 5-HTT. Rodent (Mathews et al., 2004, Qu et al., 2003, Qu et al., 2005, Dubois et al., 2006, Strosznajder et al., 1996, Basselin et al., 2009, Bambico et al., 2007, Gobbi et al., 2005, McLaughlin et al., 2012) and cell culture (Garcia and Kim, 1997, Berg et al., 1998, Felder et al., 1990) studies suggest possible mechanisms for AA - 5-HTT interactions. These putative pathways are illustrated schematically in Figure 5, and include the following observations: low 5-HTT expression elevates intrasynaptic 5-HT (Mathews et al., 2004), which binds to postsynaptic 5-HT receptors. The 5-HT2A/2C and 5-HT1A receptors have opposite effects on the balance between membrane-bound and unesterified (free) AA, presumably subserving a homeostatic function. Specifically, 5-HT2A/2C stimulates activation of cytosolic phospholipase A2 (cPLA2) (Berg et al., 1998, Qu et al., 2005, Garcia and Kim, 1997, Felder et al., 1990), triggering the release of unesterified AA from membrane phospholipids. Some of the released AA is recycled back into the membrane, a process that is stimulated by 5-HT1A receptors (Strosznajder et al., 1996). Remaining unesterified AA can decrease 5-HTT in certain brain regions (du Bois et al., 2006). AA influences on 5-HTT and depression also may be a function of AA cascade products, anandamide (N-arachidonylethanolamide) and 2-arachidonoylglycerol, binding to cannabinoid (CB1) receptors. CB1 agonists dose-dependently stimulate dorsal raphe serotonergic neurons (Bambico et al., 2007). Serotonergic neurons in dorsal raphe nucleus likewise increase firing in response to inhibition of anandamide breakdown (McLaughlin et al., 2012), which is associated with antidepressant-like effects in rodents and is blocked by CB1 antagonism (Gobbi et al., 2005). Furthermore, CB1 knockout mice exhibit decreased brain 5-HTT binding site density (Burokas et al., 2014), and both 5-HTT (Kalueff et al., 2010) and CB1 (Aso et al., 2008) rodent knockouts exhibit a depressive-like phenotype. Other possible factors regulating 5-HTT binding levels not assessed in this study include the rate of 5-HTT internalization (Rahbek-Clemmensen et al., 2014), governed partly by intrasynaptic 5-HT concentrations (Jorgensen et al., 2014), DNA methylation (Drabe et al., 2017), and gene promoter variants such as HTTLPR (Heils et al., 1996).
Figure 5. Hypothetical schematic of relationships between arachidonic acid and serotonergic systems.
Abbreviations: AA, arachidonic acid; BBB, blood-brain barrier; CB1, cannabinoid receptor 1; cPLA2, cytosolic phospholipase A2; 5-HT, 5-hydroxytryptamine, or serotonin; 5-HT2A/2C and 5-HT1A, serotonin receptor subtypes; 5-HTT, serotonin transporter. For reasons of space, citations in this figure have omitted “et al.”
In contextualizing our finding that 5-HTT binding potential is positively correlated with depression severity, we are aware of five human studies (Boileau et al., 2008, Cannon et al., 2007, Miller et al., 2013, Meyer et al., 2004, Selvaraj et al., 2011) that assessed the correlation of depression severity with [11C]DASB binding, and nine PET and single photon emission computed tomography (SPECT) studies using other ligands (described below). These studies reported a variety of results that could be related to differences in radioligands, binding potential outcome measures, brain regions studied, and/or sample characteristics. Our findings agreed with only one other study, which reported a positive correlation of depression severity with [11C]DASB BPND in patients with Parkinson’s and depression (Boileau et al., 2008). Other studies in MDD relating depression severity to [11C]DASB found inverse correlations with BPND (Cannon et al., 2007) or BPP (Selvaraj et al., 2011), and no correlation with BPND (Meyer et al., 2004) or VT/fP (Miller et al., 2013). In studies with [11C]McN5652, no correlation was seen (Ichimiya et al., 2002, Parsey et al., 2006a). Using SPECT, inverse correlations of binding with depression severity were reported with [123I]-2β-carbomethoxy-3β-(4-iodophenyl)tropane (123Iβ-CIT) at a trend level (p=0.052) (Malison et al., 1998) and significantly (p=0.02) with 123I-ADAM (Newberg et al., 2005) in a very small sample (n=7), but the latter finding was not replicated by the same team in a larger sample (n=20) (Newberg et al., 2012). Other 123I-ADAM (Herold et al., 2006, Catafau et al., 2006, Ho et al., 2013) and 123Iβ-CIT (Joensuu et al., 2007) SPECT studies likewise found no correlation with depression severity.
Some studies report lower binding specifically in depressed suicide attempters (Meyer et al., 2001, Meyer et al., 2004, Miller et al., 2013); however, only three of our patients had a history of suicide attempt, and their data did not fall at extremes of the depression severity or binding potential distributions.
Our finding of an inverted U-shaped function describing the relationship of plasma AA concentration to depression severity adds complexity to previous meta-analytic findings that depression is associated with low DHA, EPA, and total omega-3 PUFA levels but not with differences in AA or total omega-6 PUFAs (Lin et al., 2010). However, some other groups have observed not only lower omega-3 PUFAs but also lower omega-6 PUFAs, including lower AA levels, in MDD patients compared with healthy controls (Maes et al., 1999, Peet et al., 1998).
That AA plasma levels correlated significantly with BPP and BPND but not with BPF, (in which only a trend is seen) likely relates to measurement error in the free fraction assay required for determining fP and thus BPF (Innis et al., 2007).
Prior medication use does not appear to have been a confound in this study, since all patients were medication-free at the time of the scan, and the main findings were only minimally affected when we removed four patients with the most recent medication use (2 to 3 weeks prior to the scan).
Limitations
As this pilot study examined a relatively small sample, testing multiple PUFA species and binding measures, results should be regarded as preliminary. In particular, mediation analyses generally require larger sample sizes. Inadequate numbers of healthy controls with PUFA measurements were available for study; therefore, we cannot comment on whether the AA relationships to [11C]DASB binding potential may be related to the pathogenesis of major depression, although the correlation between binding potential and depression severity suggests pathophysiologic importance. Plasma concentrations of DHA, EPA and AA were assayed because these PUFAs have known clinical associations with brain health and depression. Additional PUFA species, particularly docosapentaenoic acid (DPA), may also have relevance, but we decided a priori to limit the number of PUFA species under study. Total AA levels in plasma measured in this study include AA esterified to phospholipids, cholesteryl esters and triglycerides, as well as nonesterified AA. The plasma AA measure is assumed to be a proxy for brain utilization, as AA crosses the blood-brain barrier in both esterified and unesterified forms (reviewed in (Liu et al., 2015)). Moreover, in animal experiments with radiolabeled AA, greater than 90% is taken up from plasma within 2 minutes, and brain phospholipids are virtually completely labeled after 1 minute (DeGeorge et al., 1989, Nariai et al., 1991, Washizaki et al., 1994, Rapoport, 2001). However, it is possible that more specific lipidomic indices (such as the unesterified or plasma phospholipid fractions) might reflect different physiological mechanisms and yield different results from total plasma PUFAs. Blood draws were not conducted at the time of the PET acquisition, for ethical and technical reasons, but repeated analyses after removing the most chronologically distant four data points did not change results. Blood was not specifically drawn in a fasting state. However, AA plasma levels do not vary greatly over short time periods; e.g., even supplementing with 1076 mg/d of omega-3 PUFA, which is known to decrease levels of AA, results in an average rate of change in plasma AA of only 2% per week (Schuchardt et al., 2016). We have no information concerning seasonal effects that may relate to diet or to 5-HTT. Given the small sample size, we did not include additional potential confounding variables in the model, such as tobacco use, body mass index, comorbid psychiatric conditions, or measures of socio-economic status.
A limitation of the [11C]DASB ligand is the lack of a valid reference region, since binding sites are present throughout the brain. Methods that require the presence of a valid reference region (such as SRTM), when employed using an invalid reference region instead, underestimate BPND binding potentials (Turkheimer et al., 2012); naturally, therefore, results are less accurate (Oquendo et al., 2007b, Parsey et al., 2005, Parsey et al., 2010, Ito et al., 2001). To overcome this limitation, we have employed the HYDECA method for estimating VND, and thus BPP and BPND, without the need for a reference region. Therefore, when the main statistical models were performed with HYDECA, we saw relationships (as reported in Results) that were not detectable with SRTM. Moreover, in contrast to HYDECA, SRTM with this tracer cannot generate valid estimates of BPF and BPP, which comprise the main results in this study.
Conclusions
We have demonstrated relationships between plasma AA, 5-HTT binding potential across six brain regions implicated in depression, and depression symptom severity, consistent with a novel model in which 5-HTT binding serves as a mediator for nonlinear AA effects on depression severity. Future studies with more comprehensive lipidomic measurements are needed to replicate and extend these findings.
HIGHLIGHTS.
[11C]DASB PET quantified serotonin transporter binding in major depression
Transporter binding (BPP) associated nonlinearly with plasma arachidonic acid (AA)
Plasma AA levels also associated nonlinearly with depression severity
Mediation analysis finds BPP mediates AA effects on depression severity
Lipidomic effects on serotonin neurotransmission merit further study in depression
Acknowledgements:
Role of the Funding Source:
This study was supported by NIMH grants R01MH040695 and P50MH062185 (PI:Mann), and by a grant from the National Alliance for Research on Schizophrenia and Depression (NARSAD; PI: Sublette). Funding sponsors had no role in design, analysis, interpretation of data, manuscript preparation, or decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
Dr. Mann receives royalties for the commercial use of the C-SSRS from the Research Foundation for Mental Hygiene. Dr. Oquendo receives royalties for the commercial use of the Columbia Suicide Severity Rating Scale; her family owns stock in Bristol Myers Squibb. Dr. Sullivan is currently an employee of Tonix Pharmaceuticals Inc. All other authors have no conflicts of interest to declare.
References
- Aso E, Ozaita A, Valdizan EM, Ledent C, Pazos A, Maldonado R Valverd O, 2008. BDNF impairment in the hippocampus is related to enhanced despair behavior in CB1 knockout mice. J Neurochem. 105, 565–72. [DOI] [PubMed] [Google Scholar]
- Bambico FR, Katz N, Debonnel G Gobbi G, 2007. Cannabinoids elicit antidepressant-like behavior and activate serotonergic neurons through the medial prefrontal cortex. J Neurosci. 27, 11700–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM Kenny DA, 1986. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 51, 1173–82. [DOI] [PubMed] [Google Scholar]
- Basselin M, Fox MA, Chang L, Bell JM, Greenstein D, Chen M, Murphy DL Rapoport S, 2009. Imaging Elevated Brain Arachidonic Acid Signaling in Unanesthetized Serotonin Transporter (5-HTT)-Deficient Mice. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger MJ, Simpson NR, Wang T, Van Heertum RL, Mann JJ Parsey RV, 2004. Biodistribution and radiation dosimetry of [11C]DASB in baboons. Nucl Med Biol. 31, 1097–102. [DOI] [PubMed] [Google Scholar]
- Benatti P, Peluso G, Nicolai R Calvani M, 2004. Polyunsaturated fatty acids: biochemical, nutritional and epigenetic properties. J Am Coll Nutr. 23, 281–302. [DOI] [PubMed] [Google Scholar]
- Berg KA, Maayani S, Goldfarb J Clarke WP, 1998. Pleiotropic behavior of 5-HT2A and 5-HT2C receptor agonists. Ann N Y Acad Sci. 861, 104–10. [DOI] [PubMed] [Google Scholar]
- Boileau I, Warsh JJ, Guttman M, Saint-Cyr JA, Mccluskey T, Rusjan P, Houle S, Wilson AA, Meyer JH Kish SJ, 2008. Elevated serotonin transporter binding in depressed patients with Parkinson’s disease: a preliminary PET study with [11C]DASB. Mov Disord. 23, 1776–80. [DOI] [PubMed] [Google Scholar]
- Burokas A, Martin-Garcia E, Gutierrez-Cuesta J, Rojas S, Herance JR, Gispert JD, Serra MA Maldonado R, 2014. Relationships between serotonergic and cannabinoid system in depressive-like behavior: a PET study with [11C]-DASB. J Neurochem. 130, 126–35. [DOI] [PubMed] [Google Scholar]
- Cannon DM, Ichise M, Fromm SJ, Nugent AC, Rollis D, Gandhi SK, Klaver JM, Charney DS, Manji HK Drevets WC, 2006. Serotonin transporter binding in bipolar disorder assessed using [11C]DASB and positron emission tomography. Biol Psychiatry. 60, 207–17. [DOI] [PubMed] [Google Scholar]
- Cannon DM, Ichise M, Rollis D, Klaver JM, Gandhi SK, Charney DS, Manji HK Drevets WC, 2007. Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and [11C]DASB; comparison with bipolar disorder. Biol Psychiatry. 62, 870–7. [DOI] [PubMed] [Google Scholar]
- Catafau AM, Perez V, Plaza P, Pascual JC, Bullich S, Suarez M, Penengo MM, Corripio I, Puigdemont D, Danus M, Perich J Alvarez E, 2006. Serotonin transporter occupancy induced by paroxetine in patients with major depression disorder: a 123I-ADAM SPECT study. Psychopharmacology (Berl). 189, 145–53. [DOI] [PubMed] [Google Scholar]
- Chen CT Bazinet RP, 2015. Beta-oxidation and rapid metabolism, but not uptake regulate brain eicosapentaenoic acid levels. Prostaglandins Leukot Essent Fatty Acids. 92, 33–40. [DOI] [PubMed] [Google Scholar]
- Dahlstrom M, Ahonen A, Ebeling H, Torniainen P, Heikkila J Moilanen I, 2000. Elevated hypothalamic/midbrain serotonin (monoamine) transporter availability in depressive drug-naive children and adolescents. Mol Psychiatry. 5, 514–22. [DOI] [PubMed] [Google Scholar]
- Degeorge JJ, Noronha JG, Bell J, Robinson P Rapoport SI, 1989. Intravenous injection of [1–14C]arachidonate to examine regional brain lipid metabolism in unanesthetized rats. J Neurosci Res. 24, 413–23. [DOI] [PubMed] [Google Scholar]
- Delorenzo C, Kumar JS, Mann JJ Parsey RV, 2011. In vivo variation in metabotropic glutamate receptor subtype 5 binding using positron emission tomography and [11C]ABP688. J Cereb Blood Flow Metab. 31, 2169–80. 3210337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorenzo C, Kumar JS, Zanderigo F, Mann JJ Parsey RV, 2009. Modeling considerations for in vivo quantification of the dopamine transporter using [(11)C]PE2I and positron emission tomography. J Cereb Blood Flow Metab. 29, 1332–45. 2757108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabe M, Rullmann M, Luthardt J, Boettcher Y, Regenthal R, Ploetz T, Becker GA, Patt M, Schinke C, Bergh FT, Zientek F, Hilbert A, Bresch A, Fenske W, Hankir MK, Sabri O Hesse S, 2017. Serotonin transporter gene promoter methylation status correlates with in vivo prefrontal 5-HTT availability and reward function in human obesity. Transl Psychiatry. 7, e1167 5538116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Bois TM, Deng C, Bell W Huang XF, 2006. Fatty acids differentially affect serotonin receptor and transporter binding in the rat brain. Neuroscience. [DOI] [PubMed] [Google Scholar]
- Dubois T, Deng C, Bell W Huang X, 2006. Fatty acids differentially affect serotonin receptor and transporter binding in the rat brain. Neuroscience. 139, 1397–1403. [DOI] [PubMed] [Google Scholar]
- Emerson JD Stoto MA 1983. Transforming data In: HOAGLIN DC, MOSTELLER F & TUKEY JW (eds.) Understanding Robust and Exploratory Data Analysis. New York: John Wiley. [Google Scholar]
- Felder CC, Kanterman RY, Ma AL Axelrod J, 1990. Serotonin stimulates phospholipase A2 and the release of arachidonic acid in hippocampal neurons by a type 2 serotonin receptor that is independent of inositolphospholipid hydrolysis. Proc Natl Acad Sci USA. 87, 2187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M Williams J, 1994. Structured clinical interview for DSM-IV Axis I disorders, patient edition.
- Frankle WG, Huang Y, Hwang DR, Talbot PS, Slifstein M, Van Heertum R, Abi-Dargham A Laruelle M, 2004. Comparative evaluation of serotonin transporter radioligands 11C-DASB and 11C-McN 5652 in healthy humans. J Nucl Med. 45, 682–94. [PubMed] [Google Scholar]
- Garcia MC Kim HY, 1997. Mobilization of arachidonate and docosahexaenoate by stimulation of the 5-HT2A receptor in rat C6 glioma cells. Brain Res. 768, 43–8. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V Piomelli D, 2005. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A. 102, 18620–5. 1317988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorjao R, Azevedo-Martins AK, Rodrigues HG, Abdulkader F, Arcisio-Miranda M, Procopio J Curi R, 2009. Comparative effects of DHA and EPA on cell function. Pharmacol Ther. 122, 56–64. [DOI] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J Neurol Neurosurg Psychiatry. 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D Lesch KP, 1996. Allelic variation of human serotonin transporter gene expression. J Neurochem. 66, 2621–4. [DOI] [PubMed] [Google Scholar]
- Herold N, Uebelhack K, Franke L, Amthauer H, Luedemann L, Bruhn H, Felix R, Uebelhack R Plotkin M, 2006. Imaging of serotonin transporters and its blockade by citalopram in patients with major depression using a novel SPECT ligand [123I]-ADAM. J Neural Transm (Vienna). 113, 659–70. [DOI] [PubMed] [Google Scholar]
- Ho PS, Ho KK, Huang WS, Yen CH, Shih MC, Shen LH, Ma KH Huang SY, 2013. Association study of serotonin transporter availability and SLC6A4 gene polymorphisms in patients with major depression. Psychiatry Res. 212, 216–22. [DOI] [PubMed] [Google Scholar]
- Huan M, Hamazaki K, Sun Y, Itomura M, Liu H, Kang W, Watanabe S, Terasawa K Hamazaki T, 2004. Suicide attempt and n-3 fatty acid levels in red blood cells: a case control study in China. Biol Psychiatry. 56, 490–6. [DOI] [PubMed] [Google Scholar]
- Ichimiya T, Suhara T, Sudo Y, Okubo Y, Nakayama K, Nankai M, Inoue M, Yasuno F, Takano A, Maeda J Shibuya, H, 2002. Serotonin transporter binding in patients with mood disorders: a PET study with [11C](+)McN5652. Biol Psychiatry. 51, 715–22. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF Carson RE, 2007. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 27, 1533–9. [DOI] [PubMed] [Google Scholar]
- Ito H, Sudo Y, Suhara T, Okubo Y, Halldin C Farde L, 2001. Error analysis for quantification of [(11)C]FLB 457 binding to extrastriatal D(2) dopamine receptors in the human brain. Neuroimage. 13, 531–9. [DOI] [PubMed] [Google Scholar]
- Joensuu M, Tolmunen T, Saarinen PI, Tiihonen J, Kuikka J, Ahola P, Vanninen R Lehtonen J, 2007. Reduced midbrain serotonin transporter availability in drug-naive patients with depression measured by SERT-specific [(123)I] nor-beta-CIT SPECT imaging. Psychiatry Res. 154, 125–31. [DOI] [PubMed] [Google Scholar]
- Jorgensen TN, Christensen PM Gether U, 2014. Serotonin-induced down-regulation of cell surface serotonin transporter. Neurochem Int. 73, 107–12. 4058363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV, Olivier JD, Nonkes LJ Homberg JR, 2010. Conserved role for the serotonin transporter gene in rat and mouse neurobehavioral endophenotypes. Neurosci Biobehav Rev. 34, 373–86. [DOI] [PubMed] [Google Scholar]
- Kim HW, Rapoport SI Rao JS, 2009. Altered arachidonic acid cascade enzymes in postmortem brain from bipolar disorder patients. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammertsma AA Hume SP, 1996. Simplified reference tissue model for PET receptor studies. Neuroimage. 4, 153–8. [DOI] [PubMed] [Google Scholar]
- Lehto S, Tolmunen T, Joensuu M, Saarinen PI, Vanninen R, Ahola P, Tiihonen J, Kuikka J Lehtonen, J, 2006. Midbrain binding of [123I]nor-beta-CIT in atypical depression. Prog Neuropsychopharmacol Biol Psychiatry. 30, 1251–5. [DOI] [PubMed] [Google Scholar]
- Lepage G, Levy E, Ronco N, Smith L, Galéano N Roy CC, 1989. Direct transesterification of plasma fatty acids for the diagnosis of essential fatty acid deficiency in cystic fibrosis. J Lipid Res. 30, 1483–90. [PubMed] [Google Scholar]
- Lewis MD, Hibbeln JR, Johnson JE, Lin YH, Hyun DY Loewke JD, 2011. Suicide deaths of active-duty US military and omega-3 fatty-acid status: a case-control comparison. J Clin Psychiatry. 72, 1585–90. 3259251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PY, Huang SY Su KP, 2010. A Meta-Analytic Review of Polyunsaturated Fatty Acid Compositions in Patients with Depression. Biol Psychiatry. 68, 140–147. [DOI] [PubMed] [Google Scholar]
- Liu JJ, Green P, John Mann J, Rapoport SI Sublette ME, 2015. Pathways of polyunsaturated fatty acid utilization: implications for brain function in neuropsychiatric health and disease. Brain Res. 1597, 220–46. 4339314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Christophe A, Delanghe J, Altamura C, Neels H Meltzer HY, 1999. Lowered omega3 polyunsaturated fatty acids in serum phospholipids and cholesteryl esters of depressed patients. Psychiatry Res. 85, 275–91. [DOI] [PubMed] [Google Scholar]
- Malison RT, Price LH, Berman R, Van Dyck CH, Pelton GH, Carpenter L, Sanacora G, Owens MJ, Nemeroff CB, Rajeevan N, Baldwin RM, Seibyl JP, Innis RB Charney DS, 1998. Reduced brain serotonin transporter availability in major depression as measured by [123I]-2 beta-carbomethoxy-3 beta-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry. 44, 1090–8. [DOI] [PubMed] [Google Scholar]
- Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL Andrews AM, 2004. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Methods. 140, 169–81. [DOI] [PubMed] [Google Scholar]
- Mclaughlin RJ, Hill MN, Bambico FR, Stuhr KL, Gobbi G, Hillard CJ Gorzalka BB, 2012. Prefrontal cortical anandamide signaling coordinates coping responses to stress through a serotonergic pathway. Eur Neuropsychopharmacol. 22, 664–71. 3366159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JH, Houle S, Sagrati S, Carella A, Hussey DF, Ginovart N, Goulding V, Kennedy J Wilson AA, 2004. Brain serotonin transporter binding potential measured with carbon 11-labeled DASB positron emission tomography: effects of major depressive episodes and severity of dysfunctional attitudes. Arch Gen Psychiatry. 61, 1271–9. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Ginovart N, Goulding V, Hussey D, Hood K Houle S, 2001. Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [(11)C]DASB PET imaging study. Am J Psychiatry. 158, 1843–9. [DOI] [PubMed] [Google Scholar]
- Miller JM, Everett BA, Oquendo MA, Ogden RT, Mann JJ Parsey RV, 2016. Positron emission tomography quantification of serotonin transporter binding in medication-free bipolar disorder. Synapse. 70, 24–32. 4654655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JM, Hesselgrave N, Ogden RT, Sullivan GM, Oquendo MA, Mann JJ Parsey RV, 2013. Positron emission tomography quantification of serotonin transporter in suicide attempters with major depressive disorder. Biol Psychiatry. 74, 287–95. 3725207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintun MA, Raichle ME, Kilbourn MR, Wooten GF Welch MJ, 1984. A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol. 15, 217–27. [DOI] [PubMed] [Google Scholar]
- Nariai T, Degeorge JJ, Lamour Y Rapoport SI, 1991. In vivo brain incorporation of [1–14C]arachidonate in awake rats, with or without cholinergic stimulation, following unilateral lesioning of nucleus basalis magnocellularis. Brain Res. 559, 1–9. [DOI] [PubMed] [Google Scholar]
- Newberg AB, Amsterdam JD, Wintering N, Ploessl K, Swanson RL, Shults J Alavi A, 2005. 123I-ADAM binding to serotonin transporters in patients with major depression and healthy controls: a preliminary study. J Nucl Med. 46, 973–7. [PubMed] [Google Scholar]
- Newberg AB, Amsterdam JD, Wintering N Shults J, 2012. Low brain serotonin transporter binding in major depressive disorder. Psychiatry Res. 202, 161–7. 3398160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nye JA, Purselle D, Plisson C, Voll RJ, Stehouwer JS, Votaw JR, Kilts CD, Goodman MM Nemeroff CB, 2013. Decreased brainstem and putamen SERT binding potential in depressed suicide attempters using [11C]-zient PET imaging. Depress Anxiety. 30, 902–7. [DOI] [PubMed] [Google Scholar]
- Ogden RT, 2003. Estimation of kinetic parameters in graphical analysis of PET imaging data. Stat Med. 22, 3557–68. [DOI] [PubMed] [Google Scholar]
- Ogden RT, Ojha A, Erlandsson K, Oquendo MA, Mann JJ Parsey RV, 2007. In vivo quantification of serotonin transporters using [(11)C]DASB and positron emission tomography in humans: modeling considerations. J Cereb Blood Flow Metab. 27, 205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo M, Hastings R, Huang Y, Simpson N, Ogden RT, Hu XZ, Goldman D, Arango V, Van Heertum R, Mann J Parsey R, 2007a. Brain serotonin transporter binding in depressed patients with bipolar disorder using positron emission tomography. Arch Gen Psychiatry. 64, 201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo MA, Galfalvy H, Sullivan GM, Miller JM, Milak MM, Sublette ME, Cisneros-Trujillo S, Burke AK, Parsey RV Mann JJ, 2016. Positron Emission Tomographic Imaging of the Serotonergic System and Prediction of Risk and Lethality of Future Suicidal Behavior. JAMA Psychiatry. 73, 1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo MA, Hastings RS, Huang YY, Simpson N, Ogden RT, Hu XZ, Goldman D, Arango V, Van Heertum RL, Mann JJ Parsey RV, 2007b. Brain serotonin transporter binding in depressed patients with bipolar disorder using positron emission tomography. Arch Gen Psychiatry. 64, 201–8. 3767993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey R, Arango V, Olvet D, Oquendo M, Van Heertum R John Mann J, 2005. Regional heterogeneity of 5-HT1A receptors in human cerebellum as assessed by positron emission tomography. J Cereb Blood Flow Metab. 25, 785–793. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Hastings RS, Oquendo MA, Huang YY, Simpson N, Arcement J, Huang Y, Ogden RT, Van Heertum RL, Arango V Mann JJ, 2006a. Lower serotonin transporter binding potential in the human brain during major depressive episodes. Am J Psychiatry. 163, 52–8. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Kent JM, Oquendo MA, Richards MC, Pratap M, Cooper TB, Arango V Mann JJ, 2006b. Acute Occupancy of Brain Serotonin Transporter by Sertraline as Measured by [(11)C]DASB and Positron Emission Tomography. Biol Psychiatry. 59, 821–8. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Ogden RT Mann JJ, 2003. Determination of volume of distribution using likelihood estimation in graphical analysis: elimination of estimation bias. J Cereb Blood Flow Metab. 23, 1471–8. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Ogden RT, Miller JM, Tin A, Hesselgrave N, Goldstein E, Mikhno A, Milak M, Zanderigo F, Sullivan GM, Oquendo MA Mann JJ, 2010. Higher serotonin 1A binding in a second major depression cohort: modeling and reference region considerations. Biol Psychiatry. 68, 170–8. 2900398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey RV, Slifstein M, Hwang DR, Abi-Dargham A, Simpson N, Mawlawi O, Guo NN, Van Heertum R, Mann JJ Laruelle M, 2000. Validation and reproducibility of measurement of 5-HT1A receptor parameters with [carbonyl-11C]WAY-100635 in humans: comparison of arterial and reference tisssue input functions. J Cereb Blood Flow Metab. 20, 1111–33. [DOI] [PubMed] [Google Scholar]
- Peet M, Murphy B, Shay J Horrobin D, 1998. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biological Psychiatry. 43, 315–9. [DOI] [PubMed] [Google Scholar]
- Qu Y, Chang L, Klaff J, Seemann R Rapoport S, 2003. Imaging Brain Phospholipase A2-Mediated Signal Transduction in Response to Acute Fluoxetine Administration in Unanesthetized Rats. Neuropsychopharmacology. 28, 1219–1226. [DOI] [PubMed] [Google Scholar]
- Qu Y, Villacreses N, Murphy DL Rapoport SI, 2005. 5-HT2A/2C receptor signaling via phospholipase A2 and arachidonic acid is attenuated in mice lacking the serotonin reuptake transporter. Psychopharmacology (Berl). 180, 12–20. [DOI] [PubMed] [Google Scholar]
- Rahbek-Clemmensen T, Bay T, Eriksen J, Gether U Jorgensen TN, 2014. The serotonin transporter undergoes constitutive internalization and is primarily sorted to late endosomes and lysosomal degradation. J Biol Chem. 289, 23004–19. 4132800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport S, 2001. In vivo fatty acid incorporation into brain phospholipids in relation to plasma availablity, signal transduction and membrane remodeling. J Mol Neurosci. 16, 243–261, 279–284. [DOI] [PubMed] [Google Scholar]
- Rapoport SI, 2014. Lithium and the Other Mood Stabilizers Effective in Bipolar Disorder Target the Rat Brain Arachidonic Acid Cascade. ACS Chem Neurosci. 4063504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimold M, Batra A, Knobel A, Smolka MN, Zimmer A, Mann K, Solbach C, Reischl G, Schwarzler F, Grunder G, Machulla HJ, Bares R Heinz A, 2008. Anxiety is associated with reduced central serotonin transporter availability in unmedicated patients with unipolar major depression: a [11C]DASB PET study. Mol Psychiatry. 13, 606–13, 557. [DOI] [PubMed] [Google Scholar]
- Reivich M, Amsterdam JD, Brunswick DJ Shiue CY, 2004. PET brain imaging with [11C](+)McN5652 shows increased serotonin transporter availability in major depression. J Affect Disord. 82, 321–7. [DOI] [PubMed] [Google Scholar]
- Schneck N, Miller JM, Delorenzo C, Kikuchi T, Sublette ME, Oquendo MA, Mann JJ Parsey RV, 2016. Relationship of the serotonin transporter gene promoter polymorphism (5-HTTLPR) genotype and serotonin transporter binding to neural processing of negative emotional stimuli. J Affect Disord. 190, 494–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchardt JP, Ostermann AI, Stork L, Kutzner L, Kohrs H, Greupner T, Hahn A Schebb NH, 2016. Effects of docosahexaenoic acid supplementation on PUFA levels in red blood cells and plasma. Prostaglandins Leukot Essent Fatty Acids. 115, 12–23. [DOI] [PubMed] [Google Scholar]
- Selvaraj S, Murthy NV, Bhagwagar Z, Bose SK, Hinz R, Grasby PM Cowen PJ, 2011. Diminished brain 5-HT transporter binding in major depression: a positron emission tomography study with [11C]DASB. Psychopharmacology (Berl). 213, 555–62. [DOI] [PubMed] [Google Scholar]
- Spector AA, 1999. Essentiality of fatty acids. Lipids. 34 Suppl, S1–3. [DOI] [PubMed] [Google Scholar]
- Staley JK, Sanacora G, Tamagnan G, Maciejewski PK, Malison RT, Berman RM, Vythilingam M, Kugaya A, Baldwin RM, Seibyl JP, Charney D Innis RB, 2006. Sex differences in diencephalon serotonin transporter availability in major depression. Biol Psychiatry. 59, 40–7. [DOI] [PubMed] [Google Scholar]
- Strosznajder J, Chalimoniuk M Samochocki M, 1996. Activation of serotonergic 5-HT1A receptor reduces Ca(2+)- and glutamatergic receptor-evoked arachidonic acid and No/cGMP release in adult hippocampus. Neurochem Int. 28, 439–44. [DOI] [PubMed] [Google Scholar]
- Sublette ME, Galfalvy HC, Hibbeln JR, Keilp JG, Malone KM, Oquendo MA Mann JJ, 2014. Polyunsaturated fatty acid associations with dopaminergic indices in major depressive disorder. Int J Neuropsychopharmacol. 17, 383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA Mann JJ, 2006. Omega-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. Am J Psychiatry. 163, 1100–2. [DOI] [PubMed] [Google Scholar]
- Todd Ogden R, Zanderigo F Parsey RV, 2015. Estimation of in vivo nonspecific binding in positron emission tomography studies without requiring a reference region. Neuroimage. 108, 234–42. [DOI] [PubMed] [Google Scholar]
- Tukey JW, 1957. On the Comparative Anatomy of Transformations. Annals of Mathematical Statistics. 28, 602–632. [Google Scholar]
- Turkheimer FE, Selvaraj S, Hinz R, Murthy V, Bhagwagar Z, Grasby P, Howes O, Rosso L Bose SK, 2012. Quantification of ligand PET studies using a reference region with a displaceable fraction: application to occupancy studies with [(11)C]-DASB as an example. J Cereb Blood Flow Metab. 32, 70–80. 3323353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washizaki K, Smith QR, Rapoport SI Purdon AD, 1994. Brain arachidonic acid incorporation and precursor pool specific activity during intravenous infusion of unesterified [3H]arachidonate in the anesthetized rat. J Neurochem. 63, 727–36. [DOI] [PubMed] [Google Scholar]
- Willeit M, Praschak-Rieder N, Neumeister A, Pirker W, Asenbaum S, Vitouch O, Tauscher J, Hilger E, Stastny J, Brucke T Kasper S, 2000. [123I]-beta-CIT SPECT imaging shows reduced brain serotonin transporter availability in drug-free depressed patients with seasonal affective disorder. Biol Psychiatry. 47, 482–9. [DOI] [PubMed] [Google Scholar]
- World Health Organization 2016. “Depression: let’s talk” says WHO, as depression tops list of causes of ill health.
- Zanderigo F, Mann JJ Ogden RT, 2017. A hybrid deconvolution approach for estimation of in vivo non-displaceable binding for brain PET targets without a reference region. PLoS One. 12, e0176636 5411064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanderigo F, Parsey RV Ogden RT, 2015. Model-free quantification of dynamic PET data using nonparametric deconvolution. J Cereb Blood Flow Metab. 35, 1368–79. 4528013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanderigo F, Schain M, Jj M Ogden R Application of a reference region-free HYbrid DEConvolution Approach for quantifying PET binding potentials in the absence of blood samples Proceedings of the 12th International Symposium on Functional Neuroreceptor Mapping of the Living Brain, 2018. London, United Kingdom. [Google Scholar]