Abstract
Most peptide hormones originate from secretory protein precursors synthesized within the endoplasmic reticulum (ER). In this specialized organelle, the newly-made prohormones must fold to their native state. Completion of prohormone folding usually occurs prior to migration through the secretory pathway, as unfolded/misfolded prohormones are retained by mechanisms collectively known as ER quality control. Not only do most monomeric prohormones fold properly, but many also dimerize or oligomerize within the ER. If oligomerization occurs before completion of monomer folding then when a poorly folded peptide prohormone is retained by quality control mechanisms, it may confer ER retention upon its oligomerization partners. Conversely, oligomerization between well-folded and improperly folded partners might help to override ER quality control, resulting in rescue of misfolded forms. Both scenarios appear to be possible in different animal models of endocrine disorders caused by genetic defects of protein folding in the secretory pathway. In this paper, we briefly review three such conditions, including familial neurohypophyseal diabetes insipidus, insulin-deficient diabetes mellitus, and hypothyroidism with defective thyroglobulin.
1. INTRODUCTION
Peptide hormone-secreting endocrine tissues have an especially highly developed protein secretion pathway designed for a high level of secretory protein synthesis, trafficking, processing, and storage of peptide hormones within secretory granules. These cells are among those considered as “professional secretory cells” (1), which have an extensive network of membrane-bound secretory organelles that carry their proteinaceous cargo progressively through increasingly mature stages of processing, packaging, and concentration. Throughout these stages, secretory proteins remain within the lumen of the membrane-bound organelles, and thus they communicate only indirectly with the packaging, processing, and signaling activities occurring on the cytosol side of the membrane, through proteins (and lipids) residing in the bilayer and on its cytosolic surface. The secretory cargo proteins themselves thus live two lives - one that takes place within the cell from which they are synthesized and secreted, and a second when they are finally allowed to explore the extracellular environment for which much of their biological function is encoded (2).
In the first life, within the secretory cell, the newly-made peptide prohormones and other secretory proteins must find a way to develop their native biological structure. While this is happening, there is strong reason to believe that the process of advancing through the series of intracellular compartments comprising the secretory pathway, is determined by “go / no-go” signals encoded within the structure of the young secretory proteins (3). Such proteins may need to expose anterograde transport signals that could lead to capture by a forward-going transport receptor, or they may expose structural information that triggers their retention within an individual compartment, preventing further forward transport.
The first hurdle for most newly-made secretory proteins occurs within the endoplasmic reticulum (ER). There, the majority of secretory preprohormones are injected across the ER membrane upon ribosome docking by virtue of the presence of a signal peptide (the “pre” piece) and its interaction with the signal recognition particle (SRP), followed by anchoring to the ER membrane via the SRP receptor, with guidance to the Sec61 translocon (4). This process usually begins when secretory preprohormone mRNAs have only begun to be translated; the bulk of the translocation of the growing polypeptide across the ER membrane tends to occur co-translationally (5). As it is being synthesized, the nascent secretory polypeptide chain finds itself in an entirely different environment from that of the cytosol, and it is in this new environment that preprohormones must first fold to their native conformation (6).
Many things may potentially go wrong during these early stages of peptide hormone biosynthesis. The preprohormone might not be properly or fully delivered across the ER membrane. The signal peptide (which is designed like a booster rocket, intended to be cleaved and jettisoned even as the first 80 amino acids of translation product are being injected across the ER membrane) – may not separate in a proper, timely way (7). Even if these events go well, the nascent prohormone may not find its way to the native state. The ER is a compartment filled with chaperones and processing enzymes (8). Many secretory proteins are designed to acquire N-linked glycosylation, which functions critically in ER protein folding. Additionally, the ER is a far more oxidizing milieu than the cytosol, promoting conversion of cysteine thiols into protein disulfide bonds. When those disulfide bonds are part of the native state of the mature peptide hormone then they are a stabilizing force, but they can wreak havoc if they result in intramolecular or intermolecular disulfide mispairing. There are numerous additional secretory protein structural modifications that can occur in the ER, including glucose and mannose trimming of N-linked carbohydrate side chains, proline isomerization (and hydroxylation), gamma carboxylation (of Glu residues), and many others (9).
Additionally, most peptide prohormones homo- or hetero-dimerize or oligomerize in the ER, with additional higher order assembly destined to occur in the Golgi complex or in secretory granules (10). Within granules, mature peptide hormones may be stored at concentrations that exceed 100 mg/mL – the kinds of levels that cannot be achieved in a test tube. It is likely that many peptide hormones and prohormones have been evolutionarily selected for self-association to very high concentration (in addition to their ultimate biological function) and with this in mind, it is not surprising that misfolded prohormones may retain features that both permit and promote the formation of aggregates, fibrils, and non-native polymers.
The physiological consequences of these various kinds of prohormone misfolding are as various as the biological activities of the peptide hormones themselves (11). In so-called “conformational diseases”, at a very minimum, a loss-of-function can be expected either from failure to secrete a peptide hormone, or from failure of a non-native secreted peptide hormone to fulfill its intended biological function. Beyond this, misfolded, unsecreted prohormones may accumulate in the cells from which they are synthesized, often clogging the ER and triggering a behavior known as the ER stress response (12). From there, the misfolded, unsecreted peptide prohormones may be cleared internally by protein degradative pathways such as ER-associated degradation (ERAD), or ER autophagy (ER-phagy), or other ER-to-lysosome (ERLAD) routes (13). Alternatively, the misfolded, unsecreted prohormones may accumulate at toxic levels, culminating in devastating biological consequences within the host cell, including de-differentiation, or cell death (14). In this review, we cite a few well-known examples of animal models of endocrine disorders caused by defects of protein folding in the secretory pathway, with defects that include loss-of-function, and gain-of-proteotoxic function, each leading to endocrine disease.
2.1. Familial Neurohypophyseal Diabetes Insipidus
Arginine vasopressin (AVP) is an antidiuretic hormone synthesized in magnocellular neurons of the supraoptic (SON) and paraventricular nuclei (PVN) of the hypothalamus, and delivered via axonal transport for storage in the posterior pituitary gland (15). The primary site of action of AVP is the collecting ducts of the kidney, where AVP promotes water reabsorption into the circulation. The subfornical organ (SFO) and the organum vasculosum laminae terminalis (OVLT) are osmosensors — when plasma osmolality rises, these hypothalamic nuclei send neural signals (via the nucleus medianus) to the SON and PVN, resulting in AVP secretion as well as AVP biosynthesis (16). This, along with an intact thirst mechanism, are essential to maintain water balance in the body.
Central diabetes insipidus (central DI) is caused by a deficiency of AVP secretion, and the affected patients excrete a large volume of hypotonic urine due to the insufficient water reabsorption in the renal collecting ducts. When AVP secretion is completely lost, urine excretion rates may reach up to 20 mL/min with a concentration as low as 50 mOsm/L (17). For long term management, desmopressin supplementation lowers urine volume with improved urine concentration (18).
The AVP gene encodes the signal peptide, AVP, neurophysin II (NPII) and glycopeptide (19). The prepro-AVP-NPII is converted to pro-AVP-NPII by excision of the signal peptide in the ER (20). In AVP neurons, in parallel with AVP mRNA up-regulation, the BiP mRNA (which encodes the major hsp70 family member of the ER and is highly expressed even under normal conditions) is further elevated under conditions of water-deprivation (21).
Independent of pharmacologic causes, familial neurohypophyseal diabetes insipidus (FNDI) is an autosomal dominant central DI caused by AVP gene mutations — most of which reside in the NPII region (22,23). A limited number of autopsy reports have shown that AVP-immunostained cells were reduced in the hypothalamus of patients with FNDI (24–26). From this it has been surmised that FNDI may lead to cell death of hypothalamic neurons that synthesize the protein (Figure 1). Indeed, a mouse model of FNDI was created with a C98X heterozygous nonsense mutation at the NPII locus, which is known to cause FNDI in humans (27). In these mice, accompanying the progressive polyuria was the finding that BiP protein levels were highly elevated in the AVP neurons (28). The mutant was postulated to have a high toxicity on neuronal cells in culture as demonstrated by the observation that the number of NPII immunostained cells progressively decreased over time (29).
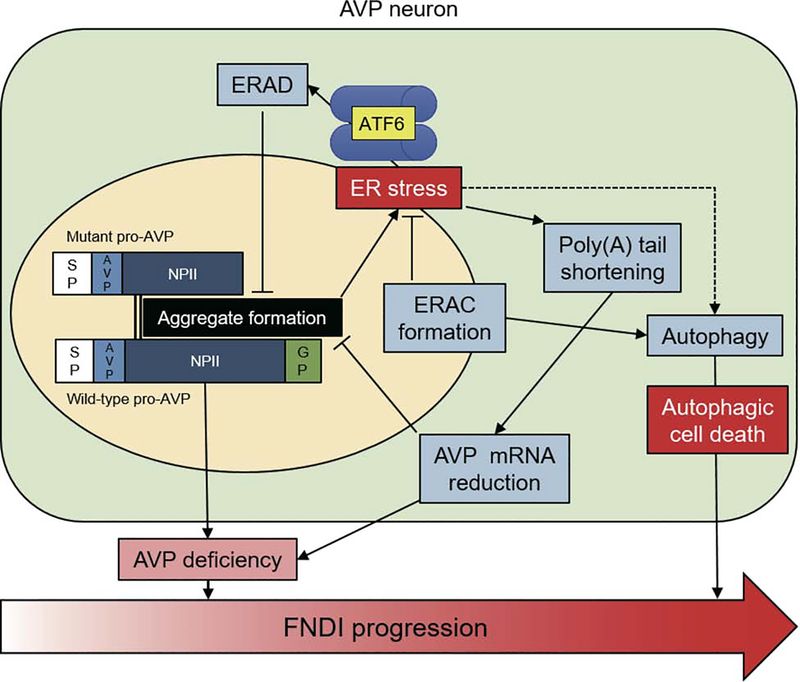
Figure 1. Conceptual diagram of FNDI progression based on misfolding of the AVP precursor protein in the ER.
In the ER, mutated pro-AVP (truncated at the NPII region) causes ER stress by forming aggregates that also encompass the non-mutant (wild-type) precursor (black box). At an early stage of the disease, ERAD, ERAC formation, autophagy, and reduction of AVP mRNA (light blue boxes) each help to prevent loss of functional AVP neurons, but ultimately, FNDI ensues as a consequence of limited AVP secretion (pink box). At the end stage of the disease, there is a net loss of immunostainable AVP neurons, which may make the central DI phenotype irreversible (large arrow at bottom).
However, another group independently created FNDI model mice with the same mutation and found that AVP neurons survived until late stages of DI. Using in situ hybridization and ultrastructural analysis in AVP producing cells, protein aggregates were observed in limited regions of the ER, which were called “ER Associated Component (ERAC)” (30,31). Aggregate formation was ameliorated by treating the mice with exogenous desmopressin, which decreases endogenous AVP expression (32). Similarly, administration of 4-phenylbutylate (4-PBA), considered to be a “chemical chaperone”, also reduced protein aggregation in the ER of AVP neurons in FNDI mice and helped to restore endogenous AVP release, ameliorating the DI phenotype (33). On the other hand, a high salt (2.0 %) diet accelerated formation of AVP protein aggregates as well as central DI (32). Interestingly, under intermittent water deprivation, protein aggregates were scattered all over the ER (34,35), accompanied by autophagy activation and autophagic cell death (Figure 1). However, in yet another related model, transgenic rats expressing the mutant AVP-C98X in their hypothalamic neurons showed an expanded ER with trapping of wild-type and mutant AVP that was targeted for lysosomal degradation by activated autophagy, but the rat model did not exhibit cell death or atrophy (36). Therefore, there remains an element of uncertainty whether the diminished hypothalamic AVP immunostaining in FNDI reflects downregulated protein expression (i.e., diminished synthesis ± enhanced degradation) or truly reflects hypothalamic cell death.
Ablation of the ATF6α gene in FNDI mice [by crossing them with ATF6α−/− mice (37,38)] resulted in diminished ERAC formation while exacerbating apparent AVP neuronal loss. These results seem to suggest that ERAC formation may serve a protective function, perhaps by isolating the aggregated protein within a subcompartment of the ER, resulting in decreased ER stress and its adverse downstream consequences.
ER-associated degradation (ERAD) is one of the main mechanisms of clearance of misfolded ER proteins, which involves degradation via cytosolic proteasomes (39). Indeed, both wild-type and mutant AVP-G57S were found to be substrates of Sel1L-Hrd1 ERAD (40,41). The core ERAD components include the E3 ubiquitin ligase HRD1 and its adaptor protein SEL1L, which are regulated by the ATF6 pathway (42). Recently, both whole-body and AVP neuron-specific SEL1L knock-out mice were found to exhibit a central DI phenotype. These results seem generally consistent with findings noted above in ATF6α deficient FNDI (C98X) mice. Interestingly, poly(A) tail length, a possible regulator of mRNA stability and translational efficacy (43–46), was shortened for the AVP mRNA in FNDI mice, and this occurred concomitant with a decreased AVP mRNA level. This decrease in mRNA level did not appear to occur as a result of nonsense-mediated mRNA decay specific to the C98X mutation, because the wild-type AVP mRNA level was also decreased (31,47). In contrast, longer poly(A) tail lengths of certain mRNAs, including known UPR genes (e.g., XBP1, CHOP and BiP) has been described under ER stress conditions (48).
2.2. Insulin-Deficient Diabetes Mellitus
Diabetes mellitus is characterized by abnormally high blood glucose level accompanied by absolute or relative insulin deficiency. A limited physiological ER stress response in pancreatic ß-cells that is concomitant with normal insulin production may be positively adaptive and may even help to support ß-cell proliferation (49), but overall, the intracellular and physiological feedback loops resulting from less insulin biosynthesis with less ER stress response may actually be even more beneficial as a stimulus to ß-cell proliferation and survival (50). Moreover, when highly increased ER stress in ß-cells is imposed by various pathological situations [such as mutant proinsulin production, ER dysfunction, or insulin resistance], these can often culminate in a profound loss of functional pancreatic ß-cell mass (51).
One of the most famous animal models of a secretory protein folding defect causing diabetes is the Akita mouse, which exhibits heterozygous expression of a missense mutant proinsulin-C96Y from one Ins2 allele leading to a defect in proinsulin disulfide bond formation (52). These animals actually express two wild-type copies of the Ins1 gene as well as one wild-type Ins2 allele; nevertheless, the animals develop insulin-deficient diabetes with a high degree of penetrance (53). In the pancreatic ß-cells of Akita mice, the misfolded mutant proinsulin is retained within the ER (54,55). The two interchain disulfide bonds of proinsulin, Cys(B19)-Cys(A20) and Cys(B7)-Cys(A7) are known to be required for proinsulin export from the ER (56). Thus, ER retention of the product of the Akita mutant proinsulin allele, which can never form the Cys(B7)-Cys(A7) disulfide bond, is expected. However, what is more remarkable is that the products of the remaining three wild-type Ins alleles are also defective for ER export in Akita islets (57). One possibility is that the misfolded mutant proinsulin could cause generalized ER dysfunction, blocking all other secretory protein traffic (58). At least in the early life of the animal, this does not seem to be the case, but rather, the misfolded mutant proinsulin directly engages innocent wild-type proinsulin “bystander” molecules in inappropriate intermolecular disulfide bonds (59), blocking the ER export of the wild-type proinsulin (60).
The problem in Akita mice has also been found in humans, with the syndrome termed Mutant Ins-gene induced Diabetes of Youth, or MIDY (59). Roughly 30 different human MIDY mutations have been reported, including an identical substitution to that found in the Akita mouse (61). The dominant-negative effect of the misfolded proinsulin on the trafficking of wild-type proinsulin is directly related to the relative expression levels of the mutant and wild-type molecules (62,63). These aberrant interactions are initiated within the ER compartment (64). Wild-type bystander proinsulin that does escape the ER and reaches the Golgi complex can still be processed to insulin and packaged in secretory granules (65,66); however, there are relatively few “successful” molecules, and there is insufficient insulin to maintain normoglycemia (67).
The Akita mutant proinsulin protein is predisposed to form disulfide-linked dimers, trimers, tetramers, and higher order complexes (58). Some of the misfolded Akita mutant proinsulin appears to be dissociated from higher order complexes with the help of the ER oxidoreductase known as PDI (68) and the atypical hsp70/helper protein known as GRP170 (69). Failure of these proteins to prevent aggregation of Akita mutant proinsulin can result in the misfolded forms accruing to enormous size; in such case, large misfolded proinsulin aggregates may be routed to lysosomal degradation by ER-phagy (70).
Misfolded Akita proinsulin accumulation in the ER provokes ER stress (59) with activation of ATF6α and Ire1α-XBP1 pathways (71). CHOP expression is also elevated in the islets of Akita mice, and when crossed with mice null for the CHOP portion of the integrated stress response pathway, Akita mice exhibited less pancreatic ß-cell death, [although the animals still proceeded to insulin-deficient diabetes (72)]. Additionally, genetic ablation of the transcription factor C/EBP in ß-cells of Akita mice resulted in improved glycemic control with an increase of ß-cell mass (73).
Independent of genetic mutations in the INS gene, a loss of function in one or more branches of the tripartite ER stress response can itself cause diabetes mellitus (74). One such branch of the ER stress response involves Perk, which is one of four kinases (and the only ER-localized kinase) that phosphorylates eIF2α to suppress general protein translation (75). Mice with global Perk-KO display early-onset diabetes mellitus (at 4 weeks of age). The exocrine and endocrine pancreas develop normally before birth and in very early postnatal life, and glucose-induced proinsulin biosynthesis is actually robust in the islets of Perk−/− mice. However, ER expansion rapidly ensues in both the acinar and islet cells of Perk−/− mice, progressing to both exocrine pancreatic insufficiency and insulin-deficient diabetes mellitus (76), along with significant skeletal disorders (77). Perk activity in these tissues seems to reflect a cell-autonomous requirement both for development and adult function (78–83). Similarly, mice with ß-cell-specific expression of a mutation at the eIF2α phosphorylation site (Ser51Ala) were also predisposed to insulin deficiency and diabetes, especially under conditions of high metabolic demand (84). In the case of both ß-cell Perk insufficiency or insufficiency of ß-cell eIF2α phosphorylation, there is strong evidence that these conditions lead, directly or indirectly, to increased proinsulin misfolding (81,84–86).
A second branch of the ER stress response involve ATF6α. Global Atf6α−/− mice exhibit essentially normal glucose tolerance on a normal chow diet. However, on a high fat diet, ATF6α-deficient mice exhibit less insulin secretion and a swollen ER in ß-cells, which accompanies higher insulin resistance with hepatic steatosis and ER stress response. Not surprisingly, when Atf6α−/− were crossed with Akita mice, the double-mutant progeny showed accelerated loss of pancreatic insulin (87).
The third and most evolutionarily conserved branch of the ER stress response is mediated by Ire1α. Mice with tamoxifen-inducible ß-cell-specific deletion of Ire1α develop hyperglycemia and hypoinsulinemia that is especially apparent upon feeding, or acute glucose stimulation (88). In this mouse model, insulin mRNA translation was reduced primarily because of defective induction of genes related to proinsulin biosynthesis, including SRP, SRP receptor, the ER translocon, signal peptidase complex, as well as many other genes associated with the function and development of the secretory pathway. These findings are supported by the work of others who reported that ß-cell-specific deletion of Ire1α impairs insulin biosynthesis leading to hyperglycemia (89). Interestingly, such mice also exhibit decreased proinsulin folding that could be attributed to lower expression of several ER oxidoreductases, including PDI, PDIR, P5, ERp44, and ERp46 (90). Ire1α works primarily through the unconventional splicing of the mRNA encoding the XBP1 transcription factor (91,92). ß-cell-specific XBPI-deficient mice exhibit modest hyperglycemia due to the decreased number of insulin granules, impaired proinsulin processing with an increase in serum proinsulin : insulin ratio, blunted glucose-stimulated insulin secretion, and inhibited ß-cell proliferation. These phenotypes might possibly also be related to defects of proinsulin folding in the secretory pathway but more likely, these ß-cells may suffer from diminished mRNA expression of many ß-cell differentiation genes as a consequence of constitutive Ire1α hyperactivation that can result in more widespread mRNA degradation by Ire1α endonuclease activity (93).
Finally, defective proinsulin folding also occurs (to a lesser degree) in ß-cells in the absence of any MIDY or known genetic defect of ß-cell ER stress response (Figure 2). Interestingly, in db/db mice, aberrant disulfide-linked proinsulin complexes – not unlike to those seen in Akita mouse islets – have been observed as one of the earliest molecular defects during the prediabetic progression to type 2 diabetes (86). The db/db animals have a global leptin receptor defect, which is also expressed in pancreatic ß-cells, but a similar mouse model bearing leptin receptor deficiency limited to the hypothalamus but not the pancreatic islets, still yields early onset proinsulin misfolding with aberrant disulfide-linked complexes (86). These recent findings support the notion that pancreatic ß-cell stress and dysfunction during the development of type 2 diabetes may represent an endocrine disorder promoted by defective folding (of proinsulin) in the secretory pathway (Figure 2).
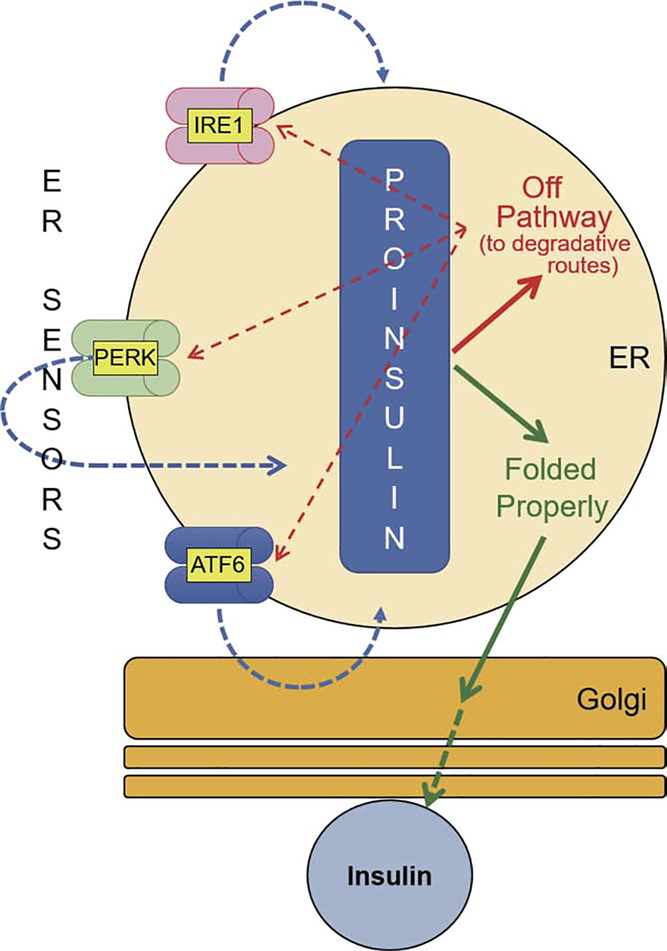
Figure 2. Conceptual diagram of the fate and sensing of proinsulin folding in pancreatic ß-cells.
Newly-synthesized proinsulin may achieve the native state (in green), and when it meets all ER quality control requirements, the native proinsulin is allowed anterograde transport through the Golgi complex to secretory granules, where insulin is made and stored for release in response to glucose challenge. Newly-synthesized proinsulin may misfold into forms bearing non-native intramolecular or intermolecular disulfide bonds. These forms are “off pathway” (in red) and it is currently unknown if they can be returned to proper folding. Additionally, similar to that seen for mutant pro-AVP (see Figure 1), misfolded proinsulin can recruit innocent bystander (wild-type) proinsulin into aberrant protein complexes. The misfolding of proinsulin in pancreatic ß-cells can be detected by ER stress sensor proteins (red dotted lines). The activities of all three ER sensors (IRE1, PERK and ATF6) contribute to a proper ER folding environment for proinsulin (blue dotted lines). When the misfolded proinsulin accumulates in the ER (as has been observed for MIDY mutants, and in early type 2 diabetes) some of the misfolded molecules are likely to be degraded via ERAD and ER-phagy, but excessive accumulation of misfolded proinsulin is likely to trigger ß-cell failure.
2.3. Congenital Hypothyroidism with Deficient Thyroglobulin Transport
Thyroglobulin (Tg), the thyroid hormone precursor encoded by the TG gene, is the most highly expressed gene product in the thyroid gland (94). The Tg protein acquires N-linked glycosylation, conformational maturation, and homodimerization in the ER. The primary functions of Tg in all vertebrates are iodide storage and thyroid hormonogenesis (95). The N-terminal two-thirds of the protein contains three regions known collectively as Tg region I-II-III (96) that include multiple cysteine-rich repeat motifs. The carboxyl-terminal region of Tg has homology with acetylcholinesterase (AChE) and is known as the Cholinesterase-Like (ChEL) domain (97–99).
Mutations of the TG gene, especially in homozygotes or compound heterozygotes, can cause congenital hypothyroidism (95,100). Curiously, however, two rodent models of congenital hypothyroidism bearing homozygous Tg missense mutations in the ChEL domain show very similar thyroid ultrastructure, but ultimately lead to interesting and important pathological differences in thyroid anatomy. The L2263P mutation in the ChEL domain causes congenital goitrous hypothyroidism in homozygous cog/cog mice (101). As a result of primary hypothyroidism, the mice are exposed to chronic TSH stimulation leading to the formation of a grossly large goiter [indeed the name “cog’ stands for congenital goiter (102)]. The cog/cog mice have a highly distended thyrocyte ER with massive accumulation of mutant Tg protein within the ER lumen (103). This is accompanied by induction of the ER stress response that dramatically upregulates the protein levels of ER molecular chaperones including GRP94, BiP, ERp72, ERp57, and calreticulin (104). Importantly, despite the defect, as the mice grow to full adulthood, they gradually achieve near-normal serum T4 levels, which parallels the growth of the large goiter (102).
The G2300R mutation in the ChEL domain of rat Tg (numbering based on the length of the mature protein — just 35 residues from the site of the mutated amino acid encoded by the cog mutation) also results in congenital hypothyroidism in rdw/rdw rats (105,106) with a highly dilated thyrocyte ER filled with mutant Tg, accompanied by absence of Tg secretion into the thyroid follicle lumen (107). Once again, a marked elevation of ER molecular chaperones GRP94, BiP and hsp70 is observed (108). However, despite highly elevated levels of circulating TSH (as a result of primary hypothyroidism), these rats develop a hypoplastic thyroid gland (109). Thus, the two single missense mutations fall closely within the same domain of Tg, yet the latter mutation has been shown to be associated with thyroid cell death suggesting a toxic gain-of-function (110), which could provide a pathophysiologic mechanism to explain the absence of goiter (Figure 3).
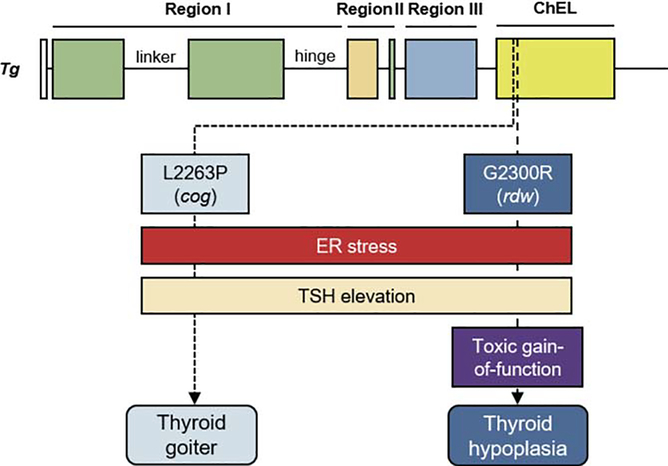
Figure 3. Conceptual diagram of the fate of the thyroid gland in rodents with homozygous expression of one of two distinct misfolded mutant Tg gene products: the cog/cog mouse, or the rdw/rdw rat.
The Tg-L2263P mutation (cog mouse) is only 35 residues away from that of the rdw rat (Tg-G2300R). Both point mutations are located in the amino-terminal portion of the ChEL domain (yellow box), and both cause severe thyroidal ER stress (red rectangle) and primary hypothyroidism with TSH elevation (beige rectangle). However, the adult cog/cog mice exhibit a significant goiter (sky blue) whereas the adult rdw/rdw rats exhibit a hypoplastic thyroid gland (dark blue). Published data to date suggest thyrocyte proliferation in cog/cog mice and thyroid cell death in rdw/rdw rats, suggesting the possibility that the Tg-G2300R mutant protein may confer a toxic gain-of-function (purple box).
Currently, 167 hypothyroidism-inducing mutations have been identified in the human TG gene (111). Although most of the patients coming to medical attention with homozygous or compound heterozygous TG mutations develop goiter, patients homozygous for G2300D (i.e., mutating the same residue as that found in rdw/rdw rats) also lack goiter development (112). These data suggest that that goitrous/non-goitrous phenotype might be linked to the biochemical or biophysical properties of the protein encoded by the particular mutant allele(s). Two human mutations replacing cysteine by either arginine or serine (C1245R and C1977S) have also been found to be retained in the ER (113). TG mutations are reported not only in human and rodents but also in Afrikander cattle (R697X) (114–117), Dutch goats (Y296X) (118–120), and other species. It is highly likely that mutant Tg retention in the ER is a common feature in most if not all forms of congenital hypothyroidism with deficient Tg.
3. DISCUSSION
In this review, we have discussed three representative protein conformational diseases of the endocrine system, including FNDI, insulin-deficient diabetes mellitus, and congenital hypothyroidism with deficient Tg. Here, we wish to highlight some of the interesting potential differences between the various conformational diseases.
There is a single AVP gene in both humans and mice (two alleles; with oxytocin encoded by a separate gene), and heterozygosity is sufficient to bring about the FNDI phenotype (22,23). There is one INS gene in humans (two alleles) but two such genes in mice (four alleles), and heterozygosity in the Ins2 locus alone is sufficient to bring about insulin-deficient diabetes mellitus in Akita mice or Munich mice (86). Although the pathogenesis of some autosomal dominant conformational diseases have been hypothetically attributed to haploinsufficiency, this idea can be excluded for either FNDI or MIDY, because other AVP and insulin heterozygous alleles that cause pure loss-of-function, lack any disease phenotype (121,122). This leaves at least two possibilities. In one case, the gene product of the mutant allele could directly associate with product of the wild-type allele, resulting in a protein complex that together is incompetent for exit from the ER, which leads to insufficient successful prohormone to move through the secretory pathway to become mature hormone. In a second case, the product of the mutant allele could form a species that brings about cytotoxicity, with either cell death or de-differentiation of the specialized secretory cells, including diminished expression of the normal allele through mRNA destabilization (123,124)(47) under ER stress — all resulting in hormonal insufficiency in the heterozygous state.
The two possibilities stated above are not mutually exclusive. In MIDY, not only is there direct evidence that misfolded mutant proinsulin directly associates with wild-type proinsulin (57), but there is also evidence that the eventual consequence of this behavior is the loss of functioning pancreatic beta cells, suggesting a state of proteotoxicity (60,125). In fact, the recruitment of wild-type proinsulin into misfolded protein complexes with mutant proinsulin implies that the ER-retained wild-type proinsulin, despite absence of any mutation, itself may be a significant contributor to beta cell proteotoxicity (65). A very similar confluence of circumstances appears to occur in FNDI, with both association between mutant and wild-type forms of pro-AVP (41) as well as an ultimate deficiency of AVP-replete neurons (126). Thus, we believe that a dominant-negative effect of the mutant protein by oligomerization with the wild-type could be a general mechanism in a variety of autosomal dominant conformational diseases (63).
Nevertheless, not all degenerative diseases caused by a mutant misfolded protein are inherited in an autosomal dominant fashion. Autosomal recessive retinitis pigmentosa (a non-endocrine conformational disease) results in progressive loss of photoreceptor cells that may lead to blindness, and several mutant genes linked to this phenotype encode proteins that traverse the secretory pathway [e.g., CRB1 (127)]. Similarly, alpha-1-antirypsin deficiency in patients bearing the Z-allele exhibits recessive inheritance such that most heterozygotes are generally healthy, yet homozygous ZZ patients commonly develop hepatocyte cell death with ultimate progression to cirrhosis (128). Thus, we in the endocrine research community should remain aware of the possibility of discovering non-dominant degenerative endocrinopathies.
The thyroglobulin (Tg) mutations described in this review highlight that a dominant versus recessive phenotype may be very much dependent upon the specific misfolding encoded by the mutant allele. Both the cog/cog mouse and the rdw/rdw rat have loss of Tg function with dramatic intracellular accumulation of Tg in the ER (129,130). Nevertheless, hypothyroidism in both cases is a recessive trait, as with most other TG mutations (95). Amazingly, in the homozygous cog/cog mouse, the thyroid gland continues to grow (129) even as evidence of chronic, continuous ER stress persists (131). In many ways, this is more remarkable than the rdw/rdw rat thyroid, which develops loss of the specialized secretory cells (132) [with a complete loss of circulating endogenous thyroxine (133)] as would be predicted from most models of cytotoxic failure from chronic continuous ER stress (134). In heterozygous rdw/+ rats, cross-dimerization between the wild-type and mutant gene products might lead to rescue of the latter (135)(63). Alternatively, in these and other endocrine proteinopathies, clean-up mechanism(s) for misfolded secretory proteins might be the critical contributor to cell survival (136) despite hormone deficiency from loss of function.
Finally, we must consider that endocrine disorders caused by defects of protein folding in the secretory pathway may yield insights not only into the pathogenesis of rare diseases, but also may provide clues about secretory cell dysfunction in common, polygenic endocrine diseases that lead to inadequate peptide hormone secretion.
ACKNOWLEDGEMENTS
This work was supported by a Young Investigator Award from the Japan Thyroid Association (to Y.M.) and NIH R01 DK40344 (to P.A.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Arvan P, Castle D. Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Biochem J 1998; 332:593–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guerriero CJ, Brodsky JL. The delicate balance between secreted protein folding and endoplasmic reticulum-associated degradation in human physiology. Physiol Rev 2012; 92:537–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barlowe C, Helenius A. Cargo Capture and Bulk Flow in the Early Secretory Pathway. Annu Rev Cell Dev Biol 2016; 32:197–222 [DOI] [PubMed] [Google Scholar]
- 4.Akopian D, Shen K, Zhang X, Shan SO. Signal recognition particle: an essential protein-targeting machine. Annu Rev Biochem 2013; 82:693–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okun MM, Eskridge EM, Shields D. Truncations of a secretory protein define minimum lengths required for binding to signal recognition particle and translocation across the endoplasmic reticulum membrane. J Biol Chem 1990; 265:7478–7484 [PubMed] [Google Scholar]
- 6.Liu M, Weiss MA, Arunagiri A, Yong J, Rege N, Sun J, Haataja L, Kaufman RJ, Arvan P Biosynthesis, structure, and folding of the insulin precursor protein. Diabetes Obes Metab 2018; 20 Suppl 2:28–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo H, Xiong Y, Witkowski P, Cui J, Wang LJ, Sun J, Lara-Lemus R, Haataja L, Hutchison K, Shan SO, Arvan P, Liu M. Inefficient translocation of preproinsulin contributes to pancreatic beta cell failure and late-onset diabetes. J Biol Chem 2014; 289:16290–16302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gidalevitz T, Stevens F, Argon Y Orchestration of secretory protein folding by ER chaperones. Biochim Biophys Acta 2013; 1833:2410–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tokunaga F, Brostrom C, Koide T, Arvan P ER-associated degradation of misfolded N-linked glycoproteins is suppressed upon inhibition of ER mannosidase I. J Biol Chem 2000; 275:40757–40764 [DOI] [PubMed] [Google Scholar]
- 10.Braakman I, Bulleid NJ. Protein folding and modification in the mammalian endoplasmic reticulum. Annu Rev Biochem 2011; 80:71–99 [DOI] [PubMed] [Google Scholar]
- 11.Kim PS, Arvan P Endocrinopathies in the family of endoplasmic reticulum (ER) storage diseases: disorders of protein trafficking and the role of ER molecular chaperones. Endocrine Reviews 1998; 19:173–202 [DOI] [PubMed] [Google Scholar]
- 12.Dufey E, Sepulveda D, Rojas-Rivera D, Hetz C. Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. 1. An overview. Am J Physiol Cell Physiol 2014; 307:C582–594 [DOI] [PubMed] [Google Scholar]
- 13.Fregno I, Molinari M. Proteasomal and lysosomal clearance of faulty secretory proteins: ER-associated degradation (ERAD) and ER-to-lysosome-associated degradation (ERLAD) pathways. Crit Rev Biochem Mol Biol 2019; 54:153–163 [DOI] [PubMed] [Google Scholar]
- 14.Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal 2014; 21:396–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bisset GW, Chowdrey HS. Control of release of vasopressin by neuroendocrine reflexes. Q J Exp Physiol 1988; 73:811–872 [DOI] [PubMed] [Google Scholar]
- 16.McKenna K, Thompson C. Osmoregulation in clinical disorders of thirst appreciation. Clin Endocrinol (Oxf) 1998; 49:139–152 [PubMed] [Google Scholar]
- 17.Baylis PH. Osmoregulation and control of vasopressin secretion in healthy humans. Am J Physiol 1987; 253:R671–678 [DOI] [PubMed] [Google Scholar]
- 18.Oiso Y, Robertson GL, Norgaard JP, Juul KV. Clinical review: Treatment of neurohypophyseal diabetes insipidus. J Clin Endocrinol Metab 2013; 98:3958–3967 [DOI] [PubMed] [Google Scholar]
- 19.Sausville E, Carney D, Battey J. The human vasopressin gene is linked to the oxytocin gene and is selectively expressed in a cultured lung cancer cell line. J Biol Chem 1985; 260:10236–10241 [PubMed] [Google Scholar]
- 20.Brownstein MJ, Russell JT, Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science 1980; 207:373–378 [DOI] [PubMed] [Google Scholar]
- 21.Hagiwara D, Arima H, Morishita Y, Goto M, Banno R, Sugimura Y, Oiso Y BiP mRNA expression is upregulated by dehydration in vasopressin neurons in the hypothalamus in mice. Peptides 2012; 33:346–350 [DOI] [PubMed] [Google Scholar]
- 22.Christensen JH, Rittig S. Familial neurohypophyseal diabetes insipidus--an update. Semin Nephrol 2006; 26:209–223 [DOI] [PubMed] [Google Scholar]
- 23.Toustrup LB, Kvistgaard H, Palmfeldt J, Bjerre CK, Gregersen N, Rittig S, Corydon TJ, Christensen JH. The Novel Ser18del AVP Variant Causes Inherited Neurohypophyseal Diabetes Insipidus by Mechanisms Shared with Other Signal Peptide Variants. Neuroendocrinology 2018; 106:167–186 [DOI] [PubMed] [Google Scholar]
- 24.Green JR, Buchan GC, Alvord EC, Swanson AG. Heredtary and idiopathic types of diabetes insipidus. Brain 1967; 90:707–714 [DOI] [PubMed] [Google Scholar]
- 25.BRAVERMAN LE, MANCINI JP, MCGOLDRICK DM. HEREDITARY IDIOPATHIC DIABETES INSIPIDUS. A CASE REPORT WITH AUTOPSY FINDINGS. Ann Intern Med 1965; 63:503–508 [DOI] [PubMed] [Google Scholar]
- 26.Nagai I, Li CH, Hsieh SM, Kizaki T, Urano Y Two cases of hereditary diabetes insipidus, with an autopsy finding in one. Acta Endocrinol (Copenh) 1984; 105:318–323 [DOI] [PubMed] [Google Scholar]
- 27.Nagasaki H, Ito M, Yuasa H, Saito H, Fukase M, Hamada K, Ishikawa E, Katakami H, Oiso Y Two novel mutations in the coding region for neurophysin-II associated with familial central diabetes insipidus. J Clin Endocrinol Metab 1995; 80:1352–1356 [DOI] [PubMed] [Google Scholar]
- 28.Russell TA, Ito M, Yu RN, Martinson FA, Weiss J, Jameson JL. A murine model of autosomal dominant neurohypophyseal diabetes insipidus reveals progressive loss of vasopressin-producing neurons. J Clin Invest 2003; 112:1697–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito M, Jameson JL. Molecular basis of autosomal dominant neurohypophyseal diabetes insipidus. Cellular toxicity caused by the accumulation of mutant vasopressin precursors within the endoplasmic reticulum. J Clin Invest 1997; 99:1897–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashi M, Arima H, Ozaki N, Morishita Y, Hiroi M, Nagasaki H, Kinoshita N, Ueda M, Shiota A, Oiso Y Progressive polyuria without vasopressin neuron loss in a mouse model for familial neurohypophysial diabetes insipidus. Am J Physiol Regul Integr Comp Physiol 2009; 296:R1641–1649 [DOI] [PubMed] [Google Scholar]
- 31.Arima H, Morishita Y, Hagiwara D, Hayashi M, Oiso Y Endoplasmic reticulum stress in vasopressin neurons of familial diabetes insipidus model mice: aggregate formation and mRNA poly(A) tail shortening. Exp Physiol 2014; 99:66–71 [DOI] [PubMed] [Google Scholar]
- 32.Hiroi M, Morishita Y, Hayashi M, Ozaki N, Sugimura Y, Nagasaki H, Shiota A, Oiso Y, Arima H. Activation of vasopressin neurons leads to phenotype progression in a mouse model for familial neurohypophysial diabetes insipidus. Am J Physiol Regul Integr Comp Physiol 2010; 298:R486–493 [DOI] [PubMed] [Google Scholar]
- 33.Tochiya M, Hagiwara D, Azuma Y, Miyata T, Morishita Y, Suga H, Onoue T, Tsunekawa T, Takagi H, Ito Y, Iwama S, Goto M, Banno R, Arima H. Chemical chaperone 4-phenylbutylate reduces mutant protein accumulation in the endoplasmic reticulum of arginine vasopressin neurons in a mouse model for familial neurohypophysial diabetes insipidus. Neurosci Lett 2018; 682:50–55 [DOI] [PubMed] [Google Scholar]
- 34.Hagiwara D, Arima H, Morishita Y, Wenjun L, Azuma Y, Ito Y, Suga H, Goto M, Banno R, Sugimura Y, Shiota A, Asai N, Takahashi M, Oiso Y Arginine vasopressin neuronal loss results from autophagy-associated cell death in a mouse model for familial neurohypophysial diabetes insipidus. Cell Death Dis 2014; 5:e1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hagiwara D, Grinevich V, Arima H. A novel mechanism of autophagy-associated cell death of vasopressin neurons in familial neurohypophysial diabetes insipidus. Cell Tissue Res 2019; 375:259–266 [DOI] [PubMed] [Google Scholar]
- 36.Si-Hoe SL, De Bree FM, Nijenhuis M, Davies JE, Howell LM, Tinley H, Waller SJ, Zeng Q, Zalm R, Sonnemans M, Van Leeuwen FW, Burbach JP, Murphy D. Endoplasmic reticulum derangement in hypothalamic neurons of rats expressing a familial neurohypophyseal diabetes insipidus mutant vasopressin transgene. FASEB J 2000; 14:1680–1684 [DOI] [PubMed] [Google Scholar]
- 37.Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, Song B, Yau GD, Kaufman RJ. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell 2007; 13:351–364 [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell 2007; 13:365–376 [DOI] [PubMed] [Google Scholar]
- 39.Qi L, Tsai B, Arvan P. New Insights into the Physiological Role of Endoplasmic Reticulum-Associated Degradation. Trends Cell Biol 2017; 27:430–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bichet DG, Lussier Y. Mice deficient for ERAD machinery component Sel1 L develop central diabetes insipidus. J Clin Invest 2017; 127:3591–3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi G, Somlo DRM, Kim GH, Prescianotto-Baschong C, Sun S, Beuret N, Long Q, Rutishauser J, Arvan P, Spiess M, Qi L. ER-associated degradation is required for vasopressin prohormone processing and systemic water homeostasis. J Clin Invest 2017; 127:3897–3912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaneko M, Yasui S, Niinuma Y, Arai K, Omura T, Okuma Y, Nomura Y A different pathway in the endoplasmic reticulum stress-induced expression of human HRD1 and SEL1 genes. FEBS Lett 2007; 581:5355–5360 [DOI] [PubMed] [Google Scholar]
- 43.Bernstein P, Ross J. Poly(A), poly(A) binding protein and the regulation of mRNA stability. Trends Biochem Sci 1989; 14:373–377 [DOI] [PubMed] [Google Scholar]
- 44.Gallie DR. The cap and poly(A) tail function synergistically to regulate mRNA translational efficiency. Genes Dev 1991; 5:2108–2116 [DOI] [PubMed] [Google Scholar]
- 45.Kuraishi T, Mizoguchi Y, Sun Y, Aoki F, Imakawa K, Sakai S. The casein mRNA decay changes in parallel with the poly(A) tail length in the mouse mammary gland. Mol Cell Endocrinol 2002; 190:101–107 [DOI] [PubMed] [Google Scholar]
- 46.Weill L, Belloc E, Bava FA, Méndez R. Translational control by changes in poly(A) tail length: recycling mRNAs. Nat Struct Mol Biol 2012; 19:577–585 [DOI] [PubMed] [Google Scholar]
- 47.Morishita Y, Arima H, Hiroi M, Hayashi M, Hagiwara D, Asai N, Ozaki N, Sugimura Y, Nagasaki H, Shiota A, Takahashi M, Oiso Y Poly(A) tail length of neurohypophysial hormones is shortened under endoplasmic reticulum stress. Endocrinology 2011; 152:4846–4855 [DOI] [PubMed] [Google Scholar]
- 48.Woo YM, Kwak Y, Namkoong S, Kristjansdottir K, Lee SH, Lee JH, Kwak H. TED-Seq Identifies the Dynamics of Poly(A) Length during ER Stress. Cell Rep 2018; 24:3630–3641.e3637 [DOI] [PubMed] [Google Scholar]
- 49.Sharma RB, O’Donnell AC, Stamateris RE, Ha B, McCloskey KM, Reynolds PR, Arvan P, Alonso LC. Insulin demand regulates β cell number via the unfolded protein response. J Clin Invest 2015; 125:3831–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szabat M, Page MM, Panzhinskiy E, Skovso S, Mojibian M, Fernandez-Tajes J, Bruin JE, Bround MJ, Lee JT, Xu EE, Taghizadeh F, O’Dwyer S, van de Bunt M, Moon KM, Sinha S, Han J, Fan Y, Lynn FC, Trucco M, Borchers CH, Foster LJ, Nislow C, Kieffer TJ, Johnson JD. Reduced insulin production relieves endoplasmic reticulum stress and induces beta cell proliferation. Cell Metab 2016; 23:179–193 [DOI] [PubMed] [Google Scholar]
- 51.Papa FR. Endoplasmic reticulum stress, pancreatic beta-cell degeneration, and diabetes. Cold Spring Harb Perspect Med 2012; 2:a007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes 1997; 46:887–894 [DOI] [PubMed] [Google Scholar]
- 53.Mathews CE, Langley SH, Leiter EH. New mouse model to study islet transplantation in insulin-dependent diabetes mellitus. Transplantation 2002; 73:1333–1336 [DOI] [PubMed] [Google Scholar]
- 54.Izumi T, Yokota-Hashimoto H, Zhao S, Wang J, Halban PA, Takeuchi T Dominant negative pathogenesis by mutant proinsulin in the Akita diabetic mouse. Diabetes 2003; 52:409–416 [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, Takata K, Koizumi A, Izumi T A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J Clin Invest 1999; 103:27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haataja L, Manickam N, Soliman A, Tsai B, Liu M, Arvan P Disulfide mispairing during proinsulin folding in the endoplasmic reticulum. Diabetes 2016; 65:1050–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu M, Hodish I, Rhodes CJ, Arvan P. Proinsulin maturation, misfolding, and proteotoxicity. Proc Natl Acad Sci U S A 2007; 104:15841–15846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Izumi T, Yokota-Hashimoto H, Zhao S, Wang J, Halban PA, Takeuchi T Dominant negative pathogenesis by mutant proinsulin in the Akita diabetic mouse. Diabetes 2003; 52:409–416 [DOI] [PubMed] [Google Scholar]
- 59.Liu M, Haataja L, Wright J, Wickramasinghe NP, Hua QX, Phillips NF, Barbetti F, Weiss MA, Arvan P. Mutant INS-gene induced diabetes of youth: proinsulin cysteine residues impose dominant-negative inhibition on wild-type proinsulin transport. PLoS One 2010; 5:e13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hodish I, Liu M, Rajpal G, Larkin D, Holz RW, Adams A, Liu L, Arvan P Misfolded proinsulin affects bystander proinsulin in neonatal diabetes. J Biol Chem 2010; 285:685–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu M, Hodish I, Haataja L, Lara-Lemus AR, Rajpal G, Wright J, Arvan P Proinsulin misfolding and diabetes: Mutant INS gene-induced Diabetes of Youth. Trends Endocrinol Metabolism 2010; 21:652–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hodish I, Absood A, Liu L, Liu M, Haataja L, Larkin D, Al-Khafaji A, Zaki A, Arvan P In vivo misfolding of proinsulin below the threshold of frank diabetes. Diabetes 2011; 60:2092–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wright J, Wang X, Haataja L, Kellogg AP Lee J, Liu M, Arvan P Dominant protein interactions that influence the pathogenesis of conformational diseases. J Clin Invest 2013; 123:3124–3134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haataja L, Snapp E, Wright J, Liu M, Hardy AB, Wheeler MB, Markwardt ML, Rizzo M, Arvan P. Proinsulin intermolecular interactions during secretory trafficking in pancreatic beta cells. The Journal of biological chemistry 2013; 288:1896–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu M, Sun J, Cui J, Chen W, Guo H, Barbetti F, Arvan P INS-gene mutations: from genetics and beta cell biology to clinical disease. Mol Aspects Med 2015; 42:3–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun J, Cui J, He Q, Chen Z, Arvan P, Liu M. Proinsulin misfolding and endoplasmic reticulum stress during the development and progression of diabetes. Mol Aspects Med 2015; 42:105–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, Takata K, Koizumi A, Izumi T A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J Clin Invest 1999; 103:27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He K, Cunningham CN, Manickam N, Liu M, Arvan P, Tsai B. PDI reductase acts on Akita mutant proinsulin to initiate retrotranslocation along the Hrd1/Sel1L-p97 axis. Mol Biol Cell 2015; 26:3413–3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cunningham CN, He K, Arunagiri A, Paton AW, Paton JC, Arvan P, Tsai B. Chaperone-driven degradation of a misfolded proinsulin mutant in parallel with restoration of wild type insulin secretion. Diabetes 2017; 66:741–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cunningham CN, Williams JM, Knupp J, Arunagiri A, Arvan P, Tsai B. Cells Deploy a Two-Pronged Strategy to Rectify Misfolded Proinsulin Aggregates. Mol Cell 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nozaki J, Kubota H, Yoshida H, Naitoh M, Goji J, Yoshinaga T, Mori K, Koizumi A, Nagata K. The endoplasmic reticulum stress response is stimulated through the continuous activation of transcription factors ATF6 and XBP1 in Ins2+/Akita pancreatic beta cells. Genes Cells 2004; 9:261–270 [DOI] [PubMed] [Google Scholar]
- 72.Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest 2002; 109:525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matsuda T, Kido Y, Asahara S, Kaisho T, Tanaka T, Hashimoto N, Shigeyama Y, Takeda A, Inoue T, Shibutani Y, Koyanagi M, Hosooka T, Matsumoto M, Inoue H, Uchida T, Koike M, Uchiyama Y, Akira S, Kasuga M. Ablation of C/EBPbeta alleviates ER stress and pancreatic beta cell failure through the GRP78 chaperone in mice. J Clin Invest 2010; 120:115–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Back SH, Kaufman RJ. Endoplasmic reticulum stress and type 2 diabetes. Annu Rev Biochem 2012; 81:767–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999; 397:271–274 [DOI] [PubMed] [Google Scholar]
- 76.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell 2001; 7:1153–1163 [DOI] [PubMed] [Google Scholar]
- 77.Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, Gannon M, Ma K, McNaughton K, Cavener DR. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol 2002; 22:3864–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang W, Feng D, Li Y, Iida K, McGrath B, Cavener DR. PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab 2006; 4:491–497 [DOI] [PubMed] [Google Scholar]
- 79.Iida K, Li Y, McGrath BC, Frank A, Cavener DR. PERK eIF2 alpha kinase is required to regulate the viability of the exocrine pancreas in mice. BMC Cell Biol 2007; 8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feng D, Wei J, Gupta S, McGrath BC, Cavener DR. Acute ablation of PERK results in ER dysfunctions followed by reduced insulin secretion and cell proliferation. BMC Cell Biol 2009; 10:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gupta S, McGrath B, Cavener DR. PERK (EIF2AK3) regulates proinsulin trafficking and quality control in the secretory pathway. Diabetes 2010; 59:1937–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang R, McGrath BC, Kopp RF, Roe MW, Tang X, Chen G, Cavener DR. Insulin secretion and Ca2+ dynamics in beta-cells are regulated by PERK (EIF2AK3) in concert with calcineurin. J Biol Chem 2013; 288:33824–33836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sowers CR, Wang R, Bourne RA, McGrath BC, Hu J, Bevilacqua SC, Paton JC, Paton AW, Collardeau-Frachon S, Nicolino M, Cavener DR. The protein kinase PERK/EIF2AK3 regulates proinsulin processing not via protein synthesis but by controlling endoplasmic reticulum chaperones. J Biol Chem 2018; 293:5134–5149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scheuner D, Vander Mierde D, Song B, Flamez D, Creemers JWM, Tsukamoto K, Ribick M, Schuit FC, Kaufman RJ. Control of mRNA translation preserves reticulum function in beta cells and maintains glucose homeostasis. Nature Medicine 2005; 11:757–764 [DOI] [PubMed] [Google Scholar]
- 85.Harding HP, Zyryanova AF, Ron D. Uncoupling proteostasis and development in vitro with a small molecule inhibitor of the pancreatic endoplasmic reticulum kinase, PERK. The Journal of biological chemistry 2012; 287:44338–44344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arunagiri A, Haataja L, Pottekat A, Pamenan F, Kim S, Zeltser LM, Paton AW, Paton JC, Tsai B, Itkin-Ansari P, Kaufman RJ, Liu M, Arvan P. Proinsulin misfolding is an early event in the progression to type 2 diabetes. eLife 2019; 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Usui M, Yamaguchi S, Tanji Y, Tominaga R, Ishigaki Y, Fukumoto M, Katagiri H, Mori K, Oka Y, Ishihara H. Atf6α-null mice are glucose intolerant due to pancreatic β-cell failure on a high-fat diet but partially resistant to diet-induced insulin resistance. Metabolism 2012; 61:1118–1128 [DOI] [PubMed] [Google Scholar]
- 88.Hassler JR, Scheuner DL, Wang S, Han J, Kodali VK, Li P, Nguyen J, George JS, Davis C, Wu SP, Bai Y, Sartor M, Cavalcoli J, Malhi H, Baudouin G, Zhang Y, Yates Iii JR, Itkin-Ansari P, Volkmann N, Kaufman RJ. The IRE1alpha/XBP1s Pathway Is Essential for the Glucose Response and Protection of beta Cells. PLoS Biol 2015; 13:e1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tsuchiya Y, Saito M, Kadokura H, Miyazaki JI, Tashiro F, Imagawa Y, Iwawaki T, Kohno K. IRE1-XBP1 pathway regulates oxidative proinsulin folding in pancreatic β cells. J Cell Biol 2018; 217:1287–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tsuchiya Y, Saito M, Kadokura H, Miyazaki JI, Tashiro F, Imagawa Y, Iwawaki T, Kohno K. IRE1-XBP1 pathway regulates oxidative proinsulin folding in pancreatic beta cells. J Cell Biol 2018; 217:1287–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 2002; 415:92–96 [DOI] [PubMed] [Google Scholar]
- 92.Lee K, Tirasophon W, Shen X, Michalak M, Prywes R, Okada T, Yoshida H, Mori K, Kaufman RJ. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev 2002; 16:452–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee AH, Heidtman K, Hotamisligil GS, Glimcher LH. Dual and opposing roles of the unfolded protein response regulated by IRE1alpha and XBP1 in proinsulin processing and insulin secretion. Proc Natl Acad Sci U S A 2011; 108:8885–8890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Di Jeso B, Arvan P. Thyroglobulin From Molecular and Cellular Biology to Clinical Endocrinology. Endocr Rev 2016; 37:2–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Citterio CE, Targovnik HM, Arvan P. The role of thyroglobulin in thyroid hormonogenesis. Nat Rev Endocrinol 2019; [DOI] [PubMed] [Google Scholar]
- 96.Lee J, Di Jeso B, Arvan P. The cholinesterase-like domain of thyroglobulin functions as an intramolecular chaperone. J Clin Invest 2008; 118:2950–2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mercken L, Simons M-J, Swillens S, Massaer M, Vassart G. Primary structure of bovine thyroglobulin deduced from the sequence of its 8,431-base complementary DNA. Nature 1985; 316:647–651 [DOI] [PubMed] [Google Scholar]
- 98.Takagi Y, Omura T, Go M. Evolutionary origin of thyroglobulin by duplication of esterase gene. FEBS Lett 1991; 282:17–22 [DOI] [PubMed] [Google Scholar]
- 99.Swillens S, Ludgate M, Mercken L, Dumont JE, Vassart G. Analysis of sequence and structure homologies between thyroglobulin and acetylcholinesterase: possible functional and clinical significance. Biochem Biophys Res Comm 1986; 137:142–148 [DOI] [PubMed] [Google Scholar]
- 100.Targovnik HM, Esperante SA, Rivolta CM. Genetics and phenomics of hypothyroidism and goiter due to thyroglobulin mutations. Mol Cell Endocrinol 2010; 322:44–55 [DOI] [PubMed] [Google Scholar]
- 101.Kim PS, Hossain SA, Park YN, Lee I, Yoo SE, Arvan P. A single amino acid change in the acetylcholinesterase-like domain of thyroglobulin causes congenital goiter with hypothyroidism in the cog/cog mouse: a model of human endoplasmic reticulum storage diseases. Proc Natl Acad Sci U S A 1998; 95:9909–9913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Adkison LR, Taylor S, Beamer WG. Mutant gene-induced disorders of structure, function and thyroglobulin synthesis in congenital goitre (cog/cog) in mice. J Endocrinol 1990; 126:51–58 [DOI] [PubMed] [Google Scholar]
- 103.Mayerhofer A, Amador AG, Beamer WG, Bartke A. Ultrastructural aspects of the goiter in cog/cog mice. J Hered 1988; 79:200–203 [DOI] [PubMed] [Google Scholar]
- 104.Kim PS, Kwon OY, Arvan P. An endoplasmic reticulum storage disease causing congenital goiter with hypothyroidism. J Cell Biol 1996; 133:517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hishinuma A, Furudate S, Oh-Ishi M, Nagakubo N, Namatame T, Ieiri T A novel missense mutation (G2320R) in thyroglobulin causes hypothyroidism in rdw rats. Endocrinology 2000; 141:4050–4055 [DOI] [PubMed] [Google Scholar]
- 106.Kim PS, Ding M, Menon S, Jung CG, Cheng JM, Miyamoto T, Li B, Furudate S, Agui T A missense mutation G2320R in the thyroglobulin gene causes non-goitrous congenital primary hypothyroidism in the WIC-rdw rat. Mol Endocrinol 2000; 14:1944–1953 [DOI] [PubMed] [Google Scholar]
- 107.Sakai Y, Yamashina S, Furudate SI. Missing secretory granules, dilated endoplasmic reticulum, and nuclear dislocation in the thyroid gland of rdw rats with hereditary dwarfism. Anat Rec 2000; 259:60–66 [DOI] [PubMed] [Google Scholar]
- 108.Oh-Ishi M, Omori A, Kwon JY, Agui T, Maeda T, Furudate SI. Detection and identification of proteins related to the hereditary dwarfism of the rdw rat. Endocrinology 1998; 139:1288–1299 [DOI] [PubMed] [Google Scholar]
- 109.Umezu M, Fujimura T, Sugawara S, Kagabu S. Pituitary and serum levels of prolactin (PRL), thyroid stimulating hormone (TSH) and serum thyroxine (T4) in hereditary dwarf rats (rdw/rdw). Jikken Dobutsu 1993; 42:211–216 [DOI] [PubMed] [Google Scholar]
- 110.Menon S, Lee J, Abplanalp WA, Yoo SE, Agui T, Furudate S, Kim PS, Arvan P. Oxidoreductase interactions include a role for ERp72 engagement with mutant thyroglobulin from the rdw/rdw rat dwarf. J Biol Chem 2007; 282:6183–6191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Citterio CE, Targovnik HM, Arvan P. The role of thyroglobulin in thyroid hormonogenesis. Nat Rev Endocrinol 2019; 15:323–338 [DOI] [PubMed] [Google Scholar]
- 112.Hishinuma A, Fukata S, Nishiyama S, Nishi Y, Oh-Ishi M, Murata Y, Ohyama Y, Matsuura N, Kasai K, Harada S, Kitanaka S, Takamatsu J, Kiwaki K, Ohye H, Uruno T, Tomoda C, Tajima T, Kuma K, Miyauchi A, Ieiri T Haplotype analysis reveals founder effects of thyroglobulin gene mutations C1058R and C1977S in Japan. J Clin Endocrinol Metab 2006; 91:3100–3104 [DOI] [PubMed] [Google Scholar]
- 113.Hishinuma A, Takamatsu J, Ohyama Y, Yokozawa T, Kanno Y, Kuma K, Yoshida S, Matsuura N, Ieiri T Two novel cysteine substitutions (C1263R and C1995S) of thyroglobulin cause a defect in intracellular transport of thyroglobulin in patients with congenital goiter and the variant type of adenomatous goiter. J Clin Endocrinol Metab 1999; 84:1438–1444 [DOI] [PubMed] [Google Scholar]
- 114.Ricketts MH, Vandenplas S, van der Walt M, van Jaarsveld PP, Bester AJ, Boyd CD. Afrikander cattle congenital goiter: size heterogeneity in thyroglobulin mRNA. Biochem Biophys Res Commun 1985; 126:240–246 [DOI] [PubMed] [Google Scholar]
- 115.Ricketts MH, Pohl V, de Martynoff G, Boyd CD, Bester AJ, Van Jaarsveld PP, Vassart G. Defective splicing of thyroglobulin gene transcripts in the congenital goitre of the Afrikander cattle. EMBO J 1985; 4:731–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ricketts MH, Simons MJ, Parma J, Mercken L, Dong Q, Vassart G. A nonsense mutation causes hereditary goitre in the Afrikander cattle and unmasks alternative splicing of thyroglobulin transcripts. Proc Natl Acad Sci U S A 1987; 84:3181–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tassi VP, Di Lauro R, Van Jaarsveld P, Alvino CG. Two abnormal thyroglobulin-like polypeptides are produced from Afrikander cattle congenital goiter mRNA. J Biol Chem 1984; 259:10507–10510 [PubMed] [Google Scholar]
- 118.Van Ommen GJ, Sterk A, Mercken LO, Arnberg AC, Baas F, De Vijlder JJ. Studies on the structures of the normal and abnormal goat thyroglobulin genes. Biochimie 1989; 71:211–221 [DOI] [PubMed] [Google Scholar]
- 119.Sterk A, van Dijk JE, Veenboer GJ, Moorman AF, de Vijlder JJ. Normal-sized thyroglobulin messenger ribonucleic acid in Dutch goats with a thyroglobulin synthesis defect is translated into a 35,000 molecular weight N-terminal fragment. Endocrinology 1989; 124:477–483 [DOI] [PubMed] [Google Scholar]
- 120.Veenboer GJ, de Vijlder JJ. Molecular basis of the thyroglobulin synthesis defect in Dutch goats. Endocrinology 1993; 132:377–381 [DOI] [PubMed] [Google Scholar]
- 121.Christensen JH, Siggaard C, Corydon TJ, Robertson GL, Gregersen N, Bolund L, Rittig S. Differential cellular handling of defective arginine vasopressin (AVP) prohormones in cells expressing mutations of the AVP gene associated with autosomal dominant and recessive familial neurohypophyseal diabetes insipidus. J Clin Endocrinol Metab 2004; 89:4521–4531 [DOI] [PubMed] [Google Scholar]
- 122.Garin I, Edghill EL, Akerman I, Rubio-Cabezas O, Rica I, Locke JM, Maestro MA, Alshaikh A, Bundak R, del Castillo G, Deeb A, Deiss D, Fernandez JM, Godbole K, Hussain K, O’Connell M, Klupa T, Kolouskova S, Mohsin F, Perlman K, Sumnik Z, Rial JM, Ugarte E, Vasanthi T, Johnstone K, Flanagan SE, Martínez R, Castaño C, Patch AM, Fernández-Rebollo E, Raile K, Morgan N, Harries LW, Castaño L, Ellard S, Ferrer J, Perez de Nanclares G, Hattersley AT, Group NDI. Recessive mutations in the INS gene result in neonatal diabetes through reduced insulin biosynthesis. Proc Natl Acad Sci U S A 2010; 107:3105–3110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol 2009; 186:323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Moore K, Hollien J. Ire1-mediated decay in mammalian cells relies on mRNA sequence, structure, and translational status. Mol Biol Cell 2015; 26:2873–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Blutke A, Renner S, Flenkenthaler F, Backman M, Haesner S, Kemter E, Landstrom E, Braun-Reichhart C, Albl B, Streckel E, Rathkolb B, Prehn C, Palladini A, Grzybek M, Krebs S, Bauersachs S, Bahr A, Bruhschwein A, Deeg CA, De Monte E, Dmochewitz M, Eberle C, Emrich D, Fux R, Groth F, Gumbert S, Heitmann A, Hinrichs A, Kessler B, Kurome M, Leipig-Rudolph M, Matiasek K, Ozturk H, Otzdorff C, Reichenbach M, Reichenbach HD, Rieger A, Rieseberg B, Rosati M, Saucedo MN, Schleicher A, Schneider MR, Simmet K, Steinmetz J, Ubel N, Zehetmaier P, Jung A, Adamski J, Coskun U, Hrabe de Angelis M, Simmet C, Ritzmann M, Meyer-Lindenberg A, Blum H, Arnold GJ, Frohlich T, Wanke R, Wolf E. The Munich MIDY Pig Biobank - A unique resource for studying organ crosstalk in diabetes. Mol Metab 2017; 6:931–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rutishauser J, Spiess M, Kopp P. Genetic forms of neurohypophyseal diabetes insipidus. Best Pract Res Clin Endocrinol Metab 2016; 30:249–262 [DOI] [PubMed] [Google Scholar]
- 127.Guo X, Li J, Wang Q, Shu Y, Wang J, Chen L, Zhang H, Shi Y, Yang J, Lu F, Jiang L, Qu C, Gong B. Identification of CRB1 mutations in two Chinese consanguineous families exhibiting autosomal recessive retinitis pigmentosa. Mol Med Rep 2019; [DOI] [PubMed] [Google Scholar]
- 128.Lomas DA, Evans DL, Finch JT, Carrell RW. The mechanism of Z alpha 1-antitrypsin accumulation in the liver. Nature 1992; 357:605–607 [DOI] [PubMed] [Google Scholar]
- 129.Adkison LR, Taylor S, Beamer WG. Mutant gene-induced disorders of structure, function and thyroglobulin synthesis in congenital goitre (cog/cog) in mice. Journal of Endocrinology 1990; 126:51–58 [DOI] [PubMed] [Google Scholar]
- 130.Mayerhofer A, Amador AG, Beamer WG, Bartke A. Ultrastructural aspects of the goiter in cog/cog mice. J Heredity 1988; 79:200–203 [DOI] [PubMed] [Google Scholar]
- 131.Kim PS, Kwon O-Y, Arvan P An endoplasmic reticulum storage disease causing congenital goiter with hypothyroidism. J Cell Biol 1996; 133:517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Umezu M, Kagabu S, Jiang J, Sato E. Evaluation and characterization of congenital hypothyroidism in rdw dwarf rats. Lab Anim Sci 1998; 48:496–501 [PubMed] [Google Scholar]
- 133.Sakai Y, Yamashina S, Furudate S. Developmental delay and unstable state of the testes in the rdw rat with congenital hypothyroidism. Dev Growth Differ 2004; 46:327–334 [DOI] [PubMed] [Google Scholar]
- 134.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta 2013; 1833:3460–3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang X, Lee J, Di Jeso B, Treglia AS, Comoletti D, Dubi N, Taylor P, Arvan P Cis and trans actions of the cholinesterase-like domain within the thyroglobulin dimer. J Biol Chem 2010; 285:17564–17573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Murrow L, Debnath J. Autophagy as a stress-response and quality-control mechanism: implications for cell injury and human disease. Annu Rev Pathol 2013; 8:105–137 [DOI] [PMC free article] [PubMed] [Google Scholar]