Abstract
Context:
The Community Preventive Services Task Force recently recommended multicomponent interventions to increase breast, cervical, and colorectal cancer screening based on strong evidence of effectiveness. This systematic review examines the economic evidence to guide decisions on the implementation of these interventions.
Evidence acquisition:
A systematic literature search for economic evidence was performed from January 2004 to January 2018. All monetary values were reported in 2016 US dollars, and the analysis was completed in 2018.
Evidence synthesis:
Fifty-three studies were included in the body of evidence from a literature search yield of 8,568 total articles. For multicomponent interventions to increase breast cancer screening, the median intervention cost per participant was $26.69 (interquartile interval [IQI] =$3.25, $113.72), and the median incremental cost per additional woman screened was $147.64 (IQI=$32.92, $924.98). For cervical cancer screening, the median costs per participant and per additional woman screened were $159.80 (IQI=$117.62, $214.73) and $159.49 (IQI=$64.74, $331.46), respectively. Two studies reported incremental cost per quality-adjusted life year gained of $748 and $33,433. For colorectal cancer screening, the median costs per participant and per additional person screened were $36.63 (IQI=$7.70, $139.23) and $582.44 (IQI=$91.10, $1,452.12), respectively. Two studies indicated a decline in incremental cost per quality-adjusted life year gained of $1,651 and $3,817.
Conclusions:
Multicomponent interventions to increase cervical and colorectal cancer screening were cost effective based on a very conservative threshold. Additionally, multicomponent interventions for colorectal cancer screening demonstrated net cost savings. Cost effectiveness for multicomponent interventions to increase breast cancer screening could not be determined owing to the lack of studies reporting incremental cost per quality-adjusted life year gained. Future studies estimating this outcome could assist implementers with decision making.
CONTEXT
In 2015, the rates of recent cancer screening test use in the U.S. were lower (71.5%, 83.0%, and 62.4%) than the Healthy People 2020 target (81.1%, 93.0%, and 70.5%) for breast, cervical, and colorectal cancers, respectively.1 In 2016, the Community Preventive Services Task Force, an independent, nonfederal panel of population health experts, recommended multicomponent interventions (MCIs) to increase screening for breast (mammography), cervical (Pap test), and colorectal cancers (fecal occult blood testing [FOBT] or colonoscopy) based on strong evidence of their effectiveness. This systematic review examines the costs and economic merits of these interventions to guide implementation decisions. Whereas previous cancer reviews2–5 have explored the economic efficiency of single interventions, this is the first systematic economic review of MCIs to increase cancer screening.
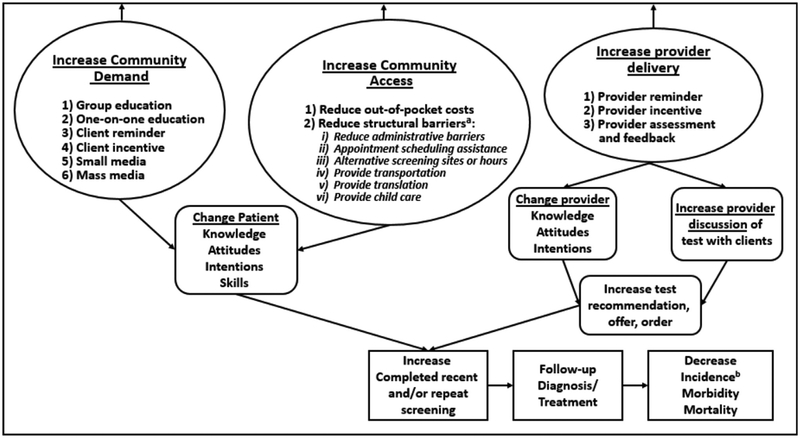
The MCIs used to promote breast, cervical, or colorectal cancer screening include any combined application of two or more single intervention components or intervention components addressing more than one structural barrier.6 Single intervention components, presented in Figure 1 and defined in Appendix Table 1 (available online), are categorized into three broad intervention strategies6:
Increasing community demand for screening. This includes client reminders, client incentives, small media, mass media, group education, and one-on-one education.
Increasing community access to screening services. This includes reducing structural barriers (appointment scheduling assistance, assistance with child care, alternative screening hours, alternative screening locations, transportation assistance, language translation services, and administrative or other barriers) and reducing client out-of-pocket costs.
Increasing provider delivery of screening services. This includes provider assessment and feedback, provider incentives, and provider reminders.
Figure 1.
Analytic framework.
a Interventions addressing multiple structural barriers are considered multicomponent.
b Reduced incidence may not apply to all cancers.
EVIDENCE ACQUISITION
Methods (Economic Measures and Analysis)
This systematic economic review focused on studies reporting cost, cost–benefit, and cost-effectiveness estimates. The intervention cost can provide program planners with an estimate of how much it would cost to implement similar interventions.7 It encompasses costs associated with intervention personnel, materials, and delivery of screening services and other intervention-related costs.7 Owing to the variation in sample size among studies, total cost was standardized to per capita cost by dividing the total intervention costs by the number of participants who received the intervention. The cost–benefit estimate, which compares the monetized value of the benefits and intervention costs, is reported as the benefit–cost ratio8:
The cost-effectiveness estimate represents the economic value of an intervention compared with an alternative.8 It is reported as an incremental cost-effectiveness ratio (ICER)8:
The change in effectiveness in the denominator of the ICER can be measured in two ways: in physical units as the number of additional individuals screened or as the quality-adjusted life years (QALYs) gained from screening. Therefore, an ICER can be reported with an intermediate or final outcome. The intermediate outcome is defined as the incremental cost per additional person screened, which is the ratio of the change in cost versus change in screenings:
The final outcome is defined as the incremental cost per QALY gained, which is the ratio of the change in cost versus change in QALYs gained8:
The QALY captures both the morbidity and mortality associated with screening as the product of life expectancy (number of years lived) and utility (health-related quality of life).9 The incremental cost per QALY gained was used to determine the overall cost effectiveness of MCIs based on an established very conservative threshold for comparison (ICER <$50,000/QALY: cost effective).10 As medians are more robust than arithmetic means, the main outcomes in this review were reported in medians accompanied by interquartile intervals (IQIs). However, in situations with only two available estimates, means were reported. The analysis was completed in 2018.
Currency Conversion and Adjustments
For non-U.S. studies, foreign currencies were converted to U.S. dollars using the Purchasing Power Parity Index from the World Bank.11 All dollar values were adjusted for inflation to 2016 U.S. dollars using the Consumer Price Index.12 For inflation adjustment, the starting year, if not specified, was assumed to be 1 year before the publication date.
Research Questions
The study aimed to answer the following research questions:
What are the costs of MCIs to increase screenings for breast, cervical, and colorectal cancers?
How do the costs of MCIs compare with their monetized benefits?
What is the incremental cost effectiveness for MCIs? How is it influenced by the baseline screening rate, type of intervention strategy, and number of intervention components?
Are MCIs cost effective compared with a very conservative threshold of $50,000/QALY?
Literature Search
The systematic literature search was performed as a two-step process: a broad search as part of a larger review to determine the effectiveness of these interventions and a focused search on economic evaluations of these interventions. The databases used for the broad search from January 2004 to November 2013 were PubMed, MEDLINE, Embase, CINAHL, PsycINFO, Cochrane Library, Web of Science, and Chronic Disease Prevention. The broad search was used to ensure that no relevant studies were missed during the focused search. The databases used for the focused economic search from January 2004 to January 2018 were EconLit, JSTOR, Scopus, and York Centre for Reviews and Dissemination. The end date for the economic search was extended to get a more recent, updated yield. The search strategy is included in Appendix Table 2 (available online).
Inclusion Criteria
The included studies:
were written in English;
were conducted in a high-income country (identified by the World Bank)13;
were MCIs to increase screening through mammography (breast cancer); Pap tests (cervical cancer); and FOBT, fecal immunochemical testing, flexible sigmoidoscopy, or colonoscopy (colorectal cancer);
reported at least one economic outcome (cost, economic benefit, cost–benefit, cost effectiveness); and
defined sources for input parameters and modeling methods with a completed sensitivity analysis for studies that modeled economic outcomes.
EVIDENCE SYNTHESIS
Literature Search Yield
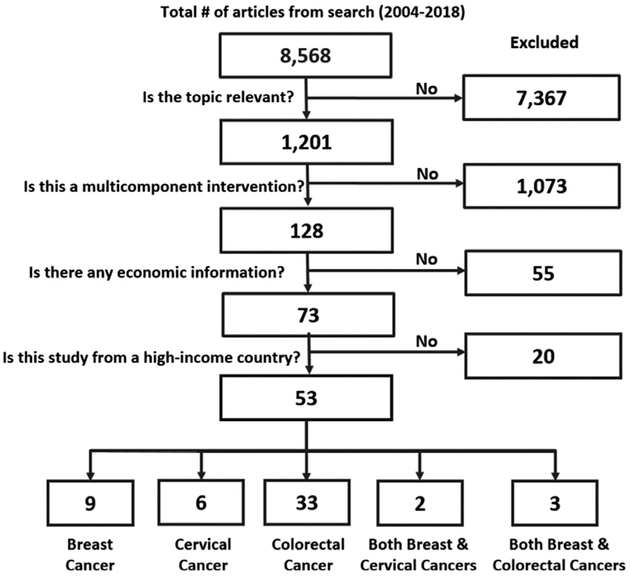
The economic literature search (January 2004–January 2018) identified 8,568 articles. The body of evidence (Figure 2) included 53 studies14–66 distributed as nine studies14,16,17,19–23,26 for breast cancer, six studies28–33 for cervical cancer, 33 studies34–66 for colorectal cancer, two studies15,25 for both breast and cervical cancers, and three studies18,24,27 for both breast and colorectal cancers.
Figure 2.
Economic literature search results.
Study Characteristics
A total of 40 studies14–21,24–27,29,31–33,35–38,40–42,45,46,48,50,52,54–65 were conducted in the U.S., and 13 studies22,23,28,30,34,39,43,44,47,49,51,53,66 were conducted in other high-income countries.13 The U.S. studies were distributed in the following geographic regions: Northeast,15,18,24,37,38,46,50,52,58,62,63 Southeast,17,18,26,27,55,64,65 Midwest,14,16,25,36,42,45,54 Southwest,19,20,29,33,35,56,59–61 and West.21,29–32,40,41,48,57 There were non-U.S. studies from Japan22 and Spain23 for breast cancer; from Japan28 and Canada30 for cervical cancer; and from Netherlands,34,39 France,47,49,53 Hong Kong,51 South Korea,66 and Italy43,44 for colorectal cancer.
Most of the studies were RCTs.14–17,20–22,24,25,29–32,34–43,45–50,52–54,56–58,62,64,66 Most included cost-effectiveness analyses, reporting ICERs,14–16,18,20–24,26,27,29–33,35–38,40,41,45,47,49,57,60,62,64–66 whereas some reported only cost estimates.17,19,25,28,34,39,42–44,46,48,54–56,58,59,61 Only one study reported cost–benefit estimates.63 Of the studies reporting cost-effectiveness estimates, the majority had comparators that did not receive an intervention (usual-care group).14–16,18,20–22,27,29–31,33,35–38,40,41,47,49,57,60,62,66 Other studies had comparators that contained a single intervention component.23,24,26,32,45,64,65 For colorectal cancer, identified studies focused on increasing colorectal cancer screening through FOBT/fecal immunochemical testing,24,35,36,39–50,52–54,56,57,59,61,62,64–66 flexible sigmoidoscopy,55 and colonoscopy.18,34,37,38,51,58,60,63
Across all three cancers, the predominant intervention strategies were a combination of increasing both community demand and community access.15,19–33,39–61,64–66 Most of the studies across all three cancers had MCIs with two intervention components.14,16,17,20,23,25–29,32,34–52,62,63,66 Other studies contained three components,19,22,24,33,53–56,64 four components,18,30,31,57–59,65 five components,15,21 six components,60 or seven components.61 For breast cancer screening, the most common intervention component was client reminders (30% of breast cancer MCI study arms).14–16,19–24,26,27 For both cervical29–32 and colorectal24,39–48,50–62,64–66 cancer screening, it was reducing structural barriers (36% of cervical and 35% of colorectal cancer MCI study arms). The most common way to reduce structural barriers was appointment scheduling assistance for breast cancer screening,15,18,24,26,27 transportation assistance for cervical cancer,30–32 and alternative screening through mailing of FOBT kits for colorectal cancer.39–48,50–55,57,59,61,62,64–66
Intervention Costs
Breast cancer.
The median intervention cost per participant was $26.69 (IQI=$3.25, $113.72; 17 study arms) across all studies,14–25 $1.49 (IQI=$0.95, $12.22; five study arms) for interventions that increased community demand,14,16,17 and $44.83 (IQI=$4.90, $133.37; 11 study arms) for interventions that increased community demand and community access (Table 1).15,19–25 The wide range for cost can be explained by the composition of intervention components. MCIs with lower cost estimates paired education with client reminders, whereas more expensive MCIs focused on reducing structural barriers. For the number of intervention components, the median cost per participant was $26.69 (IQI=$1.49, $44.83; nine study arms)14,16,17,20,23,25 and $3.32 (IQI=$3.25, $6.48; five study arms)19,22,24 for two and three components, respectively. Studies with two components reporting higher cost estimates used monetary client incentives, tailored interventions, and home visits by community health workers. Studies with three components reporting lower cost estimates used telephone calls instead of home visits.
Table 1.
Intervention Cost per Participant and Incremental Cost per Additional Person Screened for Multicomponent Interventions to Increase Breast, Cervical, and Colorectal Cancer Screening
| Type of intervention strategy and number of intervention components | Study arms, n | Median intervention cost per participant (IQI | Study arms, n | Median incremental cost per additional person screened (IQI) |
|---|---|---|---|---|
| Breast cancer | ||||
| Type of intervention strategy | ||||
| All studies | 17 | $26.69 ($3.25, $113.72) | 10 | $147.64 ($32.92, $924.98) |
| Increasing community demand strategy | 5 | $1.49 ($0.95, $12.22) | 2 | Mean: $567.82 (from two estimates: $0.45, $1,135.19) |
| Increasing community demand + community access (combined strategy) | 11 | $44.83 ($4.90, $133.37) | 8 | $147.64 ($33.54, $528.17) |
| Number of intervention components | ||||
| 2 components | 9 | $26.69 ($1.49, $44.83) | 3 | $1,229.62 ($631.73, $1,239.44) |
| 3 components | 5 | $3.32 ($3.25, $6.48) | 3 | $32.61 ($18.90, $44.29) |
| 5 components | 2 | $281.97 (from two estimates: $113.72, $450.22) | 2 | $266.83 (from two estimates: $239.30, $294.35) |
| Cervical cancer | ||||
| Type of intervention strategy | ||||
| All studies: increasing community demand + community access (combined strategy) | 10 | $159.80 ($117.62, $214.73) | 6 | $159.49 ($64.74, $331.46) |
| Number of intervention components | ||||
| 2 components | 6 | $159.80 ($147.27, $168.91) | 3 | $79.68 ($69.72, $553.57) |
| 4 components | 2 | Mean: $83.36 (from two estimates: $82.32, $84.40) | 2 | Mean: $183.25 (from two estimates: $4.32, $362.18) |
| Colorectal cancer | ||||
| Type of intervention strategy | ||||
| All studies | 42 | $36.63 ($7.70, $139.23) | 15 | $582.44 ($91.10, $1,452.12) |
| Increasing community demand strategy | 3 | $44.07 ($31.92, $46.83) | NR | NR |
| Increasing provider delivery | 2 | Mean: $366.51 (from two estimates: $108.54, $624.48) | NR | NR |
| Increasing community demand + community access (combined strategy) | 33 | $30.82 ($7.27, $94.68) | 11 | $582.44 ($51.27, $1,281.91) |
| Number of intervention components | ||||
| 2 components | 24 | $46.45 ($15.07, $118.61) | 5 | $369.18 ($42.70, $933.39) |
| 3 components | 10 | $33.23 ($7.50, $106.49) | 5 | $582.44 ($76.98, $2,017.20) |
| 4 components | 5 | $8.91 ($7.53, $264.39) | 1 | Only one estimate ($1,398.08) |
| All studies combined | ||||
| Type of intervention strategy: | ||||
| Increasing community demand strategy | 8 | $15.99 ($1.36, $32.23) | 4 | $674.36 ($160.26, $1,568.12) |
| Increasing community demand + community access (combined strategy) | 54 | $49.08 ($7.70, $163.19) | 25 | $239.30 ($42.70, $1,027.46) |
| Number of intervention components | ||||
| 2 components | 40 | $46.83 ($13.83, $149.88) | 11 | $369.18 ($51.23, $1,128.54) |
| 3 components | 16 | $19.51 ($4.95, $124.80) | 8 | $66.48 ($27.79, $941.13) |
| 4 components | 7 | $82.32 ($8.22, $174.40) | 6 | $266.83 ($239.30, $345.22) |
IQI, interquartile interval; NR, not reported.
Cervical cancer.
The median intervention cost per participant was $159.80 (IQI=$117.62, $214.73; ten study arms) across all studies, which all had combined strategies of increasing community demand and access.15,25,28–33 For two intervention components, the median cost per participant was $159.80 (IQI=$147.27, $168.91; six study arms).25,28,29,32 These studies used specialized personnel to educate and screening vouchers to reduce out-of-pocket costs.
Colorectal cancer.
The median cost per participant was $36.63 (IQI=$7.70, $139.23; 42 study arms) across all studies.18,24,34–62 The median cost per participant was $44.07 (IQI=$31.92, $46.83; three study arms) for interventions that increased community demand34,35 and $30.82 (IQI=$7.27, $94.68; 33 study arms) for interventions that increased both community demand and community access.24,39–61 Only two studies had interventions aimed at increasing provider delivery with a per capita cost of $108.54 and $624.48.36,37 For the number of intervention components, the median cost per participant was $46.45 (IQI=$15.07, $118.61; 24 study arms),34–52 $33.23 (IQI=$7.50, $106.49; ten study arms),24,42,50,53–56 and $8.91 (IQI=$7.53, $264.39; five study arms)18,57,59 for two, three, and four components, respectively.
Cost Benefit
An MCI to increase colonoscopy at three urban public hospitals used reminder telephone calls, lay health worker education, and appointment scheduling assistance by a patient navigator.63 The authors reported benefit–cost ratios >1.00 for two of three hospital sites.63 No overall cost–benefit statement can be made because only one study with mixed evidence was identified.
Incremental Cost per Additional Person Screened Breast cancer.
Across all studies, the median incremental cost per additional woman screened was $147.64 (IQI=$32.92, $924.98; ten study arms) (Table 1).14–16,20–24,26 The median incremental cost per additional woman screened was $147.64 (IQI=$33.54, $528.17; eight study arms) for studies focused on increasing both community demand and community access.15,20–24,26 The median incremental cost per additional person screened was $1,229.62 (IQI=$631.73, $1,239.44; three study arms)20,23,26 for two intervention components and $32.61 (IQI=$18.90, $44.29; three study arms)22,24 for three intervention components. There was no consistent relationship between the baseline screening rate (median, 53%; IQI=32%, 69%; six estimates) and the incremental cost per additional woman screened.14,15,22–24,26
Cervical cancer.
The median incremental cost per additional woman screened was $159.49 (IQI=$64.74, $331.46; six study arms) for all studies15,29–31 and $79.68 (IQI=$69.72, $553.57; three study arms) for studies with two intervention components.29 There was no consistent relationship between the baseline screening rate (median, 63%; IQI=54%, 76%; four estimates) and the incremental cost per additional woman screened.15,29–31
Colorectal cancer.
Across all studies, the median incremental cost per additional person screened was $582.44 (IQI=$91.10, $1,452.12; 15 study arms).24,35,36,40,41,48–50,52,53,62–65 The median incremental cost per additional person screened was $582.44 (IQI=$51.27, $1,281.91; 11 study arms) for interventions that increased both community demand and community access.24,40,41,48–50,52,53,64,65 The median incremental cost per additional person screened was $369.18 (IQI=$42.70, $933.39; five study arms) for two intervention components40,41,49,50,52 and $582.44 (IQI=$76.98, $2,017.20; five study arms) for three intervention components.24,48,50,53,64 There was no consistent relationship between the baseline screening rate (median, 44%; IQI=27%, 60%; nine estimates) and the incremental cost per additional person screened.24,35,48–50,52,62,64,65
Incremental Cost per Quality-Adjusted Life Year Gained
Breast cancer.
No studies reporting incremental cost per QALY were identified.
Cervical cancer.
Two studies reported incremental cost per QALY gained (Table 2).32,33 A community-based patient navigation intervention for Hispanic women in Texas aged ≥18 years reported an ICER of $748/QALY gained.33 An intervention that used lay health workers to motivate Vietnamese–American women in Seattle aged 20–79 years to receive screening reported an ICER of $33,433/QALY gained.32 Both estimates were considered cost effective because they were below a very conservative threshold of $50,000/QALY. Analyzing the calculation of incremental cost and QALY gained could explain the difference in these estimates. In the study by Li et al.,33 the incremental cost was $44.90, and the incremental QALY gained was 0.06 years. In the study by Scoggins and colleagues,32 the incremental cost was $117.05, and incremental QALY gained was 0.0035 years. The incremental cost reported by Scoggins et al.32 was 2.6 times higher than that reported by Li and colleagues,33 primarily because the intervention involved home visits by lay health workers. The study by Scoggins et al.32 started with the assumption of having at least one Pap test every 3 years, and in the lifetime modeling, assumed Pap test frequency would improve by 8.36%. They calculated change in QALY gained by multiplying total QALYs gained by this improvement in Pap test frequency, resulting in a lower QALY gain (0.0035 years) compared with the results reported in the study by Li and colleagues (0.06 years).33 This lower QALY value (denominator) combined with the more than twofold increase in intervention cost (numerator), resulted in a 45-fold increase in its cost-effectiveness estimate.32
Table 2.
Incremental Cost per Quality-Adjusted Life Year Gained for Multicomponent Interventions to Increase Cervical and Colorectal Cancer Screening by Study
| Study characteristics | Li (2017)33 | Scoggins (2010)32 | Wilson (2015)60 | Lee (2016)66 |
|---|---|---|---|---|
| Cancer type | Cervical cancer | Cervical cancer | Colorectal cancer | Colorectal cancer |
| Screening test | Pap test | Pap test | Colonoscopy | FOBT |
| Target population | Hispanic women in Texas aged ≥18 years | Vietnamese—American women in Seattle aged 20–79 years | Hispanic men in Texas aged ≥50 years | Hypothetical cohorts in Korea aged ≥50 years |
| Program length | 36 months | 18 months | 24 months | 12 months |
| Perspective | Societal | Societal | Societal | Societal |
| Comparator | Status quo: no intervention | Control arm: physical activity materials | Status quo: no intervention | Status quo: no intervention |
| Number of components | 3 | 2 | 6 | 2 |
| Type of intervention components | OE: personalized education MM: mass media campaign ROPC: free screening tests | OE: motivational education RSB: screening appointment scheduling assistance from bicultural, bilingual lay health workers | ROPC: free cost colonoscopy OE: educational session with graphic based presentation RSB1: transportation assistance RSB2: screening appointment scheduling assistance RSB3: extension of clinic hours to weekends and evenings CR: telephone reminders | RSB: mailed FOBT kits CR: reminder letter sent separately |
| Incremental cost (Δ cost) | $44.90 | $117.05 | $−1,164.09 | $−397.76 |
| Incremental QALY (ΔQALY) | 0.06 years | 0.0035 years | 0.305 years | 0.2409 years |
| ICER (ΔCost/ΔQALY) | $748/QALY | $33,443/QALY | $−3,817/QALY | $−1,651/QALY |
CR, client reminders; FOBT, fecal-occult blood test; ICER, incremental cost effectiveness ratio; MM, mass media; OE, one-on-one education; QALY, quality-adjusted life year; ROPC, reducing out-of-pocket costs; RSB, reducing structural barriers.
Colorectal cancer.
Two studies reported incremental cost per QALY gained.60,66 A patient navigation intervention to increase colorectal cancer screening by colonoscopy among Hispanic men in Texas aged ≥50 years reported a decline in incremental cost of $3,817/QALY gained.60 The second study was an intervention to increase colorectal cancer screening by FOBT among people in Korea aged 50 years.66 In this study by Lee and Park,66 the authors used the standard group, who were mailed FOBT with no reminder, as the comparator for calculating the ICER. In the study by Wilson et al.,60 the calculated ICER had a comparator of no intervention (status quo). As Lee and Park66 provided a regional population distribution along with intervention and status quo cost and QALYs for all the study arms, the ICER was calculated for the targeted intervention group versus the same no intervention status quo, resulting in a decline in incremental cost of $1,651/QALY gained.66 Both were modeled studies with a societal perspective and reported per capita costs and QALY gained for both intervention and comparator arms. The decline in incremental cost reported in the study by Wilson and colleagues60 was three times that in the Lee and Park66 study, whereas the incremental QALY gained was 1.3 times higher.60,66 The negative incremental cost for both studies implies that QALYs increased, whereas averted healthcare costs were higher than the intervention costs, resulting in net cost savings. As both estimates are <$50,000/QALY, MCIs to increase colorectal cancer screening are cost effective. Additionally, the two studies showed that the QALYs gained from screening were associated with treatment cost savings that outweighed the intervention costs. The estimates also indicated that treatment cost savings were higher with screening by colonoscopy than with FOBT.
DISCUSSION
Summary of Findings
The median intervention cost per participant for MCIs to increase screening was $26.69 (IQI=$3.25, $113.72; 17 study arms) for breast cancer, $159.80 (IQI=$117.62, $214.73; six study arms) for cervical cancer, and $36.63 (IQI=$7.70, $139.23; 42 study arms) for colorectal cancer. Compared with the costs for breast and colorectal cancer, the higher per capita cost for cervical cancer could be explained by the lower number of reported estimates, interventions reducing out-of-pocket costs through screening vouchers, and using specialized personnel for intervention delivery. Only one study reported monetized benefits, so no overall cost–benefit statement was made. The median incremental cost per additional person screened was $147.64 (IQI=$32.92, $924.98; ten study arms) for breast cancer, $159.49 (IQI=$64.74, $331.46; six study arms) for cervical cancer, and $582.44 (IQI=$91.10, $1,452.12; 15 study arms) for colorectal cancer. The number of reported estimates used in the median calculation varied among the different types of intervention strategies and number of components. For breast and colorectal cancer, most studies used the same combined intervention strategy. For cervical cancer, all studies had the same combined strategy, making it difficult to compare between intervention strategies. For the number of components, the per capita cost was not always higher for a greater number of components as intuitively expected. Similarly, for three components versus two components, the observed ICER was lower for breast cancer and higher for colorectal cancer. The variability could be explained by the composition and intensity of intervention components along with the materials and personnel used for its delivery.
As additional screening resulting from the intervention could be related to baseline screening rates, the relationship between the two was examined. Intuitively, with higher baseline screening rates, it might become more expensive to access the remaining hard-to-reach populations. However, there was no consistent relationship because many studies did not report baseline rates, and the rates from those that did report varied widely. No studies reported incremental cost per QALY gained for breast cancer. MCIs to increase cervical and colorectal cancer screening were determined to be cost effective as there were two studies for each that reported incremental cost per QALY below the very conservative $50,000/QALY threshold. Additionally, MCIs to increase colorectal cancer screening were cost-saving, with ICERs below $0/QALY. These studies showed that QALYs gained from screening were associated with treatment cost savings that were greater than intervention costs.60,66 Furthermore, the study that used MCIs to improve screening with colonoscopy reported greater cost savings than the study focused on screening through FOBT. The greater cost savings for colonoscopy could be attributed to its use for screening, diagnosis, and treatment, which can lead to identification and removal of polyps at earlier stages, resulting in higher averted treatment costs.
Limitations
Most studies only reported incremental cost per additional person screened, which cannot be used for a cost-effectiveness determination owing to the lack of an existing threshold. Furthermore, it was challenging to compare intermediate outcomes because of the variation among studies for intervention strategies, number of intervention components, and number of reported estimates. Some studies reported incremental costs only without sufficient information required to ascertain the per capita intervention cost.26,27,64,65 Additionally, studies did not always provide a breakdown of cost for all individual intervention components within the MCI. As the focus of this review was on the overall intervention cost of MCIs, the lack of consistent cost reporting for individual intervention components did not hinder the analysis. For incremental cost-effectiveness analysis, all included studies were experimental. Most of these studies were RCTs, whereas two studies each for breast and colorectal cancer were quasi-experimental.23,26,63,65 Although only four of 31 estimates were from quasi-experimental studies, it is important to note that these studies did not have random assignment for intervention and comparator groups.23,26,63,65 Another limitation is that each modeling study had its own specific assumptions, input, and parameter values.32,33,60,66 However, these studies performed sensitivity analyses to assess the impact of key cost and effectiveness parameters and mentioned their specific input sources and parameters derived from literature.32,33,60,66
Evidence Gaps
The lack of studies reporting incremental cost per QALY gained for MCIs to increase breast cancer screening is an evidence gap that can be filled through modeling, as it is difficult to follow patients longitudinally to obtain actual morbidity and mortality outcomes. Another evidence gap is the low number of identified economic evaluation studies that focused on an intervention strategy to increase provider delivery of services. Further research on the different types of personnel used for intervention delivery could benefit implementers.
Comparability
Both studies that reported incremental cost per QALY for cervical cancer were conducted in the U.S., adopted the same perspective, used similar modeling methods, and focused on a similarly aged target population.32,33 The main differences between the studies include ethnicity of target population, baseline screening rate, number of intervention components, intervention program length, and type of comparator.32,33 Although comparators for both studies32,33 were different, the control group in Scoggins et al.32 was treated similarly to the status quo (no intervention) comparator in the study reported by Li and colleagues33 in terms of ICER calculation. The authors state that the costs of the physical activity materials were promotional items with minimal cost, which were not included in the estimation of incremental costs.32 So, in essence, the incremental cost is the cost of the intervention, which is the same as treating the comparator control group as status quo.32 As the longevity and quality-of-life benefits of cervical cancer screening are seldom observed in a time adequate to capture lifetime impact, both studies used economic modeling methods to examine the long-term screening benefits. The study by Scoggins et al.32 constructed a state-transition Markov model with yearly intervals because screening is scheduled to occur regularly, and the researchers wanted to project benefits of screening beyond the trial to lifetime perspective. The study by Li and colleagues33 used an evidence-based microsimulation model to assess improvements in long-term patient outcomes. These modeling methods were based on actual behavioral observations, incorporating knowledge from previous cancer decision models with parameter values based on the specific program, to make informed decisions. Both studies used input parameters and utility weights from literature derived from the U.S. population and performed sensitivity analyses. For colorectal cancer, the economic finding included both colonoscopy and FOBT. Although there are differences in screening cost, the focus of the finding is on the MCIs to increase screening. Both studies reporting incremental cost per QALY had the same perspective and similar comparators and modeling types.60,66 The differences between the studies include number of intervention components, program length, target population, and location.60,66 The health utility weights used in the non-U.S. study were derived from published studies containing potentially applicable sources of North American and European populations.66 Nonetheless, it is important to note that there are sociocultural differences in this target population.66
Implementation Considerations
Understanding the target population before implementation can be helpful. One of the cost-effective studies focused on key factors that the target population considers when contemplating screening.33 Their patient navigation services leveraged behavioral economics principles to address decision making by focusing on sociocultural norms through personalized communication.33 To personalize the delivery of MCIs, personnel with the same ethnicity as the target population could be used during intervention delivery to improve participant receptiveness. One of the cost-effective studies invested in bicultural, bilingual Vietnamese–American women as lay health workers to reduce cultural barriers.32 Using personnel with familiarity to the target population provides an opportunity to personally understand concerns, address barriers, and serve as motivators to encourage screening. Furthermore, demonstrating cultural competency assists in effectively delivering relatable motivational messages.60 Cultural competency training can be provided to personnel delivering MCIs. Another consideration is behavioral variation among individuals from the same ethnic group based on prior screening history. For example, Vietnamese–American women who had never been screened did not respond as favorably to home visits when compared with Vietnamese–American women who had been screened at least once in the past.32 This indicates the importance of considering behavioral (prior history) and sociocultural factors during implementation.
For low-income, uninsured populations, it is especially important to consider structural and financial barriers. One of the cost-effective studies designed MCI for a low-income, uninsured population by examining and addressing financial and structural barriers through free screening, transportation assistance, and improved access to screening locations with flexibility of hours.60 The MCI focused on bolstering social networks through culturally appropriate communication to motivate not only the target population but also their family and friends for support. The subsequent increase in colonoscopy screening led to more cancer cases being diagnosed at earlier stages, which contributed to averted healthcare treatment costs, improved life expectancies, and QALYs gained.60 Implementing MCIs for vulnerable populations can be beneficial to healthcare organizations to reach those without access to care and avoid future treatment costs at later stages of disease. Policymakers and other entities involved in implementation would likely also value MCIs’ benefits to patients.
CONCLUSIONS
Overall, MCIs to increase cervical and colorectal cancer screening are cost effective. Furthermore, MCIs for colorectal cancer screening have demonstrated net cost savings from averted healthcare costs. More economic evaluations reporting incremental cost per QALY gained are needed to make a cost-effectiveness determination for MCIs to increase breast cancer screening.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge colleagues from the Division of Cancer Prevention and Control at CDC for guidance and subject matter expertise, staff from the CDC library for assistance with the systematic literature search, and the Community Guide Branch at CDC for scientific, dissemination, and editorial assistance.
The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry.
No financial disclosures were reported by the authors of this paper.
Footnotes
SUPPLEMENTAL MATERIAL
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016/j.amepre.2019.03.006.
REFERENCES
- 1.White A, Thompson TD, White MC, et al. Cancer screening test use -United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(8):201–206. 10.15585/mmwr.mm6608a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baron RC, Melillo S, Rimer BK, et al. Intervention to increase recommendation and delivery of screening for breast, cervical, and colorectal cancers by healthcare providers; a systematic review of provider reminders. Am J Prev Med. 2010;38(1):110–117. 10.1016/j.amepre.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 3.Baron RC, Rimer BK, Breslow RA, et al. Client-directed interventions to increase community demand for breast, cervical, and colorectal cancer screening; a systematic review. Am J Prev Med. 2008;35(1 suppl): S34–S55. 10.1016/j.amepre.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Baron RC, Rimer BK, Coates RJ, et al. Client-directed interventions to increase community access to breast, cervical, and colorectal cancer screening; a systematic review. Am J Prev Med. 2008;35(1 suppl):S56–S66. 10.1016/j.amepre.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Sabatino SA, Habarta N, Baron RC, et al. Interventions to increase recommendation and delivery of screening for breast, cervical, and colorectal cancers by healthcare providers; systematic reviews of provider assessment and feedback and provider incentives. Am J Prev Med. 2008;35(1 suppl):S67–S74. 10.1016/j.amepre.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Sabatino SA, Lawrence B, Elder R, et al. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: nine updated systematic reviews for the guide to community preventive services. Am J Prev Med. 2012;43(1):97–118. 10.1016/j.amepre.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Haddix AC, Corso PS, Gorsky RD. Costs In: Haddix AC, Teutsch SM, Corso PS, eds. Prevention Effectiveness: A Guide to Decision Analysis and Economic Evaluation. 2nd ed New York, NY: Oxford University Press, 2003:53–76. [Google Scholar]
- 8.Shih YC, Halpern MT. Economic evaluations of medical care interventions for cancer patients: how, why, and what does it mean. CA Cancer J Clin. 2008;58(4):231–244. 10.3322/CA.2008.0008. [DOI] [PubMed] [Google Scholar]
- 9.Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull. 2010;96:5–21. 10.1093/bmb/ldq033. [DOI] [PubMed] [Google Scholar]
- 10.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness-the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 11.The World Bank. Purchasing power parity index conversion. https://data.worldbank.org/indicator/pa.nus.ppp. Accessed March 21, 2017. [Google Scholar]
- 12.Bureau of Labor Statistics, U.S. Department of Labor. Consumer price index. www.bls.gov/cpi/. Accessed March 21, 2017. [Google Scholar]
- 13.The World Bank. High income countries. https://data.worldbank.org/income-level/high-income. Accessed March 21, 2017. [Google Scholar]
- 14.Slater JS, Parks MJ, Malone ME, Henly GA, Nelson CL. Coupling financial incentives with direct mail in population-based practice: a randomized trial of mammography promotion. Health Educ Behav. 2017;44(1):165–174. 10.1177/1090198116646714. [DOI] [PubMed] [Google Scholar]
- 15.Schuster AL, Frick KD, Huh BY, Kim KB, Kim M, Han HR. Economic evaluation of a community health worker-led health literacy intervention to promote cancer screening among Korean American women. J Health Care Poor Underserved. 2015;26(2):431–440. 10.1353/hpu.2015.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saywell RM Jr, Champion VL, Skinner CS, Menon U, Daggy J. A cost-effectiveness comparison of three tailored interventions to increase mammography screening. J Womens Health (Larchmt). 2004;13(8):909–918. 10.1089/jwh.2004.13.909. [DOI] [PubMed] [Google Scholar]
- 17.DeFrank JT, Rimer BK, Gierisch JM, Bowling JM, Farrell D, Skinner CS. Impact of mailed and automated telephone reminders on receipt of repeat mammograms: a randomized controlled trial. Am J Prev Med. 2009;36(6):459–467. 10.1016/j.amepre.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donaldson EA, Holtgrave DR, Duffin RA, Feltner F, Funderburk W, Freeman HP. Patient navigation for breast and colorectal cancer in 3 community hospital settings: an economic evaluation. Cancer. 2012;118(19):4851–4859. 10.1002/cncr.27487. [DOI] [PubMed] [Google Scholar]
- 19.Carkaci S, Geiser WR, Adrada BE, Marquez C, Whitman GJ. How to establish a cost-effective mobile mammography program. Am J Roentgenol. 2013;201(5):W691–W697. 10.2214/AJR.12.9825. [DOI] [PubMed] [Google Scholar]
- 20.Lairson DR, Chan W, Chang YC, del Junco DJ, Vernon SW. Cost-effectiveness of targeted versus tailored interventions to promote mammography screening among women military veterans in the United States. Eval Program Plann. 2011;34(2):97–104. 10.1016/j.evalprogplan.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naeim A, Keeler E, Bassett LW, Parikh J, Bastani R, Reuben DB. Cost-effectiveness of increasing access to mammography through mobile mammography for older women. J Am Geriatr Soc. 2009;57(2):285–290. 10.1111/j.1532-5415.2008.02105.x. [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa Y, Hirai K, Saito H, et al. Cost-effectiveness of a tailored intervention designed to increase breast cancer screening among a non-adherent population: a randomized controlled trial. BMC Public Health. 2012;12:760 10.1186/1471-2458-12-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vidal C, Garcia M, Benito L, Milà N, Binefa G, Moreno V. Use of text-message reminders to improve participation in a population-based breast cancer screening program. J Med Syst. 2014;38(9):118 10.1007/s10916-014-0118-x. [DOI] [PubMed] [Google Scholar]
- 24.Phillips L, Hendren S, Humiston S, Winters P, Fiscella K. Improving breast and colon cancer screening rates: a comparison of letters, automated phone calls, or both. J Am Board Fam Med. 2015;28(1):46–54. 10.3122/jabfm.2015.01.140174. [DOI] [PubMed] [Google Scholar]
- 25.Meghea CI, Williams KP. Aligning cost assessment with community-based participatory research: the Kin KeeperSM Intervention. Health Educ Behav. 2015;42(2):148–152. 10.1177/1090198114557126. [DOI] [PubMed] [Google Scholar]
- 26.Davis TC, Arnold CL, Bennett CL, Wolf MS, Liu D, Rademaker A. Sustaining mammography screening among the medically under-served: a follow-up evaluation. J Womens Health (Larchmt). 2015;24(4):291–298. 10.1089/jwh.2014.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis TC, Arnold CL, Wolf MS, Bennett CL, Liu D, Rademaker A. Joint breast and colorectal cancer screenings in medically underserved women. J Commun Support Oncol. 2015;13(2):47–54. 10.12788/jcso.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabuchi T, Hoshino T, Nakayama T, et al. Does removal of out-of-pocket costs for cervical and breast cancer screening work? A quasi-experimental study to evaluate the impact on attendance, attendance inequality and average cost per uptake of a Japanese Government intervention. Int J Cancer. 2013;133(4):972–983. 10.1002/ijc.28095. [DOI] [PubMed] [Google Scholar]
- 29.Lairson DR, Chang YC, Byrd TL, Lee Smith J, Fernandez ME, Wilson KM. Cervical cancer screening with AMIGAS: a cost-effectiveness analysis. Am J Prev Med. 2014;46(6):617–623. 10.1016/j.amepre.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson B, Thompson LA, Chan NL, Hislop TG, Taylor VM. Cost effectiveness of cervical cancer screening among Chinese women in North America. Asian Pac J Cancer Prev. 2007;8(2):287–293. [PubMed] [Google Scholar]
- 31.Thompson B, Carosso EA, Jhingan E, et al. Results of a randomized controlled trial to increase cervical cancer screening among rural Latinas. Cancer. 2017;123(4):666–674. 10.1002/cncr.30399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scoggins JF, Ramsey SD, Jackson JC, Taylor VM. Cost effectiveness of a program to promote screening for cervical cancer in the Vietnamese-American population. Asian Pac J Cancer Prev. 2010;11(3):717–722. [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Carlson E, Villarreal R, Meraz L, Pagán JA. Cost-effectiveness of a patient navigation program to improve cervical cancer screening. Am J Manag Care. 2017;23(7):429–434. [PubMed] [Google Scholar]
- 34.de Haan MC, Thomeer M, Stoker J, Dekker E, Kuipers EJ, van Ballegooijen M. Unit costs in population-based colorectal cancer screening using CT colonography performed in university hospitals in the Netherlands. Eur Radiol. 2013;23(4):897–907. 10.1007/s00330-012-2689-6. [DOI] [PubMed] [Google Scholar]
- 35.Misra S, Lairson DR, Chan W, et al. Cost effectiveness of interventions to promote screening for colorectal cancer: a randomized trial. J Prev Med Public Health. 2011;44(3):101–110. 10.3961/jpmph.2011.44.3.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolf MS, Fitzner KA, Powell EF, et al. Costs and cost effectiveness of a health care provider-directed intervention to promote colorectal cancer screening among Veterans. J Clin Oncol. 2005;23(34):8877–8883. 10.1200/JCO.2005.02.6278. [DOI] [PubMed] [Google Scholar]
- 37.Shankaran V, Luu TH, Nonzee N, et al. Costs and cost effectiveness of a health care provider-directed intervention to promote colorectal cancer screening. J Clin Oncol. 2009;27(32):5370–5375. 10.1200/JCO.2008.20.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schroy PC III, Glick JT, Robinson P, et al. A cost-effectiveness analysis of subject recruitment strategies in the HIPAA era: results from a colorectal cancer screening adherence trial. Clin Trials. 2009;6(6):597–609. 10.1177/1740774509346703. [DOI] [PubMed] [Google Scholar]
- 39.van Roon AH, Hol L, Wilschut JA, et al. Advance notification letters increase adherence in colorectal cancer screening: a population-based randomized trial. Prev Med. 2011;52(6):448–451. 10.1016/j.ypmed.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 40.Lee JK, Groessl EJ, Ganiats TG, Ho SB. Cost-effectiveness of a mailed educational reminder to increase colorectal cancer screening. BMC Gastroenterol. 2011;11:93 10.1186/1471-230X-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith DH, Feldstein AC, Perrin N, et al. Automated telephone calls to enhance colorectal cancer screening: economic analysis. Am J Manag Care. 2012;18(11):691–699. [PMC free article] [PubMed] [Google Scholar]
- 42.Liss DT, French DD, Buchanan DR, et al. Outreach for annual colorectal cancer screening: a budget impact analysis for community health centers. Am J Prev Med. 2016;50(2):e54–e61. 10.1016/j.amepre.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Giogi Rossi P, Grazzini G, Anti M, et al. Direct mailing of faecal occult blood tests for colorectal cancer screening: a randomized population study from Central Italy. J Med Screen. 2011;18(3):121–127. 10.1258/jms.2011.011009. [DOI] [PubMed] [Google Scholar]
- 44.Grazzini G, Ciatto S, Cislaghi C, et al. Cost evaluation in a colorectal cancer screening programme by faecal occult blood test in the District of Florence. J Med Screen. 2008;15(4):175–181. 10.1258/jms.2008.008032. [DOI] [PubMed] [Google Scholar]
- 45.Schlichting JA, Mengeling MA, Makki NM, et al. Increasing colorectal cancer screening in an overdue population: participation and cost impacts of adding telephone calls to a FIT mailing program. J Commun Health. 2014;39(2):239–247. 10.1007/s10900-014-9830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stokamer CL, Tenner CT, Chaudhuri J, Vazquez E, Bini EJ. Randomized controlled trial of the impact of intensive patient education on compliance with fecal occult blood testing. J Gen Intern Med. 2005;20(3):278–282. 10.1111/j.1525-1497.2005.40023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Broc G, Denis B, Gana K, Gendre I, Perrin P, Pascual A. Impact of the telephone motivational interviewing on the colorectal cancer screening participation. A randomized controlled study. Rev Eur Psychol Appl. 2015;65(3):133–142. 10.1016/j.erap.2015.04.002. [DOI] [Google Scholar]
- 48.Green BB, Wang CY, Anderson ML, et al. Automated intervention with stepped increases in support to increase uptake of colorectal cancer screening: a randomized trial. Ann Intern Med. 2013;158(5 Pt 1):301–311. 10.7326/0003-4819-158-5-201303050-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Mil R, Guillaume E, Guittet L, et al. Cost-effectiveness analysis of a navigation program for colorectal cancer screening to reduce social health inequalities: a French cluster randomized controlled trial. Value Health. 2018;21(6):685–691. 10.1016/j.jval.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 50.Lairson DR, Dicarlo M, Deshmuk AA, et al. Cost-effectiveness of a standard intervention versus a navigated intervention on colorectal cancer screening use in primary care. Cancer. 2014;120(7):1042–1049. 10.1002/cncr.28535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong MC, Ching JY, Chan VC, Sung JJ. The comparative cost-effectiveness of colorectal cancer screening using faecal immunochemical test vs. colonoscopy. Sci Rep. 2015;5:13568 10.1038/srep13568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lairson DR, DiCarlo M, Myers RE, et al. Cost-effectiveness of targeted and tailored interventions on colorectal cancer screening use. Cancer. 2008;112(4):779–788. 10.1002/cncr.23232. [DOI] [PubMed] [Google Scholar]
- 53.Tifratene K, Eisinger F, Rinaldi Y, Didelot R, Seitz JF. Colorectal cancer screening program: cost effectiveness of systematic recall letters. Gastroenterol Clin Biol. 2007;31(11):929–933. 10.1016/S0399-8320(07)78300-8. [DOI] [PubMed] [Google Scholar]
- 54.Baker DW, Brown T, Buchanan DR, et al. Comparative effectiveness of a multifaceted intervention to improve adherence to annual colorectal cancer screening in community health centers: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1235–1241. 10.1001/jamainternmed.2014.2352. [DOI] [PubMed] [Google Scholar]
- 55.Elmunzer BJ, O’Connell MT, Prendes S, et al. Improving access to colorectal cancer screening through medical philanthropy: feasibility of a flexible sigmoidoscopy health fair for uninsured patients. Am J Gastroenterol. 2011;106(10):1741–1746. 10.1038/ajg.2011.147. [DOI] [PubMed] [Google Scholar]
- 56.Larkey LK, Herman PM, Roe DJ, et al. A cancer screening intervention for underserved Latina women by lay educators. J Womens Health (Larchmt). 2012;21(5):557–566. 10.1089/jwh.2011.3087. [DOI] [PubMed] [Google Scholar]
- 57.Meenan RT, Anderson ML, Chubak J, et al. An economic evaluation of colorectal cancer screening in primary care practice. Am J Prev Med. 2015;48(6):714–721. 10.1016/j.amepre.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jandorf L, Stossel LM, Cooperman JL, et al. Cost analysis of a patient navigation system to increase screening colonoscopy adherence among urban minorities. Cancer. 2013;119(3):612–620. 10.1002/cncr.27759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim B, Lairson DR, Chung TH, Kim J, Shokar NK. Budget impact analysis of against Colorectal Cancer in Our Neighborhoods (ACCION): a successful community-based colorectal cancer screening program for a medically underserved minority population. Value Health. 2017;20(6):809–818. 10.1016/j.jval.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 60.Wilson FA, Villarreal R, Stimpson JP, Pagán JA. Cost-effectiveness analysis of a colonoscopy screening navigator program designed for Hispanic men. J Cancer Educ. 2015;30(2):260–267. 10.1007/s13187-014-0718-7. [DOI] [PubMed] [Google Scholar]
- 61.Shokar NK, Byrd T, Lairson DR, et al. Against colorectal cancer in our neighborhoods, a community-based colorectal cancer screening program targeting low-income Hispanics: program development and costs. Health Promot Pract. 2015;16(5):656–666. 10.1177/1524839915587265. [DOI] [PubMed] [Google Scholar]
- 62.Sequist TD, Franz C, Ayanian JZ. Cost-effectiveness of patient mailings to promote colorectal cancer screening. Med Care. 2010;48(6):553–557. 10.1097/MLR.0b013e3181dbd8eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elkin EB, Shapiro E, Snow JG, Zauber AG, Krauskopf MS. The economic impact of a patient navigator program to increase screening colonoscopy. Cancer. 2012;118(23):5982–5988. 10.1002/cncr.27595. [DOI] [PubMed] [Google Scholar]
- 64.Davis TC, Arnold CL, Bennett CL, et al. Strategies to improve repeat fecal occult blood testing cancer screening. Cancer Epidemiol Biomark Prev. 2014;23(1):134–143. 10.1158/1055-9965.EPI-13-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davis T, Arnold C, Rademaker A, et al. Improving colon cancer screening in community clinics. Cancer. 2013;119(21):3879–3886. 10.1002/cncr.28272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee KS, Park EC. Cost effectiveness of colorectal cancer screening interventions with their effects on health disparity being considered. Cancer Res Treat. 2016;48(3):1010–1019. 10.4143/crt.2015.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.