Abstract
The role of the thalamus in cortical sensory transmission is well-known, but its broader role in cognition is less appreciated. Recent studies have shown thalamic engagement in dynamic regulation of cortical activity in attention, executive control and perceptual decision making, but the circuit mechanisms underlying such functionality are unknown. Because the thalamus is composed of excitatory neurons that are devoid of local recurrent excitatory connectivity, delineating long-range, input/output connectivity patterns of single thalamic neurons is critical for building functional models. We discuss this need in relation to existing organizational schemes such as core vs. matrix and first order vs. higher order relay nuclei. We propose that a new classification is needed based on thalamocortical motifs, where structure naturally informs function. Overall, our synthesis puts understanding thalamic organization at the forefront of existing research in systems and computational neuroscience, with both basic and translational applications.
Introduction
In the context of mammalian evolution, the expansion of the cortex has been associated with the rise of higher order cognitive functions (Buckner and Krienen, 2013; Krubitzer and Prescott, 2018). In fact, human cortical expansion is hypothesized to be the core driver of our ability to reason (Mansouri et al., 2017), conceive abstract thoughts, generate language (Hage and Nieder, 2016), and build elaborate social structures (Bicks et al., 2015; Jost et al., 2018). The focus on cortical function in neuroscience and its quest for intelligence has inspired the construction of artificial neural networks that can rival human abilities on visual recognition (Yamins et al., 2014), playing video games (Silver et al., 2017) and spatial navigation (Banino et al., 2018).
In this review, we propose that the quest for neural basis of cognition requires a better understanding of the role of thalamus in cortical function, especially since thalamic evolutionary expansion parallels that of the cortex itself and is intimately involved in communication between cortical areas (Halassa and Kastner, 2017; Rikhye et al., 2018b; Sherman, 2016). The thalamus is a collection of nuclei primarily composed of excitatory neurons that project to cortex but lack local excitatory recurrent connections, a hallmark of cortical circuitry. In other words, all of the known excitatory inputs to these thalamic neurons emanate from extrathalamic sources whereas the vast majority of such inputs to cortical neurons are quite local in origin (Binzegger et al., 2009; Braitenberg and Schütz, 1998). As such, the computations performed by a thalamic neuron will be dependent on its long-range excitatory inputs (Rikhye et al., 2018b) as well as local inhibitory recurrence through the thalamic reticular nucleus (Lee et al., 2014; Pinault and Deschenes, 1998). The impact of the ensuing thalamic output on cortical computations is dependent on the connectivity patterns thalamic neurons make in cortex.
A major impediment to understanding the thalamus and its role in cortical function is the fact that our knowledge of input/output connectivity schemes across the thalamus are limited. Thus, what is needed is a complete classification of these functionally-relevant connectivity patterns, which we refer to as “thalamo-cortical motifs.” Our definition of a motif is the input/output functional architecture of a single thalamic neuron that is informative of what computation that neuron performs and how its resulting output impacts its cortical targets. As such, it includes the set of driving inputs to a single thalamic neuron, along with the precise cortical connectivity pattern of that same neuron. Of course, additional knowledge of the capacity of the input and ouptut connections for plasticity, along with the precise learning rules, is an important determinant of the types of computations performed, but we will only briefly mention this important topic here.
An important point that we would like to communicate throughout this review is that identifying the variety of thalamo-cortical motifs represents a basic classification strategy that is an essential early process in understanding any complex system, and indeed such a classification strategy has proven quite fruitful in central nervous system research more generally. For instance, early advances in retinal research have involved a classification of component cell types---photoreceptors, ganglion cells, etc., followed by a subclassification of these cells, such as rods and cones and different ganglion cell types. This type of early work paved the way for complete connectomics of the retina’s inner plexiform layer (Helmstaedter et al., 2013), which has played important roles in constraining models of retinal function, such as those involving the computation of visual motion (Borst and Helmstaedter, 2015). Similarly, and given the increasing interest in the role of thalamo-cortical interactions in cognitive function, we believe it is important to recognize that a proper classification of motifs underlying these interactions is lacking, and this is vitally needed. We argue that current attempts at classification, and there have been many, are clearly inadequate. We will review recent developments in thalamo-cortical research that highlight limitations of these existing classification schemes and discuss the sorts of data required to establish a comprehensive one. More specifically, we will argue that the diversity of thalamic functions will clarify how an organizational scheme based on input/output connectivity data at the single cell level would provide a thalamo-cortical classification system that is far more comprehensive and functionally-meaningful than currently existing ones. Lastly, we will end on how such basic understanding will propel future efforts in biomedical sciences, including clinical applications, as well as development of brain-inspired artificial neural networks with expanded cognitive capacity.
Existing Thalamocortical Classification Systems
Broad Classification: Thalamic nuclei
Perhaps the oldest thalamic classification scheme for thalamus is the use of histological criteria to divide the structure into a number of distinct nuclei. These nuclei can be segregated into an anterior group (anteriomedial, anteriolateral, anterodorsal and laterodorsal nuclei); a medial division composed of the midline group (paratenial, paraventricular and centromedian; an intralaminar group (centrolateral, centromedial and parafascicular) and the mediodorsal nucleus; a lateral division containing the ventroanterior/ventrolateral group, the ventrobasal complex (the ventral posteriomedial and ventral posterolateral nuclei), and ventromedial nucleus; and a posterior group containing the posteromedial nucleus, the lateroposterior nucleus, the pulvinar, and the medial and lateral geniculate nuclei. Abutting these nuclei laterally is a shell of inhibitory neurons, collectively known as the thalamic reticular nucleus, which is derived from the ventral thalamus (Jones, 2007). Although these nuclear divisions broadly inform function, the idea that a single thalamic nucleus performs a single function is far from accurate (Phillips et al., 2018). Instead, it is clear from studying a limited subset of thalamic nuclei that heterogeneity of circuit form is the rule rather than the exception (Rikhye et al., 2018b). This is a major thrust for our proposal for a new classification system.
Classification based solely on thalamocortical output
Core and matrix
Jones (Jones, 2001) has suggested a major thalamic classification scheme that divides thalamocortical projections into what he calls “core” and “matrix”: core neurons project in a spatially-dense and topographically restricted manner and innervate middle cortical layers, whereas matrix neurons project in a spatially-sparse and topographically diffuse manner, innervating superficial cortical layers, and largely layer 1 (Figure 1A). Also, core neurons innervate only cortex (with branches to the thalamic reticular nucleus en route to cortex), whereas matrix neurons also innervate the basal ganglia; the quantitative breakdown of individual matrix neurons preferentially innervating cortex or basal ganglia, dually innervating both structures, or forming a population that continuously represent both features remains unclear (Kuramoto et al., 2015; Nakamura et al., 2015; Ohno et al., 2012; Unzai et al., 2017). This classification refers to individual thalamocortical cells, because thalamic nuclei are composed of different proportions of core and matrix neurons. The lateral geniculate nucleus is a nucleus that contains both types (see below). However, some nuclei are chiefly core, such as the ventral posterior medial, and others, matrix, such as centroedian.
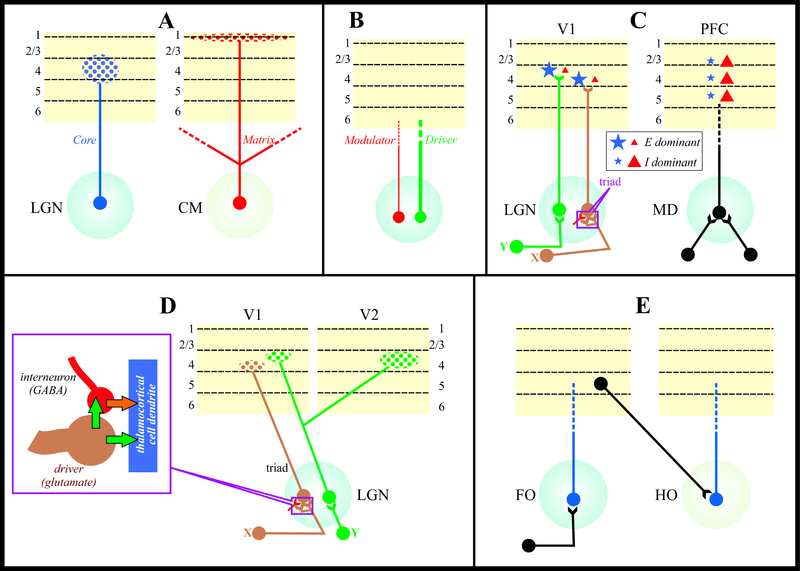
Figure 1.
Examples of various classification attempts that include partial descriptions of thalamocortical motifs. A: Core and matrix (output characteristics). The geniculocortical projection, an example of a core system, mainly targets middle cortical layers in a highly topographical manner (left), whereas the projection from the centromedian nucleus, an exemplar of a matrix system, diffusely targets upper cortical layers, chiefly layer 1 (right) and typically innervates multiple cortical areas. B: Drivers and modulators (output characteristics). Thalamocortical afferents can be either driver or modulator. C: LGN vs MD (input and output characteristics), in addition to the input differences shown in D, LGN X and Y cells receive input from very few retinal axons, often only one, and their outputs chiefly innervate excitatory cells in V1 (left), whereas some MD neurons, which receive significant convergent driver input, predominantly activate inhibitory cells in prefrontal cortex (PFC, right). D: X and Y streams through the cat’s LGN (both input and output characterization). On the input side, retinal Y axons form simple synapses onto dendritic shafts, whereas retinal X axons form triadic synaptic complexes (inset). On the output side, X axons innervate only V1, chiefly in the lower half of layer 4, whereas Y axons innervate the upper half of layer 4 and branch to innervate V2 as well. E: First and higher order (FO and HO; predominantly input characteristics) thalamocortical circuits. Driver input to FO cell derive from a a subcortical source (i.e., retina to LGN), whereas HO thalamocortical cells receive driver input from layer 5 of cortex (e.g, pulvinar from layer 5 of V1).
More recent systematic single cell fills have challenged the core/matrix dichotomy by showing a substantially richer variety of thalamocortical output types (Clasca et al., 2012; Kuramoto et al., 2017). For example, in frontal thalamic structures the same neurons can branch to exhibit spatially-diffuse projections that target middle layers, or spatially-dense projections that target superficial and deep layers simultaneously. To complicate matters even more, an individual thalamic neuron may, via a branching axon, have one or more core-like projections targeting one area and a matrix-like projection targeting another (Clasca et al., 2012). As such, it is clear that the idea of core and matrix either captures only a limited subset of thalamocortical output types or represents ends of a single continuum with considerable variability. This, compounded with the complexity added when factoring in the types of cortical neurons these thalamic outputs target suggests that there may be a wide range of functional outputs the thalamus imparts on cortical circuitry.
Drivers and modulators
Sherman and Guillery noted that glutamatergic pathways, which include thalamocortical projections, are not homogeneous but rather can be classified into two types, which they referred to as drivers and modulators (Sherman and Guillery, 1998, 2013). They further proposed that drivers carry the main information to their targets, whereas modulators perform many of the same operations as do the classical modulators (e.g., ACh, NA, 5-HT, etc.). For example, retinal input to geniculate thalamocortical cells have driver properties, and the layer 6 input from visual cortex have modulator properties, and both are glutamatergic. There is evidence that thalamocortical projections contain both types although the examples documented are too few to draw more general conclusions Figure 1B. For details of the patterns so far described, see (Lee and Sherman, 2008; Viaene et al., 2011a, b, c).
Excitatory versus inhibitory cortical targets
Of equal significance is the functional impact a thalamocortical projection has on its cortical target, which will necessarily depend on the strength and timing of connectivity to the various cell types within that particular cortical region. For example, while projections from primary sensory thalamic regions contact both excitatory and inhibitory cortical neurons, the inhibitory component is largely accounted for by connections onto parvalbumin positive interneurons (Tuncdemir et al., 2016). This can be contrasted to thalamic projections from higher order sensory nuclei, such as the posterior medial nucleus, which also targets vasoactive intestinal peptide positive interneurons (Williams and Holtmaat, 2018), known to specialize in disinhibitory control (Pi et al., 2013). In addition, purely functional evidence derived from mediodorsal thalamic projections onto the prefrontal cortex suggests that these projections are far more efficient at driving cortical inhibition compared to the inhibitory impact of a first order sensory thalamic input on its cortical targets (Schmitt et al., 2017) (Figure 1C).
Single versus multiple cortical targets
Some thalamic cells target a single cortical area (e.g., geniculate X cells in the cat; Figure 1D), whereas other send axons that branch to innervate multiple areas. This is seen both for core projections (e.g., geniculate Y cells; Figure 1D), and matrix projections (Figure 1A).
Classification based solely on input
First and higher order thalamic nuclei
Perhaps the best studied thalamic structure is the lateral geniculate nucleus, which represents the main station for visual information traveling from the retina to primary visual cortex (Sherman, 2017; Sherman and Guillery, 2013, 2014). Although structural and functional features of the lateral geniculate have been useful for interpreting data from other thalamic structures interposed between subcortical sensory inputs and primary sensory cortical areas (Lee, 2013; Reichova and Sherman, 2004), the utility to understanding thalamic function more broadly may be limited. The key limitation stems from the fact that lateral geniculate neurons are driven by retinal inputs, with cortical input derived only from layer 6 playing only a modulatory role (Briggs and Usrey, 2011; Sherman and Guillery, 1998, 2013).
In contrast, many other thalamic nuclei contain neurons that are primarily driven by layer 5 cortical inputs, potentially with varying degrees of convergence (Groh et al., 2014; Reichova and Sherman, 2004; Rovo et al., 2012; Sherman, 2016; Sherman and Guillery, 2013). Higher order relays appear to serve as a station in transthalamic corticocortical communication, as opposed to direct corticocortical pathways; often cortical areas are connected by both direct and transthalamic pathways organized in parallel (for review, see (Sherman, 2012; Sherman and Guillery, 2011)) (Figure 1D. This has provided one useful classification of thalamic nuclei: first order nuclei receive their driving input from a subcortical source, such as the retina, whereas higher order relays are driven by input from layer 5 of cortex.
One problem with this classification scheme is that whereas first order thalamic nuclei seem completely first order, meaning that all driving input emanates from subcortical sites, higher order nuclei may often contain first order elements. For instance, the pulvinar and mediodorsal nucleus both are innervated by the superior colliculus, a subcortical source, and if this collicular input contains driver afferents(Beltramo and Scanziani, 2019; Kelly et al., 2003), then these higher order nuclei would also contain first order circuitry. Another problem is that this classification does not account for a key distinguishing feature across distinct thalamic circuits; convergence. Limited convergence of driver inputs, such as retinogeniculate synapses, is well known, but in such examples, the driver inputs share basic receptive field properties, meaning that transformation of information passed on to cortex is limited. However, there is evidence for layer 5 cortical input convergence from different cortical areas onto single thalamic neurons (Groh et al., 2014; Rovo et al., 2012), and this suggests that significant transformation of information is possible. In addition, evidence for cortical input convergence onto single thalamic neurons can be inferred from functional studies involving prefrontal inputs onto mediodorsal nucleus, where thalamic neurons represent conjunctions of cortical signals (Rikhye et al., 2018a) (Figure 1C). This type of encoding is reminiscent of activity observed in the non-human primate pulvinar, where thalamic neurons reflect a transformation of cortical motion signals that are directional into a non-directional signal of confidence (Komura et al., 2013), which may be explained by cortico-thalamic convergence (Jaramillo et al., 2019). The degree of input convergence (both in terms of magnitude and type) coupled with distinct types of learning rules may endow thalamic circuits with truly distinct computational functions, which we think is critical to consider in a comprehensive classification scheme.
Triadic vs non-triadic circuitry
Triadic circuitry, which involves three synapses, is ubiquitous throughout thalamus (reviewed in (Sherman and Guillery, 2013)). Such circuitry involves the driver input. In a triad, the driver input forms two synapses, one onto the dendrite of a thalamocortical cell and the other onto a terminal from a GABAergic interneuron, and the interneuron terminal forms a synapse onto the same thalamocortical cell dendrite. Thus the three synapses are: 1) driver input onto a thalamocortical cell dendrite, 2) driver input onto an interneuronal terminal, and 3) a synapse from the same interneuronal terminal onto the same thalamocortical cell dendrite. Note that the interneuronal terminal is both presynaptic and postsynaptic; it also derives from an interneuronal dendrite rather than from the axon (Sherman, 2004).
However, many driver inputs do not enter into triadic arrangements. Instead they form simple, conventional synapses onto thalamocortical cell dendrites. An example of these two types of driver input is seen in retinal X and Y innervation of the cat’s LGN (Figure 1E): X axons innervate via triads whereas Y axons do not (Hamos et al., 1987; Wilson et al., 1984). Although the function of the triad remains unknown (but see (Sherman, 2004)) it seems likely that the presence or absence thereof is a significant classificatory parameter in defining thalamocortical motifs.
Classification of parallel thalamocortical streams
Parallel processing streams to classify thalamocortical pathways may be regarded as the ultimate form of identifying thalamocortical motifs, because at the end of the day, it seems likely that most or all such defined motifs will be seen as operating independently and in parallel with respect to one another. Unfortunately, this classification approach has been used successfully only with respect to the lateral geniculate nucleus, mainly in carnivores and primates.
In the cat, for instance, independent and parallel retino-geniculo-cortical streams known as W, X, and Y have been identified (Sherman, 1985; Stone, 1983). These start with distinct classes of retinal ganglion cell, each of which innervates a distinct class of geniculate cell for projection to visual cortex. (A comprehensive description of these pathways is beyond the scope of this account, but details can be found in (Sherman, 1985; Stone, 1983)). Differences are seen both on the inputs to geniculate cells as well as their cortical outputs, but this is best seen in the X and Y pathways involving the presence or absence of triads as noted above.
Outputs of cat geniculate X and Y cells also differ (Humphrey et al., 1985a, b) (Figure 1E). Both chiefly innervate layer 4 of V11 and as such would be considered core projections (see above). However, Y axons terminate in the upper half of layer 4, whereas X axons, the lower half. Also, X axons branch less and innervate a single patch of layer 4, whereas Y axons branch to innervate several patches, and some even innervate V22 as well. As a result, each geniculate Y axon produces more synapses and covers more territory on average than does each X axon.
W cells in retina and the lateral geniculate nucleus pose a classificatory problem, because they are probably a heterogeneous group that includes multiple classes. Thus, the classification of this group is incomplete. Nonetheless, with this proviso in mind, it is clear that W cells have yet a projection patter to cortex different from X and Y cells. W cells do not innervate layer 4 but rather chiefly innervate layers 2/3 (Boyd and Matsubara, 1996; Kawano, 1998). Some extend axon arbors into layer 1, and these then would be seen as matrix projections (see above). However, there is no innervation by the lateral geniculate nucleus of the basal ganglia, a matrix feature. These discrepancies further reinforce the notion that the core/matrix dichotomy does not adequately describe the diversity observed even in a fairly well-characterized structure such as the lateral geniculate.
Data from the monkey regarding differences among parallel retino-geniculo-cortical streams is less well documented that those in the cat, but the available data indicate a similar arrangement. In the monkey, these are called the K (for koniocellular), M (for magnocellular), and P (for parvocellular), and these seem homologous to the pathways in the cat: K homologous with W, M with Y, and P with X (Casagrande and Xu, 2004). Like the differences in cortical projection patterns in the cat, M and P chiefly target layer 4, with the M pathway terminating dorsal to the P and producing larger terminal arbors, and the K pathway seems to involve multiple classes that mainly innervate layers 2/3 with some innervation of layer 1 but no innervation of the basal ganglia (Casagrande and Xu, 2004; Ding and Casagrande, 1997).
It is thus clear that these parallel retino-geniculo-cortical streams represent distinct thalamocortical motifs. A problem is that such parallel processing has not yet been documented more generally beyond the lateral geniculate nucleus. Such further documentation is in fact a main point of this manuscript.
Toward a comprehensive classification of thalamo-cortical circuit motifs
The structural diversity of thalamocortical projections observed within and across thalamic nuclei indicates that the thalamus can influence cortical representations and dynamics in a multitude of ways. Importantly, different thalamocortical outputs from within a single nucleus can give rise to distinct computational functions partly through differences in which layers and cell types a thalamic terminal innervates in the cortical recipient region. We suggest that comprehensive mapping of the different types of thalamo-cortical output types is of critical importance. Methods of achieving this are experiencing rapid technical growth, and include high resolution single-cell terminal mapping (Lichtman et al., 2008). While traditional methods have relied on sparse labeling and complete reconstruction of single cell terminal fields, recent development in fluorescent (Chung et al., 2013; Gradinaru et al., 2007) and genetic bar coding technologies (Kebschull and Zador, 2018) may allow for deriving similar insights based on densely labeled samples, which would provide substantially higher throughput. Utilizing these insights for building functional models would also require the incorporation of ultrastructural data, which would provide a proxy for synaptic strength based on terminal size, number of vesicles and mitochondria (Cserep et al., 2018; Harris and Weinberg, 2012). Furthermore, by correlating such ultrastructural data for thalamocortical pathways identified as driver or modulator (Viaene et al., 2011a, b, c), might permit identifying such functional classes in connectomic data. Separately, determining which cortical neurons are innervated by a thalamocortical projection would benefit from the development of anterograde trans-synaptic tracing (Zingg et al., 2017). Ultimately, the combination of all these structural tools and physiological recordings will be necessary for directly testing the inferred functional models.
A comprehensive mapping of thalamocortical output types will only constitute half the battle, as the other half will require determining what the inputs are, including type, strength and degree of convergence that individual thalamic neurons receive (Figure 1A–D). Such an endeavor would benefit from monosynaptic transsynaptic retrograde labeling originating from single thalamic neurons, which currently require the use of pseudorabies viruses (Ghanem and Conzelmann, 2016). Alternatively, if bar coding methods were to be developed in which both the pre and postsynaptic neurons are identifiable, then such mapping can be performed in a high throughput manner. Similar to output mapping, input mapping would require co-registration with physiological studies to provide a blueprint for subsequent functional studies.
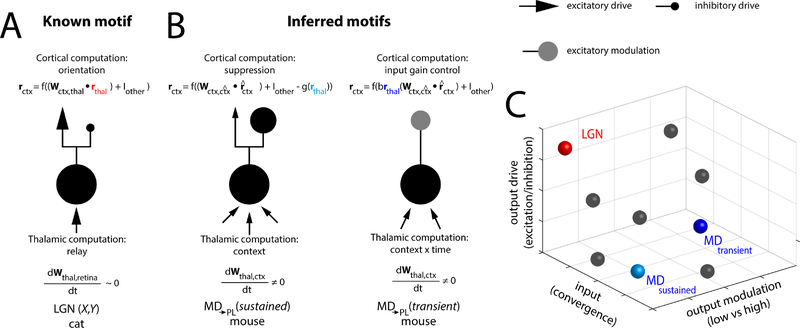
Although we can only speculate as to what this endeavor would ultimately yield, it is almost inevitable that several putative thalamocortical motifs would be identified (Figure 2A–B). One key idea that we emphasize is that these motifs are informative of computational function. For example, the fact that retinal terminals form excitatory driving inputs that exhibit little convergence onto individual geniculate neurons renders geniculate receptive fields very similar to those of retinal ganglion cells (Hubel and Wiesel, 1961; Usrey et al., 1999). Receptive fields of thalamo-recipient visual cortical neurons are built from the appropriately wired geniculate inputs, giving rise to orientation- and direction selective neural responses (Finn et al., 2007; Hubel and Wiesel, 1961; Lien and Scanziani, 2013, 2018; Usrey et al., 2000). Combining these known input/output properties, this geniculate motif intuitively supports the idea that the thalamic neurons included operate as relays since minimal alteration of the retinal inputs occur (Figure 2A). In contrast, inferred input-output connectivity patterns of a subset of neurons within the mediodorsal nucleus indicates that their interactions with the prefrontal cortex is not well-explained by a similar relay design (Figure 2B). More specifically, these mediodorsal neurons may receive highly convergent prefrontal inputs, and by adjusting the connectivity weights on these inputs, each neuron may generate a distinct conjunctive representation. Our experimental data have shown that these conjunctive representations can be understood as ones encoding a task-context. In a sense, we hypothesize that these thalamic neurons may implement a competitive Hebbian learning algorithm that allows them to capture cortical input statistics (principal components) (Diamantaras and Kung, 1996; White, 1992). As such, and solely based on their conjunctive neural responses, these thalamic neurons violate the notion of a relay. Based on other experimental findings, we also suggest that their functional impact on the prefrontal cortex deviates from that of the geniculate on primary visual cortex. More specifically, some neurons appear to efficiently drive cortical inhibition (Figure 2B, left), likely through stronger and/or more plentiful contacts onto inhibitory interneurons compared to excitatory ones. Other mediodorsal neurons appear to exert a modulatory impact on prefrontal neurons, enhancing their local functional connectivity ((Schmitt et al., 2017); Figure 2B, left). Mechanistically, this modulatory impact may be exerted through a neuromodulatory signal from thalamic terminals (Casas-Torremocha et al., 2019; Viaene et al., 2011b) or disinhibitory circuit motifs (Williams and Holtmaat, 2018), as had been shown in other thalamic structures.
Figure 2:
Towards a classification system based on thalamo-cortical motifs. In panels A and B, individual and idealized single thalamic neurons are shown to illustrate the notion of the motif being a single-cell attribute (A) Example of a well-characterized thalamocortical motif, involving the retino-geniculo-cortical pathway, with the set of computations required for retinal signal transmission. X and Y geniculate neurons of the cat receive retinal inputs with minimal convergence, and therefore show similar responses to those of the retina. An important feature of this system is that the retinogeniculate connections are relatively stable in adult animals, rendering geniculate neurons stably tuned to visual features. On the output side, because geniculate neurons providing predominantly driving excitatory inputs to visual cortex, cortical responses can be largely explained as weighted sums of the thalamic output (B)Two examples of inferred motifs within the mediodorsal thalamus of the mouse. These connections are derived from statistical dependencies between the mediodorsal neurons and prefrontal ones, which are recorded in a context-switching task (Rikhye et al., 2018). These statistical dependencies have also been tested through pathway-specific optogenetic manipulations. Mediodorsal neural types can be segregated based on inputs, which are explained by the degree of their cortical input convergence. Specifically, a subset of neurons encodes a set of task-relevant prefrontal cue-selective signals over a broad temporal scale (left), and another encoding the same type of task-relevant variable but on a shorter timescale (right). Given that these response profiles shift on a session-by-session basis depending on how the context is experimentally configured, a reasonable interpretation is that the cortico-thalamic connections are highly plastic. These same mediodorsal types also segregate based on output, with the high input convergence neurons exhibiting predominantly suppressive effects on prefrontal cortical activity, while the low input convergence neurons exhibiting predominantly modulatory excitatory effects. By modulation, we do not necessarily mean that the effect is implemented through a neuromodulator, but rather that it controls the gain of effective recurrent connections in the prefrontal cortex (see equations within the figure). (C) A putative classification space for thalamocortical motifs, with the relevant geniulate and mediodorsal types plotted within. The number and distribution of points within this space are currently unknown, but we expect that their pattern will inform function and comparisons across species.
Notation: the embedded equations describe the input-output transformations of the thalamocortical motifs. Lower boldface symbols denote vectors and upper boldface symbols denote matrices. rcortex: cortical output, f(): cortical non-linearity, r^cortex: recurrent drive, rthal: thalamic input, g(): non-linearity over thalamic input for the middle motif, b: scaling parameter for the thalamic term in the last motif. The key idea for this formalism is that the thalamic input shows up as very different terms in the cortical computation performed. This is what is precisely meant by relay vs. non-relay functions of the thalamus.
Directly testing these inferred motifs, and ultimately defining them will hopefully lead to a new ‘classification space’ where the axes are structural attributes that most explain the functional variance across thalamic circuits. In Figure 2C, we utilize attributes that we have discussed throughout this review (although, we do not know whether these will ultimately be the most meaningful), to illustrate how the geniculate and mediodorsal circuits discussed would be plotted within such a space. Other thalamic circuits would fall into different locations within this space, making clustering in this feature space a natural way to group circuits based on similarities in their input/output computations. Our guess is that these clusters (which would be informative of common computations across thalamocortical motifs) would not necessarily respect traditional nuclear boundaries.
Achieving a proper classification of thalamocortical motifs, including a hierarchical subclassification analogous to the classification of retinal ganglion cells, is of critical importance for at least two reasons. First, such a classification is a prerequisite for significantly improving our understanding of thalamocortical functioning. Second, it is also a prerequisite for establishing homologs needed for generating an appreciation of thalamocortical functioning applicable to mammals as a whole.
Classification as a prerequisite to better understand thalamocortical functioning
The structural diversity of thalamocortical projections observed within and across thalamic nuclei indicates that the thalamus can influence cortical representations and dynamics in a multitude of ways. Importantly, different thalamocortical outputs from within a single nucleus can give rise to distinct computational functions partly through differences in which layers and cell types a thalamic terminal innervates in the cortical recipient region(s). Current models of thalamocortical functioning tend to be biased by a few examples, such as the geniculocortical pathways, but we now know that thalamocortical circuitry contains considerable variability and that geniculocortical pathways represent a limited set of thalamocortical motifs. However, in addition to the diversity of thalamocortical projections is the potential diversity in inputs to thalamocortical neurons, including the type, strength, and degree of convergence that these individual neurons receive. An open question is: How many distinct types of computational functions are supported by the diversity of thalamocortical motifs, which includes an analysis of both inputs to and outputs of thalamocortical neurons? We submit that, until this answer is realized, we will lack an important insight into thalamocortical functioning.
Given the growing evidence for thalamic dysfunction across a variety of neurological (Schiff, 2008) and psychiatric (Clinton and Meador-Woodruff, 2004; Krol et al., 2018; Scheibel, 1997; Schmitt and Halassa, 2017) disorders, we predict that the result of identifying functional thalamocortical motifs will be of significant impact on public health. For example, the recognition that the lateral geniculate nucleus can be a locus for attentional control across mice (Wimmer et al., 2015), non-human primates (Briggs et al., 2013; McAlonan et al., 2006) and humans, calls for further studies regarding the dysfunction of sensory thalamic motifs in disorders with an attentional component. Which motifs are related to attentional demands and how are these affected in disorders related to attention? For another example, there is evidence of cognitive defects associated with pathology in higher order thalamic relays (Chauveau et al., 2005; Means et al., 1974; Rafal and Posner, 1987) but again, which specific motifs are involved?
Furthermore, thalamocortical research may benefit the growing synergy between neuroscience and artificial intelligence. The fact that multiple studies have shown different roles for thalamic circuits in cortical learning and dynamic switching of computational states (Rikhye et al., 2018a; Saalmann and Kastner, 2015) is of great relevance for the development of artificial intelligence architectures that can rapidly learn and flexibly switch (Masse et al., 2018). A proper classification is a prerequisite for understanding which motifs may be involved in these behaviors and how they operate, and as such, this would enhance the potential of the synergy noted above.
We recognize that achieving such a classification is a daunting task and may seem unattainable at present. However, we are experiencing an amazing period of new technology applicable to neuroscience questions such as those raised here. Tools for identifying unique neuron classes, for defining detailed connectivity at electron microscopic resolution in larger and larger volumes, for exploring functional circuitry via new imaging and recording techniques, and for transsynaptic neuroanatomical tracing are already available and can be expected to increase in quality and quantity. Whereas it may be the case that a complete classification of thalamocortical motifs is beyond our capabilities now and for the foreseeable future, we feel nonetheless that emphasizing the issue is important.
Classification as a prerequisite for establishing homologies with other mammalian species
What has not yet been stated explicitly here is that the sort of classification we describe will almost certainly depend heavily on the mouse as the model species. This is because most of the technical advances applicable to mammals and alluded to above have been pioneered in the mouse, and many of these are applicable only to this species. The challenge with the mouse as a model for neuroscience is that those of us working with this species hope to address questions that are relevant to all mammals and avoid those that apply only to mice or rodents. This is not an easy or straightforward approach.
Studies of parallel processing in the visual system across mammalian species serves as a good example for this point (see above). In the cat, W, X, and Y pathways have been documented, and these appear to be homologous, respectively, to the K, P, and M pathways in the monkey. As noted above, this has served as a partial classification of thalamocortical motifs. More to the point here, the distant evolutionary relationship between cats and monkeys suggests that these motifs are general mammalian features, and study of identified homologous thalamocortical motifs in any species can provide insights applicable to all. The mouse has now become a major model to study visual pathways, but in doing so, how do we determine the relevance of any findings to mammals more generally? An important first step would be to identify motifs in the mouse pathways, especially geniculocortical ones, that are candidates for homology to those of the cat and monkey. We cannot hope to establish geniculocortical homologies across species until we first determine how many classes exist in each species. Of course, the number of geniculocortical motifs may differ across species, because, for instance, the process of evolution may produce extra motifs found in monkey compared to those in the mouse. Nonetheless, a classification of geniculocortical motifs in the mouse should be seen as a first step in determining which, if any, are candidates for being homologous to those in the cat or monkey.
Conclusions
As we have documented above, there have been numerous attempts to classify thalamocortical motifs, but each is demonstrably quite limited. These attempts demonstrate the understood value in providing such a classification. However, this understanding has generally been implied subtly or not at all, and we feel it important to clarify rather explicitly the importance of a thorough classification of thalamocortical motifs as a prerequisite to a more complete understanding of thalamocortical functioning in health and disease. Even though the means to achieve such a complete classification are not yet available, we nonetheless argue that identifying the issue as we have attempted to do here is a useful step in the process.
Acknowledgement
We thank members of the Halassa and Sherman labs for thoughtful discussions. MMH is supported by grants from the NIH, Brain and Behavior, Klingenstein, Simons and Pew Foundations as well as the Human Frontiers Science Program. SMS is supported by grants from the NINDS (NS094184) and NEI (EY022388)
Footnotes
Declaration of Interests
The authors declare no competing interests
Also known as area 17 or primary visual cortex.
Also known as area 18 or secondary visual cortex
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banino A, Barry C, Uria B, Blundell C, Lillicrap T, Mirowski P, Pritzel A, Chadwick MJ, Degris T, Modayil J, et al. (2018). Vector-based navigation using grid-like representations in artificial agents. Nature 557, 429–+. [DOI] [PubMed] [Google Scholar]
- Beltramo R, and Scanziani M (2019). A collicular visual cortex: Neocortical space for an ancient midbrain visual structure. Science 363, 64–69. [DOI] [PubMed] [Google Scholar]
- Bicks LK, Koike H, Akbarian S, and Morishita H (2015). Prefrontal Cortex and Social Cognition in Mouse and Man. Frontiers in psychology 6, 1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binzegger T, Douglas RJ, and Martin KA (2009). Topology and dynamics of the canonical circuit of cat V1. Neural networks : the official journal of the International Neural Network Society 22, 1071–1078. [DOI] [PubMed] [Google Scholar]
- Borst A, and Helmstaedter M (2015). Common circuit design in fly and mammalian motion vision. Nature neuroscience 18, 1067–1076. [DOI] [PubMed] [Google Scholar]
- Boyd JD, and Matsubara JA (1996). Laminar and columnar patterns of geniculocortical projections in the cat: relationship to cytochrome oxidase. J Comp Neurol 365, 659–682. [DOI] [PubMed] [Google Scholar]
- Braitenberg V, and Schütz A (1998). Cortex: Statistics and Geometry of Neuronal Connectivity, Second edition edn (New York: Springer; ). [Google Scholar]
- Briggs F, Mangun GR, and Usrey WM (2013). Attention enhances synaptic efficacy and the signal-to-noise ratio in neural circuits. Nature 499, 476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F, and Usrey WM (2011). Corticogeniculate feedback and visual processing in the primate. The Journal of physiology 589, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, and Krienen FM (2013). The evolution of distributed association networks in the human brain. Trends in cognitive sciences 17, 648–665. [DOI] [PubMed] [Google Scholar]
- Casagrande VA, and Xu X (2004). Parallel visual pathways: a comparative perspective (Cambridge, MA: MIT Press; ). [Google Scholar]
- Casas-Torremocha D, Porrero C, Rodriguez-Moreno J, Garcia-Amado M, Lubke JHR, Nunez A, and Clasca F (2019). Posterior thalamic nucleus axon terminals have different structure and functional impact in the motor and somatosensory vibrissal cortices. Brain structure & function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauveau F, Celerier A, Ognard R, Pierard C, and Beracochea D (2005). Effects of ibotenic acid lesions of the mediodorsal thalamus on memory: relationship with emotional processes in mice. Behavioural brain research 156, 215–223. [DOI] [PubMed] [Google Scholar]
- Chung K, Wallace J, Kim SY, Kalyanasundaram S, Andalman AS, Davidson TJ, Mirzabekov JJ, Zalocusky KA, Mattis J, Denisin AK, et al. (2013). Structural and molecular interrogation of intact biological systems. Nature 497, 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasca F, Rubio-Garrido P, and Jabaudon D (2012). Unveiling the diversity of thalamocortical neuron subtypes. The European journal of neuroscience 35, 1524–1532. [DOI] [PubMed] [Google Scholar]
- Clinton SM, and Meador-Woodruff JH (2004). Thalamic dysfunction in schizophrenia: neurochemical, neuropathological, and in vivo imaging abnormalities. Schizophrenia research 69, 237–253. [DOI] [PubMed] [Google Scholar]
- Cserep C, Posfai B, Schwarcz AD, and Denes A (2018). Mitochondrial Ultrastructure Is Coupled to Synaptic Performance at Axonal Release Sites. Eneuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamantaras KI, and Kung SY (1996). Principal Component Neural Networks: Theory and Applications (John Wiley & Sons; ). [Google Scholar]
- Ding Y, and Casagrande VA (1997). The distribution and morphology of LGN K pathway axons within the layers and CO blobs of owl monkey V1. Visual Neurosci 14, 691–704. [DOI] [PubMed] [Google Scholar]
- Finn IM, Priebe NJ, and Ferster D (2007). The emergence of contrast-invariant orientation tuning in simple cells of cat visual cortex. Neuron 54, 137–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanem A, and Conzelmann KK (2016). G gene-deficient single-round rabies viruses for neuronal circuit analysis. Virus research 216, 41–54. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Thompson KR, Zhang F, Mogri M, Kay K, Schneider MB, and Deisseroth K (2007). Targeting and readout strategies for fast optical neural control in vitro and in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience 27, 14231–14238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh A, Bokor H, Mease RA, Plattner VM, Hangya B, Stroh A, Deschenes M, and Acsady L (2014). Convergence of cortical and sensory driver inputs on single thalamocortical cells. Cereb Cortex 24, 3167–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hage SR, and Nieder A (2016). Dual Neural Network Model for the Evolution of Speech and Language. Trends in neurosciences 39, 813–829. [DOI] [PubMed] [Google Scholar]
- Halassa MM, and Kastner S (2017). Thalamic functions in distributed cognitive control. Nature neuroscience 20, 1669–1679. [DOI] [PubMed] [Google Scholar]
- Hamos JE, Van Horn SC, Raczkowski D, and Sherman SM (1987). Synaptic circuits involving an individual retinogeniculate axon in the cat. J Comp Neurol 259, 165–192. [DOI] [PubMed] [Google Scholar]
- Harris KM, and Weinberg RJ (2012). Ultrastructure of synapses in the mammalian brain. Cold Spring Harbor perspectives in biology 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter M, Briggman KL, Turaga SC, Jain V, Seung HS, and Denk W (2013). Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature 500, 168–174. [DOI] [PubMed] [Google Scholar]
- Hubel DH, and Wiesel TN (1961). Integrative action in the cat’s lateral geniculate body. The Journal of physiology 155, 385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey AL, Sur M, Uhlrich DJ, and Sherman SM (1985a). Projection patterns of individual X- and Y-cell axons from the lateral geniculate nucleus to cortical area 17 in the cat. J Comp Neurol 233, 159–189. [DOI] [PubMed] [Google Scholar]
- Humphrey AL, Sur M, Uhlrich DJ, and Sherman SM (1985b). Termination Patterns of Individual X-Cell and Y-Cell Axons in the Visual-Cortex of the Cat - Projections to Area-18, to the 17/18 Border Region, and to Both Area-17 and Area-18. Journal of Comparative Neurology 233, 190–212. [DOI] [PubMed] [Google Scholar]
- Jaramillo J, Mejias JF, and Wang XJ (2019). Engagement of Pulvino-cortical Feedforward and Feedback Pathways in Cognitive Computations. Neuron 101, 321–336 e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG (2001). The thalamic matrix and thalamocortical synchrony. Trends in neurosciences 24, 595–601. [DOI] [PubMed] [Google Scholar]
- Jones EG (2007). The Thalamus, Second edition edn (Cambridge, U.K: Cambridge University Press; ). [Google Scholar]
- Jost JT, Sapolsky RM, and Nam HH (2018). Speculations on the Evolutionary Origins of System Justification. Evolutionary psychology : an international journal of evolutionary approaches to psychology and behavior 16, 1474704918765342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano J (1998). Cortical projections of the parvocellular laminae C of the dorsal lateral geniculate nucleus in the cat: an anterograde wheat germ agglutinin conjugated to horseradish peroxidase study. J Comp Neurol 392, 439–457. [DOI] [PubMed] [Google Scholar]
- Kebschull JM, and Zador AM (2018). Cellular barcoding: lineage tracing, screening and beyond. Nature methods 15, 871–879. [DOI] [PubMed] [Google Scholar]
- Kelly LR, Li J, Carden WB, and Bickford ME (2003). Ultrastructure and synaptic targets of tectothalamic terminals in the cat lateral posterior nucleus. J Comp Neurol 464, 472–486. [DOI] [PubMed] [Google Scholar]
- Komura Y, Nikkuni A, Hirashima N, Uetake T, and Miyamoto A (2013). Responses of pulvinar neurons reflect a subject’s confidence in visual categorization. Nature neuroscience 16, 749–755. [DOI] [PubMed] [Google Scholar]
- Krol A, Wimmer RD, Halassa MM, and Feng G (2018). Thalamic Reticular Dysfunction as a Circuit Endophenotype in Neurodevelopmental Disorders. Neuron 98, 282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer LA, and Prescott TJ (2018). The Combinatorial Creature: Cortical Phenotypes within and across Lifetimes. Trends in neurosciences 41, 744–762. [DOI] [PubMed] [Google Scholar]
- Kuramoto E, Ohno S, Furuta T, Unzai T, Tanaka YR, Hioki H, and Kaneko T (2015). Ventral medial nucleus neurons send thalamocortical afferents more widely and more preferentially to layer 1 than neurons of the ventral anterior-ventral lateral nuclear complex in the rat. Cereb Cortex 25, 221–235. [DOI] [PubMed] [Google Scholar]
- Kuramoto E, Pan S, Furuta T, Tanaka YR, Iwai H, Yamanaka A, Ohno S, Kaneko T, Goto T, and Hioki H (2017). Individual mediodorsal thalamic neurons project to multiple areas of the rat prefrontal cortex: A single neuron-tracing study using virus vectors. J Comp Neurol 525, 166–185. [DOI] [PubMed] [Google Scholar]
- Lee CC (2013). Thalamic and cortical pathways supporting auditory processing. Brain and language 126, 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, and Sherman SM (2008). Synaptic properties of thalamic and intracortical inputs to layer 4 of the first- and higher-order cortical areas in the auditory and somatosensory systems. Journal of neurophysiology 100, 317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Patrick SL, Richardson KA, and Connors BW (2014). Two functionally distinct networks of gap junction-coupled inhibitory neurons in the thalamic reticular nucleus. The Journal of neuroscience : the official journal of the Society for Neuroscience 34, 13170–13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman JW, Livet J, and Sanes JR (2008). A technicolour approach to the connectome. Nature reviews Neuroscience 9, 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien AD, and Scanziani M (2013). Tuned thalamic excitation is amplified by visual cortical circuits. Nature neuroscience 16, 1315–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien AD, and Scanziani M (2018). Cortical direction selectivity emerges at convergence of thalamic synapses. Nature 558, 80–86. [DOI] [PubMed] [Google Scholar]
- Mansouri FA, Koechlin E, Rosa MGP, and Buckley MJ (2017). Managing competing goals - a key role for the frontopolar cortex. Nature reviews Neuroscience 18, 645–657. [DOI] [PubMed] [Google Scholar]
- Masse NY, Grant GD, and Freedman DJ (2018). Alleviating catastrophic forgetting using context-dependent gating and synaptic stabilization. Proceedings of the National Academy of Sciences of the United States of America 115, E10467–E10475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, and Wurtz RH (2006). Attentional modulation of thalamic reticular neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 26, 4444–4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means LW, Harrell TH, Mayo ES, and Alexander GB (1974). Effects of dorsomedial thalamic lesions on spontaneous alternation, maze, activity and runway performance in the rat. Physiology & behavior 12, 973–979. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Hioki H, Furuta T, and Kaneko T (2015). Different cortical projections from three subdivisions of the rat lateral posterior thalamic nucleus: a single-neuron tracing study with viral vectors. The European journal of neuroscience 41, 1294–1310. [DOI] [PubMed] [Google Scholar]
- Ohno S, Kuramoto E, Furuta T, Hioki H, Tanaka YR, Fujiyama F, Sonomura T, Uemura M, Sugiyama K, and Kaneko T (2012). A morphological analysis of thalamocortical axon fibers of rat posterior thalamic nuclei: a single neuron tracing study with viral vectors. Cereb Cortex 22, 2840–2857. [DOI] [PubMed] [Google Scholar]
- Phillips JW, Schulmann A, Hara E, Liu C, Wang L, Shields B, Korff W, Lemire A, Dudman J, Nelson SB, et al. (2018). A single spectrum of neuronal identities across thalamus. bioRxiv. [Google Scholar]
- Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, and Kepecs A (2013). Cortical interneurons that specialize in disinhibitory control. Nature 503, 521–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D, and Deschenes M (1998). Anatomical evidence for a mechanism of lateral inhibition in the rat thalamus. The European journal of neuroscience 10, 3462–3469. [DOI] [PubMed] [Google Scholar]
- Rafal RD, and Posner MI (1987). Deficits in human visual spatial attention following thalamic lesions. Proceedings of the National Academy of Sciences of the United States of America 84, 7349–7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichova I, and Sherman SM (2004). Somatosensory corticothalamic projections: distinguishing drivers from modulators. Journal of neurophysiology 92, 2185–2197. [DOI] [PubMed] [Google Scholar]
- Rikhye RV, A G, and Halassa MM (2018a). Thalamic regulation of switching between cortical representations enables cognitive flexibility. Nature neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikhye RV, Wimmer RD, and Halassa MM (2018b). Toward an Integrative Theory of Thalamic Function. Annual review of neuroscience 41, 163–183. [DOI] [PubMed] [Google Scholar]
- Rovo Z, Ulbert I, and Acsady L (2012). Drivers of the primate thalamus. The Journal of neuroscience : the official journal of the Society for Neuroscience 32, 17894–17908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann YB, and Kastner S (2015). The cognitive thalamus. Frontiers in systems neuroscience 9, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel AB (1997). The thalamus and neuropsychiatric illness. The Journal of neuropsychiatry and clinical neurosciences 9, 342–353. [DOI] [PubMed] [Google Scholar]
- Schiff ND (2008). Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Annals of the New York Academy of Sciences 1129, 105–118. [DOI] [PubMed] [Google Scholar]
- Schmitt L, and Halassa M (2017). Interrogating the mouse thalamus to correct human neurodevelopmental disorders. Molecular Psychiatry 22, 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt LI, Wimmer RD, Nakajima M, Happ M, Mofakham S, and Halassa MM (2017). Thalamic amplification of cortical connectivity sustains attentional control. Nature 545, 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM (1985). Functional organization of the W-,X-, and Y-cell pathways in the cat: a review and hypothesis, Vol 11 (Orlando: Academic Press; ). [Google Scholar]
- Sherman SM (2004). Interneurons and triadic circuitry of the thalamus. Trends in neurosciences 27, 670–675. [DOI] [PubMed] [Google Scholar]
- Sherman SM (2012). Thalamocortical interactions. Current opinion in neurobiology 22, 575–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM (2016). Thalamus plays a central role in ongoing cortical functioning. Nature neuroscience 19, 533–541. [DOI] [PubMed] [Google Scholar]
- Sherman SM (2017). Circuitry of the lateral geniculate nucleus (New York: Oxford University Press; ). [Google Scholar]
- Sherman SM, and Guillery RW (1998). On the actions that one nerve cell can have on another: Distinguishing “drivers” from “modulators”. Proceedings of the National Academy of Sciences of the United States of America 95, 7121–7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, and Guillery RW (2011). Distinct functions for direct and transthalamic corticocortical connections. Journal of neurophysiology 106, 1068–1077. [DOI] [PubMed] [Google Scholar]
- Sherman SM, and Guillery RW (2013). Functional Connections of Cortical Areas: A New View from the Thalamus (Cambridge, MA: MIT Press; ). [Google Scholar]
- Sherman SM, and Guillery RW (2014). The lateral geniculate nucleus and pulvinar (Cambridge: MIT Press; ). [Google Scholar]
- Silver D, Schrittwieser J, Simonyan K, Antonoglou I, Huang A, Guez A, Hubert T, Baker L, Lai M, Bolton A, et al. (2017). Mastering the game of Go without human knowledge. Nature 550, 354–+. [DOI] [PubMed] [Google Scholar]
- Stone J (1983). The Y/X/W Classification of Cat Retinal Ganglion Cells (Springer; ). [Google Scholar]
- Tuncdemir SN, Wamsley B, Stam FJ, Osakada F, Goulding M, Callaway EM, Rudy B, and Fishell G (2016). Early Somatostatin Interneuron Connectivity Mediates the Maturation of Deep Layer Cortical Circuits. Neuron 89, 521–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unzai T, Kuramoto E, Kaneko T, and Fujiyama F (2017). Quantitative Analyses of the Projection of Individual Neurons from the Midline Thalamic Nuclei to the Striosome and Matrix Compartments of the Rat Striatum. Cereb Cortex 27, 1164–1181. [DOI] [PubMed] [Google Scholar]
- Usrey WM, Alonso JM, and Reid RC (2000). Synaptic interactions between thalamic inputs to simple cells in cat visual cortex. Journal of Neuroscience 20, 5461–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usrey WM, Reppas JB, and Reid RC (1999). Specificity and strength of retinogeniculate connections. Journal of neurophysiology 82, 3527–3540. [DOI] [PubMed] [Google Scholar]
- Viaene AN, Petrof I, and Sherman SM (2011a). Properties of the thalamic projection from the posterior medial nucleus to primary and secondary somatosensory cortices in the mouse. Proceedings of the National Academy of Sciences of the United States of America 108, 18156–18161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaene AN, Petrof I, and Sherman SM (2011b). Synaptic properties of thalamic input to layers 2/3 and 4 of primary somatosensory and auditory cortices. Journal of neurophysiology 105, 279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaene AN, Petrof I, and Sherman SM (2011c). Synaptic properties of thalamic input to the subgranular layers of primary somatosensory and auditory cortices in the mouse. The Journal of neuroscience : the official journal of the Society for Neuroscience 31, 12738–12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RH (1992). Competitive Hebbian Learning - Algorithm and Demonstrations. Neural Networks 5, 261–275. [Google Scholar]
- Williams LE, and Holtmaat A (2018). Higher-order thalamocortical inputs gate synaptic long-term potentiation via disinhibiton. bioRxiv. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Friedlander MJ, and Sherman SM (1984). Fine structural morphology of identified X- and Y-cells in the cat’s lateral geniculate nucleus. Proceedings of the Royal Society of London Series B, Biological sciences 221, 411–436. [DOI] [PubMed] [Google Scholar]
- Wimmer R, Schmitt L, Davidson T, Nakajima M, Deisseroth K, and Halassa M (2015). Thalamic control of sensory selection in divided attention. Nature 526, 705–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamins DL, Hong H, Cadieu CF, Solomon EA, Seibert D, and DiCarlo JJ (2014). Performance-optimized hierarchical models predict neural responses in higher visual cortex. Proceedings of the National Academy of Sciences of the United States of America 111, 8619–8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingg B, Chou XL, Zhang ZG, Mesik L, Liang F, Tao HW, and Zhang LI (2017). AAV-Mediated Anterograde Transsynaptic Tagging: Mapping Corticocollicular Input-Defined Neural Pathways for Defense Behaviors. Neuron 93, 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]