Abstract
Cannabis abuse commonly co-occurs with alcohol use disorder (AUD). With increased acceptance and accessibility to cannabis in the US, it is imperative to understand the psychological and neural mechanisms of concurrent alcohol and cannabis use. We hypothesized that neural alcohol-cue conditioning may extent to other drug-related stimuli, such as cannabis, and underwrite the loss of control over reward-driven behavior. Task-activated fMRI examined the neural correlates of alcohol- and cannabis-related word cues in 21 abstinent AUD and 18 control subjects. Relative to controls, AUD showed behavioral attentional biases and frontal hypoactivation to both alcohol- and cannabis-related words. This cue-elicited prefrontal hypoactivation was related to higher lifetime alcohol consumption (pcorrected < 0.02) and modulated by past cannabis use histories (p ≦ 0.001). In particular, frontal hypoactivation to both alcohol and cannabis cues was pronounced in AUD without prior cannabis exposure. Overall, frontal control mechanisms in abstinent AUD were not sufficiently engaged to override automatic alcohol and cannabis-related intrusions, enhancing the risk for relapse and potentially for alcohol and cannabis co-use with the increased social acceptance and accessibility in the US.
Keywords: Functional MRI, Addiction cues, Attentional bias, Cognitive control
1. Introduction
In the U.S., alcohol and cannabis are the two most commonly used psychoactive substances. Cannabis abuse commonly occurs with Alcohol Use Disorder (AUD) (Hayley et al., 2017), with one report estimating that over 86% of individuals with a history of cannabis use disorder also meet criteria for AUD (Stinson et al., 2006). The combined misuse of alcohol and cannabis is a major public health problem and creates a significant risk for developing a substance use disorder, especially among teens and emerging adults (De Luca et al., 2017; Haas et al., 2015; Hasin et al., 2015; Moss et al., 2014; Aloi et al., 2018). Longitudinal studies support the general comorbidity of AUD and CUD during adolescence and young adulthood (Duncan et al., 2015; Patton et al., 2007); however, as the cohort aged, the influences of AUD and CUD on each other were lessened such that adults tended toward using one preferred substance predominantly. Accordingly, adults with an AUD may also have a history of cannabis misuse in their remote past. This is important as we do not currently know how cannabis-alcohol co-use during adolescence and young adulthood may influence the development of an AUD and whether such cannabis use histories impact the neurobiological mechanisms of addictive behaviors.
With increased social acceptance and accessibility to cannabis in the U.S., it is imperative to understand the consequences of co-use with alcohol on mood, cognition, and behavior. Polysubstance use in youth (Karoly et al., 2015) is often based on availability (Moss et al., 2014) and the two most often abused substances are alcohol (75.6%) and cannabis (48.6%) (Kann et al., 2014). It can be assumed that with increased accessibility in several U.S. states, the use of cannabis will increase, either as a complement to alcohol intake or as a substitute (Guttmannova et al., 2016). In young subjects, the conjoint use of both substances has been previously associated with neuropsychological and social deficits far greater than the effects from either substance used alone (Hayley et al., 2017; Thoma et al., 2011). We are beginning to understand the effects of chronic alcohol use and the neural circuits involved and affected in individuals with AUD (e.g., Müller-Oehring et al., 2013; Schulte et al., 2012, 2017; Volkow et al., 2013) but have inconsistent findings about the effects of chronic cannabis use (Curran et al., 2016; Sagar and Gruber, 2018), and know even less about the potential interacting effects of cannabis and alcohol use. For example, chronic cannabis use has been associated with negative emotionality (Volkow et al., 2014; John and Wu, 2017) and psychotic symptoms (e.g., psychosis risk, Andréasson et al., 1987; Di Forti et al., 2019; Galvez-Buccollini et al., 2012; Van Os et al., 2002) in vulnerable individuals (Di Forti et al., 2012; Morgan et al., 2016). Yet, chronic cannabis use was also found to reduce attention deficits in ADHD (Cooper et al., 2017) and better cognition and memory in psychiatric populations (e.g., schizophrenia, Menendez-Miranda et al., 2019). In addition, acute cannabis consumption can impair episodic memory (Curran et al., 2002; Crane et al., 2013), salience processing (e.g., Wijayendran et al., 2018), and visuomotor skills in infrequent users (Battistella et al., 2013), while several other studies did not find any cannabis-related performance changes (for a review, see Sagar and Gruber, 2018). Overall, there is little evidence for persisting neuropsychological and brain functional deficits of long-term use after 28 days of abstinence (Crane et al., 2013; Mokrysz et al., 2016; Sagar and Gruber, 2018), which is in line with the finding that the density of CB1 receptors return to normal levels after ~4weeks (Hirvonen et al., 2012). Nevertheless, cannabis use has been linked to an increased risk of other substance use (Jones and McCance-Katz, 2019) and addictions (CUD: Fairman et al., 2019; AUD: John and Wu, 2017). Among the key components of addictive behaviors are a narrowed attentional focus toward the substance of abuse (e.g. Hicks et al., 2012; Field and Cox, 2008) and difficulty exerting control over reward-driven behavior (e.g., Bechara, 2005). Commensurately, both alcohol and/or cannabis abuse compromise attentional focus and executive control (Crean et al., 2011; Müller-Oehring et al., 2013), but may compromise neural systems underlying attention and behavioral control relatively independently (Aloi et al., 2018, Aloi et al., 2019; Blair et al., 2019; De Ternay et al., 2019). For example, Aloi et al. (2019) findings suggest differential impacts of alcohol and cannabis abuse on the activation of brain regions implicated in executive attention and response control: While AUD severity was negatively related to dorsolateral prefrontal cortex, anterior cingulate cortex, and precuneus responsiveness, CUD severity was positively related to task responsiveness within these regions.
Cue-related attentional mechanisms, in particular the responsiveness to alcohol-related stimuli in AUD (Fadardi and Cox, 2006), have been causally implicated in the maintenance of addictive behaviors, craving, and relapse (Field and Eastwood, 2005; Field et al., 2009; Wiers et al., 2006). Wiers and colleagues (2006) proved this experimentally by manipulating attention and biasing it either towards or away from alcohol-related stimuli in a training of heavy social drinkers. The group receiving ‘towards-alcohol attention’ training exhibited greater attentional biases to alcohol stimuli post-training, reported more subjective craving, and engaged in higher subsequent alcohol consumption. In contrast, the group undergoing ‘away-from-alcohol attention’ training showed reduced attentional biases and no change in subjective craving and alcohol consumption. Recent research is emerging on how reward circuits become hypersensitive to alcohol cues and is supportive of the incentive salience hypothesis (Robinson and Berridge, 2008). It states that initially neutral cues become incentive stimuli through their repeated pairing with the pharmacological effects of alcohol. As alcohol cue-reactivity develops through personal alcohol use (Courtney et al., 2016; Schulte et al., 2017; Vollstädt-Klein et al., 2012), the rewarding effects of alcohol become more important during the progression of alcohol addiction (Dager et al., 2014). In consequence, alcohol cues then induce craving and promote relapse (Grüsser et al., 2004; Papachristou et al., 2014; Valyear et al., 2017). Similar sensitization of the reward system through associative learning mechanisms have been proposed for other drugs including cannabis (Cousijn et al., 2013; Metrik et al., 2015). Nonetheless, investigations of AUD into the engagement of brain regions under different reward-related conditions, such as alcohol and cannabis cues, are currently missing (Weiss and Porrino, 2002). Activation of neural network responses to alcohol and cannabis cues may both increase craving (Witteman et al., 2015), initiate compulsive alcohol consumption (Stacy, 1997), and may transfer to difficulty in refraining from alcohol and other substances.
Long-term heavy alcohol use affects brain circuits involved in reward, emotion, attention, and executive control (Durazzo et al., 2011; Müller-Oehring et al., 2013; Schulte et al., 2017). Neuroadaptations in dopamine-rich midbrain-striatal and limbic systems and closely connected prefrontal control systems mediate an attentional response to alcohol in chronic heavy users (Heinz et al., 2004; Müller-Oehring et al., 2013; Schacht et al., 2013). In cannabis-dependent adults, cannabis cues also elicit neural responses in reward-related areas including the striatum, limbic (hippocampus, amygdala), insular and orbitofrontal cortices (Wetherill et al., 2015; de Sousa Fernandes Perna et al., 2017). To our knowledge, ours is the first human neuroimaging study to test the neurofunctional brain response evoked by alcohol- and cannabis-related reward cues (vs. neutral cues) in individuals with AUD and healthy controls using an addiction-Stroop task. We hypothesized that individuals with AUD, relative to controls, will show attentional bias to reward cues containing both alcohol- and cannabis-related information, and differ from controls in the brain’s neurofunctional response to alcohol and cannabis cues. At the neurofunctional level, we expected differences in AUD and control groups in attentional salience and limbic responsiveness to reward-related cues and prefrontal control network responses depending on experience and habit formation (basal ganglia/striatal responses). We further explored whether conjoint cannabis use history in AUD has an effect on cue-induced brain activity and behavior, and used age of illness onset for AUD and cannabis abuse to explore whether the timing in associative learning in the context of alcohol and cannabis cues has relevance for attentional capture mechanisms.
2. Materials and methods
2.1. Study participants
Individuals with alcohol dependence were recruited from a variety of sources including treatment centers, community programs, and also by word of mouth, internet postings, and advertisements. The control participants were volunteers from the local communities. The Institutional Review Boards at SRI International and Stanford University School of Medicine approved the study. All participants gave written informed consent for study participation.
All participants were screened by calibrated research clinicians using the Structured Clinical Interview for DSM-IV-TR (SCID) (First et al., 1998), and the implemented substance module, drug use form, and health questionnaires. The dataset comprised 21 adults who met DSM-IV-TR criteria for alcohol dependence (n = 4 women), and 18 age- matched healthy controls (n = 8 women) who did not meet criteria for alcohol abuse or dependence (American Psychiatric Association, 2000) (Table 1). By definition, individuals who met DSM-IV-TR diagnostic criteria for alcohol dependence would also meet criteria for DSM-5 criteria for AUD, therefore we use these terms interchangeably. No control participant met DSM-IV-TR criteria for alcohol, substance abuse or dependence.
Table 1.
Study Sample description: demographics and drug history.
| Demographics | CTL (n = 18) | AUD (n = 21) | p value |
|---|---|---|---|
| Women/Men (n) | 8/10 | 4/17 | 0.087 a |
| Age (yrs) | 49.6 ± 11 (29–66) | 50.3 ± 9.5 (26–68) | 0.83 |
| Education (yrs) | 16.3 ± 2.9 | 13.6 ± 2.7 | 0.005 |
| Socioeconomic Status (SES) | 24.2 ± 11.2 | 40.7 ± 18.3 | 0.002 |
| Handedness | 73.3 ± 26.2 | 51.5 ± 57.8 | 0.15 |
| Visual Acuity | 1.3 ± 0.7 | 1.3 ± 0.8 | 0.90 |
| Body Mass Index | 25.4 ± 4.2 | 27.5 ± 4.7 | 0.18 |
| Alcohol and Substance Use History | |||
| Lifetime Alcohol Consumption (kg) | 27.1 ± 24 (0–73) | 913.1 ± 816 (181–3442) | 0.0001 |
| Age at AUD Onset (yrs) | – | 26.6 ± 11 (13–48) | – |
| AUD remission (yrs) | – | 1.6 ± 2.9 (0.01–12.6) | – |
| Days since last drink | 126 ± 375 (2–1479) | 342 ± 969 (1–4551) | 0.42 |
| Alcohol Craving (ACQ-R score) | 8.2 ± 2.6 (7–17) | 10.3 ± 7 (7–30) | 0.21 |
| AUDIT score | 2.9 ± 2.3 | 15.7 ± 11.3 | 0.0001 |
| Cannabis use diagnosis (y/n) | 0/18 | 11b/10 | 0.0001a |
| CUD remission (yrs) | – | 22.5 ± 11.3 (0.8–36.5) | – |
| Age at Cannabis Onset (yrs) | – | 16.2 ± 3 (12–21) | – |
| Nicotine use diagnosis (n) | 0/18 | 11/10 | 0.0001a |
| Other substance use diagnoses c (n) | 0/18 | 15/6 | 0.0001a |
| SUD remissiond (yrs) | – | 16.8 ± 14.8 (0.5–44.9) | – |
Mean and standard deviation for each group: control subjects (CTL) and subjects diagnosed with alcohol use disorder (AUD); t-tests were applied to test for group differences; statistical significance level was set at p < 0.05 (cursive).
Chi square test.
n = 5 AUD had both cannabis and nicotine abuse/dependency.
Other substances of abuse included cocaine, amphetamines, sedatives, and opioids.
According to DSM-IV remission criteria, of the 15 AUD with past SUD, 12 were in full remission and 3 in early remission. Abbreviations: Socioeconomic Status (SES): higher scores represent lower SES (range, 11–77) (Hollingshead and Redlich, 1958), Alcohol Craving Questionnaire (ACQ-R) (Drobes and Thomas, 1999); Intelligence Quotient (IQ), number (n), years (yrs); Edinburgh Handedness Inventory: Laterality index above +40 = right-handed, below − 40 = left-handed (Oldfield, 1971).
Alcoholic and control participants were excluded at screening if they had fewer than 8 years of education or a significant history of medical (e.g., epilepsy, stroke, multiple sclerosis, uncontrolled diabetes, or loss of consciousness >30 min), psychiatric (i.e., schizophrenia or bipolar I disorder) or neurological disorder (e.g., neurodegenerative disease). An additional exclusion criterion was any DSM-IV-TR Axis I disorder in the control group. All participants underwent a semistructured timeline follow-back interview (Skinner and Sheu, 1982) to quantify lifetime alcohol consumption. Individuals with AUD did not receive cognitive bias modification training (for such trainings see Wiers et al., 2011; Wiers and Wiers, 2017) while enrolled in the study.
Table 1 summarizes AUD and control groups’ demographics, alcohol and drug histories.
Group baseline differences.
AUD and CTL groups did not significantly differ in age or sex distribution. Although individuals with AUD had fewer years of education than CTLs, AUD’s education level was on average beyond high school (>12 years).
2.1.1. Alcohol and other substance use histories in AUD (Table 1)
Regarding DSM-IV-TR alcohol dependence remission criteria in the AUD group, 3 of 21 (14.3%) were in sustained remission (> 1 year), 17 (80.9%) were in early remission (> 3 month and < 1 year), and 1 (4.8%) had current alcohol dependence (< 3 months). The average time since last met alcohol dependence DSM-IV-TR criteria was 1.6 ± 2.9 years (median = 0.6 years). Average age at AUD onset was 2.6.6 ± 11 years, the average AUD duration was 24.1 ± 12.5 years. Total lifetime alcohol consumption was 913 ± 816 kg (median = 636 kg), which is > 30 times that of the controls (mean = 27 ± 24 kg; median = 21 kg).
Eleven of 21 AUD (52%) met lifetime DSM-IV-TR criteria for cannabis abuse (n = 4) or dependence (n = 7), all were in remission: 8 in sustained remission and 3 in early remission. None had met cannabis abuse criteria in the past 0.8 years (average CUD remission was 22.5 years, median = 24 years). Age at CUD onset was on average at 16 years (median age = 17 years) and the frequency during heaviest use was on average 11.3 times per month (median = 9 times) (substance module and drug use form, SCID; First et al. 1998). Cannabis diagnosis preceded AUD diagnosis by an average of 10 years (median: 4 years; range: < 1 to 24 years) (Supplement, Figure S1). Fifteen of the 21 AUD (71%) met DSM-IV-TR criteria for substance abuse/dependence in their past (cocaine, opioids, sedatives, amphetamines) and all were in remission (Table 1). Mean time since last met SUD criteria was 16.3 years (median = 13.7 years). Of the 15 individuals with SUD, eight had used more than one other substance in the past. The most common substance of abuse was cocaine, occurring in 14 of the 15 with SUD. In all cases, alcohol was the preferred drug of choice, and all met criteria for alcohol dependence more recently than for substance abuse or dependence. In addition, Table S1 in the supplement describes the demographics, alcohol and substance use histories of AUD individuals with and without past CUD and shows that they did not differ in age, education, socioeconomic status, lifetime alcohol consumption, age at AUD onset, nicotine, or other substance use diagnoses.
2.1.2. Mood and emotion
Eight AUD subjects had a history of Major Depressive Disorder (MDD), all eight were in remission and none had current depression. No control participant had a history of MDD or current depression. In addition, no control and two AUD individuals had a history of an anxiety disorder, both AUD were in remission. Congruently, the AUD group reported more depressive symptoms (Becks Depression Inventory; Beck et al., 1996), more non-planning impulsiveness (Barratt Impulsiveness Scale; Patton et al., 1995), and higher trait anxiety, but did not differ from controls in state anxiety at the time of testing (State-Trait Anxiety Inventory; Spielberger et al., 1983) (Table 2).
Table 2.
Study sample description: cognition and emotion.
| CTL | AUD | p value | |
|---|---|---|---|
| Cognition | |||
| Verbal IQ (WTAR Standard Score) | 111.3 ± 17.2 | 105.6 ± 13.6 | 0.26 |
| DRS-2 - Total Score | 140.9 ± 1.5 | 138.8 ± 3.7 | 0.041 |
| Digit Span Forward - Total Score | 9.9 ± 1.8 | 8.8 ± 1.8 | 0.07 |
| Digit Span Backward - Total Score | 8.6 ± 3.1 | 6.8 ± 2.8 | 0.069 |
| Block Span Forward - Total Score | 10.1 ± 1.6 | 7.7 ± 1.3 | 0.0001 |
| Block Span Backward - Total Score | 8.4 ± 1.5 | 6.8 ± 1.4 | 0.002 |
| Emotion | |||
| Depressive Symptom Score (BDI-II) | 2.6 ± 2.9 | 11.7 ± 10.4 | 0.001 |
| State Anxiety Score (STAI-S) | 28.2 ± 9.4 | 31.5 ± 16.9 | 0.46 |
| Trait Anxiety Score (STAI-T) | 29.1 ± 6.7 | 39.4 ± 15.3 | 0.012 |
| Barratt Impulsiveness (Total Score) | 54.7 ± 7.7 | 64.2 ± 14 | 0.015 |
| Barratt Subscales: Attentional Impulsiveness | 13.2 ± 3.6 | 15.1 ± 4.7 | 0.16 |
| Motor Impulsiveness | 21.1 ± 3 | 23.2 ± 5 | 0.11 |
| Non-Planning Impulsiveness | 20.4 ± 4 | 25.8 ± 6.8 | 0.006 |
Mean and standard deviation for each group: control subjects (CTL) and subjects diagnosed with alcohol use disorder (AUD); Wechsler Test of Adult Reading (WTAR; Wechsler, 2001); Dementia Rating Scale (DRS-2; Jurica et al., 2001); Digit and Block Span, Wechsler Memory Scale-Revised (WMS-R; Wechsler and Stone, 1987); Becks Depression Inventory (BDI-II; Beck et al., 1996); State-Trait Anxiety Inventory (STAI; Spielberger et al., 1983); Barratt Impulsiveness scale (Patton et al., 1995); t-tests were applied to test for group differences; statistical significance level was set at p < 0.05 (cursive).
2.1.3. Cognitive ability
For the dementia rating scale (DRS-2; Jurica et al., 2001) that tested current global cognitive ability, we used the cutoff score of 136/144 for healthy controls, according to Springate et al. (2014). Although AUD had average DRS–2 scores > 136, the AUD group displayed somewhat lower DRS–2 scores than the CTL group (Table 2) but did not differ from controls in premorbid IQ (Wechsler Test of Adult Reading Standard Score; Wechsler, 2001). Relative to CTLs, individuals with AUD showed slower processing speed and working memory, in particular for visuospatial material (block span forward and backward) but not significantly for verbal material (digit span forward and backward) (Wechsler Memory Scale-Revised; Wechsler and Stone, 1987).
2.1.4. Relation of lifetime alcohol consumption to cognition and emotion variables
As expected, higher lifetime alcohol consumption was related to higher scores in the alcohol addiction screening instrument AUDIT (Babor et al., 2001) (Rho = 0.63, p < 0.0001, n = 39). Higher lifetime alcohol consumption was further associated with = = = = = = = = = = = = poorer visuo-spatial working memory performance (Rho = −0.65, p < 0.0001, n = 37), more depressive symptoms (Rho = 0.47, p= 0.002, n = 39), trait anxiety (Rho = 0.40, p = 0.013, n = 39), and impulsiveness (Rho = 0.37, p = 0.022, n = 39), in particular non-planning impulsiveness (Rho = 0.41, p = 0.009, n = 39) (all pcorrected < 0.05; Holm’s Sequential Bonferroni Procedure).
2.1.5. Influence of past cannabis diagnosis on baseline variables
Exploratory analyses (Supplement, Tables S1, S2) revealed that AUD with an additional cannabis use disorder (CUD) did not differ from AUD without CUD on key alcohol-related variables including amount of lifetime alcohol consumption (in kg), time since last drink, age at AUD onset, or in demographic variables such as age, education, socioeconomic status, or in cognitive variables such as dementia rating scale (DRS-2) scores and verbal intelligence quotients, or emotion variables such as depressive symptoms, anxiety, and impulsiveness. However, a younger age at the onset of cannabis abuse or dependency was associated with a younger age at AUD onset and with a shorter duration since last met cannabis diagnosis criteria, i.e., shorter abstinence (Supplement, Figure S1).
2.2. Addiction-Stroop color match-to-sample task
Here we used fMRI to examine the neural correlates of alcohol- and cannabis-related stimulus processing in AUD and CTL groups. An addiction-Stroop color match-to-sample task was utilized to test for attentional bias specific to alcohol and cannabis cues. The addiction-Stroop color match-to-sample task is a modification of the Stroop match-to-sample task, measuring Stroop color-word interference in combination with a color matching task in the MRI environment (e.g., Schulte et al., 2009, 2012), and then modified for measuring interference resulting from emotionally salient word content (Müller-Oehring et al., 2013; Schulte et al., 2017), and in this instance from alcohol- or cannabis-related word content, relative to neutral content (e.g., color words). All subjects performed the behavioral task during fMRI acquisition using a clinical whole-body GE 3T scanner. The task was programmed with PsyScope software (Cohen et al., 1993) and synchronized with the MRI acquisition via the fORP system interface (www.curdes.com). Stimuli were presented through a rear-projection system and viewed via a mirror attached to the head coil.
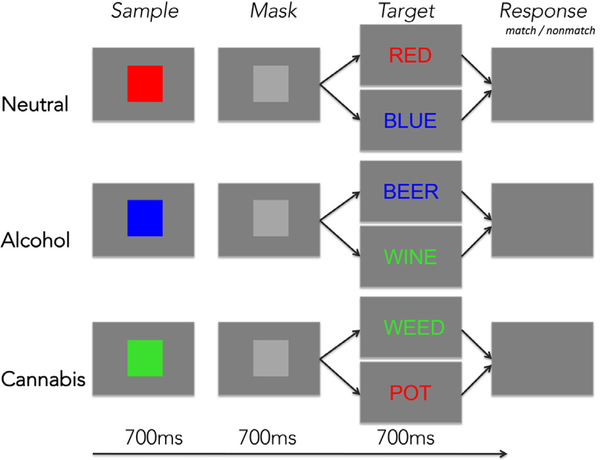
The addiction Stroop color match-to-sample task started with an instruction slide and countdown of 11 s. Task conditions were presented in a blocked design with each block lasting 17.6 s and comprising four trials. The total number of trials per run was 72; two runs were presented with an equal amount of trials in all three conditions (color, cannabis, alcohol). Each trial started with a color patch presented for 700 ms followed by a gray mask presented for 700 ms, which was followed by the target color-word Stroop stimulus presented for 700 ms and an inter-trial interval of about 2 s during which responses were recorded (Fig. 1). The task involved matching the color of a patch to the font color of a word by pressing a Yes-key for color matches and a No-key for non-matches, thereby providing measures of reaction time and accuracy for each trial. Words were color words (RED, BLUE, GREEN), alcohol-related (e.g., WINE, BEER), or cannabis-related words (e.g., WEED, POT) and written in green, red, or blue colored font. Color word presentations were always congruent (e.g. the word RED written in red font color). Test instructions were reviewed with the subject in a practice session before entering the scanner and again through the scanner’s intercom system before each run.
Fig. 1.
Addiction-Stroop color match-to-sample task paradigm.
Participants matched the color of a color sample to that of a target word by pressing a YES key for color matches and a NO key for color nonmatches. The task had three conditions for word content (Stroop): (1) color words (neutral), (2) alcohol words (alcohol), and (3) cannabis words (cannabis). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The word’s content (alcohol, cannabis) is assumed to be processed automatically and to elicit prepotent responses that need to be inhibited to correctly and efficiently perform the color-matching task (see e.g. for emotion and reward-related content: Müller-Oehring et al., 2013; Schulte et al., 2017). Effects to the alcohol or cannabis word conditions are operationalized to test ‘current need or state,’ in AUD relative to controls, and considered against conditions of ‘neutral state’ (color) conditions. At the behavioral level, attentional bias toward alcohol and cannabis cues were defined as the difference in RT between alcohol and neutral color word content (RTalcohol – RTcolor) or cannabis and neutral color word content (RTcannabis – RTcolor), calculated for correct trials, equivalent to previous reports (Müller-Oehring et al., 2013).
2.3. Magnetic resonance imaging (MRI)
2.3.1. Data acquisition and analyses
Whole-brain structural and functional MRI data were acquired using a 3T GE Discovery MR750 with an 8-channel head coil. Subject motion was minimized by following standard practices for head fixation (tight padding of the neck and at the sides of the head, head strap) and image series were inspected for residual motion. Whole-brain fMRI data were acquired with a T2*-weighted gradient echo-planar pulse sequence (2D axial, TE = 30 ms; TR = 2200 ms; flip angle = 90°; xy matrix = 60 × 60; thick = 5 mm; 36 slices; 1 NEX). A total of 288 frames were collected for task-activated fMRI data. A dual-echo fast spin echo (FSE) scan series (2D axial; TR = 8585 ms; TE = 17/102 ms; xy matrix = 256 × 192; thick = 2.5 mm; 62 slices; FOV = 240 × 240 mm2; 1 NEX) was used for spatially registering the fMRI data.
2.3.2. Image preprocessing
The fMRI analysis was done with the SPM8 software package (Wellcome Department of Cognitive Neurology, UK; http://www.fil.ion.ucl.ac.uk/spm/) and focused on the whole brain. The functional images were subjected to geometric distortion (field map) correction and the time-series were realigned to the first image as reference image to remove movement artifacts in fMRI (Friston et al., 1995). Scans exceeding movement thresholds (1 mm/TR) based on ArtRepair Software for more than 12% of frames were excluded (see also Power et al., 2010). Outliers were infrequent and randomly spread over runs. The average percentage of outlier frames was 1.75% of all frames (median = 0.7%). Groups did not differ in the number of outlier frames (F(1,37) = 1.23, ns). Identified outlier frames were excluded from the individual general linear models (GLM) and condition contrast images (see first level fMRI image analysis). The FSE structural images were co-registered to the mean unwarped and motion-corrected functional image for each subject and segmented into gray and white matter images. Functional and structural gray matter images were normalized to Montreal Neurological Institute (MNI) space assuring that the fMRI signal was confined to gray matter. Functional volumes were smoothed with a Gaussian kernel of 8 mm (FWHM).
2.3.3. First level fMRI image analysis
Individual statistics were computed using a general linear model (GLM) approach (Friston et al., 1995). For each individual, one image per contrast was computed from a design matrix that included the task conditions as explanatory variables in addition to the estimated individual movement parameters as regressors. Contrasts of interest were derived at the individual analysis level by contrasting alcohol to color word conditions, and cannabis to color word conditions. The individual contrast images were then subjected to random effects analyses for group averaging and population inference. The second level full factorial design matrix involved two factors: group (AUD, CTL) and attentional bias (alcohol, cannabis) as derived from the first-level individual contrast images (alcohol-color, cannabis-color). As implemented in SPM full-factorial modeling, t-contrasts then tested within-group and between-group activation differences for alcohol and cannabis attentional bias. Significance threshold of p < 0.05 cluster-corrected for multiple comparisons was carried out by performing Monte Carlo simulation implemented in 3dClustSim program at a voxel threshold of p = 0.001 (corrected version; https://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html).
2.4. Statistical analyses
Data analysis of task performance was conducted with the statistical software package SPSS Statistics 23.0. For the first set of analyses, repeated measures analysis of variance (ANOVA) tested group effects (AUD vs. CTL) on alcohol and cannabis cue processing during color matching. The alpha p level was set to 0.05. We extracted individual MR signal change from significant clusters (see Table 4) using the matlab-based SPM-compatible MarsBar toolbox (http://marsbar.sourceforge.net/marsbar.pdf) to test for brain-behavior relationships. Significant voxel cluster of activation, specific to each contrast, were correlated with the respective behavioral effect, alcohol and cannabis history data (lifetime alcohol consumption, craving, abstinence). Exploratory analyses examined the generalization of attentional capture from one substance class of stimuli to another by testing the interactions between cannabis and alcohol use history on attentional capture metrics. Family-wise Holm’s Sequential Bonferroni Procedure was used to correct for multiple comparisons (Holm, 1979).
Table 4.
Brain regional activation to alcohol- and cannabis-related words in contrast to neutral color words in alcohol use disorder (AUD) and control (CTL) subjects while performing an addiction Stroop color match-to-sample task. Activation clusters reported were significant at cluster-forming threshold corrected for multiple comparisons at level p < 0.05 voxels using a voxel threshold of p = 0.001.
| Region | k extent | t value | MNI coordinates | |||
|---|---|---|---|---|---|---|
| BA | x | y | z | |||
| Group differences | ||||||
| AUD < CTL | ||||||
| Alcohol (alcohol>color contrast) | ||||||
| L. dorsolateral prefrontal cortex | 9 | 130 | 3.91 | −30 | 32 | 38 |
| R. dorsolateral prefrontal cortex | 9 | 122 | 3.90 | 32 | 36 | 34 |
| L. + R. middle frontal gyrus, premotor | 6,8 | 205 | 3.85 | 2 | 28 | 40 |
| Cannabis (cannabis>color contrast) | ||||||
| R. MFG, precentral | 6,8,9 | 623 | 4.92 | 44 | 2 | 48 |
| AUD>CTL | ||||||
| no supratheshold voxel | ||||||
| Within group effects | ||||||
| AUD | ||||||
| Alcohol < Color | ||||||
| L. middle frontal gyrus, premotor | 6 | 445 | 5.60 | − 34 | 0 | 56 |
| L. + R. medial superior frontal gyrus, dACC | 8 | 702 | 4.64 | 10 | 16 | 44 |
| R. middle frontal gyrus, premotor | 6 | 174 | 4.31 | 30 | −2 | 60 |
| L. lateral middle/superior frontal gyrus | 6,8 | 150 | 4.28 | − 38 | 2 | 34 |
| R. dorsolateral prefrontal cortex | 9 | 153 | 4.09 | 34 | 36 | 30 |
| R. caudate | 123 | 3.88 | 16 | − 10 | 26 | |
| L. + R. dACC, SMA | 6,24 | 118 | 3.81 | −4 | −6 | 50 |
| Cannabis < Color (can<con) | ||||||
| R. dorsolateral prefrontal cortex, premotor | 6,9 | 605 | 4.66 | 36 | 32 | 40 |
| R. medial superior frontal gyrus | 8 | 199 | 3.95 | 8 | 30 | 40 |
| Cannabis > Color (can>con) | ||||||
| L. + R. calcarine V1, PCC | 17,23 | 242 | 3.65 | 6 | − 68 | 16 |
| CTL | ||||||
| Alcohol vs. Color | ||||||
| no suprathreshold voxels | ||||||
| Cannabis > Color (can>con) | ||||||
| L. middle, superior temporal, angular gyri | 21,39 | 202 | 4.30 | − 54 | − 42 | 8 |
| L. superior parietal lobe | 7 | 178 | 4.21 | − 26 | − 76 | 50 |
| R. middle frontal gyrus | 6,8 | 362 | 4.01 | 38 | 4 | 36 |
Abbreviations: BA = Brodman Area, L. = left, R. = right, dlPFC = dorsolateral prefrontal cortex, dACC = dorsal anterior cingulate cortex, PCC = posterior cingulate cortex.
3. Results
3.1. Addiction Stroop color match-to-sample task: behavior
3.1.1. Response speed and accuracy
To ensure that group differences in attentional bias to alcohol and cannabis cues are not due to general slowing, error rate, or a speed-accuracy tradeoff, we first compared groups on those variables.
Overall error rate was less than 3% (mean number of errors = 4, median = 4 out of 144). AUD did not significantly differ from CTL in overall response times (RT) (median RT F(1,37) = 3.26, p = 0.08), RT variation as measured by standard deviation (SD), F(1,37) = 2.63, p = 0.11), or accuracy (error rate F(1,37) = 1.84, p = 0.18; misses F (1,37) = 2.41, p = 0.13) (MANOVA Pillai’s Trace for group F (4,34) = 0.901, p = 0.47) (Table 3, Fig. 2A). Slower speed (longer RTs) correlated with more errors (rho = 0.57, p < 0.0001, n = 37). Thus, our behavioral data showed no speed-accuracy trade-off, which is indicated by a negative correlation, i.e., shorter RTs with more errors.
Table 3.
Alcohol-cannabis Stroopcolormatch-to-sample fMRI Task: Performance measures. Group means ± standard deviation for control subjects (CTL) and subjects with alcohol use disorder alcoholics (AUD).
| CTL | AUD | |
|---|---|---|
| Accuracy | ||
| Number of errors | 3.94 ± 2.6 | 5.48 ± 4.2 |
| Number of misses | 1.56 ± 2.7 | 4.6 ± 7.9 |
| Reaction time (ms) | ||
| Overall | 797 ± 139 | 897 ± 196 |
| Neutral (color) words | 808 ± 151 | 909 ± 197 |
| Cannabis-related words | 793 ± 141 | 878 ± 201 |
| Alcohol-related words | 791 ± 141 | 904 ± 209 |
| Standardized education-corrected Z-scores | ||
| Neutral (color) words | 0 ± 1.02 | −0.49 ± 1.5 |
| Cannabis-related words | 0 ± 1.03 | −0.63 ± 1.5 |
| Alcohol-related words | 0 ± 1.03 | −0.81 ± 1.6 |
Fig. 2.
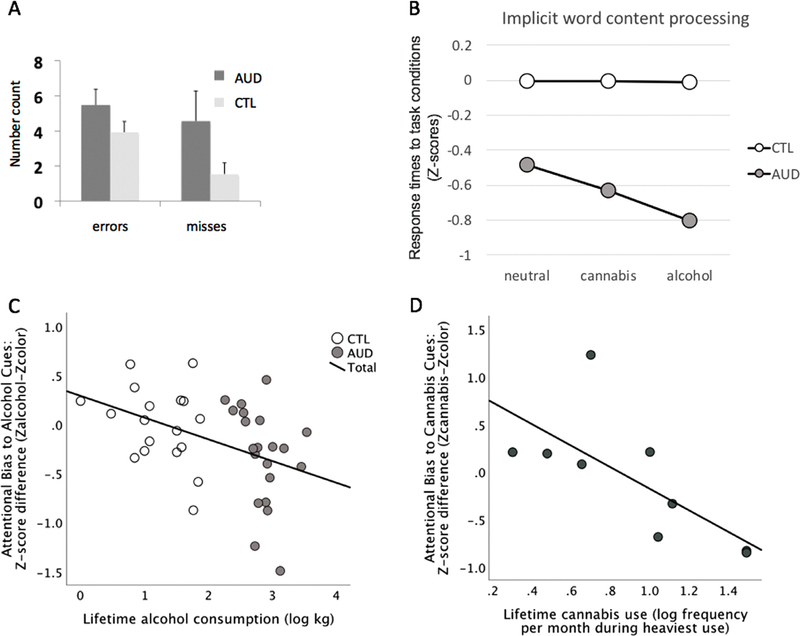
Addiction-Stroop color match-to-sample task performance.
A. Accuracy: Means and SD (standard deviations) for number of errors and misses. B. Color-matching performance for alcohol, cannabis, neutral (color) word task conditions: Education-corrected standardized Z-scores (of reaction times) depicting the deviation of AUD from the control group (CTL: mean Z = 0 ± 1 SD). C. Correlation graph depicting the relation between lifetime alcohol consumption and attentional bias to alcohol cues in AUD (using standardized education-corrected scores). D. Correlation graph depicting the relation between cannabis use frequency and attentional bias to cannabis cues in AUD-CUD (using standardized education- corrected scores). Abbreviations: AUD: alcohol use disorder, CUD: cannabis use disorder, CTL: healthy controls.
3.1.2. Attentional bias to word content
Attentional bias analyses for alcohol and cannabis word content were based on median RT measures for each condition and participant. To test whether implicit processing of reward-related word content differed between AUD and age-matched controls, and because groups differed in years of education, we used standardized education-corrected Z-scores, where the control group has a group mean of Z = 0 ± 1 standard deviation (SD) (Table 3). Groups also differed in socioeconomic status (SES). SES was determined using a two-factor scale that includes education and lifetime occupation (Hollingshead and Redlich, 1958) and can be considered a representative measure of the highest functioning achieved (Sassoon et al., 2007). Because the SES score is derived from education, correcting for SES instead of education does not significantly change the results.
A repeated-measures ANOVA with word content (neutral, alcohol, cannabis) as within subject factor and group (AUD, CTL) as between subject factor revealed a significant main effect for condition (F (1,37) = 5.069, p = 0.03), no group effect (F(1,37) = 2.387, p = 0.131), and a significant group-by-condition interaction (F (1,37) = 4.618, p = 0.038) with AUD deviating from controls for alcohol and cannabis relative to neutral word content (Fig. 2B).
3.1.3. Relation to substance use
We next tested the assumption that alcohol cue-reactivity develops through personal alcohol use and attentional bias becomes stronger during the progression of alcohol addiction. We found that higher amounts of lifetime alcohol consumption correlated with greater alcohol attentional biases, i.e., greater difference between alcohol and neutral conditions (Zalcohol–Zcolor), overall (Rho = −0.46, p = 0.002, 1-tailed, n = 39) and in AUD (Rho = −0.45, p = 0.02, 1-tailed n = 21) (Fig. 2C). We further tested if past cannabis use histories were related to attentional biases to cannabis cues and found that higher past cannabis use correlated with a greater attentional bias to cannabis word content, i.e., greater RT difference between cannabis and neutral conditions (Zcannabis–Zcolor) in AUD (Rho = −0.71, p = 0.013, 1-tailed, n = 11) (Fig. 2D). Alcohol attentional bias was not significantly related to the amount of past cannabis use (Rho = − 0.29, ns), nor was cannabis attentional bias significantly related to the amount of lifetime alcohol (Rho = −0.21, ns). Significant correlations remained significant after correction for multiple comparisons (pcorrected = 0.026; Holm’s Sequential Bonferroni Procedure; Holm, 1979).
An ANOVA exploring the generalization of attentional capture from one class of substance-related stimuli to another in interaction with cannabis and alcohol use history (groups: AUD without CUD, n = 10; AUD with CUD, n = 11) for attentional alcohol and cannabis capture metrics (conditions: Zalcohol–Zcolor; Zcannabis–Zcolor) revealed no significant difference for cue content (F(1,19) = 0.9, ns), group (F(1,19) = 1.2, ns), or group-by-cue interaction (F(1,19) = 1.5, ns).
To explore the relative importance of the timing of addiction onset (age at AUD onset, age at CUD onset) together with substance use quantity (lifetime alcohol consumption in kg, heaviest cannabis consumption in times of use per month) for alcohol and cannabis attentional bias, we used multiple regression analyses. Note that the onset of past cannabis use disorder (CUD) occurred prior to AUD onset in our sample (see Figure S1). We found that all four predictor variables together explained over 90% of the variance of alcohol attentional bias (R2 = 0.97) (F(4,8) = 30.2, p = 0.003). In particular, earlier age at AUD onset (standardized β = 0.58, p = 0.043), but later age at CUD onset (standardized β = −1.40, p = 0.004), and less heavy past cannabis use per month (standardized β = − 0.75, p = 0.005) contributed to stronger alcohol-attentional bias, over and above the contribution of the amount of lifetime alcohol consumption (kg). Correspondingly, a stronger alcohol-attentional bias (i.e., more negative Z-scores) was associated with a longer time since last met CUD diagnosis criteria (Rho = −0.66; p = 0.026; n = 11). The multiple regression analysis with cannabis attentional bias as dependent variable was not significant (F(4,8) = 1.82, p = 0.3).
3.2. fMRI activation to addiction-Stroop task conditions
3.2.1. AUD and CTL group differences
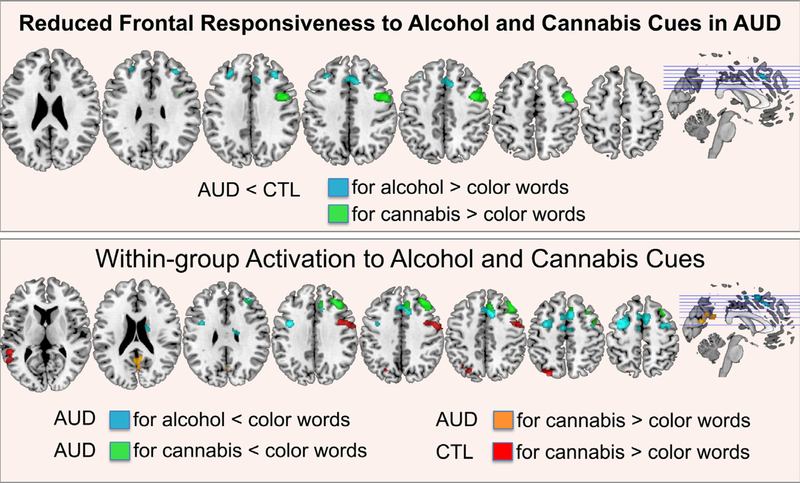
Functional brain image contrast analyses revealed group differences between AUD and CTL for implicit word content processing of alcohol- and cannabis-related words (compared to neutral color words; Table 4, Fig. 3). In particular, AUD relative to CTL activated bilateral dorsolateral prefrontal and premotor cortices less to alcohol-related words and right motor, premotor cortices less to cannabis-related word content.
Fig. 3.
Addiction-Stroop color match-to-sample task: fMRI activation.
Group activation differences (AUD vs. CTL) (upper panel) and within-group activation (AUD, CTL) (lower panel) to alcohol-Stroop (alcohol > color words) and cannabis-Stroop (cannabis > color words). Abbreviations: AUD: alcohol use disorder, CTL: healthy controls. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2.2. Within group analyses
In a comparison between neutral color words and alcohol-related words, AUD deactivated several frontal regions including medial and dorsolateral prefrontal, dorsal anterior cingulate, premotor, and supplementary motor cortices, and subcortically the right caudate nucleus. No significant activation differences between alcohol and neutral word conditions were observed in CTL. Individuals with AUD further deactivated right dorsolateral prefrontal, premotor and medial superior frontal regions less to cannabis-related than neutral words and activated posterior brain regions including primary visual areas and the posterior cingulate cortex. CTL participants activated left temporoparietal cortical and right middle frontal cortical regions to cannabis-related (vs. neutral) word content (Table 4, Fig. 3).
3.2.3. Relationship to demographics
3.2.3.1. Education, SES.
To test if group differences in (de)activation remain significant with education or SES as covariates, we used MANCOVAs with group as between-subject factor and (de)activation clusters (Table 4) as dependent variables. Group effects remained significant for all clusters with education (multivariate test Pillai’s Trace F(4,33) = 5.18; p = 0.002, = 0.39; all univariate tests p > 0.02) or SES as covariate (multivariate test Pillai’s Trace F (4,33) = 4.36; p = 0.006, = 0.35; all univariate tests p > 0.03).
3.2.3.2. Age.
In controls, older age correlated with more frontal activation to alcohol cues (R. MFG, premotor: Rho = 0.53, p = 0.025; n = 18) and less temporoparietal activation to cannabis cues (L. middle, superior temporal, angular gyri: Rho = − 0.53, p = 0.023; n = 18). Age did not correlate with imaging results in individuals with AUD (all p’s > 0.05).
3.3. Brain–Behavior relationships
3.3.1. Task performance
Cue-elicited activations (i.e., MR signal change) were extracted for each significant cluster (Table 4) and participant to test the relationship between regional brain activation and task performance. Lower activity in frontal and greater activity posterior brain regions was associated with poorer task performance in the AUD group, i.e., more errors (L. + R. MFG, premotor: Rho = −0.55, p = 0.01), greater RT variation (L. dlPFC: Rho = −0.54, p = 0.01; R. dlPFC: Rho = −0.63, p = 0.002; R. MFG, precentral: Rho = −0.57, p = 0.007; R. medial SFG: Rho = −0.59, p = 0.005; L. + R. calcarine, PCC: Rho = 0.64, p = 0.002) and longer RTs (L. dlPFC: Rho = −0.50, p = 0.02). Lower left dlPFC activity to alcohol-related cues correlated with performance Z-scores for both alcohol (Rho = 0.51, p = 0.019) and cannabis conditions (Rho = 0.56, p = 0.009). All correlations remained significant when controlling for age and survived correction for multiple comparisons (all pcorrected < 0.05; Family-wise Holm’s Sequential Bonferroni correction).
3.3.2. Impulsivity
Higher impulsivity scores were moderately correlated with greater behavioral attentional biases toward alcohol cues (all: Rho = −0.28, p = 0.045, n = 39; AUD: Rho = −0.34, p = 0.068, n = 21) and alcohol cue reactivity (all: Rho = − 0.44, p = 0.024, n = 21, one-tailed; but not in AUD: p’s > 0.05). Correlations did not survive correction for multiple comparisons. Impulsivity was not related to cannabis-related attentional bias or cue reactivity (all p’s > 0.05).
3.4. Relationship to alcohol and other drug use history
3.4.1. Cue-elicited brain activation and alcohol consumption
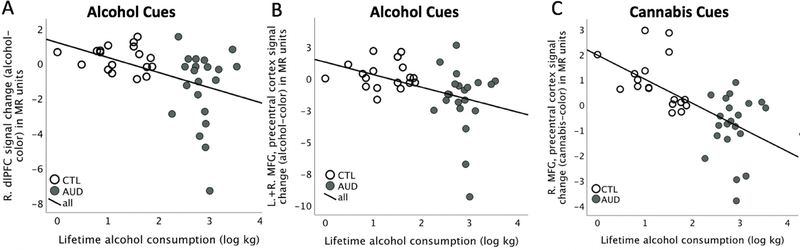
The amount of lifetime alcohol consumption correlated significantly with cue-elicited activation patterns, in particular with frontal deactivation to both alcohol cues (left dlPFC Rho = −0.40, p = 0.013; right dlPFC Rho = −0.50, p = 0.001; bilateral MFG, premotor Rho = −0.50, p = 0.001) (Fig. 4A,B) and cannabis cues (right MFG, precentral; Rho = −0.67, p < 0.0001; n = 39) (Fig. 4C) and with occipito-parietal activation to cannabis cues (calcarine, PCC Rho = 0.48, p = 0.002; n = 39). All correlations survived correction for multiple comparisons (all pcorrected < 0.02). Correlations were also confirmed when controlling for age. Finally, we correlated ‘time since last drink’ with behavioral and neural indices of attentional bias and did not find significant relationships (all p’s > 0.05).
Fig. 4.
Cue reactivity and lifetime alcohol consumption.
Graphs depicting relationships between the amount of lifetime alcohol consumption (log kg) and cue-elicited frontal (de-)activation pattern in A. dorsolateral prefrontal cortical (dlPFC) and B. bilateral middle frontal gyrus (MFG)/premotor cortical activation to alcohol cues, and C. right MFG/precentral cortical activation to cannabis cues. Abbreviations: AUD: alcohol use disorder, CTL: healthy controls.
3.4.2. Cue-elicited brain activation and other drug use
Exploratory MANOVA tested the role of past CUD for alcohol- and cannabis cue-elicited activation pattern (left and right dlPFC, bilateral MFG/premotor cortex, and right MFG/precentral gyrus; Table 4) by comparing AUD with (n = 11) and without (n = 10) past CUD histories, relative to CTL (n = 18) (Pillai’s Trace: F(8,68) = 5.35; p < 0.0001; univariate tests for group: all F(2,36) ≧ 8.58; p’s ≦ 0.001). The analysis was confirmed with MANCOVAs controlling for age (Pillai’s Trace: F (8,66) = 5.35; p < 0.0001; univariate tests for group: all F(2,35) ≧ 8.07; p’s ≦ 0.001) and lifetime alcohol consumption (log kg) (Pillai’s Trace: F(8,66) = 3.06; p = 0.005; univariate tests for group: all F(2,35) ≧ 4.21; p’s ≦ 0.023). Tukey’s HSD post-hoc analysis showed that AUD without CUD differed from AUD with CUD (all p’s < 0.02) and from CTL (all p’s < 0.001), while AUD with CUD did not differ from CTL (for all regions, p’s > 0.4). Both AUD subgroups differed significantly from CTL for cannabis cue-related activity (AUD without CUD vs. CTL: p = 0.0001; AUD with CUD vs. CTL: p = 0.02), and from each other (p = 0.025) (Supplement, Figures S2 and S3). Finally, greater cannabis-cue elicited activation in the superior parietal lobe moderately correlated with younger age at CUD onset (SPL: Rho = −0.75, pcorrected = 0.021, n = 11; controlling for age: Rhopartial(8) = −0.87, p = 0.001) and moderately with heavier past lifetime cannabis consumption (Supplement, Figure S3). Two exploratory MANCOVAs (with age and lifetime alcohol consumption as covariates) testing for subgroup effects of AUD with and without past other substance use (F(2,16) = 1.16; p = 0.56; Pillai’s Trace), and AUD smokers and non-smokers (F(2,16) = 0.46; p = 0.85; Pillai’s Trace) showed no cue-elicited activation differences between AUD subgroups for all regions (all univariate tests p’s > 0.05).
4. Discussion
Our task-activated functional neuroimaging study revealed implicit attentional bias and frontal hypoactivation to alcohol- and cannabis-related cues in abstinent AUD relative to controls using an addiction- Stroop color match-to-sample task with both alcohol and cannabis-related words. Others have reported similar results. For example, Mechin et al. (2016) recently found that decreased frontal activation of the supervisory control system was linked to appetitive reactivity to alcohol cues. Also, when differentiating heavy from light drinkers by their neural response patterns to alcohol cues, Ihssen et al. (2011) reported that responsiveness of emotion and reward brain circuitry was accompanied by reduced responses in frontal areas to cues related to higher life goals. Similarly, decreased frontal activation during an inhibitory control task was observed in heavier recent binge drinking young adults (Cohen-Gilbert et al., 2017). Disruption of prefrontal cortex function has been previously observed in individuals with AUD (Müller-Oehring et al., 2013; Schulte et al., 2012, 2017) and other addictions (Goldstein et al., 2007) and is associated with loss of control, compulsive drug taking, and the erosion of free will (Goldstein and Volkow, 2011). Although the cues in our color-matching paradigm were not relevant for task completion, the behavioral and neural responses denote that reward cues were implicitly and involuntarily processed in AUD participants and differed from controls. Frontal deactivation in response to alcohol cues has been related to relapse risk (see also, Beck et al., 2012; Courtney et al., 2016; Heinz et al., 2009; Kareken et al., 2012; Schacht et al., 2013). Considering findings on the continuance of addictive behaviors despite physical and social harm and the persistence of relapse risk long after a person has stopped drinking alcohol (Moos and Moos, 2006), our findings confirm that the activation of involuntary reward-cue-initiated capture mechanisms remains current in abstinent AUD, even under laboratory conditions. The neural correlates of persistent compulsive alcohol and drug use may be based on cues as positive reminders activating attentional capture mechanisms through long-term associative memory processes that occur in several neural circuits (de Sousa Fernandes Perna et al., 2017).
Here we studied whether such behavioral and neural response patterns can be observed for other reward cues, in particular cannabis cues, as well, even with remote cannabis use histories as in our sample. As a significant portion of individuals engage in co-use of alcohol and cannabis (Subbaraman and Kerr, 2015) and given the expanding cannabis legalization, it is of increased importance to understand how the neuropsychological mechanisms of addictive behaviors in co-users compare to those using alcohol only. Behaviorally, AUD relative to controls showed attentional biases to both alcohol and cannabis cues. These attentional biases were specific to past personal alcohol and cannabis use experiences. Alcohol was the preferred and most recently used substance in our AUD sample and accordingly, the attentional bias effect was graded and more pronounced to alcohol than cannabis cues. Also, higher lifetime alcohol consumption was related to stronger alcohol attentional bias and past heavier cannabis use to stronger cannabis attentional bias and not vice versa. We did not find an interaction between alcohol and cannabis attentional biases that would indicate a generalization of attentional capture from one substance class of stimuli to another. However, when exploring the role of the relative timing of the illness onset, we found that it mattered for alcohol attentional bias. As expected, alcohol-attentional bias was stronger with an earlier AUD onset; surprisingly, however, it was weaker with earlier CUD onset and heavier past cannabis use. As CUD onset occurred prior to AUD onset, during adolescence and young adulthood, these exploratory results may imply a directional influence of cannabis use disorder on alcohol attention capture mechanisms in AUD. Others discussed early cannabis use as a risk factor for future other substance use including alcohol (Fairman et al., 2019; Lynskey et al., 2003). However, causality cannot be implied from our data; although CUD occurred prior to AUD in our sample, other variables such as personality and a tendency to engage in risk taking behaviors could have been common underlying factors (Cavicchioli et al., 2019; Scalzo et al., 2018). In addition, the finding that AUD with and without past CUD did not differ in alcohol or drug use histories suggests that alcohol and cannabis were not used to replace each other but rather in addition to each other (Egan et al., 2019; Subbaraman, 2016).
For the neurofunctional bases of attentional capture by reward cues, AUD showed decreased frontal activation to both alcohol and cannabis-related word content relative to controls. Frontal hypoactivation correlated with task performance and specifically left dorsolateral prefrontal hypoactivation was related to behavioral measures of both alcohol- and cannabis-cue attentional biases. This suggests that frontal control mechanisms were not sufficiently engaged in AUD making it difficult to override automatic intrusions from implicit reward-related information. At first glance, this mechanism appears to be independent from the specific reward cue content. Medial and dorsolateral prefrontal hypoactivation to alcohol cues was related to higher lifetime alcohol consumption, and so was the prefrontal hypoactivation to cannabis cues. This supports the notion that cue-reactivity in AUD has developed through alcohol use, becoming stronger over time with more use, and that it can generalize to cannabis cues at the neural level, as evidenced in AUD without past cannabis use. However, neural responsiveness in AUD with past CUD did not differ from controls for alcohol cues; yet, it did for cannabis cues. The differences in neural responsiveness in AUD with and without past CUD imply that attentional biases toward reward-cues depend on past experience with that substance. Cannabis cues in our experiment likely acted as reminders and activated cannabis-related neural mechanisms through long-term associative memory processes (see de Sousa Fernandes Perna et al.,2017). Our exploratory results further showed that neural sensitization toward cannabis cues, in those with remote cannabis experience, attenuated neural responsiveness to alcohol cues. Neuroimaging work has repeatedly shown that, relative to controls, adults with heavy alcohol use histories show decreased dorsolateral and medial prefrontal activation during inhibitory control (Ahmadi et al., 2013; Claus et al., 2013; Li et al., 2009), while literature on the relationship between cannabis use and brain regions implicated in behavioral inhibition or executive control suggests increased, and potentially compensatory, recruitment of frontal and parietal regions (Aloi et al., 2018; Gruber and Yurgelun-Todd, 2005). Thus, it is possible that opposing alcohol-cannabis effects on frontal responsivity explain our observation of attenuated effects in AUD with past CUD. An alternate explanation is provided by the attentional focus hypotheses. According to Hicks et al. (2012), alcohol cues narrow the attentional mindset for individuals who are motivated to consume alcohol. In individuals with AUD, even during abstinence, the attentional scope would be narrowed toward alcohol cues and consequently elicit strong behavioral and neural responses to alcohol cues (see e.g., Hicks et al., 2012). Experiences with other drugs may broaden the attentional scope and attenuate responsiveness to alcohol cues in the presence of other reward cues, such as cannabis cues in our paradigm. Converging evidence supporting this interpretation comes from a recent event-related potentials (ERP) study that found a reduced N1 amplitude, which is a neurophysiological marker of rapid motivated attention, after manipulation of the attentional focus from a local to a global scope prior to alcohol picture presentation (Ryerson et al., 2017).
It is possible that neurochemical mechanisms of cannabis use affect associative learning, memory, and long-term neural responsiveness to alcohol cues. The effect of cannabis depends on different chemical substances. Tetrahydrocannabinol (THC) is one psychoactive ingredient in cannabis with a high density of CB-1 receptors expressed in the lateral and basal nuclei of the amygdala (Katona et al., 2001) underlying emotional behavior (Gruber et al., 2009). For example, the early exposure to THC in adolescent rodents is associated with an altered reward system that has implications for the use of other drugs later in adulthood (Pistis et al., 2004). The AUD subjects in our sample had initiated cannabis use mainly during adolescence and received the CUD diagnoses prior to being diagnosed with an AUD. If and how exactly the timing of cannabis use in humans changes behavioral and brain functional mechanisms for other substance use, such as alcohol, is unclear. Our exploratory observation, however, showed that more recent cannabis use was associated with attenuated attentional capture by alcohol cues in AUD. Similarly, cannabidiol intake was found to reverse at- tentional bias to cigarette cues in nicotine dependency (Hindocha et al., 2018).
Finally, despite the considerable heterogeneity of the effects of cannabis on brain function reported in the literature, a recent review on 29 functional neuroimaging studies on the chronic effects of cannabis (24 in adult users and 5 in adolescent users) summarizes it as compensatory brain activity in response to chronic cannabis exposure (Batalla et al., 2013). One example is an fMRI study showing greater left superior parietal cortical activity in the cannabis-using group performing a verbal working memory task at normal levels (Jager et al., 2006), consistent with the idea of compensatory neural recruitment. In our study, we observed greater left superior parietal lobe activation, similar to controls, in AUD participants with younger age at cannabis use onset. It is possible that age-related differences in cue reactivity and parietal cortical responsiveness over the course adolescent neurodevelopment may have preceded the cannabis use onset and constitute a risk factor rather than a consequence of early cannabis use (Scheinsburg et al., 2008; Tervo-Clemmens et al., 2018). Our data are consistent with epidemiological and preclinical data suggesting that cannabis initiation precedes the abuse of alcohol and other drugs (Agrawal et al., 2004; Hayley et al., 2017; Stinson et al., 2006). We also observed that a younger age at cannabis onset, i.e., during adolescence, was related to greater severity (dependence vs. abuse and more recent use) and with younger age at AUD onset. Longitudinal studies are needed to gain a deeper understanding of potential underlying developmental, socio- economical, and psychological mechanisms associated with early cannabis exposure and how it influences neural responsiveness toward other reward and addiction cues with consequences for alcohol and polysubstance use behaviors.
In AUD, attentional biases are of particular relevance for approach and avoidance behavior towards a drug and accordingly addiction treatment has started to integrate attentional bias modification trainings to reduce alcohol craving and prevent relapse (see e.g., Cox et al., 2014; Wiers et al., 2015, Heitmann et al., 2017; Luehring-Jones et al., 2017). Our analyses show that AUD participants without prior cannabis exposure also responded to cannabis-related stimuli suggesting an enhanced readiness to perceive and attend to other drug cues as well. It may be helpful to expand bias modification-trainings in alcohol addiction treatment programs to cannabis-related stimuli as a preventive measure, particularly when considering the enhanced availability of cannabis with current changes in its legalization in the U.S.
Limitations of our study are that the sample did not include individuals with acute cannabis use, and the subsample of AUD with a history of past cannabis use was small and did not permit for additional analyses to explore differences between early initiation of cannabis (i.e., age 17 or younger; Pope et al., 2003) and later onset. Additionally, investigation of performance differences by simultaneous use patterns was not explored. While there is evidence that cognitive bias modification training can attenuate alcohol attentional biases (Wiers et al., 2011; Wiers and Wiers, 2017), AUD participants were not receiving such training during our study. Studies on cannabis bias modification trainings are more inconsistent showing that a computerized approach- avoidance training reduces cannabis use in adolescent cannabis users (Jacobus et al., 2018) but does not change fMRI cannabis cue reactivity (Karoly et al., 2019). We did, however, observe that attentional biases to alcohol cues correlated with higher amounts of lifetime alcohol consumption, but not with length of alcohol abstinence. This may indicate that once an AUD is established, attentional bias may be difficult to extinguish without a specific training (Wiers et al., 2011). Another limitation is that the majority of our AUD sample had histories of other substance abuse/dependencies, a pattern often observed in U.S. AUD samples depending on the availability of other substances (e.g. Fama et al., 2019; Choi et al., 2018). In our sample, past substance use was in remission for on average 16.8 years. Hence, when controlling for remote other substance use, we did not observe significant effects on alcohol and cannabis cue reactivity. Ultimately, further research with larger sample sizes is warranted on the evolution and reversibility of long-term chronic alcohol, cannabis, and other drug effects on neural attentional capture mechanisms and how they interact with one another.
5. Conclusion
Involuntary cue-initiated attentional capture mechanisms remained current in abstinent AUD. We found attentional biases to alcohol- and cannabis-related cues and decreased frontal activation in AUD that was related to lifetime alcohol consumption and modulated by an individual’s history of cannabis abuse. Concerning the role of reward cues in the maintenance of addiction and relapse risk, our findings confirm that frontal control mechanisms in AUD are not sufficiently engaged to override automatic intrusions from implicit alcohol related information. Differential patterns of frontal dysfunction in AUD were associated with CUD histories. When processing alcohol and cannabis cues, individuals with AUD and no history of CUD had decreased responses in frontal brain regions associated with behavioral inhibition and executive attention, while AUD participants with past CUD had less pronounced frontal and increased parietal responses to reward cues. Thus, attentional bias to alcohol cues in AUD is influenced by individuals’ past cannabis use experiences. Even when CUD is in remission, cannabis cues can act as reminders activating neural response pattern through long-term associative memory processes and affect neural cue reactivity to alcohol cues in AUD patients. This is important for a mechanistic understanding of how the alcoholic brain responds to reward cues during abstinence. With the increased social acceptance and accessibility of cannabis in the U.S., attentional bias to alcohol- and cannabis- related cues as pathway to addiction could have implications for treatment programs on alcohol-cannabis co-use in AUD.
Supplementary Material
Acknowledgement
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grants Nos. AA023165, AA010723, AA012388, AA017168, and AA017923. We thank Dr. Edith Sullivan for critical reading of and valuable comments on this article. We also thank Dr. Stephanie Sassoon and Priya Asok for clinical screening of study participants.
Footnotes
Ethical statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all participants for being included in the study.
Declaration of Competing Interest
None.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.pscychresns.2019.111005.
References
- Aloi J, Meffert H, White SF, Blair KS, Hwang S, Tyler PM, Thornton LC, Crum KI, Adams KO, Killanin AD, Filbey F, Pope K, Blair RJR, 2019. Differential dysfunctions related to alcohol and cannabis use disorder symptoms in reward and error-processing neuro-circuitries in adolescents. Dev. Cog. Neurosci. 36, 200618. https://doi.org/10.10167j.dcn.2019.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, 2000. Diagnostic and Statistical Manual of Mental Disorders, 4th ed American Psychiatric Association, Washington DC. [Google Scholar]
- Agrawal A, Neale MC, Prescott CA, Kendler KS, 2004. A twin study of early cannabis use and subsequent use and abuse/dependence of other illicit drugs. Psychol. Med. 34 (7), 1227–1237. 10.1017/s0033291704002545. [DOI] [PubMed] [Google Scholar]
- Ahmadi A, Pearlson GD, Meda SA, Dager A, Potenza MN, Rosen R, Austad CS, Raskin SA, Fallahi CR, Tennen H, Wood RM, Stevens MC, 2013. Influence of alcohol use on neural response to go/no-go task in college drinkers. Neuropsychopharmacology 38, 2197–2208. 10.1038/npp.2013.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloi J, Blair KS, Crum KI, Meffert H, White SF, Tyler PM, Thornton LC, Mobley AM, Killanin AD, Adams KO, Filbey F, Pope K, Blair RJR, 2018. Adolescents show differential dysfunctions related to alcohol and cannabis use disorder severity in emotion and executive attention neuro-circuitries. Neuroimage 19, 782–792. 10.1016/j.nicl.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andréasson S, Allebeck P, Engström A, Rydberg U, 1987. Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet 2, 1483–1486. 10.1016/S0140-6736(87)92620-1. [DOI] [PubMed] [Google Scholar]
- Babor T, Higgins-Biddle JC, Saunders JB, Monteiro MG, 2001. AUDIT – The Alcohol use Disorders Identification Test: Guidelines for use in Primary Care. World Health Organizaton Department of Mental Health and Substance Abuse, Geneva. [Google Scholar]
- Batalla A, Bhattacharyya S, Yücel M, Fusar-Poli P, Crippa JA, Nogué S, Torrens M, Pujol J, Farré M, Martin-Santos R, 2013. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS ONE 8, e55821. 10.1371/journal.pone.0055821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistella G, Fornari E, Thomas A, Mall J-F, Chtioui H, Appenzeller M, Annoni J-M, Favrat B, Maeder P, Giroud C, 2013. Weed or Wheel! fMRI, Behavioural, and Toxicological Investigations of How Cannabis Smoking Affects Skills Necessary for Driving. PLoS ONE 8 (1), e52545. 10.1371/journal.pone.0052545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, 2005. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat. Neurosci. 8, 1458–1463. 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK, 1996. BDI-II, Beck Depression Inventory: Manual. Psychological Corp. Harcourt Brace, San Antonio, Tex., Boston. [Google Scholar]
- Beck A, Wüstenberg T, Genauck A, Wrase J, Schlagenhauf F, Smolka MN, Mann K, Heinz A, 2012. Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch. Gen. Psychiatry 69, 842–852. 10.1001/archgenpsychiatry.2011.2026. [DOI] [PubMed] [Google Scholar]
- Blair RJR, White SF, Tyler PM, Johnson K, Lukoff J, Leiker EK, Fibley F, Dobbertin M, Blair KS, 2019. Threat Responsiveness as a Function of Cannabis and Alcohol Use Disorder Severity. J. Child Adolesc. Psychopharmacol. 29, 526–534. 10.1089/cap.2019.0004. [DOI] [PubMed] [Google Scholar]
- Cavicchioli M, Prudenziati F, Movalli M, Ramella P, Maffei C, 2019. The severity of personality pathology: a risk factor for concurrent substance use disorders in alcohol use disorder. J. Dual. Diagn. 15, 159–171. 10.1080/15504263.2019.1612131. [DOI] [PubMed] [Google Scholar]
- Choi NG, DiNitto DM, Marti CN, 2018. A longitudinal assessment of change in marijuana use with other substance use problems. Am. J. Drug Alcohol Abuse. 44, 642–652. 10.1080/00952990.2018.1461879. [DOI] [PubMed] [Google Scholar]
- Claus ED, Feldstein Ewing SW, Filbey FM, Hutchison KE, 2013. Behavioral control in alcohol use disorders: relationships with severity. J. Stud. Alcohol Drugs 74, 141–151. 10.15288/jsad.2013.74.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J, 1993. PsyScope: a new graphic interactive environment for designing psychology experiments. Beh. Res. Methods Instrum. Comput. 25, 257–271. https://ci.nii.ac.jp/naid/10018219542/. [Google Scholar]
- Cohen-Gilbert JE, Nickerson LD, Sneider JT, Oot EN, Seraikas AM, Rohan ML, Silveri MM, 2017. College binge drinking associated with decreased frontal activation to negative emotional distractors during inhibitory control. Front. Psychol. 8, 1650 10.3389/fpsyg.2017.01650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RE, Williams E, Seegobin S, Tye C, Kuntsi J, Asherson P, 2017. Cannabinoids in attention-deficit/hyperactivity disorder: a randomised-controlled trial. Eur. Neuropsychopharmacol. 27, 795–808. 10.1016/j.euroneuro.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Schacht JP, Hutchison K, Roche DJ, Ray LA, 2016. Neural substrates of cue reactivity: association with treatment outcomes and relapse. Addict. Biol. 21, 3–22. 10.1111/adb.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Watson P, Koenders L, Vingerhoets WAM, Goudriaan AE, Wiers RW, 2013. Cannabis dependence, cognitive control and attentional bias for cannabis words. Addict. Behav. 38, 2825–2832. 10.1016/j.addbeh.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Cox WM, Fadardi JS, Intriligator JM, Klinger E, 2014. Attentional bias modification for addictive behaviors: clinical implications. CNS Spectr. 19, 215–224 10.1017/S1092852914000091. [DOI] [PubMed] [Google Scholar]
- Crane NA, Schuster RM, Fusar-Poli P, Gonzalez R, 2013. Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Neuropsychol. Rev. 23, 117–137. 10.1007/s11065-012-9222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean RD, Crane NA, Mason BJ, 2011. An Evidence Based Review of Acute and Long-Term Effects of Cannabis Use on Executive Cognitive Functions. J. Addict. Med. 5, 1–8. 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran HV, Brignell C, Fletcher S, Middleton P, Henry J, 2002. Cognitive and subjective dose-response effects of acute oral delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology (Berl) 164, 61–70. 10.1007/s00213-002-1169-0. [DOI] [PubMed] [Google Scholar]
- Curran HV, Freeman TP, Mokrysz C, Lewis DA, Morgan CJ, Parsons LH, 2016. Keep off the grass? Cannabis, cognition and addiction. Nat. Rev. Neurosci. 17, 293–306. 10.1038/nrn.2016.28. [DOI] [PubMed] [Google Scholar]
- Dager AD, Anderson BM, Rosen R, Khadka S, Sawyer B, Jiantonio-Kelly RE, Austad CS, Raskin SA, Tennen H, Wood RM, Fallahi CR, Pearlson GD, 2014. Functional magnetic resonance imaging (fMRI) response to alcohol pictures predicts subsequent transition to heavy drinking in college students. Addiction 109, 585–595. 10.1111/add.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca MA, Di Chiara G, Cadoni C, Lecca D, Orsolini L, Papanti D, Corkery J, Schifano F, 2017. Cannabis; epidemiological, neurobiological and psychopathological issues: an update. CNS Neurol. Disord. Drug Targets 16, 598–609. 10.2174/1871527316666170413113246. [DOI] [PubMed] [Google Scholar]
- de Sousa Fernandes Perna EB, Theunissen EL, Kuypers KP, Evers EA, Stiers P, Toennes SW, Witteman J, van Dalen W, Ramaekers JG, 2017. Brain reactivity to alcohol and cannabis marketing during sobriety and intoxication. Addict. Biol. 22, 823–832. 10.1111/adb.12351. [DOI] [PubMed] [Google Scholar]
- De Ternay J, Naassila M, Nourredine M, Louvet A, Bailly F, Sescousse G, Maurage P, Cottencin O, Carrieri PM, Rolland B, 2019. Therapeutic Prospects of Cannabidiol for Alcohol Use Disorder and Alcohol-Related Damages on the Liver and the Brain. Front. Pharmacol. 10, 627 10.3389/fphar.2019.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Forti M, Iyegbe C, Sallis H, Kolliakou A, Falcone MA, Paparelli A, Sirianni M, La Cascia C, Stilo SA, Marques TR, Handley R, Mondelli V, Dazzan P, Pariante C, David AS, Morgan C, Powell J, Murray RM, 2012. Confirmation that the AKT1 (rs2494732) genotype influences the risk of psychosis in cannabis users. Biol. Psychiatry 72, 811–816. 10.1016/j.biopsych.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Di Forti M, Quattrone D, Freeman TP, Tripoli G, Gayer-Anderson C, EU-GEI WP2 Group, 2019. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): a multicentre case-control study. Lancet Psychiatry 6, 427–436. 10.1016/S2215-0366(19)30048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobes DJ, Thomas SE, 1999. Assessing craving for alcohol. Alcohol. Res. Health 12, 179–186. [PMC free article] [PubMed] [Google Scholar]
- Duncan SC, Gau JM, Farmer RF, Seeley JR, Kosty DB, Lewinsohn PM, 2015. Comorbidity and temporal relations of alcohol and cannabis use disorders from youth through adulthood. Drug Alcohol Depend. 149, 80–86. 10.1016/j.drugalcdep.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A, Fryer SL, Meyerhoff DJ, 2011. Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcohol. Clin. Exp. Res. 35, 1187–1200. 10.1111/j.1530-0277.2011.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan KL, Cox MJ, Suerken CK, Reboussin BA, Song EY, Wagoner KG, Wolfson M, 2019. More drugs, more problems? Simultaneous use of alcohol and marijuana at parties among youth and young adults. Drug Alcohol Depend. 202, 69–75. 10.1016/j.drugalcdep.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadardi JS, Cox WM, 2006. Alcohol attentional bias: drinking salience or cognitive impairment? Psychopharmacology (Berl). 185, 169–178. [DOI] [PubMed] [Google Scholar]
- Fairman BJ, Furr-Holden CD, Johnson RM, 2019. When marijuana is used before cigarettes or alcohol: demographic predictors and associations with heavy use, cannabis use disorder, and other drug-related outcomes. Prev. Sci. 20, 225–233. 10.1007/s11121-018-0908-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fama R, Le Berre AP, Sassoon SA, Zahr NM, Pohl KM, Pfefferbaum A, Sullivan EV, 2019. Relations between cognitive and motor deficits and regional brain volumes in individuals with alcoholism. Brain Struct. Funct. 224, 2087–2101. 10.1007/s00429-019-01894-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Cox WM, 2008. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 97, 1–20. 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Field M, Eastwood B, 2005. Experimental manipulation of attentional bias increases the motivation to drink alcohol. Psychopharmacology (Berl) 183, 350–357. 10.1007/s00213-005-0202-5. [DOI] [PubMed] [Google Scholar]
- Field M, Munafo MR, Franken IH, 2009. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol. Bull. 135, 589–607. 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBM, 1998. Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition (SCID-I/P, Version 2.0, 8/98 revision). Biometrics Research Department, New York State Psychiatric Institute, New York, NY. [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RS, Turner R, 1995. Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage 2, 166–172. 10.1006/nimg.1995.1019. [DOI] [PubMed] [Google Scholar]
- Galvez-Buccollini JA, Proal AC, Tomaselli V, Trachtenberg M, Coconcea C, Chun J, Manschreck T, Fleming J, Delisi LE, 2012. Association between age at onset of psychosis and age at onset of cannabis use in non-affective psychosis. Schizophr. Res. 139, 157–160. 10.1016/j.schres.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, Telang F, Alia-Klein N, Volkow ND, 2007. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 144, 1153–1159. 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, 2011. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 12, 652–669. 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Rogowska J, Yurgelun-Todd DA, 2009. Altered affective response in marijuana smokers: an fmri study. Drug Alcohol Depend. 105, 139–153. 10.1016/j.drugalcdep.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA, 2005. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Res. Cogn. Brain Res. 23, 107–118. https://doi.Org/10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Grüsser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A, 2004. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology 175, 296–302. 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Guttmannova K, Lee CM, Kilmer JR, Fleming CB, Rhew IC, Kosterman R, Larimer ME, 2016. Impacts of changing marijuana policies on alcohol use in the United States. Alcohol. Clin. Exp. Res. 40, 33–46. 10.1111/acer.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas AL, Wickham R, Macia K, Shields M, Macher R, Schulte T, 2015. Identifying classes of conjoint alcohol and marijuana use in entering freshmen. Psychol. Addict. Behav. 29, 620–626. 10.1037/adb0000089. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, Jung J, Pickering RP, Ruan WJ, Smith SM, Huang B, Grant BF, 2015. Prevalence of marijuana use disorders in the United States between 2001–2002 and 2012–2013. JAMA Psychiatry 72, 1235–1242. 10.1001/jamapsychiatry.2015.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grüsser SM, Flor H, Braus DF, Buchholz HG, Gründer G, Schreckenberger M, Smolka MN, Rösch F, Mann K, Bartenstein P, 2004. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am. J. Psychiatry 161, 1783–1789. 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Hayley AC, Stough C, Downey LA, 2017. DSM-5 cannabis use disorder, substance use and DSM-5 specific substance-use disorders: evaluating comorbidity in a population- based sample. Eur. Neuropsychopharmacol. 27, 732–743. 10.1016/j.euroneuro.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Heinz A, Beck A, Grüsser SM, Grace AA, Wrase J, 2009. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addict. Biol. 14, 108–118. 10.1111/j.1369-1600.2008.00136.x. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitmann J, van Hemel-Ruiter ME, Vermeulen KM, Ostafin BD, MacLeod C, Wiers RW, DeFuentes-Merillas L, Fledderus M, Markus W, de Jong PJ, 2017. Internet-based attentional bias modification training as add-on to regular treatment in alcohol and cannabis dependent outpatients: a study protocol of a randomized control trial. BMC Psychiatry 17, 193 10.1186/s12888-017-1359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindocha C, Freeman TP, Grabski M, Stroud JB, Crudgington H, Davies AC, Das RK, Lawn W, Morgan CJA, Curran HV, 2018. Cannabidiol reverses attentional bias to cigarette cues in a human experimental model of tobacco withdrawal. Addiction. [Epub ahead of print] 10.1111/add.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks JA, Friedman RS, Gable PA, Davis WE, 2012. Interactive effects of approach motivational intensity and alcohol cues on the scope of perceptual attention. Addiction 107, 1074–1080. 10.1111/j.1360-0443.2012.03781.x. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, Pike VW, Volkow ND, Huestis MA, Innis RB, 2012. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol. Psychiatry 17, 642–649. 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S, 1979. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6, 65–70. https://www.jstor.org/stable/4615733. [Google Scholar]
- Hollingshead AB, Redlich FC, 1958. Social Class and Mental Illness: Community study. Wiley, New York, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihssen N, Cox WM, Wiggett A, Fadardi JS, Linden DE, 2011. Differentiating heavy from light drinkers by neural responses to visual alcohol cues and other motivational stimuli. Cereb. Cortex. 21, 1408–1415. 10.1093/cercor/bhq220. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Taylor CT, Gray KM, Meredith LR, Porter AM, Lia I, Castro N, Squeglia LM, 2018. A multi-site proof-of-concept investigation of computerized approach-avoidance training in adolescent cannabis users. Drug Alcohol Depend. 187, 195–204. 10.1016/j.drugalcdep.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager G, Kahn RS, van den Brink W, van Ree JM, Ramsey NF, 2006. Long-term effects of frequent cannabis use on working memory and attention: an fMRI study. Psychopharmacology (Berl) 185, 358–368. 10.1007/s00213-005-0298-7. [DOI] [PubMed] [Google Scholar]
- John WS, Wu LT, 2017. Problem alcohol use and healthcare utilization among persons with cannabis use disorder in the United States. Drug Alcohol Depend. 178, 477–484. 10.1016/j.drugalcdep.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, McCance-Katz EF, 2019. Relationship between recency and frequency of youth cannabis use on other substance use. J. Adolesc. Health 64, 411–413. 10.1016/j.jadohealth.2018.09.017. [DOI] [PubMed] [Google Scholar]
- Jurica P, Leitten C, Mattis S, 2001. Dementia Rating Scale-2: Professional Manual. Psychological Assessment Resources, Lutz, FL. [Google Scholar]
- Kann L, Kinchen S, Shanklin SL, Flint KH, Hawkins J, Harris WA, Lowry R, O’Malley Olsen E, McManus T, Chyen D, Whittle L, Taylor E, Demissie Z, Brener N, Thornton J, Moore J, Zaza S, 2014. Youth Risk Behavior Surveillance — United States, 2013. Morbidity and Mortality Weekly Report: Surveillance Summaries 63 (4), 1–168. https://www.jstor.org/stable/24806229. [PubMed] [Google Scholar]
- Kareken DA, Grahame N, Dzemidzic M, Walker MJ, Lehigh CA, O’Connor SJ, 2012. fMRI of the brain’s response to stimuli experimentally paired with alcohol intoxication. Psychopharmacology (Berl) 220, 787–797. 10.1007/s00213-011-2526-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoly HC, Bryan AD, Weiland BJ, Mayer A, Dodd A, Feldstein Ewing SW, 2015. Does incentive-elicited nucleus accumbens activation differ by substance of abuse? An examination with adolescents. Dev. Cogn. Neurosci. 16, 5–15. 10.1016/j.dcn.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoly HC, Schacht JP, Mereditha LR, Jacobus J, Tapert SF, Graya KM, Squeglia LM, 2019. Investigating a novel fMRI cannabis cue reactivity task in youth. Addict. Behav. 89, 20–28. 10.1016/j.addbeh.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF, 2001. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J. Neurosci. 21, 9506–9518. 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CR, Luo X, Yan P, Bergquist K, Sinha R, 2009. Altered impulse control in alcohol dependence: neural measures of stop signal performance. Alcohol. Clin. Exp. Res. 33, 740–750. 10.1111/j.1530-0277.2008.00891.x.Altered. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luehring-Jones P, Louis C, Dennis-Tiwary TA, Erblich J, 2017. A single session of attentional bias modification reduces alcohol craving and implicit measures of alcohol bias in young adult drinkers. Alcohol. Clin. Exp. Res. 41, 2207–2216. 10.1111/acer.13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey MT, Heath AC, Bucholz KK, Slutske WS, Madden PA, Nelson EC, Statham DJ, Martin NG, 2003. Escalation of drug use in early-onset cannabis users vs co-twin controls. JAMA 289, 427–433. 10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- Mechin N, Gable PA, Hicks JA, 2016. Frontal asymmetry and alcohol cue reactivity: influence of core personality systems. Psychophysiology 53, 1224–1231. 10.1111/psyp.12659. [DOI] [PubMed] [Google Scholar]
- Menendez-Miranda I, Garcia-Alvarez L, Garcia-Portilla MP, Gonzalez-Blanco L, Saiz PA, Bobes J, 2019. History of lifetime cannabis use is associated with better cognition and worse real-world functioning in schizophrenia spectrum disorders. Eur. Addict. Res. 25, 111–118. 10.1159/000497317. [DOI] [PubMed] [Google Scholar]
- Metrik J, Aston ER, Kahler CW, Rohsenow DJ, McGeary JE, Knopik VS, 2015. Marijuana’s Acute Effects on Cognitive Bias for Affective and Marijuana Cues. Exp. Clin. Psychopharmacol. 23, 339–350. 10.1037/pha0000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokrysz C, Landy R, Gage SH, Munafo MR, Roiser JP, Curran HV, 2016. Are IQ and educational outcomes in teenagers related to their cannabis use? A prospective cohort study. J. Psychopharmacol. 30, 159–168. 10.1177/0269881115622241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJ, Freeman TP, Powell J, Curran HV, 2016. AKT1 genotype moderates the acute psychotomimetic effects of naturalistically smoked cannabis in young cannabis smokers. Transl. Psychiatry 6, e738. 10.1038/tp.2015.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos RH, Moos BS, 2006. Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction 101, 212–222. 10.1111/j.1360-0443.2006.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss HB, Chen CM, Yi HY, 2014. Early adolescent patterns of alcohol, cigarettes, and marijuana polysubstance use and young adult substance use outcomes in a nationally representative sample. Drug Alcohol Depend. 136, 51–62. 10.1016/j.drugalcdep.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Müller-Oehring EM, Jung YC, Sullivan EV, Hawkes WC, Pfefferbaum A, Schulte T, 2013. Midbrain-driven emotion and reward processing in alcoholism. Neuropsychopharmacology 38, 1844–1853. 10.1038/npp.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC, 1971. The assessment and analysis of handedness: the edinburgh inventory. Neuropsychologia 97–113. 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES, 1995. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 51, 768–774. . [DOI] [PubMed] [Google Scholar]
- Papachristou H, Nederkoorn C, Giesen JCAH, Jansen A, HarilaosPapachristouabChantalNederkoornaJanneke C.A.H.GiesenaAnitaJansena, 2014. Cue reactivity during treatment, and not impulsivity, predicts an initial lapse after treatment in alcohol use disorders. Addict. Behav. 39, 737–739. 10.1016/j.addbeh.2013.11.027. [DOI] [PubMed] [Google Scholar]
- Patton GC, Coffey C, Lynskey MT, Reid S, Hemphill S, Carlin JB, Hall W, 2007. Trajectories of adolescent alcohol and cannabis use into young adulthood. Addiction 102, 607–615. 10.1111/j.1360-0443.2006.01728.x. [DOI] [PubMed] [Google Scholar]
- Pistis M, Perra S, Pillolla G, Melis M, Muntoni AL, Gessa GL, 2004. Adolescent exposure to cannabinoids induces long-lasting changes in the response to drugs of abuse of rat midbrain dopamine neurons. Biol. Psychiatry 56, 86–94. 10.1016/j.biopsych.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D, 2003. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 69, 303–310. 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, Petersen SE, 2010. The development of human functional brain networks. Neuron 67, 735–748. 10.1016/j.neuron.2010.08.017. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC, 2008. The incentive sensitization theory of addiction: some current issues. Phil. Trans. R. Soc. B. 363, 3137–3146. 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryerson NC, Neal LB, Gable PA, 2017. Attenuating the alcohol allure: attentional broadening reduces rapid motivational response to alcohol pictures. Psychopharmacology (Berl) 34, 1247–1254. 10.1007/s00213-017-4557-1. [DOI] [PubMed] [Google Scholar]
- Sagar KA, Gruber SA, 2018. Interactions between recreational cannabis use and cognitive function: lessons from functional magnetic resonance imaging. Ann. N. Y. Acad. Sci. 1451, 42–70. 10.1111/nyas.13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoon SA, Fama R, Rosenbloom MJ, O’Reilly A, Pfefferbaum A, Sullivan EV, 2007. Component cognitive and motor processes of the digit symbol test: differential deficits in alcoholism, HIV infection, and their comorbidity. Alcohol. Clin. Exp. Res. 31, 1315–1324. 10.1111/j.1530-0277.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- Scalzo F, Hulbert CA, Betts JK, Cotton SM, Chanen AM, 2018. Predictors of substance use in youth with borderline personality disorder. Personal. Disord. 9, 390–396. 10.1037/per0000257. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H, 2013. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict. Biol. 18, 121–133. 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Müller-Oehring EM, Vinco S, Hoeft F, Pfefferbaum A, Sullivan EV, 2009. Double dissociation between action-driven and perception-driven conflict resolution invoking anterior versus posterior brain systems. Neuroimage 48, 381–390. 10.1016/j.neuroimage.2009.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Müller-Oehring EM, Sullivan EV, Pfefferbaum A, 2012. Synchrony of corticostriatal-midbrain activation enables normal inhibitory control and conflict processing in recovering alcoholic men. Biol. Psychiatry 71, 269–278. 10.1016/j.biopsych.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinsburg AD, Brown SA, Tapert SF, 2008. The Influence of Marijuana Use on Neurocognitive Functioning in Adolescents. Curr. Drug Abuse Rev. 1, 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Jung YC, Sullivan EV, Pfefferbaum A, Serventi M, Müller-Oehring EM, 2017. The neural correlates of priming emotion and reward systems for conflict processing in alcoholics. Brain Imaging Behav. 11, 1751–1768. 10.1007/s11682-016-9651-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ, 1982. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J. Stud. Alcohol. 43, 1157–1170. 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA, 1983. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press, Palo Alto, CA. [Google Scholar]
- Springate BA, Tremont G, Papandonatos G, Ott BR, 2014. Screening for mild cognitive impairment using the dementia rating scale-2. J. Geriatr. Psychiatry Neurol. 27, 139–144. 10.1177/0891988714522700. [DOI] [PubMed] [Google Scholar]
- Stacy AW, 1997. Memory activation and expectancy as prospective predictors of alcohol and marijuana use. J. Abnorm. Psychol. 106, 61–73. https://doi.org/10.1037Z/0021-843x.106.1.61. [DOI] [PubMed] [Google Scholar]
- Stinson FS, Ruan WJ, Pickering R, Grant BF, 2006. Cannabis use disorders in the USA: prevalence, correlates and co-morbidity. Psychol. Med. 36, 1447–1460. 10.1017/S0033291706008361. [DOI] [PubMed] [Google Scholar]
- Subbaraman MS, 2016. Substitution and complementarity of alcohol and cannabis: a review of the literature. Subst. Use Misuse. 51, 1399–1414. 10.3109/10826084.2016.1170145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaraman MS, Kerr WC, 2015. Simultaneous vs. concurrent use of alcohol and cannabis in the National Alcohol Survey. Alcohol. Clin. Exp. Res. 39, 872–879. 10.1111/acer.12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo-Clemmens B, Simmonds D, Calabro FJ, Montez DF, Lekht JA, Day NL, Richardson GA, Luna B, 2018. Early Cannabis Use and Neurocognitive Risk: A Prospective Functional Neuroimaging Study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3, 713–725. 10.1016/j.bpsc.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma RJ, Monnig MA, Lysne PA, Ruhl DA, Pommy JA, Bogenschutz M, Tonigan JS, Yeo RA, 2011. Adolescent substance abuse: the effects of alcohol and marijuana on neuropsychological performance. Alcohol. Clin. Exp. Res. 35, 39–46. 10.1111/j.1530-0277.2010.01320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valyear MD, Villaruel FR, Chaudhri N, 2017. Alcohol-seeking and relapse: a focus on incentive salience and contextual conditioning. Behav. Processes. 141, 26–32. 10.1016/j.beproc.2017.04.019. [DOI] [PubMed] [Google Scholar]
- van Os J, Bak M, Hanssen M, Bijl RV, de Graaf R, Verdoux H, 2002. Cannabis use and psychosis: a longitudinal population-based study. Am. J. Epidemiol. 156, 319–327. 10.1093/aje/kwf043. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD, 2013. Unbalanced neuronal circuits in addiction. Curr. Opin. Neurobiol. 23, 639–648. 10.1016/j.conb.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Alexoff D, Logan J, Jayne M, Wong C, Tomasi D, 2014. Decreased dopamine brain reactivity in marijuana abusers is associated with negative emotionality and addiction severity. Proc. Natl. Acad. Sci. U. S. A. 111, e3149–e3156. 10.1073/pnas.1411228111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Loeber S, Richter A, Kirsch M, Bach P, von der Goltz C, Hermann D, Mann K, Kiefer F, 2012. Validating incentive salience with functional magnetic resonance imaging: association between mesolimbic cue reactivity and attentional bias in alcohol-dependent patients. Addict. Biol. 17, 807–816. 10.1111/j.1369-1600.2011.00352.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D, 2001. Wechsler Test of Adult Reading: WTAR. Psychological Corp., San Antonio, Tex. [Google Scholar]
- Wechsler D, Stone CP, 1987. Wechsler Memory Scale-Revised. Psychological Corporation. [Google Scholar]
- Weiss F, Porrino LJ, 2002. Behavioral neurobiology of alcohol addiction: recent advances and challenges. J. Neurosci. 22, 3332–3337 https://doi.org/20026359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Jagannathan K, Hager N, Childress AR, Franklin TR, 2015. Sex differences in associations between cannabis craving and neural responses to cannabis cues: implications for treatment. Exp. Clin. Psychopharmacol. 23, 238–246. 10.1037/pha0000036. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Cox WM, Field M, Fadardi JS, Palfai TP, Schoenmakers T, Stacy AW, 2006. The search for new ways to change implicit alcohol-related cognitions in heavy drinkers. Alcohol. Clin. Exp. Res. 30, 320–331. 10.1111/j.1530-0277.2006.00037.x. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Eberl C, Rinck M, Becker ES, Lindenmeyer J, 2011. Retraining automatic action tendencies changes alcoholic patients’ approach bias for alcohol and improves treatment outcome. Psychol. Sci. 22, 490–497. 10.1177/0956797611400615. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Houben K, Fadardi JS, van Beek P, Rhemtulla M, Cox WM, 2015. Alcohol cognitive bias modification training for problem drinkers over the web. Addict. Behav. 40, 21–26. 10.1016/j.addbeh.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Wiers CE, Wiers RW, 2017. Imaging the neural effects of cognitive bias modification training. Neuroimage 151, 81–91. 10.1016/j.neuroimage.2016.07.041. [DOI] [PubMed] [Google Scholar]
- Wijayendran SB, O’Neill A, Bhattacharyya S, 2018. The effects of cannabis use on salience attribution: a systematic review. Acta Neuropsychiatr. 30, 43–57. 10.1017/neu.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witteman J, Post H, Tarvainen M, de Bruijn A, Perna Ede S, Ramaekers JG, Wiers RW, 2015. Cue reactivity and its relation to craving and relapse in alcohol dependence: a combined laboratory and field study. Psychopharmacology (Berl) 232, 3685–3696. 10.1007/s00213-015-4027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.