Abstract
Several neurologic diseases exhibit different prevalence and severity in males and females, highlighting the importance of understanding the influence of biologic sex and gender. Beyond host-intrinsic differences in neurologic development and homeostasis, evidence is now emerging that the microbiota is an important environmental factor that may account for differences between men and women in neurologic disease. The gut microbiota is composed of trillions of bacteria, archaea, viruses, and fungi, that can confer benefits to the host or promote disease. There is bidirectional communication between the intestinal microbiota and the brain that is mediated via immunologic, endocrine, and neural signaling pathways. While there is substantial interindividual variation within the microbiota, differences between males and females can be detected. In animal models, sex-specific microbiota differences can affect susceptibility to chronic diseases. In this review, we discuss the ways in which neurologic diseases may be regulated by the microbiota in a sex-specific manner.
1. Acquisition, maturation, and function of the gut microbiota throughout lifespan
The intestinal microbiota is a diverse community of bacteria, archaea, viruses, and fungi that inhabit every surface of the human body, inside and out. They provide several beneficial functions, including producing vitamins, protecting from infection, regulating hormone levels, aiding in digestion, and promoting neurologi health. The composition of the microbiota is specific to the site they inhabit, i.e. oral microbiota differs from skin microbiota (Consortium, 2012a), and is shaped by environmental factors and host genetics. The total number of microbial cells within the microbiota is estimated to be equal to the cells in the human body, and the number of microbial genes is estimated to be 100 times the number of genes within the human genome.
At birth, the newborn infant is colonized by a large number of microorganisms, shaped by delivery mode (i.e. vaginal birth vs. C-section) (Dominguez-Bello et al., 2010), which then undergo a typical succession, increasing in both diversity and stability (Spor et al., 2011) until an adult-like intestinal microbiota is reached by the age of three (Yatsunenko et al., 2012). There is limited evidence that the fetus might be exposed to microbes during gestation (Aagaard et al., 2014), however the majority of the studies indicate that the fetus develops in a sterile environment (Wassenaar and Panigrahi, 2014). While there is considerable day-to-day and inter-individual variation, (Consortium, 2012a; Consortium, 2012b), the microbiota remain relatively stable in the adult until advanced aging in which reduced immune function and altered physiology can result in reduced microbiome stability (Spor et al., 2011). These changes in gut microbiota throughout lifespan have been hypothesized to play a role in neurologic diseases which present at different ages, including in childhood: autism, in early to mid-adulthood: multiple sclerosis (MS) and amyotrophic lateral sclerosis (ALS), and in the elderly Alzheimer’s disease (AD) and Parkinson’s disease (PD). Whether the age-related changes in the microbiota also affect the prevalence in males and females is unknown.
The microbiota can carry out many beneficial functions for the host and can be thought of as a virtual organ (O'Hara and Shanahan, 2006). One important role is pathogen displacement, in which non-pathogenic members microbiota outcompete virulent organisms. When the microbiota become depleted through heavy antibiotic exposure, pathogenic bacteria such as Clostridium difficile can overgrow and result in serious intestinal infection (Seekatz and Young, 2014). In addition to post-antibiotic treatment, there is also reduced colonization resistance in infancy due to an immature gut microbiota. This leaves the infant vulnerable to colonization by Clostridium botulinum, which can be frequently found in the environment. Upon colonization, C. botulinum secretes potent neurotoxins and can lead to flaccid paralysis, respiratory distress, and death (Brown and Desai, 2013; Fujinaga et al., 2013). Thus, changes in the stability of the microbiota is an important factor in preventing infection by potentially neurotoxic bacteria.
2. Microbiota metabolic and immunologic influence on the gut-brain axis.
The intestinal microbiota can influence the brain via endocrine, immune, or neural signaling pathways and alterations have been detected in several neurologic disease compared to healthy controls (Bercik et al., 2011; Collins et al., 2012; Cox and Weiner, 2018; Cryan and Dinan, 2012; Fung et al., 2017; Hsiao et al., 2013; Jangi et al., 2016; Jiang et al., 2017; Stilling et al., 2014). The microbiota encodes over 100 times more functional genes than the human genome, and microbial immunomodulatory and metabolic functions can influence the brain in homeostasis and disease (Fung et al., 2017; Luczynski et al., 2016). For example, serotonin and behavior are regulated by the microbiota in a sex-specific manner (Clarke et al., 2013), tryptophan derivatives modulate astrocyte function in neuroinflammatory disease (Rothhammer et al., 2016), and microbially-derived secondary bile acids are elevated in AD (MahmoudianDehkordi et al., 2018; Marksteiner et al., 2018).
In addition, the microbiota plays a role in metabolism by breaking down complex dietary compounds, increasing energy extraction, and the producing vitamins and essential amino acids (O'Hara and Shanahan, 2006). Disruptions in the gut microbiota from antibiotics or altered immune status can contribute to obesity and metabolic diseases (Cox and Blaser, 2013). Given that obesity is a risk factor for several neurologic diseases, it is plausible that the microbiota may indirectly contribute to neurologic diseases by altering metabolic signaling. Conversely, limiting caloric intake has been shown to improve models of neurodegenerative disease (Cignarella et al., 2018; Schafer et al., 2015a). A 30% reduction in calories from carbohydrates prevented amyloid-beta plaque accumulation in a model of Alzheimer’s disease in female, but not male mice (Schafer et al., 2015a), which was associated with sex-specific changes in amyloid-precursor processing enzymes. Reducing calories through an intermittent fasting scheme changed the microbiota and improved the clinical outcomes experimental autoimmune encephalitis (EAE), the animal model for MS. Importantly, when microbiota from intermittent-fasting mice was transferred to recipients on a normal diet, they were protected from EAE (Cignarella et al., 2018). This suggests that diets that can help prevent neurologic diseases may work in part by selecting a beneficial microbiota.
The microbiota play a critical role in regulating host immunity, with species specific effects (Hooper et al., 2012). For example, segmented filamentous bacteria can induce the development of Th17 cells that are important for mucosal immunity and protection against intestinal pathognes (Gaboriau-Routhiau et al., 2009; Ivanov et al., 2009) but is also implicated in multiple sclerosis and experimental autoimmune encephalomyelitis pathogenesis (Lee et al., 2011). T regulatory cells can be induced by diverse types of microbiota and differing mechanisms. Bacteroides fragilis have been shown to induce Tregs by a mechanism that is dependent on polysaccharide A in their cell wall, and this response can ameliorate EAE (Ochoa-Reparaz et al., 2010a). Several members of Clostridia clusters IV and XIV can also induce Tregs by secreting butyrate (Atarashi et al., 2011). In addition, microbial metabolites can influence different immune cell populations in the brain including microglia and astrocytes, which may be important in inflammatory disease (e.g. MS), neurodegenerative disease (e.g. Parkinson’s disease), or recovery from injury (e.g. traumatic brain injury).
3. The cross-talk between microbiota and sex-hormones
Hormones can modulate gut microbiota composition. There is a distinct shift in the gut microbiota in humans during pregnancy, including a decrease in alpha diversity, a decrease in butyrate producing bacteria, and an increase in lactic acid bacteria (Koren et al., 2012). Administering 17β-estradiol to mice shifts the microbiota and can ameliorate EAE (Benedek et al., 2017). However, whether the improvement in EAE is mediated solely by 17β-estradiol or in part by the microbiome is still not known. Altering hormone levels via ovariectomy or by exposure to endocrine disrupting chemicals (e.g. bisphenol A) can alter microbiome (Cox-York et al., 2015; Liu et al., 2016). Thus, the evidence that the microbiota is shaped by the host hormonal milieu provides a basis for differences in microbiota composition and function in males and females and during different reproductive stages of life.
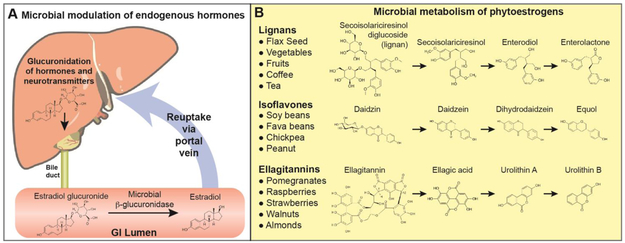
Androgens influence brain function and neurologic diseases via modulating dopaminergic signaling, mitochondria function, and immunologic signaling (Loke et al., 2015; Pinares-Garcia et al., 2018b). The microbiota can regulate the circulating level of androgens (Bokkenheuser, 1993), thus affecting important signaling molecules that could have widespread sex-specific effects in neurologic disease. The microbiota modulates the reuptake or excretion of androgens via the enterohepatic circulation (Figure 1) (Adlercreutz et al., 1976; Back et al., 1978; Chen and Madak-Erdogan, 2016; Groh et al., 1993; Markle et al., 2013; Plottel and Blaser). Hormones, as well as other host derived molecules and exogenous compounds, are inactivated by the host and tagged for gastrointestinal (GI) excretion by conjugating the molecule to glucuronic acid (Pellock and Redinbo, 2017). Several members of the gut microbiota contain β-glucuronidase enzymes that cleave the glucuronide tag, resulting in reuptake of the active hormone. Several hormones, including testosterone, 17β-estradiol, and thyroxine, as well as neurotransmitters dopamine, serotonin, and acetylcholine are excreted via glucuronide tags and can be modulated by the microbiota (Pellock and Redinbo, 2017). A diverse number of bacteria have been shown to have β-glucuronidase activity, including members of the phyla Actinobacteria (genera Bifidobacterium and Collinsella), Bacteroidetes (Bacteroides and Prevotella), Firmicutes (Bryantella, Clostridium, Enterococcus, Faecalibacterium, Lactobacillus, Roseburia, and Streptococcus), and Proteobacteria (Escherichia) (Pellock and Redinbo, 2017). Antibiotics reduce the levels of plasma and urinary estriole (Van Look et al., 1981), further supporting the concept that the gut microbiota regulate the reabsorption of hormones.
Figure 1. Microbial regulation of endocrine signaling.
A) The gut microbiota can alter the levels of circulating hormones and certain neurotransmitters by altering reabsorption rates. Estrogens, testosterone, as well as serotonin, dopamine, and acetylcholine are tagged with the addition of gluronic acid for excretion via the bile duct. In the GI tract, the intestinal microbiota β-glucuronidase activity can cleave this tag leading to reuptake via the portal vein. B) Several plant-based phytoestrogens that have can be converted into enterolactone, equol, and urolithin, which have a greater effect on estrogen signaling than their precursor molecules.
In addition to regulating hormone levels by affecting excretion and reuptake, the microbiota can modulate endocrine signaling by bioconversion of host endogenous hormones (Plottel and Blaser) or by metabolizing dietary products into estrogen mimics (Penttinen-Damdimopoulou et al., 2009). For example, the gut microbiota can convert estrone (E1, higher after menopause) to estradiol (E2, higher during reproductive years), and can convert 16-hydroxyestrone to estriol (E3, higher in pregnancy). Altered hormone levels have been associated with a number of diseases, and the microbial modulation of sex-steroids has been hypothesized to play a role in disease initiation and progression (Kwa et al., 2016).
Several plants contain phytoestrogens: isoflavones, ellagitannins, and lignans (Figure 1). These compounds can be converted by the microbiota to equol, urolithins, and enterolignans, respectively. The microbial derived products have a higher activity than the plant-based precursors (Landete et al., 2016). Interestingly, microbiota from women show a higher prevalence of bacteria that can convert the lignin secoisolariciresinol found in flax seed into the enterolignans: enterodiol and enterolactone (Clavel et al., 2005). Only approximately 1/3 of individuals harbor a microbiota that is able to convert isoflavanoids found in soy and other legumes into equol (Morton et al., 2002). This variation phytoestrogen conversion within the human population suggests that the same phytoestrogen intake could have substantially different physiologic effects, which could be dependent on the microbiota.
4. Sex-specific interaction between host and microbe
Males show increased incidence and more severe diseases in response to several pathogens, including Campylobacter jejuni, Mycobacterium tuberculosis, and the hepatitis B virus (vom Steeg and Klein, 2016). Sex-specific response to infection can be mediated by differences in immune function between males and females, the influence of sex steroids on the immune system, as well as X-linked immune genes. Females have been reported to have a 10-fold higher anti-viral response, and a greater innate, cell-mediated, and antibody responses to infection (vom Steeg and Klein, 2016). In addition, the pattern recognition receptor TLR7 is located on the X chromosome, which is associated with higher interferon alpha (INFα) secretion from dendritic cells exposed to TLR7 ligands (vom Steeg and Klein, 2016). Differences between male and female immune response to the same infectious agent suggests that underlying biologic differences can influence the magnitude and specific response to microbes.
At a homeostatic level, the gut microbiota differs between males and females, which can drive some, but not all, sex-specific changes in immunity. For example, germ-free females have higher numbers of T cell precursors in the thymus and higher type 1 interferon signaling in the ileum than males. This relationship is not altered based when male microbiota is transferred into germ-free females, indicating that these sex-based differences are a result of host intrinsic biology (Fransen et al., 2017). Conversely, transferring female microbiota to male germ-free mice results in lower FoxP3+ Treg populations in the Peyer’s patches compared to transferring male microbiota, demonstrating that sex-specific microbiota can modulate immunity. Microbiota alterations were detected between male and female mice, including higher Alistipes, Rikenella, and Porphyromonadacea in males, and higher Akkermansia and Lactobacillus in females (Fransen et al., 2017). Altogether, these studies demonstrate that biologic sex selects for individual members of the microbiota may in turn influence sex-specific immune responses.
Differences in male and female microbiota can drive chronic diseases (Fransen et al., 2017; Markle et al., 2013). For example, in the non-obese diabetic (NOD) mouse model of type 1 diabetes, females have worse disease than males, but are protected when colonized with male microbiota, demonstrating that sex-dependent changes in the microbiota can drive disease pathology (Markle et al., 2013). This protection was associated with higher levels of testosterone in female mice colonized with male microbiota. In another study using the NOD mouse, sex-specific differences in the microbiota become more prominent following puberty, and were reduced by orchidectomy in males, indicating the role that androgens have in shaping the gut microbiota (Yurkovetskiy et al., 2013).
5. Behavior
The gut microbiota is capable of synthesizing neurotransmitters and affects behavior (Collins et al., 2012), however it is still not fully understood whether these molecules are capable of entering circulation and crossing the blood brain barrier at a relevant concentration (Foster and McVey Neufeld, 2013). It has been shown that a gamma-aminobutyric acid producing Lactobacillus rhamnosus strain can reduce depressive-like behavior in mice (Bravo et al., 2011). This effect is lost when the vagus nerve is severed, indicating that the microbiota can also influence behavior via signaling through afferent nerves.
In addition, serotonin and behavior are regulated by the early-life microbiota in a sex-specific manner (Clarke et al., 2013). Both male and female germ-free mice had reduced inflammatory responses to LPS stimulation, and increased corticosterone release following stress induced by a novel environment (Clarke et al., 2013). However, male germ-free mice showed decreased brain-derived neurotrophic factor (BDNF) and increased 5-hydroxytryptamine (5-HT) in their hippocampus compared with conventional mice, whereas female mice showed no differences according to microbiota status. 5-HT, otherwise known as serotonin, is synthesized from the amino acid tryptophan, and levels in the brain relate to the amount that is in circulation. The microbiota can also utilize tryptophan, thus depleting circulating tryptophan stores. In absence of the microbiota, male mice showed elevated plasma tryptophan, however no changes were detected in females (Clarke et al., 2013), suggesting that there are sex-specific metabolic profiles of the gut microbiota.
The gut microbiota can also regulate anxiety and aggression. Mice treated with antibiotics show reduced anxiety and increased exploratory activity (Bercik et al., 2011). The gut microbiota can also mediate behavioral phenotypes observed in different strains of mice. BALBc mice show increased anxiety and reduced exploratory behavior compared to NIH Swiss Webster mice, and these behavioral characteristics can be transmitted by colonizing germ-free mice with either brave (Swiss-Webster) or timid (BALBc) microbiota (Bercik et al., 2011). This effect of the microbiota on anxiolytic responses was linked with increased BDNF expression in the brain of BALBc mice colonized with Swiss-Webster microbiota. Depleting the microbiota with antibiotics can also reduce aggressive behavior in Siberian hamsters, with greater effects in females than in males (Sylvia et al., 2017). Though females showed fewer attacks than males (15 for females vs. 25 for males), a single dose of antibiotics reduced the number of attacks in half for females and this reduction was sustained after the antibiotics were withdrawn. For males, antibiotics reduced the number of attacks only after two successive treatment periods, and the effect was only transient as the same level of aggression returned following antibiotic cessation (Sylvia et al., 2017).
6. Microbiota and Microglia
Microglia are the predominant immune cells in the brain and perform critical functions including synaptic pruning during development, defending against infectious threats, and initiating repair following injury. However, microglia can also contribute to disease pathogenesis in inflammatory and neurodegenerative diseases (Butovsky and Weiner, 2018; Hong et al., 2016; Stephan et al., 2012). Microglia are yolk-sac derived, migrate to the brain early in embryonic development (day 8.5 for mice), and are long-lived tissue resident cells (Lenz and McCarthy, 2015). Differences in male and female microglia can be detected in several brain regions, including higher numbers of microglia with an activated morphology in the pre-optic area of the male brain compared to females (Lenz and McCarthy, 2015).
Recently, it was shown that the microbiota can regulate microglia during development and their function in adulthood (Erny et al., 2015). Germ-free mice exhibited microglia with a more immature phenotype both in terms of morphology (longer cell processes and increased branching) and gene expression, including a decreased expression in inflammatory genes IL-1α, CD86, and Fcgr2β, and increased expression of genes that promote survival Sfpi1 and Csf1r measured by RNAseq. As measured by flow cytometry, germ-free microglia also had increased markers of immature cells (Csf1r, F4/80, and CD31). At a functional level, germ-free microglia had diminished responses to challenge with lipopolysaccharide (LPS) or lymphocytic choriomenigitis virus (LCMV), suggesting that the microbiota help with the maturation of microglia and their ability to respond to pathogens. In addition, adult mice treated with high dose of broad-spectrum antibiotics showed similar changes in microglia morphology and increased Csf1r, F4/80, and CD31 levels, suggesting that the microbiota not only shape microglia during embryonic development but also throughout the lifespan. These changes appeared to be mediated in part by the microbial metabolites short-chain fatty acids and their signaling through the free fatty acid receptor 2(Ffar2) (Erny et al., 2015).
Microglia function and gene expression can change throughout lifespan in a sex-specific manner (Mangold et al., 2017). For example, there is a greater increase in complement factor C1q in female mice in aging (from 3 to 24 months of age). In development, at embryonic day 18.5, female microglia show increased gene expression related to LPS and other inflammatory responses compared to males (Thion et al., 2018). Furthermore, the microbiota can shape microglia responses in development in a sex- and temporal-specific manner. At embryonic day 18.5, female microglia from germ-free mice show little difference from SPF mice (20 genes altered), whereas male mice show profound changes (1216 genes altered) (Thion et al., 2018). In adulthood, this relationship is reversed and microglia from germ-free females (433 genes), but not males (26 genes), have a markedly different gene expression compared to conventional mice. Looking at an even earlier developmental window, there was little effect of the microbiota on the microglia from embryos at day 14.5, however, both males and females were combined for this analysis, raising the question of whether differences would have been detected if biologic sex was isolated as a variable.
7. Multiple Sclerosis
Evidence is emerging to support a role of gut microbiota in the pathophysiology of multiple sclerosis (MS) (Mielcarz and Kasper, 2015; Tremlett and Waubant, 2017). We and others have shown that not only the intestinal microbiome differs between MS patients and healthy controls, but seems to be also associated with disease activity and treatment response (Cantarel et al., 2015; Chen et al., 2016; Jangi et al., 2016; Miyake et al., 2015; Tremlett et al., 2016a; Tremlett et al., 2016b). Abundance of certain gut bacteria has been shown to be associated with serum immunological markers in MS (Jangi et al., 2016; Tremlett et al., 2016b). Specifically, we found increased Akkermansia and Methanobrevibacter, and reduced Butyricimonas in MS patients, and found the strongest correlations between Akkermansia and immune gene expression in T cells and monocytes (Jangi et al., 2016).
Several studies have investigated whether the microbiota plays an active role in neuroinflammatory diseases. Germ-free mice show attenuated severity in the animal model of MS (experimental autoimmune encephalitis; EAE), and depleting the microbiota with antibiotics also ameliorates EAE symptoms, demonstrating that the gut microbiota can contribute to disease (Berer et al., 2011; Lee et al., 2011; Ochoa-Reparaz et al., 2009; Ochoa-Reparaz et al., 2010b). Colonization of GF mice either with entire microbiota or monocolonizing with Segmented Filamentous Bacteria (SFB) induced IL-17 and IFN-γ production in the spinal cord, demonstrating that one mechanism by which the microbiota contributes to EAE pathogenesis is by increasing inflammation in the CNS (Lee et al., 2011). The dietary intervention intermittent-fasting has been shown to improve EAE, decrease pro-inflammatory cytokines IL-17, IFN-γ, and GM-CSF, and shifted microbiota composition and function. Specifically, intermittent fasting increased Bacteroidaceae, Lactobacillaceae, and Prevotellaceae, increased microbial genes related to antioxidative glutathione and ketone body metabolism, and decreased inflammatory lipopolysaccharide biosynthesis. Furthermore, mice on a normal diet colonized with microbiota from mice on an intermittent fasting-diet had less severe EAE and reduced proinflammatory cytokine production, demonstrating that the diet selects for a microbiota have a direct role in EAE protection. As these studies were only done in female mice, whether this diet has a sex-specific effect is unknown.
Transferring the gut microbiota from MS patients to mice can worsen EAE, (Berer et al., 2017; Cekanaviciute et al., 2017). Interestingly, these studies also detected an increase in Akkermansia in MS patients compared to healthy controls. When germ-free mice were monocolonized with Akkermansia or other MS associated bacteria Acinetobacter (high in MS) and Parabacteroides (low in MS), there was a shift in the host immune profile (Cekanaviciute et al., 2017). It has also been shown that MS associated bacterial extracts induced a proinflammatory immune shift when co-cultured with human PBMCs (Cekanaviciute et al., 2017). In addition, two other studies have demonstrated that transferring members of the phylum Bacteroidetes, namely Prevotella histicola and Bacteroides fragilis (polysaccharide A positive strain), can ameliorate EAE (Mangalam et al., 2017; Ochoa-Reparaz et al., 2010a), indicating that there can be species specific effects which either promote disease pathogenesis or protect animals from EAE.
It is well documented that women are more susceptible to MS and autoimmune diseases in general (Bove and Chitnis, 2014; Harbo et al., 2013; Voskuhl and Gold, 2012). Several studies have shown an increase in incidence and prevalence of MS worldwide during the past decades, which is partly driven by disproportionate incidence increase in women during the recent years, increasing the female to male sex ratio (Koch-Henriksen and Sorensen, 2010; Maghzi et al., 2010; Orton et al., 2006). It is hypothesized that this rapid change is multifactorial, but mainly due to changing environmental risk factors (diet, lifestyle, reproductive habits, etc.) and that these factors affect men and women differently (Sellner et al., 2011). A growing literature links the dietary and lifestyle changes to gut microbiota (Conlon and Bird, 2014), hence it is plausible that these effect are mediated through a sex-based effect of microbiota on the risk of the disease.
Another observation which highlights the importance of sex hormones in MS, is that the disease activity subsides during pregnancy and increases in the post-partum period (Airas et al., 2011; Confavreux et al., 1998). Pregnancy alters microbiota in humans, which can drive changes in metabolism in non-pregnant mice, demonstrating that the pregnancy-microbiome has a functional role in host physiology (Koren et al., 2012; Nuriel-Ohayon et al., 2016). In EAE, the protective effect of pregnancy is mediated by estrogen signaling on T cells and astrocytes (Lelu et al., 2011; Spence et al., 2013). Administration of 17β -estradiol to non-pregnant mice to mimic the hormone levels during pregnancy and ameliorates EAE and is associated with a shifts in the microbiota (Benedek et al., 2017). Specifically, 17β-estradiol increased levels of Lactobacillaceae, a family that has several species linked with improvements in EAE (Yamashita et al., 2017). The beneficial effect of 17β -estradiol was also linked with an increase in FoxP3+ T regulatory cells and IL-10+ B regulatory cells, thus it is not clear whether the effects are through direct influence on the immune system, or whether this was mediated in part by the microbiota (Benedek et al., 2017).
In EAE, some strains of mice (e.g. SJL) show a sex-bias (Papenfuss et al., 2004), whereas others (e.g. C57/BL6) do not (Okuda et al., 2002). Interestingly, a study demonstrated that a sex-bias in spontaneous EAE development in TNFR2 knockout mice was mediated though a disruption of sex-specific microbiota (Miller et al., 2015). The complex interaction of sex hormones, the immune system, and the microbiota seems to be one of the factors responsible for the observed sex dimorphism in MS, however the direction of effects remains to be discovered.
Astrocytes are one of the most common cells in the brain and regulate homeostasis. Astrocytes play an important role in the pathophysiology of MS and their role in MS is an area of intense research (Ludwin et al., 2016). Recent data demonstrates that the microbiota can influence astrocytes and modulate EAE via aryl hydrocarbon receptor (AHR) (Rothhammer et al., 2016; Rothhammer et al., 2017). It has also been shown that the neuroprotective effects of estrogen on EAE is through estrogen receptor-α (ERα) specifically on astrocytes (Spence et al., 2011; Spence et al., 2013). AHR and ERα pathways seem to be also interconnected and affect each other (Matthews and Gustafsson, 2006). However, it remains unknown how microbiota, astrocytes, and sex hormones interact during neuroinflammation and contribute to sex dimorphism in disease. In summary based the available data, it seems that the sex hormones, astrocytes, the immune system, and gut microbiota are key players in the sex-based differences observed in the disease.
8. Alzheimer’s Disease
Alzheimer's disease (AD) affects an estimated 5.5 million Americans (Hebert et al., 2013), with higher prevalence in women than in men (Mielke et al., 2014). Furthermore, women show a faster cognitive decline and may have sex-dependent response to treatment (Li and Singh, 2014; Mazure and Swendsen, 2016). Women who are carriers of the Apoe4 allele, a risk factor for sporadic AD, are more likely to have mild cognitive impairment and AD compared to men (Altmann et al., 2014). Despite these higher rates of decline and risk of disease, women may also show increased resilience to AD pathology. A recent study examined amyloid beta and tau levels in the brain by positron emission tomography (PET) scanning in 298 cognitively normal aged men and women (55-94 years) found that women had higher levels of AD pathology, despite not having symptoms (Buckley et al., 2019). This suggests that while women may be more vulnerable to the development of AD pathology and symptoms, there may be sex-specific factors that compensate for the early stages of the disease.
AD risk can be influenced by diet, metabolism, and immunity, suggesting that factors outside the brain play a pivotal role (Bekkering et al., 2013; Devi et al., 2012; Meraz-Ríos et al., 2013; Tucsek et al., 2013; Wang et al., 2005; Wyss-Coray and Rogers, 2012). The gut microbiota influences neurologic diseases (Bercik et al., 2011; Collins et al., 2012; Cox and Weiner, 2018; Cryan and Dinan, 2012; Fung et al., 2017; Hsiao et al., 2013; Jangi et al., 2016; Jiang et al., 2017; Stilling et al., 2014) and sex-based differences in immunity (Fransen et al., 2017; Markle et al., 2013), however the extent to which it modulates AD and the observed sex-bias is unknown.
The greatest risk factor for AD is advanced age (Hebert et al., 2013; Masters et al., 2015). Age-related changes in physiology and immunity result in a destabilized microbiota (Tiihonen et al., 2010), which can be manipulated by diet (Claesson et al., 2012). The aging microbiome impairs gut barrier defense and promotes systemic immune dysfunction, including increasing interleukin (IL-6) and tumor necrosis factor-α (TNFα) and impairing macrophage phagocytosis, which can be rescued by transferring young microbiota to old mice (Thevaranjan et al., 2017).
Modulating the diet may be a therapeutic modality to control the microbiota in aging and prevent physiologic decline (Claesson et al., 2012). Recently a calorie-restriction diet has been linked with the microbiota, AD pathology, and the GI tract, with differing effects in males and females. Calorie-restriction decreases AD-like pathology in mice (Radler et al., 2014; Schafer et al., 2015a; Wang et al., 2005) and monkeys (Qin et al., 2006). Studies further showed that calorie-restriction reduced amyloid-beta (Aβ) plaque accumulation in female, but not male, Tg2576 mice (Schafer et al., 2015a). This effect was linked with lower levels of amyloid precursor protein (APP) processing enzymes that favor the pathologic Aβ40 and Aβ42 isoforms (Schafer et al., 2015a). In addition to a reduction in Aβ pathology in an animal model of AD, calorie-restriction also slowed age-related changes in hippocampal gene expression in wild-type (WT) mice (Schafer et al., 2015b).
Beyond the brain, the gut ages differently in males and females. In Drosophila, females have higher intestinal stem cell proliferation which protects them from infections compared to males at a cost of greater deterioration of the intestinal epithelium in aging (Regan et al., 2016). Calorie-restriction rescues age-related changes in gut epithelium and extends lifespan in females but has no effect on lifespan in males. Intriguingly, genetically modified males with a feminized midgut exhibit an extended lifespan on calorie-restriction, indicating that sexually-dimorphic GI tract aging critically mediates the benefits that females derive from this diet (Regan et al., 2016). In addition to changes in host physiology, lifelong calorie restriction alters the microbiota by increasing bacteria that positively correlate with lifespan and improves gut barrier function (Zhang et al., 2013), suggesting that some of the beneficial effect of calorie-restriction may be linked with the microbiota.
Animal models of AD demonstrate that the microbiota can influence pathology, and there are critical sex-specific effects. Several groups have reported altered microbiota in models of AD (Bauerl et al., 2018; Bonfili et al., 2017; Brandscheid et al., 2017; Minter et al., 2017; Sanguinetti et al., 2018; Shen et al., 2017; Wang et al., 2016), and two groups have examined the effect of manipulating the microbiota on AD pathology. Minter, et al., treated APP/PS1 mice with broad spectrum antibiotics and found decreased Aβ plaque deposition in association with the expansion of the Allobaculum, Akkermansia, and Lachnospiraceae (Minter et al., 2016). They also observed a decrease in the number of plaque-localized Iba-1+ microglia and GFAP+ astrocytes. This was observed in male but not female mice, suggesting a sex-dependent relationship between Aβ and the microbiome. In a follow-up study, they found a similar effect when animals were only treated in the early post-natal period (P14-P21) and observed increased numbers of Foxp3 Tregs and an altered circulating inflammatory mediator profile (Minter et al., 2017). Harach, et al., reported that germ-free APP/PS1 mice had less Aβ deposition and showed differences in the microbiota between compared to WT mice, including a deficit in Allobaculum (Harach et al., 2015). They also demonstrated that APP/PS1 germ-free mice colonized with WT microbiota showed decreased Aβ compared to those colonized with APP/PS1 microbiota. Like Minter, they observed a decrease in Iba-1 staining cells. They also found that IL1-β levels in the brain did not increase with age in the germ-free mice. Decreasing IL1-β levels may increase levels of the Aβ-degrading enzymes neprilysin and insulin degrading enzymes.
Microbiota alterations have been detected in AD patients. Vogt, et al. found elevated Bacteroides and decreased Ruminococcaceae in AD patients (Vogt et al., 2017). Cattaneo, et al., measured 6 bacteria by qPCR and also found changes in subjects with AD (Cattaneo et al., 2017). Although evidence suggests the microbiome may play an important role in AD, mechanisms by which this occurs are not well understood. Proper immune function in the brain is critical for the clearance of Aβ plaques in AD (Heneka et al., 2015; Town, 2010). Microglia, the key immune cells in the brain, play a critical role in AD (Butovsky and Weiner, 2018; Sarlus and Heneka, 2017; Sochocka et al., 2018). Recent studies show that microbiota shapes microglia development in a sex-dependent manner (Thion et al., 2018), that the microbiota can modulate microglia homeostasis in adulthood (Erny et al., 2015), as well as in neurodegenerative disease (Sampson et al., 2016). Furthermore, microbial metabolites, such as secondary bile acids and tryptophan derivatives have been linked with cognitive decline in AD (MahmoudianDehkordi et al., 2018) and CNS inflammation (Rothhammer et al., 2016).
9. Traumatic Brain Injury
Traumatic brain injury (TBI) is one of the leading causes of disability and neurological dysfunction among children, athletes, and veterans (Ropper, 2011). With the growing number of TBI-related hospitalizations and deaths, TBI remains an underappreciated public health concern (Wilson, 2016) while incurring huge economical and healthcare burden (Coronado et al., 2012).
There has been growing evidence on the gender and sex differences in TBI. TBI-related emergency department visits, hospitalizations and deaths are more common in males (Faul and Coronado, 2015). The sex-biased TBI epidemiology is evident in the pediatric population. In a retrospective review of the National Trauma Data Bank, pubescent patients with TBI were more likely to be males in comparison to the prepubescent cohorts suggesting the involvement of hormonal differences in TBI morbidity (Ley et al., 2013). Females are becoming increasingly engaged in the military and athletics, yet this dichotomy still show the disproportionate representation of male research subjects in both pre-clinical and clinical trials. Although women are more susceptible to the development of post-traumatic mental health disorders (Lavoie et al., 2017) with a distinct orbitofrontal functional connectivity (McGlade et al., 2015), they tend to better tolerate the physiologic consequences of traumas (Deitch et al., 2007). The conflicting data described below on the role of sex differences and temporal hormonal changes in TBI undermines the long-held belief on the impact of sex on the progression and outcomes post-TBI.
Growing research endeavors aim to uncover the role of sex differences during the pathogenesis of TBI. Inflammatory processes occurring throughout the secondary phase of brain injury involve microglial activation (Ramlackhansingh et al., 2011), destruction of cytoskeletal and cell junction proteins (Abou-El-Hassan et al., 2017), and subsequent BBB breakdown (Nokkari et al., 2018). Analyzing F2-isoprostane, glutamate and lactate revealed greater excitotoxic and ischemic events in severe TBI male rats compared to females (Wagner et al., 2004). In the same scope, completely divergent sex response exists up to 7 days post-injury where a more robust astrogliosis, microglia/macrophage phenotype and a persistent elevation of inflammatory cytokines was found in male mice compared to females (Villapol et al., 2017). Cerebral autoregulation was more disrupted in males in a TBI animal model (Armstead et al., 2010) and also in children (Tontisirin et al., 2007). Male rats had increased COX-2, neuronal cell death and subsequent inflammation in a penetrating form of TBI but with no difference in microglial activation (Gunther et al., 2015).
Several studies have investigated the involvement of hormones in sex-biased responses to TBI. In a preclinical closed-head injury model of TBI with and without 17beta-estradiol replacement, acute survival and cerebral perfusion were better in female rats (Roof and Hall, 2000) which was attributed to the neuroprotective role of 17beta-estradiol (Amantea et al., 2005; Wang et al., 2006). Indeed, 17beta-estradiol, but not 17alpha-estradiol or progesterone, demonstrated anti-inflammatory effects through a significant reduction in the release of microglial superoxide as well as microglial phagocytic activity in primary rat microglia and N9 microglial cell lines (Bruce-Keller et al., 2000). Exogenous treatment of either estrogen or progesterone reduced BBB permeability and edema formation in TBI (Mannix et al., 2014; O'Connor et al., 2005). On the other hand, orchidectomized male rats treated with testosterone, estradiol, or dihydrotestosterone had significant reduction in immunoreactive hippocampus microglia (Barreto et al., 2007) whereas ovariectomized young and aged female rats treated with raloxifene and tamoxifen, selective estrogen receptor modulators, reduced microglial activation (Barreto et al., 2014). Moreover, gonadally intact male mice had more Iba-1-positive microglial cells as well as neuronal densities in the traumatized brain parenchyma (Acaz-Fonseca et al., 2015). On the other hand, Bruce-Keller et al. later showed that biologic sex or estrogen manipulation does not alter cortical and hippocampal neuroinflammation (Bruce-Keller et al., 2007). In this study, levels of IL-6 and CCL-2 were indeed higher in normal male and female mice compared to ovariectomized mice or those treated with estrogen. Larger serial cross-sectional traumatized brain areas were seen in ovariectomized rats compared to intact females suggesting histopathological protection of endogenous female hormones in TBI (Bramlett and Dietrich, 2001). At the functional level, however, no sex difference was observed in motor and cognitive abilities after TBI (Tucker et al., 2016).
At the clinical level, sex- and gender-based studies investigating outcomes post-TBI offered inconsistent findings. A landmark study extensively analyzed adjusted severe TBI-related data of 72,294 patients from the National Trauma Database over a 5-year period showed remarkably less mortality and trauma complications in females (Berry et al., 2009). Males are more likely to sustain severe head traumas and Berry et al. demonstrated that even in severe TBI, normalized data showed that females have more favorable outcomes. Coimbra et al. analyzed various severities of TBI and reported the indifferent role of gender on posttraumatic mortality (Coimbra et al., 2003). Also, further studies showed that outcomes were worse in women than men (Bazarian et al., 2010; Farace and Alves, 2000). Given the reduced levels of estrogen after menopause, a significantly lower mortality risk was noted in peri- and post-menopausal women providing contradictory evidence that estrogen confers neuroprotection (Berry et al., 2009). In moderate-to-severe TBI, significantly better outcomes were observed in postmenopausal females further undermining the neuroprotective role of estrogen following TBI (Davis et al., 2006). Yet in all pediatric trauma cases including TBI, postpubescent sex-hormone producing female patients had improved survival rates in a manner consistent with the injury severity supporting the beneficial effects of female sex hormones (Phelan et al., 2007). Consistently, progesterone was found to decrease brain edema within 6 hours of the injury in both in vivo and clinical studies (Roof et al., 1993; Roof et al., 1996). The previous evidence that female sex hormones have neuroprotective and neurodegenerative roles due to their anti-oxidant capacity attenuating glutamate excitotoxicity and in enhancing synaptogenesis and dendritic arborization does not successfully and consistently translate to the conducted clinical big-data analyses (Stein, 2001). One of the reasons of failed clinical trials in humans (Lin et al., 2015) compared to animal models is the more complex and heterogenous nature of TBI in humans (Wright et al., 2015).
Increasing evidence has been accumulating on the involvement of the gut in TBI. Changes in white matter architecture have been linked to diet-dependent changes in gut microbiome populations (Ong et al., 2018). Cerebral lesions due to severe brain traumas cause cognitive and personality changes (Ropper and Gorson, 2007) where the microbiota recently proved to regulate behavior via the glucocorticoid receptor in the hippocampus (Luo et al., 2018). Dysautonomia following TBI is not uncommon projecting worsened outcomes (Lv et al., 2011) and is explained by a disruption of the brain-gut axis (Baguley et al., 2007). Intestinal microbiota (Sundman et al., 2017), contractility (Olsen et al., 2013) and permeability (Jin et al., 2008) is altered after TBI. At the intestinal level, ileal samples demonstrated reduced expression of expression of tight junction proteins explaining intestinal dysfunction and increased gut permeability in TBI mice (Bansal et al., 2009). Histopathological alterations of gut mucosa following TBI include epithelial shedding, villous and mucosal atrophy, focal ulcerations, vascular dilation and edema, loss of tight junctions, mitochondria damage as well as increased serum endotoxin level (Hang et al., 2003). Cellular and molecular alterations in the ileum include upregulation of NFκB (Ling et al., 2013), proinflammatory cytokines (Hang et al., 2005a), adhesion molecules (Hang et al., 2005b), T cell activation (Ma et al., 2017) and Treg suppression (Lv et al., 2011) which were reversed upon inhibition of myeloid differentiation factor 88 (Myd88) (Zhang et al., 2016). Myd88 is essential in regulating intracellular inflammatory responses induced by TLR agonists and IL-1R families (Medzhitov et al., 1998). The bidirectional neuronal, hormonal, and immunologic gut-brain crosstalk is therefore evident, and this axis underlies the pathologic changes observed in both of the brain, primarily, and secondarily in the gut (Collins et al., 2012).
Looking at traumatic injuries in general, no change in gut microbial composition was detected shortly after injury whereas enrichment of Clostridiales and Enterococcus paralleled by depletion of Bacteroidales, Fusobacteriales and Verrucomicrobiales occurred within 72 hours (Howard et al., 2017). A landmark study by Houlden et al. investigated the consequences of traumatic and ischemic brain injury on gut microbiota whereby shifts in bacterial populations correlated with injury severity (Houlden et al., 2016). In regard to bacterial composition, the Peptococcaceae and Prevotellaceae bacterial families were increased and decreased, respectively, in the ischemic model of brain injury, whereas three bacterial families were altered in TBI: Porphyromonadaceae, Firmicutes and α-Proteobacteria (Houlden et al., 2016). Secondary alteration of brain function and the stress response to tissue injury are hypothesized to be the cause of gut microbial alterations. Temporal microbiome characterization revealed key changes in phylogenetic families (decreased beneficial bacteria in the families Lachnospiraceae, Mogibacteriaceae, and Ruminococcaceae within the phylum Firmicutes, increased potentially pathogenic bacteria including Bacteroidaceae in the phylum Bacteroidetes, and Enterobacteriaceae and Pseudomonadaceae in the phylum Proteobacteria) as well as a significant decrease in α-bacterial diversity after TBI (Nicholson et al., 2018). Similarly, evaluating species-level differential abundances via clustered and annotated operational taxonomic units revealed a decrease in three species (Lactobacillus gasseri, Ruminococcus flavefaciens, Eubacterium ventriosum) and an increase in two species (Eubacterium sulci, Marvinbryantia formatexigens) (Treangen et al., 2018). Since microbial dysbiosis profiles were identified at different time points and correlated with MRI-determined lesion volume, the gut commensals may be used as a novel biomarker to classify injury severity and determine prognosis (Nicholson et al., 2018). Whether the observed dysbiosis due to brain injury contributes to the pathogenesis of TBI has yet to be investigated.
Recently, treating TBI mice with Clostridium butyricum substantially ameliorated neurological dysfunction, increased expression of tight junction proteins, and reduced brain edema and neuronal cell death (Li et al., 2018). The same study also showed that the secretion of glucagon-like peptide-1 (GLP-1), a mediator of the gut-brain axis, was increased along with an increased expression of its cerebral receptor (Li et al., 2018). Clostridium butyricum is thought to be neuroprotective due to its ability to secrete butyrate in large amounts (Liu et al., 2015). Transcending this finding to clinical trials, Brenner et al. systematically reviewed prebiotic and probiotic interventions following TBI (Brenner et al., 2017). In three severe TBI clinical studies, patients receiving a pre-biotic-containing immune enhancing nutritional formula had lower rates of bacteremia but longer intensive-care unit stay (Painter et al., 2015), patients randomized to receive glutamine and fermented milk with the probiotic strain Lactobacillus johnsonii had less sepsis rates and less time spent in critical care (Falcao de Arruda and de Aguilar-Nascimento, 2004) and finally, patients randomized to receive sachets containing Bifidobacterium longum, Lactobacillus bulgaricus, Streptococcus thermophilus had less nosocomial infections, intensive-care stay and altered Th1/Th2 cytokine profiles (Tan et al., 2011).
10. Parkinson’s Disease
Parkinson’s disease (PD) is the second most common cause of neurodegeneration of the central nervous system and the most common movement disorder. The main symptoms consist of tremor, rigidity, and bradykinesia, and dementia. The pathology is characterized by dopaminergic neurons loss in the substantia nigra and α-synuclein deposition (Tysnes and Storstein, 2017). PD is 1.5 times more frequent in males than in females (Moisan et al., 2016) and both exhibit a different clinical progression and treatment response (Dahodwala et al., 2016). This may be explained by the fact that striatal dopamine receptor 2 density and binding potential decline twice as fast in aging males (Pohjalainen et al., 1998) and that females have more dopaminergic neurons than males, as well as a greater striatal uptake (Mariani et al., 2018). In males, the gene SRY present on the chromosome Y in males can also exert specific actions on dopaminergic neurons (Pinares-Garcia et al., 2018a).
Another hypothesis is that chromosomal differences in men and women may mitigate risk in PD. Women have two mosaic-activated X-chromosomes, instead of 1 X chromosome in men, which contain several PD-susceptibility genes (Pankratz et al., 2002), including genes encoding androgen receptors, proteins involved in mitochondrial function, apoptosis, and response to hypoxia (Rocca et al., 2014). Mitochondrial function plays a key role in cellular pathways crucial for neurons survival, including cellular energy production and regulation of apoptosis, calcium homeostasis, oxidative stress (Franco-Iborra et al., 2018), and abnormalities in the electron transport chain have been found in PD patients (Mizuno et al., 1989; Parker et al., 1989). The release of mitochondrial damage-associated molecular patterns (DAMPs) can also contribute to neurodegeneration, as they can initiate proinflammatory immune responses from glial cells (Bajwa et al., 2019).
Sex steroids may also account for the lower prevalence of PD in women as estrogens exhibit a neuroprotective effect in both clinical studies and experimental animal models (Inestrosa et al., 1998). Indeed, after an early menopause (before 45 years) or bilateral oophorectomy, women have an increased risk to develop dementia (Rocca et al., 2014), suggesting that the timing of estrogen presence effect may be important (Rocca et al., 2011). Estrogens act by signaling via the mitogen-activated kinase (MAPK) pathway to protect against glutamate neurotoxicity (Singer et al., 1999), through elevation of B-cell lymphoma 2 (Bcl2) expression to reduce oxidative stress (Singer et al., 1998), and through synergy with glutathione to scavenge free radicals (Green et al., 1998; Wooten et al., 2004). Critically, estrogens seem to be able to mediate a protection against oxidative stress only for healthy cells (Tenkorang et al., 2018). Estrogens also effect dopamine transporters (Lee et al., 2015) and the levels of dopamine synthesis and function (Mariani et al., 2018), which could explain in part their neuroprotective effect for PD. Estrogens also protect against neuronal death through microglia activation toward M2 cytoprotective phenotype in the substantia nigra.
Despite some genetic risk factors (like mutations in α-synuclein or PTEN-induced kinase 1, PINK-1), the cause of PD is not fully understood. Indeed, genetic factors can be identified in less than 10% of the patients (Tysnes and Storstein, 2017). On the other hand, several environmental factors can be associated with an increased risk of PD (Breckenridge et al., 2016). For example the use of pesticides, which can drive neuroinflammation and may lead to neurodegeneration (Kanthasamy et al., 2019). There, some gender-bias could also occur because of lifestyle and occupational differences men and women, Wooten GF 2004. Because more men work in agriculture, they could have an increased exposure to pesticides (Moisan et al., 2016).
Our mucosae are one the first layer between us and the outside, and it has been shown that α-synuclein aggregates can be present in the enteric and olfactory nervous systems years and even decades before spreading via the vagal nerve to the substantia nigra, where it will lead to dopaminergic neuron death (Braak’s staging theory) (Braak et al., 2004; Perez-Pardo et al., 2017; Poirier et al., 2016). Environmental factors can enter via the olfactory or digestive tracts are hypothesized to drive to PD pathogenesis (Chen and Ritz, 2018). In support of this, systemic (Chiang et al., 2017) and gastrointestinal (Pal et al., 2015) inflammation have been observed in PD patients and correlated with the severity of the disease. Several studies reveal that prevalence of constipation was substantially higher in PD cases than in controls (Chen and Ritz, 2018; Poirier et al., 2016). A low frequency of bowel movement could predict a higher risk of developing PD in men (Abbott et al., 2001), suggesting that bowel problems can occur before the motor symptoms.. In addition, 20% of PD patients also experience other GI symptoms, including vomiting, nausea, and 80% have and hyper-salivation (Poirier et al., 2016).
Gut microbiota alterations have been detected in PD. An early study by Scheperjans et al., found markedly decreased Prevotella and increased Enterobacteriaceae, which was positively correlated with the severity of postural instability and gait difficulty, underlining the direct importance of the microbiome on PD symptoms (Scheperjans et al., 2015). Multiple additional studies have detected decreased butyrate-producing bacteria, for example from the genera Blautia, Coprococcus, and Roseburia, and Faecalibacterium and more proinflammatory Proteobacteria of the genus Ralstonia (Hill-Burns et al., 2017; Keshavarzian et al., 2015; Petrov et al., 2017). A reduction of fecal SCFA concentrations, which can stimulate GI motility, has been observed in PD patients compared to controls, which might explain some alterations of the ENS and contribute to gastrointestinal dysmotility in PD (Unger et al., 2016). This dysbiosis is hypothesized to stimulate the innate immune system and trigger inflammation-induced misfolding of α-Synuclein and thus PD pathology (Caputi and Giron, 2018; Keshavarzian et al., 2015; Petrov et al., 2017). Several studies have also found an increase in Bifidobacterium and Lactobacillus in PD patients (Gerhardt and Mohajeri, 2018; Hill-Burns et al., 2017), which would be expected to play a beneficial role. Whether this reflects a physiology shift in the microbiota to compensate with the disease or is a reflection of fiber or probiotic supplementation in the PD population is currently unknown.
Sampson et al. 2016 investigated the causal role of the microbiome on the pathogenesis in an animal model of PD (Sampson et al., 2016). Germ-free and antibiotic-treated α-synuclein overexpressing (ASO) mice) had reduced motor deficits, which they mechanistically link to microglia activation. Recolonizing mice with microbiota restored motor deficits. Strikingly, motor deficits could be induced by administering the microbial metabolites acetate, butyrate, and propionate to germ-free mice, but little effect was seen with heat killed bacteria. This suggests that the microbiota primarily contribute to PD via secretion of neuromodulatory metabolites, not antigenic stimulation in the gut. Furthermore, colonizing ASO mice with microbiota from PD patients led to worse motor dysfunction compared to colonizing with healthy control microbiota, which provides evidence that PD patients harbor a gut microbiota that contributes to disease. In an independent study, healthy fecal microbiota transplantation to a mouse model of PD reduces gut dysbiosis, alleviates physical impairment, increases dopamine pathways in the striatum and reduces microglial activation (Sun and Shen, 2018).
While there is evidence of interactions, between microbiome and Parkinson’s disease, and between PD and sex, but there are no studies that have yet investigated the sex-specific role of the microbiota in PD. However, if these interactions are important, then disease modifying therapies for the microbiota and PD that focus on the dopaminergic system, as well as anti-inflammation, antioxidants production and prevention of motor impairment (Parashar and Udayabanu, 2017) may need sex-specific approaches. In addition, the gut microbiota can play a role in therapeutic response in PD. The gut bacteria can restrict the bioavailability of levodopa, which is the primary treatment of PD, (van Kessel et al., 2019). Microbial tyrosine decarboxylases can convert L-dopa, which crosses the blood brain barrier, to dopamine, which cannot cross the blood brain barrier. An independent study has also linked the microbiota with treatment response and found Dorea and Phascolarctobacterium negatively associated with levodopa doses (Qian et al., 2018). Thus, the change in the microbiome may account for some of the variability in dosage requirements in L-dopa.
11. Summary.
There are many diverse mechanisms by which the microbiome can influence the brain, which involve metabolic, immunologic, and neurologic signaling pathways (Cox and Weiner, 2018). In addition, differences in hormone levels and immunologic responses can select for sex-specific microbiota that has been shown to mediate chronic diseases including Type 1 diabetes (Markle et al., 2013). Furthermore, microbiota from multiple sclerosis, Parkinson’s disease, and autism patients affects animal models of neurologic disease when transferred to mice, providing evidence that it can play a causal role (Berer et al., 2017; Cekanaviciute et al., 2017; Sampson et al., 2016; Sharon et al., 2018). Increase in estradiol in animal models or physiologic increases during pregnancy can modulate the microbiota and ameliorate symptoms of EAE or MS (Benedek et al., 2017; Harbo et al., 2013). However, whether this is directly mediated by the microbiota is currently unknown. Much more work is needed to establish a causal role of the microbiome in sex-specific differences in neurologic disease and to identify and the mechanisms by which they act. These will likely involve changes in hormone and immunologic signaling and have the potential to identify novel sex- and disease-specific microbiota therapeutic approaches for neurologic disease.
Highlights.
The gut microbiota can influence the brain and plays a role in neurologic disease
There is a bidirectional interaction between androgens and the microbiota
Sex-specific microbiota interactions can influence behavior and neurologic disease
Acknowledgments
Funding
This work was supported by the National Institutes of Health [Grant #:1R01NS087226]; the Brigham Health Program for Interdisciplinary Neuroscience Women’s Brain Initiative; Women’s Alzheimer’s Movement; the National MS Society and American Academy of Neurology [Grant #: FAN-1707-28798].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aagaard K, et al. , 2014. The placenta harbors a unique microbiome. Science translational medicine. 6, 237ra65–237ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott RD, et al. , 2001. Frequency of bowel movements and the future risk of Parkinson's disease. Neurology. 57, 456–462. [DOI] [PubMed] [Google Scholar]
- Abou-El-Hassan H, et al. , 2017. Degradomics in Neurotrauma: Profiling Traumatic Brain Injury. Methods Mol Biol. 1598, 65–99. [DOI] [PubMed] [Google Scholar]
- Acaz-Fonseca E, et al. , 2015. Sex differences in glia reactivity after cortical brain injury. Glia. 63, 1966–1981. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H, et al. , 1976. Intestinal metabolism of estrogens. The Journal of Clinical Endocrinology & Metabolism. 43, 497–505. [DOI] [PubMed] [Google Scholar]
- Airas L, Etemadifar M, Maghzi A-H, 2011. 1 - Pregnancy and Multiple Sclerosis In: Neurological Disorders and Pregnancy. Vol., Minagar A, ed.^ds. Elsevier, London, pp. 1–11. [Google Scholar]
- Altmann A, et al. , 2014. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol. 75, 563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amantea D, et al. , 2005. From clinical evidence to molecular mechanisms underlying neuroprotection afforded by estrogens. Pharmacol Res. 52, 119–32. [DOI] [PubMed] [Google Scholar]
- Armstead WM, et al. , 2010. Impaired cerebral blood flow autoregulation during posttraumatic arterial hypotension after fluid percussion brain injury is prevented by phenylephrine in female but exacerbated in male piglets by extracellular signal-related kinase mitogen-activated protein kinase upregulation. Crit Care Med. 38, 1868–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, et al. , 2011. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 331, 337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back D, et al. , 1978. The effect of antibiotics on the enterohepatic circulation of ethinylestradiol and norethisterone in the rat. Journal of steroid biochemistry. 9, 527–531. [DOI] [PubMed] [Google Scholar]
- Baguley IJ, et al. , 2007. The incidence of dysautonomia and its relationship with autonomic arousal following traumatic brain injury. Brain Inj. 21, 1175–81. [DOI] [PubMed] [Google Scholar]
- Bajwa E, Pointer CB, Klegeris A, 2019. The Role of Mitochondrial Damage-Associated Molecular Patterns in Chronic Neuroinflammation. Mediators of Inflammation. 2019, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal V, et al. , 2009. Traumatic brain injury and intestinal dysfunction: uncovering the neuro-enteric axis. J Neurotrauma. 26, 1353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto G, et al. , 2007. Testosterone decreases reactive astroglia and reactive microglia after brain injury in male rats: role of its metabolites, oestradiol and dihydrotestosterone. Eur J Neurosci. 25, 3039–46. [DOI] [PubMed] [Google Scholar]
- Barreto GE, Santos-Galindo M, Garcia-Segura LM, 2014. Selective estrogen receptor modulators regulate reactive microglia after penetrating brain injury. Front Aging Neurosci. 6, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerl C, et al. , 2018. Shifts in gut microbiota composition in an APP/PSS1 transgenic mouse model of Alzheimer's disease during lifespan. Lett Appl Microbiol. 66, 464–471. [DOI] [PubMed] [Google Scholar]
- Bazarian JJ, et al. , 2010. Sex differences in outcome after mild traumatic brain injury. J Neurotrauma. 27, 527–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkering P, et al. , 2013. The intricate association between gut microbiota and development of type 1, type 2 and type 3 diabetes. Expert Rev Clin Immunol. 9, 1031–41. [DOI] [PubMed] [Google Scholar]
- Benedek G, et al. , 2017. Estrogen protection against EAE modulates the microbiota and mucosal-associated regulatory cells. Journal of Neuroimmunology. 310, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P, et al. , 2011. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 141, 599–609, 609 e1-3. [DOI] [PubMed] [Google Scholar]
- Berer K, et al. , 2011. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 479, 538–41. [DOI] [PubMed] [Google Scholar]
- Berer K, et al. , 2017. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S A. 114, 10719–10724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C, et al. , 2009. The effect of gender on patients with moderate to severe head injuries. J Trauma. 67, 950–3. [DOI] [PubMed] [Google Scholar]
- Bokkenheuser V, 1993. The friendly anaerobes. Clinical Infectious Diseases. 16. [DOI] [PubMed] [Google Scholar]
- Bonfili L, et al. , 2017. Microbiota modulation counteracts Alzheimer's disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci Rep. 7, 2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove R, Chitnis T, 2014. The role of gender and sex hormones in determining the onset and outcome of multiple sclerosis. Multiple Sclerosis Journal. 20, 520–526. [DOI] [PubMed] [Google Scholar]
- Braak H, et al. , 2004. Stages in the development of Parkinson’s disease-related pathology. Cell and Tissue Research. 318, 121–134. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD, 2001. Neuropathological protection after traumatic brain injury in intact female rats versus males or ovariectomized females. J Neurotrauma. 18, 891–900. [DOI] [PubMed] [Google Scholar]
- Brandscheid C, et al. , 2017. Altered Gut Microbiome Composition and Tryptic Activity of the 5xFAD Alzheimer's Mouse Model. J Alzheimers Dis. 56, 775–788. [DOI] [PubMed] [Google Scholar]
- Bravo JA, et al. , 2011. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences. 108, 16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge CB, et al. , 2016. Association between Parkinson’s Disease and Cigarette Smoking, Rural Living, Well-Water Consumption, Farming and Pesticide Use: Systematic Review and Meta-Analysis. PLoS ONE. 11, e0151841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner LA, et al. , 2017. Growing literature but limited evidence: A systematic review regarding prebiotic and probiotic interventions for those with traumatic brain injury and/or posttraumatic stress disorder. Brain Behav Immun. 65, 57–67. [DOI] [PubMed] [Google Scholar]
- Brown N, Desai S, 2013. Infantile Botulism: A Case Report and Review. Journal of Emergency Medicine. 45, 842–845. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, et al. , 2000. Antiinflammatory effects of estrogen on microglial activation. Endocrinology. 141, 3646–56. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, et al. , 2007. Gender and estrogen manipulation do not affect traumatic brain injury in mice. J Neurotrauma. 24, 203–15. [DOI] [PubMed] [Google Scholar]
- Buckley RF, et al. , 2019. Sex Differences in the Association of Global Amyloid and Regional Tau Deposition Measured By Positron Emission Tomography in Clinically Normal Older Adults. JAMA Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Weiner HL, 2018. Microglial signatures and their role in health and disease. Nat Rev Neurosci. 19, 622–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarel BL, et al. , 2015. Gut microbiota in multiple sclerosis: possible influence of immunomodulators. J Investig Med. 63, 729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputi V, Giron MC, 2018. Microbiome-Gut-Brain Axis and Toll-Like Receptors in Parkinson's Disease. International journal of molecular sciences. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo A, et al. , 2017. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 49, 60–68. [DOI] [PubMed] [Google Scholar]
- Cekanaviciute E, et al. , 2017. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci U S A. 114, 10713–10718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Ritz B, 2018. The Search for Environmental Causes of Parkinson's Disease: Moving Forward. Journal of Parkinson's disease. 8, S9–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, et al. , 2016. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Scientific Reports. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KL, Madak-Erdogan Z, 2016. Estrogen and Microbiota Crosstalk: Should We Pay Attention? Trends in Endocrinology & Metabolism. 27, 752–755. [DOI] [PubMed] [Google Scholar]
- Chiang P-L, et al. , 2017. White matter damage and systemic inflammation in Parkinson's disease. BMC neuroscience. 18, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cignarella F, et al. , 2018. Intermittent Fasting Confers Protection in CNS Autoimmunity by Altering the Gut Microbiota. Cell Metabolism. 27, 1222–1235.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson MJ, et al. , 2012. Gut microbiota composition correlates with diet and health in the elderly. Nature. 488, 178–84. [DOI] [PubMed] [Google Scholar]
- Clarke G, et al. , 2013. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 18, 666–73. [DOI] [PubMed] [Google Scholar]
- Clavel T, et al. , 2005. Intestinal bacterial communities that produce active estrogen-like compounds enterodiol and enterolactone in humans. Appl Environ Microbiol. 71, 6077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coimbra R, et al. , 2003. Does sexual dimorphism influence outcome of traumatic brain injury patients? The answer is no! J Trauma. 54, 689–700. [DOI] [PubMed] [Google Scholar]
- Collins SM, Surette M, Bercik P, 2012. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 10, 735–42. [DOI] [PubMed] [Google Scholar]
- Confavreux C, et al. , 1998. Rate of pregnancy-related relapse in multiple sclerosis. New England Journal of Medicine. 339, 285–291. [DOI] [PubMed] [Google Scholar]
- Conlon MA, Bird AR, 2014. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 7, 17–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium, T.H.M.P., 2012a. Structure, function and diversity of the healthy human microbiome. Nature. 486, 207 – 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium, T.H.M.P., 2012b. A framework for human microbiome research. Nature. 486, 215 – 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado VG, et al. , 2012. Trends in Traumatic Brain Injury in the U.S. and the public health response: 1995-2009. J Safety Res. 43, 299–307. [DOI] [PubMed] [Google Scholar]
- Cox LM, Blaser MJ, 2013. Pathways in microbe-induced obesity. Cell Metab. 17, 883–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LM, Weiner HL, 2018. Microbiota Signaling Pathways that Influence Neurologic Disease. Neurotherapeutics. 15, 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox-York KA, et al. , 2015. Ovariectomy results in differential shifts in gut microbiota in low versus high aerobic capacity rats. Physiol Rep. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG, 2012. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 13, 701–12. [DOI] [PubMed] [Google Scholar]
- Dahodwala N, Pei Q, Schmidt P, 2016. Sex Differences in the Clinical Progression of Parkinson's Disease. Journal of obstetric, gynecologic, and neonatal nursing : JOGNN. 45, 749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DP, et al. , 2006. Traumatic brain injury outcomes in pre- and post- menopausal females versus agematched males. J Neurotrauma. 23, 140–8. [DOI] [PubMed] [Google Scholar]
- Deitch EA, et al. , 2007. Hormonally active women tolerate shock-trauma better than do men: a prospective study of over 4000 trauma patients. Ann Surg. 246, 447–53; discussion 453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L, et al. , 2012. Mechanisms underlying insulin deficiency-induced acceleration of beta-amyloidosis in a mouse model of Alzheimer's disease. PLoS One. 7, e32792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello M, et al. , 2010. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 107, 11971 – 11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erny D, et al. , 2015. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 18, 965–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcao de Arruda IS, de Aguilar-Nascimento JE, 2004. Benefits of early enteral nutrition with glutamine and probiotics in brain injury patients. Clin Sci (Lond). 106, 287–92. [DOI] [PubMed] [Google Scholar]
- Farace E, Alves WM, 2000. Do women fare worse: a metaanalysis of gender differences in traumatic brain injury outcome. J Neurosurg. 93, 539–45. [DOI] [PubMed] [Google Scholar]
- Faul M, Coronado V, 2015. Epidemiology of traumatic brain injury. Handb Clin Neurol. 127, 3–13. [DOI] [PubMed] [Google Scholar]
- Foster JA, McVey Neufeld KA, 2013. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 36, 305–12. [DOI] [PubMed] [Google Scholar]
- Franco-Iborra S, Vila M, Perier C, 2018. Mitochondrial Quality Control in Neurodegenerative Diseases: Focus on Parkinson's Disease and Huntington's Disease. Frontiers in Neuroscience. 12, 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen F, et al. , 2017. The impact of gut microbiota on gender-specific differences in immunity. Frontiers in immunology. 8, 754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga Y, Sugawara Y, Matsumura T, 2013. Uptake of Botulinum Neurotoxin in the Intestine In: Botulinum Neurotoxins. Vol., Rummel A, Binz T, ed.^ds. Springer Berlin Heidelberg, Berlin, Heidelberg, pp. 45–59. [DOI] [PubMed] [Google Scholar]
- Fung TC, Olson CA, Hsiao EY, 2017. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 20, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaboriau-Routhiau V, et al. , 2009. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 31, 677–89. [DOI] [PubMed] [Google Scholar]
- Gerhardt S, Mohajeri MH, 2018. Changes of Colonic Bacterial Composition in Parkinson's Disease and Other Neurodegenerative Diseases. Nutrients. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PS, Gridley KE, Simpkins JW, 1998. Nuclear estrogen receptor-independent neuroprotection by estratrienes: a novel interaction with glutathione. Neuroscience. 84, 7–10. [DOI] [PubMed] [Google Scholar]
- Groh H, Schade K, Hörhold-Schubert C, 1993. Steroid metabolism with intestinal microorganisms. Journal of basic microbiology. 33, 59–72. [DOI] [PubMed] [Google Scholar]
- Gunther M, et al. , 2015. COX-2 regulation and TUNEL-positive cell death differ between genders in the secondary inflammatory response following experimental penetrating focal brain injury in rats. Acta Neurochir (Wien). 157, 649–59. [DOI] [PubMed] [Google Scholar]
- Hang CH, et al. , 2003. Alterations of intestinal mucosa structure and barrier function following traumatic brain injury in rats. World J Gastroenterol. 9, 2776–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang CH, et al. , 2005a. Expressions of intestinal NF-kappaB, TNF-alpha, and IL-6 following traumatic brain injury in rats. J Surg Res. 123, 188–93. [DOI] [PubMed] [Google Scholar]
- Hang CH, et al. , 2005b. Up-regulation of intestinal nuclear factor kappa B and intercellular adhesion molecule-1 following traumatic brain injury in rats. World J Gastroenterol. 11, 1149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harach T, et al. , 2015. Reduction of Alzheimer's disease beta-amyloid pathology in the absence of gut microbiota. arXiv preprint arXiv:1509.02273. [Google Scholar]
- Harbo HF, Gold R, Tintoré M, 2013. Sex and gender issues in multiple sclerosis. Ther Adv Neurol Disord. 6, 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, et al. , 2013. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 80, 1778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Golenbock DT, Latz E, 2015. Innate immunity in Alzheimer's disease. Nat Immunol. 16, 229–36. [DOI] [PubMed] [Google Scholar]
- Hill-Burns EM, et al. , 2017. Parkinson's disease and Parkinson's disease medications have distinct signatures of the gut microbiome. Movement Disorders. 32, 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Dissing-Olesen L, Stevens B, 2016. New insights on the role of microglia in synaptic pruning in health and disease. Curr Opin Neurobiol. 36, 128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ, 2012. Interactions between the microbiota and the immune system. Science. 336, 1268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlden A, et al. , 2016. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav Immun. 57, 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard BM, et al. , 2017. Characterizing the gut microbiome in trauma: significant changes in microbial diversity occur early after severe injury. Trauma Surg Acute Care Open. 2, e000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, et al. , 2013. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 155, 1451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inestrosa NC, Marzolo M-P, Bonnefont AB, 1998. Cellular and molecular basis of estrogen's neuroprotection. Molecular Neurobiology. 17, 73–86. [DOI] [PubMed] [Google Scholar]
- Ivanov II, et al. , 2009. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 139, 485–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangi S, et al. , 2016. Alterations of the human gut microbiome in multiple sclerosis. Nature Communications. 7, 12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, et al. , 2017. The gut microbiota and Alzheimer’s disease. Journal of Alzheimer's Disease. 58, 1–15. [DOI] [PubMed] [Google Scholar]
- Jin W, et al. , 2008. Increased intestinal inflammatory response and gut barrier dysfunction in Nrf2-deficient mice after traumatic brain injury. Cytokine. 44, 135–40. [DOI] [PubMed] [Google Scholar]
- Kanthasamy A, et al. , 2019. Environmental neurotoxicant-induced dopaminergic neurodegeneration: a potential link to impaired neuroinflammatory mechanisms. Pharmacology & Therapeutics. 197, 61–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzian A, et al. , 2015. Colonic bacterial composition in Parkinson's disease. Movement Disorders. 30, 1351–1360. [DOI] [PubMed] [Google Scholar]
- Koch-Henriksen N, Sorensen PS, 2010. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 9, 520–32. [DOI] [PubMed] [Google Scholar]
- Koren O, et al. , 2012. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 150, 470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwa M, et al. , 2016. The Intestinal Microbiome and Estrogen Receptor-Positive Female Breast Cancer. J Natl Cancer Inst. 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landete JM, et al. , 2016. Bioactivation of Phytoestrogens: Intestinal Bacteria and Health. Crit Rev Food Sci Nutr. 56, 1826–43. [DOI] [PubMed] [Google Scholar]
- Lavoie S, et al. , 2017. Depression in Men and Women One Year Following Traumatic Brain Injury (TBI): A TBI Model Systems Study. Front Psychol. 8, 634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, et al. , 2015. Gender Differences in Age-Related Striatal Dopamine Depletion in Parkinson's Disease. Journal of movement disorders. 8, 130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, et al. , 2011. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 108 Suppl 1, 4615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelu K, et al. , 2011. Estrogen Receptor Alpha Signaling in T Lymphocytes Is Required for Estradiol-Mediated Inhibition of Th1 and Th17 Cell Differentiation and Protection against Experimental Autoimmune Encephalomyelitis. In: The Journal of Immunology. Vol. 187, ed.^ds., pp. 2386–2393. [DOI] [PubMed] [Google Scholar]
- Lenz KM., McCarthy MM., 2015. A starring role for microglia in brain sex differences. Neuroscientist. 21, 306–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley EJ, et al. , 2013. Gender impacts mortality after traumatic brain injury in teenagers. J Trauma Acute Care Surg. 75, 682–6. [DOI] [PubMed] [Google Scholar]
- Li H, et al. , 2018. Clostridium butyricum exerts a neuroprotective effect in a mouse model of traumatic brain injury via the gut-brain axis. Neurogastroenterol Motil. 30, e13260. [DOI] [PubMed] [Google Scholar]
- Li R, Singh M, 2014. Sex differences in cognitive impairment and Alzheimer’s disease. Frontiers in Neuroendocrinology. 35, 385–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, et al. , 2015. Efficacy of progesterone for moderate to severe traumatic brain injury: a meta-analysis of randomized clinical trials. Sci Rep. 5, 13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling HP, et al. , 2013. Expression of intestinal myeloid differentiation primary response protein 88 (Myd88) following experimental traumatic brain injury in a mouse model. J Surg Res. 179, e227–34. [DOI] [PubMed] [Google Scholar]
- Liu J, et al. , 2015. Neuroprotective Effects of Clostridium butyricum against Vascular Dementia in Mice via Metabolic Butyrate. Biomed Res Int. 2015, 412946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, et al. , 2016. Influence of Endogenous and Exogenous Estrogenic Endocrine on Intestinal Microbiota in Zebrafish. PLoS One. 11, e0163895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke H, Harley V, Lee J, 2015. Biological factors underlying sex differences in neurological disorders. Int J Biochem Cell Biol. 65, 139–50. [DOI] [PubMed] [Google Scholar]
- Luczynski P, et al. , 2016. Growing up in a bubble: using germ-free animals to assess the influence of the gut microbiota on brain and behavior. Int J Neuropsychopharmacol. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwin SK, et al. , 2016. Astrocytes in multiple sclerosis. Mult Scler. 22, 1114–24. [DOI] [PubMed] [Google Scholar]
- Luo Y, et al. , 2018. Gut microbiota regulates mouse behaviors through glucocorticoid receptor pathway genes in the hippocampus. Transl Psychiatry. 8, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv LQ, et al. , 2011. Risk factors related to dysautonomia after severe traumatic brain injury. J Trauma. 71, 538–42. [DOI] [PubMed] [Google Scholar]
- Ma EL, et al. , 2017. Bidirectional brain-gut interactions and chronic pathological changes after traumatic brain injury in mice. Brain Behav Immun. 66, 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghzi AH, et al. , 2010. Increasing female preponderance of multiple sclerosis in Isfahan, Iran: a population-based study. Mult Scler. 16, 359–61. [DOI] [PubMed] [Google Scholar]
- MahmoudianDehkordi S, et al. , 2018. Altered bile acid profile associates with cognitive impairment in Alzheimer's disease—An emerging role for gut microbiome. Alzheimer's & Dementia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangalam A, et al. , 2017. Human Gut-Derived Commensal Bacteria Suppress CNS Inflammatory and Demyelinating Disease. Cell Rep. 20, 1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangold CA, et al. , 2017. Sexually divergent induction of microglial-associated neuroinflammation with hippocampal aging. J Neuroinflammation. 14, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannix R, et al. , 2014. Sex differences in the effect of progesterone after controlled cortical impact in adolescent mice: a preliminary study. J Neurosurg. 121, 1337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani E, et al. , 2018. Sex-Specific Transcriptome Differences in Substantia Nigra Tissue: A Meta-Analysis of Parkinson’s Disease Data. Genes. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markle JG, et al. , 2013. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 339, 1084–8. [DOI] [PubMed] [Google Scholar]
- Marksteiner J, et al. , 2018. Bile acid quantification of 20 plasma metabolites identifies lithocholic acid as a putative biomarker in Alzheimer's disease. Metabolomics. 14, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters CL, et al. , 2015. Alzheimer's disease. Nat Rev Dis Primers. 1, 15056. [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA, 2006. Estrogen receptor and aryl hydrocarbon receptor signaling pathways. Nucl Recept Signal. 4, e016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazure CM, Swendsen J, 2016. Sex differences in Alzheimer’s disease and other dementias. The Lancet. Neurology. 15, 451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlade E, Rogowska J, Yurgelun-Todd D, 2015. Sex differences in orbitofrontal connectivity in male and female veterans with TBI. Brain Imaging Behav. 9, 535–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, et al. , 1998. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 2, 253–8. [DOI] [PubMed] [Google Scholar]
- Meraz-Ríos MA, et al. , 2013. Inflammatory process in Alzheimer's Disease. Frontiers in Integrative Neuroscience. 7, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielcarz DW, Kasper LH, 2015. The Gut Microbiome in Multiple Sclerosis. Curr Treat Options Neurol. 17, 18–10. [DOI] [PubMed] [Google Scholar]
- Mielke MM, Vemuri P, Rocca WA, 2014. Clinical epidemiology of Alzheimer's disease: assessing sex and gender differences. Clin Epidemiol. 6, 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PG, et al. , 2015. TNFR2 Deficiency Acts in Concert with Gut Microbiota To Precipitate Spontaneous Sex-Biased Central Nervous System Demyelinating Autoimmune Disease. J Immunol. 195, 4668–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minter MR, et al. , 2016. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer's disease. Sci Rep. 6, 30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minter MR, et al. , 2017. Antibiotic-induced perturbations in microbial diversity during post-natal development alters amyloid pathology in an aged APPSWE/PS1DeltaE9 murine model of Alzheimer's disease. Sci Rep. 7, 10411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake S, et al. , 2015. Dysbiosis in the Gut Microbiota of Patients with Multiple Sclerosis, with a Striking Depletion of Species Belonging to Clostridia XIVa and IV Clusters. PLoS One. 10, e0137429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y, et al. , 1989. Deficiencies in complex I subunits of the respiratory chain in Parkinson's disease. Biochemical and biophysical research communications. 163, 1450–5. [DOI] [PubMed] [Google Scholar]
- Moisan F, et al. , 2016. Parkinson disease male-to-female ratios increase with age: French nationwide study and meta-analysis. Journal of Neurology, Neurosurgery & Psychiatry. 87, 952–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton MS, et al. , 2002. Phytoestrogen concentrations in serum from Japanese men and women over forty years of age. J Nutr. 132, 3168–71. [DOI] [PubMed] [Google Scholar]
- Nicholson SE, et al. , 2018. Moderate Traumatic Brain Injury Alters the Gastrointestinal Microbiome in a Time-Dependent Manner. Shock. [DOI] [PubMed] [Google Scholar]
- Nokkari A, et al. , 2018. Implication of the Kallikrein-Kinin system in neurological disorders: Quest for potential biomarkers and mechanisms. Prog Neurobiol. 165-167, 26–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuriel-Ohayon M, Neuman H, Koren O, 2016. Microbial Changes during Pregnancy, Birth, and Infancy. Front Microbiol. 7, 1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor CA, Cernak I, Vink R, 2005. Both estrogen and progesterone attenuate edema formation following diffuse traumatic brain injury in rats. Brain Res. 1062, 171–4. [DOI] [PubMed] [Google Scholar]
- O'Hara A, Shanahan F, 2006. The gut flora as a forgotten organ. EMBO Rep. 7, 688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, et al. , 2009. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol. 183, 6041–50. [DOI] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, et al. , 2010a. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal immunology. 3, 487. [DOI] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, et al. , 2010b. Induction of a regulatory B cell population in experimental allergic encephalomyelitis by alteration of the gut commensal microflora. Gut Microbes. 1, 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda Y, Okuda M, Bernard CC, 2002. Gender does not influence the susceptibility of C57BL/6 mice to develop chronic experimental autoimmune encephalomyelitis induced by myelin oligodendrocyte glycoprotein. Immunology letters. 81, 25–29. [DOI] [PubMed] [Google Scholar]
- Olsen AB, et al. , 2013. Effects of traumatic brain injury on intestinal contractility. Neurogastroenterol Motil. 25, 593–e463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong IM, et al. , 2018. Gut microbiome populations are associated with structure-specific changes in white matter architecture. Transl Psychiatry. 8, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orton SM, et al. , 2006. Sex ratio of multiple sclerosis in Canada: a longitudinal study. Lancet Neurol. 5, 932–6. [DOI] [PubMed] [Google Scholar]
- Painter TJ, Rickerds J, Alban RF, 2015. Immune enhancing nutrition in traumatic brain injury - A preliminary study. Int J Surg. 21, 70–4. [DOI] [PubMed] [Google Scholar]
- Pal GD, et al. , 2015. Abnormal lipopolysaccharide binding protein as marker of gastrointestinal inflammation in Parkinson disease. Frontiers in neuroscience. 9, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankratz N, et al. , 2002. Genome screen to identify susceptibility genes for Parkinson disease in a sample without parkin mutations. American journal of human genetics. 71, 124–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papenfuss TL, et al. , 2004. Sex differences in experimental autoimmune encephalomyelitis in multiple murine strains. Journal of neuroimmunology. 150, 59–69. [DOI] [PubMed] [Google Scholar]
- Parashar A, Udayabanu M, 2017. Gut microbiota: Implications in Parkinson's disease. Parkinsonism & Related Disorders. 38, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker WD, Boyson SJ, Parks JK, 1989. Abnormalities of the electron transport chain in idiopathic parkinson's disease. Annals of Neurology. 26, 719–723. [DOI] [PubMed] [Google Scholar]
- Pellock SJ, Redinbo MR, 2017. Glucuronides in the gut: Sugar-driven symbioses between microbe and host. J Biol Chem. 292, 8569–8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttinen-Damdimopoulou PE, et al. , 2009. Dietary sources of lignans and isoflavones modulate responses to estradiol in estrogen reporter mice. Mol Nutr Food Res. 53, 996–1006. [DOI] [PubMed] [Google Scholar]
- Perez-Pardo P, et al. , 2017. The gut-brain axis in Parkinson's disease: Possibilities for food-based therapies. European Journal of Pharmacology. 817, 86–95. [DOI] [PubMed] [Google Scholar]
- Petrov VA, et al. , 2017. Analysis of Gut Microbiota in Patients with Parkinson’s Disease. Bulletin of Experimental Biology and Medicine. 162, 734–737. [DOI] [PubMed] [Google Scholar]
- Phelan HA, et al. , 2007. Use of a pediatric cohort to examine gender and sex hormone influences on outcome after trauma. J Trauma. 63, 1127–31. [DOI] [PubMed] [Google Scholar]
- Pinares-Garcia P, et al. , 2018a. Sex: A Significant Risk Factor for Neurodevelopmental and Neurodegenerative Disorders. Brain Sciences. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinares-Garcia P, et al. , 2018b. Sex: A Significant Risk Factor for Neurodevelopmental and Neurodegenerative Disorders. Brain Sci. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plottel Claudia S., Blaser Martin J., Microbiome and Malignancy. Cell Host & Microbe. 10, 324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohjalainen T, et al. , 1998. Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. The American journal of psychiatry. 155, 768–73. [DOI] [PubMed] [Google Scholar]
- Poirier A-A, et al. , 2016. Gastrointestinal Dysfunctions in Parkinson's Disease: Symptoms and Treatments. Parkinson's disease. 2016, 6762528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, et al. , 2018. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain, Behavior, and Immunity. 70, 194–202. [DOI] [PubMed] [Google Scholar]
- Qin W, et al. , 2006. Calorie restriction attenuates Alzheimer's disease type brain amyloidosis in Squirrel monkeys (Saimiri sciureus). JAD. 10, 417–422. [DOI] [PubMed] [Google Scholar]
- Radler ME, Hale MW, Kent S, 2014. Calorie restriction attenuates lipopolysaccharide (LPS)-induced microglial activation in discrete regions of the hypothalamus and the subfornical organ. Brain Behav Immun. 38, 13–24. [DOI] [PubMed] [Google Scholar]
- Ramlackhansingh AF, et al. , 2011. Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol. 70, 374–83. [DOI] [PubMed] [Google Scholar]
- Regan JC, et al. , 2016. Sex difference in pathology of the ageing gut mediates the greater response of female lifespan to dietary restriction. Elife. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Shuster LT, 2011. Oophorectomy, menopause, estrogen treatment, and cognitive aging: Clinical evidence for a window of opportunity. Brain Research. 1379, 188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, et al. , 2014. Sex and gender differences in the causes of dementia: a narrative review. Maturitas. 79, 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, Stein DG, 1993. Gender influences outcome of brain injury: progesterone plays a protective role. Brain Res. 607, 333–6. [DOI] [PubMed] [Google Scholar]
- Roof RL, et al. , 1996. Progesterone rapidly decreases brain edema: treatment delayed up to 24 hours is still effective. Exp Neurol. 138, 246–51. [DOI] [PubMed] [Google Scholar]
- Roof RL, Hall ED, 2000. Estrogen-related gender difference in survival rate and cortical blood flow after impact-acceleration head injury in rats. J Neurotrauma. 17, 1155–69. [DOI] [PubMed] [Google Scholar]
- Ropper A, 2011. Brain injuries from blasts. N Engl J Med. 364, 2156–7. [DOI] [PubMed] [Google Scholar]
- Ropper AH, Gorson KC, 2007. Clinical practice. Concussion. N Engl J Med. 356, 166–72. [DOI] [PubMed] [Google Scholar]
- Rothhammer V, et al. , 2016. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 22, 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V, et al. , 2017. Dynamic regulation of serum aryl hydrocarbon receptor agonists in MS. Neurol Neuroimmunol Neuroinflamm. 4, e359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson TR, et al. , 2016. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson's Disease. Cell. 167, 1469–1480 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti E, et al. , 2018. Microbiome-metabolome signatures in mice genetically prone to develop dementia, fed a normal or fatty diet. Sci Rep. 8, 4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarlus H, Heneka MT, 2017. Microglia in Alzheimer's disease. J Clin Invest. 127, 3240–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer MJ, et al. , 2015a. Reduction of beta-amyloid and gamma-secretase by calorie restriction in female Tg2576 mice. Neurobiol Aging. 36, 1293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer MJ, et al. , 2015b. Calorie Restriction Suppresses Age-Dependent Hippocampal Transcriptional Signatures. PLoS One. 10, e0133923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, et al. , 2015. Gut microbiota are related to Parkinson's disease and clinical phenotype. Movement Disorders. 30, 350–358. [DOI] [PubMed] [Google Scholar]
- Seekatz AM, Young VB, 2014. Clostridium difficile and the microbiota. J Clin Invest. 124, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellner J, et al. , 2011. The increasing incidence and prevalence of female multiple sclerosis—A critical analysis of potential environmental factors. Autoimmunity Reviews. 10, 495–502. [DOI] [PubMed] [Google Scholar]
- Shen L, Liu L, Ji HF, 2017. Alzheimer's Disease Histological and Behavioral Manifestations in Transgenic Mice Correlate with Specific Gut Microbiome State. J Alzheimers Dis. 56, 385–390. [DOI] [PubMed] [Google Scholar]
- Singer CA, Rogers KL, Dorsa DM, 1998. Modulation of Bcl-2 expression: a potential component of estrogen protection in NT2 neurons. Neuroreport. 9, 2565–8. [DOI] [PubMed] [Google Scholar]
- Singer CA, et al. , 1999. The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 19, 2455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sochocka M, et al. , 2018. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer's Disease-a Critical Review. Mol Neurobiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence RD, et al. , 2011. Neuroprotection mediated through estrogen receptor-alpha in astrocytes. Proc Natl Acad Sci U S A. 108, 8867–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence RD, et al. , 2013. Estrogen Mediates Neuroprotection and Anti-Inflammatory Effects during EAE through ER-alpha Signaling on Astrocytes But Not through ER-beta Signaling on Astrocytes or Neurons. In: Journal of Neuroscience. Vol. 33, ed.^ds., pp. 10924–10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spor A, Koren O, Ley R, 2011. Unravelling the effects of the environment and host genotype on the gut microbiome. Nature reviews Microbiology. 9, 279–290. [DOI] [PubMed] [Google Scholar]
- Stein DG, 2001. Brain damage, sex hormones and recovery: a new role for progesterone and estrogen? Trends Neurosci. 24, 386–91. [DOI] [PubMed] [Google Scholar]
- Stephan AH, Barres BA, Stevens B, 2012. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 35, 369–89. [DOI] [PubMed] [Google Scholar]
- Stilling RM, Dinan TG, Cryan JF, 2014. Microbial genes, brain & behaviour - epigenetic regulation of the gut-brain axis. Genes Brain Behav. 13, 69–86. [DOI] [PubMed] [Google Scholar]
- Sun M-F, Shen Y-Q, 2018. Dysbiosis of gut microbiota and microbial metabolites in Parkinson’s Disease. Ageing Research Reviews. 45, 53–61. [DOI] [PubMed] [Google Scholar]
- Sundman MH, et al. , 2017. The bidirectional gut-brain-microbiota axis as a potential nexus between traumatic brain injury, inflammation, and disease. Brain Behav Immun. 66, 31–44. [DOI] [PubMed] [Google Scholar]
- Sylvia KE, et al. , 2017. Sex-specific modulation of the gut microbiome and behavior in Siberian hamsters. Brain, Behavior, and Immunity. 60, 51–62. [DOI] [PubMed] [Google Scholar]
- Tan M, et al. , 2011. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: a prospective randomized pilot study. Crit Care. 15, R290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenkorang MA, Snyder B, Cunningham RL, 2018. Sex-related differences in oxidative stress and neurodegeneration. Steroids. 133, 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevaranjan N, et al. , 2017. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe. 21, 455–466 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thion MS, et al. , 2018. Microbiome Influences Prenatal and Adult Microglia in a Sex-Specific Manner. Cell. 172, 500–516 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen K, Ouwehand AC, Rautonen N, 2010. Human intestinal microbiota and healthy ageing. Ageing Res Rev. 9, 107–16. [DOI] [PubMed] [Google Scholar]
- Tontisirin N, et al. , 2007. Change in cerebral autoregulation as a function of time in children after severe traumatic brain injury: a case series. Childs Nerv Syst. 23, 1163–9. [DOI] [PubMed] [Google Scholar]
- Town T, 2010. Inflammation, immunity, and Alzheimer's disease. CNS Neurol Disord Drug Targets. 9, 129–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treangen TJ, et al. , 2018. Traumatic Brain Injury in Mice Induces Acute Bacterial Dysbiosis Within the Fecal Microbiome. Front Immunol. 9, 2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremlett H, et al. , 2016a. Gut microbiota composition and relapse risk in pediatric MS: A pilot study. J Neurol Sci. 363, 153–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremlett H, et al. , 2016b. Associations between the gut microbiota and host immune markers in pediatric multiple sclerosis and controls. BMC Neurol. 16, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremlett H, Waubant E, 2017. The multiple sclerosis microbiome? Ann Transl Med. 5, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker LB, Fu AH, McCabe JT, 2016. Performance of Male and Female C57BL/6J Mice on Motor and Cognitive Tasks Commonly Used in Pre-Clinical Traumatic Brain Injury Research. J Neurotrauma. 33, 880–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, et al. , 2013. Obesity in Aging Exacerbates Blood-Brain Barrier Disruption, Neuroinflammation, and Oxidative Stress in the Mouse Hippocampus: Effects on Expression of Genes Involved in Beta-Amyloid Generation and Alzheimer's Disease. J Gerontol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tysnes O-B, Storstein A, 2017. Epidemiology of Parkinson’s disease. Journal of Neural Transmission. 124, 901–905. [DOI] [PubMed] [Google Scholar]
- Unger MM, et al. , 2016. Short chain fatty acids and gut microbiota differ between patients with Parkinson's disease and age-matched controls. Parkinsonism & related disorders. 32, 66–72. [DOI] [PubMed] [Google Scholar]
- van Kessel SP, et al. , 2019. Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson's disease. Nat Commun. 10, 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Look PF, Top-Huisman M, Gnodde HP, 1981. Effect of ampicillin or amoxycillin administration on plasma and urinary estrogen levels during normal pregnancy. Eur J Obstet Gynecol Reprod Biol. 12, 225–33. [DOI] [PubMed] [Google Scholar]
- Villapol S, Loane DJ, Burns MP, 2017. Sexual dimorphism in the inflammatory response to traumatic brain injury. Glia. 65, 1423–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt NM, et al. , 2017. Gut microbiome alterations in Alzheimer's disease. Sci Rep. 7, 13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Steeg LG, Klein SL, 2016. SeXX Matters in Infectious Disease Pathogenesis. PLoS Pathog. 12, e1005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskuhl RR, Gold SM, 2012. Sex-related factors in multiple sclerosis susceptibility and progression. Nat Rev Neurol. 8, 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AK, et al. , 2004. Relationships between cerebrospinal fluid markers of excitotoxicity, ischemia, and oxidative damage after severe TBI: the impact of gender, age, and hypothermia. J Neurotrauma. 21, 125–36. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. , 2005. Caloric restriction attenuates beta-amyloid neuropathology in a mouse model of Alzheimer's disease. FASEB J. 19, 659–61. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. , 2016. The Effects of LW-AFC on Intestinal Microbiome in Senescence-Accelerated Mouse Prone 8 Strain, a Mouse Model of Alzheimer's Disease. J Alzheimers Dis. 53, 907–19. [DOI] [PubMed] [Google Scholar]
- Wang KK, et al. , 2006. Neuroprotection targets after traumatic brain injury. Curr Opin Neurol. 19, 514–9. [DOI] [PubMed] [Google Scholar]
- Wassenaar TM, Panigrahi P, 2014. Is a foetus developing in a sterile environment? Letters in applied microbiology. 59, 572–579. [DOI] [PubMed] [Google Scholar]
- Wilson MH, 2016. Traumatic brain injury: an underappreciated public health issue. Lancet Public Health. 1, e44. [DOI] [PubMed] [Google Scholar]
- Wooten GF, et al. , 2004. Are men at greater risk for Parkinson's disease than women? Journal of neurology, neurosurgery, and psychiatry. 75, 637–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DW, Yeatts SD, Silbergleit R, 2015. Progesterone in traumatic brain injury. N Engl J Med. 372, 1766–7. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Rogers J, 2012. Inflammation in Alzheimer Disease--A Brief Review of the Basic Science and Clinical Literature. Cold Spring Harbor Perspectives in Medicine. 2, a006346–a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, et al. , 2017. Lactobacillus helveticus SBT2171 Attenuates Experimental Autoimmune Encephalomyelitis in Mice. Front Microbiol. 8, 2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T, et al. , 2012. Human gut microbiome viewed across age and geography. Nature. 486, 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurkovetskiy L, et al. , 2013. Gender bias in autoimmunity is influenced by microbiota. Immunity. 39, 400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, et al. , 2013. Structural modulation of gut microbiota in life-long calorie-restricted mice. Nat Commun. 4, 2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HS, et al. , 2016. Inhibition of myeloid differentiation factor 88(MyD88) by ST2825 provides neuroprotection after experimental traumatic brain injury in mice. Brain Res. 1643, 130–9. [DOI] [PubMed] [Google Scholar]