Abstract
Background and aims
Many studies have investigated the association between the level of myeloid derived suppressor cells (MDSCs) and clinical features and prognosis of hepatocellular carcinoma (HCC), but the results remain controversial. This systematic review and meta-analysis was conducted to summarize all available data and estimate the relationship.
Methods
A comprehensive literature review was carried out using Medline, Embase and Web of Science database through December 2018 to identify relevant studies. The standardized mean difference (SMD) and the hazard ratio (HR) with 95% confidence interval (CI) were utilized for evaluating continuous outcomes and survival analysis, respectively. All statistical analyses were performed by STATA 14.0 software.
Results
A total of 13 studies with 1002 HCC patients were included in the meta-analysis. Overall, the proportion of MDSCs in HCC patients was higher than that in healthy controls (SMD = 4.49, 95% CI = 2.53–6.46, P<0.001), and patients with chronic liver disease (SMD = 3.41, 95% CI = 1.58–5.24, P<0.001). Subgroup analysis based on the phenotypes of MDSCs and geographical areas showed similar results. However, the frequency of MDSCs was not affected by the treatment with conventional approaches for HCC (SMD = -0.25, 95% CI = -0.57–0.06, P = 0.119). Moreover, increased MDSCs level was significantly associated with poorer overall survival (HR = 2.36, 95% CI = 1.70–3.29, P<0.001) and recurrence-free survival (HR = 2.72, 95% CI = 1.70–4.35, P<0.001), but not significantly correlated with any clinicopathological parameters.
Conclusion
The results of this systematic review suggest that elevated MDSCs level appears to be associated with an increased risk for disease progression and poor prognosis for HCC.
Introduction
Hepatocellular carcinoma is one of the leading causes of cancer-related death worldwide, which responsible for more than 700,000 deaths each year according to the most recent global cancer statistics in 2018[1]. Despite remarkable improvement in the diagnosis and treatment, HCC remains an intractable disease. The major risk factor of HCC is chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV) and subsequent hepatic cirrhosis[2]. The pathogenesis of HCC is not fully understood. Recently, there is a general consensus that various dysfunctions of the immune system contribute to HCC development and progression[3,4]. Therefore, it is important to understand the potential immune modulator in HCC.
Myeloid derived suppressor cells (MDSCs) are a key component of the immunosuppressive network. These cells are capable of impairing innate and adaptive immune responses through various pathways, thereby restricting antitumor immune responses[5,6]. MDSCs have been reported to involve in many carcinomas, such as lung, breast, colorectal and gastric cancers[7–9]. In the last decade, the clinical importance of MDSCs in HCC patients has been investigated, but no consensus has been reached due to multiple phenotypes of MDSCs, the different design, patients population, and so on[10–12]. Furthermore, concerning the prognosis value, MDSCs are expected to be associated with an unfavorable prognosis based on their negative immunoregulated capacity. However, this idea has been challenged by recent studies showing that MDSCs may not be a predictor for the prognosis of overall survival of breast cancer[13]. Therefore, it is desirable to demonstrate whether there is an association between MDSCs and the risk of HCC. In the present meta-analysis, we summarized the results of published studies and evaluated the available evidence to better understand the role played by MDSCs in HCC process.
Methods
Search strategy
This meta-analysis was performed according to the PRISMA guidelines. The PRISMA checklist is provided in S1 Table.
A systematical literature search was performed using the Medline, Embase and Web of Science databases to acquire eligible studies published before December 2018. The literature review was independently conducted by two investigators, and the following search terms were used: (“MDSC” OR “MDSCs” OR “Myeloid derived suppressor cell” OR “Myeloid derived suppressor cells”) and (“Hepatocellular carcinoma” OR “HCC” OR “Liver neoplasm” OR “Liver tumor” OR “Liver cancer”). Other potentially eligible studies were also checked through manual searches of reference lists of included studies.
Inclusion and exclusion criteria
The inclusion criteria were as following: studies that evaluated the level of MDSCs and/or the association between MDSCs level and HCC risk; peripheral MDSC are defined as “HLA-DRlow/−CD11b+CD33+” or “HLA-DRlow/-CD14+”; the HCC patients were clearly diagnosed; providing sufficient published data for the meta-analysis; studies were written in English. The exclusion criteria were as follows: reviews, conference abstracts, case reports, and letters; articles about cell lines or animals.
Data extraction and quality assessment
Two investigators independently extracted the data from all available studies. The discrepancies in article information were resolved via discussion and consensus.
The following information was collected from each study: first author, publication year, country, sample source, the number of participants, therapeutic method, measure method of MDSCs, markers to identify MDSCs, frequency of MDSCs, Hazard ratio (HR) with 95% confidence interval (CI), and prognostic outcome. If the HRs and 95% CIs were not reported directly in the papers, they were estimated from Kaplan-Meier curves according to the methods reported by Tierney et al.[14].The Newcastle-Ottawa Quality Assessment Scale for cross-sectional study was used to assess the quality of the included studies[15]. The scores ranged from 0 to 9, with a score ≥ 6 indicating good quality.
Statistical analysis
Standardized mean difference (SMD) and 95% CI were employed to compare the difference in MDSCs level between HCC patients and controls, or before and after therapy. Subgroup analysis was performed according to the phenotypes of MDSCs and geographical areas. HR combined with 95% CI were calculated to evaluate the association between MDSCs level and prognosis of HCC patients. Heterogeneity among the included studies was evaluated using Chi-square based Q test and I2-statistic test. A P value <0.05 was considered significant. If heterogeneity existed, a random effect model was used. Otherwise, a fixed-effect model was applied. Publication bias was assessed using Begg's funnel plot and Egger's test. All statistical analyses were performed by STATA 14.0 software.
Results
Study selection
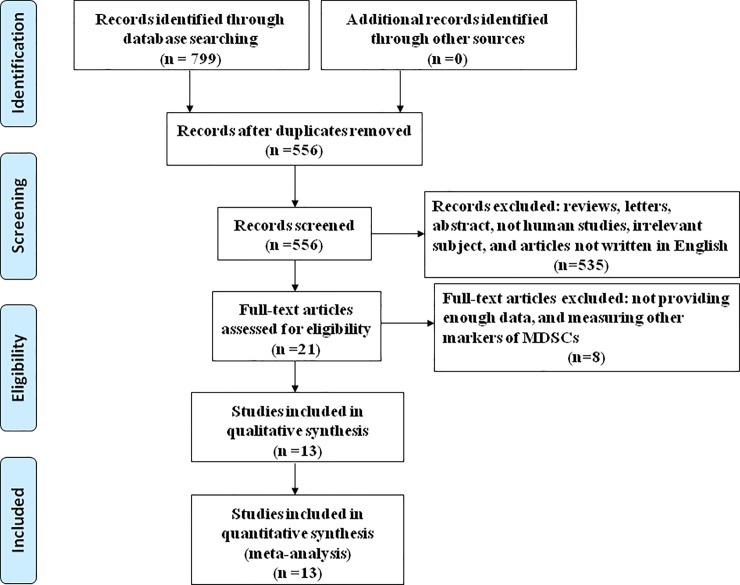
A flowchart of the study selection process was shown in Fig 1. After searching the databases, we obtained 556 potentially relevant articles without duplication. In a primary screening of titles and abstracts, 535 studies were excluded. After further evaluation of the full text, 8 articles were excluded. Finally, a total of 13 publications with 1002 HCC patients were used to perform the meta-analysis.
Fig 1. Flowchart summarizing literature search strategy and selection of studies.
Characteristics of the included studies
The main characteristics of the 13 studies[10,16–27] were summarized in Table 1 and S2 Table. All of the eligible studies were published by groups in USA, China, Japan, and Egypt between 2008 and 2018. Nine studies assessed the level of MDSCs in HCC patients and healthy controls, and 6 studies evaluated the level of MDSCs in HCC patients and patients with chronic liver disease. Additionally, 6 articles compared the level of MDSCs in patients before and after therapy, which included sorafenib, chemotherapy, radiofrequency ablation (RFA), and transarterial chemoembolization (TACE). As for the survival outcomes, 5 studies had data for overall survival (OS), 3 studies for disease-free survival (DFS)/time-to-recurrence (TTR). With regard to the determination of MDSCs, FACS was used in 11 studies, while 2 studies used quantitative real-time PCR and immunohistochemistry.
Table 1. The baseline characteristics of included studies.
| First author, year | Country | Sample source | No. of HCC | Treatment | MDSC definition | Measure method | Cut-off | Outcome |
|---|---|---|---|---|---|---|---|---|
| Elwan 2018 | Egypt | PB | 20 | NR | Lin-HLA-DR-CD11b+CD33+ | FACS | NR | NR |
| Zhou, 2018 | China | PB, Tumor tissue |
PB:26 Tissue:102 |
NR | PB:HLA-DR-CD11b+CD33+ Tissue:CD11b/CD33/CCRK |
PB:FACS Tissue:qRT-PCR |
Median | OS, RFS |
| Li, 2017 | China | PB | 55 | NR | HLA-DR-/lowCD11b+CD33+ | FACS | NR | NR |
| Deng, 2017 | China | Tumor tissue | 78 | NR | CD11b+ | IHC | Median | OS |
| Gao, 2017 | China | PB | 183 | Surgery | HLA-DR-/lowCD14+ | FACS | Median | OS,TTR |
| Iwata, 2016 | Japan | PB | 122 | RFA and TACE | PDL1+HLA-DR-/lowCD11b+CD33+CD14+ | FACS | Median | NR |
| Kalathil, 2016 | USA | PB | 19 | Sorafenib | CD11b+CD33+ | FACS | NR | NR |
| Mizukoshi, 2016 | Japan | PB | 36 | Chemotherapy | HLA-DR-/lowCD14+ | FACS | Median | NR |
| Wang, 2016 | China | PB | 92 | Radiotherapy | HLA-DR-/lowCD14+ | FACS | Average+2SD | OS |
| Arihara, 2013 | Japan | PB | 123 | NR | HLA-DR-/lowCD14+ | FACS | Average+2SD | OS, RFS |
| Kalathil, 2013 | USA | PB | 23 | NR | HLA-DR-CD11b+CD33+CD14- | FACS | NR | NR |
| Mizukoshi, 2013 | Japan | PB | 12 | RFA | HLA-DR-/lowCD14+ | FACS | NR | NR |
| Hoechst, 2008 | Germany | PB | 111 | NR | HLA-DR-/lowCD14+ | FACS | NR | NR |
Abbreviations: NR, not reported; PB, peripheral blood; IHC, immunohistochemistry; RFA, radiofrequency ablation; TACE, trans arterial chemo-embolization; FACS, fluorescence-activated cell sorting; OS, overall survival; RFS, recurrence-free survival; TTR, Time to recurrence
Proportion of peripheral MDSCs in HCC patients
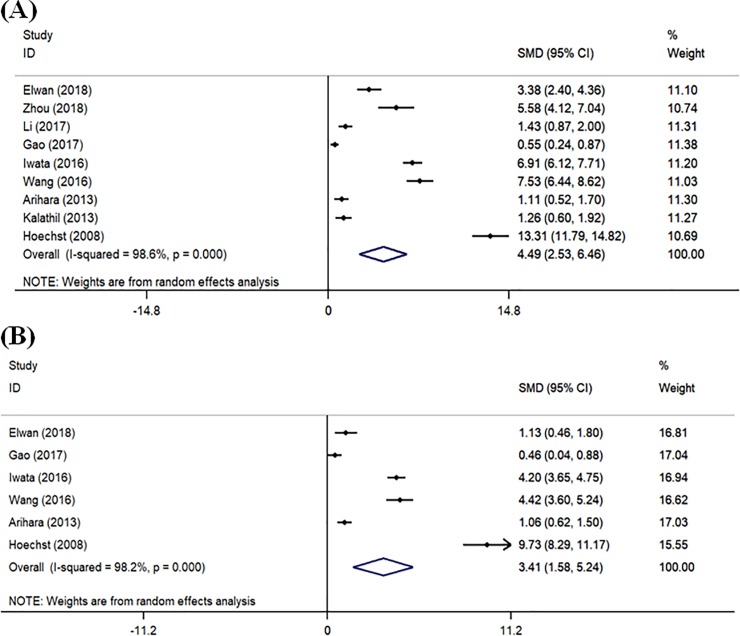
As shown in Fig 2, the pooled analysis showed that the proportion of peripheral MDSCs in HCC patients was higher than that in healthy controls (SMD = 4.49, 95% CI = 2.53–6.46, P<0.001), and patients with chronic liver disease (SMD = 3.41, 95% CI = 1.58–5.24, P<0.001). Heterogeneity assessed by I2 statics was 98.6% (P<0.001) and 98.2% (P<0.001), respectively.
Fig 2.
Forest plot of meta-analysis evaluating the proportion of MDSCs in HCC patients and healthy controls (A) or HCC patients and patients with chronic liver disease (B).
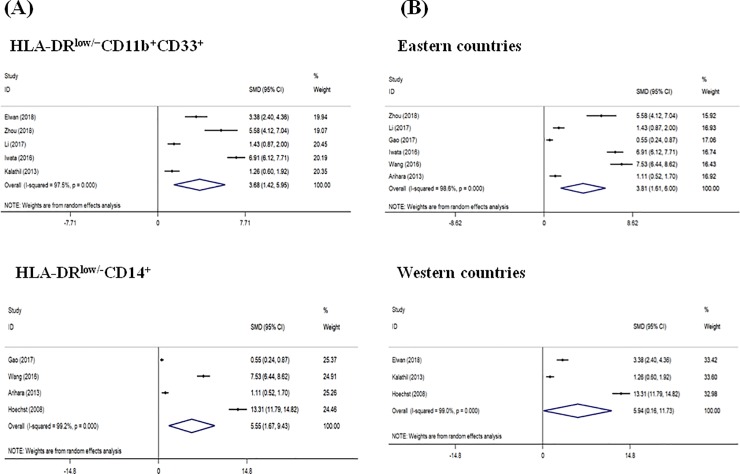
Subgroup analysis was performed according to the phenotypes of MDSCs (Fig 3A). Pooled data of 5 studies in which MDSCs were identified as “HLA-DRlow/−CD11b+CD33+” revealed that there was a significantly higher frequency of MDSCs in HCC patients compared to healthy controls (SMD = 3.68, 95% CI = 1.42–5.95, P = 0.001). There was also a significant increase in the frequency of MDSCs in HCC patients compared to healthy controls when MDSCs were identified as “HLA-DRlow/-CD14+” (SMD = 5.55, 95% CI = 1.67–9.43, P = 0.005). For subgroup analysis of the geographical areas, 6 studies were performed in Eastern countries, and the remaining 3 in Western countries (Fig 3B). The pooled analysis showed that the frequency of MDSCs was significantly higher in HCC patients compared with healthy controls in both Eastern (SMD = 3.81, 95% CI = 1.61–6.00, P = 0.001) and Western (SMD = 5.94, 95% CI = 0.16–11.73, P = 0.044) countries.
Fig 3.
Forest plot of subgroup meta-analysis evaluating the proportion of MDSCs by the phenotypes of MDSCs (A) and geographical areas (B) in HCC patients and healthy controls.
Effect of treatment on MDSCs in HCC patients
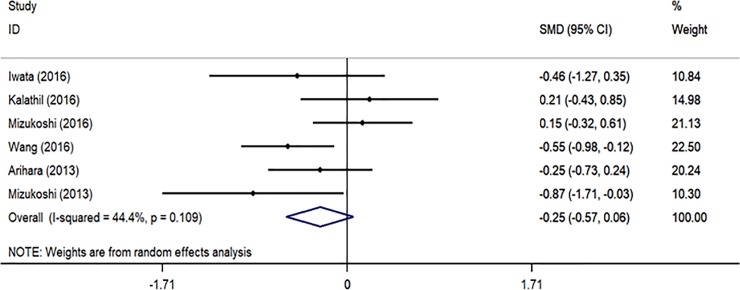
To further assess the effect of treatments on MDSCs level, we analyzed 6 studies that compared the proportion of MDSCs before and after therapy in HCC patients (Fig 4). There was no significant difference between two groups (SMD = -0.25, 95% CI = -0.57–0.06, P = 0.119). Heterogeneity assessed by I2 statics was 44.4% (P = 0.109).
Fig 4. Forest plot of meta-analysis evaluating the proportion of MDSCs before and after therapy in HCC patients.
Association between MDSCs and survival of HCC patients
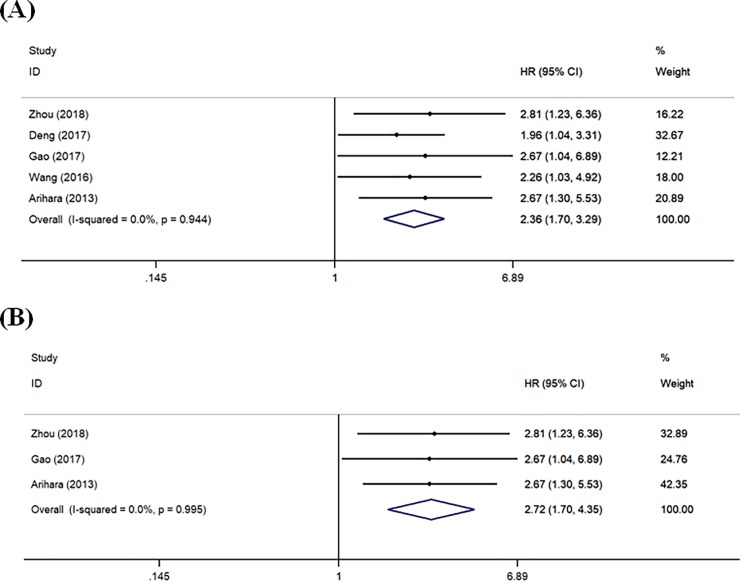
There were 5 studies reporting HRs for OS (Fig 5A). Overall, higher MDSCs level was significantly associated with poorer OS (HR = 2.36, 95% CI = 1.70–3.29, P<0.001). Heterogeneity assessed by I2 statics was 0% (P = 0.944). In addition, stratified analyses were carried out according to the specimen source. In these 5 studies, 2 used liver tissue samples and 3 used peripheral blood samples. High expression of both circulating (HR = 2.52, 95% CI = 1.58–4.00, P<0.001) and tissue-based (HR = 2.21, 95% CI = 1.38–3.55, P = 0.001) MDSCs was significantly associated with unfavorable OS of HCC patients (Data not shown).
Fig 5.
Forest plot of meta-analysis evaluating the relationship between MDSCs level and OS (A) or RFS/TTR (B) in HCC patients.
There were 3 studies reporting HRs for RFS/TTR (Fig 5B). The pooled estimate showed that HCC patients with higher MDSCs had shorter RFS/TTR (HR = 2.72, 95% CI = 1.70–4.35, P<0.001). Heterogeneity assessed by I2 statics was 0% (P = 0.995).
Correlation of MDSCs level with clinicopathological features
As shown in Table 2, MDSCs level was not significantly correlated with any clinicopathological parameters evaluated in this study, including gender, virological markers, tumor size, liver cirrhosis, number of tumors, tumor differentiation, TNM stage, vascular invasion, and lymph node metastasis.
Table 2. Main results for meta-analysis between MDSC and clinicopathological factors.
| Characteristics | No. of studies | No. of patients | Pooled OR (95%CI) | P value |
|---|---|---|---|---|
| Gender (male vs. female) |
3 | 398 | 1.620 (0.980–2.678) | 0.060 |
| HBsAg/HBV/HCV (positive vs. negative) |
2 | 275 | 2.009 (0.951–4.245) | 0.067 |
| Tumor size (cm) (>5 vs. ≤5) |
1 | 183 | 0.651 (0.351–1.211) | 0.175 |
| Liver cirrhosis (yes vs. no) |
1 | 183 | 0.888 (0.473–1.666) | 0.712 |
| Number of tumors (multiple vs solitary) |
3 | 398 | 2.067 (0.785–5.441) | 0.141 |
| Tumor differentiation (poor/moderate vs. well) |
1 | 183 | 0.677 (0.375–1.225) | 0.197 |
| TNM stage (III/IV) vs. I/II) |
2 | 215 | 1.349 (0.073–24.994) | 0.841 |
| Child-Pugh score (B+C) vs. (0+A) |
3 | 398 | 0.823 (0.139–4.869) | 0.830 |
| Vascular invasion (yes vs. no) |
1 | 183 | 2.180 (1.154–4.118) | 0.016 |
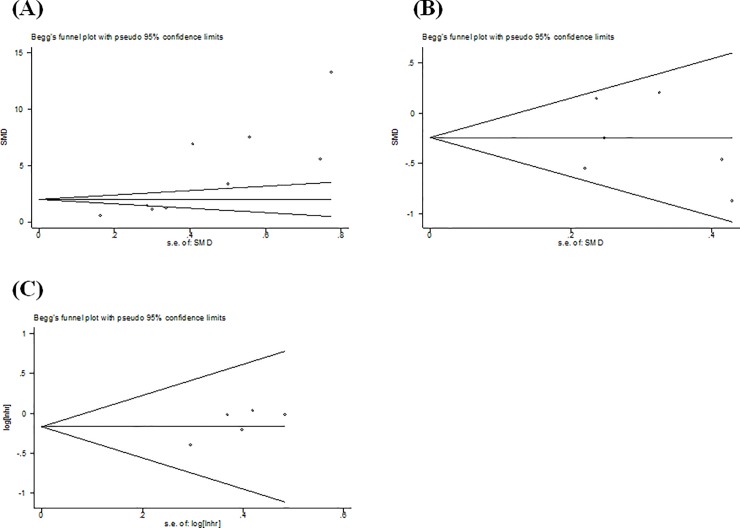
Publication bias
Begg’s test was performed to estimate the publication bias. As shown in Fig 6, there was evidence of publication bias for studies evaluating the proportion of MDSCs in HCC patients and healthy controls (P<0.001). The Trim and Fill method was applied to address this problem, and showed a pooled adjusted SMD of 1.02 (95% CI = 0.82–1.21, P<0.001), which remained statistically significant. There were no significant publication biases for studies included in the analysis of treatment effect (P = 0.669) and OS analysis (P = 0.091).
Fig 6. Funnel plot of publication bias for the studies evaluating MDSCs proportion in HCC patients and healthy controls.
(A), treatment effect (B), and overall survival (C).
Discussion
As a novel immunosuppressive cell population, MDSCs appear to facilitate tumor immune escape by inhibiting antitumor immune response[5,6,28]. Recently, more and more studies focus on the clinical role of MDSCs in HCC, but the results are controversial. The present meta-analysis summarized all relevant studies, and evaluated the proportion of MDSCs in HCC patients. Moreover, the clinicopathological and prognostic value of MDSCs for HCC was also assessed.
We firstly performed a meta-analysis of studies measuring the frequency of MDSCs in HCC patients. To our knowledge, this is the first meta-analysis to elucidate MDSCs status in such patients. Pooled data revealed that the percentage of MDSCs in HCC patients was significantly higher than that in patients with chronic liver disease and healthy controls. We also performed a meta-analysis to compare the percentage of MDSCs in patients with chronic liver disease and healthy controls, and found that patients with chronic liver disease had a significantly higher percentage of MDSCs (SMD = 2.71, 95% CI = 0.89–4.53) (Data not shown). These results suggested that increased MDSCs may contribute to the progression from chronic hepatitis to HCC.
To address the clinical heterogeneity among the studies, subgroup analyses were done according to the geographical areas. The results indicated that the association remained statistically significant. We also performed a subgroup analysis based on the markers for MDSCs. The results revealed that HCC patients had a higher proportion of MDSCs compared to healthy controls, regardless of whether MDSCs were defined as “HLA-DRlow/−CD11b+CD33+” or “HLA-DRlow/-CD14+”, two most commonly used markers of MDSCs in HCC patients. It seems that the different phenotypic markers of MDSCs do not affect the conclusion. Over the last years, there is a change in the definition of MDSCs[29,30]. At present, HLA-DRlow/−CD11b+CD33+ cells are considered to be total MDSCs, whereas HLA-DRlow/-CD14+ cells are used to identify monocytic MDSCs [31,32]. Therefore, more studies with larger sample sizes are needed to value the real status of MDSCs in patients with HCC. It is unclear the effect of HCC treatment on MDSCs level. Several studies have investigated the change of MDSCs frequency in patients receiving curative therapy, such as chemotherapy, surgical resection, sorafenib, RFA and TACE[17–20,22,23]. We summarized the results of these studies, and found that there was no significant difference in the proportion of MDSCs between before and after treatment. This finding suggested that there was only a limited effect of current clinical therapeutic options on MDSCs. Therefore, the combination of routine treatment and inhibition of MDSCs could be an effective strategy in improving clinical outcome for HCC patients. However, subgroup analysis based on every therapy could not be conducted due to the limitation of sample size. Considering that the different therapeutic approaches might have different effects on MDSCs, more clinical studies are needed to verify the above conclusion.
Abnormally increased MDSCs might contribute to poor prognosis of cancer patients. Several previous systematic reviews have evaluated the prognostic value of MDSCs in all types of solid tumors[33–35]. In these studies, the relationship between MDSCs and prognosis of HCC was also evaluated, but the number of relevant studies was very small. Different from the above studies, the present study collected more recent data and provided more comprehensive information. Our pooled data revealed that higher MDSCs level significantly correlated with short OS and RFS. We carried out the stratified analysis based on the specimen source, and found that the HRs for OS were similar between the subgroups. The results provide support that the change of circulating and tissue-based MDSCs level could be utilized as an independent factor in predicting the prognosis of HCC patients. As for the clinicopathological features, our analysis revealed that MDSCs expression did not correlate with any clinicopathological variables. This is the first meta-analysis to systematically explore the relationship between MDSCs level and clinicopathological factors of HCC patients. However, this conclusion needs to be further verified because of the small number of studies.
There were some limitations in our meta-analysis. First, the cutoff values defining high MDSCs expression were different across studies. These may contribute to the heterogeneity in our meta-analysis. Second, some studies did not provide complete data, therefore we used the extracted data. This could influence the accuracy of data. Third, the number of studies related to clinical treatment was relatively insufficient. Hence, no stratified analysis was performed. Finally, studies with positive data are more likely to be published, which may lead to overestimated correlation. Therefore, more researches are needed to verify the results obtained by the current study.
Conclusion
In conclusion, the results of this meta-analysis suggest that there is a significant association between higher MDSCs level and an increased risk and poor survival of HCC. The status of MDSCs could be important for maintaining tumor microenvironment to facilitate tumor growth. MDSCs might be utilized as a prognostic marker for HCC. Considering the phenotypical and functional heterogeneity of MDSCs, more clinical studies are necessary to verify our conclusion.
Supporting information
(DOC)
(DOCX)
Data Availability
All relevant data are within the manuscript and Supporting Information files.
Funding Statement
This work was supported by grants from the Chinese Foundation for Hepatitis Prevention and Control-TianQing Liver Disease Research Fund (No. TQGB20150231), the Natural Science Foundation of Hebei Province of China (No. H2015106081), and the Hebei Medical Research Foundation (No. 20150893). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi VK, Singh A, Dubey SK, Hetta HF, John J, Singh MP. Molecular mechanistic insight of hepatitis B virus mediated hepatocellular carcinoma. Microb Pathog. 2019;128:184–194. 10.1016/j.micpath.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 3.Roth GS, Decaens T. Liver immunotolerance and hepatocellular carcinoma: Patho-physiological mechanisms and therapeutic perspectives. Eur J Cancer. 2017;87:101–112. 10.1016/j.ejca.2017.10.010 [DOI] [PubMed] [Google Scholar]
- 4.Ringelhan M, Pfister D, O'Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19(3):222–232. 10.1038/s41590-018-0044-z [DOI] [PubMed] [Google Scholar]
- 5.Gabrilovich D, Nagaraj S. Myeloid-derived-suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009; 9:162–174. 10.1038/nri2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker KH, Beury DW, Ostrand-Rosenberg S. Myeloid-derived suppressor cells: critical cells driving immune suppression in the tumor microenvironment. Adv Cancer Res. 2015;128:95–139. 10.1016/bs.acr.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian T, Gu X, Zhang B, Liu Y, Yuan C, Shao L, Guo Y, Fan K. Increased circulating CD14(+)HLA-DR-/low myeloid-derived suppressor cells are associated with poor prognosis in patients with small-cell lung cancer. Cancer Biomark. 2015;15(4):425–432. 10.3233/CBM-150473 [DOI] [PubMed] [Google Scholar]
- 8.Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19(2):108–119. 10.1038/s41590-017-0022-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mao FY, Zhao YL, Lv YP, Teng YS, Kong H, Liu YG, Wu XL, Hao CJ, Chen W, Duan MB, Han B, Ma Q, Wang TT, Peng LS, Zhang JY, Cheng P, Su CY, Fu XL, Zou QM, Guo G, Guo XL, Zhuang Y. CD45+CD33lowCD11bdim myeloid-derived suppressor cells suppress CD8+ T cell activity via the IL-6/IL-8-arginase I axis in human gastric cancer. Cell Death Dis. 2018;9(7):763 10.1038/s41419-018-0803-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arihara F, Mizukoshi E, Kitahara M, Takata Y, Arai K, Yamashita T, Nakamoto Y, Kaneko S. Increase in CD14+HLA-DR -/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol Immunother. 2013;62(8):1421–1430. 10.1007/s00262-013-1447-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu DK, Tse AP, Xu IM, Di Cui J, Lai RK, Li LL, Koh HY, Tsang FH, Wei LL, Wong CM, Ng IO, Wong CC. Hypoxia induces myeloid-derived suppressor cell recruitment to hepatocellular carcinoma through chemokine (C-C motif) ligand 26. Nat Commun. 2017;8(1):517 10.1038/s41467-017-00530-7 [DOI] [PubMed] [Google Scholar]
- 12.Hetta HF, Zahran AM, Mansor SG, Abdel-Malek MO, Mekky MA, Abbas WA. Frequency and Implications of myeloid-derived suppressor cells and lymphocyte subsets in Egyptian patients with hepatitis C virus-related hepatocellular carcinoma. J Med Virol. 2019. July;91(7):1319–1328. 10.1002/jmv.25428 [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Yang J. Identification of CD4+CD25+CD127- regulatory T cells and CD14+HLA-DR-/low myeloid-derived suppressor cells and their roles in the prognosis of breast cancer. Biomed Rep. 2016;5(2):208–212. 10.3892/br.2016.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herzog R, Alvarez-Pasquin MJ, Diaz C, Del Barrio JL, Estrada JM, Gil A. Are healthcare workers' intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. 2013;13:154 10.1186/1471-2458-13-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Krüger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135(1):234–243. 10.1053/j.gastro.2008.03.020 [DOI] [PubMed] [Google Scholar]
- 17.Mizukoshi E, Yamashita T, Arai K, Sunagozaka H, Ueda T, Arihara F, Kagaya T, Yamashita T, Fushimi K, Kaneko S. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2013;57(4):1448–1457. 10.1002/hep.26153 [DOI] [PubMed] [Google Scholar]
- 18.Kalathil S, Lugade AA, Miller A, Iyer R, Thanavala Y. Higher frequencies of GARP(+)CTLA-4(+)Foxp3(+) T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res. 2013;73(8):2435–2444. 10.1158/0008-5472.CAN-12-3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D, An G, Xie S, Yao Y, Feng G. The clinical and prognostic significance of CD14(+)HLA-DR(-/low) myeloid-derived suppressor cells in hepatocellular carcinoma patients receiving radiotherapy. Tumour Biol. 2016;37(8):10427–10433. 10.1007/s13277-016-4916-2 [DOI] [PubMed] [Google Scholar]
- 20.Mizukoshi E, Yamashita T, Arai K, Terashima T, Kitahara M, Nakagawa H, Iida N, Fushimi K, Kaneko S. Myeloid-derived suppressor cells correlate with patient outcomes in hepatic arterial infusion chemotherapy for hepatocellular carcinoma. Cancer Immunol Immunother. 2016;65(6):715–725. 10.1007/s00262-016-1837-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalathil SG, Lugade AA, Miller A, Iyer R, Thanavala Y. PD-1+ and Foxp3+ T cell reduction correlates with survival of HCC patients after sorafenib therapy. JCI Insight. 2016;1(11). pii: e86182 10.1172/jci.insight.86182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwata T, Kondo Y, Kimura O, Morosawa T, Fujisaka Y, Umetsu T, Kogure T, Inoue J, Nakagome Y, Shimosegawa T. PD-L1+MDSCs are increased in HCC patients and induced by soluble factor in the tumor microenvironment. Sci Rep. 2016;6:39296 10.1038/srep39296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao XH, Tian L, Wu J, Ma XL, Zhang CY, Zhou Y, Sun YF, Hu B, Qiu SJ, Zhou J, Fan J, Guo W, Yang XR. Circulating CD14+ HLA-DR-/low myeloid-derived suppressor cells predicted early recurrence of hepatocellular carcinoma after surgery. Hepatol Res. 2017;47(10):1061–1071. 10.1111/hepr.12831 [DOI] [PubMed] [Google Scholar]
- 24.Deng Y, Cheng J, Fu B, Liu W, Chen G, Zhang Q, Yang Y. Hepatic carcinoma-associated fibroblasts enhance immune suppression by facilitating the generation of myeloid-derived suppressor cells. Oncogene. 2017;36(8):1090–1101. 10.1038/onc.2016.273 [DOI] [PubMed] [Google Scholar]
- 25.Li X, Xing YF, Lei AH, Xiao Q, Lin ZH, Hong YF, Wu XY, Zhou J. Neutrophil count is associated with myeloid derived suppressor cell level and presents prognostic value of for hepatocellular carcinoma patients. Oncotarget. 2017;8(15):24380–24388. 10.18632/oncotarget.15456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou J, Liu M, Sun H, Feng Y, Xu L, Chan AWH, Tong JH, Wong J, Chong CCN, Lai PBS, Wang HK, Tsang SW, Goodwin T, Liu R, Huang L, Chen Z, Sung JJ, Chow KL, To KF, Cheng AS. Hepatoma-intrinsic CCRK inhibition diminishes myeloid-derived suppressor cell immunosuppression and enhances immune-checkpoint blockade efficacy. Gut. 2018;67(5):931–944. 10.1136/gutjnl-2017-314032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elwan N, Salem ML, Kobtan A, El-Kalla F, Mansour L, Yousef M, Al-Sabbagh A, Zidan AA, Abd-Elsalam S. High numbers of myeloid derived suppressor cells in peripheral blood and ascitic fluid of cirrhotic and HCC patients. Immunol Invest. 2018;47(2):169–180. 10.1080/08820139.2017.1407787 [DOI] [PubMed] [Google Scholar]
- 28.Sarhan D, Cichocki F, Zhang B, Yingst A, Spellman SR, Cooley S, Verneris MR, Blazar BR, Miller JS. Adaptive NK Cells with Low TIGIT Expression Are Inherently Resistant to Myeloid-Derived Suppressor Cells. Cancer Res. 2016;76(19):5696–5706. 10.1158/0008-5472.CAN-16-0839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ben-Meir K, Twaik N, Baniyash M. Plasticity and biological diversity of myeloid derived suppressor cells. Curr Opin Immunol. 2018;51:154–161. 10.1016/j.coi.2018.03.015 [DOI] [PubMed] [Google Scholar]
- 30.Su L, Xu Q, Zhang P, Michalek SM, Katz J. Phenotype and Function of Myeloid-Derived Suppressor Cells Induced by Porphyromonas gingivalis Infection. Infect Immun. 2017;85(8):e00213–17. 10.1128/IAI.00213-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dumitru CA, Moses K, Trellakis S, Lang S, Brandau S. Neutrophils and granulocytic myeloid-derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol Immunother. 2012;61(8):1155–1167. 10.1007/s00262-012-1294-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125(9):3356–3364. 10.1172/JCI80005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirbod-Mobarakeh A, Mirghorbani M, Hajiju F, Marvi M, Bashiri K, Rezaei N. Myeloid-derived suppressor cells in gastrointestinal cancers: A systematic review. J Gastroenterol Hepatol. 2016;31(7):1246–1256. 10.1111/jgh.13284 [DOI] [PubMed] [Google Scholar]
- 34.Zhang S, Ma X, Zhu C, Liu L, Wang G, Yuan X. The Role of Myeloid-Derived Suppressor Cells in Patients with Solid Tumors: A Meta-Analysis. PLoS One. 2016;11(10):e0164514 10.1371/journal.pone.0164514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang PF, Song SY, Wang TJ, Ji WJ, Li SW, Liu N, Yan CX. Prognostic role of pretreatment circulating MDSCs in patients with solid malignancies: A meta-analysis of 40 studies. Oncoimmunology. 2018;7(10):e1494113 10.1080/2162402X.2018.1494113 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and Supporting Information files.