Abstract
Objective
To verify whether growth hormone receptor (GHR) gene expression plays a role in growth of children with cystic fibrosis (CF), as a consequence of the chronic inflammatory condition and malnutrition.
Design
We enrolled 49 prepubertal patients (24 males and 25 females) affected by CF in a stable clinical condition, 19 of whom had been diagnosed through newborn screening and 30 following presentation of symptoms. Patients had no significant comorbidity affecting growth or cystic fibrosis transmembrane conductance regulator (CFTR)-related diabetes requiring insulin therapy. Blood was collected during two follow-up visits to measure insulin-like growth factor (IGF-I), growth hormone-binding protein (GHBP), and GHR gene expression. Recruited as a control group were 52 healthy children, sex- and age-matched, were recruited as a control group.
Methods
We compared body mass index (BMI), height, weight, IGF-I, GHBP, and GHR gene expression values (evaluated by Chemiluminescent Immunometric assay; ELISA and real-time PCR, respectively) in CF patients diagnosed through newborn screening (NBS) or by symptoms (late diagnosis [LD]) and in healthy controls.
Results
BMI increased significantly in patients between the time of diagnosis and check-up (P<0.001), particularly in the LD group; median value was lower at diagnosis and significantly higher (P<0.001) at follow-up visits compared to controls. At initial evaluation, higher levels of IGF-I (not statistically significant) were found in both the NBS group and the LD group compared to the control group. At the second evaluation, significantly higher levels of IGF-I (P=0.003) were found in both the NBS and LD groups compared to controls; GHR mRNA expression had significantly increased (P=0.013) in LD patients compared with the first evaluation and was significantly higher in the NBS and LD groups than in controls. GHBP values had significantly increased (P=0.047) in the NBS group after one year of therapy compared to first visit levels and were significantly higher (P<0,0001) in the NBS and LD groups compared to controls.
Conclusion
In our LD patients during childhood, we observed good auxological values and a GH/IGF-I axis function within normal range for the factor evaluated. However, earlier diagnosis through NBS might further minimize and prevent growth retardation, by reducing the duration of symptoms before treatment.
Keywords: Cystic fibrosis: growth, IGF-I, Growth hormone receptor, Newborn screening
Cystic fibrosis (CF) is a genetic disorder inherited as an autosomal recessive mutation in the transmembrane conductance regulator (CFTR) gene. This gene codifies a protein, which is a chloride channel present in the apical membrane of epithelial cells, although it also has many other important regulatory roles.1,2 In fact, as CFTR is widely expressed, CF affects most organs including the lung, pancreas, liver, and kidney.3,4 Moreover, individuals with CF show a high degree of variability in disease severity, complications, and survival3 due to the fact that different CFTR mutations cause a number of defects in the CFTR protein synthesis, trafficking, or function.5
Like most chronic inflammatory diseases in childhood, CF is associated with impaired growth.2,6,7 The exact mechanisms of this effect are not known, although it is generally thought to be caused by concomitant severe disease complications due to the inflammation itself, as well as prolonged use of glucocorticoids and suboptimal nutrition.8–10 Moreover, an important correlation has been found between poor growth and reduced long-term lung function in children with CF.11
The growth hormone insulin-like growth factor (GH-IGF) axis is the most important endocrine axis involved in linear growth in children and adolescents. In children with chronic inflammation, growth failure may be affected by several mechanisms including GH\IGF-I defect, GH\IGF-I resistance, down-regulation of GH\IGF receptors, disruptions in downstream GH\IGF signaling pathways, or deregulation of IGF binding protein (IGFBPs).7 In children with CF, growth impairment may be associated with abnormally low IGF-I concentrations. In fact, IGF-I signaling is mediated by CFTR, and consequently CFTR dysfunction may impact linear growth, adding to indirect effects due to malnutrition.10,11 Furthermore, IGF-I plays an important role in inflammation and, in turn, is related to lung function and nutritional status in CF.
Management of CF has improved significantly in recent years. While infants born in the past would have been unlikely to live beyond their first year, infants today are likely to live well into adulthood, thanks to the introduction of newborn screening (NBS), more effective treatment, greater availability and improved genetic testing, using more specific and sensitive diagnostic criteria. The cornerstones of CF management are the treatment of respiratory infections, promotion of good nutrition, and an active lifestyle.12 The increased life expectancy of these patients has meant that attention is now focused on new complications of the disease, such as endocrine dysfunctions,13–15 in particular, dysfunctions involving the GH, the IGF system, thyroid hormones, insulin, and sex steroids.2
Therefore, even though the current strategy to improve growth and weight in children with CF tends to focus on correction of exocrine pancreas insufficiency and nutritional supplements, careful evaluation of other endocrine complications such as GH deficiency (GHD) should not be overlooked. In fact, the prevalence of GHD in pediatric CF patients is higher than in the general population, although it should be noted that the relevant studies were centered on children with growth delay.13,16 Furthermore, it is thought that the anabolic action of GH and IGF-I could theoretically have beneficial effects in CF patients with growth failure, regardless of GH deficiency.17
The involvement of the GH\IGF-I axis in poor linear growth in children with CF prompted us to verify whether growth hormone receptor (GHR) gene expression plays a role in the growth of children with CF, as a consequence of the chronic inflammatory condition and malnutrition. We measured in serum IGF-I and GH binding protein levels; GHR gene expression was evaluated in lymphocytes, obtained from peripheral blood of CF patients, and from age-sex-matched controls. In CF patients, we analyzed these parameters at various points: when patients were in a clinically stable condition, a few years after diagnosis, and at one year after the first evaluation. We then analyzed the values obtained together with anthropometric data and clinical activity, to discover whether they may be influenced by therapy in childhood.
Methods
Patients
We enrolled 49 consecutive CF patients (23 males and 26 females) attending regular follow-up at Bambino Gesù Children’s Hospital in Rome and Federico II University Hospital in Naples. Patients were enrolled if they were diagnosed with CF according to current diagnostic criteria,18 prepubertal, and in stable clinical condition. Exclusion criteria were diagnosis of another significant comorbidity affecting growth and CFTR-related diabetes requiring insulin therapy. Of these CF patients, 19 had been diagnosed by NBS, and 30 had been diagnosed following presentation of symptoms (late diagnoses [LD]). Spirometry was performed in patients aged 6 years or older. Mild lung disease (forced expiratory volume [FEV]1 >70%) was found in 18 patients, whereas 6 patients had moderate lung disease (FEV1 >40%<70%). None of the patients had severe lung disease (FEV1 <40%). Standard therapy for CF was prescribed (pancreatic enzymes in pancreatic insufficient patients, liposoluble vitamin supplementation in all patients, ursodeoxycholic acid in patients with CF-liver disease, and dornase alfa inhalation, inhaled antibiotics, or oral azithromycin according to the patient’s clinical condition). Table 1 shows the characteristics of the CF patients and healthy subjects.
Table 1.
Demographic and anthropometric characteristics of patients and controls.
| Characteristic | NBS (N=19) | LD-CF (N=29) | CTRL (N=52) |
|---|---|---|---|
| Age I (Years) | 5.09±0.46 | 7.16±0.43 | 8.9±0.38 |
| Height (SDS) | |||
| 0 | −0.21*(−0.72–0.88) | −0.59°(−1.85–0.34) | |
| 1 | 0.99*(0.19–1.49) | 0.35**(−0.36–1.2) | −0.84(−0.99–0.49) |
| 2 | 0.76*(0.02–0.94) | 0.29**(0.55–1.49) | |
| Weight (SDS) | |||
| 0 | −0.7(−1.38–0.48) | −1.3(−1.9–0.2) | |
| 1 | 0.63*°(0.015–1.56) | 0.44**° (0.07–1.01) | −0.97(−1.33–0.02) |
| 2 | 0.74°°(0.17–1.28) | 0.59°°(−0.2–1.2) | |
| BMI (SDS) | |||
| 0 | −0.325(−2.445–0.32) | −1.435(−4.14–2.18) | |
| 1 | 0.4°*(−0.51–1.09) | 0.09°*(−47–0.74) | −1.1(−1.59–0.2) |
| 2 | 0.1°°** (−0.4–0.86) | −0.14**(−0.6–1.18) | |
| FEV1 (%) | |||
| 1 | 88.5(80–103) | 94(83.75–108.25) | |
| 2 | 96(84.25–105.75) | 101(91–110) | |
Abbreviations: NBS, new born screening; LD-CF, late diagnosis cystic fibrosis; CTRL, controls; SDS, Standard
Deviation Score; BMI, body mass index; FEV, forced expiratory volume.
Except age (expressed as mean ± SE), all data are expressed as median value and interquartile range.
Key: *NB patients vs controls; ** LD patients vs controls °LD or NB patients at diagnosis vs LD or NB patients at first follow-up visit respectively; °° LD or NB patients at diagnosis vs LD or NB patients at second follow-up visit respectively.
0: patients at diagnosis; 1: patients at the first follow-up visit; 2: patients at the second follow-up visit.
Patients were prospectively followed for 1 year. Blood was collected during follow-up visits in clinically stable conditions (a few years after diagnosis) and again one year after the first evaluation, to measure IGF-I, GHBP and GH-R gene expression. A control group consisting of 52 healthy, sex and age-matched children was recruited (mean age: 8.95 ± 0.38 years).
Written informed consent was obtained from parents or legal guardians of all children, and patients over 13 years-of-age signed a statement of assent. The study was approved by the Ethics Committees of the centers involved in the study.
GHBP Evaluation
Serum levels of GHBP were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) (DSL-10-48100 ACTIVE hGHBP Elisa-Webster, Texas, USA). The minimum detectable concentration was 1.69 pmol/L. The intra- and inter-assay coefficients of variation were 5.59% to 4.78% and 8.36% to 5.11%, for a quality control range of 20.25 pmol/L to 198.24 pmol/L and 19.99 pmol/L to 195.78 pmol/L, respectively.
IGF-I Determination
The serum IGF-I concentration was measured using an automatic assay that utilizes a solid-phase, enzyme-labeled chemilluminescent immunometric assay (Immulite 2000 IGF-I-DPC, Los Angeles, CA and Immulite Analyzer). The intra-assay coefficients of variation were 3.9% to 2.4%, for a quality control range of 77 ng/mL to 1,358 ng/mL. IGF-I values are expressed as mean standard deviation score (SDS) according to Elmlinger, et al.19
GHR Gene Expression
Peripheral blood mononuclear cells (PBMC) of patients and age-matched controls were separated by Ficoll density gradient centrifugation using a standard procedure (centrifugation at 1800 rpm for 30 minutes at room temperature, followed by the recovery of the PBMC ring at the interface).
For real-time GHR gene expression analysis, total RNA was isolated from PBMC using RNeasy mini-columns (Qiagen, Hilden, Germany). Reverse transcriptase [RT]-PCR was carried out using the SuperScript First-Strand Synthesis System. An RNA/primer mixture containing total RNA, oligo dT (50 ng/μL), 10 mM dNTP mix, and diethyl pyrocarbonate water was prepared. The samples were incubated at 65°C for 5 minutes and then on ice for at least 1 minute. A master reaction mixture containing 10X RT buffer, 25 mM MgCl2, 0.1 M DTT, and RNaseOUT was prepared for each sample. The reaction mixture was then added to the RNA/primer mixture; samples were mixed briefly and kept at room temperature for 2 mins. SuperScript II RT (50 units) was added to each tube; the samples were mixed and incubated at 25°C for 10 mins, and the tubes were then incubated at 42°C for 50 mins, heat inactivated at 70°C for 15 mins, and chilled on ice. First strand cDNA was stored at –20°C until use for real-time PCR.
Quantitation of GHR mRNA expression was determined by quantitative real-time RT-PCR (Real-Time PCR 3500-Applied Biosystems), and assays on demand were used (Hs00174872_m1 Applied Biosystems). Normalization and validation of the data were carried out using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a housekeeping control gene. Each GHR or GAPDH probe was labeled with a fluorescent reporter (FAM). Specifically, a 25 μL volume reaction mixture containing 1.25 μL Assay, 12.5 μL Master Mix, 10.25 μL H2O and 1 μL cDNA was treated under the following conditions: 95°C for 10 mins, 95°C for 15 sec, 60°C for 1 min, for 40 cycles. Quantitative real-time PCR data were calculated using a standard curve and expressed as agGHR/5X105 agGAPDH. The amplification efficiency of GHR in relation to GAPDH mRNA expression was evaluated by analyzing the ΔCt variation with template dilutions in the 1000-fold range.
Statistical Analysis
Data are expressed as the median ± interquartile range. Statistical differences between patients and controls were determined using the Mann-Whitney U test; whereas, the non-parametric Wilcoxon test for paired samples was used to compare values in patients at baseline and after one year. Statistical differences between groups of different subjects were determined using the one-way ANOVA test when a normal distribution of data was observed, and the nonparametric Kruskall-Wallis test when data were not normally distributed. If a statistical significance was found, an adequate post-test identified which group differed from which. A value of P< 0.05 was considered statistically significant.
Results
In this study, we compared body mass index (BMI), height, weight, IGF-I, GHBP, and GHR gene expression values in CF patients diagnosed by NBS or by symptoms (LD), and in healthy controls. We evaluated length, weight and BMI (Table 1) at diagnosis, at a follow-up visit 5 years after diagnosis, in stable clinical conditions, and 1 year after the first measurement. IGF-I, GHBP and GHR gene expression was measured at two follow-up visits only: 5 years from diagnosis and 1 year after the first measurement.
Median height at time of diagnosis was higher in patients than in controls but the difference was statistically significant (P=0.005) only in NBS patients. In the LD group, height showed a significant increase (P<0,05) at the first follow-up visit compared to diagnosis. For all patients, height was significantly higher compared to the control group (P<0.0001) at the second follow-up visit (Table 1).
BMI increased significantly in patients between the time of diagnosis and check-ups (P<0.001), particularly in the LD group; the median value was lower than the controls’ value at diagnosis and significantly higher (P<0.001) at follow-up visits. The NBS group also displayed significantly higher BMI at follow-up visits compared to controls (Table 1).
Overall, patients’ weight significantly increased (P<0.002) between diagnosis and follow-up visits, but no significant differences were found between the two groups of subjects, although the median level of LD patients was consistently lower than that of NBS patients. No significant difference was found in weight between patients and controls (Table 1).
In summary, LD children were found to have reduced weight, height, and BMI values compared to NBS children. In particular, five patients were less than 2 SD for weight in the LD group compared to just one in the NBS group; four children had pathological height in the LD group, but no children were found to be less than 2 SD in the NBS group
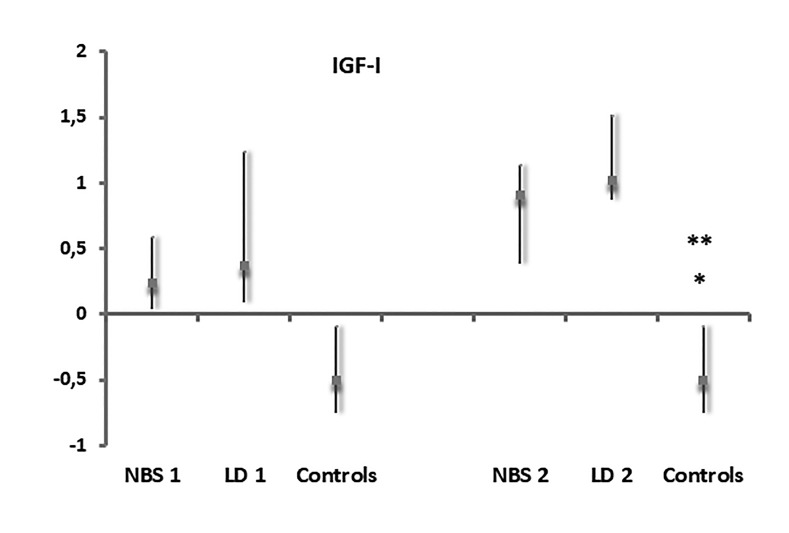
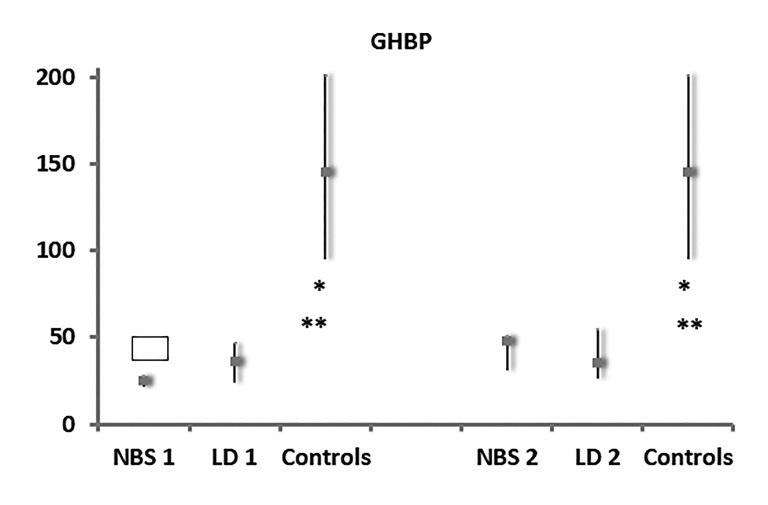
At the initial evaluation, higher levels of IGF-I were found in the NBS group (median level 0.24 SDS within an interquartile range of 0.04 to 0.59), and in the LD group (median level 0.37 SDS within an interquartile range of 0.09 to 1.24), compared to the control group (median value −0.5 SDS within an interquartile range of −0.75 to 0.09), although the differences were not statistically significant (Figure 1). On the contrary, GHBP was significantly lower (P<0.0001) in the NBS and LD groups (median 25 ng/mL within an interquartile range of 21.35 to 27.9; median 36 ng/mL within an interquartile range of 23.6 to 47, respectively) compared to controls (median: 145 ng/mL within an interquartile range of 95 to 201.25) (Figure 2).
Figure 1.
Serum values of IGF-I in NBS, LD patients and controls, at first evaluation (1) and at 1-year follow-up (2), expressed as SDS (Standard Deviation Score). *: P=0.003 Patients NBS (2) vs controls; **: P=0.003 Patients LD (2) vs controls.
Abbreviations: IGF-I, insulin-like growth factor-1; NBS, new born screening; LD, late diagnosis
Figure 2.
Serum values of GHBP in NBS, LD patients and controls, at first evaluation (1) and at 1-year follow-up (2), expressed as ng/mL. *: P<0.0001 Patients NBS (1) vs controls and patients NBS (2) vs controls; **: P<0.0001 Patients LD-CF (1) vs controls and patients LD (2) vs controls;
 : P=0.047 Patients NBS (2) vs patients NBS (1) vs Controls Abbreviations: GHBP, growth hormone binding protein; NBS, newborn screening; LD, late iagnosis; LD-CF, late diagnosis cystic fibrosis
: P=0.047 Patients NBS (2) vs patients NBS (1) vs Controls Abbreviations: GHBP, growth hormone binding protein; NBS, newborn screening; LD, late iagnosis; LD-CF, late diagnosis cystic fibrosis
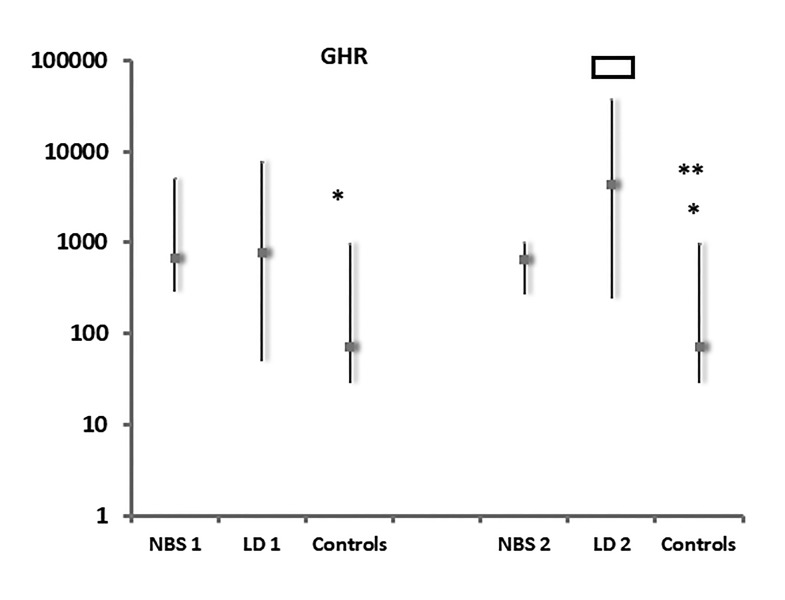
GH-R mRNA expression was significantly higher (P=0.036) in the NBS group (median value 681.3 agGHR/5X105 agGAPDH within an interquartile range of 287.36 to 5090.86) but not in the LD-CF group (median value 768.89 agGHR/5X105 agGAPDH within an interquartile range of 50.45 to 7851.79) compared to controls (median value 71.83 agGHR/5X105 agGAPDH within an interquartile range of 28.56 to 981.14) (Figure 3).
Figure 3.
GHR gene expression values in NBS, LD patients and controls, at first evaluation (1) and at 1-year follow-up (2), expressed as agGHR/5X105agGAPDH. *: P=0.036 Patients NBS (1) vs controls and P=0.022 patients NBS (2) vs controls; **:P=0.0003 Patients LD (2) vs controls.
 : P=0.013 Patients LD (2) vs patients LD (1) vs controls; Abbreviations: GHR, growth hormone receptor; NBS, newborn screening; LD, late diagnosis; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
: P=0.013 Patients LD (2) vs patients LD (1) vs controls; Abbreviations: GHR, growth hormone receptor; NBS, newborn screening; LD, late diagnosis; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
At the second evaluation, during the 1-year follow-up visit, significantly higher levels of IGF-I (P=0.003) were found in the NBS group (median level 0.91SDS within an interquartile range of 0.38 to 1.14) and in the LD group (median level 1.02 SDS within an interquartile range of 0.87 to 1.52) compared to controls.
Furthermore, GHR mRNA expression had significantly increased (P=0.013) in LD-CF patients (median value 4,338.67 agGHR/5X105 agGAPDH within an interquartile range of 240.86 to 37,690.16) in comparison with the first evaluation. Moreover, GHR mRNA expression levels were significantly higher (P=0.022) in the NBS group (median value 646.86 agGHR/5X105 agGAPDH within an interquartile range of 268.39 to 1000) and the LD group (P=0.0003) than in controls.
In addition, GHBP values had significantly increased (P=0.047) in the NBS group after one more year of therapy (median value 48 ng/mL within an interquartile range of 30.5 to 50.75) compared to first visit levels. Furthermore, GHBP levels were significantly higher (P<0,0001) in the NBS and LD groups (median value 35 ng/mL within an interquartile range of 25.8 to 54.8) compared to controls.
Discussion
Growth failure is a common feature in many children with chronic diseases such as CF. Both altered GH metabolism and organ resistance to GH have been implicated as major contributors of growth retardation in CF subjects.20 Chronic undernutrition and inflammatory cytokines are the principal and interrelated determinants of GH resistance, whose negative effect may be compounded by long-term steroid therapy. On the contrary, there is evidence that higher serum IGF-I levels are associated with better health in CF, because they reflect a link between nutritional sufficiency and lung function in CF.11
Before NBS, many patients showed persistent growth failure due to a delay in the diagnosis of CF. The advent of NBS improved clinical outcomes of CF patients, in particular regarding weight and stature.21 Therefore, in our study we considered both children with CF who underwent NBS and those who were diagnosed later. We analyzed the differences in weight, height, and BMI at diagnosis and again some years later, when they were in a clinically stable condition, to confirm data on benefits of NBS. Furthermore, we evaluated some parameters relating to the GH/IGF-I axis (GHR gene expression, IGF-I, and GHBP) to verify if significant differences exist among children diagnosed by NBS, subjects diagnosed by symptoms, and controls.
Our results showed differences in height, weight, and BMI between NBS children and LD children at diagnosis. Even though these differences were not statistically significant at median level, LD children were found to have reduced weight, height, and BMI values compared to NBS children. In particular, five patients were less than 2 SD for weight in the LD group compared to just one in the NBS group; four children had pathological height in the LD group, but no children were found to be less than 2 SD in the NBS group. These data show that the LD children were at a disadvantage compared to the NBS group at diagnosis, probably because of the longer interval between the onset of symptoms and diagnosis in this group. Some years later, the children in both these groups showed significantly higher weight-for-age values compared with the values recorded at diagnosis, as well as significantly higher BMI and height compared to healthy controls, thanks to appropriate and prompt nutritional intervention; although, the LD group’s values continue to be lower than those of the NBS children. Darrah, et al22 identified a relationship between birth weight of newborns affected by CF and later childhood pulmonary function. In contrast to these findings, we did not find any significant correlation between weight at diagnosis and pulmonary function expressed as FEV1 (%). The different sampling of subjects and the longer time between diagnosis and the evaluation of lung function may explain the differences in our results.
Previous studies21 showed that despite NBS and prompt nutritional intervention, a sub-group of patients with CF was unable to overcome growth deficits, suggesting that other factors contributed to poor weight gain and linear growth. Hence, we evaluated IGF-I, GHR gene expression, and GHBP levels during childhood, when patients were in clinically stable condition. We found no significant differences in these parameters between NBS and LD children, but there were significantly higher values of IGF-I and GHR expression and lower levels of GHBP in CF patients compared to healthy controls. In particular, in the NBS patients, GHR gene expression and serum GHBP values were already significantly different at the first visit compared to controls. Therefore, in our LD patients during childhood, we observed a good auxological condition and a GH/IGF-I axis function in normal range regarding factor evaluated. However, earlier diagnosis with NBS might further minimize and prevent growth retardation, ie, in height and weight, by reducing the duration of symptoms before treatment, as opposed to late diagnosis based on symptom presentation and treatment aimed at rectifying the growth inhibiting effects of malnutrition and inflammatory cytokines.
Furthermore, our data show that GHBP level and GHR function are not closely correlated, as demonstrated also in other physiological and pathological conditions.23 GHR regulation and its cleavage to GHBP is tissue-specific. Therefore, it is possible that, in our patients, these mechanisms lead to an increase in GHR availability on the cell’s surface, thus improving GH action.24 Further studies are mandatory to confirm our results and improve the quality of life of CF sufferers.
Acknowledgements
The authors are grateful to Sheila Margaret McVeigh for the English revision of the paper. The authors would like to thank the staff of the Adolfo Ferrata Medical Library at the University of Pavia (Italy) for their invaluable assistance.
Footnotes
Financial Support: The charity “Il bambino e il suo pediatra”, via XX Settembre n.28, 28066 Galliate (Novara) supported the work presented in this paper.
References
- 1.Bernardi DM, Ribeiro AF, Mazzola TN, Vilela MMS, Sgarbieri VC. The impact of cystic fibrosis on the immunologic profile of pediatric patients. J Pediatr (Rio J). 2013;89(1):40 47. [DOI] [PubMed] [Google Scholar]
- 2.Cirillo F, Lazzeroni P, Sartori C, Street ME. Inflammatory diseases and growth: effects on the GH-IGF axis and on growth plate. Int J Mol Sci. 2017;18(9):pii: E1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009;373(9678):1891–1904. [DOI] [PubMed] [Google Scholar]
- 4.Bush A, Bilton D, Hodson M, eds. Hodson and Geddes’ Cystic Fibrosis. 4th ed Boca Raton, FL: CRC Press; 2015. [Google Scholar]
- 5.Castellani C, Cuppens H, Macek M, Jr, et al. ,. Consensus on the use and interpretation of cystic fibrosis mutation analysis in clinical practice. J Cyst Fibros. 2008;7(3):179–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bozzola E, Pagani S, Meazza C, et al. Changes in growth hormone receptor gene expression during therapy in children with juvenile idiopathic arthritis. Horm Res Paediatr. 2012;77(1):52–58. [DOI] [PubMed] [Google Scholar]
- 7.Pagani S, Bozzola E, Strisciuglio C, et al. Growth hormone receptor gene expression increase reflects nutritional status improvement in patients affected by Crohn’s disease. Front Pediatr. 2018;6:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong SC, Dobie R, Altowati MA, Werther GA, Farquharson C, Ahmed SF. Growth and growth hormone-insulin like growth factor 1 axis in children with chronic inflammation: Current evidence, gaps in knowledge, and future directions. Endocr Rev. 2016;37(1):62 110. [DOI] [PubMed] [Google Scholar]
- 9.Sanderson IR. Growth problems in children with IBD. Nat Rev Gastroenterol Hepatol. 2014;11(10):601–610. [DOI] [PubMed] [Google Scholar]
- 10.Stalvey MS, Pace J, Niknian M, et al. Growth in Prepubertal Children With Cystic Fibrosis Treated With Ivacaftor. Pediatrics. 2017;139(2):e20162522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gifford AH, Nymon AB, Ashare A. Serum insulin-like growth factor-1 (IGF-1) during CF pulmonary exacerbation: trends and biomarker correlations. Pediatr Pulmonol. 2014;49(4):335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santos V, Cardoso AV, Lopes C, Azevedo P, Gamboa F, Amorim A. Cystic fibrosis – Comparison between patients in paediatric and adult age. Rev Port Pneumol (2006). 2017;23(1):17–21. [DOI] [PubMed] [Google Scholar]
- 13.Pascucci C, De Biase RV, Savi D, et al. Deregulation of the growth hormone/insulin-like growth factor-1 axis in adults with cystic fibrosis. J Endocrinol Invest. 2018;41(5):591 596. [DOI] [PubMed] [Google Scholar]
- 14.Yankaskas JR, Marshall BC, Sufian B, Simon RH, Rodman D. Cystic fibrosis adult care: consensus conference report. Chest. 2004;125(1Suppl):1S–39S. [DOI] [PubMed] [Google Scholar]
- 15.Nick JA, Rodman DM. Manifestations of cystic fibrosis diagnosed in adulthood. Curr Opin Pulm Med. 2005;11(6):513–518. [DOI] [PubMed] [Google Scholar]
- 16.Ciro DO, Padoan R, Blau H, et al. Growth retardation and reduced growth hormone secretion in cystic fibrosis. Clinical observations from three CF centers. J Cyst Fibros. 2013;12(2):165–169. [DOI] [PubMed] [Google Scholar]
- 17.Blackman SM, Tangpricha V. Endocrine Disorders in Cystic Fibrosis. Pediatr Clin North Am. 2016;63(4):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrell PM, White TB, Ren CL, et al. Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation. J Pediatr. 2017;181:S4–S15.e1. [DOI] [PubMed] [Google Scholar]
- 19.Elmlinger MW, Kühnel W, Weber MM, Ranke MB. Reference ranges for two automated chemiluminescent assays for serum insulin-like growth factor I (IGF-I) and IGF-binding protein 3 (IGFBP-3). Clin Chem Lab Med. 2004;42(6):654–664. [DOI] [PubMed] [Google Scholar]
- 20.Kyle UG, Shekerdemian LS, Coss-Bu JA. Growth failure and nutrition considerations in chronic childhood wasting diseases. Nutr Clin Pract. 2015;30(2):227–238. [DOI] [PubMed] [Google Scholar]
- 21.Leung DH, Heltshe SL, Borowitz D, et al. Effects of Diagnosis by Newborn Screening for Cystic Fibrosis on Weight and Length in the First Year of Life. JAMA Pediatr. 2017;171(6):546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darrah R, Nelson R, Damato EG, Decker M, Matthews A, Hodges CA. Growth Deficiency in Cystic Fibrosis is Observable at Birth and Predictive of Early Pulmonary Function. Biol Res Nurs. 2016;18(5):498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kissmeyer-Nielsen P, Christensen H, Laurberg S. Trophic effects of biosynthetic growth hormone on normal and defunctioned left colon in rats. Scand J Gastroenterol. 1995;30(3):246–251. [DOI] [PubMed] [Google Scholar]
- 24.Flores-Morales A, Greenhalgh CJ, Norstedt G, Rico-Bautista E. Negative regulation of growth hormone receptor signaling. Mol Endocrinol. 2006;20(2):241–253. [DOI] [PubMed] [Google Scholar]