Abstract
Objective
Surveillance of antimicrobial resistance patterns on a local level can reveal paradigms not obvious on a regional or national scale. Data collection from this perspective may potentially impact local prescribing patterns and empiric treatment guidelines. The objective of this study was to establish a baseline Staphylococcus aureus antibiogram for the state of Wisconsin and to elucidate potential geographic and demographic factors associated with antimicrobial resistance.
Design
Multi-center laboratory surveillance, with testing at a single site utilizing standardized media and susceptibility testing protocols.
Methods
309 isolates of clinically-significant S. aureus were collected from hospital microbiology laboratories across Wisconsin in 2018, with distribution across seven geographic regions. Each isolate was tested using reference broth microdilution methods against a panel of 15 antimicrobial agents. Percentage susceptibility data, as well as median and 90th percentile minimum inhibitory concentration (MIC) values, were computed for each antimicrobial agent as a function of geographic region or demographic category.
Results
Increased resistance to penicillin (≥ 86.0% of isolates), erythromycin (≥ 56.8%), cefoxitin (≥ 45.5%), levofloxacin (≥ 25.0%), and clindamycin (≥ 20.5%) was observed in the Southcentral, Lake Winnebago, and Southeast regions of Wisconsin. In addition, isolates phenotypically classified as methicillin-resistant S. aureus (MRSA) were found to have increased rates of resistance to clindamycin, erythromycin, and levofloxacin as compared to S. aureus isolates susceptible to cefoxitin. S. aureus isolates demonstrated nearly 100% in vitro susceptibility to ceftaroline, dalbavancin, and telavancin. Statewide S. aureus isolates exhibited a vancomycin MIC90 of 1 μg/mL. S. aureus isolates from patients aged 20–39 years were more likely to demonstrate cefoxitin resistance when compared to other age groups (P ≤ 0.03), while isolates from patients ≥ 80 years were more likely to exhibit resistance to levofloxacin and clindamycin (P ≤ 0.046).
Conclusions
Several antimicrobial agents continue to demonstrate in vitro efficacy against clinical isolates of S. aureus (including MRSA) throughout Wisconsin, including three agents with recently-published susceptibility testing guidelines. However, continued surveillance efforts may be necessary in the Lake Winnebago, Southeast, and Southcentral regions to further assess higher rates of resistance to a number of antimicrobial agents.
Keywords: Staphylococcus aureus, Antimicrobial resistance, Surveillance
According to the World Health Organization, significant increases in antimicrobial resistance have occurred in recent years.1 In addition to agents of healthcare-associated infection such as extended-spectrum β-lactamase-producing Enterobacterales, carbapenemase-producing organisms, and vancomycin-resistant enterococci,2–4 emerging agents such as Candida auris5 and antibiotic-resistant Neisseria gonorrhoeae6 are impacting clinical management of patients and economics of healthcare.7 This has resulted in a need for novel antimicrobial agents and new means of treatment for disease states involving these resistant strains.8 However, antimicrobial research and development efforts may not come to fruition due to cost considerations or discovery of unexpected toxicity profiles. As a result, development efforts have largely turned to antimicrobial compounds already in use, with the possibility of using them in new combinations (often paired with β-lactamase inhibitor compounds) to enhance their efficacy. Due to the paucity of novel antimicrobial agents, antimicrobial resistance surveillance can be a means of assessing agents that are currently utilized.9 It is important to study these strains of increasingly-resistant bacteria due to potentially novel resistance mechanisms and the potential of developing resistance to alternative therapeutic options.10
Staphylococcus aureus is a common human pathogen, with increased antimicrobial resistance complicating therapeutic measures in recent years. In 2014, portions of Southeast Asia, the Western Pacific, and other regions of the world reported greater than 80% of S. aureus infections having a methicillin-resistant S. aureus (MRSA) phenotype.11 In the United States, MRSA was deemed a serious antibiotic resistance threat by the Centers for Disease Control and Prevention in a 2013 report.12 Studies have demonstrated that MRSA isolation rates have declined in recent years, yet additional adjunctive surveillance and prevention may be necessary to further decrease the spread of this pathogen. Between 2005–2008, Diekema, et al13 documented international MRSA rates of 44.2%. By 2016, this rate declined to 39.0%. A study by Sader, et al14 reported a shift in MRSA rates from 50.0% to 42.2% between 2010 and 2016 in United States hospitals. Landrum, et al15 reported decreased trending between 2005 and 2010 for community-onset MRSA bacteremia, hospital-onset MRSA bacteremia, and community-onset skin and soft tissue infection due to MRSA among United States military personnel. By monitoring antibiotic resistance on a local level, healthcare professionals can make informed decisions regarding antimicrobial therapy and infection prevention.16–18
In 2014, the Wisconsin Clinical Laboratory Network (WCLN) undertook an effort to monitor statewide antimicrobial resistance patterns.19 This means of monitoring involved a compilation of antibiograms submitted on a voluntary basis. Two years later,16,17 the Surveillance of Wisconsin Organisms for Trends in Antimicrobial Resistance and Epidemiology (SWOTARE) program initiated an improved understanding of frank and emerging resistance by both determining percentage susceptibility data and calculating minimum inhibitory concentration (MIC) frequency distributions by Wisconsin region.
The purpose of this SWOTARE program investigation was to establish a Wisconsin S. aureus antibiogram and to elucidate potential geographic and demographic factors associated with antimicrobial resistance. Clinically-significant S. aureus isolates collected throughout the state were tested in a central laboratory using a standardized method. The presented antibiogram and associated ancillary data can serve as a baseline for future monitoring and surveillance of S. aureus antimicrobial resistance patterns throughout the state of Wisconsin.
Materials and Methods
Region Demarcation
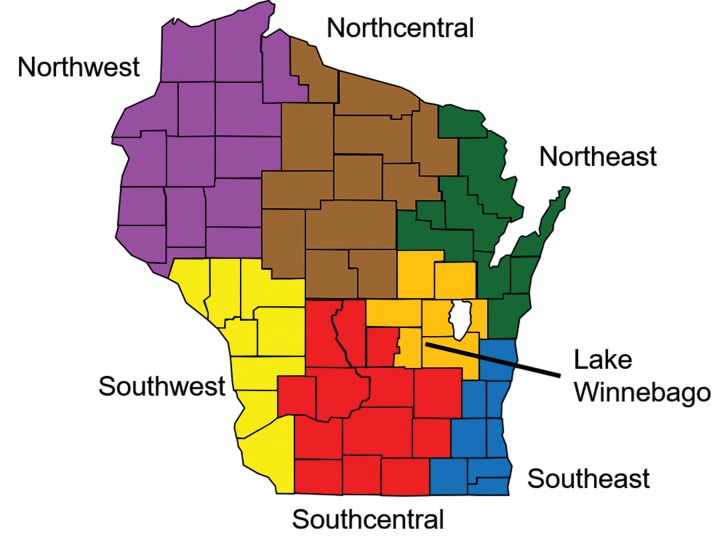
The seven bioterrorism preparedness regions of the WCLN, as originally defined in 2001, served as the basis for geographic comparison within this study. Population density for each region was determined by querying 2010 United States Census population data for each county within a region (Figure 1), with that sum divided by the aggregate land area of counties located in that region (https://legis.wisconsin.gov/lrb/blue-book/).
Figure 1.
Distribution of seven Wisconsin geographic regions defined by the SWOTARE program, 2018.
Study Site Recruitment
Three study sites were chosen from each region for provision of bacterial isolates. To prevent potential bias from larger population centers, two hospital microbiology laboratories from more rural areas and one from an area of higher population within the region were selected. Study locations included microbiology laboratories in Eau Claire, Spooner/Ashland, and St. Croix Falls/Amery (Northwest region); Stevens Point, Marshfield, and Weston (Northcentral region); Green Bay (two locations) and Manitowoc (Northeast region); La Crosse, Platteville/Prairie du Chien, and Viroqua (Southwest region); Madison, Janesville/Monroe, and Fort Atkinson (Southcentral region); Appleton, Neenah, and Fond du Lac (Lake Winnebago region); and, Milwaukee, West Allis, and West Bend (Southeast region).
Selection of Isolates
Study sites were requested to forward 14–15 clinically-significant isolates of S. aureus to a centralized testing laboratory. Isolates were collected in consecutive (non-duplicate) fashion regardless of cefoxitin susceptibility result. This instruction attempted to prevent a bias toward MRSA within the collection. Any duplicate or nonviable isolates were excluded from the study. In addition, study sites were asked to provide limited demographic information related to age, gender, specimen source of isolate, and location of healthcare encounter. Access to protected health information for the purpose of surveillance was granted by the Marquette University Institutional Review Board. Because of the lack of direct involvement in the collection of specimens and because of the utilization of de-identified isolates from routine clinical care, the SWOTARE program was not considered to be actively engaged in human research subjects’ research by the Marquette University Institutional Review Board.
Test Performance
Broth microdilution antimicrobial susceptibility testing was performed and interpreted using standards published by the Clinical and Laboratory Standards Institute (CLSI).20,21 Each isolate was tested against the antimicrobials listed in Table 1 in serial two-fold concentration dilutions that extended beyond CLSI breakpoints for susceptibility and resistance (when appropriate). Identification of MRSA phenotype occurred via cefoxitin susceptibility testing20,22 on the basis of increased induction of mecA activity. Isolates with a penicillin MIC of ≤ 0.12 μg/mL were subjected to nitrocefin-based β-lactamase testing, with follow-up zone edge testing for β-lactamase-negative isolates.20 Isolates demonstrating a phenotype of erythromycin resistance (or intermediate resistance) and clindamycin susceptibility were subjected to inducible clindamycin resistance testing.20,23,24 Cefoxitin and vancomycin susceptibility testing results were determined following a full 24-hour incubation.
Table 1.
Wisconsin Staphylococcus aureus antibiogram with delineation into methicillin-susceptible S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA), 2018
| Percentage Susceptible | ||||
|---|---|---|---|---|
|
| ||||
| Antimicrobial Agent | MSSA (n=194) | MRSA (n=115) | Total (n=309) | |
| Beta-lactams | FOX | 100 | 0.0 | 62.8 |
| PEN | 17.0 | 0.0 | 11.0 | |
| TAR | 100 | 99.1 | 99.7 | |
|
| ||||
| Macrolide | ERY | 67.0 | 16.5 | 48.2 |
|
| ||||
| Lincosamide | CLI | 81.4 | 63.5 | 74.8 |
|
| ||||
| Aminoglycoside | GEN | 98.5 | 100 | 99.0 |
|
| ||||
| Fluoroquinolone | LEV | 88.7 | 49.6 | 74.1 |
|
| ||||
| Glyco/lipopeptides | VAN | 100 | 100 | 100 |
| DAP | 100 | 99.1 | 99.7 | |
| TEL | 100 | 99.1 | 99.7 | |
| DAL | 100 | 100 | 100 | |
|
| ||||
| Tetracyclines | TET | 93.8 | 93.0 | 93.5 |
| DOX | 95.9 | 95.7 | 95.8 | |
|
| ||||
| Others | LZD | 100 | 100 | 100 |
| T/S | 99.5 | 97.4 | 98.7 | |
Abbreviations: FOX, cefoxitin; PEN, penicillin; TAR, ceftaroline; ERY, erythromycin; CLI, clindamycin; GEN, gentamicin; LEV, levofloxacin; VAN, vancomycin; DAP, daptomycin; TEL, telavancin; DAL, dalbavancin; TET, tetracycline; DOX, doxycycline; LZD, linezolid; T/S, trimethoprim-sulfamethoxazole
Data Analysis
Percentage susceptible, intermediate, and resistant values, as well as median MIC (MIC50) and 90th percentile MIC (MIC90) determinations were made on a statewide or geographic basis. To characterize geographic variation, the statewide mean susceptibility percentage for a given organism/antimicrobial combination established a baseline value. An interval of 5% on either side of that mean represented normal distribution. Region-specific values ≥ 5% less than the state mean indicated areas with increased resistance. Region-specific values ≥ 5% greater than the state mean indicated less resistance potential. The significance test of proportions determined if differences in susceptibility percentage among epidemiologic comparisons were significant. The alpha level was set at 0.05 before the investigations commenced, and all P values are two-tailed.
Results and Discussion
Patient Demographics, Specimen Source, and Patient Location
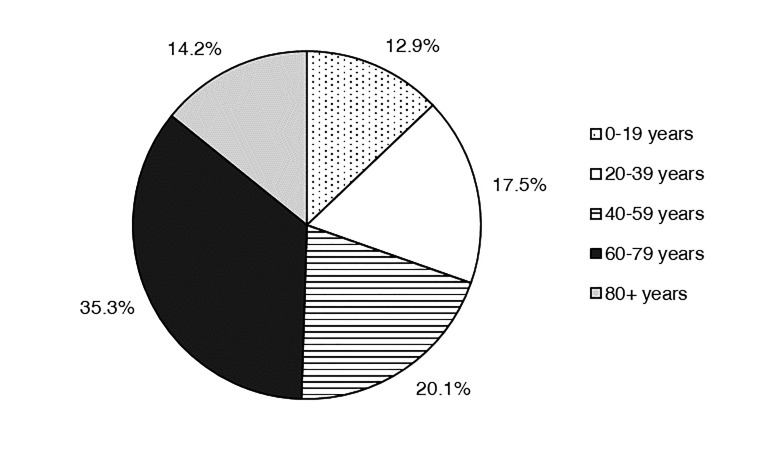
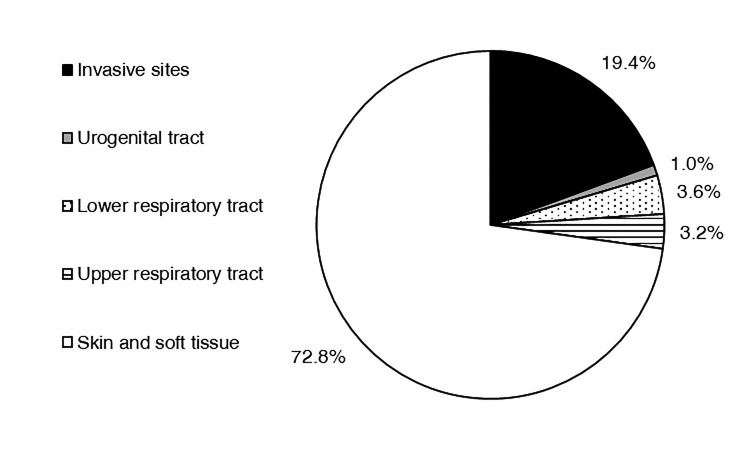
In 2018, 309 isolates of S. aureus were submitted to the surveillance program. Of this total, 155 (50.2%) were isolated from males. The highest percentage of isolates was derived from patients between the ages of 60–79 years (35.3%; Figure 2). Other age groups contributed between 12.9% and 20.1% of isolates. Most isolates emanated from inpatient care (42.4%) or outpatient (40.1%) encounters. The mean percentage of isolates emanating from inpatient collections as a function of individual study site was 39.9 ± 5.82% (median 33.3%). Only 54 isolates (17.5%) resulted from emergency department visits. Of the 309 isolates, 72.8% came from a skin and soft tissue source (178 isolates from wounds, 26 from abscesses, 21 from tissue; Figure 3). Invasive sources comprised 19.4% of the total isolates (50 from blood, 9 from aspirates, 1 from bone), and 3.6% were derived from the lower respiratory tract (8 from sputum, 3 from semi-invasive respiratory procedures).
Figure 2.
Distribution of Wisconsin Staphylococcus aureus surveillance isolates on the basis of patient age, 2018.
Figure 3.
Distribution of Wisconsin Staphylococcus aureus surveillance isolates on the basis of specimen source, 2018.
S. aureus Antibiogram
A S. aureus antibiogram for the state of Wisconsin is presented in Table 1. Across the state, gentamicin and trimethoprim-sulfamethoxazole continue to maintain potency against both MRSA and methicillin-susceptible S. aureus (MSSA) in vitro, with ≥ 98.7% susceptibility (95% confidence interval [CI] ≥ 97.4, 100). Tetracyclines demonstrated slightly less potency against MSSA and MRSA. Other Gram-positive agents such as vancomycin and linezolid demonstrated 100% in vitro susceptibility across Wisconsin. The vancomycin MIC90 value for Wisconsin S. aureus isolates was 1 μg/mL, which represented at least two 2-fold dilutions below the CLSI intermediate interpretive range of 4–8 μg/mL.20
With respect to doxycycline, tetracycline, and trimethoprim-sulfamethoxazole, 0.2% to 2.1% differences in percentage susceptible values for MSSA and MRSA isolates were observed statewide. However, a distinct difference between MSSA and MRSA phenotypes was observed in terms of erythromycin, levofloxacin, and clindamycin susceptibility. Susceptibility percentages decreased by 50.5% (erythromycin), 39.1% (levofloxacin), and 17.9% (clindamycin) upon phenotypic classification as MRSA (Table 1). Similar increases in macrolide and fluoroquinolone resistance rates within a MRSA phenotype were reported from a recent ocular pathogen surveillance effort in the United States.25 In clinical practice, utilization of levofloxacin for staphylococcal infections is often limited to specific entities and would be used in combination with rifampin.26
Geographic Variation in Wisconsin S. aureus Resistance
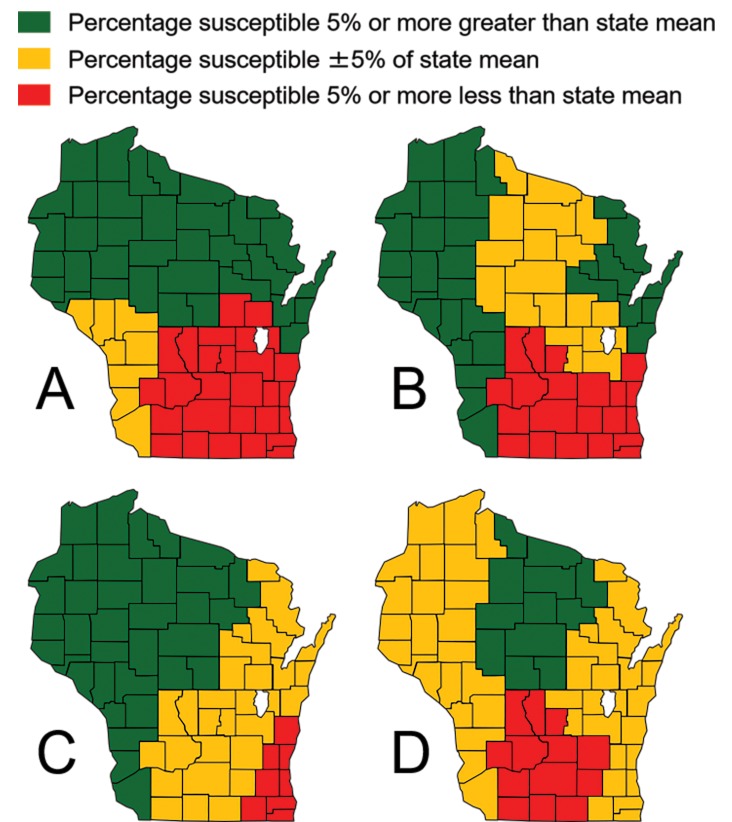
Outside of penicillin, the only agents S. aureus isolates throughout Wisconsin demonstrate appreciable in vitro resistance to include clindamycin (74.8% susceptibility; CI 70.0, 79.6), levofloxacin (74.1% susceptibility; CI 69.2, 79.0), and erythromycin (48.2% susceptibility; CI 42.6, 53.8) (Table 1). The identification of clindamycin-resistant isolates was augmented by a positive inducible clindamycin resistance result in 31.4% of 118 statewide isolates eligible for testing. Of Wisconsin isolates tested, 37.2% (CI 31.8, 42.6) were phenotypically classified as MRSA, with the highest rates in the Lake Winnebago (50.0%; CI 35.2, 64.8), Southeast (46.5%; CI 31.6, 61.4), and Southcentral (45.5%; CI 30.8, 60.2) regions (Figure 4A). Remaining agents exhibited strong in vitro efficacy in the context of both MSSA and MRSA.
Figure 4.
Wisconsin geographic differences in Staphylococcus aureus susceptibility to (A) cefoxitin, (B) levofloxacin, (C) erythromycin, and (D) clindamycin, 2018.
Emerging doxycycline resistance may be evident among S. aureus isolates in Southwest Wisconsin. While the percentage of isolates susceptible to this agent in this region exhibited only a 4.7% deviation from the state mean (Table 2), a distinct difference in MIC90 value was observed. The Southwest region MIC90 value of 4 μg/mL was at least three 2-fold dilution values higher than the state MIC90 value. This is important, as a doxycycline MIC of 4 μg/mL is at the upper limit of the CLSI susceptible interpretive category.20 Analysis of MIC frequency distributions (in addition to susceptibility percentages) can be helpful in predicting future trends in resistance patterns, particularly when these MIC values still lie within a CLSI susceptible interpretive category.
Table 2.
MIC50, MIC90, and percentage susceptibility (%S) of Wisconsin Staphylococcus aureus isolates to doxycycline, stratified by geographic region, 2018
| Northwest | Northcentral | Northeast | Southwest | Southcentral | Lake Winnebago | Southeast | Wisconsin | |
|---|---|---|---|---|---|---|---|---|
| n | 45 | 43 | 45 | 45 | 44 | 44 | 43 | 309 |
| MIC50 | ≤ 0.5 | ≤ 0.5 | ≤ 0.5 | ≤ 0.5 | ≤ 0.5 | ≤ 0.5 | ≤ 0.5 | ≤ 0.5 |
| MIC90 | ≤ 0.5 | ≤ 0.5 | ≤ 0.5 | 4 | ≤ 0.5 | ≤ 0.5 | ≤ 0.5 | ≤ 0.5 |
| %S | 95.6 | 95.3 | 100 | 91.1 | 95.5 | 100 | 93 | 95.8 |
Past data from the United States27 introduced the incidence of doxycycline resistance in MRSA phenotypes that exhibited decreased susceptibility to oxacillin, erythromycin, and levofloxacin. Within this phenotype, which represented 31% of MRSA isolates surveyed, 4.2% of isolates were resistant to doxycycline. Jones, et al28 utilized the doxycycline susceptible breakpoint of 4 μg/mL to report 96.2% and 99.2% susceptibility rates for worldwide MRSA and MSSA isolates, respectively. These values were 5% greater than corresponding tetracycline susceptibility rates. MIC frequency distribution analysis of Wisconsin data regarding another alternative S. aureus therapeutic agent, trimethoprim-sulfamethoxazole, did not reveal potential emerging trends of resistance (data not illustrated). Additional investigation into this doxycycline/S. aureus paradigm from the Southwest region may be warranted to further elucidate this potential emerging resistance profile.
In addition to an increased frequency of MRSA, S. aureus isolates derived from the Southeast, Southcentral, and Lake Winnebago regions exhibited increased resistance to levofloxacin (Figure 4B), erythromycin (Figure 4C), and clindamycin (Figure 4D) when compared to other Wisconsin regions. In these regions, increased surveillance efforts through the SWOTARE program and concomitant adjustments to antimicrobial stewardship programs have the potential to impact future resistance profiles. Investigations are in progress to assess provider prescribing patterns within these regions of Wisconsin and to determine any correlation with known resistance patterns.
Hicks, et al29 reported significant antibiotic prescription burden within outpatient settings in the United States, with over 260 million courses being prescribed by clinicians in 2011. Agents within seven antimicrobial classes (including penicillins, macrolides, fluoroquinolones, and tetracyclines) accounted for 94% of total outpatient prescriptions. Antimicrobial resistance surveillance is particularly necessary in the Midwest region of the United States, as an average of 897 outpatient prescriptions per 1000 persons was issued in this 12-state region in 2011, second only to the southern United States (931 prescriptions per 1000 persons).29 Analyzing local patterns of resistance by geographic region, patient population, and practitioner specialty could provide insight into antimicrobial resistance beyond that relative to inpatient populations or healthcare-associated infections. The SWOTARE program, with its inherent design allowing for participation by rural healthcare facilities, can assist in future analyses.
In this study, three agents with recently established CLSI guidelines20,30,31 were tested against each S. aureus isolate to contribute to the baseline antibiogram (Table 3). Telavancin does not currently possess a resistant interpretive breakpoint;20 however, one isolate was found to be non-susceptible to this agent (surgical tissue isolate from Southcentral region, MIC of 0.25 μg/mL). MIC50 and MIC90 values were consistent throughout Wisconsin for dalbavancin (both 0.06 μg/mL). S. aureus susceptibility to telavancin and ceftaroline demonstrated some variation with respect to MIC values; however, none exceeded the CLSI susceptible breakpoint. Telavancin MIC50 and MIC90 values were calculated at either 0.06 μg/mL or 0.12 μg/mL on a geographic basis. Ceftaroline MIC50 and MIC90 values ranged from ≤ 0.12 μg/mL to 0.5 μg/mL across the state; > 95% of all isolates tested susceptible to these three agents in each region. One daptomycin non-susceptible isolate (MIC of 2 μg/mL) was identified from the Southwest region of the state.
Table 3.
MIC50, MIC90, and percent susceptibility (%S) of Wisconsin Staphylococcus aureus isolates to telavancin (TEL), dalbavancin (DAL), and ceftaroline (TAR), stratified by geographic region, 2018
| TEL | Northwest | Northcentral | Northeast | Southwest | Southcentral | Lake Winnebago | Southeast | Wisconsin |
|---|---|---|---|---|---|---|---|---|
| n | 45 | 43 | 45 | 45 | 44 | 44 | 43 | 309 |
| MIC50 | 0.06 | 0.12 | 0.06 | 0.06 | 0.06 | 0.06 | 0.12 | 0.06 |
| MIC90 | 0.12 | 0.12 | 0.12 | 0.06 | 0.12 | 0.12 | 0.12 | 0.12 |
| %S | 100 | 100 | 100 | 100 | 97.7 | 100 | 100 | 99.7 |
|
| ||||||||
| DAL | Northwest | Northcentral | Northeast | Southwest | Southcentral | Lake Winnebago | Southeast | Wisconsin |
| n | 45 | 43 | 45 | 45 | 44 | 44 | 43 | 309 |
| MIC50 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 |
| MIC90 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 |
| %S | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
|
| ||||||||
| TAR | Northwest | Northcentral | Northeast | Southwest | Southcentral | Lake Winnebago | Southeast | Wisconsin |
| n | 45 | 43 | 45 | 45 | 44 | 44 | 43 | 309 |
| MIC50 | 0.25 ≤ | 0.12 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| MIC90 | 0.5 | 0.25 | 0.25 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| %S | 100 | 100 | 100 | 100 | 97.7 | 100 | 100 | 99.7 |
Demographic Variation S. aureus Resistance in Wisconsin
In addition to geographic variability associated with MRSA in Wisconsin, we also observed differences as a function of patient age. Patients aged 20–39 years generated the highest proportion of S. aureus isolates with a MRSA phenotype (57.4%; Table 4), while MRSA rates in patients aged 0–19 years, 40–59 years, and 60–79 years approximated 30% (P ≤ 0.03). These findings extend those derived from a United States tertiary care center in which MRSA was detected more frequently in patients under the age of 24 when compared to other age groups.32 These authors hypothesized that increased risk of MRSA among younger individuals may be related to increased participation in what was termed risky activity (such as team sports). One past study33 reported the median age of community-acquired MRSA infection to be significantly less than that in cases of healthcare-associated MRSA infection. Our findings contrast a European study that reported an increased proportion of MRSA in elderly patients when compared to younger patients.34 Further investigation may be warranted in subsequent SWOTARE collections to elucidate a role for patient age in terms of risk for MRSA infection in Wisconsin.
Table 4.
Comparative Wisconsin Staphylococcus aureus resistance to cefoxitin (FOX), levofloxacin (LEV), erythromycin (ERY), clindamycin (CLI), doxycycline (DOX), and trimethoprim-sulfamethoxazole (T/S), stratified by age group, 2018
| Percentage Resistant to Antimicrobial Agents | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Age group | FOX | LEV | ERY | CLI | DOXa | T/S |
| 0–19 | 35.0 | 15.0 | 55.0 | 20.0 | 2.5 | 0.0 |
| 20–39 | 57.4b | 24.1 | 51.9 | 16.7 | 1.9 | 1.9 |
| 40–59 | 30.7 | 21.0 | 43.6 | 21.0 | 4.8 | 1.6 |
| 60–79 | 30.3 | 28.4 | 42.7 | 28.4 | 5.5 | 1.8 |
| 80+ | 40.9 | 38.6c | 56.8 | 36.3d | 4.6 | 0.0 |
Percentages reflect doxycycline intermediate resistance (MIC 8 μg/mL); isolates with MIC ≥ 16 μg/mL were not observed.
P ≤ 0.03 vs. 0–19 years, 40–59 years, 60–79 years
P ≤ 0.046 vs 40–59 years, 0–19 years
P = 0.026 vs. 20–39 years
Other differences were noted with levofloxacin and clindamycin. An increased proportion of levofloxacin-resistant S. aureus from patients aged 80 or older was observed compared to patients aged 0–19 or 40–59 years (P ≤ 0.046; Table 4). This elderly population also exhibited an increased percentage of S. aureus resistant to clindamycin (36.3%) when compared to 20–39 year olds (16.7%, P = 0.026). Moreover, when compared to the 40–59 year-old population, resistance rates trended higher in the 80+ years group (P = 0.08). These data extend findings from David, et al35 who demonstrated increased S. aureus resistance to clindamycin in elderly patients when compared to pediatric patients. Moreover, findings from pediatric patients treated in United States military facilities from 2005–2014 documented annual clindamycin resistance rates between 9.3% and 14.4%.36 However, recent pediatric data from the Baltimore, Maryland area collected over a 12-year interval reported an increase in clindamycin resistance rate from 21% to 38% in MRSA clinical isolates and from 5% to 40% in MSSA isolates.37 Sutter et al.36 also noted significant decreases in MSSA susceptibility to clindamycin over time when compared to pediatric MRSA isolates. As such, revisiting these demographic studies in subsequent Wisconsin surveillance efforts may be warranted. In the context of additional alternative staphylococcal regimens, trimethoprim-sulfamethoxazole resistance rates and distributions of doxycycline intermediate MIC values did not demonstrate variation across age groupings (P ≥ 0.36 and P ≥ 0.28, respectively; Table 4).
No significant differences were noted with respect to cefoxitin, levofloxacin, erythromycin, and clindamycin resistance rates in isolates of S. aureus when stratified by inpatient, outpatient, or emergency department encounter. Resistance rates ranged from 32.8% to 41.0% between the three facility groupings for cefoxitin (P ≥ 0.18), 21.4% to 30.3% for levofloxacin (P ≥ 0.10), 46.7% to 50.0% for erythromycin (P ≥ 0.68), and 17.9% to 27.9% for clindamycin (P ≥ 0.15). These data further suggest that potential outpatient bias factored little into observations made in the study.
Limitations
Limitations to the desired two rural laboratory/one larger-population center laboratory approach to study site recruitment included regions with fewer hospital microbiology laboratories and regions with increased population density. The Southeast, Northeast, and Lake Winnebago regions were represented by fewer than two rural laboratories. The Northeast region of Wisconsin has experienced a movement toward hospital microbiology laboratory consolidation over the past decade. With respect to the Lake Winnebago and Southeast regions, population density figures of 151.4 and 670.8 inhabitants/square mile were calculated, respectively. In contrast, three of the Wisconsin regions exhibited population density data of <50 inhabitants/square mile. As further evidence of measures taken to ensure a representative sampling that was not biased toward heavily urban centers during this 2018 surveillance effort, 38% of participating hospital microbiology laboratories were derived from municipalities with populations < 15,000 (per 2010 United States census data), with an aggregate 58% of participants derived from municipalities with populations < 35,000.
A second limitation is related to potential S. aureus clonality contributing to noted geographic variation. We were unable to investigate this possibility during this initial surveillance effort. As mentioned previously, approximately one-half of isolates were derived from hospital laboratories in rural areas of the state; these laboratories do not have the capacity to perform molecular epidemiologic studies. In addition, the limited demographic information requested of the study sites did not inquire about potential mini-outbreak status of isolates. Moreover, limited demographic information (as allowed by Institutional Review Board) did not allow for provision of patient identifiers, as such, retrospective investigation of potential mini-outbreak status could not be facilitated.
Conclusion
While limited to an extent by numbers of isolates collected in the initial year of SWOTARE S. aureus surveillance, presented data identified both geographic and demographic-based differences in antimicrobial resistance among Wisconsin S. aureus isolates. By carefully analyzing regional data, trends can be realized that are not apparent at a national level. In addition, regions of the state that warrant increased vigilance and intervention can be identified in efforts to mitigate antimicrobial resistance. Continued future surveillance efforts may reveal additional trends and contribute to stewardship and intervention opportunities at the local level.
Acknowledgments
The authors are grateful to the following individuals for collection of isolates throughout 2018:
Sonja Alt, Monroe
Jorn Bansberg, Viroqua
Sherry Barta, Green Bay
Eric Beck, PhD, West Allis
Tim Block, West Bend
Becky Brooks, Stevens Point
Tracy Felland, Janesville
Thomas Fritsche, MD, PhD, Marshfield
Ben Kaetterhenry, Appleton
Debra Kieler, Platteville
Timothy Kramme, Milwaukee
Joshua Kropp, Weston
Kathy Lang, Ashland
Debbie Maedke, Manitowoc
Brooke Olson, Marshfield
Mattie Pitts, Spooner
Ray Podzorski, PhD, Madison
Lori Reed, Amery
Andrea Roder, Green Bay
Karen Siebers, Neenah
Brian Simmons, Prairie du Chien
Mary A. Smith, St. Croix Falls
Frances Spray-Larson, PhD, Fort Atkinson
Janelle Stearns, Eau Claire
Sarah Stoner, La Crosse
Ellen Wirtz, Fond du Lac
Footnotes
Financial Support: The research received support from the Wisconsin Department of Health Services, Division of Public Health (ELC Project K2: HAI Coordinated Prevention and Stewardship).
References
- 1.World Health Organization. Antibiotic Resistance. 2018. Available at: www.who.int/news-room/fact-sheets/detail/antibiotic-resistance Accessed April 18, 2019.
- 2.Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol. 2013;34(1):1–14. [DOI] [PubMed] [Google Scholar]
- 3.Magill SS, O’Leary E, Janelle SJ, et al. Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med. 2018;379(18):1732–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomson KS. The immaculate carbapenemase study. J Clin Microbiol. 2017;55(6):1608 1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamoth F, Kontoyiannis DP. The Candida auris alert: facts and perspectives. J Infect Dis. 2018;217(4):516–520. [DOI] [PubMed] [Google Scholar]
- 6.Day MJ, Spiteri G, Jacobsson S, et al. Stably high azithromycin resistance and decreasing ceftriaxone susceptibility in Neisseria gonorrhoeae in 25 European countries, 2016. BMC Infect Dis. 2018;18(1):609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts RR, Hota B, Ahmad I, et al. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis. 2009;49(8):1175–1184. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. WHO publishes list of bacteria for which new antibiotics are urgently needed. 2017. Available at: www.who.int/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-areurgently-needed Accessed March 20, 2019.
- 9.Munson E. Biographical feature: Clyde Thornsberry, Ph.D. J Clin Microbiol. 2016;54(2):250 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest. 2003;111(9):1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Antimicrobial resistance: global report on surveillance. 2014. Available at: https://www.who.int/drugresistance/documents/surveillancereport/en/ Accessed April 25, 2019.
- 12.U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States. 2013. Available at: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf Accessed April 18, 2019.
- 13.Diekema DJ, Pfaller MA, Shortridge D, Zervos M, Jones RN. Twenty-year trends in antimicrobial susceptibilities among Staphylococcus aureus from the SENTRY antimicrobial surveillance program. Open Forum Infect Dis. 2019;6(Suppl 1):S47–S53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sader HS, Mendes RE, Streit JM, Flamm RK. Antimicrobial susceptibility trends among Staphylococcus aureus isolates from U.S. hospitals: results from 7 years of the ceftaroline (AWARE) surveillance program, 2010–2016. Antimicrob Agents Chemother. 2017;61(9):e01043–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landrum ML, Neumann C, Cook C, et al. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005–2010. JAMA. 2012;308(1):50–59. [DOI] [PubMed] [Google Scholar]
- 16.Munson E, Zeman H, Hueppchen E. Surveillance of Wisconsin Organisms for Trends in Antimicrobial Resistance and Epidemiology (SWOTARE): epidemiologic correlates for 2016 surveillance isolates. Gundersen Medical Journal. 2017;10(1):41–48. [Google Scholar]
- 17.Munson E, Hueppchen E, Zeman H. Surveillance of Wisconsin Organisms for Trends in Antimicrobial Resistance and Epidemiology (SWOTARE): introduction to the program and summary of 2016 geographic variation. WMJ. 2018;117(3):116–121. [PubMed] [Google Scholar]
- 18.Zeman H, Martin R, Munson E, Schulte R. Surveillance of Wisconsin Organisms for Trends in Antimicrobial Resistance and Epidemiology (SWOTARE): summary of 2017 findings with focus on changing resistance patterns in selected antimicrobial classes. Gundersen Medical Journal. In press. [Google Scholar]
- 19.Munson E, Block TK, Bowles EJ, et al. Surveillance of Wisconsin antibacterial susceptibility patterns. WMJ. 2016;115(1):29–36. [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing, M100. 28th informational supplement. Wayne, PA: CLSI; 2018. [Google Scholar]
- 21.Clinical and Laboratory Standards Institute (CLSI). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, M07. 10th ed Wayne, PA: CLSI; 2015. [Google Scholar]
- 22.McKinney TK, Sharma VK, Craig WA, Archer GL. Transcription of the gene mediating methicillin resistance in Staphylococcus aureus (mecA) is corepressed but not coinduced by cognate mecA and beta-lactamase regulators. J Bacteriol. 2001;183(23):6862–6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prabhu K, Rao S, Rao V. Inducible clindamycin resistance in Staphylococcus aureus isolated from clinical samples. J Lab Physicians. 2011;3(1):25–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiebelkorn KR, Crawford SA, McElmeel ML, Jorgensen JH. Practical disk diffusion method for detection of inducible clindamycin resistance in Staphylococcus aureus and coagulase-negative staphylococci. J Clin Microbiol. 2003;41(10):4740–4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asbell PA, Sanfilippo CM, Pillar CM, DeCory HH, Sahm DF, Morris TW. Antibiotic resistance among ocular pathogens in the United States: five-year results from the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) surveillance study. JAMA Ophthalmol. 2015;133(12):1445–1454. [DOI] [PubMed] [Google Scholar]
- 26.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–e55. [DOI] [PubMed] [Google Scholar]
- 27.McDougal LK, Fosheim GE, Nicholson A, et al. Emergence of resistance among USA300 methicillin-resistant Staphylococcus aureus isolates causing invasive disease in the United States. Antimicrob Agents Chemother. 2010;54(9):3804–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones RN, Stilwell MG, Wilson ML, Mendes RE. Contemporary tetracycline susceptibility testing: doxycycline MIC methods and interpretive criteria (CLSI and EUCAST) performance when testing Gram-positive pathogens. Diagn Microbiol Infect Dis. 2013;76(1):69–72. [DOI] [PubMed] [Google Scholar]
- 29.Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60(9):1308–1316. [DOI] [PubMed] [Google Scholar]
- 30.Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing, M100. 23rd informational supplement. Wayne, PA: CLSI; 2013. [Google Scholar]
- 31.Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing, M100. 26th informational supplement. Wayne, PA: CLSI; 2016. [Google Scholar]
- 32.Jacobus CH, Lindsell CJ, Leach SD, Fermann GJ, Kressel AB, Rue LE. Prevalence and demographics of methicillin resistant Staphylococcus aureus in culturable skin and soft tissue infections in an urban emergency department. BMC Emerg Med. 2007;7(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290(22):29762984. [DOI] [PubMed] [Google Scholar]
- 34.Pomorska-Wesołowska M, Różańska A, Natkaniec J, et al. Longevity and gender as the risk factors of methicillin-resistant Staphylococcus aureus infections in southern Poland. BMC Geriatr. 2017;17(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.David MZ, Crawford SE, Boyle-Vavra S, Hostetler MA, Kim DC, Daum RS. Contrasting pediatric and adult methicillin-resistant Staphylococcus aureus isolates. Emerg Infect Dis. 2006;12(4):631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sutter DE, Milburn E, Chukwuma U, Dzialowy N, Maranich AM, Hospenthal DR. Changing susceptibility of Staphylococcus aureus in a US pediatric population. Pediatrics. 2016;137(4):e20153099. [DOI] [PubMed] [Google Scholar]
- 37.Khamash DF, Voskertchian A, Tamma PD, Akinboyo IC, Carroll KC, Milstone AM. Increasing clindamycin and trimethoprim-sulfamethoxazole resistance in pediatric Staphylococcus aureus infections. J Pediatric Infect Dis Soc. 2019;8(4):351–353. [DOI] [PubMed] [Google Scholar]