Abstract
Objectives
This study investigated the sex differences in the associations between ideal cardiovascular health (CVH), measured by the American Heart Association’s Life’s Simple 7 metrics, and cardiovascular disease (CVD)-related biomarkers among an ethnically diverse cohort of women and men free of clinical CVD at baseline.
Setting
We analysed data from the Multi-Ethnic Study of Atherosclerosis conducted in six centres across the USA (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles, California; New York, New York; and St Paul, Minnesota).
Participants
This is a cross-sectional study of 5379 women and men, aged 45–84 years old. Mean age (SD) was 62 (10), 52% were women, 38% White, 11% Chinese American, 28% Black and 23% Hispanic.
Primary measures
The seven metrics (smoking, body mass index, physical activity, diet, total cholesterol, blood pressure and blood glucose) were each scored as 0 points (poor), 1 point (intermediate) or 2 points (ideal). The total CVH score ranged from 0 to 14. The CVD-related biomarkers studied were high-sensitivity C-reactive protein, D-dimer, fibrinogen, homocysteine, high-sensitivity cardiac troponin T, N-terminal pro B-type natriuretic peptide (NT-proBNP) and interleukin 6. We examined the association between the CVH score and each biomarker using multivariable linear regression, adjusting for age, race/ethnicity, education, income and health insurance status.
Results
Higher CVH scores were associated with lower concentrations of all biomarkers, except for NT-proBNP where we found a direct association. There were statistically significant interactions by sex for all biomarkers (p<0.001), but results were qualitatively similar between women and men.
Conclusion
A more favourable CVH score was associated with lower levels of multiple CVD-related biomarkers for women and men, except for NT-proBNP. These data suggest that promotion of ideal CVH would have similarly favourable impact on the reduction of biomarkers of CVD risk for both women and men.
Keywords: biomarkers, cardiovascular disease, ideal cardiovascular health metrics, life’s simple 7, sex, gender
Strengths and limitations of this study.
Use of a large and diverse study sample that enabled stratification by sex, race/ethnicity and age.
Use of validated survey instruments and standardised methods for data collection allowed for comparison with other studies.
Study population included adults between the ages of 45 and 84 years, which limits the generalisability of our findings to younger or older age groups.
Cross-sectional design cannot establish temporality or causation.
Introduction
The ideal cardiovascular health (CVH) construct, defined as meeting specific criteria for seven health behaviours and health factors called the Life’s Simple 7 (LS7) metrics, was introduced by the American Heart Association (AHA) to decrease the burden of cardiovascular disease (CVD).1 This was a shift towards primordial prevention—focusing on wellness rather than disease.2 Biomarkers, which are often used in conjunction with traditional risk factors, are subclinical indicators of physiological and pathological processes3 and serve as useful tools in facilitating early detection and prognostication of CVD.4 Although prior studies have examined the relationship of biomarkers with incident CVD, few have focused on biomarkers and measures of cardiovascular wellness. Not surprisingly, the studies that have examined the association between ideal CVH and subclinical biomarkers of disease have shown an inverse relationship.5 For example, in a prior analysis from the Multi-Ethnic Study of Atherosclerosis (MESA), poor CVH was found to be associated with higher levels of GlycA (a novel inflammatory marker) and higher levels of traditional inflammatory markers [high-sensitivity C-reactive protein (hsCRP), interleukin 6 (IL-6), fibrinogen and D-dimer].6 However, research on the sex differences of the relationship of CVH with CVD-related biomarkers is sparse.7
Women are known to have higher levels of hsCRP8 9 and N-terminal pro B-type natriuretic peptide (NT-proBNP)10 than men even after accounting for cardiometabolic risk factors, while troponin levels are higher in men than women.11 12 Thus, understanding sex differences in the relationship of ideal CVH measures with biomarkers is an important intermediate step in explaining sex differences in clinical CVD.
This study aimed to examine the sex differences in the associations between ideal CVH and CVD-related biomarkers among women and men free of clinical CVD in an ethnically diverse cohort. We hypothesised that better CVH would be associated with lower concentrations of CVD-related biomarkers especially in women.
Methods
Study population
As previously described, MESA is a longitudinal study of 6814 adult women and men between the ages of 45 and 84 years. The study participants, with no previous history of clinical CVD at baseline, were recruited from six centres (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles, California; New York, New York; and St Paul, Minnesota) in the USA between July 2000 and August 2002.13 Among participants, 38% were White, 11% Chinese American, 28% Black and the remaining 23% were Hispanic. Informed consent was provided by all participants. The MESA protocol was approved by the institutional review boards (IRB) of all the recruitment centres. At the Johns Hopkins field centre where this analysis was conducted, the IRB approval number was NA_00030361.
Baseline information was collected using standardised questionnaires, physical examinations and fasting laboratory blood draw. For the current analyses, we included 5379 participants from the MESA baseline examination after excluding participants with missing information for the CVD biomarkers and LS7 metrics.
Assessment of biomarkers
We examined biomarkers that were measured at baseline. Fasting blood samples were drawn, processed and stored using standardised procedures.14 HsCRP, D-dimer, fibrinogen, IL-6 and homocysteine levels were analysed at the laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, Vermont, USA). Serum levels of hsCRP (mg/L) were measured using the BNII nephelometer (Dade Behring, Deerfield, Illinois, USA). Analytical intra-assay coefficients of variation (CV) of hsCRP ranged from 2.3% to 4.4%, and inter-assay CV ranged from 2.1% to 5.7%.6 15 D-dimer (μg/mL) was measured with an immunoturbidimetric assay (Liatest D-DI; Diagnostica Stago, Parsippany, New Jersey, USA) which was used on a Sta-R analyser (Diagnostica Stago, Parsippany, New Jersey, USA). The lower detection limit of D-dimer assay was 0.01 µg/mL.6 Serum fibrinogen (mg/dL) was measured by immunoprecipitation of fibrinogen antigen using the BNII nephelometer (N Antiserum to Human Fibrinogen; Dade Behring, Deerfield, Illinois, USA). The intra-assay and inter-assay CV were 2.7% and 2.6%, respectively.6 15 Serum IL‐6 (pg/mL) was measured by ultrasensitive ELISA (Quantikine HS Human IL‐6 Immunoassay; R&D Systems, Minneapolis, Minnesota). The analytical CV was 6.3%.15 Plasma homocysteine (μmol/L) was measured using a fluorescence polarisation immunoassay with the IMx analyser (Abbott Diagnostics, Abbott Park, Illinois, USA). The CV was 4.5%.16
The assays for high-sensitivity cardiac troponin T (hs-cTnT, ng/L) and NT-proBNP (pg/mL) were performed at the Veteran’s Affairs San Diego Healthcare System (La Jolla, California, USA) and measured in serum using the Elecsys 2010 system (Roche Diagnostics, Indianapolis, Indiana, USA). For hs-cTnT, the inter-assay CV observed for the MESA cohort measurements were 3.6% at 28 ng/L and 2.0% at 2154 ng/L.17 For NT-proBNP, the intra-assay and inter-assay CV were as follows: at 175 pg/mL, 2.7% and 3.2%; at 355 pg/mL, 2.4% and 2.9%; at 1068 pg/mL, 1.9% and 2.6%; and at 4962 pg/mL, 1.8% and 2.3%, respectively.10
Assessment of cardiovascular health
Cardiovascular health was assessed at baseline using the LS7 metrics based on AHA criteria.1 A detailed assessment can be found in the supplementary material (online Supplementary Methods).
bmjopen-2019-031414supp001.pdf (129.9KB, pdf)
Assessment of covariates
Sociodemographic factors included as covariates are age, sex, race/ethnicity, education, income and health insurance. Age was examined as a continuous variable in the multivariable models but dichotomised as <65 and≥65 years for subgroup testing. Race/ethnicity had four groups: White, Chinese American, Black and Hispanic. We had nine categories for education and 13 categories for income (online supplementary table 1), which were used in our multivariable models; however, they were dichotomised as ≥bachelor’s degree and <bachelor’s degree; ≥$40 000 and < $40 000, respectively, for descriptive statistics. ‘Yes’ or ‘No’ responses were given for health insurance status.
Statistical analyses
The characteristics of the study participants were reported for the overall population and by sex. Categorical variables were presented as frequencies with percentages, and continuous variables were presented as means with SD. We compared the baseline characteristics of participants by sex, using analysis of variance and χ2 tests as appropriate. The CVD-related biomarkers were natural logarithmically transformed for the analyses because distribution was skewed. The LS7 metrics were each defined as ideal, intermediate and poor,1 and their distribution was reported by sex, as shown in online supplementary tables 2,3. Points were awarded to each category of the LS7 metrics, with 0 indicating poor; 1, intermediate; and 2, ideal. The points were summed, yielding a total CVH score ranging from 0 to 14.18 As previously reported, total CVH scores of 0–8, 9–10 and 11–14 were considered as inadequate, average and optimal CVH, respectively.19–21
Using linear regression models, we estimated the crude beta coefficients and corresponding 95% confidence intervals (CIs) for the associations between the CVH score (assessed as a continuous variable) and CVD-related biomarkers (log-transformed, assessed as a continuous variable) in the overall cohort and by sex (model 1). We adjusted for sociodemographic factors [age (continuous), sex (for overall cohort), race/ethnicity (4 categories), education (9 categories), income (13 categories) and health insurance status (yes/no)] in model 2 and reported the adjusted beta coefficients. We examined the interaction of the CVH score categories with sex for all six biomarkers by including interaction terms in model 2.
The associations between the LS7 metrics and CVD-related biomarkers were examined by comparing the intermediate and ideal categories of the metrics to the poor category. We reported only the adjusted model for women and men. In supplementary analyses, we examined the association between the CVH score and CVD-related biomarkers stratified by race/ethnicity and age (<65 and ≥65 years) within each sex, using multivariable linear regression models. For statistical analyses, STATA V.15.0 was used (StataCorp LP, College Station, Texas, USA) and an alpha level of <0.05 (two-sided) was considered statistically significant.
Patient and public involvement
Neither patients nor the public were involved in the conduct of this research. We did not invite patients or the public to comment on the study design nor did we consult them to develop patient-related outcomes or interpret the results of this study. We did not invite patients or the public to contribute to the writing or editing of this document for readability or accuracy.
Results
Baseline characteristics of participants are shown in table 1. Over half of the participants were women (52%), and the mean age (SD) was 62 (10) years. Women had higher hsCRP, D-dimer, fibrinogen, NT-proBNP and IL-6 levels, while men had higher hs-cTnT and homocysteine levels. Women were less likely to be physically active and were more likely to have higher systolic blood pressure as well as higher healthy diet score and total cholesterol levels (table 1 and online supplementary table 3).
Table 1.
Characteristics of study participants
| Total (n=5379) | Women (n=2775) | Men (n=2604) | P value | |
| Age, mean (SD), years | 62 (10) | 62 (10) | 62 (10) | 0.67 |
| Age, years | ||||
| <65, n (%) | 3013 (56) | 1559 (56) | 1454 (56) | 0.80 |
| ≥65, n (%) | 2366 (44) | 1216 (44) | 1150 (44) | |
| Race/Ethnicity | ||||
| White, n (%) | 2150 (40) | 1092 (39) | 1058 (41) | |
| Chinese–American, n (%) | 733 (14) | 372 (13) | 361 (14) | 0.17 |
| Black, n (%) | 1253 (23) | 681 (25) | 572 (22) | |
| Hispanic, n (%) | 1243 (23) | 630 (23) | 613 (24) | |
| Education | ||||
| ≥Bachelor’s degree, n (%) | 1929 (36) | 824 (30) | 1105 (42) | <0.001 |
| <Bachelor’s degree, n (%) | 3450 (64) | 1951 (70) | 1499 (58) | |
| Income | ||||
| ≥$40 000, n (%) | 2648 (49) | 1162 (42) | 1486 (57) | <0.001 |
| <$40 000, n (%) | 2731 (51) | 1613 (58) | 1118 (43) | |
| Health insurance | ||||
| Yes, n (%) | 4871 (91) | 2511 (90) | 2360 (90) | 0.86 |
| No, n (%) | 508 (9) | 264 (10) | 244 (10) | |
| Biomarkers, mean (95% CI) | ||||
| hsCRP (mg/L) | 3.7 (3.5, 3.8) | 4.5 (4.2, 4.7) | 2.8 (2.6, 3.0) | <0.001 |
| D-dimer (μg/mL) | 0.37 (0.34, 0.39) | 0.38 (0.35, 0.41) | 0.35 (0.32, 0.39) | 0.29 |
| Fibrinogen, mg/dL | 345.2 (343.2, 347.1) | 358.0 (355.2,360.8) | 331.5 (328.8, 334.1) | <0.001 |
| Homocysteine (μmol/L) | 9.3 (9.2, 9.4) | 8.7 (8.6, 8.8) | 10.0 (9.9, 10.1) | <0.001 |
| hs-cardiac troponin T (ng/L) | 6.6 (6.4, 6.8) | 5.2 (5.0, 5.4) | 8.1 (7.7, 8.5) | <0.001 |
| NT-proBNP (pg/mL) | 100.8 (94.2, 107.4) | 114.0 (108.1, 119.8) | 86.8 (74.8, 98.9) | <0.001 |
| *IL-6 (pg/mL) | 1.5 (1.5, 1.6) | 1.6 (1.5, 1.6) | 1.5 (1.4, 1.5) | 0.002 |
| LS7 metrics | ||||
| Current smoking, n (%) | 671 (12) | 303 (11) | 368 (14) | <0.001 |
| Body mass index (kg/m2) | 28 (5) | 29 (6) | 28 (4) | <0.001 |
| Physical activity (MET-min/week) | 401 (589) | 338 (490) | 468 (672) | <0.001 |
| Healthy diet score (0–5) | 1.6 (0.9) | 1.7 (0.9) | 1.4 (0.9) | <0.001 |
| Total cholesterol (mg/dL) | 194 (36) | 200 (36) | 189 (35) | <0.001 |
| Systolic blood pressure (mm Hg) | 126 (21) | 127 (23) | 125 (19) | 0.03 |
| Diastolic blood pressure (mm Hg) | 72 (10) | 69 (10) | 75 (9) | <0.001 |
| Fasting blood glucose (mg/dL) | 97 (31) | 95 (29) | 100 (32) | <0.001 |
| CVH score | ||||
| Inadequate | 2509 (47) | 1284 (46) | 1225 (47) | |
| Average | 1772 (33) | 915 (33) | 857 (33) | 0.78 |
| Optimal | 1098 (20) | 576 (21) | 522 (20) | |
*For IL-6, total sample size = 5279; women, n = 2733; men, n = 2546.
CVH, cardiovascular health; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; LS7, Life’s Simple 7;NT-proBNP, N-terminal pro B-type natriuretic peptide.
The associations between the total CVH score and the CVD-related biomarkers are reported in table 2. After adjusting for sociodemographic factors (model 2), higher CVH scores were associated with lower concentrations of all of the CVD-related biomarkers except for NT-proBNP, where CVH was associated with a higher concentration. For example, in the overall cohort, a one-unit increment in the CVH score corresponded to a 0.13 mg/L lower log (hsCRP) concentration and a 0.04 ng/L lower log(hs-cTnT) concentration, but a 0.02 pg/mL higher log (NT-proBNP) concentration.
Table 2.
The associations between CVH score and CVD-related biomarkers
| Total, n=5379 | |||||||
| hsCRP (mg/L) |
D-dimer (μg/mL) |
Fibrinogen (mg/dL) |
Homocysteine (μmol/L) |
hs-cTnT (ng/L) |
NT-ProBNP (pg/mL) |
IL-6* (pg/mL) |
|
| Model 1 | −0.16
(−0.17,−0.14) |
−0.06
(−0.07,−0.05) |
−0.02
(−0.03,−0.02) |
−0.02
(−0.02,−0.01) |
−0.05
(−0.06,−0.05) |
0.00004 (−0.01, 0.01) |
−0.09
(−0.10,−0.08) |
| Model 2 | −0.13
(−0.14,− 0.12) |
−0.03
(−0.04,−0.02) |
−0.02
(−0.02,−0.02) |
−0.01
(−0.01,−0.01) |
−0.04
(−0.05,−0.03) |
0.02
(0.01,0.03) |
−0.07
(−0.08,−0.06) |
| Women, n=2775 | |||||||
| Model 1 | −0.18
(−0.20,−0.16) |
−0.07
(−0.09,−0.06) |
−0.03
(−0.03,−0.03) |
−0.02
(−0.03,−0.02) |
−0.04
(−0.05,− 0.04) |
0.01 (−0.01,0.03) |
−0.11
(−0.12,−0.10) |
| Model 2 | −0.16
(−0.18,−0.14) |
−0.03
(−0.05,−0.02) |
−0.02
(−0.03,−0.02) |
−0.01
(−0.02,−0.01) |
−0.03
(−0.04,−0.02) |
0.03
(0.01,0.04) |
−0.08
(−0.09,−0.07) |
| Men, n=2604 | |||||||
| Model 1 | −0.13
(−0.15,−0.11) |
−0.04
(−0.05,−0.02) |
−0.02
(−0.02,−0.02) |
−0.01
(−0.02,−0.01) |
−0.07
(−0.08,−0.06) |
−0.01 (−0.03, 0.01) |
−0.07
(−0.08,−0.06) |
| Model 2 | −0.10
(−0.12,−0.08) |
−0.02
(−0.03,−0.001) |
−0.02
(−0.02,−0.01) |
−0.01
(−0.01,−0.004) |
−0.06
(−0.07,−0.05) |
0.005 (−0.01, 0.02) |
−0.05
(−0.06,−0.04) |
*For IL-6, total sample size = 5279; women, n = 2733; men, n = 2546.
CVD, cardiovascular disease;CVH, cardiovascular health; hsCRP, high-sensitivity C-reactive protein; hs-cTnT, high-sensitivity cardiac troponin T; IL-6, interleukin 6; NT-proBNP, N-terminal pro B-type natriuretic peptide.
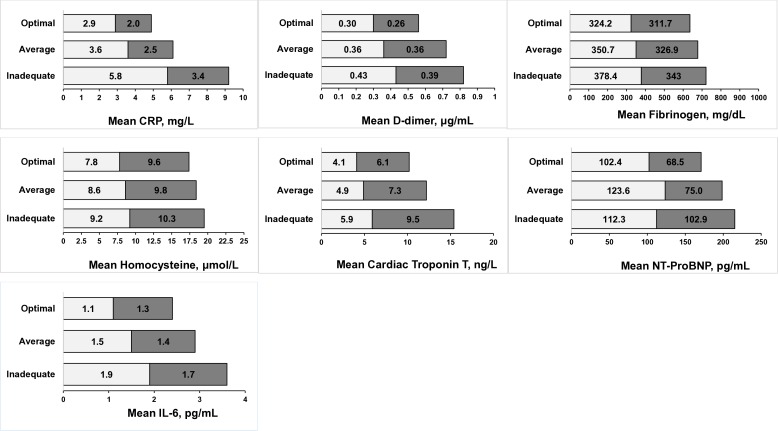
For all CVD-related biomarkers, there was a significant interaction for CVH with sex at p<0.001. For a unit increase in CVH score, the magnitude of concentrations was marginally lower for hsCRP, D-dimer and IL-6 in women compared with men, while for hs-cTnT, the magnitude of concentration was lower in men compared with women. There was no sex difference in the magnitude of concentrations of fibrinogen and homocysteine (table 2). Figure 1 illustrates the sex-stratified mean biomarker concentrations by categories of the total CVH score. For all the biomarkers, participants with optimal scores had the smallest mean values.
Figure 1.
Sex-stratified mean biomarker levels by cardiovascular health score categories (inadequate (0–8), average (9–10) and optimal (11–14)). Mean values for biomarkers were not log-transformed. Lighter colour=women; darker colour=men. hsCRP, high-sensitivity C-reactive protein; hs-cTnT, high-sensitivity cardiac troponin T; NT-proBNP, N-terminal pro B-type natriuretic peptide; IL-6, interleukin 6.
The associations between the LS7 metrics and CVD-related biomarkers (log-transformed) in women and men are reported in tables 3 and 4. For the ideal category of smoking, lower concentrations of D-dimer, fibrinogen, homocysteine, NT-proBNP and IL-6 were found in men but only lower concentrations of homocysteine and IL-6 were found in women. For ideal smoking status, the magnitude of concentration of homocysteine was marginally lower in women than men, while the magnitude of concentration of IL-6 was marginally lower in men than women. For the ideal category of body mass index (BMI), lower concentrations of all biomarkers except for NT-proBNP were found in women, whereas in men, lower concentrations of hsCRP, D-dimer, fibrinogen, hs-cTnT and IL-6 were found. Both sexes had higher concentrations of NT-proBNP for ideal BMI. Additionally, for ideal BMI, the magnitudes of concentration of hsCRP, D-dimer, fibrinogen and IL-6 were lower in women than men but hs-cTnT was lower in men. For the ideal category of physical activity, lower concentrations of hsCRP, fibrinogen, hs-cTnT and IL-6 were found in women, while lower concentrations of fibrinogen, homocysteine, hs-cTnT and IL-6 were found in men. For ideal physical activity, the magnitudes of concentration of fibrinogen and hs-cTnT were marginally similar in women and men. The magnitude of concentration of IL-6 was marginally lower in men. An ideal diet score was associated with lower concentrations of hsCRP and IL-6 in women. For the ideal category of total cholesterol, lower concentrations of hs-cTnT were found in men, while lower concentrations of fibrinogen and higher concentrations of NT-proBNP and IL-6 were found in women and men. For total cholesterol, the magnitudes of concentration of fibrinogen and IL-6 were marginally lower in women than men, while the magnitude of concentration of NT-proBNP was higher in women than men, although CIs between women and men overlapped.
Table 3.
The associations between LS7 metrics and CVD-related biomarkers in women, n=2775
| hsCRP (mg/L) |
D-dimer (μg/mL) |
Fibrinogen (mg/dL) |
Homocysteine (μmol/L) |
hs-cTnT (ng/L) |
NT-ProBNP (pg/mL) |
IL-6* (pg/mL) |
|
| Smoking | |||||||
| Poor | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Intermediate | −0.07 (−0.51, 0.38) |
0.11 (−0.22, 0.44) |
0.03 (−0.05, 0.11) |
0.03 (−0.08, 0.14) |
0.005 (−0.17, 0.18) |
0.003 (−0.35, 0.36) |
0.03 (−0.22, 0.29) |
| Ideal | 0.02 (−0.12, 0.16) |
0.03 (−0.07, 0.13) |
−0.01 (−0.03, 0.02) |
−0.08
(–0.11,– 0.04) |
−0.01 (−0.07, 0.04) |
0.07 (−0.04, 0.18) |
−0.10
(–0.18,–0.02) |
| Body mass index | |||||||
| Poor | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Intermediate | −0.61
(–0.71,–0.51) |
−0.17
(–0.25,–0.10) |
−0.08
(–0.10,–0.07) |
−0.04
(–0.07,–0.02) |
−0.07
(–0.11,–0.03) |
0.06 (−0.03, 0.14) |
−0.34
(–0.40,–0.29) |
| Ideal | −1.15
(–1.25,–1.04) |
−0.32
(–0.40,–0.23) |
−0.14
(–0.16,–0.12) |
−0.06
(–0.08,–0.03) |
−0.09
(–0.14,–0.05) |
0.26
(0.17,0.35) |
−0.61
(–0.67,–0.55) |
| Physical activity | |||||||
| Poor | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Intermediate | −0.06 (−0.19, 0.07) |
0.05 (−0.05, 0.15) |
0.01 (−0.02, 0.03) |
−0.03
(–0.07,–0.00) |
−0.02 (−0.07, 0.03) |
0.01 (−0.09, 0.12) |
0.01 (−0.07, 0.08) |
| Ideal | −0.16
(–0.27,–0.06) |
−0.04 (−0.11, 0.04) |
−0.03
(–0.05,–0.02) |
−0.02 (−0.05, 0.00) |
−0.05
(–0.09,–0.005) |
0.04 (−0.05, 0.12) |
−0.10
(–0.16,–0.04) |
| Diet | |||||||
| Poor | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Intermediate | −0.16
(–0.25,–0.07) |
−0.02 (−0.09, 0.04) |
−0.01 (−0.02, 0.01) |
−0.002 (−0.02, 0.02) |
−0.01 (−0.05, 0.02) |
−0.01 (−0.09, 0.06) |
−0.10
(–0.15,–0.05) |
| Ideal | −0.40
(−0.74,−0.07) |
−0.09 (−0.33, 0.16) |
−0.02 (−0.07, 0.04) |
−0.07 (−0.16, 0.01) |
−0.11 (−0.24, 0.02) |
−0.05 (−0.32, 0.21) |
0.24
(−0.43,−0.06) |
| Total cholesterol | |||||||
| Poor | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Intermediate | 0.02 (−0.11, 0.14) |
−0.04 (−0.13, 0.05) |
−0.02 (−0.04, 0.00) |
−0.0001 (−0.03, 0.03) |
−0.01 (−0.06, 0.04) |
0.12
(0.02,0.21) |
0.09
(0.02,0.16) |
| Ideal | −0.08 (−0.21, 0.04) |
−0.02 (−0.11, 0.08) |
−0.06
(−0.08,−0.04) |
0.01 (−0.02, 0.04) |
−0.006 (−0.04, 0.06) |
0.24
(0.14,0.34) |
0.13
(0.06,0.20) |
| Blood pressure | |||||||
| Poor | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Intermediate | −0.13
(−0.24,−0.03) |
−0.05 (−0.13, 0.03) |
−0.003 (−0.02, 0.02) |
−0.005 (−0.03, 0.02) |
−0.09
(−0.13,−0.04) |
−0.28
(−0.37,−0.20) |
−0.07
(−0.13,−0.01) |
| Ideal | −0.45
(−0.56,−0.35) |
−0.11
(-0.19,−0.03) |
−0.02
(−0.04,−0.002) |
−0.04
(−0.07,−0.01) |
−0.12
(−0.16,−0.08) |
−0.30
(−0.39,−0.21) |
−0.21
(−0.27,−0.15) |
| Blood glucose | |||||||
| Poor | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Intermediate | −0.04 (−0.22, 0.14) |
0.01 (−0.13, 0.14) |
−0.01 (−0.04, 0.02) |
0.04 (−0.01, 0.08) |
−0.16
(−0.23,−0.09) |
0.10 (−0.04, 0.25) |
−0.0002 (−0.10, 0.10) |
| Ideal | −0.43
(−0.57,−0.28) |
−0.03 (−0.14, 0.08) |
−0.07
(−0.09,−0.04) |
0.002 (−0.03, 0.04) |
−0.19
(−0.25,−0.13) |
0.29
(0.17,0.40) |
−0.28
(−0.36,−0.20) |
*For IL-6, sample size=2733.
CVD, cardiovascular disease;hsCRP, high-sensitivity C-reactive protein; hs-cTnT, high-sensitivity cardiac troponin T;IL-6, interleukin 6; LS7, Life’s Simple 7; NT-proBNP, N-terminal pro B-type natriuretic peptide.
Table 4.
The associations between LS7 metrics and CVD-related biomarkers in men, n=2604
| hsCRP (mg/L) |
D-dimer (μg/mL) |
Fibrinogen (mg/dL) |
Homocysteine (μmol/L) |
hs-cTnT (ng/L) |
NT-ProBNP (pg/mL) |
IL-6* (pg/mL) |
|
| Smoking | |||||||
| Poor | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Intermediate | −0.16 (−0.50, 0.17) |
−0.17 (−0.45, 0.11) |
−0.04 (−0.10, 0.02) |
−0.16
(−0.25,−0.07) |
−0.07 (−0.25, 0.12) |
−0.52
(−0.86,−0.19) |
−0.21
(−0.42,−0.005) |
| Ideal | −0.27 (−0.39, 0.15) |
−0.17
(-0.27,- 0.07) |
−0.05
(−0.07,−0.03) |
−0.05
(−0.08,−0.02) |
0.02 (−0.04, 0.08) |
−0.15
(−0.27,−0.03) |
−0.19
(−0.27,−0.12) |
| Body mass index | |||||||
| Poor | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Intermediate | −0.50
(−0.59,−0.40) |
−0.11
(−0.20,−0.03) |
−0.06
(−0.08,−0.05) |
−0.01 (−0.04, 0.01) |
−0.17
(−0.23,−0.12) |
0.07 (−0.02, 0.17) |
−0.30
(−0.36,−0.24) |
| Ideal | −0.80
(−0.92,−0.69) |
−0.17
(−0.26,−0.07) |
−0.08
(−0.10,−0.06) |
−0.02 (−0.05, 0.01) |
−0.29
(−0.35,−0.23) |
0.25
(0.14,0.36) |
−0.37
(−0.43,−0.30) |
| Physical activity | |||||||
| Poor | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Intermediate | 0.09 (−0.04, 0.23) |
−0.06 (−0.17, 0.06) |
−0.002 (−0.03, 0.02) |
−0.01 (−0.05, 0.02) |
−0.07 (−0.14, 0.01) |
0.03 (−0.10, 0.17) |
−0.02 (−0.10, 0.07) |
| Ideal | −0.03 (−0.14, 0.07) |
−0.05 (−0.13, 0.04) |
−0.02
(−0.04,−0.004) |
−0.02
(−0.05,−0.00) |
−0.07
(−0.13,−0.02) |
0.05 (−0.05, 0.15) |
−0.14
(−0.20,−0.07) |
| Diet | |||||||
| Poor | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Intermediate | −0.11
(−0.19,−0.02) |
0.002 (−0.07, 0.07) |
−0.01 (−0.03, 0.00) |
−0.003 (−0.02, 0.02) |
−0.02 (−0.07, 0.02) |
0.04 (−0.04, 0.12) |
−0.05
(−0.10,−0.003) |
| Ideal | −0.57 (−1.25, 0.11) |
−0.50 (−1.07, 0.07) |
0.01 (−0.12, 0.14) |
0.06 (−0.11, 0.24) |
−0.17 (−0.54, 0.20) |
0.31 (−0.36, 0.98) |
−0.14 (−0.56, 0.27) |
| Total cholesterol | |||||||
| Poor | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Intermediate | −0.03 (−0.17, 0.11) |
0.01 (−0.11, 0.12) |
−0.04
(−0.06,−0.01) |
−0.003 (−0.04, 0.03) |
−0.10
(−0.18,−0.02) |
−0.09 (−0.22, 0.05) |
0.08 (−0.01, 0.16) |
| Ideal | −0.01 (−0.15,0.13) |
0.05 (−0.06, 0.17) |
−0.05
(−0.08,−0.03) |
−0.006 (−0.04, 0.03) |
−0.10
(−0.17,−0.02) |
0.19
(0.05,0.32) |
0.17
(0.08,0.25) |
| Blood pressure | |||||||
| Poor | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Intermediate | −0.10 (−0.20, 0.01) |
−0.06 (−0.14, 0.03) |
−0.01 (−0.03, 0.004) |
−0.03
(−0.06,−0.005) |
−0.12
(−0.18,−0.07) |
−0.37
(−0.47,−0.27) |
−0.07
(−0.13,−0.01) |
| Ideal | −0.23
(−0.34,−0.13) |
−0.01 (−0.10, 0.08) |
−0.03
(−0.05,−0.01) |
−0.04
(−0.07,−0.02) |
−0.22
(−0.27,−0.16) |
−0.40
(−0.50,−0.30) |
−0.09
(−0.16,−0.03) |
| Blood glucose | |||||||
| Poor | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Intermediate | 0.07 (−0.08, 0.22) |
0.03 (−0.09, 0.16) |
−0.01 (−0.04, 0.02) |
0.04 (−0.003, 0.08) |
−0.23
(−0.31,−0.15) |
−0.06 (−0.21, 0.09) |
−0.04 (−0.14, 0.05) |
| Ideal | −0.17
(−0.30,−0.04) |
0.04 (−0.07, 0.15) |
−0.03
(−0.06,−0.01) |
0.01 (−0.02, 0.05) |
−0.36
(−0.43,−0.29) |
0.04 (−0.09, 0.17) |
−0.15
(−0.23,−0.07) |
*For IL-6, n=2546.
CVD, cardiovascular disease;hsCRP, high-sensitivity C-reactive protein; hs-cTnT, high-sensitivity cardiac troponin T;IL-6, interleukin 6; LS7, Life’s Simple 7; NT-proBNP, N-terminal pro B-type natriuretic peptide.
For ideal blood pressure, a lower concentration of all biomarkers was found in women, whereas in men, a lower concentration was observed for all biomarkers except D-dimer. Additionally, for ideal blood pressure, the magnitudes of concentration of fibrinogen, hs-cTnT and NT-proBNP were lower in men than women, while the magnitude of concentration of IL-6 was lower in women than men. For ideal blood glucose levels, a higher concentration of NT-proBNP was found in women, while a lower concentration of hsCRP, fibrinogen, hs-cTnT and IL-6 was observed in both sexes. For ideal blood glucose, the magnitudes of concentration of hsCRP, fibrinogen and IL-6 were lower in women than men.
The supplementary analyses show the associations between CVH and CVD-related biomarkers stratified by race/ethnicity and age for women and men. The results were similar for both sexes and mostly showed a statistically significant lower concentration of CVD-related biomarkers for a unit increment in CVH score. Among White and Chinese American women as well as women <65 years old, a unit increment in CVH score was associated with higher concentrations of NT-proBNP (Online supplementary tables S4-S7).
Discussion
In this cross-sectional analysis of 5379 adult women and men free of clinical CVD at baseline, after adjusting for sociodemographic factors, we found an inverse association between the CVH score and most of the CVD-related biomarkers. Higher CVH scores were associated with lower concentrations of all of the CVD-related biomarkers in women and men, except for NT-proBNP that showed a direct relationship. We found a similarly inverse relationship between the LS7 metrics and CVD-related biomarkers except for NT-proBNP where the associations were both direct and inverse. Additionally, we observed a direct association between ideal cholesterol and IL-6 in both sexes. In the stratified analyses by race/ethnicity and age, the associations observed were similar for both sexes.
Our results are similar to a study conducted to investigate the association between CVH metrics and biomarkers (hsCRP and homocysteine) among 3009 Chinese adults between the ages of 24 and 85 years, without a history of CVD.7 In that study, after adjusting for age, sex and education, a unit increment in CVH score was inversely related to biomarker concentration (CRP: −0.182 (−0.652, −0.457); homocysteine: −0.092 (−0.930, −0.426)). A similar association was found in women and men, although the association was stronger in women.
A cross-sectional study of 2680 participants from the Framingham Heart Study also examined the association between CVH and CVD-related biomarkers (BNP, CRP, D-dimer, fibrinogen and homocysteine).22 Similar to our findings in MESA, the Framingham researchers found that the CVH score had a direct association with higher circulating concentrations of natriuretic peptides but was inversely related to blood concentrations of the other biomarkers examined, after adjusting for age and sex.22 For a unit increase in CVH score, the beta coefficients for the biomarkers were as follows: BNP, 0.057 pg/mL (0.035, 0.080); CRP, −0.248 mg/L (−0.279, −0.217); D-dimer, −0.030 ng/mL (−0.046, −0.014); fibrinogen, −0.028 mg/dL (−0.033, −0.023) and homocysteine, −0.021 mmol/L (−0.029, −0.012). The authors concluded that the inverse association of CVH with incident CVD events was at least partly attributable to the favourable relationship of CVH and subclinical biomarkers of risk.22 Notably, none of the aforementioned studies examined for effect modification by sex in the association of CVH with subclinical biomarkers, as we newly present here. One prior study conducted in a Chinese population7 did stratify the association between CVH and biomarkers by sex; however, they did not test for effect modification. In contrast to our study, that study did not include D-dimer, fibrinogen, hs-cTnT, NT-proBNP and IL-6 in their analysis.7
Our main finding showed a better CVH score was associated with lower concentrations of all CVD-related biomarkers (except NT-proBNP) in both women and men. Despite statistically significant interactions by sex for the total CVH score, qualitatively the magnitude of lower concentrations for these biomarkers per one unit increment in CVH were generally similar among women and men. However, for the metric of ideal BMI, the magnitude of lower concentrations of hsCRP, D-dimer, fibrinogen and IL-6 per unit of CVH was greater in women than men. In the univariate analysis, women in this study had slightly higher BMI than men. Studies have shown that oestrogen and adipose tissue may increase the circulating levels of inflammatory biomarkers,9 23 and thus a more favourable BMI might have greater impact on these biomarker concentrations in women than men.
Additionally, we noted that in women, ideal BMI, a health behaviour, was associated with a greater magnitude of reduction in hsCRP, D-dimer, fibrinogen and IL-6 compared with ideal blood pressure, ideal blood glucose (health factors) while in men, the same association was observed for hsCRP and IL-6. This may suggest that attaining ideal health behaviours such as ideal BMI may lead to more reductions in the biomarkers of CVD risk compared with ideal health factors. However, more elaborate studies would be needed to explore these findings so definite conclusions can be reached because of the importance of biomarkers such as hsCRP, fibrinogen and IL-6 in mediating the relationship between CVH and CVD.24 For example, in a prior study of over 9300 men followed for 10 years, individuals with ideal CVH had lower risk for all CVD subtypes examined and the lower risk of coronary heart disease was mediated in part through lower inflammatory and hemostatic factors.24
In our univariate analyses, homocysteine concentrations were higher in men which may be attributable to a higher prevalence of smoking and poorer healthy diet score.25 In the adjusted regression analyses, a unit increment in CVH score corresponded to a slightly lower concentration of homocysteine in women. In addition, the higher prevalence of smoking found in men in this study may be responsible for their higher baseline hs-cTnT concentrations.26 Although in adjusted regression analysis, the magnitude of concentration of hs-cTnT per 1-unit increment in CVH score was lower in men. Moreover, we found that ideal cholesterol was directly associated with IL-6 in both sexes. Although this finding has been previously documented among healthy individuals, other studies have reported an inverse association in pathological conditions, which according to prior research may suggest polymorphism in the IL-6 gene differentially affects lipid metabolism.27
Interestingly, a better CVH score was associated with higher concentrations of NT-proBNP, particularly in women. At first this may seem paradoxical, as in the setting of disease states, BNP levels are frequently elevated. However, in normal states, NT-proBNP actually plays a favourable cardioprotective role by inhibiting cardiac hypertrophy and fibrosis as well as promoting vasodilation and natriuresis. In patients with heart failure, there is relative BNP deficiency and BNP resistance, resulting in a compensatory increase in NT-proBNP concentrations to restore homeostasis.28 We found that baseline concentrations of NT-proBNP were higher in women than men, as previously reported in MESA,10 although average concentrations for both sexes were within normal limits in this cohort free of clinical heart failure at baseline. Other previous studies have also reported higher NT-proBNP concentrations in women,22 29 as well as a prior analysis in the MESA cohort that showed a more androgenic (‘male-like’) sex hormone profile was associated with lower NT-proBNP concentrations.10
The sex-specific differences observed in the association of CVH and CVD-related biomarkers may reflect different pathways of CVD risk. Additional research that explain the potential sex-specific mechanisms underlying the association between CVH and CVD-related biomarkers may improve our knowledge of the development of CVD in women and men.30 An understanding of these pathways may also help clinicians tailor interventions specific to the prevention and treatment of CVD risk factors in women and men.30 Our study emphasises the importance of promoting ideal CVH, which may be more beneficial in women, particularly with research showing that women have poorer cardiovascular outcomes compared with men. Encouraging the attainment of ideal CVH may reverse this trend and lead to a decrease in CVD burden.
In the interpretation of our findings, some limitations should be noted. First, neither temporality nor causal inferences between the association of CVH and CVD-related biomarkers can be determined because of the cross-sectional study design. Second, we cannot rule out recall bias from the use of self‐administered questionnaires to collect data on smoking, diet and physical activity. Third, the findings of this study may not be generalisable to younger people or adults of very advanced age because our participants were between ages of 45 and 84 years old. Fourth, multiple statistical tests were performed and some findings might be expected to occur by chance; however, our findings were generally consistent between women and men and across age and race/ethnic subgroups. Lastly, CVH was assessed once at baseline and may not be representative of the future CVH status of study participants.
Conclusions
We found that more favourable CVH scores were associated with lower concentrations of CVD-related biomarkers in both women and men, except for NT-proBNP which showed a direct relationship. These favourable associations of CVH with biomarkers of risk may be an intermediary step in the prevention of clinical CVD events. Overall, our findings were qualitatively similar between the sexes and suggest that promotion of ideal CVH would have similarly favourable impact on the reduction of biomarkers of risk among women and men. However, long-term outcome studies are needed to improve our understanding of the underlying sex-specific mechanisms and the clinical implications of these findings.
Supplementary Material
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the Multi-Ethnic Study of Atherosclerosis for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Twitter: @ErinMichos
Contributors: Author contributions, study conception and design: O Osibogun, O Ogunmoroti and EDM. Acquisition of data and analysis: O Osibogun and O Ogunmoroti. Interpretation of data: O Osibogun, O Ogunmoroti, MT, EB and EDM. Drafting of manuscript: O Osibogun, O Ogunmoroti and EDM. Critical revision and approval of final version submitted: O Osibogun, O Ogunmoroti, MT, EB and EDM.
Funding: The Multi-Ethnic Study of Atherosclerosis is supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169 and HHSN268201500003I from the National Heart, Lung, and Blood Institute (NHLBI) and by grants UL1-RR-024156 and UL1-RR-025005 from the National Center for Research Resources (NCRR). Dr Michos is supported by the (unrestricted) Blumenthal Scholars Fund in Preventive Cardiology at Johns Hopkins University.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The MESA protocol was approved by the institutional review boards of all the recruitment centers.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data may be obtained from a third party and are not publicly available.
References
- 1. Lloyd-Jones DM, Hong Y, Labarthe D, et al. . Defining and setting national goals for cardiovascular health promotion and disease reduction: the American heart association's strategic impact goal through 2020 and beyond. Circulation 2010;121:586–613. 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 2. Labarthe DR. From cardiovascular disease to cardiovascular health: a quiet revolution? Circ Cardiovasc Qual Outcomes 2012;5:e86–92. 10.1161/CIRCOUTCOMES.111.964726 [DOI] [PubMed] [Google Scholar]
- 3. Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001;69:89–95. 10.1067/mcp.2001.113989 [DOI] [PubMed] [Google Scholar]
- 4. Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation 2006;113:2335–62. 10.1161/CIRCULATIONAHA.104.482570 [DOI] [PubMed] [Google Scholar]
- 5. Polonsky TS, Ning H, Daviglus ML, et al. . Association of cardiovascular health with subclinical disease and incident events: the Multi‐Ethnic study of atherosclerosis. J Am Heart Assoc 2017;6 10.1161/JAHA.116.004894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benson E-MA, Tibuakuu M, Zhao D, et al. . Associations of ideal cardiovascular health with GlycA, a novel inflammatory marker: the multi-ethnic study of atherosclerosis. Clin Cardiol 2018;41:1439–45. 10.1002/clc.23069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y-Q, Wang C-F, Zhu L, et al. . Ideal cardiovascular health and the subclinical impairments of cardiovascular diseases: a cross-sectional study in central South China. BMC Cardiovasc Disord 2017;17:269 10.1186/s12872-017-0697-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Libby P, Ridker PM, Hansson GK, et al. . Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol 2009;54:2129–38. 10.1016/j.jacc.2009.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garcia VP, Rocha HNM, Sales ARK, et al. . Sex differences in high sensitivity C-reactive protein in subjects with risk factors of metabolic syndrome. Arq Bras Cardiol 2016;106:182–7. 10.5935/abc.20160027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ying W, Zhao D, Ouyang P, et al. . Sex hormones and change in N-terminal pro-B-type natriuretic peptide levels: the multi-ethnic study of atherosclerosis. J Clin Endocrinol Metab 2018;103:4304–14. 10.1210/jc.2018-01437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gore MO, Seliger SL, Defilippi CR, et al. . Age- and sex-dependent upper reference limits for the high-sensitivity cardiac troponin T assay. J Am Coll Cardiol 2014;63:1441–8. 10.1016/j.jacc.2013.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Greene DN, Tate JR. Establishing consensus-based, assay-specific 99th percentile upper reference limits to facilitate proper utilization of cardiac troponin measurements. Clin Chem Lab Med 2017;55:1675–82. 10.1515/cclm-2017-0067 [DOI] [PubMed] [Google Scholar]
- 13. Bild DE, et al. Multi-Ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–81. 10.1093/aje/kwf113 [DOI] [PubMed] [Google Scholar]
- 14. Cushman M, Cornell ES, Howard PR, et al. . Laboratory methods and quality assurance in the cardiovascular health study. Clin Chem 1995;41:264–70. [PubMed] [Google Scholar]
- 15. Whelton SP, Narla V, Blaha MJ, et al. . Association between resting heart rate and inflammatory biomarkers (high-sensitivity C-reactive protein, interleukin-6, and fibrinogen) (from the multi-ethnic study of atherosclerosis). Am J Cardiol 2014;113:644–9. 10.1016/j.amjcard.2013.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perng W, Villamor E, Shroff MR, et al. . Dietary intake, plasma homocysteine, and repetitive element DNA methylation in the multi-ethnic study of atherosclerosis (MESA). Nutrition, Metabolism and Cardiovascular Diseases 2014;24:614–22. 10.1016/j.numecd.2013.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seliger SL, Hong SN, Christenson RH, et al. . High-Sensitive cardiac troponin T as an early biochemical signature for clinical and subclinical heart failure: MESA (multi-ethnic study of atherosclerosis). Circulation 2017;135:1494–505. 10.1161/CIRCULATIONAHA.116.025505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lloyd-Jones DM. Improving the cardiovascular health of the US population. JAMA 2012;307:1314–6. 10.1001/jama.2012.361 [DOI] [PubMed] [Google Scholar]
- 19. Ogunmoroti O, Utuama OA, Michos ED, et al. . Does education modify the effect of ethnicity in the expression of ideal cardiovascular health? the Baptist health South Florida employee study. Clin Cardiol 2017;40:1000–7. 10.1002/clc.22757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ogunmoroti O, Michos ED, Aronis KN, et al. . Life's simple 7 and the risk of atrial fibrillation: the multi-ethnic study of atherosclerosis. Atherosclerosis 2018;275:174–81. 10.1016/j.atherosclerosis.2018.05.050 [DOI] [PubMed] [Google Scholar]
- 21. Osibogun O, Ogunmoroti O, Spatz ES, et al. . Is self-rated health associated with ideal cardiovascular health? the multi-ethnic study of atherosclerosis. Clin Cardiol 2018;41:1154–63. 10.1002/clc.22995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xanthakis V, Enserro DM, Murabito JM, et al. . Ideal cardiovascular health: associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham offspring study. Circulation 2014;130:1676–83. 10.1161/CIRCULATIONAHA.114.009273 [DOI] [PubMed] [Google Scholar]
- 23. Rudnicka AR, Rumley A, Whincup PH, et al. . Sex differences in the relationship between inflammatory and hemostatic biomarkers and metabolic syndrome: British 1958 birth cohort. J Thrombosis Haemost 2011;9:2337–44. 10.1111/j.1538-7836.2011.04517.x [DOI] [PubMed] [Google Scholar]
- 24. Gaye B, Tafflet M, Arveiler D, et al. . Ideal cardiovascular health and incident cardiovascular disease: heterogeneity across event subtypes and mediating effect of blood biomarkers: the prime study. J Am Heart Assoc 2017;6. doi: 10.1161/JAHA.117.006389. [Epub ahead of print: 17 Oct 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J 2015;14:6 10.1186/1475-2891-14-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miyazaki T, Ashikaga T, Ohigashi H, et al. . Impact of smoking on coronary microcirculatory resistance in patients with coronary artery disease. Int Heart J 2015;56:29–36. 10.1536/ihj.14-189 [DOI] [PubMed] [Google Scholar]
- 27. Zhang B, Li X-L, Zhao C-R, et al. . Interleukin-6 as a predictor of the risk of cardiovascular disease: a meta-analysis of prospective epidemiological studies. Immunol Invest 2018;47:689–99. 10.1080/08820139.2018.1480034 [DOI] [PubMed] [Google Scholar]
- 28. Daniels LB, Maisel AS, peptides N. Natriuretic peptides. J Am Coll Cardiol 2007;50:2357–68. 10.1016/j.jacc.2007.09.021 [DOI] [PubMed] [Google Scholar]
- 29. Lew J, Sanghavi M, Ayers CR, et al. . Sex-Based differences in cardiometabolic biomarkers. Circulation 2017;135:544–55. 10.1161/CIRCULATIONAHA.116.023005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garcia M, Mulvagh SL, Merz CNB, et al. . Cardiovascular disease in women: clinical perspectives. Circ Res 2016;118:1273–93. 10.1161/CIRCRESAHA.116.307547 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-031414supp001.pdf (129.9KB, pdf)