Abstract
Purpose
The Neonatal Intensive Care Unit Hospital Exposures and Long-Term Health (NICU-HEALTH) longitudinal preterm birth cohort studies the impact of the NICU exposome on early-life development. NICU-HEALTH collects multiple biospecimens, complex observational and survey data and comprehensive multisystem outcome assessments to allow measurement of the impact of modifiable environmental exposures during the preterm period on neurodevelopmental, pulmonary and growth outcomes.
Participants
Moderately preterm infants without genetic or congenital anomalies and their mothers are recruited from an urban academic medical centre level IV NICU in New York City, New York, USA. Recruitment began in 2011 and continues through multiple enrolment phases to the present with goal enrolment of 400 infants. Follow-up includes daily data collection throughout the NICU stay and six follow-up visits in the first 2 years. Study retention is 77% to date, with the oldest patients turning age 8 in 2019.
Findings to date
NICU-HEALTH has already contributed significantly to our understanding of phthalate exposure in the NICU. Phase I produced the first evidence of the clinical impact of phthalate exposure in the NICU population. Further study identified specific sources of exposure to clinically relevant phthalate mixtures in the NICU.
Future plans
Follow-up from age 3 to 12 is co-ordinated through integration with the Environmental Influences on Child Health Outcomes (ECHO) programme. The NICU-HEALTH cohort will generate a wealth of biomarker, clinical and outcome data from which future studies of the impact of early-life chemical and non-chemical environmental exposures can benefit. Findings from study of this cohort and other collaborating environmental health cohorts will likely translate into improvements in the hospital environment for infant development.
Trial registration numbers
This observational cohort is registered with ClinicalTrials.gov (NCT01420029 and NCT01963065).
Keywords: NICU, neonatal intensive care unit, children’s environmental health, prematurity, longitudinal birth cohort
Strengths and limitations of this study.
The Neonatal Intensive Care Unit Hospital Exposures and Long-Term Health (NICU-HEALTH) cohort is the first comprehensive longitudinal preterm birth cohort with a primary focus on NICU-based environmental exposures.
The NICU-HEALTH study comprises a large cohort of moderately preterm children with biological samples linked to detailed demographic and clinical data regarding the intensive care course and longitudinal follow-up to age 3 years using standardised assessment measures and survey tools.
Mothers of preterm infants are also enrolled, and they provide both their own biospecimens and significant information about stressful life events, depressive symptoms and dietary history during pregnancy, during the NICU stay and after NICU discharge.
NICU-HEALTH takes an exposomics approach throughout the highly controlled and continuously observed NICU hospitalisation, collecting data about the physical, chemical and social environment, in addition to biobanking specimens for multi-omic analyses.
Although rates of medical morbidity related to prematurity among moderately preterm infants are low, confounding by indication can be challenging in some NICU-HEALTH analyses, requiring complex statistical techniques.
Introduction
While preterm infants now have high rates of survival,1 they continue to experience significant neurodevelopmental impairments linked to preterm birth. Even ‘mature’ preterm infants born at 28–36 weeks’ gestation have significantly higher rates of behavioural, cognitive and psychiatric deficits compared with term-born peers.2–7 Beyond neurodevelopmental abnormalities, children born preterm demonstrate elevated rates of lung disease8 9 and maladaptive growth.10 11 ‘Prematurity’, however, is not uniformly predictive or well-understood in the causal pathway of morbidity. The heightened risk of lifelong multisystem dysfunction associated with prematurity is only partially explained by severity of illness in infancy.9–15 In fact, traditional perinatal risk factors—gestational age (GA), for example—have little prognostic value.16 Although children born at the limits of viability or who suffer severe neonatal illness often have predictably poor outcome, the aetiology of significant deficits seen in the large population of moderately preterm infants with benign medical history remains poorly understood. Alterations in developmental trajectory, rather than focal end organ injury following preterm birth, are implicated.6 17
Early-life environmental exposures can alter developmental trajectories in critical and often unexpected ways to produce clinically important outcomes years later. Numerous prospective birth cohorts, often drawn from communities with high pollutant burden, have used maternal biomarkers as estimates of fetal exposure to explore the influence of the third trimester environment on long-term child health outcomes. Third trimester fetal life, a critical period for brain and lung development as well as for metabolic programming, is now known to be particularly sensitive to environmental perturbations.
Whether the normal developmental trajectory is impacted by environmental toxicants in the neonatal intensive care unit (NICU) has not been rigorously studied. In addition to providing life-sustaining treatments, the NICU confers significant exposure to chemical plasticisers, heavy metals, potentially toxic stress, social isolation and other environmental factors shown to be detrimental to brain development in studies of term-born fetuses and infants.18–21 We believe that opportunities exist to improve outcomes of preterm infants by optimising the NICU from an environmental health perspective.
Cohort description
The NICU Hospital Exposures and Long-Term Health (NICU-HEALTH) longitudinal preterm birth cohort is based in the premise that modifiable environmental exposures in the NICU contribute to developmental deficits in children born preterm. The NICU-HEALTH infrastructure facilitates detailed study of the NICU exposome and comprehensive assessment of early developmental progress, allowing us to measure the impact of modifiable environmental exposures during the preterm period on multisystem outcomes.
Study aims
NICU-HEALTH is a prospective environmental health cohort focused on the large population of moderately preterm infants.22 Moderately preterm infants require extended hospitalisation in the NICU following birth, but have low rates of physiological derangement, sepsis, intraventricular haemorrhage or other medical predictors of poor outcome. Nonetheless, they have elevated rates of adverse neurobehavioural, pulmonary and growth outcomes. The goal of NICU-HEALTH is to determine the role of potentially modifiable NICU environmental factors that contribute to long-term neurodevelopmental, pulmonary and growth deficits of NICU graduates. To do this, we collect data throughout the NICU stay, with daily record of equipment and medication exposure, procedural experience and potentially stressful events. We collect a variety of biospecimens and evaluate multisystem outcomes longitudinally to provide a comprehensive cognitive, motor, behavioural, pulmonary and anthropomorphic phenotype through early childhood. Extensive maternal survey data and maternal biomarkers allow for estimation of the in utero environment. Longitudinal study visits through childhood allow for long-term follow-up (figure 1). NICU-HEALTH data analyses focus on identifying sources of NICU-based toxicants that can be mitigated.
Figure 1.
Participants are followed with serial biomarker and survey data as well as serial subjective and objective measures of development. EEG, electroencephalogram; CBCL, Child Behavior Checklist; IBQR, Infant Behavior Questionnaire-Revised; NICU, Neonatal Intensive Care Unit; NNS, non-nutritive suck; VRM, visual recognition memory.
Study population
Participant recruitment and informed consent
Mothers of eligible infants are approached for enrolment soon after NICU admission at the Mount Sinai Hospital. Initial verbal consent permits non-invasive collection of valuable biospecimens in the immediate period after birth; full informed consent during the infant’s first week of life facilitates linkage of these specimens with comprehensive clinical data available prospectively and from maternal and infant medical records. Detailed survey work and objective assessments are conducted while the infant is hospitalised, such that loss to follow-up for early data is low. Our research team has pioneered collection techniques for preterm infants; the collected volume of biospecimens such as urine and saliva exceeds those of published studies.23 24
The NICU-HEALTH cohort displays racial, ethnic and socioeconomic diversity (table 1). Almost half of NICU-HEALTH participants report racial and/or ethnic minority status. This is more racial and ethnic diversity than the birth population at our hospital, which is 22% non-white. The per cent of participants of low socioeconomic status in our cohort is similar to that of our hospital population. Longitudinal follow-up of the NICU-HEALTH cohort is conducted through participation in the Environmental Influences on Child Health Outcomes (ECHO) programme.25
Table 1.
NICU-HEALTH enrolment, 2011 to date
| Number of infants enrolled | 275 |
| Mean birth weight (g) of survivors to NICU discharge | 1348 |
| Mean gestational age (weeks) of survivors to NICU discharge | 30.2 |
| Multiple births (% of cohort) | 49 |
| Child’s sex (% female) | 50 |
| Per cent of cohort reporting racial/ethnic minority status | 49 |
| Per cent of cohort with income ≤200% US federal poverty line | 31 |
NICU, neonatal intensive care unit; NICU-HEALTH, Neonatal Intensive Care Unit Hospital Exposures and Long-Term Health.
Phase I of NICU-HEALTH enrolled neonates with birth weight less than 1500 g born September 2011 through July 2013. Phase I focused on organic chemical exposure with biospecimen collection limited to urine, a single neurodevelopmental outcome assessment before NICU discharge and enrolment of infants but not mothers. Phase II enrolment commenced in March 2015 and continues through early 2019. Phase II switched to GA-based enrolment criteria (28–33 weeks) to decrease the incidence of major morbidities of extreme prematurity in the cohort. Phase II expanded focus, with enrolment of both infants and mothers, banking of infant urine, stool, saliva, hair and blood, as well as maternal blood, hair and breast milk. Mothers complete comprehensive surveys including evaluation of maternal stress, mental health and IQ. Multiple infant outcome assessments are completed including dense-array electroencephalogram (EEG) and co-ordinated follow-up via multiple contacts during the first 2 years of life (table 2). Phase III will be launched in 2020 and will add a dedicated study visit at 7–8 months corrected age to complete objective assessments of memory, attention, social cognition and non-nutritive suck (NNS). Phase III will also include ECHO study visits for NICU-HEALTH participants aged 3–10.
Table 2.
Selected NICU-HEALTH study procedures
| Data/specimen/assessment | NICU | 2–4 months | 7–8 months | 12 months | 2 years | 3 years | 4 years | 5 years | 6 years | 7 years | |
| Eligibility screen/informed consent | First week | ||||||||||
| Biospecimens | Urine | Qweek | X | X* | X | X* | X* | X* | X* | X* | |
| Meconium/stool | Qweek | ||||||||||
| Hair—mother | X | ||||||||||
| Hair—child | X | X* | X | X | X* | X* | |||||
| Infant blood | X | ||||||||||
| Breast milk | Qweek | X | X* | ||||||||
| Child saliva | Qweek | X* | X | ||||||||
| Primary teeth | X* | X* | X* | X* | |||||||
| Clinical data | Weight | Qday | X | X* | X | X | X* | X* | X* | X* | X* |
| Length/height | Qweek | X | X* | X | X | X* | X* | X* | X* | X* | |
| Head circumference | Qweek | X | X* | X | X | X* | X* | X* | X* | X* | |
| Cranial ultrasound | 2–3x | ||||||||||
| Pubertal development/tanner staging | X* | X* | |||||||||
| Surveys | Medical history | Qday | X | X* | X | X | X* | X* | X* | X* | X* |
| Medical equipment exposure | Qday | ||||||||||
| Visitor interactions | Qday | ||||||||||
| Neonatal Infant Stressor Scale114 | Qday | ||||||||||
| Maternal depression (EPDS)115 | X | X | X | ||||||||
| Perceived Stress Scale116 | X | X | X | ||||||||
| Life events (CRYSIS-R)117 | X | X | X | ||||||||
| Maternal IQ (Raven)118 | X | ||||||||||
| Maternal diet119 | X | X | X* | X | X | ||||||
| Infant diet | X | X* | X | X | |||||||
| Maternal PTSD (PCL-C)120 | X* | X | |||||||||
| ELEAT Pregnancy Questionnaires121 | X* | ||||||||||
| ELEAT Parent Questionnaires121 | X* | X* | X* | ||||||||
| Outcomes | Dense-array EEG | X | |||||||||
| NICU Network Neurobehavioral Scale | X | ||||||||||
| Infant Behavior Questionnaire—Revised122 | X | ||||||||||
| Childhood Behavior Questionnaire123 | X | ||||||||||
| Video-based attention tasks | X* | ||||||||||
| Non-nutritive suck assessment | X* | X* | |||||||||
| Child Behavior Checklist123 | X | ||||||||||
| NIH Toolbox Cognition Battery124 | X* | X* | X* | ||||||||
| NIH Toolbox Motor Battery124 | |||||||||||
| Bayley-III125 | X | ||||||||||
| Brief Respiratory Questionnaire/spirometry126 | X* | X* | |||||||||
| Strengths and Difficulties Questionnaire127 | X* | X* | X* | ||||||||
| Adaptive Behavior Assessment System128 | X* | X* | X* | ||||||||
| PROMIS Physical Function—Mobility129 | X* | ||||||||||
Age at assessment is corrected age from due date.
*For implementation in NICU-HEALTH phase III.
EEG, electroencephalogram; EPDS, Edinburgh Postnatal Depression Scale; NICU, neonatal intensive care unit; NICU-HEALTH, Neonatal Intensive Care Unit Hospital Exposures and Long-Term Health; PCL-C, Post-traumatic stress disorder CheckList - Civilian version; PTSD, Post-traumatic stress disorder; Q, every.
Since all infants born prior to 35 weeks gestation require NICU hospitalisation and since healthy term-born children are at a different stage of development in the ex utero environment than preterm infants, there is no non-NICU ‘control’ arm for NICU-HEALTH. We rely on exposure variability within our cohort (figure 2) to meet our aims. Data collection through all phases includes direct observation of medical equipment exposures; phases II and III add prospective record of stressful events and observations of infants’ social interactions. Longitudinal follow-up is achieved at clinically indicated visits to the NICU-Follow Up Program (FUP) and through the ECHO programme. The FUP is a clinical programme that conducts developmental and nutritional screening and offers family support and early intervention to all NICU graduates born before 33 weeks, and thus serves the entire NICU-HEALTH study population. The FUP sees children at 2–6 month intervals from NICU discharge to age 3 with retention greater than 85%. Biospecimens collected after NICU discharge will be analysed for community-based phthalate and stress exposure—important confounders of our primary assessment of the impact of NICU-based exposures on outcomes.
Figure 2.
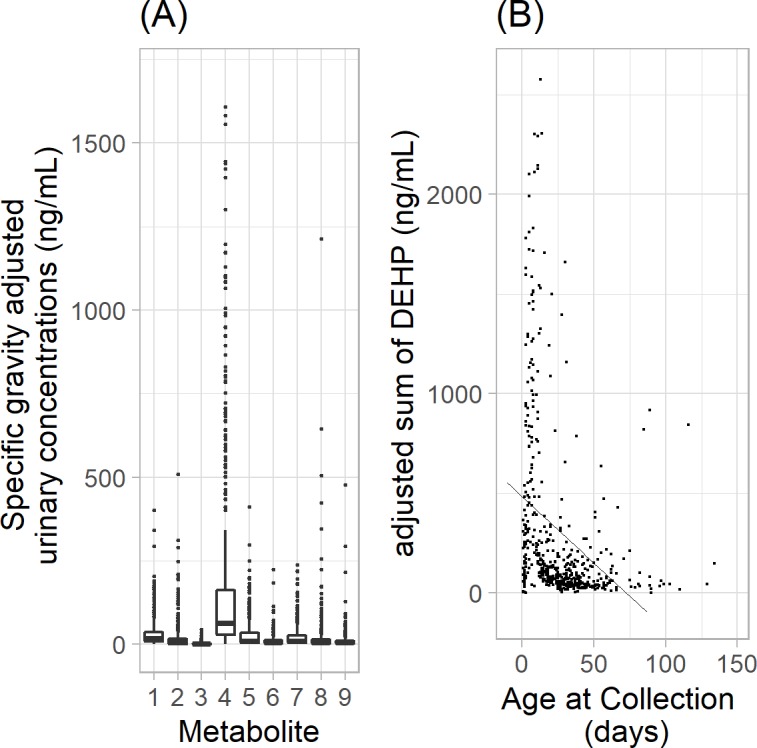
Distribution of representative phthalate biomarkers in NICU-HEALTH analysed to date. (A) Boxes mark 25th percentile, median and 75th percentile; bars mark 5th and 95th percentile. Interquartile ranges vary from 3-fold to 16-fold. (B) Phthalate exposure (represented by the sum of DEHP metabolites) decreases with chronological age. As preterm infants mature, they require less phthalate-exposing medical support. DEHP, di(2-ethylhexyl)phthalate; NICU-HEALTH, Neonatal Intensive Care Unit Hospital Exposures and Long-Term Health.
NICU-HEALTH enrolled 81 infants in phase I; phase II has enrolled 194 participants to date with planned enrolment of 225. We plan to continue enrolment to achieve a combined target over three phases of 400 infants.
Biospecimens
Infant urine
Urine collection is performed weekly in the NICU as previously described.26 We place pre-screened cotton balls in the diaper; those not contaminated with stool are retained for urine extraction. Urine is squeezed from the cotton, refrigerated immediately and frozen at −80°C. Urine is similarly collected at follow-up visits. Urine can be used to identify organic chemicals and metals.
Child and maternal hair
A small segment of hair is cut from the nape of the neck of participant infants and mothers during the NICU hospitalisation. Maternal hair is collected to capture prenatal exposures based on known hair growth rates and the duration of gestation. Infant hair is sampled immediately before NICU discharge to capture exposures during the hospitalisation. Hair is again collected from the child at the 2-year-old follow-up visit. Hair is stored dry in paper envelopes at room temperature. Hair provides a measure of temporally accumulated exposure and can be used to identify distinct periods of cortisol elevation27 or chemical exposure.28 Hair collected during follow-up visits will allow estimation of post-NICU exposures to include as model covariates.29
Saliva: stress biomarkers in preterm infants
Biologically active free cortisol can be measured in all fluids; salivary cortisol reflects levels of free cortisol in blood.30 Infants do not develop diurnal cortisol cycling until 44–48 weeks’ postmenstrual age.31 32 A saliva collection swab (Salmetrics, Carlsbad, California) is placed in the infant’s mouth before feeding, then centrifuged. Saliva is stored frozen at −80°C pending batched cortisol immunoassay.30
Meconium
Meconium/stool is collected weekly from the diaper of NICU-HEALTH participant infants during the NICU hospitalisation. Specimens are transferred from the diaper to pre-screened polypropylene collection cups and immediately frozen at −80°C. Stool specimens are appropriate for organic or inorganic biomarker analysis and certain microbiome assessments.
Blood
Blood spots are collected on Whatman cards from infant heel-stick specimens at the time of clinically indicated phlebotomy in the week before NICU discharge. Cards are stored at room temperature and are appropriate for a wide variety of analyses. In phase III, cards will be stored frozen to allow future metabolomics studies.
Additional specimen collection in NICU-HEALTH phase III will include saliva for nucleic acids, toenails for chemical exposure and teeth for re-creation of the prenatal and early-life exposome.
Clinical data
Outcome assessments
Serial neurodevelopmental assessments through NICU-HEALTH (table 2) allow development of a neurophenotype through childhood including behavioural, cognitive and motor domains affected by environmental exposures.19 33–41 We also collect longitudinal data on respiratory support during hospitalisation, respiratory diagnoses and growth parameters. Non-standard phenotyping methods are detailed below.
NICU Network Neurobehavioral Scale
The NICU Network Neurobehavioral Scale (NNNS), a standardised exam of infant behaviour, motor function and stress response42 43 reported as 13 summary scores, is associated with motor, cognitive and behavioural function in childhood.44 It is an established method for early detection of attention and motor deficits in preterm and toxin-exposed populations.45–47
Dense-array EEG
EEG is an objective measure of infant neurological function. Comfortable, commercially available dense-array mesh caps (Electrical Geodesics, Eugene, Oregon) can be placed on an infant in 3 min.48 Dense-array EEG can detect varying electrocortical power that increases with development. This test quantifies crucial milestones for early neurodevelopment49 including the development of functional connectivity,48 50 visual attention, recognition memory51 and processing pathways for visual50 and language information.52
(Phase III) Memory, attention and social cognition tasks
NICU-HEALTH will soon include objective assessments of visual attention, information processing speed and working memory at 7–8 months’ corrected age.53–55 These novel assessments adapt existing psychological tasks using a standardised video-based approach with infrared eye-tracking technology to record and objectively quantify infant looking behaviour.
The attention and visual recognition memory tasks use a paired-comparison paradigm53–57 with pairs of faces and of coloured geometric shapes.54 Eye fixations are tracked using an infrared eye tracker (SR Research, Canada) to determine total looking time at novel stimuli, number and average duration of fixations and fixation shift rate between stimuli.54
The third task tests Theory of Mind (ToM), the ability to infer other people’s intentions and beliefs and use them to predict behaviour. It is one aspect of cognition impaired in autism.58 59 Infants possess ToM abilities that traditional ‘false-belief’ tasks are not sensitive enough to capture.60 61 Our innovative task is based on the one developed by Kovács et al.62
(Phase III) Non-nutritive suck
NNS is one of the earliest motor skills in human development.63 Sucking and feeding require co-ordination and neural integration across more than 26 muscle pairs and more than 5 cranial nerves.64 65 As such, abnormalities in sucking and swallowing are considered markers of neonatal brain injury,66 67 and delayed sucking and feeding occurs in approximately 35%–48% of infants with neonatal brain injuries.68 Therefore, sucking and feeding behaviours can reflect development of central nervous system and potential neonatal brain injury, serving as a potential prognostic tool for detecting future developmental delays.69 70 During a 5 min assessment with a dedicated pacifier-pressure transducer, we will measure NNS cycles per burst, cycles per minute, amplitude, burst per minute, frequency and burst duration.
Patient and public involvement
Although there was no formal involvement of NICU parents or the general public in the development of the research question and outcome measures chosen for NICU-HEALTH, clinicians within the research team identified the research question and outcomes as being important for NICU families from informal discussions with parents of NICU patients. Phase III of NICU-HEALTH will be informed by the ECHO programme and it has both a formal Stakeholder Engagement committee and a Burden Task Force to gather data about participant feedback on the experience of executing the study protocol. Data from outreach efforts by these groups will help shape future additions of the NICU-HEALTH protocol.
Planned statistical analyses
NICU-HEALTH was designed with two explicit goals: to quantify the impact of NICU-based phthalate exposures on neurodevelopment and to facilitate and investigate the role of NICU-based environmental exposures in the development of preterm infants across multiple organ systems. To address our complex data structure, we employ two statistical approaches commonly in NICU-HEALTH analyses: weighted quantile sum (WQS) regression, which allows for objective consideration of multiple concurrent exposures, and latent class analysis (LCA), which allows for grouping of participants by similar performance across multiple scales of complex neurodevelopmental assessment tools.
WQS regression creates an empirically weighted index that identifies ‘bad actors’ based on non-negligible weights and yields an estimated mixture effect of the association between the exposure index and an outcome.71 72 Two steps are used to estimate a weighted index of standardised concentrations (eg, scored into quartiles) in a nonlinear model with a link function g(μ) to accommodate continuous, binary or count data: (A) across 100 bootstrap samples and (B) testing for the significance of the constructed WQS in a generalised linear model of the outcome. A test for the significance of β1 is a test for a mixture effect, which may be subthreshold for individual components, in the direction associated with the parameter estimate; detection of a signal in the opposite direction is possible by estimating the weighted index with a constraint on β1 to be <0 or >0. These constraints reduce ill-conditioning due to the complex correlations among the components. The strategy is robust to the correlation patterns in terms of sensitivity and specificity for identifying bad actors. The construction of weighted exposure indices can additionally be stratified by sex to test the effects of sex-specific mixtures in an integrated model, while also allowing for adjustment by additional relevant covariates.73
LCA is a probabilistic, model-based variant of traditional non-hierarchical cluster analysis44 74 which we and other groups have used to classify participants into discrete data-driven groups based on the performance on multiple neurodevelopmental assessment tool subscales. The LCA can be used as a single neurodevelopmental outcome in multinomial logistic regression modelling to reveal associations between NICU-based exposures and the latent class. As in our prior work,75 LCA can also facilitate probabilistic modelling of the association between a neurophenotype and concurrent NICU-based exposure to varying levels of multiple exposures.
Sample size
Sample size and power estimates are based on preliminary data from the NICU-HEALTH cohort. As one of the central goals of NICU-HEALTH is to facilitate study of the clinical impact of NICU-based environmental exposures, it is not powered on a single hypothesis. We propose to enrol 400 infants to achieve >80% power to detect differences in age-appropriate neurophenotypes. This number of participants was extrapolated from preliminary data on the relationship between NICU-based phthalate exposure on NNNS76 performance (table 3) as described in Stroustrup et al,26 and pilot data on the relationship between phthalate exposure and performance on the Bayley Scales of Infant and Toddler Development77 or the Child Behavior Checklist.78 Four hundred participants is an ample size to allow separate analyses of boys and girls for those exposures/outcomes known to be sexually dimorphic, for accommodation of the non-independence among twins in our population, and to account for an estimated 20% loss to follow-up. Published studies of environmental chemical or stress exposure in the NICU by other groups are based on cohorts of 6–63 participants.23 24 79–88 Analogous studies of prenatal exposures on term-born infants are based on 150–400 participants.89–91
Table 3.
Sample size estimation for sexually dimorphic outcomes with NICU-HEALTH twin rate and estimated 20% loss to follow-up
| Exposure | Representative NNNS Outcome Scales |
Power 0.7 | Power 0.8 | Power 0.9 |
| Phthalate mixture | NNNS Attention | 190 | 229 | 293 |
| NNNS Handling | 226 | 276 | 351 | |
| NNNS Non-Optimal Reflexes | 265 | 323 | 408 | |
| NNNS Regulation | 331 | 400 | 511 | |
| NNNS Excitability | 384 | 466 | 596 |
NICU-HEALTH, Neonatal Intensive Care Unit Hospital Exposures and Long-Term Health; NNNS, NICU Network Neurobehavioral Scale.
Findings to date
NICU-HEALTH has already contributed significantly to our understanding of phthalate exposure in the NICU. Phase I produced the first evidence of the clinical impact of phthalate exposure in the NICU population.26 Further study identified specific sources of exposure to clinically relevant phthalate mixtures in the NICU.92
For these studies, we applied a mixture-based outcome-driven approach, WQS regression,71 to assess the impact of concurrent exposure to multiple phthalates on performance on the NNNS. WQS generates a single index of exposure for the mixture allowing for an estimation of an overall mixture effect in a regression analysis. We used the geometric mean of the multiple concentration-adjusted measures of each monoester species to estimate NICU-based exposure for each infant. We then derived multiple WQS indices based on mixtures of monoester exposure, each weighted for a single NNNS summary scale adjusted for relevant covariates. Adjusted WQS regression indicated a significant association between NICU-based exposure to specific phthalate mixtures and improved performance on the NNNS Attention, Handling and Non-Optimal Reflexes summary scales.26
As NNNS performance improves with maturity, ‘better’ summary scale performance can be interpreted as attainment of neurodevelopmental milestones earlier than expected. Other studies have also reported a link between environmental exposures during the third trimester neurodevelopmental window and more rapid behavioural maturation. Specifically, Posner et al93 found that term-born infants exposed to elevated maternal stress in late pregnancy demonstrated behavioural and neuroanatomical phenotypes expected for children of an older age. They hypothesised that the stressful in utero environment during the third trimester period of development provoked rapid maturation as a protective mechanism for an anticipated later-life stress. When followed into middle childhood, these children demonstrated phenotypes of inattention and hyperactivity, recapitulating the recognised association between prenatal exposure to stress and poor behavioural outcome in middle childhood.94–97 Early ‘hyper-attention’ became maladaptive with age.
As our goal of risk reduction in the NICU necessitates identifying specific sources of exposure, we sought to identify sources of the clinically relevant phthalate mixture exposure we previously identified.26 92 In models accounting for concurrent equipment use, exposure to respiratory support was associated with DEHP biomarkers 50%–136% higher in exposed compared with unexposed infants (p=0.007–0.036). Phthalate mixtures clinically relevant to neurobehavioural development were significantly associated with non-invasive respiratory support.92 This finding allows efforts to mitigate exposure to clinically relevant phthalate mixtures through improvements in medical material composition. Future study of the NICU-HEALTH cohort could inform relatively inexpensive NICU interventions (eg, use of medical materials that do not leach neurotoxic chemicals, guidance on infant stress reduction) with significant potential to reduce lifelong morbidities common among NICU graduates.
Strengths and limitations
The scientific premise of our work, that NICU-based environmental exposures contribute to the abnormal development of preterm children, is supported by data linking exposures during the period of development that occurs while preterm infants are in the NICU with outcomes in term-born populations94 98–108; the heightened exposure to specific toxicants in the NICU79 82 88 109–111 and our own group’s early findings.26 92 Traditional NICU neurodevelopmental research has focused on medical complications without accounting for the role of the NICU environment6 7 16 112 113 and has failed to yield highly predictive outcome models. There are no prospective studies on the long-term neurodevelopmental impact of common, coincident NICU-based environmental exposures on the vulnerable and growing population of preterm infants. As NICU practice is constantly evolving, continued study of relevant materials and practice patterns are necessary to provide risk modification in the dynamic real-world setting.
Our cohort does have some limitations. Our patient population, preterm infants cared for in an academic level IV NICU, may not be representative of the entire NICU population. As data on ‘typical’ community-based exposure to phthalates in early infancy in non-NICU and non-preterm populations is not available—the youngest patients with phthalate biomarker measures in the National Health and Nutrition Examination Survey, for example, aged 6—comparison to a relevant non-NICU group is not possible. Confounding by indication presents an additional challenge in data analyses. Beyond phase I, we enrolled infants with low risk of serious morbidities of prematurity but predictably long NICU hospitalisation—those born after 28-0/7 to 32-6/7 weeks GA. This population requires care with a wide variety of medical equipment that is likely to convey exposure to chemical plasticisers. This equipment is needed to support immaturity in the absence of significant illness or physiological derangement. Analysis of data collected to date reveals no association between severity of illness at birth and biomarkers of exposure to phthalates, the family of organic chemicals most studied in our cohort to date (figure 3). Additionally, major morbidities of prematurity (sepsis, bronchopulmonary dysplasia, necrotising enterocolitis, intraventricular haemorrhage, retinopathy of prematurity) are not associated with phthalate biomarkers, nor is GA at birth. Nonetheless, we will continue to take an agnostic approach to all data interpretation, with comprehensive examination of indications that might explain any associations between the target exposures and outcomes.
Figure 3.
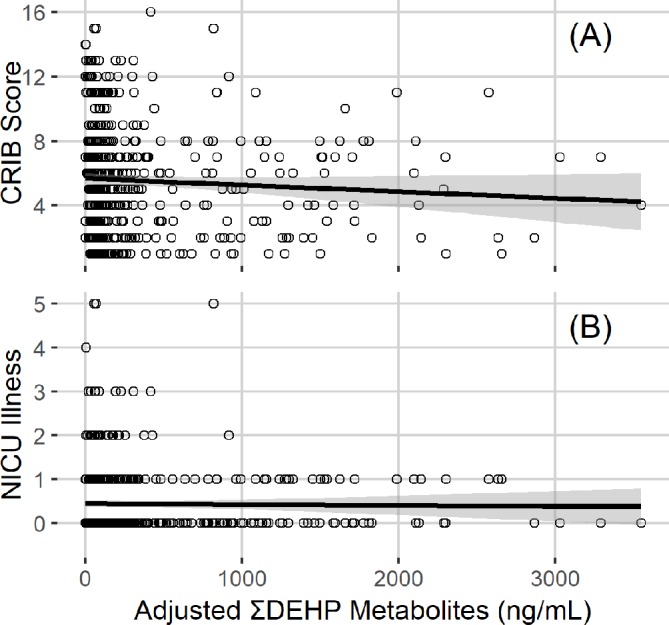
LOESS fit with SE bars showing the absence of significant relationship between NICU-based DEHP exposure and either (A) severity of illness at birth (CRIB score) or (B) NICU-based illness. DHEP, di(2-ethylhexyl)phthalate; LOESS, LOcally WEighted Scatter-plot Smoother; NICU, neonatal intensive care unit.
Collaboration
Requests for collaboration, either sample analyses or data analyses using the NICU-HEALTH data repository, can be made in writing to the principal investigator once the primary analyses planned have been completed. The NICU-HEALTH study management group will evaluate the request and if written approval is provided, a prespecified analytical plan will be requested.
Future directions
The NICU-HEALTH cohort will generate a wealth of biomarker, clinical and outcome data from which future studies of the impact of early-life chemical and non-chemical environmental exposures can benefit. We anticipate future analyses of the data and biospecimens collected, as well as future longitudinal follow-up of the NICU-HEALTH cohort beyond middle childhood. Findings from study of this cohort and other collaborating environmental health cohorts will likely translate into improvements in the hospital environment for infant development.
Supplementary Material
Footnotes
Collaborators: Requests for collaboration, either sample analyses or data analyses using the NICU-HEALTH data repository, can be made in writing to the principal investigator once the primary analyses planned have been completed. The NICU-HEALTH study management group will evaluate the request and if written approval is provided, a prespecified analytical plan will be requested.
Contributors: AS and JBB designed the study, facilitated and co-ordinated the samples and data collection. AS, JBB and EAS obtained the clinical data. AS, JBB, AA, EZ and JRI designed and performed clinical phenotyping. SAB, PCC and CG designed and performed the statistical analysis plan. SSA and MA designed and conducted the environmental chemical analysis plan. AS drafted this manuscript, and all authors made significant contributions to this manuscript and have read and approved the final version of it.
Funding: NICU-HEALTH is supported by the National Institutes of Health for the Environmental Influences on Child Health Outcomes (ECHO) programme through co-operative agreement UH3OD023320. Additional past funding for this cohort came through pilot grants from the Passport Foundation and the Mount Sinai Children’s Environmental Health Center, a National Institute of Environmental Health Sciences (NIEHS) mentored award K23ES022268 to Dr. Stroustrup, NIEHS centre grant P30ES023515 and the primary phase of the ECHO programme UG3OD023320. The study funders did not and will not have a role in or authority over study design; collection, management, analysis and interpretation of data; writing of reports or the decisions to submit reports for publication.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study has been continuously approved by the Icahn School of Medicine at Mount Sinai Program for the Protection of Human Subjects since 2011 (GCO 11-0664 and 12 -0332).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1. Patel RM. Short- and long-term outcomes for extremely preterm infants. Am J Perinatol 2016;33:318–28. 10.1055/s-0035-1571202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahlsson F, Kaijser M, Adami J, et al. . School performance after preterm birth. Epidemiology 2015;26:106–11. 10.1097/EDE.0000000000000171 [DOI] [PubMed] [Google Scholar]
- 3. de Jong M, Verhoeven M, van Baar AL. School outcome, cognitive functioning, and behaviour problems in moderate and late preterm children and adults: a review. Semin Fetal Neonatal Med 2012;17:163–9. 10.1016/j.siny.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 4. Downey LC, O'Shea TM, Allred EN, et al. . Antenatal and early postnatal antecedents of parent-reported attention problems at 2 years of age. J Pediatr 2015;166:20–5. 10.1016/j.jpeds.2014.08.004 [DOI] [PubMed] [Google Scholar]
- 5. Guy A, Seaton SE, Boyle EM, et al. . Infants born late/moderately preterm are at increased risk for a positive autism screen at 2 years of age. J Pediatr 2015;166:269–75. 10.1016/j.jpeds.2014.10.053 [DOI] [PubMed] [Google Scholar]
- 6. Johnson S, Marlow N. Preterm birth and childhood psychiatric disorders. Pediatr Res 2011;69:11R–18. 10.1203/PDR.0b013e318212faa0 [DOI] [PubMed] [Google Scholar]
- 7. Montagna A, Nosarti C. Socio-emotional development following very preterm birth: pathways to psychopathology. Front Psychol 2016;7:80 10.3389/fpsyg.2016.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Islam JY, Keller RL, Aschner JL, et al. . Understanding the short- and long-term respiratory outcomes of prematurity and bronchopulmonary dysplasia. Am J Respir Crit Care Med 2015;192:134–56. 10.1164/rccm.201412-2142PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McEvoy CT, Aschner JL. The natural history of bronchopulmonary dysplasia: the case for primary prevention. Clin Perinatol 2015;42:911–31. 10.1016/j.clp.2015.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scharf RJ, Stroustrup A, Conaway MR, et al. . Growth and development in children born very low birthweight. Arch Dis Child Fetal Neonatal Ed 2016;101:F433–8. 10.1136/archdischild-2015-309427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vasylyeva TL, Barche A, Chennasamudram SP, et al. . Obesity in prematurely born children and adolescents: follow up in pediatric clinic. Nutr J 2013;12:150 10.1186/1475-2891-12-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andrews B, Lagatta J, Chu A, et al. . The nonimpact of gestational age on neurodevelopmental outcome for ventilated survivors born at 23-28 weeks of gestation. Acta Paediatr 2012;101:574–8. 10.1111/j.1651-2227.2012.02609.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Conrad AL, Richman L, Lindgren S, et al. . Biological and environmental predictors of behavioral sequelae in children born preterm. Pediatrics 2010;125:e83–9. 10.1542/peds.2009-0634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pérez-Yarza EG, Moreno-Galdó A, Ramilo O, et al. . Risk factors for bronchiolitis, recurrent wheezing, and related hospitalization in preterm infants during the first year of life. Pediatr Allergy Immunol 2015;26:797–804. 10.1111/pai.12414 [DOI] [PubMed] [Google Scholar]
- 15. Vollsæter M, Skromme K, Satrell E, et al. . Children born preterm at the turn of the millennium had better lung function than children born similarly preterm in the early 1990s. PLoS One 2015;10:e0144243 10.1371/journal.pone.0144243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Linsell L, Malouf R, Morris J, et al. . Prognostic factors for poor cognitive development in children born very preterm or with very low birth weight: a systematic review. JAMA Pediatr 2015;169:1162–72. 10.1001/jamapediatrics.2015.2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kapellou O, Counsell SJ, Kennea N, et al. . Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med 2006;3:e265 10.1371/journal.pmed.0030265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bucci M, Marques SS, Oh D, et al. . Toxic stress in children and adolescents. Adv Pediatr 2016;63:403–28. 10.1016/j.yapd.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 19. Huang H-B, Chen H-Y, Su P-H, et al. . Fetal and childhood exposure to phthalate diesters and cognitive function in children up to 12 years of age: Taiwanese maternal and infant cohort study. PLoS One 2015;10:e0131910 10.1371/journal.pone.0131910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sheikh MA. Childhood physical maltreatment, perceived social isolation, and internalizing symptoms: a longitudinal, three-wave, population-based study. Eur Child Adolesc Psychiatry 2018;27:481–91. 10.1007/s00787-017-1090-z [DOI] [PubMed] [Google Scholar]
- 21. Tzanoulinou S, Sandi C. The programming of the social brain by stress during childhood and adolescence: from rodents to humans. Curr Top Behav Neurosci 2017;30:411–29. 10.1007/7854_2015_430 [DOI] [PubMed] [Google Scholar]
- 22. Trembath AN, Payne AH, Colaizy TT, et al. . The problems of moderate preterm infants. Semin Perinatol 2016;40:370–3. 10.1053/j.semperi.2016.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kahraman A, Başbakkal Z, Yalaz M, et al. . The effect of nesting positions on pain, stress and comfort during heel lance in premature infants. Pediatr Neonatol 2018;59:352–9. 10.1016/j.pedneo.2017.11.010 [DOI] [PubMed] [Google Scholar]
- 24. Vittner D, McGrath J, Robinson J, et al. . Increase in oxytocin from Skin-to-Skin contact enhances development of parent-infant relationship. Biol Res Nurs 2018;20:54–62. 10.1177/1099800417735633 [DOI] [PubMed] [Google Scholar]
- 25. Paneth N, Monk C. The importance of cohort research starting early in life to understanding child health. Curr Opin Pediatr 2018;30:292–6. 10.1097/MOP.0000000000000596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stroustrup A, Bragg JB, Andra SS, et al. . Neonatal intensive care unit phthalate exposure and preterm infant neurobehavioral performance. PLoS One 2018;13:e0193835 10.1371/journal.pone.0193835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kirschbaum C, Tietze A, Skoluda N, et al. . Hair as a retrospective calendar of cortisol production-Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology 2009;34:32–7. 10.1016/j.psyneuen.2008.08.024 [DOI] [PubMed] [Google Scholar]
- 28. Alves A, Kucharska A, Erratico C, et al. . Human biomonitoring of emerging pollutants through non-invasive matrices: state of the art and future potential. Anal Bioanal Chem 2014;406:4063–88. 10.1007/s00216-014-7748-1 [DOI] [PubMed] [Google Scholar]
- 29. Hinkelmann K, Muhtz C, Dettenborn L, et al. . Association between childhood trauma and low hair cortisol in depressed patients and healthy control subjects. Biol Psychiatry 2013;74:e15–17. 10.1016/j.biopsych.2013.04.021 [DOI] [PubMed] [Google Scholar]
- 30. Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology 1994;19:313–33. 10.1016/0306-4530(94)90013-2 [DOI] [PubMed] [Google Scholar]
- 31. Ivars K, Nelson N, Theodorsson A, et al. . Development of salivary cortisol circadian rhythm in preterm infants. PLoS One 2017;12:e0182685 10.1371/journal.pone.0182685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stalder T, Bäumler D, Miller R, et al. . The cortisol awakening response in infants: ontogeny and associations with development-related variables. Psychoneuroendocrinology 2013;38:552–9. 10.1016/j.psyneuen.2012.07.015 [DOI] [PubMed] [Google Scholar]
- 33. Derauf C, LaGasse L, Smith L, et al. . Infant temperament and high-risk environment relate to behavior problems and language in toddlers. J Dev Behav Pediatr 2011;32:125–35. 10.1097/DBP.0b013e31820839d7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lien Y-J, Ku H-Y, Su P-H, et al. . Prenatal exposure to phthalate esters and behavioral syndromes in children at 8 years of age: Taiwan maternal and infant cohort study. Environ Health Perspect 2015;123:95–100. 10.1289/ehp.1307154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roen EL, Wang Y, Calafat AM, et al. . Bisphenol A exposure and behavioral problems among inner city children at 7-9 years of age. Environ Res 2015;142:739–45. 10.1016/j.envres.2015.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chuang C-H, Jeng S-F, Hsieh W-S, et al. . Maternal psychosocial factors around delivery, and the behavior of 2-year-old children. Pediatr Int 2011;53:656–61. 10.1111/j.1442-200X.2010.03315.x [DOI] [PubMed] [Google Scholar]
- 37. Grizenko N, Shayan YR, Polotskaia A, et al. . Relation of maternal stress during pregnancy to symptom severity and response to treatment in children with ADHD. J Psychiatry Neurosci 2008;33:10–16. [PMC free article] [PubMed] [Google Scholar]
- 38. Téllez-Rojo MM, Cantoral A, Cantonwine DE, et al. . Prenatal urinary phthalate metabolites levels and neurodevelopment in children at two and three years of age. Sci Total Environ 2013;461-462:386–90. 10.1016/j.scitotenv.2013.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Whyatt RM, Liu X, Rauh VA, et al. . Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environ Health Perspect 2012;120:290–5. 10.1289/ehp.1103705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Coletti MF, Caravale B, Gasparini C, et al. . One-year neurodevelopmental outcome of very and late preterm infants: risk factors and correlation with maternal stress. Infant Behav Dev 2015;39:11–20. 10.1016/j.infbeh.2015.01.003 [DOI] [PubMed] [Google Scholar]
- 41. Zhu P, Sun M-S, Hao J-H, et al. . Does prenatal maternal stress impair cognitive development and alter temperament characteristics in toddlers with healthy birth outcomes? Dev Med Child Neurol 2014;56:283–9. 10.1111/dmcn.12378 [DOI] [PubMed] [Google Scholar]
- 42. Lester BM, Tronick EZ, Brazelton TB. The neonatal intensive care unit network neurobehavioral scale procedures. Pediatrics 2004;113:641–67. [PubMed] [Google Scholar]
- 43. Tronick E, Lester BM. Grandchild of the NBAS: the NICU network neurobehavioral scale (NNNS): a review of the research using the NNNS. J Child Adolesc Psychiatr Nurs 2013;26:193–203. 10.1111/jcap.12042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu J, Bann C, Lester B, et al. . Neonatal neurobehavior predicts medical and behavioral outcome. Pediatrics 2010;125:e90–8. 10.1542/peds.2009-0204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lester BM, Tronick EZ, LaGasse L, et al. . The maternal lifestyle study: effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics 2002;110:1182–92. 10.1542/peds.110.6.1182 [DOI] [PubMed] [Google Scholar]
- 46. Montirosso R, Del Prete A, Bellù R, et al. . Level of NICU quality of developmental care and neurobehavioral performance in very preterm infants. Pediatrics 2012;129:e1129–37. 10.1542/peds.2011-0813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smith JR, McGrath J, Brotto M, et al. . A randomized-controlled trial pilot study examining the neurodevelopmental effects of a 5-week M Technique intervention on very preterm infants. Adv Neonatal Care 2014;14:187–200. 10.1097/ANC.0000000000000093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fifer WP, Grieve PG, Grose-Fifer J, et al. . High-density electroencephalogram monitoring in the neonate. Clin Perinatol 2006;33:679–91. 10.1016/j.clp.2006.06.011 [DOI] [PubMed] [Google Scholar]
- 49. Benasich AA, Gou Z, Choudhury N, et al. . Early cognitive and language skills are linked to resting frontal gamma power across the first 3 years. Behav Brain Res 2008;195:215–22. 10.1016/j.bbr.2008.08.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tremblay E, Vannasing P, Roy M-S, et al. . Delayed early primary visual pathway development in premature infants: high density electrophysiological evidence. PLoS One 2014;9:e107992 10.1371/journal.pone.0107992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Reynolds GD, Richards JE. Cortical source localization of infant cognition. Dev Neuropsychol 2009;34:312–29. 10.1080/87565640902801890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ortiz-Mantilla S, Hämäläinen JA, Musacchia G, et al. . Enhancement of gamma oscillations indicates preferential processing of native over foreign phonemic contrasts in infants. J Neurosci 2013;33:18746–54. 10.1523/JNEUROSCI.3260-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rose SA, Feldman JF. Prediction of IQ and specific cognitive abilities at 11 years from infancy measures. Dev Psychol 1995;31:685–96. 10.1037/0012-1649.31.4.685 [DOI] [Google Scholar]
- 54. Rose SA, Feldman JF, Jankowski JJ. Attention and recognition memory in the 1st year of life: a longitudinal study of preterm and full-term infants. Dev Psychol 2001;37:135–51. 10.1037/0012-1649.37.1.135 [DOI] [PubMed] [Google Scholar]
- 55. Rose SA, Feldman JF, Jankowski JJ. Processing speed in the 1st year of life: a longitudinal study of preterm and full-term infants. Dev Psychol 2002;38:895–902. 10.1037/0012-1649.38.6.895 [DOI] [PubMed] [Google Scholar]
- 56. Rose SA, Feldman JF. Memory and speed: their role in the relation of infant information processing to later IQ. Child Dev 1997;68:630–41. 10.2307/1132115 [DOI] [PubMed] [Google Scholar]
- 57. Rose SA, Feldman JF, Jankowski JJ. Infant visual recognition memory: independent contributions of speed and attention. Dev Psychol 2003;39:563–71. 10.1037/0012-1649.39.3.563 [DOI] [PubMed] [Google Scholar]
- 58. Leslie AM, Frith U. Autistic children's understanding of seeing, knowing and believing. Br J Dev Psychol 1988;6:315–24. 10.1111/j.2044-835X.1988.tb01104.x [DOI] [Google Scholar]
- 59. Senju A, Southgate V, White S, et al. . Mindblind eyes: an absence of spontaneous theory of mind in Asperger syndrome. Science 2009;325:883–5. 10.1126/science.1176170 [DOI] [PubMed] [Google Scholar]
- 60. Onishi KH, Baillargeon R. Do 15-month-old infants understand false beliefs? Science 2005;308:255–8. 10.1126/science.1107621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Senju A, Southgate V, Snape C, et al. . Do 18-month-olds really attribute mental states to others? A critical test. Psychol Sci 2011;22:878–80. 10.1177/0956797611411584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kovács Ágnes Melinda, Téglás E, Endress AD. The social sense: susceptibility to others' beliefs in human infants and adults. Science 2010;330:1830–4. 10.1126/science.1190792 [DOI] [PubMed] [Google Scholar]
- 63. Wolff PH. The serial organization of sucking in the young infant. Pediatrics 1968;42:943–56. [PubMed] [Google Scholar]
- 64. Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: normal and abnormal. Phys Med Rehabil Clin N Am 2008;19:691–707. 10.1016/j.pmr.2008.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Travers JB. Motor control of feeding and drinking : Squire LR, Encyclopedia of neuroscience. Amsterdam; Boston: Elsevier/Academic Press, 2009: 1001–7. [Google Scholar]
- 66. Bingham PM. Deprivation and dysphagia in premature infants. J Child Neurol 2009;24:743–9. 10.1177/0883073808329530 [DOI] [PubMed] [Google Scholar]
- 67. Poore M, Barlow SM, Wang J, et al. . Respiratory treatment history predicts suck pattern stability in preterm infants. J Neonatal Nurs 2008;14:185–92. 10.1016/j.jnn.2008.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Slattery J, Morgan A, Douglas J. Early sucking and swallowing problems as predictors of neurodevelopmental outcome in children with neonatal brain injury: a systematic review. Dev Med Child Neurol 2012;54:796–806. 10.1111/j.1469-8749.2012.04318.x [DOI] [PubMed] [Google Scholar]
- 69. Adams-Chapman I, Bann CM, Vaucher YE, et al. . Association between feeding difficulties and language delay in preterm infants using Bayley Scales of Infant Development—Third edition. J Pediatr 2013;163:680–5. 10.1016/j.jpeds.2013.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Malas K, Trudeau N, Chagnon M, et al. . Feeding–swallowing difficulties in children later diagnosed with language impairment. Dev Med Child Neurol 2015;57:872–9. 10.1111/dmcn.12749 [DOI] [PubMed] [Google Scholar]
- 71. Carrico C, Gennings C, Wheeler DC, et al. . Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J Agric Biol Environ Stat 2015;20:100–20. 10.1007/s13253-014-0180-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gennings C, Carrico C, Factor-Litvak P, et al. . A cohort study evaluation of maternal PCB exposure related to time to pregnancy in daughters. Environ Health 2013;12 10.1186/1476-069X-12-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Brunst KJ, Sanchez Guerra M, Gennings C, et al. . Maternal lifetime stress and prenatal psychological functioning and decreased placental mitochondrial DNA copy number in the prism study. Am J Epidemiol 2017;186:1227–36. 10.1093/aje/kwx183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hagenaars JA, McCutcheon AL. Applied latent class analysis. Cambridge; New York: Cambridge University Press, 2002. [Google Scholar]
- 75. Stroustrup A, Hsu H-H, Svensson K, et al. . Toddler temperament and prenatal exposure to lead and maternal depression. Environ Health 2016;15 10.1186/s12940-016-0147-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lester BM, Andreozzi-Fontaine L, Tronick E, et al. . Assessment and evaluation of the high risk neonate: the NICU Network Neurobehavioral Scale. J Vis Exp 2014. doi: 10.3791/3368. [Epub ahead of print: 25 Aug 2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bayley N. Bayley scales of infant and toddler development. 3rd edn San Antonio, TX: 2006. [Google Scholar]
- 78. Assessment ASoEB Child behavior checklist 2019, 2019. Available: https://aseba.org/preschool/ [Accessed 25 Sep 2019].
- 79. Calafat AM, Needham LL, Silva MJ, et al. . Exposure to di-(2-ethylhexyl) phthalate among premature neonates in a neonatal intensive care unit. Pediatrics 2004;113:e429–34. 10.1542/peds.113.5.e429 [DOI] [PubMed] [Google Scholar]
- 80. Calafat AM, Weuve J, Ye X, et al. . Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect 2009;117:639–44. 10.1289/ehp.0800265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cong X, Wu J, Vittner D, et al. . The impact of cumulative pain/stress on neurobehavioral development of preterm infants in the NICU. Early Hum Dev 2017;108:9–16. 10.1016/j.earlhumdev.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Green R, Hauser R, Calafat AM, et al. . Use of di(2-ethylhexyl) phthalate-containing medical products and urinary levels of mono(2-ethylhexyl) phthalate in neonatal intensive care unit infants. Environ Health Perspect 2005;113:1222–5. 10.1289/ehp.7932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Karle VA, Short BL, Martin GR, et al. . Extracorporeal membrane oxygenation exposes infants to the plasticizer, di(2-ethylhexyl)phthalate. Crit Care Med 1997;25:696–703. 10.1097/00003246-199704000-00023 [DOI] [PubMed] [Google Scholar]
- 84. Plonait SL, Nau H, Maier RF, et al. . Exposure of newborn infants to di-(2-ethylhexyl)-phthalate and 2-ethylhexanoic acid following exchange transfusion with polyvinylchloride catheters. Transfusion 1993;33:598–605. 10.1046/j.1537-2995.1993.33793325058.x [DOI] [PubMed] [Google Scholar]
- 85. Provenzi L, Giusti L, Fumagalli M, et al. . Pain-related stress in the neonatal intensive care unit and salivary cortisol reactivity to socio-emotional stress in 3-month-old very preterm infants. Psychoneuroendocrinology 2016;72:161–5. 10.1016/j.psyneuen.2016.07.010 [DOI] [PubMed] [Google Scholar]
- 86. Su P-H, Chang Y-Z, Chang H-P, et al. . Exposure to di(2-ethylhexyl) phthalate in premature neonates in a neonatal intensive care unit in Taiwan. Pediatr Crit Care Med 2012;13:671–7. 10.1097/PCC.0b013e3182455558 [DOI] [PubMed] [Google Scholar]
- 87. von Rettberg H, Hannman T, Subotic U, et al. . Use of di(2-ethylhexyl)phthalate-containing infusion systems increases the risk for cholestasis. Pediatrics 2009;124:710–6. 10.1542/peds.2008-1765 [DOI] [PubMed] [Google Scholar]
- 88. Weuve J, Sánchez BN, Calafat AM, et al. . Exposure to phthalates in neonatal intensive care unit infants: urinary concentrations of monoesters and oxidative metabolites. Environ Health Perspect 2006;114:1424–31. 10.1289/ehp.8926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gray SAO, Jones CW, Theall KP, et al. . Thinking across generations: unique contributions of maternal early life and prenatal stress to infant physiology. J Am Acad Child Adolesc Psychiatry 2017;56:922–9. 10.1016/j.jaac.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jensen SKG, Pangelinan M, Björnholm L, et al. . Associations between prenatal, childhood, and adolescent stress and variations in white-matter properties in young men. Neuroimage 2018;182:389–97. 10.1016/j.neuroimage.2017.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yolton K, Xu Y, Strauss D, et al. . Prenatal exposure to bisphenol A and phthalates and infant neurobehavior. Neurotoxicol Teratol 2011;33:558–66. 10.1016/j.ntt.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Stroustrup A, Bragg JB, Busgang SA, et al. . Sources of clinically significant neonatal intensive care unit phthalate exposure. J Expo Sci Environ Epidemiol. In Press 2018. doi: 10.1038/s41370-018-0069-2. [Epub ahead of print: 21 Sep 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Posner J, Cha J, Roy AK, et al. . Alterations in amygdala-prefrontal circuits in infants exposed to prenatal maternal depression. Transl Psychiatry 2016;6:e935 10.1038/tp.2016.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Glover V. Prenatal stress and its effects on the fetus and the child: possible underlying biological mechanisms. Adv Neurobiol 2015;10:269–83. 10.1007/978-1-4939-1372-5_13 [DOI] [PubMed] [Google Scholar]
- 95. Grizenko N, Fortier Marie-Ève, Gaudreau-Simard M, et al. . The effect of maternal stress during pregnancy on IQ and ADHD symptomatology. J Can Acad Child Adolesc Psychiatry 2015;24:92–9. [PMC free article] [PubMed] [Google Scholar]
- 96. Scheinost D, Kwon SH, Lacadie C, et al. . Prenatal stress alters amygdala functional connectivity in preterm neonates. Neuroimage Clin 2016;12:381–8. 10.1016/j.nicl.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Udagawa J, Hino K. Impact of maternal stress in pregnancy on brain function of the offspring. Nihon Eiseigaku Zasshi 2016;71:188–94. 10.1265/jjh.71.188 [DOI] [PubMed] [Google Scholar]
- 98. Barrett ES, Parlett LE, Sathyanarayana S, et al. . Prenatal stress as a modifier of associations between phthalate exposure and reproductive development: results from a multicentre pregnancy cohort study. Paediatr Perinat Epidemiol 2016;30:105–14. 10.1111/ppe.12264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Capron LE, Glover V, Pearson RM, et al. . Associations of maternal and paternal antenatal mood with offspring anxiety disorder at age 18 years. J Affect Disord 2015;187:20–6. 10.1016/j.jad.2015.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Engel SM, Zhu C, Berkowitz GS, et al. . Prenatal phthalate exposure and performance on the Neonatal Behavioral Assessment Scale in a multiethnic birth cohort. Neurotoxicology 2009;30:522–8. 10.1016/j.neuro.2009.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hohwü L, Henriksen TB, Grønborg TK, et al. . Maternal salivary cortisol levels during pregnancy are positively associated with overweight children. Psychoneuroendocrinology 2015;52:143–52. 10.1016/j.psyneuen.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 102. Chiu Y-HM, Coull BA, Cohen S, et al. . Prenatal and postnatal maternal stress and wheeze in urban children: effect of maternal sensitization. Am J Respir Crit Care Med 2012;186:147–54. 10.1164/rccm.201201-0162OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Miodovnik A, Engel SM, Zhu C, et al. . Endocrine disruptors and childhood social impairment. Neurotoxicology 2011;32:261–7. 10.1016/j.neuro.2010.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Stout SA, Espel EV, Sandman CA, et al. . Fetal programming of children's obesity risk. Psychoneuroendocrinology 2015;53:29–39. 10.1016/j.psyneuen.2014.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Strømmen K, Lyche JL, Blakstad EW, et al. . Increased levels of phthalates in very low birth weight infants with septicemia and bronchopulmonary dysplasia. Environ Int 2016;89-90:228–34. 10.1016/j.envint.2016.01.024 [DOI] [PubMed] [Google Scholar]
- 106. Whyatt RM, Perzanowski MS, Just AC, et al. . Asthma in inner-city children at 5-11 years of age and prenatal exposure to phthalates: the Columbia Center for Children's Environmental Health Cohort. Environ Health Perspect 2014;122:1141–6. 10.1289/ehp.1307670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Whyatt RM, Rundle AG, Perzanowski MS, et al. . Prenatal phthalate and early childhood bisphenol A exposures increase asthma risk in inner-city children. J Allergy Clin Immunol 2014;134:1195–7. 10.1016/j.jaci.2014.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wright RJ. Psychological stress: a social pollutant that may enhance environmental risk. Am J Respir Crit Care Med 2011;184:752–4. 10.1164/rccm.201106-1139ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Aschner JL, Anderson A, Slaughter JC, et al. . Neuroimaging identifies increased manganese deposition in infants receiving parenteral nutrition. Am J Clin Nutr 2015;102:1482–9. 10.3945/ajcn.115.116285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Mörelius E, He H-G, Shorey S. Salivary cortisol reactivity in preterm infants in neonatal intensive care: an integrative review. Int J Environ Res Public Health 2016;13. doi: 10.3390/ijerph13030337. [Epub ahead of print: 18 Mar 2016]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Srinath BK, Shah J, Kumar P, et al. . Kangaroo care by fathers and mothers: comparison of physiological and stress responses in preterm infants. J Perinatol 2016;36:401–4. 10.1038/jp.2015.196 [DOI] [PubMed] [Google Scholar]
- 112. Ochiai M, Ichiyama M, Iwayama M, et al. . Longitudinal study of very low birth weight infants until 9 years of age; attention deficit hyperactivity and autistic features are correlated with their cognitive functions. Early Hum Dev 2015;91:783–6. 10.1016/j.earlhumdev.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 113. Poggi C, Giusti B, Gozzini E, et al. . Genetic contributions to the development of complications in preterm newborns. PLoS One 2015;10:e0131741 10.1371/journal.pone.0131741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Newnham CA, Inder TE, Milgrom J. Measuring preterm cumulative stressors within the NICU: the neonatal infant stressor scale. Early Hum Dev 2009;85:549–55. 10.1016/j.earlhumdev.2009.05.002 [DOI] [PubMed] [Google Scholar]
- 115. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry 1987;150:782–6. 10.1192/bjp.150.6.782 [DOI] [PubMed] [Google Scholar]
- 116. Ingram PB, Clarke E, Lichtenberg JW. Confirmatory factor analysis of the perceived stress scale-4 in a community sample. Stress Health 2016;32:173–6. 10.1002/smi.2592 [DOI] [PubMed] [Google Scholar]
- 117. Berry C, Shalowitz M, Quinn K, et al. . Validation of the crisis in family Systems-Revised, a contemporary measure of life stressors. Psychol Rep 2001;88:713–24. 10.2466/pr0.2001.88.3.713 [DOI] [PubMed] [Google Scholar]
- 118. Bilker WB, Hansen JA, Brensinger CM, et al. . Development of abbreviated nine-item forms of the raven's standard progressive matrices test. Assessment 2012;19:354–69. 10.1177/1073191112446655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lipton LR, Brunst KJ, Kannan S, et al. . Associations among prenatal stress, maternal antioxidant intakes in pregnancy, and child temperament at age 30 months. J Dev Orig Health Dis 2017;8:638–48. 10.1017/S2040174417000411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Marshall GN. Posttraumatic stress disorder symptom checklist: factor structure and English-Spanish measurement invariance. J Trauma Stress 2004;17:223–30. 10.1023/B:JOTS.0000029265.56982.86 [DOI] [PubMed] [Google Scholar]
- 121. Schmidt RJ, Walker CK, Bennett D, et al. . The early life exposures assessment tool (ELEAT) Davis, Ca: University of California, Davis; 2015, 2018. Available: https://eleat.ucdavis.edu/ [Accessed 11 May 2018].
- 122. Gartstein MA, Rothbart MK. Studying infant temperament via the revised infant behavior questionnaire. Infant Behavior and Development 2003;26:64–86. 10.1016/S0163-6383(02)00169-8 [DOI] [Google Scholar]
- 123. Achenbach System of Empirically Based Assessment. Preschool (Ages 1-1/2–5) Assessments Burlington, VT: ASEBA; 2016, 2016. Available: http://www.aseba.org/preschool.html [Accessed 4 Jul 2016].
- 124. HealthMeasures.net Intro to NIH Toolbox Chicago, IL: Northwestern University; 2018, 2018. Available: http://www.healthmeasures.net/explore-measurement-systems/nih-toolbox [Accessed 11 May 2018].
- 125. Bayley N. Bayley scales of infant and toddler development. 3rd edn San Antonio, TX: The Psychological Corporation, 2006. [Google Scholar]
- 126. Bonner S, Matte T, Rubin M, et al. . Validating an asthma case detection instrument in a head start sample. J Sch Health 2006;76:471–8. 10.1111/j.1746-1561.2006.00144.x [DOI] [PubMed] [Google Scholar]
- 127. Goodman R. The strengths and difficulties questionnaire: a research note. J Child Psychol Psychiatry 1997;38:581–6. 10.1111/j.1469-7610.1997.tb01545.x [DOI] [PubMed] [Google Scholar]
- 128. Wei Y, Oakland T, Algina J. Multigroup confirmatory factor analysis for the adaptive behavior assessment system-II parent form, ages 5–21. Am J Ment Retard 2008;113:178–86. 10.1352/0895-8017(2008)113[178:MCFAFT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 129. HealthMeasures.net Intro to PROMIS Chicago, IL: Northwestern University; 2018, 2018. Available: http://www.healthmeasures.net/explore-measurement-systems/promis [Accessed 11 May 2018].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



