Abstract
Objectives
To provide evidence for targeted smoking cessation policy, the aim of this study was to compare pregnancy outcomes of Aboriginal mothers who reported not smoking during pregnancy with Aboriginal mothers who reported smoking during pregnancy.
Design
Population based retrospective cohort study using linked data.
Setting
New South Wales, the most populous Australian state.
Population
18 154 singleton babies born to 13 477 Aboriginal mothers between 2010 and 2014 were identified from routinely collected New South Wales datasets. Aboriginality was determined from birth records and from four linked datasets through an Enhanced Reporting of Aboriginality algorithm.
Exposure
Not smoking at any time during pregnancy.
Main outcome measures
Unadjusted and adjusted relative risks (aRR) and 95% CIs from modified Poisson regression were used to examine associations between not smoking during pregnancy and maternal and perinatal outcomes including severe morbidity, inter-hospital transfer, perinatal death, preterm birth and small-for-gestational age. Population attributable fractions (PAFs) were calculated using adjusted relative risks.
Results
Compared with babies born to mothers who smoked during pregnancy, babies born to non-smoking mothers had a lower risk of all adverse perinatal outcomes including perinatal death (aRR=0.58, 95% CI 0.44 to 0.76), preterm birth (aRR=0.58, 95% CI 0.53 to 0.64) and small-for-gestational age (aRR=0.35, 95% CI 0.32 to 0.39). PAFs (%) were 27% for perinatal death, 26% for preterm birth and 48% for small-for-gestational-age. Compared with women who smoked during pregnancy (n=8919), those who did not smoke (n=9235) had a lower risk of being transferred to another hospital (aRR=0.76, 95% CI 0.66 to 0.89).
Conclusions
Babies born to women who did not smoke during pregnancy had a lower risk of adverse perinatal outcomes. Rates of adverse outcomes among Aboriginal non-smokers were similar to those among the general population. These results quantify the proportion of adverse perinatal outcomes due to smoking and highlight why effective smoking cessation programme are urgently required for this population.
Keywords: pregnancy, smoking, Aboriginal health, preterm birth, stillbirth, linked data
Strengths and limitations of this study.
The first study to examine the association between not smoking in pregnancy and pregnancy outcomes among Aboriginal women.
A large population-based cohort study using whole-of-population linked data.
To improve ascertainment of Aboriginal status, which is under-recorded on routinely collected health datasets, we linked four databases and applied an Enhanced Reporting of Aboriginality algorithm.
The inclusion of population attributable fractions quantifies the potential reduction in adverse perinatal outcomes if it was possible to reduce the smoking during pregnancy rate to zero.
Data on history, heaviness, or passive smoking were not available, nor were data on some potential confounders such as alcohol consumption.
Introduction
In 2008 the Australian federal, state and territory governments committed to reducing the national adult daily smoking rate by 2018, including halving the Aboriginal adult smoking rate.1 Although smoking rates have substantially declined over this time, they remain high among pregnant Aboriginal women. In 2016, 41% of all pregnant Aboriginal women reported smoking at some time during their pregnancy compared with just 7% of non-Aboriginal women.2 Smoking during pregnancy is the ‘most important preventable risk factor for maternal and infant health’,3 thus smoking cessation for pregnant Aboriginal women remains a key priority for New South Wales (NSW) Health.4 For the purposes of this study, Aboriginal and/or Torres Strait Islander people were considered together in one group. The reason for this was the small proportion of Torres Strait Islander people living in NSW (an estimated 2.6% of all females of Aboriginal and/or Torres Strait Islander descent5) and that some people were recorded as both. We respectfully use the term Aboriginal as Aboriginal people are the original inhabitants of NSW.6
Australia’s anti-tobacco campaigns and smoking cessation strategies are among the most comprehensive in the world, and there is growing evidence that programmes specifically targeted to Aboriginal Australians are more effective.7 There have been several campaigns to promote smoking cessation among pregnant Aboriginal mothers with varying efficacy.8 To date these have been grounded in evidence from a general population. Although the benefits of not smoking during pregnancy are unlikely to be any different for Aboriginal mothers from the general population, quantifying the benefits of not smoking among Aboriginal mothers may be regarded as more relevant by this population and thus have the potential to influence smoking cessation. The benefits of not smoking during pregnancy are well established,9–13 but no previous studies have demonstrated associations between not smoking in pregnancy and positive pregnancy outcomes among Aboriginal women. This study aims to compare pregnancy outcomes of mothers who reported not smoking during pregnancy with those who reported any smoking during pregnancy from the Aboriginal population of NSW. Findings from this study will provide the most relevant evidence to date for pregnant Aboriginal women.
Methods
Study population and data sources
The study population consisted of all singleton babies born to Aboriginal women residing in NSW between 1 January 2010 and 31 December 2014 and their mothers. This population-based retrospective cohort study used linked data from routinely collected NSW datasets. The study population was identified from all records in the NSW Perinatal Data Collection (‘birth data’) for the period 1 January 2010 to 31 December 2014. All births in the population, including births at NSW public and private hospitals and home births are recorded in the birth data. This surveillance system includes all live births and stillbirths of at least 400 g birth weight or at least 20 weeks gestation.14
All deaths within NSW are registered in the Registry of Births, Deaths and Marriages and fact of death was retrieved from these data between 1 January 2010 and 31 December 2015. Public and private hospital admission records were drawn from the NSW Admitted Patient Data Collection (‘hospital data’) for admissions from 1 September 2009 to 31 December 2014. An additional 4 months of hospital data were retrieved prior to the start of the study period to allow for admissions to hospital for births early in 2010. Diagnoses coded in the hospital data are applied according to the International Classification of Diseases, Australian Modification (ICD-10-AM). Records within and across all datasets were probabilistically linked using personal identifiers by the NSW Centre for Health Record Linkage with an estimated false linkage rate of less than 5 per 1000 records.15 Hospital birth records were those where the birth was recorded to have occurred between the mother’s admission and discharge dates using the linked birth data. It’s estimated that 96% of records from the birth data link to the mother’s and infant’s hospital records from the birth.16
Aboriginal women were defined as those who were recorded as Australian Aboriginal in the birth data or who were assigned Aboriginal status according to the Enhanced Reporting of Aboriginality (ERA) algorithm.
Enhanced Reporting of Aboriginality
It is widely acknowledged that Aboriginal status is under-recorded on routinely collected health datasets nationwide.17 Enhancement of reporting of Aboriginal people using linked records creates a statistical construct that results in improved information about Aboriginal people. It does not define a person as being Aboriginal, nor does it replace efforts to improve the overall quality of recording Aboriginal status at the point of care.
Information surrounding individuals’ Aboriginal status was pooled via linkage of the birth data, NSW Registry of Births, Deaths and Marriages birth registrations, hospital data and the NSW Emergency Department Data Collection. Using this information, a weight of evidence surrounding a woman’s Aboriginal status was determined by a multistage median algorithm.18 Since multiple datasets were used and some women had multiple records in each of these datasets, the algorithm initially assigned a separate status for each woman and dataset. Aboriginal or Torres Strait Islander status was assigned to a mother if: one or two linked records were available and at least one reported her as Aboriginal; three or more linked records were available and at least two reported her as Aboriginal. A comparable algorithm using dataset-specific statuses instead of records was used to determine the inclusion of each woman in the study population.
The enhanced reporting of Aboriginality is a technique used by many research groups.19–21 Although this combination of datasets and algorithm has not been used before, similar methods have been found to minimise the risk of incorrect inclusion while capturing more women than simply relying on a single record.22 Details on the algorithm, the data used and the mothers identified through the ERA have been described in more detail elsewhere.23
Exposure
The exposure of interest for this study was not smoking at any time during pregnancy. Mothers who reported not smoking during pregnancy will henceforth be referred to as non-smokers and those who reported any smoking during pregnancy are referred to as smokers. To increase ascertainment, birth data and mother’s hospital birth record(s) were used to assign smoking status. If the birth data indicated that a mother smoked at any time during her pregnancy and/or recorded her as a current smoker within the hospital birth record(s) (according to the ICD-10-AM diagnosis codes Z72.0 and F17) then she was considered to be a smoker. The sensitivity and specificity of current smoking from the most recent separation in the hospital data is estimated to be 58.5% and 98.4%, respectively.24 Where a mother had multiple hospital records associated with the birth and those records contradicted each other according to smoking status, her smoking status defaulted to that recorded in the birth data.
Outcomes
Maternal outcomes were identified using the birth data and the mother’s hospital birth record and included two binary outcomes: severe maternal morbidity and inter-hospital transfer. Severe maternal morbidity was defined using a validated composite indicator that captures a broad range of diagnoses and procedures such as cardiac arrest, renal failure or assisted ventilation.25 Mothers requiring inter-hospital transfer were defined as those with at least one record with a mode of separation indicating transfer or where multiple hospitalisation records were present with differing hospital codes.
Perinatal outcomes, including birth outcomes and those occurring within the first 28 days of life for the baby were retrieved from the birth data and the baby’s linked hospital and birth registration records. These included perinatal death (stillbirth and neonatal death), preterm birth (<37 completed weeks of gestation), and small for gestational age (birth weight <3rd and/or 10th percentile for sex and age26). Admissions to a special care nursery (SCN) or neonatal intensive care unit (NICU) were assessed among an eligible population of babies born in a hospital classified as level 3 or above (NSW Ministry of Health’s Guide to the Role Delineation of Hospitals) or a private hospital. Severe neonatal morbidity, measured according to a validated composite indicator,27 was assessed among all live births.
Covariates
Maternal age and parity were reported according to the birth data. The mother’s chronic conditions, hypertension and diabetes information were obtained from the birth data and the hospital birth record(s). We used the broad category of any hypertension rather than the specific categories of chronic hypertension, pregnancy hypertension, pre-eclampsia and eclampsia, as there is known misclassification among types of hypertension.28 The NSW ranking of the Australian Bureau of Statistics 2011 Socio-Economic Index for Areas (SEIFA) Index of Relative Socio-Economic Disadvantage and the 2011 Remoteness Areas were used to assess the mother’s relative socioeconomic status and access to services, respectively. Where available, the mother’s 2011 Statistical Local Area (SLA) according to her birth data was used to assign these measures. Otherwise, and for all babies born in 2010, the mother’s 2010 SLA was used. Hospital type is an indicator of the size of a hospital and its location (urban or regional)29 and was assigned using the hospital code recorded in the birth data.
Statistical analyses
The study population was described using frequencies and percentages by potential confounders and the mother’s smoking status. Summary statistics were calculated by mother’s smoking status to investigate the associations between smoking during pregnancy and maternal and child outcomes. To estimate the unadjusted and adjusted relative risk (RR) of binary outcomes while accounting for the correlation within the data (some mothers had more than one baby during the study period), an extension to the modified Poisson regression30 was used with an unstructured correlation matrix. Those observations where data were missing for an outcome were excluded from analysis for that outcome. SAS for Windows V.9.4 was used for all data manipulation and analysis.
In view of the established causal relationship between smoking and adverse perinatal outcomes, we quantified the proportion and number of adverse perinatal outcomes that would not have occurred in this population if all the mothers had been non-smokers during pregnancy. We used the formula: Population attributable fraction (PAF) =[Ps(RRs−1)]/RRs, where Ps is the proportion of babies with the outcome whose mothers smoked and RRs is the adjusted RR for smokers. The RRs is the inverse of the RR for non-smokers.
Patient and public involvement
An Aboriginal advisory committee was consulted prior to submission of the study proposal to ethics committees and throughout the process. The committee provided guidance on presentation and interpretation of results. It was of particular importance to members of the committee that the results were framed positively, that is, the benefits of not smoking, rather than the risks of smoking. It was also important to committee members that all comparisons were among Aboriginal women and that Aboriginal women were not compared with non-Aboriginal women. There are plans to develop culturally appropriate educational material based on the results of this research and in collaboration with Aboriginal Health Workers and others involved in the care of Aboriginal women who are pregnant or may be planning a pregnancy.
Results
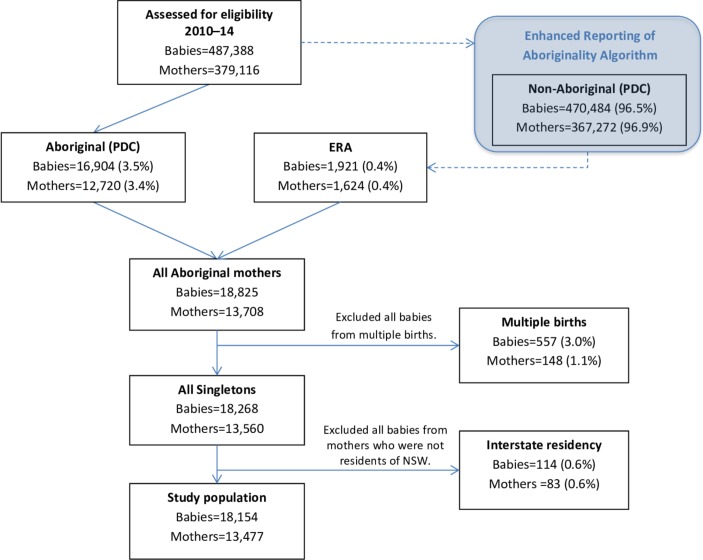
Following exclusion of duplicates (n=76), a total of 487 388 babies were born to 379 116 mothers in NSW and were assessed for inclusion in this study. Records for 16 904 babies born to 12 720 mothers who were recorded as Aboriginal in the birth data were available for analysis. An additional 1921 babies born to 1624 mothers were identified as eligible for inclusion in the study using the ERA. Of the total 18 825 babies, 557 were from a multiple birth and 114 were born to mothers who were not residents of NSW. These babies did not meet the eligibility criteria and were excluded. Thus, the final study population consisted of 18 154 singleton babies born to 13 477 Aboriginal mothers. Figure 1 outlines the flow of participants in this study.
Figure 1.
Flow diagram of mothers and babies eligible for inclusion in the final study population. ERA, Enhanced Reporting of Aboriginality; NSW, New South Wales.
Among the study population, 9235 (51%) babies were born to non-smoking mothers and 8919 (49%) were born to smoking mothers (table 1). Only two per cent of all linked records had contradictory smoking statuses from the birth and hospital data. For comparison, when smoking status was assigned only according to the birth data, 52% of babies were born to non-smoking mothers and 48% were born to smoking mothers. Mothers who reported not smoking at any time during their pregnancy were generally less disadvantaged than their smoking counterparts; approximately 8.1% of non-smoking mothers were in the highest SEIFA quintile, compared with just 4.1% of smoking mothers. Non-smoking mothers were older, lived in less remote regions and had fewer previous pregnancies than smoking mothers. The number of non-smoking mothers with hypertension (1106) was almost double that of smoking mothers (578) and slightly more non-smoking mothers had diabetes (table 1).
Table 1.
Demographics at the time of birth of all Aboriginal or Torres Strait Islander mothers who gave birth to at least one singleton baby in New South Wales (NSW) between 2010 and 2014 reported for all births and by smoking status during pregnancy
| All births | Non-smoking | Smoking | ||||
| n=18 154 | Nns=9235 (51%) | Ns=8919 (49%) | ||||
| n | % | n | % | n | % | |
| Year of baby’s birth | ||||||
| 2010 | 3487 | 19 | 1740 | 50* | 1747 | 50* |
| 2011 | 3380 | 19 | 1638 | 48* | 1742 | 52* |
| 2012 | 3680 | 20 | 1833 | 50* | 1847 | 50* |
| 2013 | 3716 | 20 | 1944 | 52* | 1772 | 48* |
| 2014 | 3891 | 21 | 2080 | 53* | 1811 | 47* |
| Maternal age | ||||||
| Under 20 | 3214 | 18 | 1568 | 17 | 1646 | 19 |
| 20–24 | 6014 | 33 | 2983 | 32 | 3031 | 34 |
| 25–29 | 4608 | 25 | 2381 | 26 | 2227 | 25 |
| 30–34 | 2729 | 15 | 1455 | 16 | 1274 | 14 |
| 35 and over | 1589 | 8.8 | 848 | 9.2 | 741 | 8.3 |
| Total | 18 154 | 100 | 9235 | 100 | 8919 | 100 |
| Parity | ||||||
| 0 | 6259 | 35 | 3720 | 40 | 2539 | 29 |
| 1 | 4709 | 26 | 2589 | 28 | 2120 | 24 |
| 2 | 3107 | 17 | 1490 | 16 | 1617 | 18 |
| 3+ | 4072 | 22 | 1431 | 16 | 2641 | 30 |
| Total | 18 147 | 100 | 9230 | 100 | 8917 | 100 |
| SEIFA IRSD quintiles† | ||||||
| First—most disadvantaged | 4827 | 27 | 2131 | 23 | 2696 | 30 |
| Second | 3674 | 20 | 1887 | 21 | 1787 | 20 |
| Third | 5375 | 30 | 2806 | 31 | 2569 | 29 |
| Fourth | 3068 | 17 | 1617 | 18 | 1451 | 16 |
| Fifth—least disadvantaged | 1115 | 6.2 | 748 | 8.1 | 367 | 4.1 |
| Total | 18 059 | 100 | 9189 | 100 | 8870 | 100 |
| Remoteness area | ||||||
| Major cities | 4193 | 23 | 2246 | 24 | 1947 | 22 |
| Inner regional | 6147 | 34 | 3310 | 36 | 2837 | 32 |
| Outer regional | 6097 | 34 | 2966 | 32 | 3131 | 35 |
| Remote | 1027 | 5.7 | 421 | 4.6 | 606 | 6.8 |
| Very remote | 595 | 3.3 | 245 | 2.7 | 350 | 4.0 |
| Total | 18 059 | 100 | 9188 | 100 | 8871 | 100 |
| Hospital level | ||||||
| Tertiary | 4099 | 23 | 2108 | 23 | 1991 | 22 |
| Small and medium urban | 308 | 1.7 | 178 | 1.8 | 130 | 1.5 |
| Large urban | 2895 | 16 | 1607 | 9 | 1288 | 14 |
| Small regional | 3441 | 19 | 1519 | 16 | 1922 | 22 |
| Medium regional | 3042 | 17 | 1550 | 17 | 1492 | 17 |
| Large regional | 3897 | 21 | 1896 | 21 | 2001 | 22 |
| Private | 336 | 1.7 | 323 | 3.2 | 13 | 0.2 |
| Other | 136 | 0.7 | 54 | 0.6 | 82 | 0.9 |
| Total | 18 154 | 100 | 9235 | 100 | 8919 | 100 |
| Chronic conditions‡ | ||||||
| Yes | 343 | 1.9 | 147 | 1.6 | 196 | 2.2 |
| Total | 18 154 | 100 | 9235 | 100 | 8919 | 100 |
| Any hypertension | ||||||
| Yes | 1684 | 9.3 | 1106 | 12 | 578 | 6.5 |
| Total | 18 154 | 100 | 9235 | 100 | 8919 | 100 |
| Any diabetes | ||||||
| Yes | 1413 | 7.8 | 804 | 8.7 | 609 | 6.5 |
| Total | 18 154 | 100 | 9235 | 100 | 8919 | 100 |
*Percentage of all births within each year.
†Socio-Economic Index for Areas—Index of Relative Socio-Economic Disadvantage (SEIFA IRSD). When ranking areas within NSW in order of their relative disadvantage, the lowest 20% (most disadvantaged) fall in the first quintile and the highest 20% (least disadvantaged) fall in fifth quartile.
‡Chronic conditions encompasses renal, cardiac, thyroid, asthma, psychiatric, and other autoimmune conditions.40
The majority (70%) of mothers only had one baby during the study period however a substantial number had multiple: 25% had two, 4.4% had three and 0.4% had four. For 564 (4%) mothers, their smoking status changed between pregnancies, 6814 (53%) mothers reported not smoking in all pregnancies during the study period and 6099 (47%) consistently reported smoking. Of the 564 mothers whose smoking status changed between pregnancies, 266 (47%) changed from smoking to non-smoking, 271 (48%) changed from non-smoking to smoking in all subsequent pregnancies and 27 (5%) moved between smoker and non-smoker status.
The rate of severe maternal morbidity was low (<3%) and not significantly different between smoking and non-smoking mothers (table 2). The rate of inter-hospital transfer was lower in the non-smoking group at 3.7% compared with the smoking group (5.1%), with an adjusted RR of RR=0.76 (95% CI 0.66 to 0.89).
Table 2.
Frequencies of maternal outcomes at the time of birth of all Aboriginal mothers by smoking status during pregnancy
| All births | Non-smoking | Smoking | Unadjusted | Adjusted | ||||
| n=18 154 | Nns=9235 | Ns=8919 | RR (95% CI) | RR (95% CI) | ||||
| n | % | n | % | n | % | |||
| Severe maternal morbidity | ||||||||
| Yes | 523 | 2.9 | 257 | 2.8 | 266 | 3.0 | 0.94 (0.79 to 1.12) | 0.92* (0.77 to 1.11) |
| Inter-hospital transfer | ||||||||
| Yes | 793 | 4.4 | 337 | 3.7 | 456 | 5.1 | 0.73 (0.63 to 0.84) | 0.76† (0.66 to 0.89) |
*Adjusted for maternal age, any hypertension, any diabetes, parity and socioeconomic status (Socio-Economic Index for Areas (SEIFA)).
†Adjusted for maternal age, any hypertension, any diabetes, parity and remoteness area.
RR, relative risk.
Adverse perinatal outcomes occurred less frequently among babies born to non-smoking mothers (table 3). Perinatal deaths were rare in both populations; however, the rate was lower in the non-smoking group with perinatal death occurring in 1.0% of babies born to non-smoking mothers, compared with 1.8% in smoking mothers. Also, severe neonatal morbidity and admission to SCN or NICU was less frequent in babies born to non-smoking mothers when compared with those born to smoking mothers. Overall, the gestational age of babies from the non-smoking group was closer to term than those from the smoking group; more babies born to non-smoking mothers (66%) were born between 39 and 41 weeks than those born to smoking mothers (55%). Preterm birth was considerably less frequent among babies born to mothers who did not smoke during pregnancy; 8.2% of births to non-smoking mothers were preterm compared with 14% from smoking mothers. Similarly, babies born to non-smoking mothers were less often small for gestational age, with 2.0% and 7.0% of these babies having a birth weight below the 3rd and 10th percentiles, respectively compared with 7.3% and 20% of babies of smoking mothers. All RRs were less than 1, suggesting a reduced risk of all adverse outcomes among babies born to non-smoking mothers when compared with those born to smoking mothers. Of note were the RRs for perinatal death (RR=0.58, 95% CI 0.44 to 0.76), preterm birth (RR=0.58, 95% CI 0.53 to 0.64) and small for gestational age (<10th percentile; RR=0.35, 95% CI 0.32 to 0.39). As indicated by the PAFs (%) in table 3, more than a quarter of the perinatal deaths and preterm births were attributable to smoking and almost half the small for gestational age births. Among this cohort of babies, this equates to 68 perinatal deaths, 540 preterm births and 1131 small for gestational age (<10th percentile) babies attributable to smoking.
Table 3.
Frequencies of perinatal outcomes among all babies born to Aboriginal or Torres Strait Islander mothers by maternal smoking status
| NSW population 14 |
All births | Non-smoking | Smoking | Unadjusted | Adjusted* | PAF (%) | ||||
| n=18 154 | Nns=9235 | Ns=8919 | RR (95% CI) | RR (95% CI) | ||||||
| % | n | % | n | % | n | % | ||||
| Preterm birth (<37 weeks) | ||||||||||
| Yes | 8 | 2045 | 11 | 760 | 8.2 | 1285 | 14 | 0.59 (0.54 to 0.64) |
0.58 (0.53 to 0.64) |
26 |
| Total | 18 154 | 100 | 9235 | 100 | 8919 | 100 | ||||
|
Small for gestational age
(<3rd population percentile) |
||||||||||
| Yes | 3 | 835 | 4.6 | 183 | 2.0 | 652 | 7.3 | 0.28 (0.23 to 0.32) |
0.27 (0.23 to 0.32) |
57 |
| Total | 18 132 | 100 | 9229 | 100 | 8903 | 100 | ||||
|
Small for gestational age
(<10th population percentile) |
||||||||||
| Yes | 10 | 2381 | 13 | 641 | 7.0 | 1740 | 20 | 0.36 (0.33 to 0.39) |
0.35 (0.32 to 0.39) |
48 |
| Total | 18 132 | 100 | 9229 | 100 | 8903 | 100 | ||||
| Severe neonatal morbidity | Among live births only | |||||||||
| Yes | 5 | 1470 | 8.2 | 636 | 6.9 | 834 | 9.5 | 0.74 (0.67 to 0.81) |
0.70 (0.63 to 0.77) |
17 |
| Total | 17 978 | 100 | 9169 | 100 | 8809 | 100 | ||||
| Admission to SCN or NICU† | ||||||||||
| Yes | 15 | 3957 | 22 | 1645 | 18 | 2312 | 26 | 0.70 (0.66 to 0.75) |
0.66 (0.63 to 0.70) |
20 |
| Total | 17 809 | 100 | 9059 | 100 | 8750 | 100 | ||||
| Perinatal death | Rate per 1000 total births | |||||||||
| Yes | 8 | 254 | 14 | 92 | 10‡ | 162 | 18‡ | 0.54 (0.42 to 0.70) |
0.58 (0.44 to 0.76) |
27 |
| Stillborn | 6 | 162 | 60 | 6.5‡ | 102 | 11‡ | 0.57 (0.41 to 0.78) |
0.60 (0.43 to 0.84) |
20 | |
| Rate per 1000 live births | ||||||||||
| Neonatal death | 2 | 92 | 32 | 3.5§ | 60 | 6.8§ | 0.50 (0.33 to 0.78) |
0.54 (0.34 to 0.86) |
30 | |
*Adjusted for maternal age, any hypertension, any diabetes, parity and socioeconomic status (Socio-Economic Index for Areas (SEIFA)).
†Admission to Special Care Nursery (SCN) or Neonatal Intensive Care Unit (NICU) was restricted to those babies recorded as being born in a hospital of maternity service level 3 or higher or a private hospital.
‡Rate per 1000 total births.
§Rate per 1000 live births.
PAF, Population Attributable Fraction; RR, relative risk.
Discussion
This study of a recent population of pregnant Aboriginal women clearly demonstrates improved pregnancy outcomes among Aboriginal mothers who reported not smoking during pregnancy when compared with Aboriginal mothers who reported smoking during pregnancy. Benefits of not smoking were found for all the perinatal outcomes we examined. We also found non-smoking mothers had a 24% lower risk of being transferred to another hospital during the birth admission than smoking mothers of similar demographics. Inter-hospital transfers may be due to complications arising before, during or after the birth. This means women are less likely to be away from their family and country during this challenging time. Although a slightly lower risk of severe maternal morbidity was found in the non-smoking group, there was not sufficient evidence to suggest a true difference existed as the CI included 1 (RR=0.92, 95% CI 0.77 to 1.11). Other risk factors may be more strongly associated with severe maternal morbidity than smoking.
Among babies born to mothers of a similar age, with similar pre-existing conditions (any diabetes or hypertension), parity and socio-economic status, those with a non-smoking mother had a 42% less risk of perinatal death and preterm birth, 65% less risk of being small-for-gestational age (<10th percentile), 30% less risk of severe neonatal morbidity, and 34% less risk of being admitted to a SCN or NICU than those born to a mother who smoked at any time during her pregnancy. The reductions in adverse outcomes for babies born to non-smoking mothers were statistically and clinically significant and remained so even after adjustment. Encouragingly, despite some rates being marginally higher, overall very little difference exists between the rates of adverse perinatal outcomes among the non-smoking Aboriginal mothers in this study and the overall NSW population of mothers giving birth in 2014, of whom 9.3% reported smoking and 3.9% were recorded as Aboriginal.14 The high PAFs for the adverse perinatal outcomes highlight the enormous potential for health improvements in this population. Over a quarter of the perinatal deaths and preterm births were attributable to smoking. Being born small for gestational age is associated with short and long-term health sequelae, and these risks are even greater for babies born with a birth weight less than the third percentile for gestational age and sex. The PAF (%) was highest (57%) for being born with a birth weight less than the third percentile. Almost half (48%) the babies born small for gestational age (<10th percentile) could have had a normal birth weight (≥10th percentile) in the absence of smoking. Our results are consistent with a recent study of a cohort of 697 003 children born in Scotland from 1997 to 2009.31 In addition to the adverse perinatal outcomes attributable to smoking, this study followed children until 5 years of age and found that maternal smoking during pregnancy also increased the risk of the child being hospitalised with acute respiratory infections, bronchiolitis, asthma and bacterial meningitis.31
As expected, and similar to findings from other studies,9 31 mothers from the non-smoking group were less disadvantaged, older, resided in less remote regions and had fewer previous pregnancies than those from the smoking group. Diabetes and hypertension were more prevalent among non-smoking mothers than smoking mothers. The small difference in prevalence of diabetes (8.7% vs 6.5%) could be due to the non-smoking group being slightly older than the smoking group. However, the prevalence of hypertension in non-smoking mothers was almost double that of smoking mothers (12% vs 6.5%). While this finding may surprise some, it is consistent with findings from previous studies.32–35 A systematic review of 48 studies concluded that smoking during pregnancy reduces the risk of pre-eclampsia by up to 50% and that there is a dose–response relationship.33 Similar results have been reported when the outcome includes gestational hypertension as well as pre-eclampsia, and the protective effect appears to continue even after women quit smoking later in pregnancy.35 This protective effect may be mediated via the biological effects of carbon monoxide that is formed during smoking.34 However, when pre-eclampsia does occur, the outcomes are much worse for babies whose mothers smoked.32 Although pre-eclampsia is associated with adverse pregnancy outcomes, and smoking reduces the incidence of pre-eclampsia, the net effect of smoking is still a worsening of pregnancy outcomes and there are dose-dependent increases in perinatal deaths and SGA babies among mothers who smoke.32 Hence, these findings in no way indicate any benefit to mothers or babies if the mother smokes during pregnancy.
As well as being a national health priority in Australia, reducing smoking during pregnancy is a key performance indicator in the annual service agreements between the NSW Ministry of Health and Local Health Districts.36 As part of this commitment, the Quit for New Life programme was established in 2013 with the aim to support women having an Aboriginal baby to quit smoking. The programme was integrated into Aboriginal Maternal and Infant Health Services and has supported over 2500 pregnant women, 950 postnatal women and 1650 cohabitants in their quit attempt.37 However, further efforts including health professional training, expansion to other maternal health services and community programme, and improved data collection and reporting are required to reduce the prevalence of smoking in pregnancy in this population. Investment to discourage women, especially young women, from taking up smoking and encouraging and appropriately supporting smokers to quit need to remain priorities.
Health professionals have a critical role in communicating the benefits of not smoking during pregnancy found in this study. However, some practitioners perceive intervention to be ineffective and thus may not raise this issue with their patients.38 The highly relevant evidence from this study may increase the salience of the issue and provide further motivation for health professionals to consistently ask and advise about smoking.
While the health impacts of smoking on maternal and child health are well known,9–13 this study provides local information that can be used to further engage Australian health professionals and community members on the benefits of not smoking. Building on the strength and resilience of Aboriginal people is an important foundation for efforts to reduce smoking among this population.39 Using local evidence on the benefits of not smoking during pregnancy has the potential to reframe health messages for women, their families and communities and to mobilise community action to achieve better health outcomes.
Strengths and limitations
This is the first study we are aware of that examines associations between smoking in pregnancy and adverse pregnancy outcomes exclusively among Aboriginal and/or Torres Strait Islander women. This was a large population-based study. Using data linkage, we were able to capture more women through the ERA, further increasing our sample size. Despite the unavailability of information surrounding some potential confounders, including individual level socioeconomic status, our findings were consistent with those among other populations from the literature.9–13 Limited data on the heaviness of smoking during pregnancy meant that potential dose effects could not be calculated. However, new data around quitting in pregnancy is available from 2016 onward so there is potential for future work to examine this phenomenon further. Similarly, no information was available on the mother’s history of smoking, exposure to environmental tobacco smoke or alcohol consumption and so effects from longer term smoking and potential confounding from alcohol consumption could not be accounted for. A lack of data surrounding history and heaviness of smoking means that the treatment effects estimated in this study are likely to be biassed toward the null and thus underestimate the true benefits of not smoking in pregnancy. Under-ascertainment of smoking status would similarly bias toward the null. Mothers who smoked in one pregnancy but not in a subsequent pregnancy were classified as non-smokers in the subsequent pregnancy. If these mothers were more likely to have worse outcomes in the subsequent pregnancy compared with never smoking mothers, this would also bias towards the null. However, any effect would be negligible due to the very low numbers (<2% of the study population).
Conclusions
Babies born to Aboriginal mothers who did not smoke during pregnancy were at a significantly reduced risk of adverse perinatal outcomes compared with those born to smoking mothers of similar demographics. Rates of these adverse outcomes among Aboriginal women who did not smoke were very similar to those among the general NSW population.
These results reinforce the importance of targeted smoking cessation policy for Aboriginal women. Barriers to smoking cessation in this population are complex and it is vital that this evidence is provided concurrently with sufficient support to enable Aboriginal women to quit smoking. Distributing this information in isolation runs the risk of furthering shame and stress experienced by pregnant women and may discourage them from seeking further help, highlighting the importance of systematic approaches to encourage and support Aboriginal women to quit smoking.
Supplementary Material
Acknowledgments
The authors would like to thank the members of the Aboriginal Advisory Committee who provided valuable advice, the NSW Ministry of Health for providing access to the datasets used and the Centre for Health Record Linkage for linking these datasets.
Footnotes
Contributors: JM and AM had the initial idea for this study. ST wrote the study proposal and was responsible for the ethics application and revisions to the manuscript. CM undertook all analyses, with guidance from ST, II and DR, and drafted the manuscript. JBF, JMM, JM, AM and ST all contributed to the design of the study and, with DM, the interpretation of the results. All authors commented on drafts and read and approved the final manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. CM and ST are guarantors.
Funding: This work was supported by a NSW Ministry of Health Prevention Research Support Programme grant This work was completed while Carol McInerney was employed as a trainee on the NSW Biostatistics Training Programme funded by the NSW Ministry of Health. She undertook this work whilstwhile based at the Women and Babies Research, Kolling Institute, Northern Sydney Local Health District. Carol McInerney and Siranda Torvaldsen are supported by the NSW Ministry of Health Prevention Research Support Programme grant. The funder had no role in the study design, analysis or interpretation of the data or in the writing of the report. However, the NSW Ministry of Health requires all Biostatistics Trainees to seek their approval before submitting a manuscript for publication.
Competing interests: CM and ST’s salaries came from a Prevention Research Support Programme grant from the NSW Ministry of Health, no other relationships or activities that could appear to have influenced the submitted work.
Patient consent for publication: Not required.
Ethics approval: Ethics approval for this study was given by the Aboriginal Health and Medical Research Council of New South Wales, Australia (HREC reference number: 1326/17) and was exempt from informed consent requirements as there was no contact with the study population and the authors only had access to de-identified data.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available.
References
- 1. Department of Health Tobacco Control - key facts and figures, 2018. Available: http://www.health.gov.au/internet/publications/publishing.nsf/Content/tobacco-control-toc
- 2. Centre for Epidemiology and Evidence Nsw mothers and babies 2016. Sydney: NSW Ministry of Health, 2017. [Google Scholar]
- 3. Gould GS, Cadet-James Y, Clough AR. Getting over the shock: taking action on Indigenous maternal smoking. Aust J Prim Health 2016;22:276–82. 10.1071/PY15066 [DOI] [PubMed] [Google Scholar]
- 4. NSW Ministry of Health Snapshot of tobacco strategy 2012-2017, 2017. [Google Scholar]
- 5. Australian Bureau of Statistics Estimates of Aboriginal and Torres Strait Islander Australians, 2013. Available: http://www.abs.gov.au/ausstats/abs@.nsf/mf/3238.0.55.001
- 6. NSW Department of Health Communicating positively - A guide to appropriate Aboriginal terminology. Sydney, Australia: NSW Department of Health, 2004. [Google Scholar]
- 7. Carson KV, Brinn MP, Peters M, et al. Interventions for smoking cessation in Indigenous populations. Cochrane Database Syst Rev 2012;118 10.1002/14651858.CD009046.pub2 [DOI] [PubMed] [Google Scholar]
- 8. Passey ME, Sanson-Fisher RW, Stirling JM. Supporting pregnant Aboriginal and Torres Strait Islander women to quit smoking: views of antenatal care providers and pregnant Indigenous women. Matern Child Health J 2014;18:2293–9. 10.1007/s10995-013-1373-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine & Tobacco Res 2004;6:125–40. 10.1080/14622200410001669187 [DOI] [PubMed] [Google Scholar]
- 10. Marufu TC, Ahankari A, Coleman T, et al. Maternal smoking and the risk of still birth: systematic review and meta-analysis. BMC Public Health 2015;15:239 10.1186/s12889-015-1552-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mendelsohn C, Gould GS, Oncken C. Management of smoking in pregnant women. Aust Fam Physician 2014;43:46–51. [PubMed] [Google Scholar]
- 12. Salihu HM, Wilson RE. Epidemiology of prenatal smoking and perinatal outcomes. Early Hum Dev 2007;83:713–20. 10.1016/j.earlhumdev.2007.08.002 [DOI] [PubMed] [Google Scholar]
- 13. US Department of Health and Human Services The health consequences of Smoking—50 years of progress: a report of the surgeon General. Atlanta, GA: US Department of Health and Human Services CfDCaP, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, editor, 2014. [Google Scholar]
- 14. Centre for Epidemiology and Evidence Nsw mothers and babies 2014. Sydney, Australia: NSW Ministry of Health, 2016. [Google Scholar]
- 15. Centre for Health Record Linkage [Internet] Quality assurance, 2018. Available: http://www.cherel.org.au/quality-assurance
- 16. Bentley JP, Ford JB, Taylor LK, et al. Investigating linkage rates among probabilistically linked birth and hospitalization records. BMC Med Res Methodol 2012;12 10.1186/1471-2288-12-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Australian Institute of Health and Welfare and Australian Bureau of Statistics National best practice guidelines for data linkage activities relating to Aboriginal and Torres Strait Islander people (contract NO: cat. No. IHW 74). Canberra, Australia: AIHW, 2012. [Google Scholar]
- 18. Christensen D, Davis G, Draper G, et al. Evidence for the use of an algorithm in resolving inconsistent and missing Indigenous status in administrative data collections. Aust J Soc Issues 2014;49:423–43. 10.1002/j.1839-4655.2014.tb00322.x [DOI] [Google Scholar]
- 19. Population and Public Health Division Improved reporting of Aboriginal and Torres Strait Islander peoples on population datasets in New South Wales using record linkage–a feasibility study. Sydney, Australia: NSW Ministry of Health, 2012. [Google Scholar]
- 20. Xu F, Sullivan EA, Madden RC, et al. Improvement of maternal Aboriginality in NSW birth data. BMC Med Res Methodol 2012;12:8 10.1186/1471-2288-12-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taylor LK, Bentley J, Hunt J, et al. Enhanced reporting of deaths among Aboriginal and Torres Strait Islander peoples using linked administrative health datasets. BMC Med Res Methodol 2012;12 10.1186/1471-2288-12-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gibberd AJ, Simpson JM, Eades SJ. Use of family relationships improved consistency of identification of Aboriginal people in linked administrative data. J Clin Epidemiol 2017;90:144–55. 10.1016/j.jclinepi.2017.06.021 [DOI] [PubMed] [Google Scholar]
- 23. McInerney C, Ibiebele I, Torvaldsen S, et al. Defining a study population using enhanced reporting of Aboriginality and the effects on study outcomes. International Journal of Population Data Science. In Press 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Havard A, Jorm LR, Lujic S. Risk adjustment for smoking identified through tobacco use diagnoses in hospital data: a validation study. PLoS One 2014;9:e95029 10.1371/journal.pone.0095029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roberts CL, Cameron CA, Bell JC, et al. Measuring maternal morbidity in routinely collected health data: development and validation of a maternal morbidity outcome indicator. Med Care 2008;46:786–94. 10.1097/MLR.0b013e318178eae4 [DOI] [PubMed] [Google Scholar]
- 26. Dobbins TA, Sullivan EA, Roberts CL, et al. Australian National birthweight percentiles by sex and gestational age, 1998-2007. Med J Aust 2012;197:291–4. 10.5694/mja11.11331 [DOI] [PubMed] [Google Scholar]
- 27. Lain SJ, Algert CS, Nassar N, et al. Incidence of severe adverse neonatal outcomes: use of a composite indicator in a population cohort. Matern Child Health J 2012;16:600–8. 10.1007/s10995-011-0797-6 [DOI] [PubMed] [Google Scholar]
- 28. Roberts CL, Bell JC, Ford JB, et al. The accuracy of reporting of the hypertensive disorders of pregnancy in population health data. Hypertension in Pregnancy 2008;27:285–97. 10.1080/10641950701826695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Falster MO, Roberts CL, Ford J, et al. Development of a maternity hospital classification for use in perinatal research. N S W Public Health Bull 2012;23:12–16. 10.1071/NB11026 [DOI] [PubMed] [Google Scholar]
- 30. Zou GY, Donner A. Extension of the modified poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res 2013;22:661–70. 10.1177/0962280211427759 [DOI] [PubMed] [Google Scholar]
- 31. Lawder R, Whyte B, Wood R, et al. Impact of maternal smoking on early childhood health: a retrospective cohort linked dataset analysis of 697 003 children born in Scotland 1997–2009. BMJ Open 2019;9:e023213 10.1136/bmjopen-2018-023213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cnattingius S, Mills JL, Yuen J, et al. The paradoxical effect of smoking in preeclamptic pregnancies: smoking reduces the incidence but increases the rates of perinatal mortality, abruptio placentae, and intrauterine growth restriction. Am J Obstet Gynecol 1997;177:156–61. 10.1016/S0002-9378(97)70455-1 [DOI] [PubMed] [Google Scholar]
- 33. England L, Zhang J. Smoking and risk of preeclampsia: a systematic review. Front Biosci 2007;12:2471–83. 10.2741/2248 [DOI] [PubMed] [Google Scholar]
- 34. Karumanchi SA, Levine RJ. How does smoking reduce the risk of preeclampsia? Hypertension 2010;55:1100–1. 10.1161/HYPERTENSIONAHA.109.148973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang J, Klebanoff MA, Levine RJ, et al. The puzzling association between smoking and hypertension during pregnancy. Am J Obstet Gynecol 1999;181:1407–13. 10.1016/S0002-9378(99)70384-4 [DOI] [PubMed] [Google Scholar]
- 36. New South Wales Health Nsw health service agreement, 2017. Available: https://www.health.nsw.gov.au/Performance/Documents/service-agreement-generic.pdf
- 37. Centre for Population Health Quit for new life: new South Wales health, 2018. Available: https://www.health.nsw.gov.au/tobacco/Pages/quit-for-new-life.aspx
- 38. The Royal Australian College of General Practitioners Supporting smoking cessation, 2014. Available: https://www.racgp.org.au/your-practice/guidelines/smoking-cessation/the-role-of-health-professionals/
- 39. Aboriginal Health & Medical Research Council of NSW and NSW Ministry of Health The ATRAC framework: a strategic framework for Aboriginal tobacco resistance and control in NSW. Sydney, Australia, 2014. [Google Scholar]
- 40. Chen JS, Roberts CL, Simpson JM, et al. Use of hospitalisation history (lookback) to determine prevalence of chronic diseases: impact on modelling of risk factors for haemorrhage in pregnancy. BMC Med Res Methodol 2011;11 10.1186/1471-2288-11-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.