Abstract
Introduction
Worldwide, 2 million patients aged 18–50 years suffer a stroke each year, and this number is increasing. Knowledge about global distribution of risk factors and aetiologies, and information about prognosis and optimal secondary prevention in young stroke patients are limited. This limits evidence-based treatment and hampers the provision of appropriate information regarding the causes of stroke, risk factors and prognosis of young stroke patients.
Methods and analysis
The Global Outcome Assessment Life-long after stroke in young adults (GOAL) initiative aims to perform a global individual patient data meta-analysis with existing data from young stroke cohorts worldwide. All patients aged 18–50 years with ischaemic stroke or intracerebral haemorrhage will be included. Outcomes will be the distribution of stroke aetiology and (vascular) risk factors, functional outcome after stroke, risk of recurrent vascular events and death and finally the use of secondary prevention. Subgroup analyses will be made based on age, gender, aetiology, ethnicity and climate of residence.
Ethics and dissemination
Ethical approval for the GOAL study has already been obtained from the Medical Review Ethics Committee region Arnhem-Nijmegen. Additionally and when necessary, approval will also be obtained from national or local institutional review boards in the participating centres. When needed, a standardised data transfer agreement will be provided for participating centres. We plan dissemination of our results in peer-reviewed international scientific journals and through conference presentations. We expect that the results of this unique study will lead to better understanding of worldwide differences in risk factors, causes and outcome of young stroke patients.
Keywords: young adults, stroke, recurrent vascular events, global cohort, meta-analysis, risk factors, prognosis
Strengths and limitations of this study.
By combining existing individual data sets, the Global Outcome Assessment Life-long after stroke in young adults initiative will be the largest study on young patients with stroke ever, with more than 10 000 patients already included.
Sufficient statistical power due to large individual data set for subanalysis on stroke subtypes, gender, ethnicity, climate.
Risk of misclassification and missing data due to the use of existing data gathered with varying protocols and cohorts.
Variability in diagnostic workup and non-obligatory imaging-based confirmation of stroke due to long inclusion period and varying cohorts.
Mainly hospital-based cohorts are included, risking inclusion bias.
Background
Worldwide, 2 million patients aged 18–50 years suffer a stroke each year.1 2 Due to physical, cognitive and emotional post-stroke consequences faced by individual patients after stroke that often occur early in life, our societies face high socioeconomic costs.1–3 The absolute number of young patients who live with the consequences of stroke is expected to increase rapidly due to a rising incidence in ischaemic stroke and increasing long-term survival.1 2
Patients with stroke at young age comprise a heterogeneous group with many different underlying causes. Furthermore, the aetiology remains unknown in one-third of all young stroke patients.4 Information regarding causative risk factors and aetiology and about long-term prognosis including the risk of recurrence or death, the potential of recovery and the range of sequelae is scarce and mainly based on smaller studies.5 Most previous studies of young patients with ischaemic stroke comprised less than 1000 patients, with even smaller numbers in aetiologic subgroups. Studies regarding young patients with intracerebral haemorrhage are even smaller. In addition, only few studies have taken ethnicity, geographical region and climate of residence into account.1–3 Finally, the optimal secondary prevention strategy for young stroke patients is poorly defined as numbers of young patients are low in randomised trials on secondary prevention.6 As a consequence, guidelines on secondary prevention or counselling after stroke do not provide specific information for individual young patients.
Information regarding specific subgroups of young ischaemic stroke patients and larger numbers of young intracerebral haemorrhage patients are long awaited and can be helpful in treating and counselling individual patients. Recent reviews have stressed the importance of initiating large collaborative studies in order to develop reliable prognostic models based on clinical and demographic features, diagnostics and genetics and establish stroke guidelines specifically for patients at young age.6–8
We therefore launched the ‘Global Outcome Assessment Life-long after stroke in young adults’ (GOAL) initiative to collect individual patient data worldwide, with the aim of performing an individual patient data meta-analysis. The GOAL initiative aims to investigate the aetiology, risk factors, functional outcome, risk of recurrent vascular events and death after stroke on young age with special emphasis on ethnic and regional variation.
Methods
Study objective
The GOAL initiative aims to collect individual patient data from young stroke cohorts that included consecutive patients. We aim to perform an individual patient data meta-analysis to assess aetiology, risk factors, functional outcome and prognosis in patients aged 18 up to and including 50 (18–50) years with an ischaemic stroke or intracerebral haemorrhage (ICH).
Specific study questions
What are the risk factors for stroke at young age?
What are the causes of stroke in young patients aged 18–50 years?
What is the functional outcome after stroke in patients aged 18–50 years?
What is the cumulative risk of recurrent vascular events and of death in young stroke patients?
What are the differences in risk factors and causes of stroke, case fatality and prognosis between patients with different clinical (eg, stroke subtype) and demographic characteristics (eg, age, sex, ethnicity and climate)?
Patient eligibility
Inclusion criteria
Ischaemic stroke or intracerebral haemorrhage, according to the definition of the WHO.9
Age 18–50 years.
Available individual patient data including at least one of the following variables of interest: risk factors of stroke, cause of stroke, functional outcome or follow-up data regarding recurrent events and death.
Exclusion criteria
Traumatic intracerebral haematoma.
Intracerebral haemorrhage or ischaemic stroke due to intracerebral malignancy.
Subarachnoid haemorrhage.
Cerebral venous thrombosis with or without brain ischaemia/cerebral haemorrhage.
Any iatrogenic stroke as a result of surgery or any other medical interventions.
Retinal infarction.
Definition of stroke
Stroke is defined as a rapidly evolving focal neurological deficit, without positive phenomena such as twitches, jerks or myoclonus, with no other cause, and symptoms persisting for more than 24 hours.9 Stroke will be further divided into intracerebral haemorrhage and ischaemic stroke based on neuroimaging. Haemorrhagic transformation of an ischaemic stroke will be classified as an ischaemic stroke.
Study design
The GOAL initiative is an international multicenter consortium in which individual patient data will be collected from young stroke cohorts. The relevant cohort studies were identified through a systematic search of PubMed using the following Mesh Major Topics: ‘Young Adult’, ‘Stroke’, ‘Risk Factors’, ‘Stroke/etiology’, ‘Prognosis’ and ‘Secondary Prevention’ (online supplementary appendix 1). References of relevant studies were examined to detect other potential cohorts of interest. Prospective and retrospective, as well as hospital-based and population-based cohorts were considered eligible for enrolment if the patients would meet our inclusion criteria. The principal investigators were contacted and informed about the study with the request to participate. Furthermore, when participants indicated they knew of other cohorts or collaborators who might be interested in participating, we contacted these centres. A specific website for potential participants was developed to provide more information about the aims of the project (www.goalinitiative.org). An overview of the participating centres is listed in table 1 and figure 1. Analyses and results on baseline variables will be conducted and before March 2020. Furthermore, the GOAL study strives to be as inclusive as possible. Therefore, we have set an ongoing open invitation for all researchers in the field who are interested in participating. We do this with the aim of setting up a worldwide young stroke consortium with an ongoing data collection and data analyses on a broad range of aspects of young stroke. Therefore, we cannot indicate a precise time period for conducting all GOAL-related studies.
Table 1.
Overview of participating cohorts in alphabetical order
| Country | Population | Study period | IS patients | ICH patients | Design | Patient selection | Stroke definition |
| Australia | Hospital-based multicenter | 2006–2010 | 306 | N.A. | Retrospective | Consecutive | WHO |
| Australia | Hospital-based multicenter | 2006–2018 | N.A. | 100* | Retrospective | Consecutive | Radiological |
| Austria | Hospital-based | 2008–2017 | 334 | 21 | Retrospective | Consecutive | Radiological |
| Belgium (BeFas and MiFas) |
Hospital-based multicenter | 2007–2008 | 447 | 49 | Prospective and retrospective | Consecutive | WHO |
| Brazil | Hospital-based | 2008–2012 | 135 | N.A. | Retrospective | Consecutive | WHO |
| Canada—Toronto Sunnybrook Health Sciences Centre |
Hospital-based multicenter | 118 | 16 | Prospective | Consecutive | WHO | |
| Canada—Toronto University Health Network |
Hospital-based | 2011–2018 | 350* | * | Prospective and retrospective | Consecutive | WHO |
| Costa-Rica | Hospital-based | 2012–2018 | 166 | 47 | Prospective and retrospective | Consecutive | WHO |
| Estonia | Hospital-based multicenter | 2003–2012 | * | * | Retrospective | Consecutive | WHO |
| Finland (HYSR) |
Hospital-based | 1994–2007 | 1004 | N.A. | Retrospective | Consecutive | WHO (modified) |
| Finland | Hospital-based | 2000–2010 | N.A. | 330* | Retrospective | Consecutive | Radiological |
| France | Hospital-based | 2006–2010 | 291 | N.A. | Prospective | Consecutive | Radiological |
| Germany | Hospital-based | 2007–2012 2017–2018 |
80 | N.A. | Prospective and retrospective | Consecutive | Radiological |
| India | Hospital-based | 1988–1997 | 206 | 23 | Prospective | Consecutive | WHO |
| Israel | Hospital-based multicenter | 2007–2017 | 326 | N.A. | Prospective and retrospective | Consecutive | WHO |
| Italy (IPSYS) |
Hospital-based multicenter | 2000–2013 | 2147 | N.A. | Prospective | Consecutive | Radiological |
| Japan (FSR) |
Hospital-based multicenter |
2009–2018 | * | * | Prospective and retrospective | Consecutive | WHO |
| Malaysia | Hospital-based | 177 | N.A. | Retrospective | Consecutive | Radiological | |
| Mexico—Mexico City | Hospital-based | 1990–2017 | 1383 | 566* | Prospective | Consecutive | Radiological |
| Mexico—Guadalajara | Hospital-based | 2017–ongoing | 15 | N.A. | Retrospective and prospective | Consecutive | Radiological |
| Mongolia | Hospital-based multicenter |
2012–2017 | * | * | Retrospective | Consecutive | WHO |
| Nigeria/Ghana (SIREN-study) |
Hospital-based multicenter |
245 | 270 | Case–control | Consecutive | Radiological | |
| The Netherlands (FUTURE-study) |
Hospital-based | 1980–2010 | 451 | 69 | Retrospective | Consecutive | WHO |
| New Zealand | Hospital-based | 2004–2009 | 128 | N.A. | Retrospective | Consecutive | WHO |
| Norway (NOR-SYS) |
Hospital-based | 2010–2015 | 149 | N.A. | Prospective | Consecutive | Radiological |
| Portugal | Hospital-based | 2009–2018 | 164 | N.A. | Prospective and retrospective | Consecutive | Radiological |
| Republic of Korea (SKY-study) |
Hospital-based multicenter | 2014–2016 | 166 | N.A. | Prospective | Consecutive | Radiological |
| South Africa | Hospital-based | 2003–2016 | 88 | N.A. | Retrospective | Consecutive | WHO |
| Sweden | Hospital-based | 1998–2007 | 502 | N.A. | Prospective | Consecutive | WHO |
| Switzerland—Bern (SYSS) |
Hospital-based multicenter | 2008–2012 | 399 | N.A. | Prospective | Consecutive | Radiological |
| Switzerland—Luzern | Hospital-based | 2016–2018 | 57* | Retrospective | Consecutive | Radiological | |
| Taiwan | Hospital-based | 1997–2001 | 590 | N.A. | Retrospective | Consecutive | Radiological |
| Turke | Hospital-based | 2017–ongoing | 102 | 6 | Retrospective | Consecutive | WHO |
| UK—Chertsey | Hospital-based | 2015–2018 | 100* | 20* | Retrospective | Consecutive | WHO/radiological |
| UK—Stockton on Tees | Hospital-based | 2013–2018 | 180* | 15* | Prospective and retrospective | Consecutive | WHO/radiological |
| USA—Baltimore–Washington region | Hospital-based multicenter | 1992–2008 | 889 | N.A. | Retrospective | Not consecutive | WHO |
| USA—Tampa | Hospital-based | 381 | 30 | Prospective and retrospective | Consecutive | WHO | |
| UnitedArab Emirates | Hospital-based | 2015–2018 | 174* | 79* | Retrospective | Consecutive | WHO |
Numbers of patients are the result of present received data. Definite numbers could differ from the numbers stated in this table as some cohorts/registers are still ongoing and will be providing additional data concerning newly included patients.
*Data not yet received.
BeFaS, Belgian Fabry Study; FSR, Fukuoka Stroke Registry; FUTURE, Follow-Up of Transient ischaemic attack and stroke patients and Unelucidated Risk factor Evaluation study; HYSR, Helsinki Young Stroke Registry; ICH, intracerebral haemorrhage; IPSYS, Italian Project on Stroke in Young Adults; IS, ischaemic stroke; MiFaS, Middelheim Fabry Study; N.A., not applicable; NOR-SYS, Norwegian Stroke in the Young Study; radiological, radiologically confirmed stroke; SIREN, Stroke Investigative Research and Educational Network; SKY-study, Stroke in Korean Young adults study; SYSS, Swiss Young Stroke Study.
Figure 1.
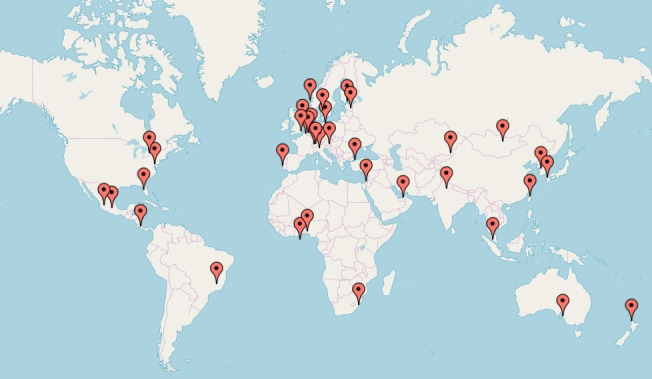
Map with participating countries (April 2019).
bmjopen-2019-031144supp001.pdf (43.4KB, pdf)
Data collection and storage
Participating centres will be requested to transfer their anonymised coded data electronically, according to current laws and legislation concerning research conduct at each participating research centre, to the GOAL-research team of the department of Neurology, Radboudumc, Nijmegen. Co-authors who met the ICMJE criteria are listed as authors. Other contributors can be found in online supplementary appendix 2. Each participating centre will provide their data by using an encrypted excel sheet containing pre-specified variables of interest. The key linking anonymised data to individual patients will remain at the participating centres. All received data will be entered in a uniform database in IBM SPSS Statistics V.22 and stored on secured servers at the co-ordinating study centre, and will only be accessible to designated researchers working under the supervision of the study co-ordinator. All data will be processed, stored and destroyed after end of the study according to European Union General Data Protection Regulation.
bmjopen-2019-031144supp002.pdf (62KB, pdf)
The data will be verified, before entering the data in the uniform database, on data completeness and missing data. In case of missing data or inconsistencies with published articles, the GOAL research team will contact the study investigators to resolve these issues. Data will be stored for at least 15 years.
Patient and public involvement
Still, information about the aetiology and prognosis is uncertain for many young stroke patients. For both young stroke patients and many clinicians treating this patient group, the study questions as described earlier are very relevant and have high priority.
No patients were involved in the design, recruitment or conduction of the study. Study results will be published in peer-reviewed journals and be communicated to country-specific young stroke communities by participating authors.
Sample size and power calculation
Based on our literature search and consequently estimated available patient data, we aim to include at least 10 000 patients, as this size will allow assessing causes of, risk factors for and prognosis of stroke at young age in meaningful subgroups.
Baseline variables
Data for each individual patient is collected from the hospital at index stroke. Baseline data will include demographic characteristics, medical history including medication used on admission and data of diagnostic workup within 1 month after the index stroke (table 2).
Table 2.
Definitions of baseline demographics and risk factors
| Variable or risk factor | Definition |
| Sex | Male/female |
| Age | 18–50 years |
| Ethnicity |
|
| Prior stroke or TIA | Stroke prior to the index stroke is defined according to the same criteria as the index stroke; a rapidly evolving focal neurological deficit, without positive phenomena such as twitches, jerks or myoclonus, with no other than vascular cause, with symptoms persisting for more than 24 hours.9 Stroke will be further divided into intracerebral haemorrhage and ischaemic stroke based on neuroimaging. Haemorrhagic transformation of an ischaemic stroke will be classified as an ischaemic stroke. TIA is defined as a history of an episode of focal cerebral dysfunction lasting <24 hours without evidence of corresponding ischaemic lesion in earlier or present imaging studies. |
| Hypertension | A history of hypertension was defined as its presence either in the patients’ medical history, or when identified during admission for the index event after the acute phase within the first month after stroke. Hypertension was defined as the use of antihypertensive medication and/or systolic blood pressure of 140 mm Hg or greater and/or diastolic blood pressure of 90 mm Hg or greater. |
| DM | A history of DM was defined as its presence either in the patients’ medical history, or when identified during admission for the index event. Diabetes was defined as the use of diabetic medication and/or a fasting (defined as no caloric intake for at least 8 hours) plasma glucose >7 mmol/L and/or 2 h PG ≥11.1 mmol/L during OGTT and/or HbA1C≥6.5% (48 mmol/mol) and/or symptoms of hyperglycaemia or hyperglycaemic crisis and a random glucose >11.1 mmol/L.20 |
| Dyslipidaemia | A history of dyslipidaemia was defined as its presence either in the patients’ medical history, or when identified during admission for the index event. Dyslipidaemia was defined as use of statins and/or cholesterol level ≥5.0 mmol/L (193 mg/dL) and/or low-density lipoprotein level ≥3.0 mmol/L (116 mg/dL) and/or high-density lipoprotein level <1.0 mmol/L (39 mg/dL) and/or triglyceride level ≥1.7 mmol/L (150 mg/dL). |
| AF | A history of AF (chronic/paroxysmal) was defined as its presence either in the patients’ medical history, or when identified during admission for the index event. Atrial fibrillation will be defined as diagnosis based on ECG findings. |
| PFO | A presence of PFO was defined based on documentation in medical records, or when identified during hospitalisation for the index event. PFO will be defined as PFO with or without atrial septum aneurysm, as identified on TTE or TEE with or without contrast. |
| CAD | CAD included myocardial infarction and/or angina pectoris. A history of myocardial infarction or angina pectoris was defined as its presence either in the patients’ medical history, or when identified during admission for the index event. |
| Heart failure | A history of heart failure was defined as its presence either in the patients’ medical history, or when identified during admission for the index event. Heart failure was defined as ejection fraction <55%, reported on echocardiogram. |
| PAD | A history of PAD was defined as its presence either in the patients’ medical history, or when identified during admission for the index event. |
| Obesity | Obesity was defined as a body mass index greater than 30 kg/m2, measured during admission for the index event or when reported by the patient. |
| Migraine | A history of migraine was defined as its presence either in the patients’ medical history or when identified during hospitalisation for the index event. Migraine was defined according to the International Headache Society criteria.21 |
| Hormone replacement therapy | Use of oral or non-oral hormone replacement therapy at admission for the index event. |
| Oral contraceptives | Use of oral contraceptive pills at time of stroke onset. |
| Recent or acute infection | Signs or laboratory findings indicative of infection at admission or reported symptoms of any infectious disease during the month prior to stroke, or as concluded by institution of cohort. |
| Ever smoking | Any current or former smoker. |
| Heavy drinking | Heavy drinking was defined as the consumption of more than 21 units a week for men and 14 units a week for women, identified at admission for the index event. |
| Illicit recent drug use | Within the month prior to stroke. |
| Family history of stroke | History of ischaemic/haemorrhagic stroke or TIA in a first-degree relative. |
| Index stroke related to pregnancy |
|
| Pregnancy-related complications during any pregnancy |
|
AF, atrial fibrillation; CAD, coronary artery disease; DM, diabetes mellitus; HbA1c, glycosylated haemoglobin, type A1c; OGTT, oral glucose tolerance test;PAD, peripheral artery disease; PFO, patent foramen ovale;2 h PG, 2 hours post glucose; HELLP syndrome, haemolysis elevated liver enzymes and low platelets syndrome; TEE, transoesophageal echocardiogram;TIA, transient ischaemic attack; TTE, transthoracic echocardiogram.
In addition, the season of the index event and climate of the country or region of origin will be registered. The climate will be classified according to the Köppen-Geiger climate classification system,10 and the season of index stroke according to the date of admission (table 3).
Table 3.
Classification of climates and seasons
| Climate of country of origin/city of study hospital |
Köppen-Geiger climate classification system A (Tropical)
B (Arid)
C (Temperate)
D (Continental)
E (Polar)
|
| Season of index stroke | Northern hemisphere countries (Austria, Belgium, Canada, Estonia, Finland, France, Germany, Ghana, India, Israel, Italy, Japan, Mexico, Mongolia, The Netherlands, Nigeria, Norway, Portugal, Republic of Korea, Sweden, Switzerland, Taiwan, Turkey, UK, USA, United
Arab Emirates) :
Southern hemisphere countries (Australia, Brazil, Costa Rica, New Zealand, South Africa):
|
The severity of stroke is assessed with the National Institutes of Health Stroke Scale (NIHSS) score,11 and the functional performance with modified Ranking Scale (mRS)12 right after a stroke. The mRS will also be assessed during follow-up, preferably at 3 months and when available later during follow-up.
Secondary prevention at admission is categorised as antihypertensive medication, HMG-CoA (β-Hydroxy β-methylglutaryl-CoA) reductase inhibitors (statins) or other cholesterol-lowering medication, platelet aggregation inhibitors (antiplatelets), oral anticoagulants (vitamin K antagonists or direct oral anticoagulants).
Death will be analysed based on occurrence within 30 days (case fatality) or thereafter.
Outcomes at baseline
Outcomes will include: cause of stroke, both individually assessed and according to the trial of ORGg 10172 in Acute Stroke Treatment (TOAST) criteria13 for ischaemic stroke and SMASH-U14 for ICH, stratified for age, sex, ethnicity and climate. Other outcomes will include frequency and distribution of vascular risk factors, stroke severity at baseline by using the NIHSS, functional neurological outcome at baseline by using the mRS, case fatality and the use of secondary prevention at baseline. Considering that not all patients have had an imaging-confirmed stroke, subgroup analyses will be done in patients with and without imaging-confirmed stroke when analysing the above-mentioned outcomes.
Risk factors
Cardiovascular risk factors include conventional risk factors, as described according to the 2014 guidelines of the American Stroke Association15: hypertension, diabetes, atrial fibrillation (AF), dyslipidaemia and cigarette smoking. Additionally, we will include the following other vascular risk factors and diseases: a history of cardiovascular diseases, patent foramen ovale, heavy drinking, illicit recent drug use, obesity, hormone replacement therapy and recent or acute infection. Previous cardiovascular diseases will include prior stroke or transientischaemic attack (TIA), ischaemic heart disease, heart failure and peripheral artery disease (PAD). Table 2 summarises the collected vascular risk factors and their definitions/operationalisation.
Causes
Ischaemic stroke: Causes of stroke are defined according to the TOAST classification.13 Causes of stroke defined according to Causative Classification System of ischaemic stroke16 and ASCO classification17 will, when available, also be collected. The participating centres will also be requested to state, if known, the precise cause of the stroke (eg, dissection, vasculitis, haematological disorder, cardiac condition).
Intracerebral haemorrhage: Location of ICH (lobar/deep, supratentorial/infratentorial) will be collected. Also the ICH volume, calculated by using the ABC/2 method from the axial CT images,18 will be collected. The cause of intracerebral haemorrhage will be preferably defined according to the SMASH-U14. Aetiology as defined by the H-ATOMIC classification will, when available, also be collected.14 The precise cause of ICH (as possibly identified with neuroimaging of the intracranial vasculature) will also be requested.
Outcome during follow-up
Follow-up evaluation of use of secondary prevention, recurrence of vascular events and death has been collected differently across the various studies: either in person, by telephone interviews with patients or relatives, by collecting hospital/general practitioner medical records or discharge diagnosis ICD codes.
The following cardiovascular risk factors will be collected at follow-up, whenever available, according to the same definitions described at baseline and in table 2: hypertension, diabetes mellitus, dyslipidaemia, cigarette smoking, obesity, AF, heavy drinking and illicit drug use.
Functional performance will be assessed with the mRS score and data on the use of secondary prevention medication will be collected from the medical records files.
Outcomes will include recurrent vascular events and all-cause death. Recurrent vascular events are defined as occurrence of any of the following events: TIA (defined as in table 2), ischaemic stroke and ICH (defined similarly as baseline events) and other vascular ischaemic events, when available. Other vascular ischaemic events of interest include angina pectoris, myocardial infarction (defined by symptoms of cardiac ischaemia with electrocardiographic changes corresponding to myocardial necrosis with or without cardiac biomarker elevation or pathological evidence of infarction according to the universal definition of myocardial infarction,19 peripheral artery disease including revascularisation procedures (coronary artery bypass grafting, percutaneous transluminal coronary angioplasty, carotid endarterectomy or other peripheral arterial revascularisation procedures). All outcomes had to be confirmed by physicians from the appropriate specialty through medical records, or through the appropriate ICD code.
Statistical analysis
We aim to perform statistical analyses with IBM SPSS Statistics or R (most recent versions). A p value of<0.05 with correction for multiple testing when appropriate will be considered statistically significant, and/or a 95% CI not containing 1. Subanalysis to identify differences in demographic characteristics including gender, age, ethnicity, pregnancy-related stroke, climate and season at time of admission will be performed.
Differences between groups will be compared using ANOVA test, Student’s t-test for continuous variables and χ2 test for categorical variables. Univariate multivariable logistic regression analysis will be performed to determine risk factors and potential trigger factors. In case of missing data at baseline, and if considered necessary, multiple imputation will be used. The cumulative risk of death and vascular events will be calculated with Kaplan–Meier survival analysis. Differences in survival between different subgroups will be assessed using Log-Rank tests. In the analysis for vascular events, patients who have died or were lost to follow-up will be censored from the last available follow-up. We will use Cox regression models to obtain HRs and their corresponding 95% CIs to calculate the risk of death and recurrent vascular events between different aetiologies and demographic characteristics while adjusting for confounders. Only patients for whom follow-up data is available will be included for these separate analyses.
Discussion
The GOAL initiative aims to investigate the causes and risk factors of ischaemic stroke and ICH in young patients, aged 18 to 50 years, to determine functional outcome, and the risk of new or recurrence of vascular events, and to study variation according to aetiological subgroups, geographical region, continent and ethnicity. The most important strength of our study is the participation of to date 30 stroke centres throughout the world, from 29 different countries across all continents (figure 1). By combining existing individual data sets, the GOAL initiative will be the largest study on young patients with stroke ever, with more than 10 000 patients already included. This large set of individual patient data provides sufficient statistical power to not only reliably quantify the differences in risk factors and aetiology of stroke between men and women, different age groups, ethnic subgroups and possibly search for differences between climates of residence, but also assess the risk of recurrent vascular events.
The study has also its limitations. There will be a risk of misclassification of aetiology, risk factors and events of interest during follow-up, as we will make use of already existing data that have been collected according to various local protocols that will not be completely identical. We will harmonise variables across studies as much as possible. There may also be a risk of missing data, as we have included studies that were designed prior to the GOAL initiative, and therefore did not include all variables of interest. Furthermore, our study will cover a long time period in which data collection took place. This may lead to variability in the diagnostic workup and differences in brain imaging protocols due to adjusted guidelines and improvements in imaging techniques. For instance, not all patients will have undergone complete cardiac examination (eg, both transthoracic and transoesophageal echocardiography, prolonged ECG), which may lead to an underestimation of the frequency of cardio-embolic strokes. Moreover, included cohorts are mainly hospital-based. In addition, not all cohorts will have had the same inclusion criteria, which may lead to an underestimation of case fatality. Finally, imaging-based confirmation of the stroke was neither mandatory nor available in all cohorts, although very few patients were diagnosed with stroke based on clinical symptoms alone, and all patients included were identified and treated as strokes by their main physicians.
In conclusion, the GOAL initiative will include data of at least 10 000 patients with a stroke at young age from six continents, providing sufficient patient numbers to allow for individual patient data meta-analysis. The size of this study will allow for detailed description of the global distribution of causes and risk factors, and for the quantification of the cumulative risk of outcomes.
The GOAL initiative explicitly reaches out to other researchers and aims to become a platform that facilitates future collaborative research in the area of stroke at young age. We envision enriching the cohort with genetic and imaging data and long-term outcomes.
Supplementary Material
Acknowledgments
We would like to thank all patients who originally signed informed consent and thank all physicians of participating centres who helped during the original data collection (online supplementary appendix 2).
Footnotes
Twitter: @teddyyhwu
MAJ and MMvD contributed equally.
Correction notice: This article has been corrected since it was published. Author initials and affiliations have been updated.
Contributors: All authors made a substantial contribution to the concept and design of the work; acquisition, analysis and interpretation of data. ME and FEdL designed the study protocol, ME, MJ, MvD, and FEdL drafted the article. KA, AA, AA, MA, MB, MB, RFRB, BC, EC, BD, SD, AD, CE, EE, SF-H, FF, AF, TG, GG, MH, TH, CJ, KJ, MK, YSK, TK, SK, TK, KK, JK, T-HL, DL, NM, NM-M, JPM, MM, VM, VM, MOO, VP, MP, BP-I, AP, JLR-S, BS, FS, RS, KST, DT, TT, VT, AT, MV-B, RV, TW, NY, UW-A, AP and JP revised the article critically for important intellectual content and approved the version to be published. All authors take public responsibility for appropriate portions of the content. FEdL is the guarantor of the study protocol.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Map disclaimer: The depiction of boundaries on the map(s) in this article does not imply the expression of any opinion whatsoever on the part of BMJ (or any member of its group) concerning the legal status of any country, territory, jurisdiction or area or of its authorities. The map(s) are provided without warranty of any kind, either express or implied.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The study will be conducted following the principles of the Declaration of Helsinki (version 60, 19 October 2013) and in accordance with the Law for Human Research. The Medical Review Ethics Committee region Arnhem-Nijmegen approved the study protocol. When necessary, national or local institutional review boards reviewed the study protocol and approved data transfer.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Barker-Collo S, Bennett DA, Krishnamurthi RV, et al. . Sex differences in stroke incidence, prevalence, mortality and disability-adjusted life years: results from the global burden of disease study 2013. Neuroepidemiology 2015;45:203–14. 10.1159/000441103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. . Global and regional burden of stroke during 1990–2010: findings from the global burden of disease study 2010. The Lancet 2014;383:245–55. 10.1016/S0140-6736(13)61953-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feigin VL, Krishnamurthi RV, Parmar P, et al. . Update on the global burden of ischemic and hemorrhagic stroke in 1990–2013: the GBD 2013 study. Neuroepidemiology 2015;45:161–76. 10.1159/000441085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffiths D, Sturm J. Epidemiology and etiology of young stroke. Stroke Res Treat 2011;2011:1–9. 10.4061/2011/209370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maaijwee NAMM, Rutten-Jacobs LCA, Schaapsmeerders P, et al. . Ischaemic stroke in young adults: risk factors and long-term consequences. Nat Rev Neurol 2014;10:315–25. 10.1038/nrneurol.2014.72 [DOI] [PubMed] [Google Scholar]
- 6.Putaala J. Ischemic stroke in the young: current perspectives on incidence, risk factors, and cardiovascular prognosis. Eur Stroke J 2016;1:28–40. 10.1177/2396987316629860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferro JM, Massaro AR, Mas J-L. Aetiological diagnosis of ischaemic stroke in young adults. Lancet Neurol 2010;9:1085–96. 10.1016/S1474-4422(10)70251-9 [DOI] [PubMed] [Google Scholar]
- 8.Ekker MS, Boot EM, Singhal AB, et al. . Epidemiology, aetiology, and management of ischaemic stroke in young adults. Lancet Neurol 2018;17:790–801. 10.1016/S1474-4422(18)30233-3 [DOI] [PubMed] [Google Scholar]
- 9.Aho K, Harmsen P, Hatano S, et al. . Cerebrovascular disease in the community: results of a WHO Collaborative study. Bull World Health Organ 1980;58:113–30. [PMC free article] [PubMed] [Google Scholar]
- 10.Peel MC, Finlayson BL, McMahon TA. Updated world map of the Köppen-Geiger climate classification. Hydrol Earth Syst Sci 2007. [Google Scholar]
- 11.Brott T, Adams HP, Olinger CP, et al. . Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864–70. 10.1161/01.STR.20.7.864 [DOI] [PubMed] [Google Scholar]
- 12.van Swieten JC, Koudstaal PJ, Visser MC, et al. . Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–7. 10.1161/01.STR.19.5.604 [DOI] [PubMed] [Google Scholar]
- 13.Adams HP, Bendixen BH, Kappelle LJ, et al. . Classification of subtype of acute ischemic stroke. definitions for use in a multicenter clinical trial. TOAST trial of ORG 10172 in acute stroke treatment. Stroke 1993;24:35–41. 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 14.Martí-Fàbregas J, Prats-Sánchez L, Guisado-Alonso D, et al. . SMASH-U versus H-ATOMIC: a head-to-head comparison for the etiologic classification of intracerebral hemorrhage. J Stroke Cerebrovasc Dis 2018;27:2375–80. 10.1016/j.jstrokecerebrovasdis.2018.04.026 [DOI] [PubMed] [Google Scholar]
- 15.Kernan WN, Ovbiagele B, Black HR, et al. . Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2160–236. 10.1161/STR.0000000000000024 [DOI] [PubMed] [Google Scholar]
- 16.Ay H, Furie KL, Singhal A, et al. . An evidence-based causative classification system for acute ischemic stroke. Ann Neurol 2005;58:688–97. 10.1002/ana.20617 [DOI] [PubMed] [Google Scholar]
- 17.Amarenco P, Bogousslavsky J, Caplan LR, et al. . New approach to stroke subtyping: the A-S-C-O (phenotypic) classification of stroke. Cerebrovasc Dis 2009;27:502–8. 10.1159/000210433 [DOI] [PubMed] [Google Scholar]
- 18.Kothari RU, Brott T, Broderick JP, et al. . The ABCs of measuring intracerebral hemorrhage volumes. Stroke 1996;27:1304–5. 10.1161/01.STR.27.8.1304 [DOI] [PubMed] [Google Scholar]
- 19.Thygesen K, Alpert JS, White HD, et al. . Universal definition of myocardial infarction. Circulation 2007;116:2634–53. 10.1161/CIRCULATIONAHA.107.187397 [DOI] [PubMed] [Google Scholar]
- 20.Fox CS, Golden SH, Anderson C, et al. . Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American heart association and the American diabetes association. Diabetes Care 2015;38:1777–803. 10.2337/dci15-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Headache Classification Subcommittee of the International Headache Society The International classification of headache disorders: 2nd edition. Cephalalgia 2004;24 Suppl 1:9–160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-031144supp001.pdf (43.4KB, pdf)
bmjopen-2019-031144supp002.pdf (62KB, pdf)


